Abstract
The hepatitis A virus cellular receptor 1 (HAVCR1/TIM1), a member of the T-cell immunoglobulin mucin (TIM) family, is an important atopy susceptibility gene in humans. The exact natural function of HAVCR1/TIM1 and the inverse association between HAV infection and prevention of atopy are not well understood. To identify natural ligands of human HAVCR1/TIM1, we used an expression cloning strategy based on the binding of dog cells transfected with a human lymph node cDNA library to a HAVCR1/TIM1 Fc fusion protein. The transfected cells that bound to the human HAVCR1/TIM1 Fc contained cDNA of human immunoglobulin alpha 1 heavy (Igα1) and lambda light (Igλ) chain and secreted human IgA1λ antibody that bound to the cell surface. Cotransfection of the isolated Igα1 and Igλ cDNAs to naïve dog cells resulted in the secretion of IgA1λ that bound to HAVCR1/TIM1 Fc but not to a poliovirus receptor Fc fusion protein in a capture enzyme-linked immunosorbent assay. The interaction of HAVCR1/TIM1 with IgA was inhibited by monoclonal antibodies (MAbs) against Igα1 and Igλ, excess IgA1λ, or anti-HAVCR1/TIM1 MAb. IgA did not inhibit HAV infection of African green monkey cells, suggesting that the IgA and the virus binding sites are in different epitopes on HAVCR1/TIM1. IgA enhanced significantly the neutralization of HAV by HAVCR1/TIM1 Fc. Our results indicate that IgA1λ is a specific ligand of HAVCR1/TIM1 and that their association has a synergistic effect in virus-receptor interactions.
The hepatitis A virus (HAV) cellular receptor 1 (HAVCR1/TIM1) is a type 1 integral membrane glycoprotein consisting of a characteristic six-cysteine immunoglobulin (Ig)-like domain extended above the cell surface by a mucin-like domain that contains a variable number of threonine, serine, and proline (TSP) hexameric repeats (19). The monkey (19) and human (13) HAVCR1/TIM1 were the first identified members of the T-cell immunoglobulin mucin (TIM) family, an immunologically important group of receptors (22, 28, 29, 32) that is conserved in vertebrates. Although HAV is a hepatotropic virus that causes acute hepatitis in humans, infection with HAV has been shown to greatly reduce the risk of developing asthma and allergy in humans (26, 27). Because the gene encoding HAVCR1/TIM1 has been shown to be an important asthma and allergy susceptibility gene in humans (14, 15, 29, 30), it appears that HAVCR1/TIM1 plays a critical role in regulating T-cell differentiation (29) and the development of atopy (30). However, the precise immunological mechanisms by which HAV infection prevents atopy and the exact mechanisms by which HAVCR1/TIM1 functions normally in the absence of HAV infection to regulate immune responses are not fully understood.
In mice, Tim-1 has been shown to be an important T-cell costimulatory molecule, which is preferentially expressed on T helper 2 (Th2) cells (48). Cross-linking of mouse Tim-1 enhances T-cell proliferation and cytokine production and prevents the induction of respiratory tolerance, resulting in airway hyperreactivity, a cardinal feature of asthma (48). Tim-1 costimulation requires its cytoplasmic tail and a conserved tyrosine that can be phosphorylated (8). In humans, HAVCR1/TIM1 is expressed in Th2 cell lines, is associated with remission in patients with multiple sclerosis (21), and is highly expressed in kidneys (19) primarily after injury (16) or in tumors (50). Recently, mouse Tim-4, a TIM family member expressed on antigen-presenting cells (APCs), has been shown to be a ligand for Tim-1 (31). However, whether human TIM4, the ortholog of mouse Tim-4, functions as a ligand of human HAVCR1/TIM1 is not known.
Using an expression cloning strategy with a soluble form of the HAVCR1/TIM1 containing the HAVCR1/TIM1 Ig variable-like (IgV) region fused to the Fc fragment of a human IgG1 antibody [HAVCR1/TIM1(IgV)-Fc], we identified IgAλ as a specific ligand of HAVCR1/TIM1. The interaction between HAVCR1/TIM1 and IgAλ is specific, since it was blocked with monoclonal antibody (MAb) to immunoglobulin alpha 1 heavy (Igα1) or lambda light (Igλ) chain, with anti-HAVCR1/TIM1 MAb, or by treatment with excess IgA1λ antibody but not with IgM. More interestingly, binding of IgA to HAVCR1/TIM1 enhanced the virus-receptor interaction. Although HAVCR1/TIM1 is sufficient for binding and alteration of HAV particles (43, 44), steps that are required for cell entry, it is possible that IgA may play a role in vivo by enhancing the interaction of the virus with the receptor under nonfavorable infection conditions such as low receptor levels. These results contribute to our understanding of the role of HAVCR1/TIM1 in the pathogenesis of HAV and provide insight into the possible natural function of HAVCR1/TIM1 in humans and the mechanisms by which HAVCR1/TIM1 may regulate the development of immune responses and atopy.
MATERIALS AND METHODS
Cells and virus.
Chinese hamster ovary (CHO) cells deficient in the enzyme dihydrofolate reductase were obtained from the American Type Culture Collection (ATCC). Perro6D cells derived from canine osteogenic sarcoma D-17 cells (ATCC) transfected with EBNA-1 cDNA are resistant to the antibiotic G418 and have an increased transfection efficiency for episomal plasmids containing an Epstein-Barr virus P1 origin of replication (46).
African green monkey kidney cells of the GL37 strain (47) (GL37 cells) were grown in complete medium containing Eagle's minimal essential medium (EMEM) and 10% fetal bovine serum (FBS).
The cell culture-adapted HM-175 strain of HAV derived from infectious cDNA (7) and passaged 100 times in BS-C1 cells was grown in GL37 cells.
Antibodies and purified human immunoglobulins.
Mouse anti-human Igα1 (IgA1), Igλ, and Igκ MAbs were obtained from Southern Biotechnology, Inc. The anti-human IgA MAb was labeled with anti-mouse Fc R-phycoerythrin (RPE)-labeled Fab fragments using Zenon technology as suggested by the manufacturer (Molecular Probes and Invitrogen). Anti-FLAG peptide MAb M2 was purchased from Sigma Co. Anti-human integrin α3 MAb was purchased from Invitrogen, Inc. Unlabeled goat anti-human IgG or IgA antibodies and peroxidase-labeled goat anti-mouse IgG, anti-human IgA, and anti-human IgM antibodies were purchased from Kirkegaard & Perry Laboratories (KPL), Inc.
Purified human secretory IgA (sIgA) and human IgM were purchased from Serotec, Inc. Purified human myeloma IgA1λ, IgMλ, and Igλ were purchased from The Binding Site, Inc.
Fusion proteins.
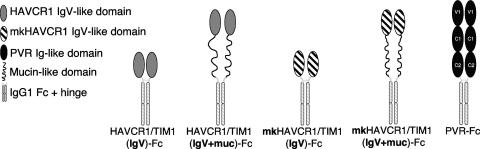
The schematic representations of the Fc fusion proteins used in this work are in Fig. 1. All fusion proteins contain the same Fc and hinge fragments of human IgG1 (Fc tail) and were constructed as described previously (43) with minor modifications. For this work, we constructed HAVCR1/TIM1(IgV)-Fc, which contains the Ig-like region and three TSP repeats of the mucin-like region of HAVCR1/TIM1 tagged at the N terminus with peptide DTKDDDK (FLAG) fused to the Fc tail. We also constructed HAVCR1/TIM1(IgV+muc)-Fc, which contains the whole ectodomain (Ig-like plus mucin-like regions) of human HAVCR1/TIM1 fused to the Fc tail. We also used the following three previously constructed Fc fusion proteins: mkHAVCR1/TIM1(IgV)-Fc, which contains the Ig-like region and two TSP repeats of the mucin-like region of monkey HAVCR1/TIM1 tagged at the N terminus with peptide DTKDDDK (FLAG) fused to the Fc tail (43); mkHAVCR1/TIM1(IgV+muc)-Fc, which contains the Ig-like region and 27 TSP repeats of the mucin-like region of the monkey HAVCR1/TIM1 tagged at the N terminus with peptide DTKDDDK (FLAG) (44); and PVR-Fc, which contains the ectodomain of the poliovirus receptor fused to the Fc tail (43). All these Fc fusion proteins were produced in stably transfected CHO cells and purified in protein A columns as described previously (43).
