Abstract
Antipathogen immune responses create a balance between immunity, tolerance, and immune evasion. However, during gene therapy most viral vectors are delivered in substantial doses and are incapable of expressing gene products that reduce the host's ability to detect transduced cells. Gene transfer efficacy is also modified by the in vivo transduction of dendritic cells (DC), which notably increases the immunogenicity of virions and vector-encoded genes. In this study, we evaluated parameters that are relevant to the use of canine adenovirus serotype 2 (CAV-2) vectors in the clinical setting by assaying their effect on human monocyte-derived DC (hMoDC). We compared CAV-2 to human adenovirus (HAd) vectors containing the wild-type virion, functional deletions in the penton base RGD motif, and the CAV-2 fiber knob. In contrast to the HAd type 5 (HAd5)-based vectors, CAV-2 poorly transduced hMoDC, provoked minimal upregulation of major histocompatibility complex class I/II and costimulatory molecules (CD40, CD80, and CD86), and induced negligible morphological changes indicative of DC maturation. Functional maturation assay results (e.g., reduced antigen uptake; tumor necrosis factor alpha, interleukin-1β [IL-1β], gamma interferon [IFN-γ], IL-10, IL-12, and IFN-α/β secretion; and stimulation of heterologous T-cell proliferation) were also significantly lower for CAV-2. Our data suggested that this was due, in part, to the use of an alternative receptor and a block in vesicular escape. Additionally, HAd5 vector-induced hMoDC maturation was independent of the aforementioned cytokines. Paradoxically, an HAd5/CAV-2 hybrid vector induced the greatest phenotypical and functional maturation of hMoDC. Our data suggest that CAV-2 and the HAd5/CAV-2 vector may be the antithesis of Adenoviridae immunogenicity and that each may have specific clinical advantages.
Dendritic cells (DC) play a pivotal role in orchestrating and bridging innate, adaptive, and memory immunity (22, 43). Novel subtypes are also continuously being identified, ranging from Langerhans cells (myeloid) to plasmacytoids (lymphoid) (11). Each subtype can be artificially divided into immature or mature, which are characterized by phenotypically and functionally distinct characteristics. Immature DC sense their environment via nonspecific phagocytosis and recognize pathogens through separate signals for antigen uptake using C-type lectin and cellular activation of pattern recognition receptors. Danger signals, such as proinflammatory cytokines (tumor necrosis factor alpha [TNF-α] and alpha/beta interferon [IFN-α/β]), necrotic cells, and bacterial and viral residues, promote DC maturation. Pathogen residues are detected via evolutionarily conserved pattern recognition receptors, such as Toll-like receptors that recognize conserved microbe-associated molecules called “pathogen-associated molecular patterns” (80). During maturation, DC lose the ability to take up antigens, change their morphology, and migrate towards the lymphoid compartments. Once there, matured DC are primed for antigen-specific naïve T-cell presentation and stimulation via the expression of major histocompatibility complex (MHC) class I/II and costimulatory molecules (5). In addition to their central role in priming the naïve response, DC also efficiently restimulate the memory T-cell (TM) response.
Viral infection of DC can exert contrasting effects on antigen presentation, leading to immunity, tolerance, or viral latency. DC infected with influenza virus are able to bypass the recruitment for CD40 signals provided by CD4+ T cells for the generation of a cytotoxic T-lymphocyte (CTL) response (9, 21, 63). In contrast, viruses that poorly infect DC (e.g., human papillomavirus, herpes simplex virus type 1, and Epstein-Barr virus) can also induce an antiviral CTL response via antigen cross-presentation, although this generates an inefficient memory response (23, 42, 46, 59, 75, 78). Measles and vaccinia virus infection of human monocyte-derived DC (hMoDC) induces apoptosis and in turn decreases the T-cell response (18, 24).
Adenoviridae are nonenveloped icosahedral particles with 26- to 42-kb double-stranded DNA genomes (31). Surprisingly, Adenoviridae probably share an ancient common ancestor with bacteriophages of the Tectiviridae family (1, 6, 7). Several years ago we began developing vectors derived from canine adenovirus serotype 2 (CAV-2) (39, 41, 71-74), one of the greater than 60 nonhuman Adenoviridae. In the central nervous systems (CNS) of several species, CAV-2 vectors preferentially transduce neurons and lead to an efficient level of axoplasmic transport (73). Helper-dependent (HD) CAV-2 vectors also lead to long-term transgene expression in the CNS (74) and respiratory tracts (38) of immunocompetent rodents without immunosuppression. Our data and those from studies using HD human adenovirus (HAd) vectors (3) suggest that HD CAV-2 vectors could be used for the long-term treatment of some global neurodegenerative disorders (40, 54). Because Ad-induced morbidity is relatively species specific, vectors derived from nonhuman Adenoviridae might be more clinically relevant, based in part on the potential lack of memory immunity (8, 55-57), than those derived from HAds.
Several groups have studied viral vector-DC interaction. Most of these reports can be divided roughly into those that study genetic modification of DC to induce tumor- or pathogen-specific cellular responses and those that assay the interaction of DC with viral vectors to predict, understand, and limit the potential immune response following in vivo gene transfer. Here we compared CAV-2 vector transduction and effects on the phenotype and function of hMoDC to those of HAd and adeno-associated virus (AAV) vectors. In contrast to three HAd-based vectors harboring the most pertinent modifications, CAV-2 poorly induced the phenotypical and functional maturation of hMoDC. Our data suggested that this was due to an alternative receptor use and a postinternalization block in vesicular escape. Second, we found that HAd type 5 (HAd5) vector-induced maturation of hMoDC was TNF-α and IFN-α/β independent. Paradoxically, a hybrid HAd5/CAV-2 vector (Ad5Luc1-CK) was appreciably more efficient at transduction and inducing hMoDC maturation. Together, our data suggest that Ad5Luc1-CK and CAV-2, whose characteristics resemble those of AAV vectors, may be the antithesis of the classic understanding of Adenoviridae immunogenicity. Each vector may have specific (i.e., long-term gene transfer versus vaccine-targeted vectors) clinical advantages.
MATERIALS AND METHODS
Donor cells and culture conditions.
hMoDC were isolated (from >40 donors), purified, differentiated, characterized, and cultured as previously described (57). The purity of the selected populations was >95% as assayed by flow cytometry with human anti-CD11c and anti-CD14 antibodies. All hMoDC stimulations were performed at 7 days postisolation. Differentiated hMoDC were cultured in complete medium (RPMI 1640 medium [Invitrogen, Auckland, New Zealand] supplemented with 10% [vol/vol] fetal calf serum [FCS] (BioWest), 20 ng/ml interleukin-4 [IL-4], and 50 ng/ml granulocyte-macrophage colony-stimulating factor [Sigma-Aldrich, France]).
Antibodies.
Anti-HLA-DR antibody (Ab) (immu-357; Immunotech, France); anti-CD11c allophycocyanin-conjugated (B-Ly6) and anti-CD14 fluorescein isothiocyanate (FITC)-conjugated (M5E2) Abs (Miltenyi Biotec); anti-HLA-A, -B, and -C FITC-conjugated (G46-2.6), anti-CD40 (5C3), anti-CD80 (L307.4), anti-CD83 (HB15e), anti-CD86 (2331 FUN-1), anti-HLA-DR (G86-6), anti-CCR7 (3D12) phycoerythrin-conjugated Abs (BD PharMingen); and anti-Ad5Hex (AB1056) and anti-Rab5 Abs (Sigma) were obtained from the indicated sources. Anti-LAMP-2 (H4B4) was obtained from K Jensen at the Developmental Studies Hybridoma Bank (University of Iowa). Polyclonal rabbit anti-CAV-2 antibodies were produced in house by injecting New Zealand White rabbits with CsCl-purified CAVGFP. Anti-CAV-2 antibodies were affinity purified using CAVGFP virions covalently linked to a CNBr-activated Sepharose column.
Vectors and viruses.
CAVβgal, CAVDsRed, CAVGFP, Adβgal, AdRFP, and AdGFP have been described previously (39-41). Briefly, AdGFP, AdRFP, Ad5Luc1 (26), and Adβgal are E1/E3-deleted HAd5 vectors and contain an enhanced green fluorescent protein (EGFP), lacZ, luciferase or DsRed expression cassette. CAVGFP, CAVDsRed, and CAVβgal are E1-deleted CAV-2 vectors containing the same EGFP, DsRed2, and lacZ expression cassettes. Ad5Luc1-CK (26) is an HAd5 vector expressing luciferase and containing the HAd5 fiber shaft and the CAV-2 fiber knob. AdΔRGD is an HAd2 virion containing an RGD-to-RGE mutation in the penton base (4). AdΔRGDGFP is an HAd5 vector containing a deletion of the RGD motif and containing an EGFP expression cassette in the E3 region (67). Ad-488 is AdGFP labeled with Alexa-488, and CAV-Cy3 is CAVGFP labeled with Cy3 (10). HAd5wt is the wild-type human Ad serotype 5 purchased from ATCC. The vectors and viruses used in this study are described in Table 1.
TABLE 1.
Vectors and viruses
| Virus or vector | Serotype | Deletion (Δ) and/or substitution(s) | Transgene | Postpurification modification | Reference |
|---|---|---|---|---|---|
| CAVGFP | CAV-2 | ΔE1 | EGFP | 41 | |
| CAVβgal | CAV-2 | ΔE1 | lacZ | 41 | |
| CAVDsRed | CAV-2 | ΔE1 | DsRed2 | 39 | |
| CAV-Cy3 | CAV-2 | ΔE1 | EGFP | Cy3 label | 71 |
| AdGFP | HAd5 | ΔE1/3 | EGFP | 41 | |
| AdRFP | HAd5 | ΔE1/3 | DsRed | 39 | |
| Ad5Luc1 | HAd5 | ΔE1/3 | Luciferase | 26 | |
| HAd5wt | HAd5 | Wild type | |||
| Adβgal | HAd5 | ΔE1/3 | lacZ | 41 | |
| Ad-488 | HAd5 | ΔE1/3 | EGFP | Alexa-488 label | |
| Ad5Luc1-CK | HAd5 | ΔE1/3, HAd5 fiber knob replaced by CAV-2 knob | Luciferase | 26 | |
| AdΔRGDGFP | HAd5 | ΔE1/3, penton base-deleted RGD motif | EGFP | 67 | |
| AdΔRGD | HAd2 | RGD to RGE mutation in penton base | 4 | ||
| AAV1 | 1 | Δ viral coding regions | EGFP | ||
| AAV2 | 2 | Δ viral coding regions | EGFP |
CAVGFP has a physical particle (pp)-to-infectious unit (IU) ratio of <3:1; AdGFP and AdΔRGDGFP have pp/IU ratios of approximately 10:1; CAVβgal has a pp/IU ratio of approximately 10:1; Adβgal, CAVDsRed, and AdRFP have pp/IU ratios of approximately 20:1. Titers were determined as described previously (41). All HAd vectors and viruses were propagated in 911 cells. CAV-2 vectors were propagated in DKCre or DKZeo cells (72). All vectors and viruses were purified by double banding on CsCl density gradients (41) and were endotoxin free. AAV serotype 1 (AAV1) and AAV2 vectors had titers of 1 × 1011 pp/ml (pp/IU ratio of approximately 20:1) and 2 × 1012 pp/ml (pp/IU ratio of approximately 30:1), respectively. Each multiplicity of infection is given in pp/cell.
