Abstract
We previously demonstrated that replication-competent adenovirus (Ad)-simian immunodeficiency virus (SIV) recombinant prime/protein boost regimens elicit potent immunogenicity and strong, durable protection of rhesus macaques against SIVmac251. Additionally, native Tat vaccines have conferred strong protection against simian/human immunodeficiency virus SHIV89.6P challenge of cynomolgus monkeys, while native, inactivated, or vectored Tat vaccines have failed to elicit similar protective efficacy in rhesus macaques. Here we asked if priming rhesus macaques with replicating Ad-human immunodeficiency virus (HIV) tat and boosting with the Tat protein would elicit protection against SHIV89.6P. We also evaluated a Tat/Env regimen, adding an Ad-HIV env recombinant and envelope protein boost to test whether envelope antibodies would augment acute-phase protection. Further, expecting cellular immunity to enhance chronic viremia control, we tested a multigenic group: Ad-HIV tat, -HIV env, -SIV gag, and -SIV nef recombinants and Tat, Env, and Nef proteins. All regimens were immunogenic. A hierarchy was observed in enzyme-linked immunospot responses (with the strongest response for Env, followed by Gag, followed by Nef, followed by Tat) and antibody titers (with the highest titer for Env, followed by Tat, followed by Nef, followed by Gag). Following intravenous SHIV89.6P challenge, all macaques became infected. Compared to controls, no protection was seen in the Tat-only group, confirming previous reports for rhesus macaques. However, the multigenic group blunted acute viremia by approximately 1 log (P = 0.017), and both the multigenic and Tat/Env groups reduced chronic viremia by 3 and 4 logs, respectively, compared to controls (multigenic, P = 0.0003; Tat/Env, P < 0.0001). The strikingly greater reduction in the Tat/Env group than in the multigenic group (P = 0.014) was correlated with Tat and Env binding antibodies. Since prechallenge anti-Env antibodies lacked SHIV89.6P-neutralizing activity, other functional anti-Env and anti-Tat activities are under investigation, as is a possible synergy between the Tat and Env immunogens.
AIDS vaccines have been under development for more than 20 years, yet an efficacious vaccine remains elusive (13). Since attenuated or inactivated human immunodeficiency virus (HIV) vaccines lack the requisite safety for human use, alternative strategies have focused on viral subunits as vaccine candidates. HIV Tat, the transactivator protein essential for viral infectivity and pathogenesis, is a logical choice for AIDS vaccine design. Tat is expressed early in the viral life cycle; consequently, Tat-specific immune responses elicited by prophylactic vaccines can potentially have a critical impact on HIV transmission and replication. Although Tat exhibits variability among HIV clades, key immunogenic and functional domains appear to be conserved (7, 40). In fact, cross-reactivity of anti-Tat antibodies in sera of patients from multiple clades has been reported (7). Further, conformational antibodies elicited by the full-length Tat protein as an immunogen have shown reactivity against nonhomologous Tat variants (39).
Tat may also serve therapeutic vaccine strategies. Tat is released by HIV-infected cells and taken up by bystander cells, where it is translocated to the nucleus (15). This extracellular Tat exhibits multiple functions contributing to immune suppression and pathogenesis (see reference 45 for a review). Among critical properties are modulation of expression of cellular genes, including transcription factors and cytokines, up-regulation of CCR5 and CXCR4 expression (24), and induction of apoptosis in T cells and macrophages (12, 28). Tat bound to cell surfaces has also been shown to enhance the infectivity of HIV and promote rapid spreading of the virus by interacting with gp120 (33). Anti-Tat antibody could inhibit this extracellular spread and help control effects on bystander cells. Paradoxically, Tat has recently been shown to exert an antiapoptotic effect on infected cells by modifying the expression of several cytoskeletal proteins (11). This may promote long-term survival of HIV-infected CD4+ T cells, turning them into reservoirs for continuous viral production. Cellular immune responses to Tat and other viral antigens could help eliminate such reservoirs.
Tat also influences the immune system and acts as an adjuvant. The Tat protein is known to alter major histocompatibility complex (MHC) class I expression on the cell surface (26) and helps facilitate MHC class I presentation of antigens (16, 38) by modifying the immunoproteasome (18, 47). Tat enhances cellular immune responses to coadministered antigens (59) and exhibits autoadjuvanticity by eliciting antibody responses in the absence of an exogenous adjuvant (25). Thus, Tat should be a potent immunogen. In fact, both Tat vaccines and native Tat expressed during HIV infection are immunogenic, and the immune responses elicited appear to contribute to protection. Both anti-Tat antibodies and Tat-specific cytotoxic T lymphocytes have been associated with slow progression to AIDS in infected individuals (46, 48, 53, 56), and Tat-specific cytotoxic T lymphocytes have been associated with control of viral replication during the acute phase of simian immunodeficiency virus (SIV) infection (2). Notably, phase I clinical trials of a native Tat vaccine have established safety and immunogenicity for both uninfected and HIV-infected volunteers, including development of Tat antibodies in all subjects and Tat-specific cellular immune responses in more than 80% of the volunteers (14, 14a).
In support of targeting Tat as a vaccine immunogen, Tat-based vaccines, both the native protein and plasmid DNA bearing tat, have shown long-term protective efficacy against simian/human immunodeficiency virus (SHIV) SHIV89.6P challenge in a cynomolgus monkey model (8, 9, 31). Surprisingly, however, the inactivated or native Tat protein did not protect rhesus macaques against the same virus challenge administered intrarectally or intravenously (43, 51). Similarly, vaccination with Tat peptides resulted in only one of seven rhesus macaques being protected against intrarectal SHIVBX08 challenge (3), and a replication-defective adenovirus (Ad) type 5 (Ad5)-HIV tat vaccine was ineffective in protecting rhesus macaques against intravenously administered SHIV89.6P (30). However, the combination of Tat with other regulatory and/or structural gene products in multigenic vaccines has improved protective efficacy against both SHIV and SIV challenges in the rhesus macaque system (10, 21). The contribution of each vaccine component has yet to be clarified.
We have pursued a vaccine approach based on replication-competent Ad recombinants with the rationale that the replicating vector will elicit more-persistent immune responses and at the same time induce mucosal immunity by allowing the vectored vaccine to reach its target epithelial cells at mucosal inductive sites. In fact, we have shown that in chimpanzees, Ad-HIV env/rev recombinant priming followed by boosting with the oligomeric gp140ΔV2 protein elicits better cellular immunity and primes higher-titer antibody responses, including antibodies able to neutralize primary isolates and exert antibody-dependent cellular cytotoxicity (ADCC) activity across HIV clades (19, 44). Further, a multigenic Ad-SIV recombinant priming envelope subunit boosting approach elicited potent protection in 39% of vaccinated macaques against the virulent SIVmac251 virus (42). Durable protection in 73% of these protected animals was subsequently demonstrated (32). These results have provided the basis for moving the replication-competent Ad-HIV recombinant approach into phase I human trials.
In this study we addressed whether priming with a replicating Ad-HIV tat recombinant vaccine followed by boosting with the Tat protein would lead to improved protection against SHIV89.6P challenge in the rhesus macaque model. In addition, we evaluated a Tat/Env combined prime/boost regimen, with the rationale that induction of antibodies against the early Tat protein as well as antienvelope antibodies would lead to better control of acute-phase viremia. Finally we included a multigenic immunization group including Tat, Env, Gag, and Nef immunogens in order to elicit broad cellular immunity for control of chronic as well as acute-phase viremia.
MATERIALS AND METHODS
Immunogens.
Plasmid VRC5201 pVR1012X/s 89.6Pgp140(del F/CL del H IS/h), containing the HIV89.6P gp140 gene with codons optimized for expression in human cells and with the fusion and cleavage domains and the interspace between heptads 1 and 2 deleted, has been described previously (27) and was kindly provided by Gary Nabel, Vaccine Research Center, NIAID, NIH. Construction of a replication-competent Ad5 host-range mutant (Ad5hr) recombinant containing this gene, abbreviated Ad5hr-HIV89.6Pgp140ΔCFI, will be described separately (T. Demberg et al., unpublished data). Additional replication-competent Ad5hr recombinants used encoded wild-type HIVIIIB tat (59), SIV239 gag (58), or SIV239 nefΔ1-13 (41). The empty Ad5hrΔE3 vector served as a control. All recombinants were administered in phosphate-buffered saline (PBS). Protein boosts included native HIVIIIB Tat (Advanced BioScience Laboratories, Inc., Kensington, MD) reconstituted at 4 μg/μl in degassed PBS containing 0.1% bovine serum albumin and mixed with 1/10-volume alum just before use, the SHIV89.6P gp140ΔCFI protein (Demberg et al., unpublished), and SIV239 Nef (Advanced BioScience Laboratories, Inc.). The last proteins were both prepared with a 1/10-volume monophosphoryl lipid A stable emulsion (MPL-SE) (Corixa, Hamilton, MT). The envelope protein was administered together with 50 μg/dose of MPL-SE and the Nef protein with 25 μg/dose MPL-SE.
Immunization and challenge of macaques.
