Abstract
Histo-blood group antigen (HBGA) phenotypes have been associated with susceptibility to human noroviruses (HuNoVs). Our aims were: (i) to determine the patterns of A/H HBGA expression in buccal and intestinal tissues of gnotobiotic (Gn) pigs; (ii) to determine if virus-like particles (VLPs) of HuNoV genogroup I (GI) and GII bind to A- or H-type tissues; (iii) to compare A/H expression and VLP binding patterns and confirm their binding specificities by blocking assays; (iv) to develop a hemagglutination inhibition test using buccal cells from live pigs to determine the Gn pig's A/H phenotype and to match viral strains with previously determined HuNoV VLP binding specificities; and (v) to determine the A/H phenotypes and compare these data to the infection outcomes of a previous study of 65 Gn pigs inoculated with HuNoV GII/4 strain HS66 and expressing A and/or H or neither antigen on their buccal and intestinal tissues (S. Cheetham, M. Souza, T. Meulia, S. Grimes, M. G. Han, and L. J. Saif, J. Virol. 80:10372-10381, 2006). We found that the HuNoV GI/GII VLPs of different clusters bound to tissues from four pigs tested (two A+ and two H+). The GI/1 and GII/4 VLPs bound extensively to duodenal and buccal tissues from either A+ or H+ pigs, but surprisingly, GII/1 and GII/3 VLPs bound minimally to the duodenum of an A+ pig. The VLP binding was partially inhibited by A-, H1-, or H2-specific monoclonal antibodies, but was completely blocked by porcine mucin. Comparing the A/H phenotypes of 65 HS66-inoculated Gn pigs from our previous study, we found that significantly more A+ and H+ pigs (51%) than non-A+ and non-H+ pigs (12.5%) shed virus. From the 22 convalescent pigs, significantly more A+ or H+ pigs (66%) than non-A+ or H+ pigs (25%) seroconverted.
Noroviruses (NoVs) are classified into five genogroups (G) (35). Strains in genogroup I (GI), GII, and GIV cause gastroenteritis in humans, but GII strains have also been detected in swine, suggesting a zoonotic potential (33). The GIII NoVs include two bovine strains, and GV comprises of a murine virus.
Recently, different susceptibilities of humans to NoV infection, depending on their histo-blood group antigen (HBGA) phenotypes, have been reported (3, 13). The HBGAs are terminal disaccharides added in a stepwise manner to precursor carbohydrate chains by the action of different glycosyltransferases (24). Inactivating mutations in the glycosyltransferase gene at the ABO(H) locus results in the O phenotype that represents the H precursor without any further carbohydrate addition; thus, presence of the H antigen with absence of A or B antigens corresponds to the O phenotype. The addition of different terminal disaccharides to the H chain results in either the A or B antigen. Although these antigens were first described on the surface of human red blood cells (RBCs), their expression occurs throughout the body. The FUT2 gene codes for a glycosyltransferase that determines the secretor (Se) phenotype of an individual, and when active (Se+), this enzyme mediates the expression of the ABO(H) antigens on mucosal epithelial cells and their secretion into body fluids (24). The activity of the FUT2 gene has been linked to the different susceptibilities of individuals to Norwalk virus (NV), a GI NoV, with Se+ volunteers being 40 times more likely to become Norwalk virus infected than nonsecretor (Se−) individuals (17). About 20% of individuals have FUT2-inactivating mutations, resulting in an Se− phenotype which results in resistance to Norwalk virus infection (20, 32).
A hemaglutination assay using human and chimpanzee RBCs established that NV virus-like particles (VLPs) differentially agglutinate RBCs of different blood groups, agglutinating all A, O, and AB samples but only 4 of 14 type B samples (14). These results correspond to the findings obtained from a Norwalk virus challenge study of volunteers (12). Also, NV VLPs bind to HBGAs present on gastroduodenal epithelial cells of Se+ but not Se− individuals (20). In vitro studies have also shown different HBGA binding patterns for other human NoV (HuNoV) VLPs from both GI and GII (8). For this reason, it is necessary to use VLPs of different genogroups and clusters to determine possible binding of HuNoV VLPs to porcine tissues.
The expression of HBGAs on RBCs is a recent evolutionary event, being present only in humans and anthropoid apes (23). However, pigs express A and H antigens on their tissues (26) and also have a gene homolog to the human FUT2 gene, the gene expressing GDP-l-fucose:β-d-galactoside α-1-2-l-fucosyltransferase (30). Swine also express A, H, or I antigens in their gut epithelial brush border (1) (the I antigen lacks the terminal fucose residue that characterizes the H antigens and therefore fails to react with monoclonal antibodies [MAbs] to A or H antigens). Therefore, VLPs from various HuNoV strains might bind to swine tissues expressing A or H antigens and, if so, this binding should be blocked by A- or H-specific MAbs or mucins containing these carbohydrates, which may aid in confirming their binding specificities.
In humans, histo-blood group typing is readily performed using human RBCs. This method is not reliable for pigs, as the A/H antigen levels present on swine RBCs are low (34). Thus, a more reliable test is needed to determine the pig's A/H phenotype for HuNoV studies prior to inoculation, to match the porcine A/H phenotype with comparable phenotype-specific HuNoV strains and to evaluate the roles of these antigens in the differential susceptibilities of swine to HuNoV strains.
Previously, in our HuNoV pathogenesis study, we observed that 44% of the 65 gnotobiotic (Gn) pigs orally inoculated with the HS66 strain of HuNoV shed virus in their feces and 59% seroconverted upon exposure to the HuNoV strain (4). The strain used in that study, HS66, belongs to GII/4, the most common cluster circulating in humans worldwide. In this study, we examined the A/H phenotype of the 65 HS66-inoculated pigs by indirect immunofluorescence (IF) and compared these data to virus infection results to determine the relationship between A/H phenotype and susceptibility to HS66 infection. We observed that pigs that expressed either the A or H antigen on the intestinal mucosa had significantly higher rates of diarrhea and seroconversion in response to the NoV strain, and fecal virus shedding was also significantly higher. Therefore, in pigs as well as humans, HuNoV VLP binding may be indicative of susceptibility to NoV infection.
MATERIALS AND METHODS
Animals.
In the present study, archival paraffin-embedded duodenal and buccal tissues of 79 Gn pigs from our previous pathogenesis study of an HuNoV GII/4 strain in the Gn pig model (4) were typed for their A and/or H antigen expression (Fig. 1). These 79 pigs (5- to 50-day-old Hampshire breed), included the 65 virus-inoculated pigs and 14 mock-inoculated controls, but only the tissues of the 65 inoculated pigs were considered in relation to the infection outcome (Fig. 1). A subset of the Gn pigs (n = 38), as well as two sows (from the Hampshire herd), were tested for their expression of HBGAs on buccal epithelial cells from the live pigs (Fig. 1). Ten additional age-matched conventional pigs from a different herd (Berkshire and Landrace cross-breeds) were also tested for their A/H phenotype to address whether A/H phenotype frequency distribution was dependent on the herd and breed of origin (Fig. 1). Tissues from 4 of the 14 mock-inoculated Gn pigs were also used in the VLP binding and blocking assays.