Transduction assays.
Immature hMoDC (1 × 105 cells) were washed with phosphate-buffered saline (PBS) and resuspended in 1 ml of complete medium containing 102, 103, 104, 2.5 × 104, or 5 × 104 vector pp/cell. hMoDC were incubated for 24 h (or for 48 to 72 h [not shown]) at 37°C and then analyzed by flow cytometry using a FACSCalibur. We performed data analysis using CellQuest software. The level of hMoDC transduction is reported as the percentage of GPF-positive or red fluorescent protein (RFP)-positive cells. The transduction efficacy of Ad5Luc1-CK versus Ad5Luc1 was determined using standard luminometric readouts. Transduction assays were performed at least in triplicate.
Expression of costimulatory molecules.
hMoDC (1 × 106 cells) were incubated in 1 ml of complete medium with 2.5 × 104 pp/cell of AdΔRGD, Ad5Luc1-CK, CAVβgal, or Adβgal; 2.5 × 104 pp/cell of AAV1 or AAV2; or 50 ng/ml lipopolysaccharide (LPS) (E. coli 0127 B5; Sigma-Aldrich). After 48 h, 1 × 105 hMoDC were washed and incubated for 20 min on ice with anti-CD11c, anti-MHC class I, anti-MHC class II, anti-CD40, anti-CD80, anti-CD83, anti-CD86, and anti-CCR7 antibodies. Background fluorescence was measured using control immunoglobulin (Ig) isotype. Cells were washed with PBS and analyzed by flow cytometry using a FACSCalibur, and the data analysis was performed using CellQuest software. hMoDC surface markers were assayed from 10 to 15 donors.
Binding and internalization of CAV-2 and HAd5.
To analyze the binding of HAd5 and CAV-2 virions, 5 × 105 hMoDC were incubated with 104 pp/cell of Ad-488 or CAV-Cy3 for 20 min at 4°C or incubated under the same conditions with an anti-HLA-DR antibody for membrane staining. Background fluorescence was measured using an Ig isotype control Ab. Cells were washed with PBS and then analyzed by flow cytometry. To evaluate the internalization of CAV-2, 5 × 105 hMoDC/well were incubated with 104 pp/cell of Ad-488 or CAV-Cy3 at 4°C to allow vector attachment but not internalization. The cells were then rinsed twice with fresh medium and incubated at 37°C for 1 h. Cells were fixed in 4% paraformaldehyde (PFA)-PBS and labeled with anti-MHC class I or II antibodies to mark the membrane. Internalization was assayed using confocal laser-scanning fluorescence microscopy (CLSM) (Zeiss LMS 510 META).
Endosomal membrane penetration.
hMoDC (2.5 × 105 cells) were mixed at 4°C in 500 μl of RPMI-2% bovine serum albumin with either 20 μg of purified recombinant glutathione S-transferase (GST) or purified recombinant GST fused to a nuclear localization signal (GST-NLS), and 2.5 × 104 pp/cell of AdGFP or CAVGFP. The hMoDC/virus/protein complex was incubated for 30 min to allow virus attachment. The cells were then shifted to 37°C for 1 h. hMoDC were washed once in RPMI-2% bovine serum albumin to remove unbound vector and incubated for another 15 min at 37°C to allow vectors and the recombinant proteins to enter the cells. Following additional washing steps with RPMI and PBS, the hMoDC were fixed in 4% PFA-PBS. Internalized GST was detected using a goat anti-GST Ab and a Cy3-coupled secondary anti-goat Ab. Cells were permeabilized for 15 min using 0.05% saponin and 10% FCS in PBS. The antibodies were added and the incubation continued for 1 h for the primary and 1 h for the secondary antibody. Control cells were kept at 4°C during the assay. Cells were counterstained with Hoechst dye to detect the nucleus and analyzed using CLSM.
Identification of vesicular compartments.
hMoDC (2.5 × 105 cells) in 500 μl of RPMI were incubated with 2.5 × 104 pp/cell of AdGFP or CAVGFP on ice for 60 min with gentle agitation. The tubes were then placed in a prewarmed 37°C heating block for 5 or 60 min. The hMoDC were then pelleted by centrifugation, rinsed in ice-cold PBS, and fixed in 250 μl of 4% PFA-PBS for 15 min at room temperature. The cells were pelleted, rinsed in PBS, and permeabilized using 200 μl of 0.05% saponin and 10% FCS in PBS for 15 min. The antibodies were added and the incubation continued for 1 h for the primary and 1 h for the secondary antibody. Cells were analyzed using CLSM. Control cells were kept at 4°C throughout the experiment. The results are representative of two individual experiments.
Cytokine detection.
hMoDC (1 × 106 cells/ml) (n = 10) were incubated in 1 ml with 103 or 2.5 × 104 pp/cell of CAVGFP, AdGFP, HAd5wt, AAV1, AAV2, Ad5Luc1-CK, AdΔRGD, or LPS for 48 or 72 h. Supernatants were collected and analyzed for the presence of TNF-α, IL-12p70, IL-1β, IFN-γ, and IL-10 by standard enzyme-linked immunosorbent assay (PharMingen). IFN-α/β detection was performed as previously described using HL116 cells (17). Briefly, serial dilutions of supernatant from treated hMoDC were incubated with HL116 cells (36), which contain a luciferase cDNA under the control of the IFN-α/β-inducible promoter. Luciferase activity was used as an indirect measurement of IFN-α/β levels. Control cells were kept at 4°C.
hMoDC antigen uptake.
Immature hMoDC (2.5 × 105 cells) were incubated with 2.5 × 104 pp/cell of CAVβgal, Adβgal, Ad5Luc1-CK, or AdΔRGD for 48 h. The cells were then incubated at 4°C or 37°C for 20 min with 1 mg/ml FITC-labeled dextran, fixed with 4% PFA-PBS, washed three times with PBS, and immediately analyzed by flow cytometry. Experiments were performed twice in triplicate. Nonspecific binding of dextran to hMoDC was controlled by incubation at 4°C (not shown).
Mixed-lymphocyte reaction.
Immature hMoDC from a single donor were incubated with 2.5 × 104 pp/cell of CAVGFP, AdGFP, AAV1, AAV2, AdΔRGD, Ad5Luc1-CK, or LPS for 48 h. CD4+ T lymphocytes from multiple donors were negatively sorted with the MACS system (Miltenyi Biotec). hMoDC were cocultured with a mix of 1 × 105 allogeneic CD4+ T lymphocytes from two donors. The cultures were incubated in 96-U-well trays in RPMI 1640-HEPES-10% FCS-antibiotics for 7 to 9 days at 37°C with 5% CO2. T-cell proliferation was determined by [3H]thymidine (Amersham Pharmacia, Piscataway, NJ) incorporation. One μCi/well of [3H]thymidine was added for the last 18 h of culture, and the plates were then stored at −20°C. Incorporated radioactivity in cells was counted using a liquid scintillation counter system (Packard TopCount). Proliferation assays for each condition were performed at least in triplicate and repeated four times.
SEM of hMoDC.
Immature hMoDC were seeded on 18-mm glass coverslips precoated with poly-d-lysine and then incubated with LPS or with 2.5 × 104 pp/cell of AdGFP or CAVGFP. hMoDC were prepared for scanning electron microscopy (SEM) analysis at 48 h postincubation. Cells were pelleted (300 × g for 5 min), washed with PBS, and fixed at room temperature for 1 h using glutaraldehyde (3.3%) in milloning phosphate buffer (pH 7.2). Fixed samples were dehydrated using a graded ethanol series (30 to 100%), followed by critical-point drying with CO2. Subsequently, the samples were sputter coated with appropriate 10-nm-thick gold film and then examined by SEM (Hitachi 400) using a lens detector with an acceleration voltage of 10 kV at calibrated magnifications.
RESULTS
CAV-2 is internalized but does not transduce hMoDC. (i) Transduction of hMoDC.
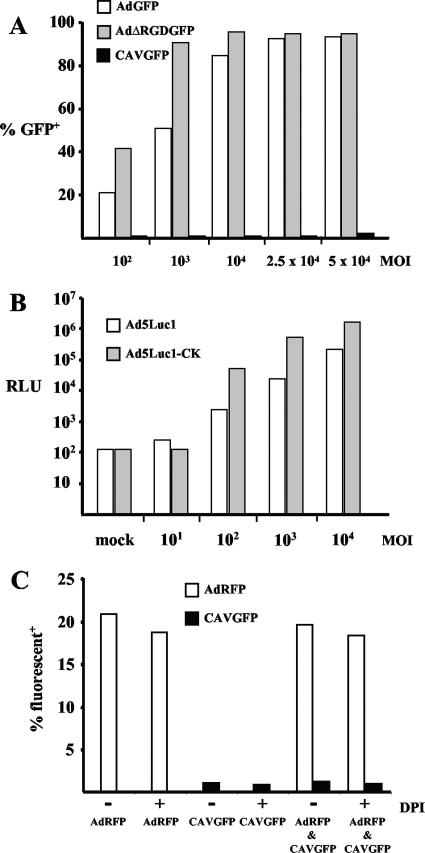
Ex vivo gene transfer to DC has attracted significant interest due to the potential to stimulate antigen-specific immunity for cancer or infectious diseases therapy (44, 52). Numerous reports have demonstrated that HAd vectors may be the most efficient tool to genetically modify hMoDC ex vivo. We therefore compared CAV-2 vector transduction to that of HAd5 and AAV vectors. Initially, we incubated immature (and LPS-matured [not shown]) hMoDC with CAVGFP or AdGFP. Similar to results from several other groups (for example, see references 61 and 76), we found that AdGFP led to a dose- and time-dependent transduction efficiency (Fig. 1A): the percent GFP-positive cells and the mean fluorescence index (MFI) increased with higher multiplicities of infection. We also found a donor-dependent transduction efficiency ranging from 20 to 90% when using AdGFP. In contrast, when immature (or LPS-matured [not shown]) hMoDC were incubated with CAVGFP, the transduction efficiency was negligible (<2%) in all donors (n = 30) (Fig. 1A), even at 50,000 pp/cell and 72 h postincubation (not shown). Similar to the results from Xin et al. (83), we found poor (<5%) transduction of human DC with 2.5 × 104 pp/cell of AAV vectors expressing GFP (not shown).