The 27 Mamu A*01-negative juvenile Indian rhesus macaques used in this study were housed at the Washington National Primate Research Center (Seattle, WA). Two of the 27 animals were B*17 positive, one in the Ad Tat group and the other in the Ad Tat/Env group. The care and maintenance of the animals were in compliance with established regulations. The animal protocol received approval from the Washington National Primate Research Center Animal Care and Use Committee prior to study initiation. The immunization schedule, vaccine dosages, and routes of administration are outlined in Table 1. Peripheral blood samples were obtained prior to immunization and 2 and 10 weeks following each immunization for evaluation of immune responses. At week 50, the macaques were challenged intravenously with 30 50% monkey infective doses of a rhesus peripheral blood mononuclear cell (PBMC)-grown SHIV89.6P stock, kindly provided by Norman L. Letvin and Keith Reimann, Beth Israel Deaconess Medical Center, Harvard Medical School.
TABLE 1.
Immunization and challenge regimen
| Immunization group | Macaques | Priming immunogensa (wk 0, i.n.; wk 12, i.t.) | Booster immunogensb (wk 24 and 36) | Challengec (wk 50, i.v.) |
|---|---|---|---|---|
| I (Ad Tat) | 4 female, 4 male | Ad5hr-HIVIIIBtat | HIVIIIB Tat | SHIV89.6P |
| II (Ad Tat/Env) | 4 female, 4 male | Ad5hr-HIVIIIBtat + Ad5hr-HIV89.6Pgp140ΔCFI | HIVIIIB Tat + HIV89.6P gp140ΔCFI | SHIV89.6P |
| III (Ad Tat/Env/Gag/Nef) | 5 female, 3 male | Ad5hr-HIVIIIBtat + Ad5hr-HIV89.6Pgp140ΔCFI + Ad5hr-SIV239gag + Ad5hr-SIV239nefΔ1-13 | HIVIIIB Tat + HIV89.6P gp140ΔCFI + SIV239 Nef | SHIV89.6P |
| IV (Controls) | 2 female, 1 male | Ad5hrΔE3 vector | MPL-SE and Alum | SHIV89.6P |
Recombinant dose: 5 × 108 PFU each, made up to a total Ad5hr dose of 2 × 109 PFU with Ad5hrΔE3 vector as necessary. i.n., intranasal; i.t., intratracheal.
HIVIIIB Tat, 10 μg administered subcutaneously in alum; HIV89.6P gp140ΔCFI protein, 100 μg administered intramuscularly in MPL-SE; SIV239 Nef, 20 μg administered intramuscularly in MPL-SE.
SHIV89.6P, 30 50% monkey infective doses administered intravenously (i.v.) in PBS.
Cellular immune responses.
Enzyme-linked immunospot (ELISPOT) assays were used to evaluate virus-specific gamma interferon (IFN-γ)-secreting cells. PBMC were isolated from peripheral blood by Ficoll gradient centrifugation and used fresh in all assays. For assessment of Tat-specific IFN-γ-secreting cells, 23 15-mer HIV clade B consensus Tat peptides with an 11-amino-acid overlap were used (AIDS Research and Reference Reagent Program, NIAID, NIH). PBMC (100 μl at dilutions of 1 × 106 and 5 × 105 cells/ml) were distributed in triplicate wells of 96-well plates and stimulated overnight by adding a single pool of Tat peptides to give a final concentration of 1 μg/ml for each peptide. Concanavalin A (5 μg/ml) and R10 medium (RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 1 mM l-glutamine) were positive and negative controls, respectively. ELISPOT responses were detected using the monkey IFN-γ ELISPOT kit from U-CyTech Biosciences (Utrecht, The Netherlands) according to the manufacturer's protocol following transfer of the stimulated cells into the coated plates, except that the incubation time was shortened to 5 h and KPL wash buffer (Kirkegaard and Perry Labs, Gaithersburg, MD) was used. Spots were counted, and spots in control wells were subtracted. Results are reported as spot-forming cells (SFC)/million PBMC. A positive Tat-specific response was defined as greater than 10 spots above the mean SFC plus 2 standard deviations exhibited by control macaques at each time point.
The procedure used in assessing ELISPOT responses to SIV Gag, SIV Nef, and SHIV89.6P Env peptides has been described previously (50). SIV Gag and Nef peptides were 15-mers overlapping by 11 amino acids. SHIV89.6P Env peptides were 20-mers, overlapping by 10. All three peptide sets were obtained from the AIDS Research and Reference Reagent Program, NIAID, NIH. Phytohemagglutinin-M (5 μg/ml) was used as the positive control. Results were calculated as described above after eliminating any values in which the background spots with medium alone were ≥125. A positive response was defined as greater than 20 SFC above the control mean plus 2 standard deviations. For evaluating IFN-γ-secreting cells specific for SHIV89.6P Env, the peptides were divided into two pools for stimulation of PBMC. Subsequently, background spots were subtracted from each pool and the sum of net SFC are reported.
Lymphoproliferative responses were evaluated with fresh PBMC. The cells (3 × 106/ml) were distributed in 100-μl aliquots into triplicate wells of a 96-flat-well plate (NUNC) and stimulated by adding 100 μl of R10 medium containing 4 μg SHIV89.6P gp140, SIV p27, or SIV Nef or 1 μg of HIVIIIB Tat, oxidized by exposure to light at room temperature (59). A further stimulus was aldrithiol-2-inactivated SHIV89.6P (Ald-SHIV) (4 μg), kindly provided by Jeffrey Lifson and Julian Bess (NCI-Frederick, Frederick, MD). PBMC were incubated at 37°C in 5% CO2 for 5 days. On the fifth day, the cells were pulsed with [3H]thymidine (1 μCi/well) and incubated overnight at 37°C. Cells were harvested the next day using a Perkin-Elmer filter mat harvester. The filter mats were sealed and thymidine incorporation was measured using a Perkin-Elmer MicroBeta TriLux beta counter. The stimulation index (SI) was determined by dividing the mean of experimental counts per minute by the mean counts per minute of the respective negative control. A positive SI was defined as greater than the mean SI plus 2 standard deviations of the control macaques at individual time points with a value of at least 2.0.
Intracellular cytokine staining.
Intracellular staining was performed at weeks 0, 14, and 38 with freshly isolated PBMC in order to detect HIV Tat-, SHIV89.6P Env-, SIV Nef-, and SIV Gag-specific IFN-γ-secreting CD8+ and CD4+ central and effector memory T cells. PBMC (1 × 106) in 1 ml R10 medium were stimulated with pools of Env, Nef, Gag, or Tat peptides (1 μg/ml each peptide) for 6 h at 37°C and 5% CO2. Unstimulated PBMC served as the negative control and concanavalin A-stimulated PBMC as the positive control. One hour into the incubation, 4 μl of Golgi-Stop (BD Pharmingen) was added to all tubes. Following stimulation, the cells were transferred into fluorescence-activated cell sorting tubes (BD) and washed twice with PBS (Invitrogen). A cocktail of the following surface antibodies was added: CD4-PerCP (clone L200; BD Pharmingen), CD95-APC (clone DX2; BD Pharmingen), and CD28-fluorescein isothiocyanate (clone CD28.2; BD Pharmingen) or CD8β-phycoerythrin (PE) (clone 2ST8.5H7; Beckmann-Coulter) together with CD95-PE-Cy5 and CD28-fluorescein isothiocyanate, each in the amount recommended by the manufacturer. The cells were incubated in the dark for 25 min at room temperature, washed with PBS, and fixed in 125 μl of Fix and Perm solution A (Invitrogen) for 15 min. After further washing, the cells were incubated in 125 μl Fix and Perm solution B containing 5 μl of the anti-IFN-γ-allophycocyanin (CD8 staining) or the anti-IFN-γ-PE (CD4 staining)-coupled antibody (clone B27; BD Pharmingen) for 25 min in the dark at room temperature. The cells were washed in PBS and stored in PBS containing 1% paraformaldehyde at 4°C. Analysis was performed on a BD FACScalibur using Cellquest software. A minimum of 100,000 events were acquired. A positive response was defined as an increase in the IFN-γ-positive percentage in stimulated PBMC over that in unstimulated PBMC that was significant at the two-tailed α = 0.05 level by the continuity-adjusted chi-square test. The response comparison was excluded from the analysis if the harmonic mean of the gated central or effector memory event numbers in the comparison was less than 300, due to the substantial loss of power for detecting a response.
Humoral immune responses.
Binding antibodies to HIV Tat, SHIV gp140, SIV p27, and SIV Nef were assessed by enzyme-linked immunosorbent assay as described previously (6, 7). The titer of antibody was defined as the reciprocal of the serum dilution at which the optical density of the test serum was two times greater than that of the negative control serum diluted 1:50.
Neutralizing antibodies were assessed using the TZM-bl assay and PBMC-grown SHIV89.6P as described elsewhere (29). Neutralizing titers are expressed as the reciprocal of the serum dilution at which relative luminescence units were reduced 50% compared to results for virus control wells (no test sample).
Virologic assays.
The nucleic acid sequence-based amplification technique (NASBA) was used to determine viral loads in plasma as described previously (49). The standard sensitivity threshold of this enhanced chemiluminescence-based assay is <2,000 viral RNA copies/input volume of plasma. Where necessary, a real-time NASBA assay with a sensitivity of <50 copies/input volume was used as described previously (32) to evaluate plasma from macaques that were consistently negative by the enhanced chemiluminescence-based assay.
Statistical analyses.