FIG. 1.

Experimental design summary.
A/H phenotyping by IF of intestinal and buccal tissues in paraffin sections.
Archival paraffin blocks containing samples of intestinal and buccal tissues were sectioned. Buccal tissues constituted complete resections of the cheek tissue, including the inside and outside of the mouth epithelia, but the cross section of this tissue was oriented to be exposed on the paraffin block. The collected portions of the duodenum jejunum and ileum were described previously (4). Slides were kept at 60°C for 20 min, deparaffinized in xylene twice for 5 min, and rehydrated through a graded ethanol series. To unmask the antigens, proteinase K (DAKO, Carpinteria, CA) treatment was applied for 3 min, and then the slides were washed in phosphate-buffered saline (PBS) buffer (10 mM potassium phosphate, 150 mM NaCl, pH 7.4) and blocked with PBS containing 1% normal goat serum for 20 min at room temperature (RT). Then the slides were incubated with MAb to human blood group A (undiluted), derived from a single clone line, Birma-I (Immucor, Norcross, GA); BG-4 MAb to human H1 (1:100) (Signet); or BRIC 231 MAb (1:100) (Biogenesis, United Kingdom), which recognizes the human H2 antigen, for the detection and subtyping of A, H1, and H2 antigens. A goat anti-mouse immunoglobulin G (IgG) conjugated to Alexa488 at a 1:500 dilution (Invitrogen, Carlsbad, CA) was used as a secondary antibody for 1 h at RT. Cell nuclei were counterstained with propidium iodide (Invitrogen) at a concentration of 3 μg/ml, and the slides were then observed using a Leica TCS-SP laser-scanning confocal microscope (Leica, Wetzlar, Germany).
NoV VLP binding and blocking assays using tissue slides.
Tissues from control pigs (C1 to C4) were used for the HuNoV binding and blocking assays. The slides were deparaffinized and the antigens unmasked as described above. The slides were incubated with NoV VLPs of NV, GI/1 (kindly provided by M. K. Estes, Baylor College of Medicine, TX); Desert Shield virus (DSV), GI/3; Hawaii virus (HV), GII/1; Toronto virus (TV), GII/3 (kindly provided by S. Monroe, Centers for Disease Control); MD145, GII/4 (kindly provided by K. Green, National Institutes of Health); or HS66, GII/4 (produced in our lab) at a concentration of 5 μg/ml in PBS buffer and incubated at RT overnight. Unfortunately, no porcine NoV VLPs were available for these studies. Detection of the VLPs was performed using rabbit or guinea pig antiserum specific to each VLP (1:500) (also provided by Estes, Monroe, and Green) followed by the goat anti-rabbit or anti-guinea pig IgG Alexa488 at a 1:400 dilution (Invitrogen) for 1 h at RT. Cell nuclei were counterstained with propidium iodine. For the blocking assays, prior to the incubation with the NoV VLPs, slides were first incubated with anti-A-, anti-H1-, or anti-H2-specific MAbs as described for the A/H typing assay and then incubated with the above VLPs. A control experiment included an additional step of preincubation of each VLP type with its specific VLP antiserum using the same conditions (time and temperature) and concentrations as described above.
For the inhibition experiments using mucins from porcine stomach (type III) and from bovine submaxillary glands (type 1-S) (Sigma, St. Louis, MO), a concentration of 1 μg/ml mucins was incubated with the VLP for 1 h at 37°C prior to being added to deparaffinized and rehydrated slides. Prior to their use, mucins were first typed for their content of A/H1/H2 carbohydrates by an enzyme-linked immunosorbent assay (ELISA). Briefly, duplicate wells were coated with porcine and bovine mucins (1 μg/ml) in coating buffer (0.05 M carbonate buffer, pH 9.6) and plates were incubated at 4°C overnight. Two percent skim milk in PBS buffer was added for 1 h at 37°C. After being washed, the slides were incubated with MAb to type A (1:10), MAb BG-4 to H1 (1:100), or MAb BRIC 231 to H2 (1:100) primary antibodies for 2 h at 37°C. A goat anti-mouse IgG conjugated to horseradish peroxidase (1:2,000 dilution) (DAKO) was used as a secondary antibody for 1 h at RT, and the assay was developed with tetramethylbenzidine (KPL, Gaithersburg, MD) following the manufacturer's instructions. Plates were washed four times between each step with PBS containing 0.5% Tween 20. Positive samples were those with an absorbance equal to or greater than the cutoff value, which was defined as the mean of the negative control wells (no primary antibody and primary MAb to an unrelated epitope [spike protein of transmissible gastroenteritis virus 25C9]) (27) plus three times the standard deviation.
HI test for A/H typing of pig buccal cells.
A hemagglutination inhibition (HI) test (Fig. 2) was developed to determine the pig's A/H phenotype by using parakeratinized oral squamous epithelium (buccal) cells collected from live pigs. The HI procedure was adapted from a previously described method (9). Briefly, sterile cotton-tipped swabs were used to vigorously swab the inside of the mouths of live pigs and were then placed in a 15-ml tube containing PBS (pH 7.4). After the swabs were twirled and removed from the tubes, the tubes were centrifuged at 120 × g for 15 min at 4°C. The supernatant was discarded and the pelleted cells were washed twice in PBS. The buccal cell numbers were standardized to 1.5 × 105 cells per well after adjusting the dilution according to the cell count in a Neubauer counting chamber and were added to duplicate wells of V-shaped microtiter plates (Nalge, Nunc, Rochester, NY). Next, MAb Bric231 to H2 (1:50) or the MAb to A (1:10) was added to the wells and the plates were incubated for 1 h at 37°C. Human RBCs of A or O (H) types (Immucor) were diluted to 0.5% in Veronal buffer and then type A RBCs were incubated for 2 h at 4°C and type H RBCs were incubated overnight at 4°C. Subsequently, the plates were read visually and the results were recorded. The method and interpretation of results are outlined in Fig. 2. Human RBCs, as well as parakeratinized epithelial cells from the oral mucosa, have low H1 but high H2 levels on their surface; therefore, the test was standardized using the MAb to H2. Although the MAb to H2 cross-reacts with A1 RBCs (as described by the manufacturer), this outcome can be clearly identified: A+ pigs are reactive only with MAb to A, whereas H+ pigs are reactive with MAbs to both A and H2 (Fig. 3).
FIG. 2.
Schematic of results of hemagglutination inhibition test using buccal cells to determine the A/H phenotypes of live pigs.
FIG. 3.

Hemagglutination inhibition test results. Pig buccal cells (columns) were loaded in duplicate wells. The top two rows have anti-A MAb and A+ human RBCs. The bottom two rows have anti-H2 MAb and H+ human RBCs. The anti-H2 MAb cross-reacts with A1+ RBCs (a human subtype) according to the manufacturer; therefore, H+ was reactive only with anti-H2 MAb, whereas A+ could be reactive with both MAbs. The controls (*) included human buccal cells of known A+ and H+ phenotypes and PBS.
Statistical analysis.