FIG. 1.
Transduction efficiency of hMoDC. (A) Immature hMoDC were incubated with AdGFP, AdΔRGDGFP, or CAVGFP. These transduction data are from a typical donor with no noteworthy gene transfer (<1%) detected with CAVGFP. (B) Transduction efficacy of Ad5Luc1-CK versus Ad5Luc1. RLU, relative light units. (C) hMoDC were incubated with (i) 2.5 × 104 pp/cell of AdRFP or CAVGFP with or without DPI or (ii) 2.5 × 104 pp/cell of AdRFP and 2.5 × 104 pp/cell of CAVGFP with or without DPI. No detectable increase of CAVGFP-mediated gene transfer was found in the presence of AdRFP, in the presence of DPI, or in the presence of both. Similar results were found when using vectors carrying the swapped fluorescent transgenes (i.e., AdGFP and CAVDsRed) (not shown).
Due to the lack of (i) an identifiable integrin-interacting motif (e.g., the highly conserved RGD motif in the HAd penton base), (ii) a possible heparan sulfate glycosaminoglycan-interacting motif (14) on the exterior of the CAV-2 virion (71), and (iii) coxsackievirus and adenovirus receptor (CAR) expression by MoDC, it was not surprising to find poor hMoDC CAV-2 transduction efficiency. To determine if the penton base RGD motif plays a role in transduction, we incubated hMoDC with AdΔRGDGFP (67). We found a similar transduction efficiency using AdΔRGDGFP compared to AdGFP (Fig. 1A). These data suggested that the lack of an RGD motif in the CAV-2 penton base was not responsible for the poor hMoDC transduction efficiency.
To test the role of the fiber knob, we also compared the transduction efficiency of Ad5Luc1 to that of Ad5Luc1-CK, an HAd5 virion harboring the CAV-2 fiber knob. We found that Ad5Luc1-CK transduction led to 10-fold-higher transgene expression compared to that with an isogenic ΔE1/3 HAd5 control (Fig. 1B). These data suggested that the CAV-2 fiber knob (26) was not responsible for the poor hMoDC transduction. Second, these data demonstrated that Ad5Luc1-CK was appreciably more efficient at transducing hMoDC than HAd5 vectors.
(ii) Coinfection and inhibition of endosomal alkalinization.
In epithelial cells, CAV-2 vector trafficking (from postbinding to infectious particle release) closely resembled that found with HAd5 vectors (10). While CAR-mediated clathrin-dependent endocytosis is the best characterized internalization mechanism, macropinocytosis is a major endocytic pathway in several cell types, including DC. Meier et al. showed that HAd2 virion binding induced macropinocytosis and that HAd2 virion endosomal escape increased macropinosomal leakage (48). Based on the possibility that the CAV-2 block was due to the failure of endosomal or macropinosomal escape, we hypothesized that if CAV-2 and HAd5 vectors were coincubated with hMoDC, we would see CAV-2-mediated gene transfer. hMoDC were therefore coincubated with AdRFP and CAVGFP (Fig. 1C). We found only RFP-positive cells, suggesting that CAV-2 and HAd5 virions were not internalized in the same endocytic vesicles or that there exists an additional block postinternalization.
To further test a possible postinternalization block, we asked if CAV-2 disassembly was sensitive to endosomal acidification. In epithelial cells, efficient HAd5 virion escape depends on endosome acidification. We previously showed that CAVβgal vector transduction of epithelial cells was inhibited by NH4Cl, suggesting that CAV-2 endosomal escape is pH dependent (10). In addition, DC and neutrophils do not reduce the phagosome environment but rather increase the pH. In DC, diphenyl iodonium (DPI) prevents the oxidation of endocytic vesicles (62). If HAd5 or CAV-2 virions were sequestered in endocytic vesicles or prevented from dissociating in an oxidizing environment, DPI might increase (in the case of AdGFP) or permit (in the case of CAVGFP) transduction. In this context, we previously found that the CAV-2 virion is more heat (72) stable than HAd5. However, we found that pre- or coincubation with 10 nM DPI with hMoDC had no significant effect on AdRFP or CAVGFP transduction (Fig. 1C).
Combined, these data are consistent with the cotransduction data suggesting that CAV-2 and HAd5 virions were interacting with different internalization pathways in MoDC. However, our data do not exclude the possibility that some internalization pathways are shared. Furthermore, our assays do not distinguish between failure to escape from vesicles (e.g., macropinosome) and the possibility that CAV-2 is unable to dissociate in this environment, or another possible downstream block. Further studies will be needed to determine if the neutral or oxidizing environment does not, for example, activate the CAV-2 protease, which in turn does not initiate virion disassembly.
(iii) Attachment and internalization in hMoDC.
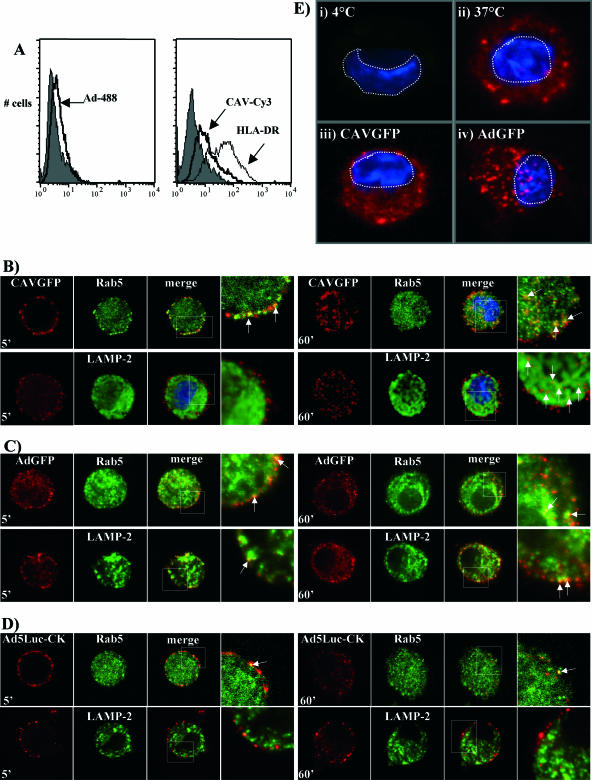
Although CAV-2 vectors poorly transduced hMoDC, this does not a priori mean that the virions are not taken up by immature hMoDC. Murine bone marrow-derived DC (mBMDC), for example, are poorly transduced by HAd5 vectors but are readily matured following coincubation (58). In contrast, murine lung DC are readily infected by HAd5 vectors but apparently show no signs of phenotypic or functional maturation (77). To determine if the lack of transduction was due to the lack of attachment, hMoDC were incubated with Ad-488 or CAV-Cy3 (10) and attachment quantified by flow cytometry (Fig. 2A). hMoDC incubated with either Ad-488 or CAV-Cy3 showed an increase of fluorescence corresponding to the binding of each virion to the cells. The level of Ad-488 binding to hMoDC appeared to mirror results obtained by Worgall et al. using HAd5 vectors and mBMDC (81). We also found no notable difference in Ad-488 or CAV-Cy3 binding compared to that when each vector was incubated alone with hMoDC (data not shown).
FIG. 2.
Binding and internalization of CAV-2 and HAd5 virions. (A) Vector binding was analyzed by flow cytometry: hMoDC were incubated with Ad-488, CAV-Cy3, or an anti-HLA-DR antibody for membrane staining. Background fluorescence using an isotopic control antibody is in gray. (B to D) hMoDC were incubated with the adenovirus vector at 4°C, and internalization was induced by increasing the temperature to 37°C. Vector internalization was stopped at 5 or 60 min and the cells assayed by immunofluorescence using antiadenovirus, anti-RAb5, and anti-LAMP-2 antibodies. The vectors are in red. The endosomal markers are in green. The third panel of each set shows the merge of the first and second panels. Colocalization is indicated by white arrows in the fourth panel, which corresponds to an enlargement of the white square in the merged panel. (B) hMoDC incubated with CAVGFP; (C) hMoDC incubated with AdGFP; (D) hMoDC incubated with Ad5Luc1-CK. The images show an internal 2-μm-thick focal plane. (E) Lysis of vesicles containing vector. The nuclei are stained with DAPI (4′,6′-diamidino-2-phenylindole) (in blue), and the GST-NLS proteins are in red. (i) hMoDC incubated with AdGFP and GST-NLS at 4°C, demonstrating background staining and temperature-dependent uptake; (ii) hMoDC incubated with GST-NLS at 37°C, demonstrating fluid phase- and temperature-dependent uptake; (iii) hMoDC incubated with GST-NLS and CAVGFP at 37°C; (iv) hMoDC incubated with GST-NLS and AdGFP at 37°C. Note the presence of nuclear accumulation of GST-NLS with AdGFP but not CAVGFP. Cells are representative of greater than 20 analyzed. Controls using GST without an NLS (not shown) showed no nuclear accumulation under any conditions.
To qualitatively assess the internalization of CAV-2 and HAd5 virions, hMoDC were incubated with either Ad-488 or CAV-Cy3 and internalization was evaluated by CLSM. Ad-488- and CAV-Cy3-associated fluorescence was observed inside the cell, with a distribution characterized by large, bright spots (not shown) suggesting vesicular localization. We also noted that cells generally contained more internalized Ad-488 than CAV-Cy3. To identify these vesicles, we incubated cells with CAVGFP, AdGFP, or Ad5Luc1-CK and assayed for the colocalization of early (Rab5) and late (LAMP-2) endosome vesicle markers. Under our conditions, at 5 min CAVGFP efficiently accumulated in early but not late endosomes (Fig. 2B). At 60 min, CAV-2 could still be found in early endosomes; however, the majority of CAVGFP virions appeared to be associated with LAMP-2-positive vesicles.