Differences in peak acute viremia levels and median chronic viral loads over weeks 6 through 24 were analyzed using one-way analysis of variance in combination with Dunnett's test. CD4 counts were square root transformed and similarly analyzed at individual times, with maximum P values reported. For the comparison of antibody titers, the two-tailed exact Wilcoxon rank sum test was used, as well as the Kruskal-Wallis test for simultaneous comparison of the three immunization groups and the exact Wei-Johnson test for two-group comparisons over multiple time points.
RESULTS
Prechallenge ELISPOT responses.
The Ad-HIV tat recombinant was immunogenic, as shown by positive responses in 62.5 to 87.5% of macaques in the three immunization groups (Table 2). Overall, however, the number of Tat-specific IFN-γ-secreting cells induced was low, as was the frequency of positive responses. At the eight prechallenge time points tested, only 17 to 25% of the immunized macaques exhibited positive responses to Tat, regardless of immunization group.
TABLE 2.
Prechallenge ELISPOT responsesa
| Macaque group | Response to antigen
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tat
|
Nef
|
Gag
|
Env
|
|||||||||
| Peak (mean) | Responders (%) | Frequency (mean %) | Peak (mean) | Responders (%) | Frequency (mean %) | Peak (mean) | Responders (%) | Frequency (mean %) | Peak (mean) | Responders (%) | Frequency (mean %) | |
| I (Ad Tat) | 124 | 87.5 | 25 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| II (Ad Tat/Env) | 28 | 62.5 | 20 | NA | NA | NA | NA | NA | NA | 1,005 | 100 | 56 |
| III (Ad Tat/Env/Gag/Nef) | 45 | 75 | 17 | 115 | 100 | 23 | 380 | 100 | 53 | 1,946 | 100 | 58 |
Peak, mean of peak positive responses, weeks 0 to 50; responders, percentage of macaques exhibiting a positive response, weeks 0 to 50; frequency, mean of frequency of positive responses for each macaque over the eight time points evaluated, weeks 0 to 50; NA, not applicable.
The Ad5hr-SIV nefΔ1-13 recombinant elicited positive responses for 100% of macaques immunized (group III) (Table 2), although the number of SFC was also low, and the frequency of positive responses was comparable to that seen for Ad5hr-HIV tat. The cellular immune response to SIV Gag indicated that the Ad5hr-SIV gag recombinant was equally immunogenic, with 100% of macaques responding. In contrast, however, the Gag response was stronger than the Nef response, both in number of SFC and in frequency of responses, with an increase to 53% in the group of macaques immunized with the multigenic Ad recombinants.
Macaques in groups II and III primed with Ad5hr-HIV89.6Pgp140ΔCFI and boosted with the homologous protein exhibited the highest peak ELISPOT responses (Table 2). All immunized macaques were positive responders, and the frequency of positive responses was approximately 50% in both immunization groups.
Prechallenge proliferative responses.
As illustrated in Table 3, the three immunization regimens elicited strong proliferative responses against the immunizing antigens. The Tat immunogens elicited SI values somewhat lower than responses against the p27, Nef, and gp140ΔCFI proteins, but the percentage of responders was similar, as was the frequency of positive responses. The highest SI were observed against Ald-SHIV in the two groups of macaques immunized with the HIV89.6P gp140ΔCFI immunogens.
TABLE 3.
Prechallenge proliferative responsesa
| Macaque group | Response to antigen
|
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tat
|
Nef
|
p27
|
gp140
|
Ald-SHIV
|
|||||||||||
| Peak (mean SI) | Responders (%) | Freq. (mean %) | Peak (mean SI) | Responders (%) | Freq. (mean %) | Peak (mean SI) | Responders (%) | Freq. (mean %) | Peak (mean SI) | Responders (%) | Freq. (mean %) | Peak (mean SI) | Responders (%) | Freq. (mean %) | |
| I (Ad Tat) | 5.3 | 87.5 | 14.3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| II (Ad Tat/Env) | 10.2 | 50 | 37.5 | NA | NA | NA | NA | NA | NA | 7.6 | 62.5 | 25 | 3.4 | 100 | 23.4 |
| III (Ad Tat/Env/Gag/Nef) | 6.5 | 75 | 22.9 | 24.2 | 87.5 | 19.6 | 9.7 | 50 | 21.9 | 11.1 | 75 | 22.9 | 8.0 | 87.5 | 46.4 |
Peak, mean of peak positive responses, weeks 0 to 50; responders, percentage of macaques exhibiting a positive response, weeks 0 to 50; Freq., mean of frequency of positive responses for each macaque over the eight time points evaluated, weeks 0 to 50; NA, not applicable.
Prechallenge memory cells.
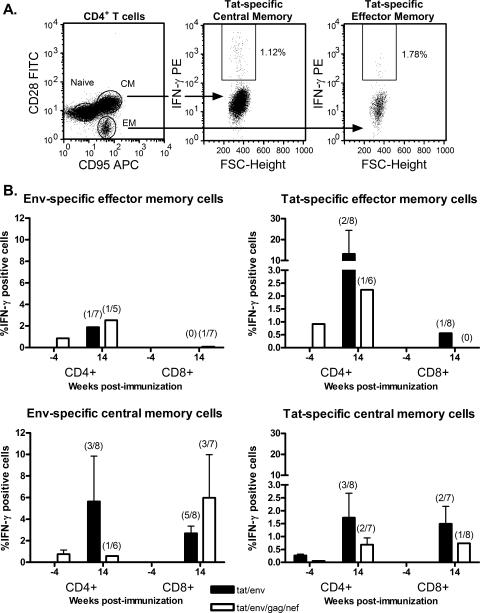
Central and effector CD4+ and CD8+ memory T cells, specific for HIV Tat, HIV Env, SIV Gag, or SIV Nef as appropriate for the various immunization groups, were evaluated in PBMC obtained prior to immunization and 2 weeks after both the second Ad recombinant priming immunizations and the second protein boosts. Cells obtained from rectal biopsies were also assayed, but the number of cells obtained from the biopsies was too small to produce reliable results (data not shown). Tat-specific memory cells above prebleed values were not detected in PBMC of macaques immunized with the Tat-only regimen at either time point (data not shown). However, both central and effector CD4+ and CD8+ central memory T cells specific for Tat or Env were detected in the other two experimental groups after the second Ad recombinant immunizations (Fig. 1). Tat- and Env-specific effector memory CD4+ T cells were exhibited by a low percentage of macaques (14 to 25%) in both groups. A somewhat greater percentage of macaques exhibited central memory CD4+ T cells specific for both Tat and Env (17 to 38%). The immunization regimens also elicited both Tat- and Env-specific CD8+ central memory T cells. The greatest percentage of positive responders was seen for Env-specific CD8+ central memory T cells in both the macaques immunized with Tat and Env vaccines (63%) and those that received multigenic vaccines (43%), perhaps reflecting the greater number of T-cell epitopes in the larger Env protein than in the Tat protein. The percentages of positive responders for Tat-specific CD8+ central memory cells in both immunization groups ranged from 13 to 29%. Env-specific CD8+ effector memory T cells were not detected, and only one macaque exhibited a positive Tat-specific CD8+ effector memory T-cell response. Of interest, the memory responses tended to be higher overall for the Tat/Env immunization group compared to the multigenic group, but this difference was not statistically significant. In addition, by 38 weeks postimmunization, the memory cells were no longer detectable among the PBMC (data not shown).
FIG. 1.
Vaccine-induced CD4+ and CD8+ memory T cells specific for HIV Tat and HIV Env in peripheral blood. The gating strategy used to identify virus-specific effector and central memory T cells is illustrated in panel A, showing a representative staining of Tat-specific CD4+ memory T cells. Tat-stimulated cells were gated by forward scatter (FSC) and side scatter as lymphocytes. The lymphocytic population was further gated for CD8 or CD4. The CD4-positive population illustrated was then gated for CD28 and CD95, distinguishing CD95− CD28+ naive cells, CD95+ CD28− effector memory cells, and CD95+ CD28+ central memory cells (first frame). The double-positive population of CD4+ T cells was gated for IFN-γ secretion, showing 1.12% Tat-specific central memory cells (second frame). The CD95+ CD28− population of CD4+ T cells was also gated for IFN-γ secretion, showing 1.78% Tat-specific effector memory cells (third frame). In panel B, the mean percentages of IFN-γ-positive central and effector memory T-cells in PBMC of responder macaques are shown for week −4 (prebleed) and week 14 (2 weeks following the second Ad recombinant immunizations) samples. The values above the bars represent the percentages of macaques exhibiting a positive response. Error bars represent standard errors of the means.
Gag-specific memory cell responses in the animals that received the multigenic vaccines were not evident at the time points tested, and high background levels were observed in cells of control animals following staining for Nef-specific responses, so positive results were not attained.
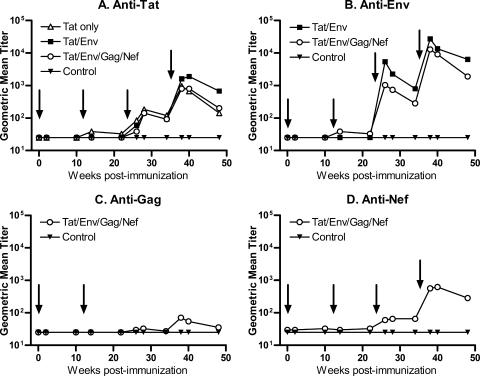
Prechallenge antibody responses.