The Fisher's exact test was performed to assess the statistical significance of the proportion of A- or H-phenotype pigs or the A/H-expressing compared to the non-A/H-expressing phenotypes that seroconverted, shed virus, and/or had diarrhea upon exposure to HS66 (see Table 4). Correlations between A/H expression and viral shedding, diarrhea, and seroconversion were analyzed by the use of Pearson's correlation coefficient. Statistical significance was assessed at a P value of <0.05. Statistical Analysis System (SAS Institute, Inc., Cary, NC) was used to analyze the data.
TABLE 4.
Summary of results and percentages from 2 and 3
| Phenotype (no.) | No. (%) responding to HS66 inoculation with:
|
||
|---|---|---|---|
| Seroconversion | Viral shedding | Diarrhea | |
| A (43) | 9/12 (75%) | 26/43 (60%) | 35/43 (81%) |
| H (14) | 3/6 (50%) | 3/14 (21%) | 11/14 (79%) |
| ? (8) | 1/4 (25%) | 1/8 (12.5%) | 3/8 (37.5%) |
RESULTS
A/H antigen distribution in swine intestinal and buccal tissue sections of gnotobiotic pigs assessed by IF.
Monoclonal antibodies to human HBGAs (A, H1, and H2) recognized the swine homologs. The distribution of the A and H antigens in the intestinal and buccal tissues varied widely among the 79 Gn pigs tested, but general patterns could be identified. As depicted in the IF results, in A+ pigs, the apical surface of some enterocytes, the whole cytoplasm in goblet cells as well as crypt cells, and Brunner glands in duodenal tissue reacted strongly with the MAb to type A (Fig. 4A). The jejunum and ileum tissues of some pigs were negative, whereas in others they were positive but with fewer reactive cells, goblet cells, and enterocytes (not shown). The presence of HBGAs in sections of the distal small intestine did not relate to the Gn pigs' ages examined at 5 to 50 days postderivation. The majority of A+ pigs also expressed H antigens on enterocytes or crypt cells (Fig. 4B and C) or in their Brunner glands (not shown). In the buccal tissues, A antigen expression was observed in the spinous cell layer (see Fig. 6A), in the minor salivary glands, and in the duct linings (not shown).
FIG. 4.
Results of IF assay for A/H phenotyping. (A to C) A+ pig phenotype (pig 52; see Table 3); (D to F) H+ pig phenotype (pig 28; see Table 3). (A) The duodenal tissue of an A+ pig showed signal in goblet cells (arrowhead), the surface of enterocytes (thin arrow), and some crypts (thick arrow) with MAb to type A. (B) The same A+ pig tissue showed signal in some surface enterocytes (thin arrow) but not in goblet cells or crypts with MAb to H1. (C) The same A+ pig tissue showed a localized reaction with enterocytes (thin arrow) and had a few crypt cells stain (thick arrow) with MAb to H2. (D) The duodenal tissue of an H+ pig showed no reaction with MAb to type A. (E) The duodenal tissue from the same H+ pig showed no reaction with MAb to H1. (F) The duodenal tissue from the same H+ pig showed reactions in goblet cells (arrowhead), the surface of enterocytes (thin arrow), and some crypt cells (thick arrow) with MAb to H2. Bars, 100 μm.
FIG. 6.
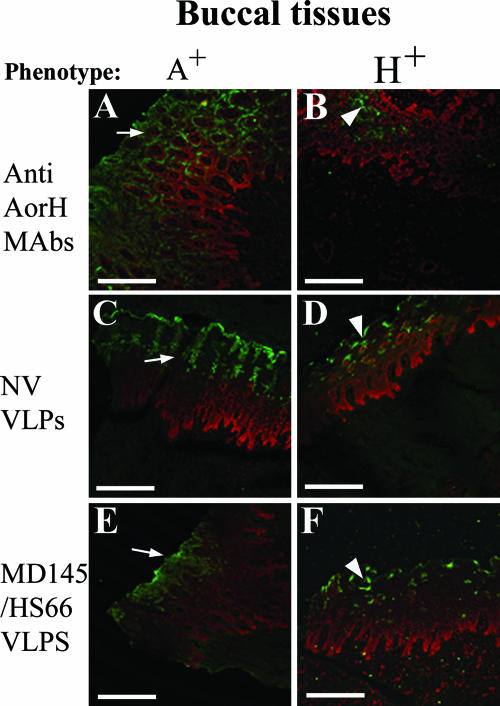
Results of IF of VLP binding in buccal tissues. Arrows denote spinous cell layers, white pointers denote spinous parabasal cell layer transitions, black arrows denote buccal salivary glands, and white arrows denote salivary ducts. (A) In the parakeratinized stratified squamous epithelium of the oral mucosa (buccal tissue) from an A+ pig, MAb to A antigen reacted with the spinous layer. (B) Buccal tissue from an H+ pig reacted deeper in the parabasal cell layer with MAb to type H2. (C) NV VLPs bound to A+-phenotype pig buccal tissue (that also expresses H2) at the spinous layer. (D) NV VLPs bound deeper, at the spinous parabasal layer transition, to H+-phenotype pig buccal tissue. (E) MD145 VLPs (as well as HS66) bound to A+-phenotype pig buccal tissue (also expressing H2) at the superficial spinous cell layer. (F) MD145 VLPs (as well as HS66) bound deeper and less extensively to H+-phenotype pig buccal tissue. Bars, 100 μm.
In the H+ pigs, enterocyte apical surfaces, goblet-cell cytoplasm, and crypt cells (Fig. 4F) and Brunner glands (not shown) reacted with the H MAbs. The H+ pigs did not express any A antigen (Fig. 4D), but H1 and H2 expression varied in their distributions and amounts in the duodenal and buccal tissues within each pig with pigs expressing either H1 or H2 (Fig. 4E and F). In the buccal tissues, H2 was strongly expressed in the parabasal cell layer (see Fig. 6B) and the duct linings and weakly in the minor salivary glands (not shown). H1 was expressed in either case in the same location as H2 (duct linings) or in the same tissue but with a different pattern (spinous layer), and in some cases, H1 was not expressed (data not shown).
NoV GI and GII VLP binding to swine buccal and intestinal tissues.
The VLP binding assays and blocking assays were performed on duodenal and buccal tissues from four mock-inoculated Gn pigs of known phenotype (two A+ and two H+).
Binding of HuNoV to tissues of A+ phenotype.
The NV (GI/1), HV (GII/1), TV (GII/3), HS66, and MD145 (both GII/4) VLPs attached only to the duodenum (Fig. 5A, C, E, and G) and not to distal regions of the small intestine of A+ pigs (not shown). The DSV VLPs did not attach to any sections on the A+ pigs (not shown). The HS66 VLPs had regional and tissue distributions similar to those of the MD145 VLPs (Table 1). The VLPs of each genotype showing binding attached with a unique pattern to the same A+ pig tissue in consecutive sections from the same block (Fig. 5A, C, E, and G). The binding was extensive for NV, HS66, and MD145 VLPs (Fig. 5A and G) in both A+ pigs and more limited for HV, and only a few cells stained for TV in only one of the A+ pigs (Fig. 5C and E) (Table 1). Binding to the parakeratinized stratified squamous epithelium of the oral mucosa of an A+ phenotype pig was extensive on the portion of the spinous layer where cells are flat (more superficial) for NV, MD145, and HS66 VLPs (Fig. 6 C and E).