FIG. 1.
Soluble receptor constructs. Shown is a schematic representation of Fc fusion proteins used in the screening and evaluation of HAVCR1/TIM1 ligands. Constructs containing the human HAVCR1/TIM1 (13) Ig-like (IgV) domain or whole ectodomain (IgV plus mucin) fused to the Fc and hinge regions of human IgG1 were termed HAVCR1/TIM1(IgV)-Fc and HAVCR1/TIM1(IgV+muc)-Fc, respectively. Similar Fc fusion proteins containing the monkey HAVCR1/TIM1 Ig-like domain, mkHAVCR1/TIM1(IgV)-Fc, or the Ig-like domain plus the 27 hexameric repeats of the mucin domain, mkHAVCR1/TIM1(IgV+muc)-Fc, were reported previously (43, 44). The PVR-Fc construct containing the whole poliovirus receptor ectodomain (IgV, IgC1, and IgC2 domains) fused to the same Fc tail (43) was used as a negative control.
Generation of monoclonal antibodies against human HAVCR1/TIM1.
Anti-HAVCR1/TIM1 hybridomas were generated by immunization of BALB/cByJ mice subcutaneously with HAVCR1/TIM1(IgV)-Fc (Fig. 1) in complete Freund's adjuvant and boosting of them multiple times with HAVCR1/TIM1(IgV)-Fc in phosphate-buffered saline (PBS) (100 μg). One day following the last boost, lymph node cells were fused with NS1 myeloma cells and cloned, and the hybridomas were screened by cell surface staining of HAVCR1/TIM1-transfected CHO cells and screened for lack of reactivity with untransfected cells. Ten anti-HAVCR1/TIM1 specific hybridomas were subcloned. Hybridoma 3D1 (mouse IgG1κ) was chosen for further analysis on the basis of blocking the interaction of HAVCR1/TIM1 with an unidentified ligand and for robust staining.
Expression cloning of human HAVCR1 ligands.
A human lymph node cDNA library (Edge Biosystems, Inc) constructed in pEAK8, an episomal shuttle vector containing prokaryotic ColE1 and eukaryotic Epstein-Barr virus P1 origins of replication and selectable markers for ampicillin and puromycin, was transfected into Perro6D cells, and transfectants were selected with puromycin. Briefly, Perro6D monolayers grown in 150-cm2 flasks were transfected with 2 μg of purified DNA from the human lymph node cDNA library or control pEAK8 and 30 μl of Fugene 6 as recommended by the manufacturer (Roche). Transfectants were selected with 2 μg/ml of puromycin, detached from the plates with 0.5 mM EDTA in PBS, and panned (1, 19, 41) three times into polystyrene petri dishes coated with purified soluble HAVCR1/TIM1(IgV)-Fc or PVR-Fc. To enrich for cells that did not bind to the Fc tail, transfectants were panned in the presence of excess of PVR-Fc and depleted of FcγRII (CD32)-positive cells using anti-mouse IgG paramagnetic beads bound to anti-human CD32 MAb and magnetic separation. To rescue episomal plasmids from Perro6D-clone 1 cells, Hirt supernatant DNA (17) was prepared and used to transform Escherichia coli XL10-GOLD ultracompetent cells as suggested by the manufacturer (Stratagene). Ampicillin-resistant colonies were selected, plasmids were purified, and nucleotide sequence of the inserted cDNAs was obtained by automated sequencing using the ABI Prism BigDye Terminator Cycle Sequencing Ready Reaction kit (Applied Biosystems) and the ABI Prism (model 3100) analyzer (Applied Biosystems). The obtained nucleotide sequences were compared to GenBank using the BLAST program.
Bead binding assays.
Purified soluble receptor, HAVCR1/TIM1(IgV)-Fc or PVR-Fc, was covalently coupled to 6-μm blue carboxylated microparticles using the carbodiimide kit as suggested by the manufacturer (Polyscience, Inc.). For binding assays, cell monolayers were grown in 12-well plates and incubated with coupled beads in PBS-2% FBS for 30 to 45 min at room temperature. Unbound beads were washed extensively, and monolayers were examined under an inverted microscope at ×50 to ×100. For binding inhibition assays, coated beads were treated with 5 μg of purified MAbs or purified human myeloma immunoglobulins (IgA1λ, IgMλ, and Igλ) before being added to cell monolayers.
Immunochemical detection.
Expression of human CD32 on Perro6D cell transfectants and IgAλ on Perro6D-clone 1 cells was analyzed by a cell surface enzyme-linked immunosorbent assay (ELISA) on live cells grown in 96-well plates (18).
For the capture ELISA, 96-well ELISA plates were coated with 1 μg/ml goat anti-human IgA in bicarbonate buffer, washed, and blocked with 5% bovine serum albumin in PBS. Twofold dilutions of supernatants of dog cell transfectants grown in the presence of 20 μg/ml puromycin were titrated on the plates in duplicate. Binding of IgA to the plates was detected by staining with 0.33 μg/ml mouse anti-human IgA MAb and 0.5 μg/ml goat anti-mouse peroxidase-labeled antibody. Plates were developed with TMB One-Component (KPL Inc.) substrate and read at 450 nm in an ELISA plate reader.
In vitro receptor binding assay.
For the in vitro assay to study binding of human IgA to HAVCR1/TIM1, HAVCR1/TIM1(IgV)-Fc or PVR-Fc (1 μg/ml) was captured on 96-well plates (Nunc, Inc.) coated with 1 μg/ml goat anti-human IgG1 Fc. Twofold dilutions of supernatants of dog cell transfectants were titrated in duplicate on the plates. After incubation at room temperature for 1 h, plates were washed extensively and stained with peroxidase-labeled anti-human IgΑ for 1 h at room temperature. Similarly, to analyze whether IgA also binds to monkey HAVCR1/TIM1, mkHAVCR1/TIM1(IgV)-Fc, HAVCR1/TIM1(IgV)-Fc, or PVR-Fc (1 μg/ml) was captured on the 96-well plates, and sIgA or control IgM was titrated on the plates and stained with peroxidase-labeled anti-human anti-IgΑ or anti-IgΜ, respectively.
Plates were washed extensively, One-Component TMB was added, the reaction was stopped with acid treatment as suggested by the manufacturer (KPL Inc.), and absorbance at 450 nm was determined in an ELISA plate reader.
Flow cytometry.
Flow cytometry was done in a FACSCalibur cytometer, and data were processed with the CellQuest program (Becton Dickinson). Cells were stained with HAVCR1/TIM1(IgV)-Fc or PVR-Fc labeled with RPE-Fab to human Fc using the Zenon technology as recommended by the manufacturer (Molecular Probes and Invitrogen, Inc.).
HAV infection in the presence of IgA.
Absence of anti-HAV antibodies in the sIgA preparation was analyzed by incubating 105 50% tissue culture infective doses (TCID50) of HAV with 10 μg of healthy human sIgA or IgM (negative control) in 0.5 ml of EMEM-10% FBS for 2 h at 37°C. Samples were diluted one-fourth in EMEM-10% FBS and filtered through an 0.22-μm sterile filter. Residual HAV infectivity was titrated by an endpoint dilution ELISA. To do so, 10-fold dilutions of the virus were titrated in 96-well plates containing GL37 cells using eight repetitions per dilution. After incubation for 10 days at 35°C under 5% CO2, cells were fixed with 90% methanol and stained with anti-HAV antibodies, a secondary peroxidase-labeled antibody, and TMB One-Component substrate (KPL Inc.) as previously described (43). Viral titers were assessed using the Reed and Muench method (36).
To analyze the effect of IgA in virus infection, confluent monolayers of GL37 cells in six-well plates were treated with 1, 5, or 10 μg of sIgA in 0.5 ml of cell culture medium or mock treated. After 30 min at room temperature, 5 × 105 TCID50 of HAV was added per well and the plates were incubated at 35°C under 5% CO2. After 3 h of adsorption, monolayers were washed three times, complete medium was added, and plates were incubated at 35°C under CO2. At different times postinfection, plates containing the GL37 cell monolayers were frozen at −70°C. HAV was released from the cells by three freeze-and-thaw cycles, cell debris was pelleted, and virus in the supernatants was titrated by an endpoint dilution ELISA in GL37 cells as described above.
Neutralization of HAV by soluble receptors.