Serum antibody titers to each of the immunizing antigens are summarized in Fig. 2. Only one macaque in group I primed with Ad5hr-HIV tat developed binding antibodies to Tat following the second Ad recombinant immunization (Fig. 2A), but all immunized macaques exhibited Tat-specific binding antibodies following the protein boosts. At week 48, 2 weeks prior to challenge, macaques in group II immunized with both HIV Tat and HIV Env immunogens exhibited significantly higher Tat-specific binding titers compared to macaques in group I immunized only with Tat vaccines (P = 0.015) and to macaques in group III, immunized with multigenic vaccines (P = 0.028).
FIG. 2.
Vaccine-induced antibody responses prior to challenge. Geometric mean titers for each immunization group are shown for the four immunogens. Arrows indicate time of Ad recombinant immunizations at weeks 0 and 12 and Env, Tat, and Nef protein boosts at weeks 24 and 36.
Macaques in group II also showed consistently higher gp140ΔCFI binding titers over the course of immunization than group III macaques, also immunized with the Env immunogens, although only group III macaques developed antibodies after the second Ad recombinant priming (Fig. 2B). Group II macaques also exhibited a significantly elevated binding titer at week 48, just before challenge, in comparison to the group III titer (P = 0.020).
Macaques in the multigenic group III also received Ad recombinant encoding SIV gag and SIV nef and additionally were boosted with the Nef protein. Consequently, they developed antibody responses to both these antigens. Anti-Gag antibody titers were modest in view of the lack of protein boosting (Fig. 2C). However, moderate titers of antibody to SIV Nef were induced in group III macaques after the Nef protein boost (Fig. 2D). None of the control macaques developed virus-specific binding antibodies prior to challenge.
Neutralizing antibodies.
Neutralizing antibodies against SHIV89.6P were evaluated in sera of group II and III macaques 2 weeks following the second protein booster immunizations and at week 48, 2 weeks prior to challenge. All sera, including those of controls, were negative except for two in the multigenic group III, one of which exhibited a 50% endpoint titer of 23 at week 38 and the other a titer of 26 at week 48 (data not shown).
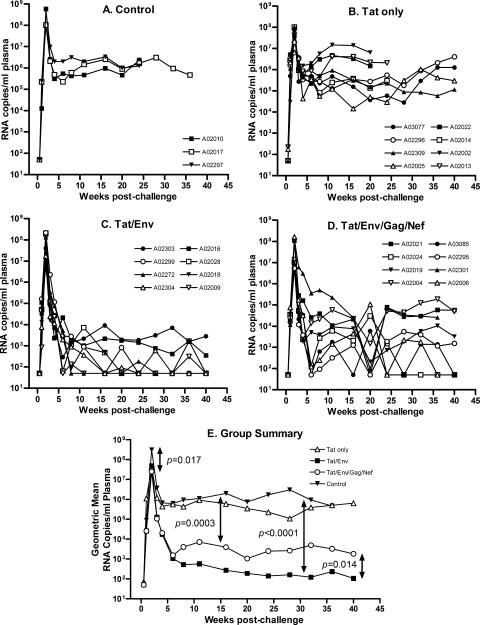
Challenge with SHIV89.9P.
At week 50, the macaques were challenged intravenously with SHIV89.6P. All became infected (Fig. 3). Acute viremia, 2 weeks postchallenge, reached a geometric mean of 3 × 108 copies/ml plasma in the control animals (Fig. 3A and E). Group I macaques, immunized with only Tat vaccines, did not exhibit statistically significant reductions in either acute or chronic phase viremia compared to controls (Fig. 3B and E). In contrast, the multigenic group III had significantly reduced acute viremia compared to the controls (P = 0.017), and both group II (immunized with Tat and Env) and group III exhibited significant reductions in chronic viremia, with some macaques exhibiting undetectable viral loads (<50 copies/ml) on several occasions (Fig. 3C and D). A significant three-log reduction in chronic viremia was observed for the multigenic group III in comparison to controls (P = 0.0003) and a four-log reduction for group II macaques (P < 0.0001). Overall, group II macaques, immunized with Tat and Env vaccines, controlled chronic viremia significantly better than group III macaques, displaying a one-log-lower viral burden during the chronic phase of infection (P = 0.014) (Fig. 3E).
FIG. 3.
Plasma viremia following challenge with SHIV89.6P. Panels A to D show viral loads of the individual macaques. Panel E summarizes geometric mean viral loads for the four immunization groups.
The analysis of viral burdens is completed through week 40 postchallenge. However, the macaques are also being monitored for survival. As of 48 weeks postchallenge, all three control macaques and six of eight macaques in the Tat-only group have been euthanized due to AIDS. In contrast, none of the macaques in the Tat/Env and multigenic groups have progressed to AIDS, and all remain alive. Thus, the control of chronic viremia seen in these two groups is associated with slow disease progression.
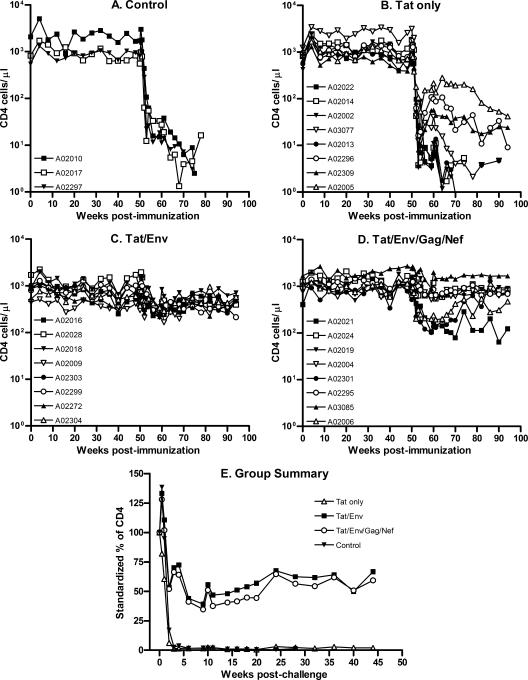
Dual-tropic SHIV89.6P targets naive CD4+ T cells. Therefore, as expected following the intravenous challenge, the control macaques became severely CD4 depleted within the first 2 weeks of challenge (Fig. 4A). Note that the absolute CD4 counts are plotted on a logarithmic scale to accommodate the wide range of CD4 counts seen prior to challenge. CD4+ T-cell loss in the three immunization groups mirrored the viral burdens. Macaques in group I immunized with Tat vaccines only (Fig. 4B) exhibited a CD4 depletion pattern similar to that of control animals. Both exhibited mean absolute numbers of CD4+ T cells below 100 cells/μl by week 3 postchallenge. In contrast, loss of CD4+ cells in group II and III macaques was less severe. The mean absolute CD4+ T-cell counts never dropped below 250 cells/μl for either group. Further, significant differences in the number of CD4+ cells throughout the chronic infection phase were seen between the control animals and group II and III macaques (P ≤ 0.0089 and P ≤ 0.0006, respectively). Group II macaques exhibited lower CD4+ T-cell counts at the time of challenge than the other groups (P = 0.0046 by the Kruskal-Wallis test) and in general had lower counts throughout the prechallenge period. Therefore, the better preservation of CD4+ T cells by group II macaques following challenge is best illustrated by standardizing CD4 counts postchallenge to prechallenge values (Fig. 4E). However, a significant difference in CD4 counts between groups II and III was not observed.
FIG. 4.
CD4+ T-cell counts in peripheral blood. CD4+ cells were monitored throughout the immunization period and postchallenge. Absolute CD4 counts are shown in panels A to D. In panel E, postchallenge CD4 counts of individual macaques were standardized to the respective geometric mean of prechallenge values. Shown are the geometric mean percent CD4 values for the four immunization groups over the postchallenge period.
Postchallenge immune responses.
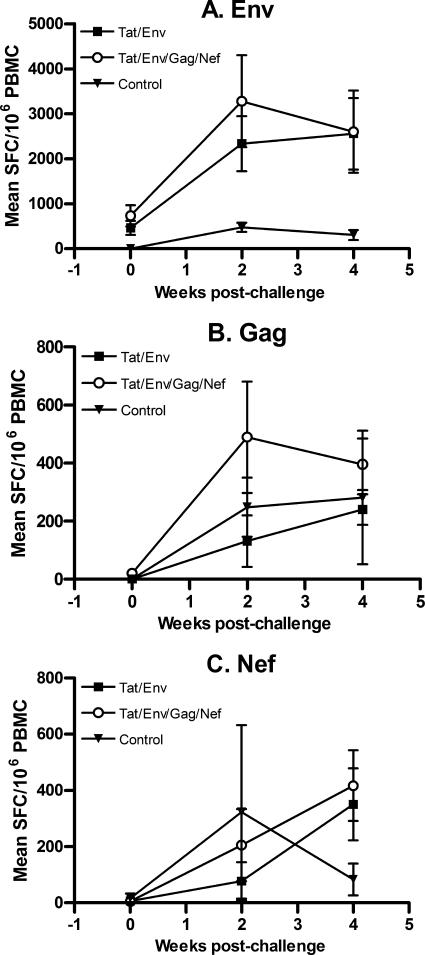
To further elucidate immune responses possibly associated with the better protection seen in groups II and III, cellular and humoral immunity were further investigated postchallenge. As shown in Fig. 5, an anamnestic response to Env peptides was exhibited by both groups of macaques 2 weeks postchallenge, with marked increases in numbers of IFN-γ-secreting cells (Fig. 5A). No apparent difference was observed between the two groups of macaques, however. Strong anamnestic responses to SIV Gag and Nef in comparison to results with control macaques were not observed (Fig. 5B and C). Additionally, no anamnestic responses to Env, Gag, or Nef were detected in proliferative assays, nor were they observed in ELISPOT or T-cell proliferation assays specific for Tat (data not shown).