FIG. 5.
Results of IF of VLP binding in duodenal tissues of control pigs (mock-inoculated) of A+ and H+ phenotypes: arrowheads denote goblet cells, thin arrows denote the surface of enterocytes, thick arrows denote crypts, and black arrows denote Brunner glands. (A) Tissue of A+ pig with NV (GI/1) VLPs that bound to some goblet cells, the surface of some enterocytes, and, minimally, to crypts. (B) Tissue of H+ pig with NV VLPs that bound to some goblet cells and, extensively, to enterocyte surfaces as well as Brunner glands. (C) Tissue of A+ pig with HV (GII/1) VLPs that reacted within certain areas of the intestinal section, where signal was observed in some goblet cells and enterocytes. (D) Tissue of H+ pig with HV VLPs showing reactivity in enterocytes but not with goblet cells. (E) Tissue of A+ pig with TV (GII/3) VLPs that bound in a few locations to some enterocytes. (F) Tissue of H+ pig with TV VLPs showing more extensive binding to enterocytes. (G) Tissue of A+ pig with MD145 (GII/4) VLPs, as well as HS66 VLPs (not shown), that bound to scattered enterocytes and Brunner glands. (H) Tissue of H+ pig with MD145 (same for HS66) VLPs that bound more extensively with enterocytes than with (few) goblet cells and Brunner glands. Bars, 100 μm.
TABLE 1.
Results of the qualitative analysis of the NoV VLP binding and A or H MAb blocking assays done on paraffin sections of control duodenal and buccal tissues of A+ and H+ Gn pigs
| Pig; A/H phenotype | Type of tissue | MAb used to block | Results with HuNoV VLP of indicated genogroupa
|
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GI/1
|
GI/3
|
GII/1
|
GII/3
|
GII/4
|
|||||||||||||||
| NV binding assay | NV blocking assay | Expected resultc | DSV Binding assay | DSV Blocking assay | Expected resultc | HV binding assay | HV blocking assay | Expected resultc | TV binding assay | TV blocking assay | Expected resultc | HS66 binding assay | HS66 blocking assay | MD145 binding assay | MD145 blocking assay | Expected resultc | |||
| C1; A+ (H1+H2+)b | Duodenal | Anti-A | + | NB | NB | − | na | na | ++ | NB | NB | + | NB | NB | ++ | PB | + | PB | PB |
| Anti-H1 | + | NB | PB | − | na | na | ++ | NB | NB | + | PB | NB | ++ | PB | + | PB | PB | ||
| Anti-H2 | + | NB | PB | − | na | na | ++ | NB | NB | + | PB | NB | ++ | PB | + | PB | PB | ||
| C2; A+ (H1+H2+) | Duodenal | Anti-A | ++ | NB | NB | − | na | na | − | na | NB | − | na | NB | + | NB | + | NB | PB |
| Anti-H1 | ++ | PB | PB | − | na | na | − | na | NB | − | na | NB | + | NB | + | NB | PB | ||
| Anti-H2 | ++ | PB | PB | − | na | na | − | na | NB | − | na | NB | + | PB | + | NB | PB | ||
| Buccal | Anti-A | + | PB | NB | − | na | na | − | na | NB | − | na | NB | + | NB | + | NB | PB | |
| Anti-H1 | + | PB | PB | − | na | na | − | na | NB | − | na | NB | + | NB | + | PB | PB | ||
| Anti-H2 | + | NB | PB | − | na | na | − | na | NB | − | na | NB | + | PB | + | PB | PB | ||
| C3; H2+ (A−H1−) | Duodenal | Anti-A | + | NB | NB | − | na | na | − | na | NB | +/− | NB | NB | ++ | NB | ++ | NB | NB |
| Anti-H1 | + | NB | NB | − | na | na | − | na | NB | +/− | PB | NB | ++ | PB | ++ | PB | PB | ||
| Anti-H2 | + | NB | PB | − | na | na | − | na | NB | +/− | PB | NB | ++ | PB | ++ | PB | PB | ||
| Buccal | Anti-A | + | NB | NB | +/− | NB | na | − | na | NB | +/− | NB | NB | ++ | NB | ++ | NB | NB | |
| Anti-H1 | + | NB | NB | +/− | NB | na | − | na | NB | +/− | PB | NB | ++ | PB | ++ | PB | PB | ||
| Anti-H2 | + | NB | PB | +/− | PB | na | − | na | NB | +/− | PB | NB | ++ | PB | ++ | PB | PB | ||
| C4; H1+ (A−H2−) | Duodenal | Anti-A | ++ | NB | NB | − | na | na | − | na | NB | − | na | NB | + | NB | + | NB | NB |
| Anti-H1 | ++ | NB | PB | − | na | na | − | na | NB | − | na | NB | + | PB | + | PB | PB | ||
| Anti-H2 | ++ | NB | NB | − | na | na | − | na | NB | − | na | NB | + | PB | + | PB | PB | ||
| Buccal | Anti-A | ++ | NB | NB | − | na | na | − | na | NB | − | na | NB | + | NB | + | NB | NB | |
| Anti-H1 | ++ | NB | PB | − | na | na | − | na | NB | − | na | NB | + | PB | + | PB | PB | ||
| Anti-H2 | ++ | NB | NB | − | na | na | − | na | NB | − | na | NB | + | PB | + | PB | PB | ||
NB, no blocking; NP, partial blocking; na, not applicable because of no binding. The plus and minus signs are qualitative assessments of the presence or absence of binding. Double plus signs indicate more extensive binding, whereas the combination of plus and minus signs refers to poor binding. (Antigens bound by reference strains in the indicated genogroups were previously determined; specific reference strains are in Discussion: GI/1 binds to H types 1, 2, and 3 and Led,b,y; GI/3 was not tested; GII/1 binds to Leb; GII/3 binds to Leb; and GII/4 binds to A, B, H types 1 and 3, and Leb.)
Buccal tissue not done.
Expected blocking result from reference binding in vitro.
Binding results of HuNoV to tissues of H+ phenotype.
In the H+ pigs, NV, HS66, and MD145 VLPs bound extensively to H1- and H2-expressing duodenal (Fig. 5B and H) and buccal tissue sections at the spinous-parabasal cell layer junction (Fig. 6D and F) (Table 1), whereas HV and TV VLPs bound less extensively to both buccal (photos not shown) and intestinal tissues (Table 1 and Fig. 5D and F). Qualitatively, more NV VLPs bound to enterocytes in tissues from the pig expressing H1, whereas HS66 and MD145 VLPs bound more to the enterocytes of the pig expressing mainly H2 (Table 1).
Blocking of VLP binding to the buccal and intestinal tissues.