Soluble receptor-mediated neutralization of HAV was performed as described previously (44) with minor modifications. Briefly, 105 TCID50 of HAV was incubated with normal human sIgA (5 μg), control normal human IgG (5 μg), or medium in 0.5 ml of EMEM-10% FBS for 2 h at 37°C. For neutralization with the short form of the HAV receptor, 40 μg of mkHAVCR1/TIM1(IgV)-Fc or negative-control PVR-Fc was added and incubated overnight at 4°C. For neutralization with the long form of the HAV receptor, 4 μg of mkHAVCR1/TIM1(IgV+muc)-Fc or PVR-Fc was added and incubated overnight at 4°C. Residual infectious HAV was titrated in 96-well plates containing GL37 cells using eight repetitions per 10-fold dilution. After 3 h of adsorption, wells were washed three times with EMEM-10% FBS, and plates were incubated for 10 days at 35°C under 5% CO2. Cells were fixed, and HAV titers were determined by ELISA as described above using the Reed and Muench method (36).
Statistical analysis.
P values between two treatments were calculated using an unpaired t test.
RESULTS
Expression cloning of HAVCR1/TIM1 ligands.
The only known ligand of HAVCR1/TIM1 is HAV, which usurps this receptor to enter the cell (12, 13, 19). To identify natural ligands of HAVCR1/TIM1, we used an expression cloning strategy in which Perro6D cells, a dog osteosarcoma cell line developed in our lab that is highly transfectable and allows maintenance of episomal plasmids containing a P1 origin of replication (46), were transfected with a human lymph node cDNA library cloned into the episomal shuttle vector pEAK8. We used conditions that allowed incorporation of 1 to 10 plasmids per cell and selected cell transfectants with 2 μg/ml puromycin. The Perro6D-transfected cells were panned on petri dishes coated with soluble forms of HAVCR1/TIM1 shown in Fig. 1. These fusion proteins included a construct called HAVCR1/TIM1(IgV)-Fc, in which the Ig-like region of HAVCR1/TIM1 was fused to human IgG1 Fc, as well as HAVCR1/TIM1(IgV+muc)-Fc, in which both the Ig-like and mucin regions of HAVCR1/TIM1 were fused to human IgG1 Fc. As a negative control, we used PVR-Fc (43), a construct containing the complete ectodomain of the poliovirus receptor consisting of three Ig domains fused to human IgG1 Fc.
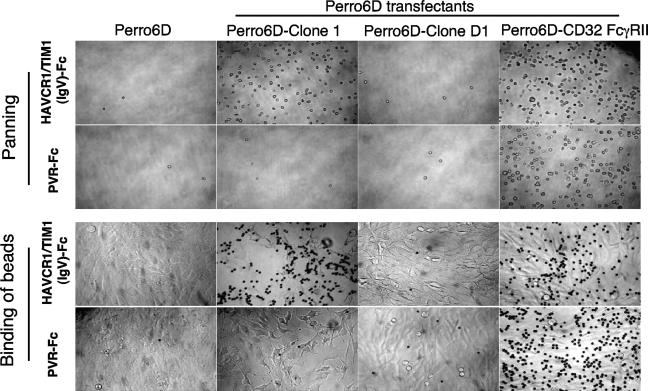
To enrich for cells that bound specifically to the HAVCR1/TIM1 Ig-like region and not to the Fc tail, the transfectants were first treated with excess PVR-Fc, which contains the same Fc tail as does HAVCR1/TIM1(IgV)-Fc, and then panned to petri dishes coated with HAVCR1/TIM1(IgV)-Fc. We further depleted transfected cells expressing the FcγRII receptor (CD32) by magnetically depleting cells that were stained with anti-human CD32 MAb. After this enrichment process, 20 transfected Perro6D cell clones were isolated and analyzed for the expression of HAVCR1/TIM1 ligands (Fig. 2). We tested the Perro6D cell clones by panning them on coated petri dishes and binding them to color latex beads coated with soluble receptors. The panning and latex bead assays gave similar results. In spite of our efforts, 10 of the transfected Perro6D cell clones expressed CD32 and bound HAVCR1/TIM1(IgV)-Fc and PVR-Fc (clone Perro6D-CD32 FcγRII is an example). Nine of the clones expressed neither CD32 nor bound soluble receptors (Perro6D-clone D1 is an example). However, one clone, termed Perro6D-clone 1, specifically bound to HAVCR1/TIM1(IgV)-Fc but not to PVR-Fc, did not express CD32, and expressed a ligand of HAVCR1/TIM1.
FIG. 2.
Expression cloning of HAVC1/TIM1 ligand. Cloning of Perro6D cells expressing an HAVCR1/TIM1 ligand is shown. Upper panels: naïve Perro6D cells (column 1), Perro6D-clone 1 cells expressing an HAVCR1/TIM1 ligand (column 2), Perro6D-clone D1 cells expressing an irrelevant human cDNA(s) (column 3), and Perro6D-CD32 FcγRIII cells expressing human CD32 (FcγRII) (column 4) were panned over plates coated with HAVCR1/TIM1(IgV)-Fc (first row) or with PVR-Fc (second row). Perro6D-clone 1 cells bound only to plates coated with HAVCR1/TIM1(IgV)-Fc, indicating that these cells express an HAVCR1/TIM1 ligand. Negative-control Perro6D and Perro6D-clone D1 cells did not bind to plates, and positive-control Perro6D-CD32 FcγRII cells bound to both plates via their Fc receptors. Images were obtained with an inverted microscope at an original magnification of ×50. Images are representative of three independent experiments. Lower panels: beads coated with HAVCR1/TIM1(IgV)-Fc (third row) or control PVR-Fc (fourth row) were incubated with naïve or transfected Perro6D cells attached to plates. Beads coated with HAVCR1/TIM1(IgV)-Fc, but not with PVR-Fc, bound to Perro6D-clone 1 cells expressing an HAVCR1/TIM1 ligand but not to naïve Perro6D and Perro6D-clone D1 cells. Both beads bound to Perro6D-CD32 FcγRIII cells via Fc receptors. Binding was performed for 30 to 45 min followed by extensive washing. Images were obtained with an inverted microscope at an original magnification of ×100. Images are representative of five independent experiments.
Human IgA1λ is expressed at the cell surface of Perro6D-clone 1 cells.
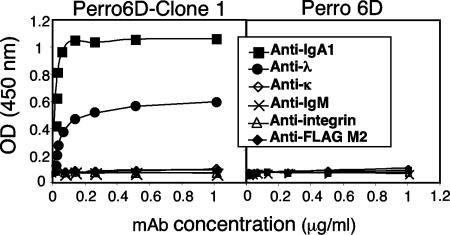
To identify the HAVCR1/TIM1 ligand in the Perro6D-clone 1 cells, we transformed E. coli with episomal DNA extracted from Perro6D-clone 1 cells. Nucleotide sequence analysis of the rescued plasmids revealed that they encoded full-length human immunoglobulin heavy α1 and light λ chains. A cell surface ELISA (Fig. 3) showed that Perro6D-clone 1 cells expressed human IgA1 and Igλ at the cell surface. Perro6D cells did not react with the MAbs raised against human IgA1 or Igλ. The dog cells, Perro6D-clone 1 and Perro6D cells, did not react with MAbs raised against human Igκ light chain or IgM. As expected, negative-control anti-human integrin and anti-FLAG MAbs did not stain the dog cells. These results confirmed that Perro6D-clone 1 cells expressed human IgA1λ at the cell surface.
FIG. 3.
Perro6D-clone 1 cells express IgAλ at the cell surface. Cell surface ELISA was performed with Perro6D-clone 1 cells (left panel) and untransfected Perro6D cells (right panel) stained with MAbs to Igα1 heavy chain (IgA), Igλ light chain, Igκ light chain, Igμ (IgM) heavy chain, human integrin α3, or M2 MAb to the FLAG epitope (as control). Values represent mean optical densities (OD) at 450 nm and are representative of three experiments.
HAVCR1/TIM1 binds specifically to IgA1λ expressed at the cell surface of Perro6D-clone 1 cells.