FIG. 5.
Cellular anamnestic responses postchallenge. ELISPOT responses to Env, Gag, and Nef peptide pools are illustrated for the time of challenge and up to 4 weeks postchallenge. Error bars represent standard errors of the means.
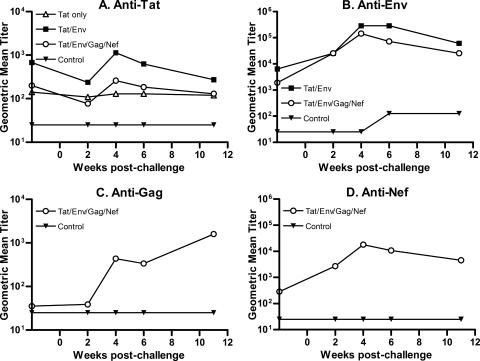
Binding antibodies to Tat and Env were already elevated in group II macaques immunized with Tat and Env vaccines at week 48 prior to challenge, and by week 4 postchallenge, group II macaques again exhibited elevated titers compared to group III macaques that received the multigenic vaccines (Fig. 6A and B). The higher anti-Env titers in the Tat/Env group compared with the multigenic group persisted, with a significant difference still seen at weeks 6 to 11 postchallenge (P = 0.0044, Wei-Johnson test). Binding antibodies to SIV Gag and Nef were strongly boosted postchallenge, but these antibodies did not result in better protection for the group III macaques relative to results for the group II animals during the chronic phase (Fig. 6C and D). The extent to which they may have contributed to the significant reduction in acute viremia in the multigenic group is not known.
FIG. 6.
Antibody anamnestic responses postchallenge. Titers of binding antibody to the indicated proteins are shown 2 weeks prior to challenge and 11 weeks after challenge.
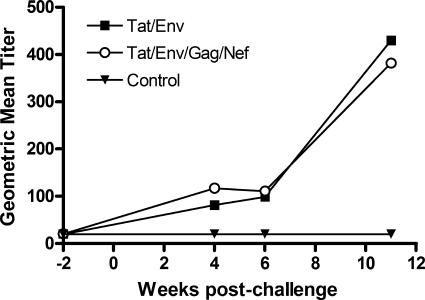
While neutralizing antibodies were not detected prior to challenge, they quickly developed in the macaques immunized with Tat/Env and the multigenic vaccines postchallenge, with higher titers compared to those that developed in control animals (Fig. 7). No difference in neutralizing titer was seen between the two groups, however, so this parameter could not explain the better challenge outcome of the group II macaques.
FIG. 7.
Neutralizing antibody against SHIV89.6P. The development of neutralizing antibody is illustrated 2 weeks prior to challenge and 11 weeks after challenge.
DISCUSSION
All three replicating Ad-recombinant prime/protein boost regimens were immunogenic, eliciting both humoral and cellular immune responses. Cellular immunity was evidenced by HIV- or SIV-specific ELISPOT, T-cell proliferative, and CD4+ and CD8+ memory cell responses, although HIV-Tat-specific ELISPOT responses were low and relatively infrequent (Table 2). This was unexpected in view of our earlier studies with mice showing strong induction of IFN-γ-secreting cells by the Ad5hr-HIV tat recombinant (59). That the recombinant elicited a lesser cell-mediated immune response in macaques perhaps reflects the multiple differences between mouse and human immunology (36), here extended to nonhuman primates. Other studies with rhesus macaques using vectored vaccine candidates have reported widely varying ELISPOT responses to Tat immunogens (30, 52). This variability may result from the different vaccine regimens but could also reflect differences in MHC haplotypes. An immunodominant SIV Tat epitope, responsible for most Tat reactivity, has been defined for Mamu-A*01 rhesus macaques (37), but HIV Tat T-cell epitopes have not been similarly mapped for nonhuman primates. In contrast to the Tat-specific ELISPOT data, the numbers of Env- Gag-, and Nef-specific IFN-γ-secreting cells were comparable to results reported earlier with macaques following priming with Ad-SIV recombinants and boosting with SIV envelope protein subunits (41, 58).
Unlike the ELISPOT results, T-cell proliferative responses to Tat were comparable to those elicited by the Env, Gag, and Nef immunogens (Table 3). Thus, the Tat immunization regimen did elicit cellular immunity. A previous study with cynomolgus monkeys reported strong vaccine-induced cellular immunity to Tat, evidenced both by T-cell proliferative responses and measurements of tumor necrosis factor alpha secretion (8). It is possible that we would have obtained a greater percentage of positive Tat-specific ELISPOT responses had additional cytokines been evaluated. Further, the previous multiple systemic immunizations (nine) with biologically active Tat protein compared to the regimen used here consisting of two mucosal priming immunizations with Ad-HIV tat followed by two native Tat protein boosts may account in part for the different outcomes. With regard to other immunogens, the greatest percentage of macaques exhibiting a positive T-cell proliferative response to HIV envelope was seen following stimulation with Ald-SHIV, suggesting that the prime/boost regimen using Ad5hr-HIV89.6Pgp140ΔCFI and the HIV89.6P gp140ΔCFI protein induced immune responses specific for native envelope structure on the surface of SHIV virions.
Antigen-specific binding antibodies were also induced by the vaccine regimen, and titers were significantly elevated after each protein booster immunization (Fig. 2). In this regard, the boosting of anti-Gag antibodies following each administration of the Env, Tat, and Nef proteins perhaps reflects a helper effect due to the proteins themselves or the adjuvant (5). The increases seen in the anti-Gag titer also suggest the continued presence of the replication-competent Ad5hr-SIV gag recombinant.
The mechanism(s) responsible for induction of higher levels of anti-Tat and anti-Env binding antibodies in the Tat/Env group than in the Tat-alone group or the Tat/Env/Gag/Nef group is unknown. Unless recombinant priming played a significant role, a synergistic effect at the site of inoculation is unlikely. The Ad recombinants were administered in a single pool; however, the Tat protein booster was administered subcutaneously in alum and the gp140ΔCFI protein was separately administered intramuscularly in MPL-SE. Antigenic competition may have contributed to induction of lower-titer envelope antibodies in the multigenic group but cannot explain the lower anti-Tat titers in the Tat-only group. The lack of induction of neutralizing antibodies to the SHIV89.6P challenge strain prior to virus exposure is similar to results obtained previously with vectored vaccines expressing a SHIV89.6P envelope immunogen (27, 34).
The lack of protection elicited by the Tat-only prime/boost regimen was not unexpected in view of earlier results in which vaccination with the native or inactivated Tat protein or with a replication-defective Ad5-HIV tat vaccine either failed to protect or partially protected rhesus macaques against a SHIV89.6P challenge administered intrarectally (43) or intravenously (30, 51). The negative results continue to contrast with results of studies of cynomolgus monkeys that have exhibited strong, long-lasting protection against SHIV89.6P infection following vaccination with the native Tat protein or Tat plasmid DNA vaccines (8, 9, 31). The different outcomes might result from species differences in immunologic and/or virologic responses following vaccination and challenge which are currently under investigation.
Similar to results of other studies of Mamu-A*01-negative rhesus macaques challenged intravenously with SHIV89.6P, we did not achieve sterilizing immunity. However, rapid control of viremia was observed in the Ad Tat/Env and Ad Tat/Env/Gag/Nef groups (Fig. 3C to E). The extent of viremia reduction achieved during the chronic phase of infection was highly favorable in comparison to findings of earlier studies. The overall level of protection achieved in this study may have been influenced by our use of replicating Ad-SIV recombinants. We have previously reported enhanced immunogenicity elicited by replication-competent Ad-HIV recombinants compared to that with matched replication-defective Ad-HIV recombinants (44). As shown in Fig. 3E, chronic viremia was reduced 3 logs in the multigenic group and 4 logs in the Tat/Env group. In contrast, macaques immunized three times with replication-defective Ad5-HIVJR-fl gp140 plus Ad5-SIV gag exhibited an approximate 2-log reduction in set-point viremia (30), while a regimen composed of three plasmid DNAs bearing SIV gag, pol, and nef followed by replication-defective Ad5 recombinants bearing HIVHXB2/Bal gp140 and SIV gag/pol elicited an approximate 3-log reduction in chronic viremia (27). In the latter study, inclusion of an envelope component, in addition to Gag, Pol, and Nef, led to enhanced viremia control. The outcome was similar to that of our multigenic group in extent of viremia reduction. Strikingly, envelope immunogens played a major role in our group II macaques. The envelope components in combination with Tat vaccines led to a further 1-log enhancement of protective efficacy in comparison to the multigenic group.