In contrast to CAVGFP, we found that AdGFP could be found sporadically in early endosomes at 5 and 60 min (Fig. 2C). At 5 min, we occasionally detected AdGFP in late vesicles. At 60 min AdGFP was more frequently found in late vesicles. Because of the noteworthy transduction efficiency of Ad5Luc1-CK, we also assayed its intracellular location. The only notable colocalization we detected with Ad5Luc1-CK was in Rab5-positive vesicles at 5 min. Together these data suggested that CAV-2 was more efficiently kept in Rab5-positive vesicles and also targeted to late endosomes, while AdGFP and Ad5Luc1-CK were initially associated with Rab5 and probably escaped into the cytoplasm before being targeted to late endosomes.
To address the postinternalization transduction block by another approach, we coincubated hMoDC with CAVGFP or AdGFP and with GST containing an NLS (GST-NLS). If the vectors escape from the endosomal compartments, the cointernalized GST-NLS would be released into the cytoplasm and then accumulate in the nucleus due to the NLS. Using this approach, we found that, unlike the case for AdGFP, we were unable to detect significant accumulation of GST-NLS in the nuclei of CAVGFP-treated cells (Fig. 2E).
We concluded that, like AdGFP and AAV1 and -2 vectors, immature hMoDC can bind and internalize CAV-2 virions. However, the principle means of internalization is likely to differ from that used by HAd5 virions. In addition, the lack of hMoDC transduction by CAVGFP was due, in part, to poor escape from vesicular compartments.
Phenotypic maturation of hMoDC. (i) Induction of MHC class I/II and costimulatory molecules.
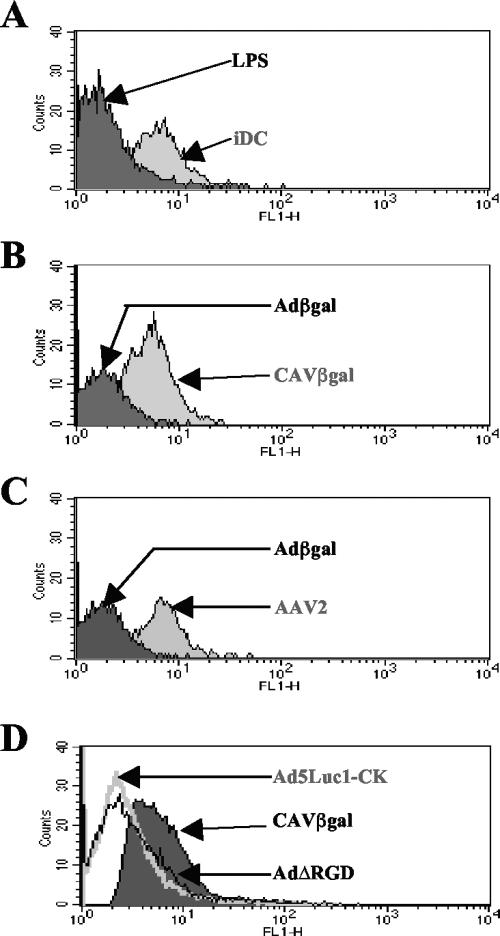
As mentioned above, transduction or internalization does not a priori correlate with vector-induced DC maturation. An advantageous characteristic for long-term clinical gene transfer would be the lack of DC maturation. This could be beneficial in numerous situations where immunosuppression could be reduced or avoided. In contrast, a vector that efficiently matures DC has numerous advantages for vaccination strategies. We therefore incubated immature hMoDC with the three HAd5-based vectors, AAV serotypes 1 and -2 (not shown), or CAVβgal and assayed for a change in the expression of costimulatory and MHC class I and II molecules (Fig. 3).
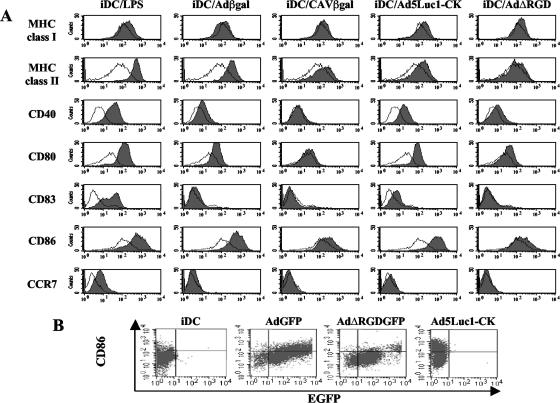
FIG. 3.
hMoDC phenotype after vector exposure. (A) hMoDC were either mock treated or incubated with LPS, Adβgal, CAVβgal, Ad5Luc1-CK, or AdΔRGD. The hMoDC were then labeled with the appropriate antibodies and analyzed by flow cytometry. In each panel the mock-treated immature DC (iDC) are in white, and the stimulus-treated cells are shaded. We found no modification of hMoDC phenotype when incubation was with 103 pp/cell of CAVβgal or Adβgal (data not shown). We detected a modest increase in MHC class I/II expression in 1/10 donors when using the highest dose of CAVβgal. The reason for this modest interdonor variation is unknown. No significant increase was seen for any of the stimuli after 72 h versus 48 h of exposure (not shown). (B) hMoDC were incubated with AdGFP, AdΔRGDGFP, or Ad5Luc1-CK for 24 h and then incubated with an anti-CD86 (or anti-CD80 [not shown]) and analyzed by flow cytometry.
In 10/12 donors, hMoDC incubated with the HAd-based vectors showed an upregulation of MHC class I/II molecules and costimulatory molecules CD40, CD80 (B7.1), and CD86 (B7.2). In our assays, CD83 (HB15) induction was detected only in 2/12 donors. Similarly, CCR7, a hallmarks of DC activation (22, 43), was modestly upregulated with the highest doses of Adβgal in a few donors. Our results are similar to the results obtained by others (16, 61, 70, 76), demonstrating that HAd5-based vectors partially mature hMoDC.
In contrast to the case with Adβgal, hMoDC incubated with all doses of CAVβgal or AAV vectors (not shown) showed no marked upregulation of costimulatory molecules or MHC class I/II molecules in most donors (Fig. 3A). Notably, the donor shown was the highest responder for CAVβgal. Similarly, in contrast to the results of others using murine bone marrow-derived DC (58), we found that HAd5 virions containing a mutation in the penton base RGD motif, i.e., AdΔRGD (or AdΔRGDGFP [not shown]) induced an upregulation of CD40 and CD80 in 4/5 donors. In the donor shown, the increase was similar to that seen for Adβgal. However, the level of CD86 was not markedly modified. These data accentuate the difference between human MoDC and murine BMDC and suggest that integrin interaction plays a more complex role in the maturation of hMoDC than previously noted. Finally, we consistently found that Ad5Luc1-CK induced a higher level of expression of the costimulatory molecules than all the other vectors. These data are also coherent with the transduction efficacy of Ad5Luc1-CK.
Finally, we assayed the level of CD86 (and CD80 [not shown]) induction in AdGFP-transduced hMoDC. We found a tendency of the AdGFP-transduced cells with increasing GFP levels to express increasing levels of CD86 (Fig. 3B). This trend was not as striking with AdΔRGDGFP in spite of greater than 80% of the MoDC being transduced. Similar to the case for AdGFP, approximately 50% of the MoDC incubated with Ad5Luc1-CK had increased levels of CD86. Further analysis will be needed to determine if these data demonstrate that attachment, internalization, endosomal escape, and transcription of the vector genome all contribute individually to hMoDC maturation or that the cells with the highest GFP levels also had the most vector genome copies per cell.
Together these data demonstrated that CAV-2 poorly induced the expression of costimulatory markers indicative of DC maturation, while Ad5Luc1-CK was the most potent inducer of maturation.
(ii) Induction of cytokine release.
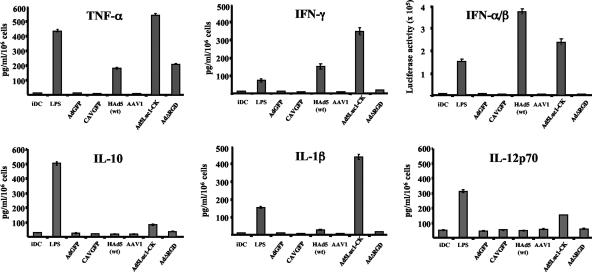
There is some divergence concerning the cytokines induced following incubation of hMoDC with HAd5 virions (61, 70, 76, 86). TNF-α, IL-1β, and IFN-γ are proinflammatory cytokines. Type I IFNs are classically considered crucial in the innate antiviral responses and induce variable effects on DC that are based on the antigens and the cytokine environment (34, 35, 79). IL-12 is currently considered a key factor in driving DC to induce a TH1-type response, and its absence induces a TH2 phenotype. IL-10 secretion may prevent a TH1 response while skewing it towards TH0 (15), prevent spontaneous maturation of DC (at least in vitro), and increase its own production (12, 34).
We therefore repeated these assays and compared CAVGFP, AdGFP, AdΔRGD, Ad5Luc1-CK, AAV1 and -2, and HAd5wt. We found no induction of cytokine release from hMoDC after CAVGFP or AAV1 and -2 exposure (Fig. 4). Equally important, we found no detectable increase in cytokine release at 2 or 3 days postincubation with AdGFP. In contrast, Ad5Luc1-CK induced noteworthy secretion of all cytokines tested, which is coherent with the increased transduction efficiency and level of costimulatory molecule expression. Furthermore, Ad5Luc1-CK was the only vector to induce an upregulation of IFN-γ receptor (not shown), suggesting an autocrine-induced hMoDC maturation. Another noteworthy exception was the induction of TNF-α by AdΔRGD, suggesting a possible role of the integrin-interacting motif in the modification of type I IFN (IFN-α and -β) and IFN-γ secretion. More analysis will be needed to understand this effect.
FIG. 4.
Cytokine release after vector exposure. Immature hMoDC (iDC) were incubated with LPS, AdGFP, CAVGFP, HAd5wt, AAV1 (results for AAV2 were identical [not shown]), Ad5Luc1-CK, or AdΔRGD. Supernatants were collected, and cytokine expression was quantified using standard enzyme-linked immunosorbent assay (except for IFN-α/β; see Materials and Methods). No significant difference in the cytokine release was detected between 48 and 72 h postexposure (data not shown). Error bars indicate standard deviations.