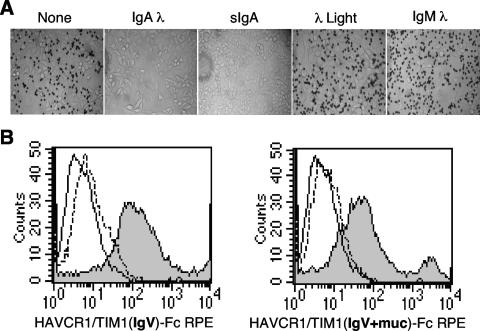
To assess the specificity of the binding of HAVCR1/TIM1 to IgA1λ, we blocked the binding of HAVCR1/TIM1(IgV)-Fc coated beads to Perro6D-clone 1 cells with an excess of different human immunoglobulins. This experiment showed that myeloma IgA1λ and sIgA, a purified commercially available form of IgA that contains Igλ and Igκ light chains, completely blocked binding of HAVCR1/TIM1(IgV)-Fc beads to Perro6D-clone 1 cells whereas IgMλ did not affect binding of the beads (Fig. 4A). Human myeloma Igλ did not block binding of the HAVCR1/TIM1(IgV)-Fc beads to Perro6D-clone 1 cells, suggesting that the interaction of HAVCR1/TIM1 with IgA requires both the heavy and light immunoglobulin chains. We also found that anti-human IgA1 and Igλ blocked approximately 50% and 75% of the binding of HAVCR1/TIM1(IgV)-Fc coated beads to Perro6D-clone 1 cells, respectively, whereas negative-control MAbs had no effect (data not shown). Finally, flow cytometry analysis showed that anti-HAVCR1/TIM1 MAb 3D1 raised against the Ig-like region of HAVCR1/TIM1 almost completely blocked binding of RPE-labeled HAVCR1/TIM1(IgV)-Fc and HAVCR1/TIM1(IgV+muc)-Fc to Perro6D-clone 1 cells (Fig. 4B), which indicated that the 3D1 epitope is involved in the interaction of HAVCR1/TIM1 with IgAλ. Taken together, these experiments proved that IgAλ binds specifically to the Ig-like domain of HAVCR1/TIM1. Further work will be required to determine whether IgA1κ, IgA2λ, or IgA2κ as well as other human immunoglobulins is also a ligand of HAVCR1/TIM1.
FIG. 4.
IgAλ is a specific ligand of HAVCR1/TIM1. Binding of HAVCR1/TIM1 to IgA was inhibited by excess IgA and anti-HAVCR1/TIM1 mAb. (A) IgA blocks binding of HAVCR1/TIM1(IgV)-Fc to Perro6D-clone 1 cells. Binding assay was performed as in Fig. 2 (lower panels) but in the presence of excess purified human immunoglobulins. HAVCR1/TIM1(IgV)-Fc coated beads were incubated with Perro6D-clone 1 cells bound to plates in the presence of excess IgA1λ, sIgA, human myeloma Igλ light chain, IgMλ, or medium (None). Human myeloma IgA1λ or sIgA but not human myeloma Igλ light chain or IgMλ blocked binding of HAVCR1/TIM1(IgV)-Fc coated beads to Perro6D-clone 1 cells compared to medium-only untreated control. Data are representative of three experiments. Micrographs were taken with an inverted microscope at ×100. (B) Anti-HAVCR1/TIM1 MAb blocks binding of HAVCR1/TIM1 Fc fusion proteins to clone 1 cells. Perro6D-clone 1 cells were stained with HAVCR1/TIM1(IgV)-Fc RPE or HAVCR1/TIM1(IgV+muc)-Fc RPE in the absence (filled histogram) or presence (continuous line) of anti-HAVCR1/TIM1 MAb. Perro6D-clone 1 cells were stained with isotypic control PVR-Fc RPE (broken lines). HAVCR1/TIM1 fusion proteins with or without the mucin region bound to Perro6D-clone 1 cells specifically via the IgV domain since the anti-HAVCR1/TIM1 MAb blocked binding of both constructs. Control PVR-Fc, which contains the same Fc tail, did not bind to Perro6D-clone 1 cells. Data are representative of three experiments.
Human IgA1λ alone is sufficient for binding to HAVCR1/TIM1.
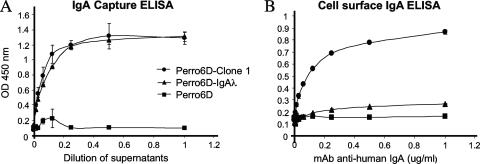
To test whether IgAλ expressed in the Perro6D-clone 1 cells was sufficient for binding to HAVCR1/TIM1 and to confirm that no additional molecules from human or dog origin were required for the interaction, we cotransfected Perro6D cells with the plasmids coding for Igα1 and Igλ rescued from Perro6D-clone 1 cells. Cell transfectants were selected with 2 μg/ml puromycin and termed Perro6D-IgA1λ. To increase the expression of IgA, cells were grown in 20 μg/ml puromycin. A capture ELISA (Fig. 5A) showed that Perro6D-IgA1λ and Perro6D-clone 1 cells but not Perro6D cells secreted IgA1λ into the cell culture medium. However, a cell surface ELISA (Fig. 5B) showed that Perro6D-clone 1 but not Perro6D-IgA1λ expressed IgA1λ at the cell surface. These results indicated that Perro6D-clone 1 cells contain an IgA binding molecule at the cell surface not present in the majority of the Perro6D cells. This unexpected characteristic of binding of IgA1λ to the cell surface allowed us to select the Perro6D-clone 1 cells by panning to petri dishes coated with HAVCR1/TIM1(IgV)-Fc.
FIG. 5.
Expression of IgA in Perro6D cells transfected with plasmids coding for Igα1 and Igλ. Perro6D cells were cotransfected with Igα1 and Igλ plasmids isolated from Perro6D-clone 1 cells, and transfectants were selected with puromycin and termed Perrro6D-IgA1λ cells. (A) An IgA capture ELISA detected secretion of IgA into the cell culture supernatant of Perrro6D-IgA1λ and positive-control Perro6D-clone 1 cells (IgA capture ELISA). (B) A cell surface IgA ELISA detected IgA on the cell surface of Perro6D-clone 1 cells but not Perro6D-IgAλ cells. Negative-control Perro6D cells did not secrete human IgA or express it at the cell surface. Data represent mean optical densities (OD) at 450 nm ± standard deviations and are representative of two experiments.
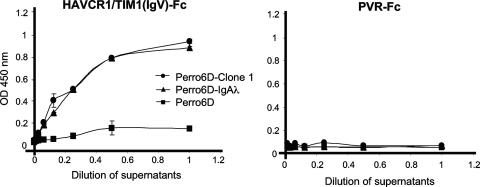
Since our results showed that IgA1λ bound to the cell surface of Perro6D-clone 1 cells but not to the cell surface of Perro6D-IgA1λ, it was of interest to determine whether IgA1λ was sufficient for binding to HAVCR1/TIM1. We set up an in vitro binding ELISA to analyze binding of IgA to HAVCR1/TIM1(IgV)-Fc captured on ELISA plates coated with goat anti-human IgG Fc (Fig. 6). IgA1λ secreted by Perro6D-IgA1λ and Perro6D-clone 1 cells bound to HAVCR1/TIM1(IgV)-Fc but not to control PVR-Fc. Similarly, human sIgA and myeloma IgAλ bound to HAVCR1/TIM1(IgV)-Fc but not to PVR-Fc (Fig. 7A). These data indicated that IgA1λ is sufficient for binding to HAVCR1/TIM1 and further confirmed that IgAλ is indeed a natural ligand of this receptor.
FIG. 6.
In vitro receptor binding assay. Binding of IgA1λ to HAVCR1/TIM1(IgV)-Fc or PVR-Fc captured on ELISA plates is shown. Supernatants of Perro6D-clone 1, Perrro6D-IgA1λ, and Perro6D cells were titrated on the ELISA plates containing the captured soluble receptors. Binding of IgA1λ to the captured receptors was assessed by staining with peroxidase-labeled anti-human Igα1 antibody. IgA1λ in supernatants of Perro6D-clone 1 and Perrro6D-IgA1λ cells bound to HAVCR1/TIM1(IgV)-Fc but not PVR-Fc. Supernatants of Perro6D cells produced background binding levels. Data represent mean optical densities (OD) at 450 nm ± standard deviations and are representative of three experiments.
FIG. 7.