In view of the better protection observed for group II macaques, immunized with Tat and Env vaccines, than with to group III macaques, immunized with multigenic immunogens, investigation of possible immunologic correlates of protection is of interest. No consistent pattern of cellular immunity to Tat or Env was observed between the two groups with regard to either ELISPOT or proliferative responses which could explain the better challenge outcome of the Tat/Env group (Tables 1 and 2). Further, group III macaques exhibited cellular responses to Nef and Gag antigens, not present in the group II macaques, yet the latter exhibited better control of viremia. A tendency toward elevated memory cell responses was observed in the peripheral blood of macaques in the Tat/Env group compared to the multigenic group (Fig. 1). However, the positive responses were relatively infrequent and were not detected during the latter part of the immunization regimen. It is possible that memory cells migrated to tissue sites prior to challenge, where they might have influenced the challenge outcome. Future studies should investigate this question in greater depth. However, anamnestic cellular immune responses observed in peripheral blood postchallenge were not significantly different between the two groups (Fig. 5).
A difference between the Tat/Env and multigenic groups was seen, however, in antibody responses. At the time of challenge, group II macaques exhibited significantly higher titers of anti-Tat and anti-Env binding antibody than group III macaques (Fig. 2A and B). But neither Gag nor Nef antibodies, elicited in the multigenic group of macaques, can explain the greater protective efficacy observed in the Tat/Env immunization group.
With regard to the anti-Tat antibodies, the higher titers for Group II macaques persisted postchallenge (Fig. 6A), although the statistically significant difference present just prior to challenge was not maintained. Whether these binding antibodies possess functional activity is a subject of ongoing investigation. Various functions have been ascribed to Tat antibody, including neutralization of Tat activity in a virus rescue assay (8), inhibition of Tat-mediated apoptosis (4), and inhibition of Tat-mediated transactivation in a long-terminal-repeat-dependent chloramphenicol acetyltransferase assay (3). While Tat antibodies cannot block HIV entry into cells, they can modulate the multitude of Tat activities that occur following infection, due in large part to the ability of released extracellular Tat to enter neighboring cells (15, 16). Tat enhances CD95/CD178 (Fas/Fas-L)-mediated apoptosis in T cells (35, 55) and upregulates tumor necrosis factor-related apoptosis-inducing ligand in macrophages (57), thus inducing bystander killing, whereas Tat-expressing cells are protected from apoptosis. Released Tat may contribute to the spread of the ongoing infection due to interaction with gp120 on cell surfaces (33). Functional anti-Tat antibodies present at the time of virus exposure and subsequently maintained postchallenge may therefore contribute importantly to viremia control. In this regard, Tat antibodies have been associated with control of CD4+ reservoir cells in peripheral blood following challenge of Chinese rhesus macaques with SHIVBX08 (54). A protective role of Tat antibodies is further supported by their association with slow progression or nonprogression to AIDS (14a, 46, 48, 56). The higher anti-Tat antibody titers exhibited here by the Tat/Env group of macaques in comparison to the multigenic group may therefore explain in part their significantly better control of chronic viremia.
Anti-Tat antibody was certainly not sufficient to control the SHIV89.6P challenge in rhesus macaques, as evidenced by the lack of control in the Tat-only group. However, in concert with higher antienvelope antibody titers in group II macaques, enhanced protective efficacy was achieved. This protection was not attributable to SHIV89.6P-neutralizing antibodies, which were lacking in both groups at the time of challenge. In fact, postchallenge neutralizing antibody also did not contribute to the difference in chronic viremia seen between the Tat/Env and multigenic groups, since similar titers were induced following infection. However, nonneutralizing antibodies, levels of which were higher both at the time of challenge and in the weeks following, may have contributed to the protective outcomes by one or more mechanisms. Nonneutralizing antienvelope antibodies can protect macrophages and immature dendritic cells from HIV type 1 infection by the binding of antibody-virion complexes to FcγRI and FcγRII receptors in vitro, with subsequent internalization and degradation of the virus (22, 23). Nonneutralizing antienvelope antibodies may mediate ADCC, leading to killing of virus-infected cells. Such antienvelope binding antibodies mediating ADCC have been significantly correlated with reduced acute viremia (20). Additional mechanisms that have been associated with protection include antibody-dependent cell-mediated viral inhibition (17) and complement-mediated viral lysis (1). The extent to which these potential mechanisms may have contributed to the improved outcome of the Tat/Env group of macaques remains to be clarified. While neutralizing antibodies can prevent infection, nonneutralizing binding antibodies can play a significant role in controlling subsequent infection. In view of the persistently higher levels of antienvelope binding antibodies in the Tat/Env group than in the multigenic group, significantly elevated both at the time of challenge and postchallenge, this hypothesis deserves further exploration.
Overall, the potential synergy between Tat and Env immunogens in eliciting enhanced protective efficacy suggests such combination vaccines should be further explored for both prophylactic and therapeutic applications. Investigation of the mechanism(s) responsible for the outcome observed here may provide useful information for improved vaccine design.
Acknowledgments
We thank Gary Nabel, VRC, NIAID, NIH, for providing the SHIV89.6P gp140ΔCFI gene; Keith Reimann and Norman L. Letvin for the SHIV89.6P challenge stock; Jeffrey Lifson and Julian Bess for aldrithiol-2-inactivated SHIV89.6P; Nancy Miller for helpful discussion; and Kristine Aldrich, Thanhdao Nguyen, and Randy McLain for technical assistance. The following reagents were provided by the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: complete peptide sets for HIV consensus B Tat, SHIV89.6P Env, SIV Gag, and SIV Nef.
This work was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, and by the NIH, NIAID, Simian Vaccine Evaluation contract N01-AI-15431 to the University of Washington.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Aasa-Chapman, M. M., S. Holuigue, K. Aubin, M. Wong, N. A. Jones, D. Cornforth, P. Pellegrino, P. Newton, I. Williams, P. Borrow, and A. McKnight. 2005. Detection of antibody-dependent complement-mediated inactivation of both autologous and heterologous virus in primary human immunodeficiency virus type 1 infection. J. Virol. 79:2823-2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 3.Belliard, G., B. Hurtrel, E. Moreau, B. A. Lafont, V. Monceaux, B. Roques, C. Desgranges, A. M. Aubertin, R. Le Grand, and S. Muller. 2005. Tat-neutralizing versus Tat-protecting antibodies in rhesus macaques vaccinated with Tat peptides. Vaccine 23:1399-1407. [DOI] [PubMed] [Google Scholar]
- 4.Belliard, G., A. Romieu, J. F. Zagury, H. Dali, O. Chaloin, R. Le Grand, E. Loret, J. P. Briand, B. Roques, C. Desgranges, and S. Muller. 2003. Specificity and effect on apoptosis of Tat antibodies from vaccinated and SHIV-infected rhesus macaques and HIV-infected individuals. Vaccine 21:3186-3199. [DOI] [PubMed] [Google Scholar]
- 5.Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. [DOI] [PubMed] [Google Scholar]
- 6.Buge, S. L., E. Richardson, S. Alipanah, P. Markham, S. Cheng, N. Kalyan, C. J. Miller, M. Lubeck, S. Udem, J. Eldridge, and M. Robert-Guroff. 1997. An adenovirus-simian immunodeficiency virus env vaccine elicits humoral, cellular, and mucosal immune responses in rhesus macaques and decreases viral burden following vaginal challenge. J. Virol. 71:8531-8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butto, S., V. Fiorelli, A. Tripiciano, M. J. Ruiz-Alvarez, A. Scoglio, F. Ensoli, M. Ciccozzi, B. Collacchi, M. Sabbatucci, A. Cafaro, C. A. Guzman, A. Borsetti, A. Caputo, E. Vardas, M. Colvin, M. Lukwiya, G. Rezza, and B. Ensoli. 2003. Sequence conservation and antibody cross-recognition of clade B human immunodeficiency virus (HIV) type 1 Tat protein in HIV-1-infected Italians, Ugandans, and South Africans. J. Infect. Dis. 188:1171-1180. [DOI] [PubMed] [Google Scholar]
- 8.Cafaro, A., A. Caputo, C. Fracasso, M. T. Maggiorella, D. Goletti, S. Baroncelli, M. Pace, L. Sernicola, M. L. Koanga-Mogtomo, M. Betti, A. Borsetti, R. Belli, L. Akerblom, F. Corrias, S. Butto, J. Heeney, P. Verani, F. Titti, and B. Ensoli. 1999. Control of SHIV-89.6P-infection of cynomolgus monkeys by HIV-1 Tat protein vaccine. Nat. Med. 5:643-650. [DOI] [PubMed] [Google Scholar]
- 9.Cafaro, A., F. Titti, C. Fracasso, M. T. Maggiorella, S. Baroncelli, A. Caputo, D. Goletti, A. Borsetti, M. Pace, E. Fanales-Belasio, B. Ridolfi, D. R. Negri, L. Sernicola, R. Belli, F. Corrias, I. Macchia, P. Leone, Z. Michelini, P. ten Haaft, S. Butto, P. Verani, and B. Ensoli. 2001. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 19:2862-2877. [DOI] [PubMed] [Google Scholar]
- 10.Caputo, A., R. Gavioli, and B. Ensoli. 2004. Recent advances in the development of HIV-1 Tat-based vaccines. Curr. HIV Res. 2:357-376. [DOI] [PubMed] [Google Scholar]
- 11.Coiras, M., E. Camafeita, T. Urena, J. A. Lopez, F. Caballero, B. Fernandez, M. R. Lopez-Huertas, M. Perez-Olmeda, and J. Alcami. 2006. Modifications in the human T cell proteome induced by intracellular HIV-1 Tat protein expression. Proteomics 6 Suppl. 1:S63-S73. [DOI] [PubMed] [Google Scholar]
- 12.Dockrell, D. H., A. D. Badley, J. S. Villacian, C. J. Heppelmann, A. Algeciras, S. Ziesmer, H. Yagita, D. H. Lynch, P. C. Roche, P. J. Leibson, and C. V. Paya. 1998. The expression of Fas ligand by macrophages and its upregulation by human immunodeficiency virus infection. J. Clin. Investig. 101:2394-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duerr, A., J. N. Wasserheit, and L. Corey. 2006. HIV vaccines: new frontiers in vaccine development. Clin. Infect. Dis. 43:500-511. [DOI] [PubMed] [Google Scholar]
- 14.Ensoli, B. 2006. Prophylactic and therapeutic phase I trials with the active Tat protein vaccine: results of the interim analysis, abstr., p. 190. Keystone Symposia on HIV Pathogenesis and HIV Vaccines, Keystone, CO, 27 March to 2 April 2006.