(iii) hMoDC morphological changes.
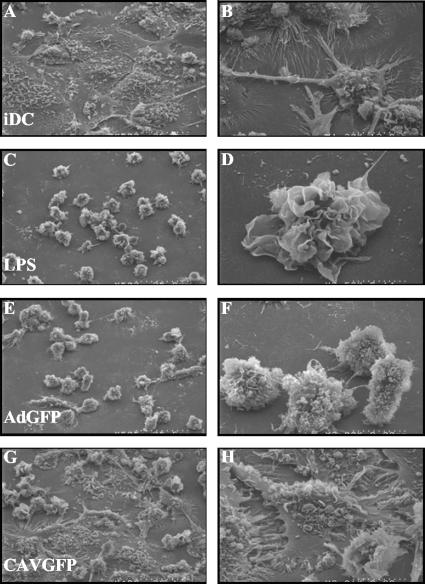
The functional differences between immature and mature DC are also characterized by dramatic changes in morphology. Immature DC are large, flat cells with few and small dorsal ruffles. In contrast, mature DC are small, round, irregularly shaped cells with numerous large dorsal ruffles and a notable increase in the surface area. We therefore incubated hMoDC with CAVGFP or AdGFP and assayed for the qualitative induction of morphological changes. We found that the majority of the hMoDC incubated with CAVGFP (Fig. 5G and H) resembled immature DC (Fig. 5A and B). In contrast, hMoDC incubated with AdGFP (Fig. 5E and F) resembled LPS-matured DC (Fig. 5C and D). Together, these data are consistent with our results assaying the phenotypical changes induced by HAd5 and CAV-2 vectors. We concluded that CAV-2 vectors poorly induced morphological changes indicative of DC maturation.
FIG. 5.
SEM of hMoDC incubated with LPS, AdGFP, or CAVGFP. (A and B) Mock-treated immature hMoDC (iDC); (C and D) LPS-treated hMoDC; (E and F) AdGFP-treated hMoDC (2.5 × 104 pp/cell); (G and H) CAVGFP-treated (2.5 × 104 pp/cell). Panels A, C, E, and G are at a magnification of ×500; panels B, D, F, and H are at a higher magnification. Under these conditions, iDC incubated with 103 pp/cell of CAVGFP or AdGFP induced no identifiable modification of immature DC morphology (not shown).
hMoDC functional maturation. (i) hMoDC antigen uptake.
Immature DC efficiently capture antigens mainly through macropinocytosis and mannose receptor-mediated endocytosis, but they lose this function during maturation (19, 20, 64). We qualitatively measured hMoDC antigen uptake by incubating cells with FITC-dextran (Fig. 6). Mock-treated hMoDC took up the dextran, consistent with functional immaturity, whereas LPS-matured hMoDC lost the antigen uptake ability (low MFI) (Fig. 6A). Immature DC incubated with CAVβgal or AAV1 or -2 vectors retained antigen uptake ability (high MFI), whereas incubation with Adβgal, AdΔRGD, and Ad5Luc1-CK induced functional hMoDC maturation (Fig. 6B to D). These results demonstrated that hMoDC incubated with a large dose of CAV-2 virions keep some functional characteristics indicative of immature DC, whereas HAd5-based vectors triggered the maturation of hMoDC.
FIG. 6.
FITC-dextran uptake by hMoDC following incubation with vector. Immature hMoDC (iDC) were mock treated or incubated with LPS or the vectors. hMoDC were then incubated with FITC-dextran and phagocytosis analyzed by flow cytometry. To simply the presentation, two or three samples are compared in order to demonstrate the relative difference between the stimuli. (A) Mock versus LPS treated; (B) CAVβgal versus Adβgal-treated; (C) Adβgal versus AAV2 treated (AAV1 gave similar results); (D) CAVβgal versus Ad5Luc1-CK versus AdΔRGD treated. Experiments were performed in triplicate, and the results are representative of those for all donors.
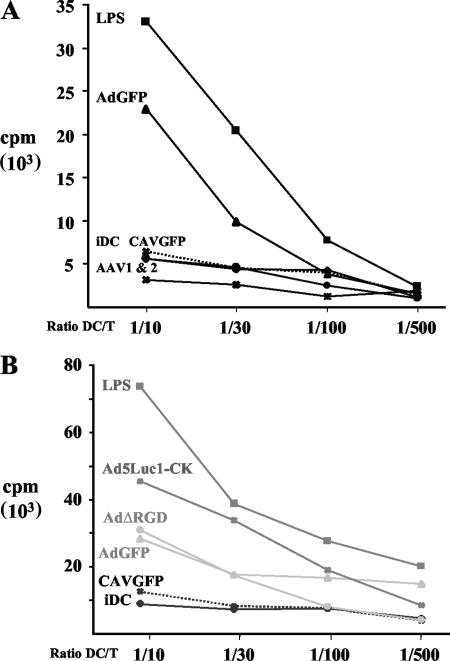
(ii) Allogeneic T-cell proliferation.
A semiquantitative functional assay to determine the maturation of hMoDC is their ability to stimulate antigen-independent T-cell proliferation. Modest induction of costimulatory molecules on DC can lead to interaction between DC and T cells and in turn induce allogeneic T cells to proliferate. As described above, we compared the vectors for their ability to induce maturation of hMoDC into functional antigen-presenting cells. Consistent with the results from others (16, 44), hMoDC incubated with AdGFP induced a dose-dependent T-cell proliferation (not shown). Consistent with the interdonor AdGFP transduction efficiency of hMoDC, we found a modest interdonor variation in T-cell proliferation. However, within each MoDC donor the relative levels of T-cell proliferation induced by the vectors were reproducible: CAVGFP and AAV1 and -2 vector stimulation led to near-background levels (Fig. 7A), while AdGFP and AdΔRGD induced proliferation of approximately 50% of that of the positive control (Fig. 7B). Consistent with the above phenotypical and functional assays, Ad5Luc1-CK induced the greatest T-cell proliferation in all donors tested.
FIG. 7.
T-cell proliferation induced by hMoDC maturation. Immature hMoDC (iDC) were incubated with LPS or viral vectors and then incubated with increasing ratios of allogeneic CD4+ T lymphocytes. (A) CAVGFP (crosses and dotted line), AdGFP (triangles), AAV1 (asterisks), AAV2 (circles), mock treated (diamonds), and LPS treated (squares); (B) CAVGFP (crosses dotted line), AdGFP (triangles), AdΔRGD (circles), Ad5Luc1-CK (asterisks), mock treated (diamonds), and LPS treated (squares). Proliferation was measured using a [3H]thymidine pulse. The values represent the mean from three to six wells.
Together, these data demonstrated that while CAV-2 poorly induced the functional maturation of hMoDC, a hybrid HAd5/CAV-2 vector efficiently transduced and matured hMoDC.
DISCUSSION
Understanding viral vector interactions with DC may enable us to understand the response following in vivo gene transfer and improve ex vivo use to induce tumor- or pathogen-specific cellular responses. The initial goal of this study was to examine the direct interactions of CAV-2 vectors with hMoDC. As this study evolved, we also tried to understand how HAd5 vectors induced hMoDC maturation. Using binding and transduction assays, we found that CAV-2 was able to attach to, and be internalized by, immature hMoDC (Fig. 2). However, gene transfer (transduction) could not be detected even at high doses and with prolonged incubation (Fig. 1). The CAV-2 virion postinternalization block appeared to be due to an entry pathway that differed from that of HAd5 virions, which may have sequestered CAV-2 in early and late endosomal vesicles (Fig. 2). Because transduction capacity does not a priori correlate with activation, we assayed for a CAV-2 virion-induced increases in costimulatory marker expression (Fig. 3), cytokine secretion (Fig. 4), and morphological changes indicative of DC maturation (Fig. 5). We then assayed functional features of hMoDC maturation such as heterologous T-cell proliferation (Fig. 7) and hMoDC antigen phagocytosis (Fig. 6). Combined, our data demonstrated that, in contrast to the case for HAd5-based vectors and similar to that for two AAV serotypes, high doses of ΔE1 CAV-2 vectors poorly induced the phenotypic and functional maturation of hMoDC.
CAV-2 immunogenicity.
We previously found that ΔE1 CAV-2 vectors were less immunogenic than ΔE1/E3 HAd5 vectors in the immunologically naïve rodent CNS and respiratory tract (38, 74). In the rat CNS, we detected fewer infiltrating CD4+ and CD8+ cells at an equivalent number of injected particles (74). We hypothesized that this was due to a combination of factors: the lack of transduction of CNS immune mediator cells (micro- and macroglia) (2), the dispersion of the vector from the site of injection via axoplasmic retrograde transport, and possibly a lower innate immune response due to the lack of an integrin-interacting motif in the CAV-2 virion (10, 71). Following intranasal instillation in mice, CAV-2 vectors also led to a lower level of TNF-α secretion than HAd5 vectors (38). The data presented here are consistent with our previous data and may provide a partial explanation for the reduced adaptive immune response in some tissues: poor DC transduction and maturation lead to a lower adaptive response in naïve hosts.
We also reported that sera from approximately 98% of a random cohort did not contain significant titers of CAV-2-neutralizing Abs (NAbs) (41). However, NAbs are only one obstacle to efficient long-term gene transfer. We predicted (55) that a TM response against virion proteins, which would be poorly blunted by many immunosuppressive drugs (29), will lead to deleterious side effects in some patients (39). Recently, we assayed CAV-2-induced human TM proliferation and activation (57). Fewer than half of the cohort harbored proliferating CD4+ TMs directed against the CAV-2 virion proteins (versus >85% against HAd5 vectors). Furthermore, the CAV-2 responders had a 10-fold-lower level of TM activation than the HAd5 vector responders. In these studies, we could not detect CD8+ TMs in any donors. The pertinence of the TM data and the data shown here is the significant role that DC play in the stimulation of TMs. The “vector-DC-TM” interaction could be relevant in the numerous situations where viral vector-mediated gene transfer is performed in patients with a significant memory antivirion response.
Receptors and internalization.
The lack of transduction and maturation of human DC may make CAV-2 vectors safer and more clinically applicable when long-term transgene expression is needed. Our transduction data are consistent with our current understanding of CAV-2 vector tropism (26, 39, 71, 73), which may be CAR dependent. CAR, a widely expressed cell adhesion molecule, is involved in the initial attachment of many Ads in some cell types (71, 85). Notably, CAR has never been found on any DC subtypes. In some CAR-negative cells the complement receptor CR3 (CD11b/CD18, αMβ2), other integrins, and heparan sulfate glycosaminoglycans (via a KKTK motif in the HAd5 fiber shaft) may function as Adenoviridae receptors (14, 33, 53).