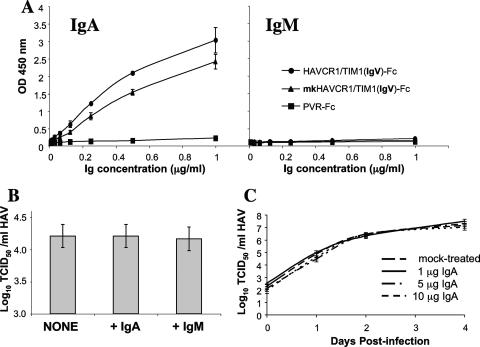
Infection of African green monkey kidney cells in the presence of IgA. (A) Monkey HAVCR1/TIM1 binds IgA. mkHAVCR1/TIM1(IgV)-Fc, HAVCR1/TIM1(IgV)-Fc, or PVR-Fc was captured on ELISA plates coated with anti-IgG Fc antibodies. sIgA (IgA) or negative-control IgM was titrated on the ELISA plates containing the captured soluble receptors. Binding of sIgA or IgM to the captured receptors was assessed by staining with peroxidase-labeled anti-human IgΑ or IgM antibody, respectively. Data represent mean optical densities (OD) at 450 nm ± standard deviations and are representative of two experiments. (B) Lack of neutralizing anti-HAV antibodies in the preparation of sIgA. HAV (105 TCID50) was mixed with 10 μg of sIgA (+IgA), 10 μg of IgM (+IgM), or medium (NONE) and titrated on 96-well plates containing GL37 cells. Viral titers were determined by ELISA using the method of Reed and Muench (36). Data are representative of two experiments and show the mean TCID50/ml and standard deviations in bars. (C) Presence of IgA does not affect HAV infection. GL37 cells in six-well plates were treated with 1, 5, or 10 μg of sIgA or mock treated for 30 min at room temperature; infected with 5 × 105 TCID50 of HAV for 3 h; and washed three times. At different times postinfection, plates were frozen, HAV was released from the cells by three freeze-and-thaw cycles, and the virus was titrated on 96-well plates containing GL37 cells. Viral titers were determined as for panel B. Data are representative of two experiments and show the mean TCID50/ml and standard deviations in bars.
Binding of IgA to HAVCR1/TIM1 does not block HAV infection.
Our finding that IgA is a ligand of HAVCR1/TIM1 raised the possibility that IgA could block the interaction of HAV with HAVCR1/TIM1. Since we previously showed that GL37 cells are infected with HAV via the monkey homolog of HAVCR1/TIM1 (19), mkHAVCR1/TIM1, we used GL37 cells to address this question. We first determined in a capture ELISA that mkHAVCR1/TIM1, which shares a high degree of homology with human HAVCR1/TIM, also binds IgA (Fig. 7A). HAVCR1/TIM1(IgV)-Fc and mkHAVCR1/TIM1(IgV)-Fc bound similar levels of sIgA whereas control PVR-Fc did not bind sIgA, and none of the Fc fusion proteins bound control IgM. We then verified that our preparation of sIgA did not contain anti-HAV antibodies that could neutralize the virus (Fig. 7B). HAV (105 TCID50) was treated with 10 μg of human sIgA or control IgM or mock treated, incubated at 37°C for 2 h, and titrated on 96-well plates containing monolayers of GL37 cells. After 10 days of incubation at 35°C, cells were fixed with methanol and stained with anti-HAV antibodies (46). Similar HAV titers were obtained with all treatments, which indicated that the preparation of sIgA used in our experiments did not contain anti-HAV neutralizing antibodies. We finally analyzed whether IgA can block HAV infection (Fig. 7C). GL37 cells were treated with 1, 5, or 10 μg of sIgA for 30 min and then infected with HAV at a multiplicity of infection of 1 to 5 TCID50 per cell. Viral titers were determined at different times postinfection by an endpoint dilution ELISA. This one-step growth curve analysis showed that HAV grew similarly in the presence and in the absence of sIgA, which indicated that IgA did not affect HAV infection of GL37 cells.
Human IgA enhances the virus-receptor interaction.
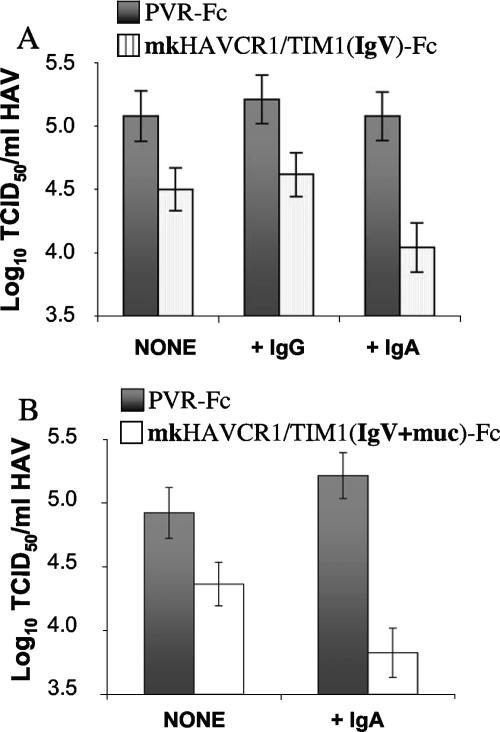
Our finding that IgA did not block HAV infection in GL37 cells suggested that IgA and HAV may bind to different epitopes on HAVCR1/TIM1. Since HAV grows poorly in cell culture and tends to bind nonspecifically to cells and surfaces, it is extremely difficult to perform binding assays and almost impossible to quantitate meaningful results. Therefore, we used a soluble receptor neutralization assay that mimics the initial steps in HAV infection (43, 44) to further characterize the interaction of HAV with HAVCR1/TIM1 in the presence of IgA. We previously showed that mkHAVCR1/TIM1(IgV)-Fc (Fig. 1) neutralizes approximately 0.5 log of HAV infectivity by blocking receptor binding sites on the viral particles whereas mkHAVCR1/TIM1(IgV+mucin)-Fc (Fig. 1), which contains the 27 repeats of the mucin domain, neutralizes up to 1.5 to 2 log of HAV by altering the viral particles (44). Here, we analyzed the effect of IgA in the neutralization of HAV by these two mkHAVCR1/TIM1 soluble receptor forms (Fig. 8). As expected, 40 μg mkHAVCR1/TIM1(IgV)-Fc in the absence of IgG or IgA (NONE) neutralized approximately 0.5 log HAV compared to control PVR-Fc (P < 0.0001) (Fig. 8A). Treatment with 5 μg of IgG had no effect on the HAV titers (compare +IgG with NONE). However, in the presence of 5 μg sIgA, mkHAVCR1/TIM1(IgV)-Fc neutralized approximately 1 log of HAV, which was significantly higher than in the presence of control IgG (P < 0.0001). We then tested HAV neutralization by mkHAVCR1/TIM1(IgV+mucin)-Fc in the presence of sIgA (Fig. 8B). We used a suboptimal nonsaturating concentration of 4 μg mkHAVCR1/TIM1(IgV+mucin)-Fc within the linear range of neutralization (44) (NONE) that, as expected, resulted in a 0.5-log reduction in the HAV titer compared to PVR-Fc (P < 0.0001). In the presence of 5 μg sIgA, mkHAVCR1/TIM1(IgV+mucin)-Fc neutralized approximately 1.5 log of HAV, which represented a significant increase in neutralization compared to neutralization in the absence of IgA (P < 0.0001). The significant increase in the receptor-mediated neutralization of HAV clearly shows that IgA enhanced the interaction of HAV with HAVCR1/TIM1.
FIG. 8.
Neutralization of HAV by soluble HAVCR1/TIM1 in the presence of sIgA. IgA enhanced significantly the neutralization of HAV by mkHAVCR1/TIM1 Fc fusion proteins. (A) HAV (105 TCID50) was incubated with 5 μg of purified human sIgA (+IgA), 5 μg of purified human IgG (+IgG), or medium (NONE) overnight at 4°C and then treated with 40 μg of mkHAVCR1/TIM1(IgV)-Fc (striped histogram) or PVR-Fc (solid histogram) for 2 h at 37°C. (B) HAV (105 TCID50) was incubated with 5 μg of purified human sIgA (+IgA) or medium (NONE) overnight at 4°C and then treated with 4 μg of mkHAVCR1/TIM1(IgV+muc)-Fc (open histogram) or PVR-Fc (solid histogram) for 2 h at 37°C. Residual infectious HAV was titrated on GL37 cells. Viral titers were determined by an endpoint dilution ELISA using the method of Reed and Muench (36). Data are from one experiment representative of three experiments and show the mean TCID50/ml and standard deviations in bars.