- 14a.Ensoli, B., V. Fiorelli, F. Ensoli, A. Cafaro, F. Titti, S. Butto, P. Monini, M. Magnani, A. Caputo, and E. Garaci. 2006. Candidate HIV-1 Tat vaccine development: from basic science to clinical trials. AIDS 20:2245-2261. [DOI] [PubMed]
- 15.Ensoli, B., L. Buonaguro, G. Barillari, V. Fiorelli, R. Gendelman, R. A. Morgan, P. Wingfield, and R. C. Gallo. 1993. Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67:277-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fanales-Belasio, E., S. Moretti, F. Nappi, G. Barillari, F. Micheletti, A. Cafaro, and B. Ensoli. 2002. Native HIV-1 Tat protein targets monocyte-derived dendritic cells and enhances their maturation, function, and antigen-specific T cell responses. J. Immunol. 168:197-206. [DOI] [PubMed] [Google Scholar]
- 17.Forthal, D. N., G. Landucci, K. S. Cole, M. Marthas, J. C. Becerra, and K. Van Rompay. 2006. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J. Virol. 80:9217-9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gavioli, R., E. Gallerani, C. Fortini, M. Fabris, A. Bottoni, A. Canella, A. Bonaccorsi, M. Marastoni, F. Micheletti, A. Cafaro, P. Rimessi, A. Caputo, and B. Ensoli. 2004. HIV-1 tat protein modulates the generation of cytotoxic T cell epitopes by modifying proteasome composition and enzymatic activity. J. Immunol. 173:3838-3843. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Roman, V. R., R. H. Florese, B. Peng, D. C. Montefiori, V. S. Kalyanaraman, D. Venzon, I. Srivastava, S. W. Barnett, and M. Robert-Guroff. 2006. An adenovirus-based HIV subtype B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype B HIV strains. J. Acquir. Immune. Defic. Syndr. 43:270-277. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Roman, V. R., L. J. Patterson, D. Venzon, D. Liewehr, K. Aldrich, R. Florese, and M. Robert-Guroff. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J. Immunol. 174:2185-2189. [DOI] [PubMed] [Google Scholar]
- 21.Hel, Z., W. P. Tsai, E. Tryniszewska, J. Nacsa, P. D. Markham, M. G. Lewis, G. N. Pavlakis, B. K. Felber, J. Tartaglia, and G. Franchini. 2006. Improved vaccine protection from simian AIDS by the addition of nonstructural simian immunodeficiency virus genes. J. Immunol. 176:85-96. [DOI] [PubMed] [Google Scholar]
- 22.Holl, V., M. Peressin, T. Decoville, S. Schmidt, S. Zolla-Pazner, A. M. Aubertin, and C. Moog. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J. Virol. 80:6177-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holl, V., M. Peressin, S. Schmidt, T. Decoville, S. Zolla-Pazner, A. M. Aubertin, and C. Moog. 2006. Efficient inhibition of HIV-1 replication in human immature monocyte-derived dendritic cells by purified anti-HIV-1 IgG without induction of maturation. Blood 107:4466-4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, L., I. Bosch, W. Hofmann, J. Sodroski, and A. B. Pardee. 1998. Tat protein induces human immunodeficiency virus type 1 (HIV-1) coreceptors and promotes infection with both macrophage-tropic and T-lymphotropic HIV-1 strains. J. Virol. 72:8952-8960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kittiworakarn, J., A. Lecoq, G. Moine, R. Thai, E. Lajeunesse, P. Drevet, C. Vidaud, A. Menez, and M. Leonetti. 2006. HIV-1 Tat raises an adjuvant-free humoral immune response controlled by its core region and its ability to form cysteine-mediated oligomers. J. Biol. Chem. 281:3105-3115. [DOI] [PubMed] [Google Scholar]
- 26.Leifert, J. A., P. D. Holler, S. Harkins, D. M. Kranz, and J. L. Whitton. 2003. The cationic region from HIV tat enhances the cell-surface expression of epitope/MHC class I complexes. Gene Ther. 10:2067-2073. [DOI] [PubMed] [Google Scholar]
- 27.Letvin, N. L., Y. Huang, B. K. Chakrabarti, L. Xu, M. S. Seaman, K. Beaudry, B. Korioth-Schmitz, F. Yu, D. Rohne, K. L. Martin, A. Miura, W. P. Kong, Z. Y. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 29.Li, M., F. Gao, J. R. Mascola, L. Stamatatos, V. R. Polonis, M. Koutsoukos, G. Voss, P. Goepfert, P. Gilbert, K. M. Greene, M. Bilska, D. L. Kothe, J. F. Salazar-Gonzalez, X. Wei, J. M. Decker, B. H. Hahn, and D. C. Montefiori. 2005. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J. Virol. 79:10108-10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang, X., D. R. Casimiro, W. A. Schleif, F. Wang, M. E. Davies, Z. Q. Zhang, T. M. Fu, A. C. Finnefrock, L. Handt, M. P. Citron, G. Heidecker, A. Tang, M. Chen, K. A. Wilson, L. Gabryelski, M. McElhaugh, A. Carella, C. Moyer, L. Huang, S. Vitelli, D. Patel, J. Lin, E. A. Emini, and J. W. Shiver. 2005. Vectored Gag and Env but not Tat show efficacy against simian-human immunodeficiency virus 89.6P challenge in Mamu-A*01-negative rhesus monkeys. J. Virol. 79:12321-12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maggiorella, M. T., S. Baroncelli, Z. Michelini, E. Fanales-Belasio, S. Moretti, L. Sernicola, A. Cara, D. R. Negri, S. Butto, V. Fiorelli, A. Tripiciano, A. Scoglio, A. Caputo, A. Borsetti, B. Ridolfi, R. Bona, P. ten Haaft, I. Macchia, P. Leone, M. R. Pavone-Cossut, F. Nappi, M. Ciccozzi, J. Heeney, F. Titti, A. Cafaro, and B. Ensoli. 2004. Long-term protection against SHIV89.6P replication in HIV-1 Tat vaccinated cynomolgus monkeys. Vaccine 22:3258-3269. [DOI] [PubMed] [Google Scholar]
- 32.Malkevitch, N. V., L. J. Patterson, M. K. Aldrich, Y. Wu, D. Venzon, R. H. Florese, V. S. Kalyanaraman, R. Pal, E. M. Lee, J. Zhao, A. Cristillo, and M. Robert-Guroff. 2006. Durable protection of rhesus macaques immunized with a replicating adenovirus-SIV multigene prime/protein boost vaccine regimen against a second SIV(mac251) rectal challenge: role of SIV-specific CD8+ T cell responses. Virology 353:83-98. [DOI] [PubMed] [Google Scholar]
- 33.Marchio, S., M. Alfano, L. Primo, D. Gramaglia, L. Butini, L. Gennero, E. De Vivo, W. Arap, M. Giacca, R. Pasqualini, and F. Bussolino. 2005. Cell surface-associated Tat modulates HIV-1 infection and spreading through a specific interaction with gp120 viral envelope protein. Blood 105:2802-2811. [DOI] [PubMed] [Google Scholar]
- 34.Mascola, J. R., A. Sambor, K. Beaudry, S. Santra, B. Welcher, M. K. Louder, T. C. Vancott, Y. Huang, B. K. Chakrabarti, W. P. Kong, Z. Y. Yang, L. Xu, D. C. Montefiori, G. J. Nabel, and N. L. Letvin. 2005. Neutralizing antibodies elicited by immunization of monkeys with DNA plasmids and recombinant adenoviral vectors expressing human immunodeficiency virus type 1 proteins. J. Virol. 79:771-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCloskey, T. W., M. Ott, E. Tribble, S. A. Khan, S. Teichberg, M. O. Paul, S. Pahwa, E. Verdin, and N. Chirmule. 1997. Dual role of HIV Tat in regulation of apoptosis in T cells. J. Immunol. 158:1014-1019. [PubMed] [Google Scholar]
- 36.Mestas, J., and C. C. W. Hughes. 2004. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172:2731-2738. [DOI] [PubMed] [Google Scholar]
- 37.Mothe, B. R., H. Horton, D. K. Carter, T. M. Allen, M. E. Liebl, P. Skinner, T. U. Vogel, S. Fuenger, K. Vielhuber, W. Rehrauer, N. Wilson, G. Franchini, J. D. Altman, A. Haase, L. J. Picker, D. B. Allison, and D. I. Watkins. 2002. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 76:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moy, P., Y. Daikh, B. Pepinsky, D. Thomas, S. Fawell, and J. Barsoum. 1996. Tat-mediated protein delivery can facilitate MHC class I presentation of antigens. Mol. Biotechnol. 6:105-113. [DOI] [PubMed] [Google Scholar]