Incubation of the slides with MAbs to A, H1, or H2 antigens prior to the addition of NV VLPs did not block VLP binding in tissues of either of the two H+ pigs or in one of the two A+ pigs (Table 1, pigs C1, C3, and C4). However, the MAbs partially blocked (50% reduction) the NV VLPs from binding to the tissue sections of an A+ pig also expressing H1 and H2 when using the H1 and H2 MAbs for the duodenal tissue and A and H1 MAbs in the buccal tissue (Table 1, pig C2). In contrast, the DSV VLPs were inhibited from binding to the buccal tissue duct lining by the H2 MAb, whereas the H1 MAb had no effect (Table 1, pig C3). HV VLP binding to an A+ pig was not affected by prior incubation with A, H1, or H2 MAbs (Table 1, pig C1), whereas there was a 50% reduction of TV VLP binding by the H1 and H2 MAbs in tissues of both the A+ and H+ pigs (Table 1, pigs C1 and C3). For the HS66 and MD145 VLPs, incubation with MAbs to A, H1, or H2 reduced binding by 30% in the duodenal tissue of one of the A+ pigs (Table 1, pig C1) but the results for the other A+ pig showed that the H2 MAb only partially blocked the VLP binding (Table 1, pig C2). When the A MAb was used in tissues of one of the A+ pigs, the signal was reduced by 30% in the villus epithelium, but not in the crypts (not shown). In the case of the H+ pigs, the H1 and H2 MAbs reduced the binding of the HS66 and MD145 GII/4 VLPs to the duodenal tissue sections (Table 1, pigs C3 and C4). The attachment of HS66 and MD145 VLPs to buccal epithelium and minor salivary gland tissues was not prevented by incubation with type A MAb, but it was decreased by incubation with H1 and H2 MAbs prior to VLP addition in the H+ pigs (Table 1, pigs C3 and C4). When a mixture of MAbs (to A, H1, and H2) was used, more extensive blocking (80%) was observed (not shown), suggesting that other factors may be involved in NoV binding to tissues of pigs.
As a control, we also incubated each VLP type with a specific antibody to each VLP prior to their addition to the sections. After the washing and completion of the assays, no VLPs were observed binding to any of the tissues, confirming the ability of antibodies to each specific VLP to block binding of the VLPs to the intestinal and buccal sections.
The mucins of porcine and bovine origin were typed for HBGAs in an ELISA (coating with the mucin and detecting with HBGA MAbs). Both the bovine and porcine mucin contained high amounts of A antigen, whereas only porcine mucin had H antigens. Levels of H antigens in the porcine mucin were lower than bovine A antigen levels. The blocking experiment using mucin was performed with NV (GI/1) and MD145 (GII/4) VLPs on duodenum tissue sections of pig C2, with an A+ (H1+H2+) phenotype, and pig C3, with an H2+ (A−H1−) phenotype (Table 1). Complete blocking was observed for both VLPs in both phenotypes using the porcine mucin, whereas bovine mucin did not block binding of either VLP to either of the HBGA phenotypes.
Hemagglutination inhibition test to determine pigs' A/H phenotypes.
The HI test using buccal cells of live pigs was rapid and reliable in determining their A/H phenotypes. Of the 38 Gn pigs tested at different ages (a subset of 38 of the 79 piglets in the pathogenesis study, 6 to 50 days old) and 2 sows from the same herd, 23 were A+ and 8 were H+, with 9 unclassified. Most of the unclassified pigs were only a few days old. Sampling of these pigs several weeks later permitted the phenotype determination in some cases (three pigs). For some pigs whose A/H phenotype was inconclusive by the HI test, the phenotypes were confirmed by IF of their intestinal tissues after euthanasia. As described by others (14), low temperature and neutral pH were essential for agglutination to occur. It is unknown why the H reaction needed a longer time to agglutinate the RBCs, but it could be related to the density of the antigen (18).
A/H phenotyping of the pigs from the HuNoV pathogenesis study (4).
To determine the A/H phenotypes of the 79 Gn pigs from the HuNoV pathogenesis study (65 HuNoV-inoculated and 14 mock-inoculated control pigs), archival samples were tested by IF (Tables 2, 3, and 4). Forty-eight (60%) of the Gn pigs (n = 79) had an A+ (H+) (and in a few cases, H−) phenotype, 21 (27%) had an H+ (A−) phenotype, and the type could not be determined in 10 pigs (13%). Most of the Gn pigs that came from a single closed herd of Hampshire breed were A+. When 10 conventional cross-bred Berkshire and Landrace pigs from another herd were tested, 6 (60%) were H+, 2 (20%) were A+, and 2 (20%) were A and H negative.
TABLE 2.
Data for pigs euthanized in the acute phase: viral shedding and diarrhea in relation to A/H phenotype
| Pig | Inoculuma | Phenotypeb | Viral sheddingc | Diarrhead |
|---|---|---|---|---|
| 1 | P0, oral | A | no | yes |
| 4 | P0, oral | A | yes | yes |
| 14 | P0, oral | A | yes | yes |
| 15 | P0, oral | A | yes | yes |
| 16 | P0, oral | A | yes | no |
| 18 | P0, oral | ? | yes | no |
| 19 | P0, oral | A | yes | yes |
| 20 | P0, oral | H | yes | yes |
| 22 | P0, oral | H | yes | yes |
| 23 | P0, oral | H | yes | yes |
| 28 | P0, oral | H | no | no |
| 29 | P0, oral | A | no | yes |
| 30 | P0, oral | A | no | yes |
| 31 | P0, oral | A | no | yes |
| 34 | P0, oral | A | no | no |
| 35 | P0, oral | A | yes | yes |
| 36 | P0, oral | A | yes | yes |
| 47 | P0, oral | A | yes | yes |
| 54 | P0, oral | H | no | yes |
| 56 | P0, oral | A | no | yes |
| 59 | P0, oral | A | no | yes |
| 67 | P0, oral | ? | no | no |
| 68 | P0, oral | A | yes | yes |
| 69 | P0, oral | A | yes | yes |
| 70 | P0, oral | A | yes | no |
| 71 | P0, oral | A | yes | yes |
| 8 | P1, oral | H | no | yes |
| 25 | P1, oral | H | no | yes |
| 26 | P1, oral | A | no | yes |
| 27 | P1, oral | H | no | yes |
| 43 | P1, oral | A | no | yes |
| 44 | P1, oral | A | no | yes |
| 52 | P1, oral | A | yes | yes |
| 62 | P1, oral | A | no | yes |
| 63 | P1, oral | A | no | yes |
| 75 | P1, oral | A | no | no |
| 76 | P1, oral | A | yes | no |
| 37 | P2, oral | A | yes | yes |
| 39 | P2, oral | A | yes | yes |
| 40 | P2, oral | A | yes | yes |
| 64 | P2, oral | A | no | no |
| 72 | P2, oral | ? | no | no |
| 73 | P2, oral | ? | no | no |
P0, original human fecal sample; P1, Gn pig intestinal contents after one passage; P2, Gn pig intestinal contents after two passages.
Histo-blood group phenotypes. A = A+, H = H+, and ? = Non-A/H.
Defined as shed virus in rectal swab samples detected by reverse transcription-PCR for 2 or more days.
Defined as ≥2 days of diarrhea.
TABLE 3.