DISCUSSION
The cell entry and pathogenic process of HAV are poorly understood. Although it is well established that HAV is transmitted through the fecal-oral route, it is unknown whether there is a primary site of HAV replication in the digestive system or whether the input virus is transported to the blood and then reaches the liver, its target organ (45). HAV initially infects Kupffer cells, which are liver-resident macrophages, and then extends to the hepatocytes (2, 25, 42). We have previously shown that HAVCR1/TIM1 is a receptor for HAV (13, 19), but the exact role of this receptor in the pathogenesis of HAV needs to be fully elucidated. Soluble receptor forms of HAVCR1/TIM1 bind, alter, and neutralize HAV particles (43, 44), which indicated that additional coreceptors are not required for the initial steps in HAV cell entry. Since IgA increased the receptor-mediated neutralization of HAV (Fig. 4), it appears that additional factors may enhance the cell entry process of HAV. Several possible mechanisms by which IgA enhances the interaction of HAV with HAVCR1/TIM1 can be envisioned. For instance, binding of IgA to HAVCR1/TIM1 may expose epitopes or provide scaffolding to the HAVCR1/TIM1 Ig-like virus binding domain, enhancing the virus-receptor interaction. We have previously shown that mkHAVCR1/TIM1(IgV)-Fc cannot neutralize more than 0.5 log of HAV (43). However, in the presence of IgA, neutralization of HAV by mkHAVCR1/TIM1(IgV)-Fc increased significantly (Fig. 8A) to levels not reached with this soluble receptor form alone. mkHAVCR1/TIM1(IgV+mucin)-Fc, which contains a longer mucin with 27 hexameric repeats, neutralizes HAV more efficiently than mkHAVCR1/TIM1(IgV)-Fc and induces the alteration of the viral particles (44), suggesting that the mucin domain provides structural support for the Ig-like domain of HAVCR1/TIM1 and/or binds to the viral particles. Since IgA also enhanced the neutralization of mkHAVCR1/TIM1(IgV)-Fc, it is possible that IgA and the mucin domain may play a similar role, providing the necessary scaffolding to the Ig-like domain of HAVCR1/TIM1. Interestingly, IgA also enhanced neutralization of HAV by a suboptimal concentration of mkHAVCR1/TIM1(IgV+mucin)-Fc (Fig. 8B) but not under saturating conditions (data not shown). Since HAVCR1/TIM1 is expressed at low levels in most tissues (13, 19), except in testis and kidneys (19) mainly after injury (16) or in tumors (50), it is possible that binding of IgA to HAVCR1/TIM1 may enhance its HAV receptor function under the physiological conditions encountered by the virus. Moreover, the interaction of IgA with HAVCR1/TIM1 may play an important role in the pathogenesis of HAV by enhancing infection of cells that contain IgA and IgA receptors at the cell surface such as Kupffer cells and hepatocytes. Structural analysis of HAVCR1/TIM1 alone and complexed with IgA and HAV will provide a better understanding of the tripartite interaction of HAV, HAVCR1/TIM1, and IgA and its role in the pathogenesis of HAV.
The role of HAVCR1/TIM1 in the pathogenesis of HAV has not been fully investigated due to the lack of a small animal model for HAV and the difficulty of working with primates, the only available animal model for HAV. We have established that HAVCR1/TIM1 mediates cell entry of HAV in African green monkey kidney cells (19) and that Fc fusion proteins of this receptor bind and alter HAV particles (43, 44). Since HAVCR1/TIM1 is expressed in human liver (3, 13, 19) and derived cell lines (G. G. Kaplan et al., unpublished results) as well as in many other tissues (13, 19), HAVCR1/TIM1 may play a role in the pathogenesis of HAV. It has been suggested that the asialoglycoprotein receptor may play a role in virus entry to the liver in the presence of IgA anti-HAV antibodies by mediating the uptake of IgA-virus immune complexes (10) and that the antivectorial translocalization of IgA-coated HAV particles occurs in polarized epithelial cells via an IgA receptor (9). However, there is plenty of evidence indicating that anti-HAV antibodies are not required for HAV infection. Indeed, HAV grown in cell culture in the absence of anti-HAV antibodies is infectious, and direct transfection of HAV infectious cDNA/cRNA into marmoset livers results in HAV infection in the absence of anti-HAV antibodies (11). Since HAVCR1/TIM1 and the asialoglycoprotein receptor are both IgA receptors, it is possible that they may play a role in relapsing HAV infection (40), which occurs in the presence of anti-HAV antibodies in approximately 10% of hepatitis A cases.
In contrast to the increasing understanding of the role of mouse TIM family members in the immune response, very little is known about the immune function and natural ligands of the human members of the family, which include HAVCR1, HAVCR2, and TIMD4 (22), which code for HAVCR1/TIM1, hTIM3, and hTIM4, respectively. In mice, studies indicate that Tim-1, Tim-2, Tim-3, and Tim-4 are expressed in different cell types and have distinct but important functions, while the roles of Tim-5, Tim-6, Tim-7, and Tim-8 are not known. Murine Tim-1 functions as a critical costimulatory molecule on CD4+ T cells that regulates Th1/Th2 cell differentiation, tolerance, and airway hyperreactivity (48). Mouse Tim-2 is upregulated in Th2 cells, may be involved in peripheral tolerance by providing an inhibitory signal to downregulate Th2 responses, and may regulate Th2 responses during autoimmune inflammation (5). Two different ligands of Tim-2 have been identified that may have immune relevance: semaphorin 4A (23) and H-ferritin (6). Mouse Tim-3 is expressed only in differentiated Th1 cells and regulates Th1-mediated auto- and alloimmune responses (33, 37, 38). Galectin 9 has been identified as a ligand of mouse Tim-3, and their interaction suppresses Th1 responses (53). Mouse Tim-4, which is expressed in APCs, including mature dendritic cells, binds to mouse Tim-1, delivering a positive signal that costimulates T-cell proliferation (31). Interestingly, we did not detect binding of HAVCR1/TIM1(IgV)-Fc to cells expressing human TIM4 at the cell surface (data not shown), suggesting that, in contrast to the murine TIMs, human TIM4 is not a ligand of HAVCR1/TIM1.
While our current studies reveal a somewhat surprising interaction between HAVCR1/TIM1 and IgA, interactions among immunoglobulin superfamily members are fairly common. For example, immunoglobulin superfamily members CAR (coxsackievirus and adenovirus receptor) and CD4 both bind immunoglobulins and like HAVCR1/TIM1 also function as viral receptors (4, 24).
Human IgA exists in multiple forms of two subclasses: IgA1, the most abundant serum subclass produced in the bone marrow as a monomer, and IgA2, the predominant mucosal subclass found in a polymeric form synthesized by local plasma cells before being secreted (20). The sIgA is a dimeric form that contains two additional polypeptides, the J chain and the secretory component, and functions at the first line of defense against pathogens. The function of serum IgA is poorly understood: it is not usually involved in humoral immune responses; does not activate complement; and regulates IgG-mediated phagocytosis, chemotaxis, bactericidal activity, oxidative burst, and cytokine release (20, 49). Serum IgA has both anti- (51, 52) and proinflammatory (49) effects. IgA is thought to be involved in suppressing the development of atopy, but the molecular mechanisms involved in this process are poorly understood (35). For instance, IgA deficiency is associated with increased susceptibility to autoimmune and allergic disorders (39), and expression of the myeloid cell-specific IgA Fc receptor (CD89 or FcαRI) is upregulated in eosinophils from allergic patients (34). It is possible that cells expressing HAVCR1/TIM1 or natural soluble forms of this receptor (3, 13, 19) may cross-link IgA on APCs and send a signal via FcαRI, resulting in the inhibition of the development of atopy. Since HAVCR1/TIM1 is a significant atopy susceptibility gene (30), it is possible that different allelic forms of HAVCR1/TIM1 have different effects on APC activation. The mechanisms by which IgA and HAVCR1/TIM1 regulate the immune response are poorly understood. Our finding that IgA is a ligand of HAVCR1/TIM1 provides a link between these two molecules that may help us to understand their involvement in the regulation of the immune response. However, further research will be required to understand the immunological relevance of such an interaction.