- 39.Opi, S., J. M. Peloponese, Jr., D. Esquieu, J. Watkins, G. Campbell, J. De Mareuil, K. T. Jeang, D. L. Yirrell, P. Kaleebu, and E. P. Loret. 2004. Full-length HIV-1 Tat protein necessary for a vaccine. Vaccine 22:3105-3111. [DOI] [PubMed] [Google Scholar]
- 40.Pantano, S., and P. Carloni. 2005. Comparative analysis of HIV-1 Tat variants. Proteins 58:638-643. [DOI] [PubMed] [Google Scholar]
- 41.Patterson, L. J., N. Malkevitch, J. Pinczewski, D. Venzon, Y. Lou, B. Peng, C. Munch, M. Leonard, E. Richardson, K. Aldrich, V. S. Kalyanaraman, G. N. Pavlakis, and M. Robert-Guroff. 2003. Potent, persistent induction and modulation of cellular immune responses in rhesus macaques primed with Ad5hr-simian immunodeficiency virus (SIV) env/rev, gag, and/or nef vaccines and boosted with SIV gp120. J. Virol. 77:8607-8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patterson, L. J., N. Malkevitch, D. Venzon, J. Pinczewski, V. R. Gomez-Roman, L. Wang, V. S. Kalyanaraman, P. D. Markham, F. A. Robey, and M. Robert-Guroff. 2004. Protection against mucosal simian immunodeficiency virus SIV(mac251) challenge by using replicating adenovirus-SIV multigene vaccine priming and subunit boosting. J. Virol. 78:2212-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pauza, C. D., P. Trivedi, M. Wallace, T. J. Ruckwardt, H. Le Buanec, W. Lu, B. Bizzini, A. Burny, D. Zagury, and R. C. Gallo. 2000. Vaccination with tat toxoid attenuates disease in simian/HIV-challenged macaques. Proc. Natl. Acad. Sci. USA 97:3515-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng, B., L. R. Wang, V. R. Gomez-Roman, A. Davis-Warren, D. C. Montefiori, V. S. Kalyanaraman, D. Venzon, J. Zhao, E. Kan, T. J. Rowell, K. K. Murthy, I. Srivastava, S. W. Barnett, and M. Robert-Guroff. 2005. Replicating rather than nonreplicating adenovirus-human immunodeficiency virus recombinant vaccines are better at eliciting potent cellular immunity and priming high-titer antibodies. J. Virol. 79:10200-10209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peruzzi, F. 2006. The multiple functions of HIV-1 Tat: proliferation versus apoptosis. Front. Biosci. 11:708-717. [DOI] [PubMed] [Google Scholar]
- 46.Re, M. C., M. Vignoli, G. Furlini, D. Gibellini, V. Colangeli, F. Vitone, and M. La Placa. 2001. Antibodies against full-length Tat protein and some low-molecular-weight Tat-peptides correlate with low or undetectable viral load in HIV-1 seropositive patients. J. Clin. Virol. 21:81-89. [DOI] [PubMed] [Google Scholar]
- 47.Remoli, A. L., G. Marsili, E. Perrotti, E. Gallerani, R. Ilari, F. Nappi, A. Cafaro, B. Ensoli, R. Gavioli, and A. Battistini. 2006. Intracellular HIV-1 Tat protein represses constitutive LMP2 transcription increasing proteasome activity by interfering with the binding of IRF-1 to STAT1. Biochem. J. 396:371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rezza, G., V. Fiorelli, M. Dorrucci, M. Ciccozzi, A. Tripiciano, A. Scoglio, B. Collacchi, M. Ruiz-Alvarez, C. Giannetto, A. Caputo, L. Tomasoni, F. Castelli, M. Sciandra, A. Sinicco, F. Ensoli, S. Butto, and B. Ensoli. 2005. The presence of anti-Tat antibodies is predictive of long-term nonprogression to AIDS or severe immunodeficiency: findings in a cohort of HIV-1 seroconverters. J. Infect. Dis. 191:1321-1324. [DOI] [PubMed] [Google Scholar]
- 49.Romano, J. W., R. N. Shurtliff, E. Dobratz, A. Gibson, K. Hickman, P. D. Markham, and R. Pal. 2000. Quantitative evaluation of simian immunodeficiency virus infection using NASBA technology. J. Virol. Methods 86:61-70. [DOI] [PubMed] [Google Scholar]
- 50.Santra, S., D. H. Barouch, B. Korioth-Schmitz, C. I. Lord, G. R. Krivulka, F. Yu, M. H. Beddall, D. A. Gorgone, M. A. Lifton, A. Miura, V. Philippon, K. Manson, P. D. Markham, J. Parrish, M. J. Kuroda, J. E. Schmitz, R. S. Gelman, J. W. Shiver, D. C. Montefiori, D. Panicali, and N. L. Letvin. 2004. Recombinant poxvirus boosting of DNA-primed rhesus monkeys augments peak but not memory T lymphocyte responses. Proc. Natl. Acad. Sci. USA 101:11088-11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silvera, P., M. W. Richardson, J. Greenhouse, J. Yalley-Ogunro, N. Shaw, J. Mirchandani, K. Khalili, J. F. Zagury, M. G. Lewis, and J. Rappaport. 2002. Outcome of simian-human immunodeficiency virus strain 89.6p challenge following vaccination of rhesus macaques with human immunodeficiency virus Tat protein. J. Virol. 76:3800-3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smith, J. M., R. R. Amara, H. M. McClure, M. Patel, S. Sharma, H. Yi, L. Chennareddi, J. G. Herndon, S. T. Butera, W. Heneine, D. L. Ellenberger, B. Parekh, P. L. Earl, L. S. Wyatt, B. Moss, and H. L. Robinson. 2004. Multiprotein HIV type 1 clade B DNA/MVA vaccine: construction, safety, and immunogenicity in macaques. AIDS Res. Hum Retrovir. 20:654-665. [DOI] [PubMed] [Google Scholar]
- 53.van Baalen, C. A., O. Pontesilli, R. C. Huisman, A. M. Geretti, M. R. Klein, F. de Wolf, F. Miedema, R. A. Gruters, and A. D. Osterhaus. 1997. Human immunodeficiency virus type 1 Rev- and Tat-specific cytotoxic T lymphocyte frequencies inversely correlate with rapid progression to AIDS. J. Gen. Virol. 78:1913-1918. [DOI] [PubMed] [Google Scholar]
- 54.Watkins, J. D., S. Lancelot, G. R. Campbell, D. Esquieu, J. de Mareuil, S. Opi, S. Annappa, J. P. Salles, and E. P. Loret. 2006. Reservoir cells no longer detectable after a heterologous SHIV challenge with the synthetic HIV-1 Tat Oyi vaccine. Retrovirology 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 56.Zagury, J. F., A. Sill, W. Blattner, A. Lachgar, H. Le Buanec, M. Richardson, J. Rappaport, H. Hendel, B. Bizzini, A. Gringeri, M. Carcagno, M. Criscuolo, A. Burny, R. C. Gallo, and D. Zagury. 1998. Antibodies to the HIV-1 Tat protein correlated with nonprogression to AIDS: a rationale for the use of Tat toxoid as an HIV-1 vaccine. J. Hum. Virol. 1:282-292. [PubMed] [Google Scholar]
- 57.Zhang, M., X. Li, X. Pang, L. Ding, O. Wood, K. Clouse, I. Hewlett, and A. I. Dayton. 2001. Identification of a potential HIV-induced source of bystander-mediated apoptosis in T cells: upregulation of TRAIL in primary human macrophages by HIV-1 tat. J. Biomed. Sci. 8:290-296. [DOI] [PubMed] [Google Scholar]
- 58.Zhao, J., Y. Lou, J. Pinczewski, N. Malkevitch, K. Aldrich, V. S. Kalyanaraman, D. Venzon, B. Peng, L. J. Patterson, Y. Edghill-Smith, R. Woodward, G. N. Pavlakis, and M. Robert-Guroff. 2003. Boosting of SIV-specific immune responses in rhesus macaques by repeated administration of Ad5hr-SIVenv/rev and Ad5hr-SIVgag recombinants. Vaccine 21:4022-4035. [DOI] [PubMed] [Google Scholar]
- 59.Zhao, J., R. Voltan, B. Peng, A. Davis-Warren, V. S. Kalyanaraman, W. G. Alvord, K. Aldrich, D. Bernasconi, S. Butto, A. Cafaro, B. Ensoli, and M. Robert-Guroff. 2005. Enhanced cellular immunity to SIV Gag following coadministration of adenoviruses encoding wild-type or mutant HIV Tat and SIV Gag. Virology 342:1-12. [DOI] [PubMed] [Google Scholar]