To better understand CAV-2 biology, we included in our assays two HAd-based vectors containing modifications in two of the major external virion proteins involved in internalization. One vector contained a functional deletion in the penton base RGD motif, and the other replaced the CAV-2 knob on the HAd5 fiber. These vectors were chosen because CAV-2 lacks a known integrin-interacting motif (10, 71) and the hybrid HAd5/CAV-2 vector allowed us to assay the role of the knob (26). The transduction data with AdΔRGDGFP suggested that the penton base RGD motif is not involved in the use of integrins and that this is not the primary reason that CAV-2 poorly transduced DC. Like the HAd5 fiber knob, the CAV-2 knob also has high affinity (approximately 1 nM for CAV-2 and approximately 8 nM for HAd5) to CAR (66), uses CAR to transduce cells (71), and does not use CD46 (unpublished data), a receptor for some species B and D HAds (25, 65, 82).
Fiber knob swap experiments (49), as well as studies using non-HAd5 serotypes, demonstrated that Adenoviridae internalization rates and trafficking are variable in epithelial cells (47, 68). Glasgow et al. found that the in vitro tropism of Ad5Luc1-CK poorly mimicked that of CAV-2 or HAd5 vectors and that it conferred a context-specific tropism (26). It is likely that the combination of CAV-2 knob-specific attachment to another cell surface moiety in synergy with the HAd5 virion use of integrins and/or heparan sulfate (14) increased the Ad5Luc1-CK-induced hMoDC transduction and maturation. In light of these data, it is probable that the CAV-2 knob interacts with other cell surface proteins, carbohydrates, or lipids. This is consistent with our assays of cotransduction and disruption of endosomal pH (Fig. 1C and 2D) and colocalization assays (Fig. 2C and F), which suggested that CAV-2 and HAd5 virions were being internalized in separate vesicles.
Finally, the notable interdonor variation in hMoDC transduction efficiency with AdGFP suggested that another receptor could be playing a role. The most conspicuous difference between donor hMoDC is the MHC haplotype. The discordant data suggesting that the MHC class I molecule is a functional Adenoviridae receptor may need further examination (13, 30).
Human versus murine DC.
While there are many conserved characteristics between the several DC subtypes and species, it is unlikely that they will all respond identically to different stimuli (11, 69). In addition, some characteristics and conclusions obtained using murine DC have been attributed to human DC. Unfortunately, there is a poor consensus concerning HAd5 vector interaction with hMoDC, possibly due to the readout and confusion and extrapolation between models (murine versus human and bone marrow versus monocyte derived versus plasmacytoid) and the paucity of data concerning the input dose.
Rea et al. showed that HAd vectors induced hMoDC maturation without polarization towards a TH1-inducing subset (61) because of the lack of IL-12 expression. In contrast, Tan et al. reported HAd5 vector-induced hMoDC maturation with IL-12 production (76). Tan et al. also found an increased level of IL-1β, IL-6, IL-8, and TNF-α secretion from immature hMoDC at 5 days postincubation (76), while others found poor cytokine expression, in particular that of TNF-α, IL-12, and IL-10, at 2 to 5 days postincubation (61, 70, 86). In our hands, HAd5 vector-induced hMoDC maturation appeared to be TNF-α as well as IL-12, IL-10, IL-1β, IFN-γ, or IFN-α/β independent.
In an elegant study, Jooss et al. found that AAV2 vector-transduced cells escape immune surveillance, in part, because AAV2 poorly infects murine DC following intramuscular injection (37). Lack of AAV-mediated transduction of murine DC in this model poorly induced an antitransgene response, whereas HAd5 vector-mediated DC transduction did induce a response (84). However, hMoDC appear to be susceptible to AAV2 transduction ex vivo, although a significant and unexamined donor-dependent variability (from 2 to 55%) was reported (60). Moreover, the mechanism of the mBMDC activation and maturation by HAd5 vectors has also been controversial. Molinier-Frenkel et al. reported that the maturation of mBMDC by HAd5 virion was mediated by the fiber knob (50). Others reported that HAd5 vector-induced mBMDC activation and maturation were due to the phosphatidylinositol 3-kinase/Akt/TNF-α activation, which was due to the RGD-integrin interaction (58). Hensley et al. also showed that murine DC maturation is dependent on type I IFN signaling via phosphatidylinositol 3-kinase (27).
Here we found (i) that the HAd5 penton base RGD motif was not primarily responsible for hMoDC maturation and (ii) that the CAV-2 fiber knob in its native context had no effect on hMoDC maturation but that (iii) the CAV-2 fiber knob on HAd5 virion increased hMoDC maturation. Because our data demonstrated a notable difference between the murine and human models, we incubated mBMDC with CAV-2 and HAd5 vectors and compared TNF-α expression and the induction of CD80 and CD86. In three of four separate assays, we found no marked difference between the maturation induced by either vector; i.e., CAV-2 appeared to induce mBMDC maturation. Again, this is inconsistent with the RGD-dependent maturation of mBMDC. Further analysis will be needed to understand this discrepancy.
Clinical relevance.
One can potentially cause more immediate harm via an acute or persistent vector-induced immune response than by the normal progression of most disorders. Although the innate immune response of CAV-2 gene transfer has not been tested exhaustively, Morante-Oria et al. and Hnasko et al. found that CAV-2 vector transduction did not significantly disrupt the normal physiology of differentiating mouse neural cells (28, 51). During vector-mediated gene transfer, the early direct interactions between the virion and the cell are conserved, but most vectors have lost some downstream immunosuppressive activities (e.g., inhibition of type I IFN expression).
Second, a reductionist approach used in many biological assays poorly mimics the complex in vivo environment where DC and viral vectors meet. Serum components (in particular, ubiquitous cross-reacting anti-HAd Ig and complement), necrotic cellular debris, TMs, fragile diseased tissue and other factors will work in synergy with the innate immune response to accentuate the effects of DC maturation. Transduced cells could induce cytokine secretion and/or die because of necrosis or apoptosis due to virion internalization. These stimuli will certainly modify the functional state of the local DC and immune response and heighten the immunogenicity of the vector and transgene product. A recent phase I/II trial with AAV2 vectors elegantly confirmed this caveat (45). Previous preclinical tests with AAV2-mediated factor IX gene transfer in immunologically naïve animals showed promising results (see references in reference 45). However, in the phase I/II trial a cytotoxic TM response destroyed AAV2-transduced hepatocytes expressing factor IX. The cytotoxic TM response was also more rapid in patients with the highest NAb titers. It is likely that AAV virion-Ig complexes interacted with DC and played a role in the outcome of this trial.
Lack of vector-induced DC maturation may limit an adaptive response as well as the memory response following in vivo gene transfer. Our data demonstrated that CAV-2 vectors appear to pose fewer risks associated with the induction of a DC-mediated immune response. A potential future direction is to understand how CAV-2 interacts with a more complex mix of human immune components (56). For example, could nonneutralizing anti-CAV-2 antibodies form immune complexes with the CAV-2 virion, and could these significantly modify the biology of DC maturation?
Finally, our data suggest that Ad5Luc1-CK may be a potent tool for vaccinations. However, while ex vivo transduction of human DC can lead to CTL generation in vitro (16), more efficient transduction of mBMDC did not lead to an increase in the in vivo cellular immune response (32). We suggest that increased transduction associated with increased maturation is the key to ex vivo use of DC for vaccination therapies. Further tests will be needed to understand how Ad5Luc1-CK induces hMoDC maturation. We believe that the increased binding, endosome escape, and/or transduction of Ad5Luc1-CK led to increased DC maturation. It is also tempting to speculate that the upregulation of the IFN-γ receptor was responsible for the hyperinduction of hMoDC maturation. Our data demonstrate that CAV-2 vectors and a CAV-2/HAd5 hybrid differ notably from the traditional concept of Adenoviridae immunogenicity and may have specific clinical advantages.
Acknowledgments
We thank P. Freimuth and A. Lieber for the ΔRGD vectors, M. Piechaczyk for the AAV vectors, V. Pinet for performing the IL-1β assay, A. Savina for the DPI, and H. Issel for performing the proliferation assay. We acknowledge the technical assistance of Montpellier Rio Imaging, F. Tribillac, and C. Cazevieille for the ultrastructure analysis and G. Uze and D. Monneron for help with the IFN-α/β assay. We thank the members of the EKL and IGMM for helpful comments on the manuscript.
This work was supported by the Association Vaincre les Maladies Lysosomales, the Association Française contre les Myopathies, the Association pour la Recherche sur le Cancer, Vaincre la Mucoviscidose, and the Fondation pour la Recherche Medicale. M.P. and N.S. are AFM/VML fellows, and H.W. and E.J.K. are INSERM fellows.
The French authors have no conflicting financial interests.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Abrescia, N. G., J. J. Cockburn, J. M. Grimes, G. C. Sutton, J. M. Diprose, S. J. Butcher, S. D. Fuller, C. San Martin, R. M. Burnett, D. I. Stuart, D. H. Bamford, and J. K. Bamford. 2004. Insights into assembly from structural analysis of bacteriophage PRD1. Nature 432:68-74. [DOI] [PubMed] [Google Scholar]
- 2.Aloisi, F. 2001. Immune function of microglia. Glia 36:165-179. [DOI] [PubMed] [Google Scholar]
- 3.Amalfitano, A., and R. J. Parks. 2002. Separating fact from fiction: assessing the potential of modified adenovirus vectors for use in human gene therapy. Curr. Gene Ther. 2:111-133. [DOI] [PubMed] [Google Scholar]
- 4.Bai, M., B. Harfe, and P. Freimuth. 1993. Mutations that alter an Arg-Gly-Asp (RGD) sequence in the adenovirus type 2 penton base protein abolish its cell-rounding activity and delay virus reproduction in flat cells. J. Virol. 67:5198-5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 6.Benson, S. D., J. K. Bamford, D. H. Bamford, and R. M. Burnett. 2004. Does common architecture reveal a viral lineage spanning all three domains of life? Mol. Cell 16:673-685. [DOI] [PubMed] [Google Scholar]
- 7.Benson, S. D., J. K. Bamford, D. H. Bamford, and R. M. Burnett. 1999. Viral evolution revealed by bacteriophage PRD1 and human adenovirus coat protein structures. Cell 98:825-833. [DOI] [PubMed] [Google Scholar]
- 8.Both, G. W. 2002. Xenogenic adenoviruses, p. 447-479. In D. Curiel and J. Douglas (ed.), Adenoviral vectors for gene therapy. Academic Press, San Diego, CA.