In summary, using an expression cloning strategy, we identified IgA1λ as a specific ligand of HAVCR1/TIM1. Further, the association between IgA1λ and HAVCR1/TIM1, which could be specifically blocked by MAb to IgA1, Igλ light chain, or HAVCR1/TIM1 or by treatment with excess IgA1λ, enhanced the interaction of HAVCR1/TIM1 with HAV. Our results suggest that the interaction of IgA with HAVCR1/TIM1 may play a role in the pathogenesis of HAV, enhancing viral cell entry in cells expressing low levels of HAVCR1/TIM1. Finally, our results indicate that the interaction between HAVCR1/TIM1 and IgA represents a new pathway for the regulation of the immune responses by TIM family members.
Acknowledgments
This work was supported by the National Institutes of Health (PO1 AI54456 to D.T.U., R.H.D., and G.J.F.) and the Food and Drug Administration (core funding from CBER to G.G.K.).
We declare that we have no competing financial interests.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Aruffo, A., and B. Seed. 1987. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc. Natl. Acad. Sci. USA 84:8573-8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asher, L. V., L. N. Binn, T. L. Mensing, R. H. Marchwicki, R. A. Vassell, and G. D. Young. 1995. Pathogenesis of hepatitis A in orally inoculated owl monkeys (Aotus trivirgatus). J. Med. Virol. 47:260-268. [DOI] [PubMed] [Google Scholar]
- 3.Bailly, V., Z. Zhang, W. Meier, R. Cate, M. Sanicola, and J. V. Bonventre. 2002. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J. Biol. Chem. 277:39739-39748. [DOI] [PubMed] [Google Scholar]
- 4.Carson, S. D., and N. M. Chapman. 2001. Coxsackievirus and adenovirus receptor (CAR) binds immunoglobulins. Biochemistry 40:14324-14329. [DOI] [PubMed] [Google Scholar]
- 5.Chakravarti, S., C. A. Sabatos, S. Xiao, Z. Illes, E. K. Cha, R. A. Sobel, X. X. Zheng, T. B. Strom, and V. K. Kuchroo. 2005. Tim-2 regulates T helper type 2 responses and autoimmunity. J. Exp. Med. 202:437-444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, T. T., L. Li, D. H. Chung, C. D. Allen, S. V. Torti, F. M. Torti, J. G. Cyster, C. Y. Chen, F. M. Brodsky, E. C. Niemi, M. C. Nakamura, W. E. Seaman, and M. R. Daws. 2005. TIM-2 is expressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. J. Exp. Med. 202:955-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, J. I., J. R. Ticehurst, S. M. Feinstone, B. Rosenblum, and R. H. Purcell. 1987. Hepatitis A virus cDNA and its RNA transcripts are infectious in cell culture. J. Virol. 61:3035-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Souza, A. J., T. B. Oriss, K. J. O'Malley, A. Ray, and L. P. Kane. 2005. T cell Ig and mucin 1 (TIM-1) is expressed on in vivo-activated T cells and provides a costimulatory signal for T cell activation. Proc. Natl. Acad. Sci. USA 102:17113-17118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dotzauer, A., M. Brenner, U. Gebhardt, and A. Vallbracht. 2005. IgA-coated particles of Hepatitis A virus are translocalized antivectorially from the apical to the basolateral site of polarized epithelial cells via the polymeric immunoglobulin receptor. J. Gen. Virol. 86:2747-2751. [DOI] [PubMed] [Google Scholar]
- 10.Dotzauer, A., U. Gebhardt, K. Bieback, U. Gottke, A. Kracke, J. Mages, S. M. Lemon, and A. Vallbracht. 2000. Hepatitis A virus-specific immunoglobulin A mediates infection of hepatocytes with hepatitis A virus via the asialoglycoprotein receptor. J. Virol. 74:10950-10957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emerson, S. U., M. Lewis, S. Govindarajan, M. Shapiro, T. Moskal, and R. H. Purcell. 1992. cDNA clone of hepatitis A virus encoding a virulent virus: induction of viral hepatitis by direct nucleic acid transfection of marmosets. J. Virol. 66:6649-6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feigelstock, D., P. Thompson, P. Mattoo, and G. G. Kaplan. 1998. Polymorphisms of the hepatitis A virus cellular receptor 1 in African green monkey kidney cells result in antigenic variants that do not react with protective monoclonal antibody 190/4. J. Virol. 72:6218-6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feigelstock, D., P. Thompson, P. Mattoo, Y. Zhang, and G. G. Kaplan. 1998. The human homolog of HAVcr-1 codes for a hepatitis A virus cellular receptor. J. Virol. 72:6621-6628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao, P. S., R. A. Mathias, B. Plunkett, A. Togias, K. C. Barnes, T. H. Beaty, and S. K. Huang. 2005. Genetic variants of the T-cell immunoglobulin mucin 1 but not the T-cell immunoglobulin mucin 3 gene are associated with asthma in an African American population. J. Allergy Clin. Immunol. 115:982-988. [DOI] [PubMed] [Google Scholar]
- 15.Graves, P. E., V. Siroux, S. Guerra, W. T. Klimecki, and F. D. Martinez. 2005. Association of atopy and eczema with polymorphisms in T-cell immunoglobulin domain and mucin domain-IL-2-inducible T-cell kinase gene cluster in chromosome 5 q 33. J. Allergy Clin. Immunol. 116:650-656. [DOI] [PubMed] [Google Scholar]
- 16.Han, W. K., V. Bailly, R. Abichandani, R. Thadhani, and J. V. Bonventre. 2002. Kidney injury molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 62:237-244. [DOI] [PubMed] [Google Scholar]
- 17.Hirt, B. 1967. Selective extraction of polyoma DNA from infected mouse cell cultures. J. Mol. Biol. 26:365-369. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan, G., A. Levy, and V. R. Racaniello. 1989. Isolation and characterization of HeLa cell lines blocked at different steps in the poliovirus life cycle. J. Virol. 63:43-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaplan, G., A. Totsuka, P. Thompson, T. Akatsuka, Y. Moritsugu, and S. M. Feinstone. 1996. Identification of a surface glycoprotein on African green monkey kidney cells as a receptor for hepatitis A virus. EMBO J. 15:4282-4296. [PMC free article] [PubMed] [Google Scholar]
- 20.Kerr, M. A. 1990. The structure and function of human IgA. Biochem. J. 271:285-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khademi, M., Z. Illes, A. W. Gielen, M. Marta, N. Takazawa, C. Baecher-Allan, L. Brundin, J. Hannerz, C. Martin, R. A. Harris, D. A. Hafler, V. K. Kuchroo, T. Olsson, F. Piehl, and E. Wallstrom. 2004. T cell Ig- and mucin-domain-containing molecule-3 (TIM-3) and TIM-1 molecules are differentially expressed on human Th1 and Th2 cells and in cerebrospinal fluid-derived mononuclear cells in multiple sclerosis. J. Immunol. 172:7169-7176. [DOI] [PubMed] [Google Scholar]
- 22.Kuchroo, V. K., D. T. Umetsu, R. H. DeKruyff, and G. J. Freeman. 2003. The TIM gene family: emerging roles in immunity and disease. Nat. Rev. Immunol. 3:454-462. [DOI] [PubMed] [Google Scholar]
- 23.Kumanogoh, A., S. Marukawa, K. Suzuki, N. Takegahara, C. Watanabe, E. Ch'ng, I. Ishida, H. Fujimura, S. Sakoda, K. Yoshida, and H. Kikutani. 2002. Class IV semaphorin Sema4A enhances T-cell activation and interacts with Tim-2. Nature 419:629-633. [DOI] [PubMed] [Google Scholar]
- 24.Lenert, P., and M. Zanetti. 1991. CD4/immunoglobulin interaction: implications for immune physiology and autoimmunity. Int. Rev. Immunol. 7:237-244. [DOI] [PubMed] [Google Scholar]