Data for pigs euthanized in the convalescent phase: shedding, diarrhea, and seroconversion in relation to A/H phenotype
| Pig | Inoculuma | Phenotypeb | Sheddingc | Diarrhead | Seroconversione |
|---|---|---|---|---|---|
| 2 | P0, oral | ? | no | yes | Neg |
| 3 | P0, i.v. | A | yes | yes | Pos |
| 17 | P0, oral | ? | yes | yes | Pos |
| 21 | P0, oral | H | yes | no | Neg |
| 32 | P0, oral | A | yes | no | Neg |
| 33 | P0, oral | A | yes | no | Neg |
| 46 | P0, oral | A | yes | yes | Pos |
| 48 | P0, oral | A | yes | yes | Pos |
| 53 | P0, oral | A | yes | no | Pos |
| 57 | P0, oral | A | no | yes | Pos |
| 58 | P0, oral | H | no | yes | Pos |
| 7 | P1, oral | H | no | yes | Pos |
| 9 | P1, i.v. | A | no | yes | Pos |
| 10 | P1, oral | H | no | yes | Neg |
| 24 | P1, oral | ? | no | no | Neg |
| 45 | P1, oral | ? | no | yes | Neg |
| 51 | P1, oral | A | yes | yes | Pos |
| 77 | P1, oral | H | yes | yes | Pos |
| 38 | P2, oral | A | yes | yes | Neg |
| 66 | P2, oral | H | no | no | Neg |
| 65 | P2, oral | A | no | yes | Pos |
| 74 | P2, oral | A | yes | yes | Pos |
P0, original human fecal sample; P1, Gn pig intestinal contents after one passage; P2, Gn pig intestinal contents after two passages; i.v., intravenous.
Histo-blood group phenotypes. A = A+, H = H+, and ? = Non-A/H.
Defined as shed virus in rectal swab samples detected by reverse transcription-PCR for 2 or more days.
Defined as ≥2 days of diarrhea.
Seroconversion tested by IgG antibody ELISA using NoV GII/4 VLPs as antigen. Neg, negative; Pos, positive.
When comparing the A/H phenotypes to the infection outcomes of the 65 pigs previously inoculated with the HS66 strain (Tables 2, 3, and 4) (4), virus-infected pigs included both type A+ and H+ pigs. Seroconversion (measured by detection of HS66-specific IgG by antibody ELISA) occurred in 12 of 18 (66%) A+/H+ pigs but only 1 of 4 (25%) of the non-A+/H+ pigs from the 22 convalescent pigs tested (P = 0.0045) (Tables 3 and 4). When A+ and H+ phenotypes were compared, 9 of 12 and 3 of 6 pigs seroconverted, respectively. There was no statistically significant difference in the latter comparison (P = 0.36) (Tables 3 and 4). Virus shedding was detected in significantly more (P = 0.043) (29/57 [51%]) of the A+/H+ pigs than in the non-A+/H+ pig group (1/8 [12.5%]). Twenty-six of 43 (60%) A+ pigs shed virus in their feces compared to 3 of 14 (21%) H+ phenotype pigs (Tables 2, 3, and 4). The differences observed between fecal virus-shedding rates and A and H HBGA phenotypes were not statistically significant (P = 0.079). However, there was a significant difference for the incidence of diarrhea in the A+/H+ group (46/57 [81%]) compared to its incidence in the non-A+/H+ pigs (37.5%) (P = 0.043). The difference was not significant when analyzing the A+ and H+ pig phenotypes separately, where 35 of 43 A+ and 11 of 14 H+ pigs had diarrhea (P = 0.26). Therefore, the expression of A and/or H antigens in Gn pigs was associated with significantly higher rates of diarrhea and seroconversion in response to HuNoV. Fecal virus-shedding rates were also significantly higher in the A+/H+ pigs than in the non-A+/H+ pigs (P = 0.043) (Tables 2, 3, and 4).
DISCUSSION
The distribution of HBGAs in the pigs was similar to their expression patterns in humans as described previously (24). In humans, expression of ABO(H) on the villas surface is confined almost exclusively to Se+ (24). According to this information, the majority of the Gn pigs in our pathogenesis study were likely Se+, because they expressed A or H antigens on the surface epithelia of the intestine. However, there is no information on inactivation mutations in the porcine FUT2 gene to be evaluated by PCR in order to confirm our findings. Of the fewer pigs that were negative for A and antigens (10 Gn and 2 conventional pigs), an Se− phenotype was less probable, because neither A nor H antigens were seen in the Brunner glands of the intestine that in humans are independent of the FUT2 gene. Most likely, these pigs could have been of the I phenotype (for which we did not test).
Sixty percent of the pigs studied from our previous pathogenesis study were type A+, and 27% were type H+. The proportion of the A/H phenotypes in the swine population has not been reported. Our limited information suggests that A/H allelic frequencies may vary depending on the breed and/or herd.
In addition to the expression of the putative NoV receptors in the pig gastrointestinal tract as shown in the present study, the detection of NoV antibodies in swine, with more than 50% of the pigs having antibodies to GI and GII HuNoVs (6), raises questions about the possible role of pigs as reservoirs for NoV strains transmissible to humans. It has been reported that the VLPs from a swine NoV strain (SW918) did not bind to human saliva, but no data are available on the binding of human VLPs to pig saliva (6). We are currently testing the HuNoV VLPs for their binding to pig saliva. Unfortunately, no porcine NoV VLPs are currently available to test their binding patterns.
Typing of the ABO(H) antigens in human oral squamous epithelium tissue has been performed by immunohistochemistry (22), as was recently also described for monkeys and pigs (2). Similarly, our typing assay using buccal cells, described herein, permits A/H typing of live pigs in a fast and simple test. This test can help to optimize the Gn pig model to study the pathogenesis of other HuNoVs. In the case of inoculation with HS66, pigs expressing either A or H phenotypes became infected. This may not be the case for other NoV strains, so determination of the pig's A/H histo-phenotype would permit matching the porcine phenotype with the comparable phenotype-specific HuNoV strains prior to pig inoculation.
Norwalk virus and its VLPs bind H and Le antigens (7, 14). That Norwalk virus and its VLPs bind H and Le antigens was determined by in vitro studies (HA, HI, and treatment with periodate and synthetic carbohydrates) but also corroborated by human challenge studies (17). Both GI and GII HuNoVs have demonstrated differential binding patterns for Le, Se, and ABO(H) in vitro (10, 11, 29) and also in vivo (8). The binding patterns for VLPs of reference strains have been previously determined: NV (GI/1) binds H type 1, H type 2, H type 3, Lewis d (Led), Leb, and Ley; Lordsdale virus (GII/4) binds Leb, A/B, H1, and H3; HV (GII/1) binds Leb; TV (GII/3) binds Leb; and DSV (GI/3) was not tested (8). Strains that cluster together tend to have similar binding patterns, although no binding predictions can be made within genotypes or between them (11). Therefore, we expected the NoV GII/4 strain HS66, like the Lordsdale and MD145 GII/4 strains, to bind to both A and H in humans, but we did not know if the pig's A and H carbohydrates were similar enough in structure to be recognized by the HS66 VLPs or virus. We confirmed the binding of HS66 VLPs to the paraffin-embedded intestinal tissues of both A- and H-positive pigs.