- 9.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent TH1 polarization. Nat. Immunol. 1:305-310. [DOI] [PubMed] [Google Scholar]
- 10.Chillon, M., and E. J. Kremer. 2001. Trafficking and propagation of canine adenovirus vectors lacking a known integrin-interacting motif. Hum. Gene Ther. 12:1815-1823. [DOI] [PubMed] [Google Scholar]
- 11.Colonna, M., B. Pulendran, and A. Iwasaki. 2006. Dendritic cells at the host-pathogen interface. Nat. Immunol. 7:117-120. [DOI] [PubMed] [Google Scholar]
- 12.Corinti, S., C. Albanesi, A. la Sala, S. Pastore, and G. Girolomoni. 2001. Regulatory activity of autocrine IL-10 on dendritic cell functions. J. Immunol. 166:4312-4318. [DOI] [PubMed] [Google Scholar]
- 13.Davison, E., I. Kirby, T. Elliott, and G. Santis. 1999. The human HLA-A*0201 allele, expressed in hamster cells, is not a high-affinity receptor for adenovirus type 5 fiber. J. Virol. 73:4513-4517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5 and 2-host cell interactions. Virology 268:382-390. [DOI] [PubMed] [Google Scholar]
- 15.De Smedt, T., M. Van Mechelen, G. De Becker, J. Urbain, O. Leo, and M. Moser. 1997. Effect of interleukin-10 on dendritic cell maturation and function. Eur. J. Immunol. 27:1229-1235. [DOI] [PubMed] [Google Scholar]
- 16.Diao, J., J. A. Smythe, C. Smyth, P. B. Rowe, and I. E. Alexander. 1999. Human PBMC-derived dendritic cells transduced with an adenovirus vectorinduce cytotoxic T-lymphocyte responses against a vector-encoded antigen in vitro. Gene Ther. 6:845-853. [DOI] [PubMed] [Google Scholar]
- 17.Dondi, E., E. Pattyn, G. Lutfalla, X. Van Ostade, G. Uzé, S. Pellegrini, and J. Tavernier. 2001. Down-modulation of type 1 interferon responses by receptor cross-competition for a shared Jak kinase. J. Biol. Chem. 276:47004-47012. [DOI] [PubMed] [Google Scholar]
- 18.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 19.Engering, A. J., M. Cella, D. Fluitsma, M. Brockhaus, E. C. Hoefsmit, A. Lanzavecchia, and J. Pieters. 1997. The mannose receptor functions as a high capacity and broad specificity antigen receptor in human dendritic cells. Eur. J. Immunol. 27:2417-2425. [DOI] [PubMed] [Google Scholar]
- 20.Engering, A. J., M. Cella, D. M. Fluitsma, E. C. Hoefsmit, A. Lanzavecchia, and J. Pieters. 1997. Mannose receptor mediated antigen uptake and presentation in human dendritic cells. Adv. Exp. Med. Biol. 417:183-187. [DOI] [PubMed] [Google Scholar]
- 21.Fonteneau, J. F., M. Gilliet, M. Larsson, I. Dasilva, C. Munz, Y. J. Liu, and N. Bhardwaj. 2003. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 101:3520-3526. [DOI] [PubMed] [Google Scholar]
- 22.Foti, M., F. Granucci, and P. Ricciardi-Castagnoli. 2004. A central role for tissue-resident dendritic cells in innate responses. Trends Immunol. 25:650-654. [DOI] [PubMed] [Google Scholar]
- 23.Freigang, S., D. Egger, K. Bienz, H. Hengartner, and R. M. Zinkernagel. 2003. Endogenous neosynthesis vs. cross-presentation of viral antigens for cytotoxic T cell priming. Proc. Natl. Acad. Sci. USA 100:13477-13482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaggar, A., D. M. Shayakhmetov, and A. Lieber. 2003. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 9:1408-1412. [DOI] [PubMed] [Google Scholar]
- 26.Glasgow, J. N., E. J. Kremer, A. Hemminki, G. P. Siegal, J. T. Douglas, and D. T. Curiel. 2004. An adenovirus vector with a chimeric fiber derived from canine adenovirus type 2 displays novel tropism. Virology 324:103-116. [DOI] [PubMed] [Google Scholar]
- 27.Hensley, S. E., W. Giles-Davis, K. C. McCoy, W. Weninger, and H. C. Ertl. 2005. Dendritic cell maturation, but not CD8+ T cell induction, is dependent on type I IFN signaling during vaccination with adenovirus vectors. J. Immunol. 175:6032-6041. [DOI] [PubMed] [Google Scholar]
- 28.Hnasko, T. S., F. A. Perez, A. D. Scouras, E. A. Stoll, S. D. Gale, S. Luquet, P. E. Phillips, E. J. Kremer, and R. D. Palmiter. 2006. Cre recombinase-mediated restoration of nigrostriatal dopamine in dopamine-deficient mice reverses hypophagia and bradykinesia. Proc. Natl. Acad. Sci. USA 103:8858-8863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ho, S., N. Clipstone, L. Timmermann, J. Northrop, I. Graef, D. Fiorentino, J. Nourse, and G. R. Crabtree. 1996. The mechanism of action of cyclosporin A and FK506. Clin. Immunol. Immunopathol. 80:S40-S45. [DOI] [PubMed] [Google Scholar]
- 30.Hong, S. S., L. Karayan, J. Tournier, D. T. Curiel, and P. A. Boulanger. 1997. Adenovirus type 5 fiber knob binds to MHC class I alpha2 domain at the surface of human epithelial and B lymphoblastoid cells. EMBO J. 16:2294-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horwitz, M. 1996. Adenoviruses, p. 2149-2171. In B. Fields and D. Knipe (ed.), Fields virology, vol. 2. Raven Press, Philadelphia, PA. [Google Scholar]
- 32.Hsu, C., M. Boysen, L. D. Gritton, P. D. Frosst, G. R. Nemerow, and D. J. Von Seggern. 2005. In vitro dendritic cell infection by pseudotyped adenoviral vectors does not correlate with their in vivo immunogenicity. Virology 332:1-7. [DOI] [PubMed] [Google Scholar]
- 33.Huang, S., T. Kamata, Y. Takada, Z. M. Ruggeri, and G. R. Nemerow. 1996. Adenovirus interaction with distinct integrins mediates separate events in cell entry and gene delivery to hematopoietic cells. J. Virol. 70:4502-4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ito, T., R. Amakawa, M. Inaba, S. Ikehara, K. Inaba, and S. Fukuhara. 2001. Differential regulation of human blood dendritic cell subsets by IFNs. J. Immunol. 166:2961-2969. [DOI] [PubMed] [Google Scholar]
- 35.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 36.Jaitin, D. A., L. C. Roisman, E. Jaks, M. Gavutis, J. Piehler, J. Van der Heyden, G. Uze, and G. Schreiber. 2006. Inquiring into the differential action of interferons (IFNs): an IFN-α2 mutant with enhanced affinity to IFNAR1 is functionally similar to IFN-β. Mol. Cell. Biol. 26:1888-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jooss, K., Y. Yang, K. J. Fisher, and J. M. Wilson. 1998. Transduction of dendritic cells by DNA viral vectors directs the immune response to transgene products in muscle fibers. J. Virol. 72:4212-4223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keriel, A., C. Rene, C. Galer, J. Zabner, and E. J. Kremer. 2006. Canine adenovirus vectors for lung-directed gene transfer: efficacy, immune response, and duration of transgene expression using helper-dependent vectors. J. Virol. 80:1487-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kremer, E. J. 2004. CAR chasing: canine adenovirus vectors—all bite and no bark? J. Gene Med. 6(Suppl. 1):S139-S151. [DOI] [PubMed] [Google Scholar]
- 40.Kremer, E. J. 2005. Gene transfer to the central nervous system: current state of the art of the viral vectors. Curr. Genomics 6:13-39. [Google Scholar]
- 41.Kremer, E. J., S. Boutin, M. Chillon, and O. Danos. 2000. Canine adenovirus vectors: an alternative for adenovirus-mediated gene transfer. J. Virol. 74:505-512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruse, M., O. Rosorius, F. Kratzer, G. Stelz, C. Kuhnt, G. Schuler, J. Hauber, and A. Steinkasserer. 2000. Mature dendritic cells infected with herpes simplex virus type 1 exhibit inhibited T-cell stimulatory capacity. J. Virol. 74:7127-7136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 44.Leen, A. M., U. Sili, B. Savoldo, A. M. Jewell, P. A. Piedra, M. K. Brenner, and C. M. Rooney. 2004. Fiber-modified adenoviruses generate subgroup cross-reactive, adenovirus-specific cytotoxic T lymphocytes for therapeutic applications. Blood 103:1011-1019. [DOI] [PubMed] [Google Scholar]
- 45.Manno, C. S., V. R. Arruda, G. F. Pierce, B. Glader, M. Ragni, J. Rasko, M. C. Ozelo, K. Hoots, P. Blatt, B. Konkle, M. Dake, R. Kaye, M. Razavi, A. Zajko, J. Zehnder, H. Nakai, A. Chew, D. Leonard, J. F. Wright, R. R. Lessard, J. M. Sommer, M. Tigges, D. Sabatino, A. Luk, H. Jiang, F. Mingozzi, L. Couto, H. C. Ertl, K. A. High, and M. A. Kay. 2006. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat. Med. 12:342-347. [DOI] [PubMed] [Google Scholar]
- 46.Matthews, K., C. M. Leong, L. Baxter, E. Inglis, K. Yun, B. T. Backstrom, J. Doorbar, and M. Hibma. 2003. Depletion of Langerhans cells in human papillomavirus type 16-infected skin is associated with E6-mediated down regulation of E-cadherin. J. Virol. 77:8378-8385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Medina-Kauwe, L. K. 2003. Endocytosis of adenovirus and adenovirus capsid proteins. Adv. Drug Deliv. Rev. 55:1485-1496. [DOI] [PubMed] [Google Scholar]
- 48.Meier, O., K. Boucke, S. V. Hammer, S. Keller, R. P. Stidwill, S. Hemmi, and U. F. Greber. 2002. Adenovirus triggers macropinocytosis and endosomal leakage together with its clathrin-mediated uptake. J. Cell Biol. 158:1119-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miyazawa, N., P. L. Leopold, N. R. Hackett, B. Ferris, S. Worgall, E. Falck-Pedersen, and R. G. Crystal. 1999. Fiber swap between adenovirus subgroups B and C alters intracellular trafficking of adenovirus gene transfer vectors. J. Virol. 73:6056-6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Molinier-Frenkel, V., A. Prevost-Blondel, S. S. Hong, R. Lengagne, S. Boudaly, M. K. Magnusson, P. Boulanger, and J. G. Guillet. 2003. The maturation of murine dendritic cells induced by human adenovirus is mediated by the fiber knob domain. J. Biol. Chem. 278:37175-37182. [DOI] [PubMed] [Google Scholar]