- 25.Mathiesen, L. R., J. Drucker, D. Lorenz, J. A. Wagner, R. J. Gerety, and R. H. Purcell. 1978. Localization of hepatitis A antigen in marmoset organs during acute infection with hepatitis A virus. J. Infect. Dis. 138:369-377. [DOI] [PubMed] [Google Scholar]
- 26.Matricardi, P. M., F. Rosmini, L. Ferrigno, R. Nisini, M. Rapicetta, P. Chionne, T. Stroffolini, P. Pasquini, and R. D'Amelio. 1997. Cross sectional retrospective study of prevalence of atopy among Italian military students with antibodies against hepatitis A virus. BMJ 314:999-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matricardi, P. M., F. Rosmini, V. Panetta, L. Ferrigno, and S. Bonini. 2002. Hay fever and asthma in relation to markers of infection in the United States. J. Allergy Clin. Immunol. 110:381-387. [DOI] [PubMed] [Google Scholar]
- 28.McIntire, J. J., D. T. Umetsu, and R. H. DeKruyff. 2004. TIM-1, a novel allergy and asthma susceptibility gene. Springer Semin. Immunopathol. 25:335-348. [DOI] [PubMed] [Google Scholar]
- 29.McIntire, J. J., S. E. Umetsu, O. Akbari, M. Potter, V. K. Kuchroo, G. S. Barsh, G. J. Freeman, D. T. Umetsu, and R. H. DeKruyff. 2001. Identification of Tapr (an airway hyperreactivity regulatory locus) and the linked Tim gene family. Nat. Immunol. 2:1109-1116. [DOI] [PubMed] [Google Scholar]
- 30.McIntire, J. J., S. E. Umetsu, C. Macaubas, E. G. Hoyte, C. Cinnioglu, L. L. Cavalli-Sforza, G. S. Barsh, J. F. Hallmayer, P. A. Underhill, N. J. Risch, G. J. Freeman, R. H. DeKruyff, and D. T. Umetsu. 2003. Immunology: hepatitis A virus link to atopic disease. Nature 425:576. [DOI] [PubMed] [Google Scholar]
- 31.Meyers, J. H., S. Chakravarti, D. Schlesinger, Z. Illes, H. Waldner, S. E. Umetsu, J. Kenny, X. X. Zheng, D. T. Umetsu, R. H. DeKruyff, T. B. Strom, and V. K. Kuchroo. 2005. TIM-4 is the ligand for TIM-1, and the TIM-1-TIM-4 interaction regulates T cell proliferation. Nat. Immunol. 6:455-464. [DOI] [PubMed] [Google Scholar]
- 32.Meyers, J. H., C. A. Sabatos, S. Chakravarti, and V. K. Kuchroo. 2005. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol. Med. 11:362-369. [DOI] [PubMed] [Google Scholar]
- 33.Monney, L., C. A. Sabatos, J. L. Gaglia, A. Ryu, H. Waldner, T. Chernova, S. Manning, E. A. Greenfield, A. J. Coyle, R. A. Sobel, G. J. Freeman, and V. K. Kuchroo. 2002. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415:536-541. [DOI] [PubMed] [Google Scholar]
- 34.Monteiro, R. C., R. W. Hostoffer, M. D. Cooper, J. R. Bonner, G. L. Gartland, and H. Kubagawa. 1993. Definition of immunoglobulin A receptors on eosinophils and their enhanced expression in allergic individuals. J. Clin. Investig. 92:1681-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pilette, C., S. R. Durham, J. P. Vaerman, and Y. Sibille. 2004. Mucosal immunity in asthma and chronic obstructive pulmonary disease: a role for immunoglobulin A? Proc. Am. Thorac. Soc. 1:125-135. [DOI] [PubMed] [Google Scholar]
- 36.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent end points. Am. J. Hyg. 27:493-497. [Google Scholar]
- 37.Sabatos, C. A., S. Chakravarti, E. Cha, A. Schubart, A. Sanchez-Fueyo, X. X. Zheng, A. J. Coyle, T. B. Strom, G. J. Freeman, and V. K. Kuchroo. 2003. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat. Immunol. 4:1102-1110. [DOI] [PubMed] [Google Scholar]
- 38.Sanchez-Fueyo, A., J. Tian, D. Picarella, C. Domenig, X. X. Zheng, C. A. Sabatos, N. Manlongat, O. Bender, T. Kamradt, V. K. Kuchroo, J. C. Gutierrez-Ramos, A. J. Coyle, and T. B. Strom. 2003. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 4:1093-1101. [DOI] [PubMed] [Google Scholar]
- 39.Schaffer, F. M., R. C. Monteiro, J. E. Volanakis, and M. D. Cooper. 1991. IgA deficiency. Immunodefic. Rev. 3:15-44. [PubMed] [Google Scholar]
- 40.Schiff, E. R. 1992. Atypical clinical manifestations of hepatitis A. Vaccine 10:S18-S20. [DOI] [PubMed] [Google Scholar]
- 41.Seed, B., and A. Aruffo. 1987. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc. Natl. Acad. Sci. USA 84:3365-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibayama, T., H. Kojima, M. Ashida, S. Hirose, A. Sato, T. Kamimura, C. Hamada, Y. Shimizu, S. Suzuki, and F. Ichida. 1985. Localization of hepatitis A virus in marmoset liver tissue during the acute phase of experimental infection. Gastroenterol. Jpn. 20:564-572. [DOI] [PubMed] [Google Scholar]
- 43.Silberstein, E., G. Dveksler, and G. G. Kaplan. 2001. Neutralization of hepatitis A virus (HAV) by an immunoadhesin containing the cysteine-rich region of HAV cellular receptor-1. J. Virol. 75:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silberstein, E., L. Xing, W. van de Beek, J. Lu, H. Cheng, and G. G. Kaplan. 2003. Alteration of hepatitis A virus (HAV) particles by a soluble form of HAV cellular receptor 1 containing the immunoglobin- and mucin-like regions. J. Virol. 77:8765-8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stapleton, J. T. 1995. Host immune response to hepatitis A virus. J. Infect. Dis. 171(Suppl. 1):S9-S14. [DOI] [PubMed] [Google Scholar]
- 46.Thompson, P., J. Lu, and G. G. Kaplan. 1998. The Cys-rich region of hepatitis A virus cellular receptor 1 is required for binding of hepatitis A virus and protective monoclonal antibody 190/4. J. Virol. 72:3751-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Totsuka, A., and Y. Moritsugu. 1994. Hepatitis A vaccine development in Japan, p. 509-513. In K. Nishioka, H. Suzuki, S. Mishiro, and T. Oda (ed.), Viral hepatitis and liver disease. Springer-Verlag, Tokyo, Japan.
- 48.Umetsu, S. E., W. L. Lee, J. J. McIntire, L. Downey, B. Sanjanwala, O. Akbari, G. J. Berry, H. Nagumo, G. J. Freeman, D. T. Umetsu, and R. H. DeKruyff. 2005. TIM-1 induces T cell activation and inhibits the development of peripheral tolerance. Nat. Immunol. 6:447-454. [DOI] [PubMed] [Google Scholar]
- 49.van Egmond, M., C. A. Damen, A. B. van Spriel, G. Vidarsson, E. van Garderen, and J. G. van de Winkel. 2001. IgA and the IgA Fc receptor. Trends Immunol. 22:205-211. [DOI] [PubMed] [Google Scholar]
- 50.Vila, M. R., G. G. Kaplan, D. Feigelstock, M. Nadal, J. Morote, R. Porta, J. Bellmunt, and A. Meseguer. 2004. Hepatitis A virus receptor blocks cell differentiation and is overexpressed in clear cell renal cell carcinoma. Kidney Int. 65:1761-1773. [DOI] [PubMed] [Google Scholar]
- 51.Wolf, H. M., M. B. Fischer, H. Puhringer, A. Samstag, E. Vogel, and M. M. Eibl. 1994. Human serum IgA downregulates the release of inflammatory cytokines (tumor necrosis factor-alpha, interleukin-6) in human monocytes. Blood 83:1278-1288. [PubMed] [Google Scholar]
- 52.Wolf, H. M., I. Hauber, H. Gulle, A. Samstag, M. B. Fischer, R. U. Ahmad, and M. M. Eibl. 1996. Anti-inflammatory properties of human serum IgA: induction of IL-1 receptor antagonist and Fc alpha R (CD89)-mediated down-regulation of tumour necrosis factor-alpha (TNF-alpha) and IL-6 in human monocytes. Clin. Exp. Immunol. 105:537-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu, C., A. C. Anderson, A. Schubart, H. Xiong, J. Imitola, S. J. Khoury, X. X. Zheng, T. B. Strom, and V. K. Kuchroo. 2005. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 6:1245-1252. [DOI] [PubMed] [Google Scholar]