Of relevance to our results for pigs, the FUT3 gene, which encodes a fucosyltransferase that produces the Le antigens, has not been identified in mammals other than hominids (19). Therefore, we did not expect VLPs with Le specificity to bind to our pig tissues. However, in our study, HV and TV VLPs that were previously described to bind only to Leb bound to A+-phenotype pig tissue and, to a lesser extent, to H+-phenotype pig intestinal tissues. Most mammals express HBGAs from the ABO(H) gene family, with some variations (19). Therefore, it is possible that pigs also have variations in a Lewis-like gene product. Our results also suggest that additional factors may be present that permit VLP binding to cells of the duodenum that are lacking in the distal small intestine, as the presence of A and H antigen alone did not guarantee VLP binding. The binding of HS66 VLP to only the duodenum of some pigs and not to the jejunum and ileum may be explained by the fact that many pigs expressed HBGAs mainly in the duodenum. This limited distribution of VLPs was surprising because, in our previous study of the pathogenesis of HuNoV in the Gn pigs (4), we observed HS66-infected cells in both the duodenum and the jejunum but only in a few cells in the ileum. These interpretations were further supported by the results of our blocking assays using MAbs to the A, H1, and H2 antigens, where blocking was incomplete with the MAbs used either separately or in combination. However, these results might indicate either that the epitope targeted by the MAb is different from the one used by the VLPs for binding or that other molecules are involved. In spite of the extensive data relating the ABO(H) and Se phenotypes to susceptibility or resistance to NoV, it remains unclear whether NoVs utilize HBGAs as primary receptors or coreceptors (8). In addition, some NoV GII strains (rUEV, rKAV, and rCHV) may bind two different receptors, the HBGAs and the heparan sulfate proteoglycan (28). Our data for both the distribution of the A/ H antigens compared to the distribution of VLP binding (to duodenal tissue) and the incomplete blocking of the VLP binding by A, H1, or H2 or a combination of these supports the existence of more than one cell receptor for NoVs.
Mucins in human breast milk from Se+ individuals could inhibit the binding of Norwalk virus VLPs to their carbohydrate ligands (25). Norwalk virus VLPs bound to porcine but not to bovine mucin (31). Mucins contain HBGAs, and goblet cells contain these mucins. Interestingly, goblet cell depletion has been described in children with intestinal transplants with osmotic or secretory diarrhea and NoV infections (15, 16, 21). However, in our pathogenesis study, NoV infection was not detected in this cell type, suggesting that goblet cell depletion could represent part of a host innate defense mechanism (5). More experiments are needed to substantiate this possibility.
In summary, our study demonstrated that pigs could be typed for the expression of HBGAs in their mucosal tissues and that the expression of A/H antigens in pigs had similar distribution to that in humans. We also observed that there was complete agreement between the expression of HBGAs in the buccal mucosa (determined by IF or HI) and the intestinal mucosa. Therefore, determination of the A/H phenotype is possible prior to NoV inoculation of live pigs. The VLP binding assays corroborated that NoV VLP binding specificities vary among virus genotypes in pig tissues as they do in human tissues and for in vitro binding of synthetic oligosaccharides. In addition, although some VLPs bound as expected (NV, MD145, and HS66), HV and TV VLPs also, surprisingly, bound to pig tissues, suggesting that their binding target in pigs could differ from their target in humans. Blocking assays using MAbs to A and H antigens indicated that it is likely that more than one oligosaccharide epitope is involved in VLP binding to the cell surface, and probably this epitope is present in porcine mucin, which was capable of totally blocking VLP binding to tissue slides. Finally, our data further support the findings of our previous work where we used Gn pigs as a model to study the pathogenesis of human NoVs, and in the present report, we determined that infection outcomes relate to HBGA expression in swine as in humans. To our knowledge this is the first study to compare HBGA expression to NoV infection response in the pig.
Acknowledgments
We thank M. G. Han and M. Azevedo for their suggestions and S. Monroe, K. Green, and M. K. Estes for the reagents they provided. We also thank J. Hanson and R. McCormick for the animal care and Andrea Kaszas and David Fulton in the Molecular and Cellular Imaging Center, who provided technical assistance. We thank Ken Theil and Daral Jackwood for reviewing the manuscript and Mark Kuhlenschmidt for his expert advice.
Salaries and research support were provided by state and federal funds appropriated to the Ohio Agricultural Research and Development Center, the Ohio State University. This work was supported by the National Institute of Allergies and Infectious Diseases, National Institutes of Health grant AI49742.
Footnotes
Published ahead of print on 10 January 2007.
REFERENCES
- 1.Balanzino, L. E., J. L. Barra, C. G. Monferran, and F. A. Cumar. 1994. Differential interaction of Escherichia coli heat-labile toxin and cholera toxin with pig intestinal brush border glycoproteins depending on their ABH and related blood group antigenic determinants. Infect. Immun. 62:1460-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Busch, J., S. Specht, M. Ezzelarab, and D. K. Cooper. 2006. Buccal mucosal cell immunohistochemistry: a simple method of determining the ABH phenotype of baboons, monkeys, and pigs. Xenotransplantation 13:63-68. [DOI] [PubMed] [Google Scholar]
- 3.Cheetham, S. 2006. Pathogenesis of HuNoV in the gnotobiotic pig model. Doctoral thesis. The Ohio State University, Columbus.