- 51.Morante-Oria, J., A. Carleton, B. Ortino, E. J. Kremer, A. Fairen, and P. M. Lledo. 2003. Subpallial origin of a population of projecting pioneer neurons during corticogenesis. Proc. Natl. Acad. Sci. USA 100:12468-12473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nabel, G. J. 2004. Genetic, cellular and immune approaches to disease therapy: past and future. Nat. Med. 10:135-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nemerow, G. R. 2000. Cell receptors involved in adenovirus entry. Virology 274:1-4. [DOI] [PubMed] [Google Scholar]
- 54.Neufeld, E. F. 1991. Lysosomal storage diseases. Annu. Rev. Biochem. 60:257-280. [DOI] [PubMed] [Google Scholar]
- 55.Paillard, F. 1997. Advantages of nonhuman adenoviruses versus human adenoviruses. Hum. Gene Ther. 8:2007-2009. [DOI] [PubMed] [Google Scholar]
- 56.Perreau, M., and E. J. Kremer. 2006. The conundrum between immunological memory to adenovirus and their use as vectors in clinical gene therapy. Mol. Biotechnol. 34:247-256. [DOI] [PubMed] [Google Scholar]
- 57.Perreau, M., and E. J. Kremer. 2005. Frequency, proliferation, and activation of human memory T cells induced by a nonhuman adenovirus. J. Virol. 79:14595-14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Philpott, N. J., M. Nociari, K. B. Elkon, and E. Falck-Pedersen. 2004. Adenovirus-induced maturation of dendritic cells through a PI3 kinase-mediated TNF-alpha induction pathway. Proc. Natl. Acad. Sci. USA 101:6200-6205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pollara, G., K. Speidel, L. Samady, M. Rajpopat, Y. McGrath, J. Ledermann, R. S. Coffin, D. R. Katz, and B. Chain. 2003. Herpes simplex virus infection of dendritic cells: balance among activation, inhibition, and immunity. J. Infect. Dis. 187:165-178. [DOI] [PubMed] [Google Scholar]
- 60.Ponnazhagan, S., G. Mahendra, D. T. Curiel, and D. R. Shaw. 2001. Adeno-associated virus type 2-mediated transduction of human monocyte-derived dendritic cells: implications for ex vivo immunotherapy. J. Virol. 75:9493-9501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rea, D., F. H. Schagen, R. C. Hoeben, M. Mehtali, M. J. Havenga, R. E. Toes, C. J. Melief, and R. Offringa. 1999. Adenoviruses activate human dendritic cells without polarization toward a T-helper type 1-inducing subset. J. Virol. 73:10245-10253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reeves, E. P., H. Lu, H. L. Jacobs, C. G. Messina, S. Bolsover, G. Gabella, E. O. Potma, A. Warley, J. Roes, and A. W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature 416:291-297. [DOI] [PubMed] [Google Scholar]
- 63.Ridge, J. P., F. Di Rosa, and P. Matzinger. 1998. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393:474-478. [DOI] [PubMed] [Google Scholar]
- 64.Sallusto, F., M. Cella, C. Danieli, and A. Lanzavecchia. 1995. Dendritic cells use macropinocytosis and the mannose receptor to concentrate macromolecules in the major histocompatibility complex class II compartment: downregulation by cytokines and bacterial products. J. Exp. Med. 182:389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Segerman, A., J. P. Atkinson, M. Marttila, V. Dennerquist, G. Wadell, and N. Arnberg. 2003. Adenovirus type 11 uses CD46 as a cellular receptor. J. Virol. 77:9183-9191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seiradake, E., H. Lortat-Jacob, O. Billet, E. J. Kremer, and S. Cusack. 2006. Structural and mutational analysis of human Ad37 and canine adenovirus 2 fibre heads in complex with the D1 domain of CAR. J. Biol. Chem. 281:33704-33716. [DOI] [PubMed] [Google Scholar]
- 67.Shayakhmetov, D. M., A. M. Eberly, Z. Y. Li, and A. Lieber. 2005. Deletion of penton RGD motifs affects the efficiency of both the internalization and the endosome escape of viral particles containing adenovirus serotype 5 or 35 fiber knobs. J. Virol. 79:1053-1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shayakhmetov, D. M., Z. Y. Li, V. Ternovoi, A. Gaggar, H. Gharwan, and A. Lieber. 2003. The interaction between the fiber knob domain and the cellular attachment receptor determines the intracellular trafficking route of adenoviruses. J. Virol. 77:3712-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shortman, K., and Y. J. Liu. 2002. Mouse and human dendritic cell subtypes. Nat. Rev. Immunol. 2:151-161. [DOI] [PubMed] [Google Scholar]
- 70.Smith, S. G., P. M. Patel, P. J. Selby, and A. M. Jackson. 2001. The response of human dendritic cells to recombinant adenovirus, recombinant Mycobacterium bovis Bacillus Calmette Guerin and biolistic methods of antigen delivery: different induction of contact-dependent and soluble signals. Immunol. Lett. 76:79-88. [DOI] [PubMed] [Google Scholar]
- 71.Soudais, C., S. Boutin, S. S. Hong, M. Chillon, O. Danos, J. M. Bergelson, P. Boulanger, and E. J. Kremer. 2000. Canine adenovirus type 2 attachment and internalization: coxsackievirus-adenovirus receptor, alternative receptors, and an RGD-independent pathway. J. Virol. 74:10639-10649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soudais, C., S. Boutin, and E. J. Kremer. 2001. Characterisation of cis-acting sequences involved in the canine adenovirus packaging domain. Mol. Ther. 3:631-640. [DOI] [PubMed] [Google Scholar]
- 73.Soudais, C., C. Laplace-Builhe, K. Kissa, and E. J. Kremer. 2001. Preferential transduction of neurons by canine adenovirus vectors and their efficient retrograde transport in vivo. FASEB J. 15:2283-2285. [DOI] [PubMed] [Google Scholar]
- 74.Soudais, C., N. Skander, and E. J. Kremer. 2004. Long-term in vivo transduction of neurons throughout the rat central nervous system using novel helper-dependent CAV-2 vectors. FASEB J. 18:391-393. [DOI] [PubMed] [Google Scholar]
- 75.Subklewe, M., C. Paludan, M. L. Tsang, K. Mahnke, R. M. Steinman, and C. Munz. 2001. Dendritic cells cross-present latency gene products from Epstein-Barr virus-transformed B cells and expand tumor-reactive CD8(+) killer T cells. J. Exp. Med. 193:405-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tan, P. H., S. C. Beutelspacher, S. A. Xue, Y. H. Wang, P. Mitchell, J. C. McAlister, D. F. Larkin, M. O. McClure, H. J. Stauss, M. A. Ritter, G. Lombardi, and A. J. George. 2005. Modulation of human dendritic-cell function following transduction with viral vectors: implications for gene therapy. Blood 105:3824-3832. [DOI] [PubMed] [Google Scholar]
- 77.Thiele, A. T., T. L. Sumpter, J. A. Walker, Q. Xu, C. H. Chang, R. L. Bacallao, R. Kher, and D. S. Wilkes. 2006. Pulmonary immunity to viral infection: adenovirus infection of lung dendritic cells renders T cells nonresponsive to interleukin-2. J. Virol. 80:1826-1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tindle, R. W. 2002. Immune evasion in human papillomavirus-associated cervical cancer. Nat. Rev. Cancer 2:59-65. [DOI] [PubMed] [Google Scholar]
- 79.Tough, D. F. 2004. Type I interferon as a link between innate and adaptive immunity through dendritic cell stimulation. Leuk. Lymphoma 45:257-264. [DOI] [PubMed] [Google Scholar]
- 80.Underhill, D. M., A. Ozinsky, A. M. Hajjar, A. Stevens, C. B. Wilson, M. Bassetti, and A. Aderem. 1999. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature 401:811-815. [DOI] [PubMed] [Google Scholar]
- 81.Worgall, S., A. Busch, M. Rivara, D. Bonnyay, P. L. Leopold, R. Merritt, N. R. Hackett, P. W. Rovelink, J. T. Bruder, T. J. Wickham, I. Kovesdi, and R. G. Crystal. 2004. Modification to the capsid of the adenovirus vector that enhances dendritic cell infection and transgene-specific cellular immune responses. J. Virol. 78:2572-2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wu, E., S. A. Trauger, L. Pache, T. M. Mullen, D. J. von Seggern, G. Siuzdak, and G. R. Nemerow. 2004. Membrane cofactor protein is a receptor for adenoviruses associated with epidemic keratoconjunctivitis. J. Virol. 78:3897-3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xin, K. Q., H. Mizukami, M. Urabe, Y. Toda, K. Shinoda, A. Yoshida, K. Oomura, Y. Kojima, M. Ichino, D. Klinman, K. Ozawa, and K. Okuda. 2006. Induction of robust immune responses against human immunodeficiency virus is supported by the inherent tropism of adeno-associated virus type 5 for dendritic cells. J. Virol. 80:11899-11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang, Y., S. E. Haecker, Q. Su, and J. M. Wilson. 1996. Immunology of gene therapy with adenoviral vectors in mouse skeletal muscle. Hum. Mol. Genet. 5:1703-1712. [DOI] [PubMed] [Google Scholar]
- 85.Zhang, Y., and J. M. Bergelson. 2005. Adenovirus receptors. J. Virol. 79:12125-12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhong, L., A. Granelli-Piperno, Y. Choi, and R. M. Steinman. 1999. Recombinant adenovirus is an efficient and non-perturbing genetic vector for human dendritic cells. Eur. J. Immunol. 29:964-972. [DOI] [PubMed] [Google Scholar]