- 4.Cheetham, S., M. Souza, T. Meulia, S. Grimes, M. G. Han, and L. J. Saif. 2006. Pathogenesis of a genogroup II human norovirus in gnotobiotic pigs. J. Virol. 80:10372-10381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delmotte, P., S. Degroote, M. D. Merten, I. Van Seuningen, A. Bernigaud, C. Figarella, P. Roussel, and J. M. Perini. 2001. Influence of TNF-alpha on the sialylation of mucins produced by a transformed cell line MM-39 derived from human tracheal gland cells. Glycoconj. J. 18:487-497. [DOI] [PubMed] [Google Scholar]
- 6.Farkas, T., S. Nakajima, M. Sugieda, X. Deng, W. Zhong, and X. Jiang. 2005. Seroprevalence of noroviruses in swine. J. Clin. Microbiol. 43:657-661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington, P. R., L. Lindesmith, B. Yount, C. L. Moe, and R. S. Baric. 2002. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J. Virol. 76:12335-12343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harrington, P. R., J. Vinje, C. L. Moe, and R. S. Baric. 2004. Norovirus capture with histo-blood group antigens reveals novel virus-ligand interactions. J. Virol. 78:3035-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoet, A. E., K. O. Cho, K. O. Chang, S. C. Loerch, T. E. Wittum, and L. J. Saif. 2002. Enteric and nasal shedding of bovine torovirus (Breda virus) in feedlot cattle. Am. J. Vet. Res. 63:342-348. [DOI] [PubMed] [Google Scholar]
- 10.Huang, P., T. Farkas, S. Marionneau, W. Zhong, N. Ruvoen-Clouet, A. L. Morrow, M. Altaye, L. K. Pickering, D. S. Newburg, J. LePendu, and X. Jiang. 2003. Noroviruses bind to human ABO, Lewis, and secretor histo-blood group antigens: identification of 4 distinct strain-specific patterns. J. Infect. Dis. 188:19-31. [DOI] [PubMed] [Google Scholar]
- 11.Huang, P., T. Farkas, W. Zhong, M. Tan, S. Thornton, A. L. Morrow, and X. Jiang. 2005. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J. Virol. 79:6714-6722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hutson, A. M., R. Atmar, D. Y. Graham, and M. K. Estes. 2002. Norwalk virus infection and disease is associated with ABO histo-blood group type. J. Infect. Dis. 185:1335-1337. [DOI] [PubMed] [Google Scholar]
- 13.Hutson, A. M., R. L. Atmar, and M. K. Estes. 2004. Norovirus disease: changing epidemiology and host susceptibility factors. Trends Microbiol. 12:279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hutson, A. M., R. L. Atmar, D. M. Marcus, and M. K. Estes. 2003. Norwalk virus-like particle hemagglutination by binding to H histo-blood group antigens. J. Virol. 77:405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaufman, S. S., N. K. Chatterjee, M. E. Fuschino, M. S. Magid, R. E. Gordon, D. L. Morse, B. C. Herold, N. S. LeLeiko, A. Tschernia, S. S. Florman, G. E. Gondolesi, and T. M. Fishbein. 2003. Calicivirus enteritis in an intestinal transplant recipient. Am. J. Transplant. 3:764-768. [DOI] [PubMed] [Google Scholar]
- 16.Kaufman, S. S., N. K. Chatterjee, M. E. Fuschino, D. L. Morse, R. A. Morotti, M. S. Magid, G. E. Gondolesi, S. S. Florman, and T. M. Fishbein. 2005. Characteristics of human calicivirus enteritis in intestinal transplant recipients. J. Pediatr. Gastroenterol. Nutr. 40:328-333. [DOI] [PubMed] [Google Scholar]
- 17.Lindesmith, L., C. Moe, S. Marionneau, N. Ruvoen, X. Jiang, L. Lindblad, P. Stewart, J. LePendu, and R. Baric. 2003. Human susceptibility and resistance to Norwalk virus infection. Nat. Med. 9:548-553. [DOI] [PubMed] [Google Scholar]
- 18.Marionneau, S., F. Airaud, N. V. Bovin, J. L. Pendu, and N. Ruvoen-Clouet. 2005. Influence of the combined ABO, FUT2, and FUT3 polymorphism on susceptibility to Norwalk virus attachment. J. Infect. Dis. 192:1071-1077. [DOI] [PubMed] [Google Scholar]
- 19.Marionneau, S., A. Cailleau-Thomas, J. Rocher, B. Le Moullac-Vaidye, N. Ruvoen, M. Clement, and J. Le Pendu. 2001. ABH and Lewis histoblood group antigens, a model for the meaning of oligosaccharide diversity in the face of the changing world. Biochimie 83:565-573. [DOI] [PubMed] [Google Scholar]
- 20.Marionneau, S., N. Ruvoen, B. Le Moullac-Vaidye, M. Clement, A. Cailleau-Thomas, G. Ruiz-Palacois, P. Huang, X. Jiang, and J. Le Pendu. 2002. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology 122:1967-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morotti, R. A., S. S. Kaufman, T. M. Fishbein, N. K. Chatterjee, M. E. Fuschino, D. L. Morse, and M. S. Magid. 2004. Calicivirus infection in pediatric small intestine transplant recipients: pathological considerations. Hum. Pathol. 35:1236-1240. [DOI] [PubMed] [Google Scholar]
- 22.Noda, H., M. Yokota, S. Tatsumi, and S. Sugiyama. 2002. Determination of ABO blood grouping from human oral squamous epithelium by the highly sensitive immunohistochemical staining method EnVision+. J. Forensic Sci. 47:341-344. [PubMed] [Google Scholar]
- 23.Oriol, R., J. Le Pendu, and R. Mollicone. 1986. Genetics of ABO, H, Lewis, X and related antigens. Vox Sang. 51:161-171. [DOI] [PubMed] [Google Scholar]
- 24.Ravn, V., and E. Dabelsteen. 2000. Tissue distribution of histo-blood group antigens. APMIS 108:1-28. [DOI] [PubMed] [Google Scholar]
- 25.Ruvoen-Clouet, N., E. Mas, S. Marionneau, P. Guillon, D. Lombardo, and J. Le Pendu. 2006. Bile-salt-stimulated lipase and mucins from milk of ‘secretor’ mothers inhibit the binding of Norwalk virus capsids to their carbohydrate ligands. Biochem. J. 393:627-634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rydberg, L., J. Molne, V. Strokan, C. T. Svalander, and M. E. Breimer. 2001. Histo-blood group A antigen expression in pig kidneys—implication for ABO incompatible pig-to-human xenotransplantation. Scand. J. Urol. Nephrol. 35:54-62. [DOI] [PubMed] [Google Scholar]
- 27.Shoup, D. I., D. E. Swayne, D. J. Jackwood, and L. J. Saif. 1996. Immuno-histochemistry of transmissible gastroenteritis virus antigens in fixed paraffin-embedded tissues. J. Vet. Diagn. Invest. 8:161-167. [DOI] [PubMed] [Google Scholar]
- 28.Tamura, M., K. Natori, M. Kobayashi, T. Miyamura, and N. Takeda. 2004. Genogroup II noroviruses efficiently bind to heparan sulfate proteoglycan associated with the cellular membrane. J. Virol. 78:3817-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan, M., and X. Jiang. 2005. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 13:285-293. [DOI] [PubMed] [Google Scholar]
- 30.Thurin, J., and M. Blaszczyk-Thurin. 1995. Porcine submaxillary gland GDP-l-fucose: β-d-galactoside α-2-l-fucosyltransferase is likely a counterpart of the human secretor gene-encoded blood group transferase. J. Biol. Chem. 270:26577-26580. [DOI] [PubMed] [Google Scholar]
- 31.Tian, P., M. Brandl, and R. Mandrell. 2005. Porcine gastric mucin binds to recombinant norovirus particles and competitively inhibits their binding to histo-blood group antigens and Caco-2 cells. Lett. Appl. Microbiol. 41:315-320. [DOI] [PubMed] [Google Scholar]
- 32.Voet, D., and J. G. Voet. 1995. Biochemistry, vol. 1, 2nd ed., p. 301-304. John Wiley & Sons, New York, NY. [Google Scholar]
- 33.Wang, Q. H., M. G. Han, S. Cheetham, M. Souza, J. A. Funk, and L. J. Saif. 2005. Porcine noroviruses related to human noroviruses. Emerg. Infect. Dis. 11:1874-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamamoto, F., and M. Yamamoto. 2001. Molecular genetics basis of porcine histo-blood group AO system. Blood 97:3308-3310. [DOI] [PubMed] [Google Scholar]
- 35.Zheng, D. P., T. Ando, R. L. Fankhauser, R. S. Beard, R. I. Glass, and S. S. Monroe. 2006. Norovirus classification and proposed strain nomenclature. Virology 346:312-323. [DOI] [PubMed] [Google Scholar]