Abstract
Recently we have shown that influenza A virus infection leads to activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and that this cellular reaction is dependent on the expression of the viral nonstructural protein 1 (NS1). These data also suggested that PI3K activation confers a virus-supporting activity at intermediate stages of the infection cycle. So far it is not known which process is regulated by the kinase that supports virus replication. It is well established that upon infection with influenza A virus, the expression of the viral NS1 keeps the induction of beta interferon and the apoptotic response within a tolerable limit. On a molecular basis, this activity of NS1 has been suggested to preclude the activation of cellular double-stranded RNA receptors as well as impaired modulation of mRNA processing. Here we present a novel mode of action of the NS1 protein to suppress apoptosis induction. NS1 binds to and activates PI3K, which results in the activation of the PI3K effector Akt. This leads to a subsequent inhibition of caspase 9 and glycogen synthase-kinase 3β and limitation of the virus-induced cell death program. Thus, NS1 not only blocks but also activates signaling pathways to ensure efficient virus replication.
Influenza virus infection results in the activation of a variety of intracellular signaling pathways that are in part required to mount an antiviral response to infection but also may be exploited by the virus to support its replication (18, 19). A recent addition to these influenza virus-induced signaling mediators is phosphatidylinositol 3-kinase (PI3K) and its downstream effector Akt/protein kinase B (PKB) (12). PI3K consists of regulatory (p85) and enzymatic (p110) subunits, each existing in several isoforms. The active enzyme exhibits both a protein kinase and a lipid kinase activity (9). The kinase controls various cellular processes, such as metabolic regulation, cell growth, proliferation, and survival (3, 5, 14). The consequence of PI3K activation is the generation of phosphatidylinositol 3,4,5-trisphosphate from phosphatidylinositol 4,5-bisphosphate in the membrane, which functions as a second messenger to recruit pleckstrin homology domain-containing proteins, such as Akt (also known as PKB) and phosphoinositide-dependent kinase 1. Akt/PKB is a major PI3K effector and becomes further activated by phosphorylation at Thr308 and at Ser473 (3).
With regard to the function of the kinase in virus-infected cells, it has been recently shown that PI3K is involved in double-stranded RNA (dsRNA)-induced activation of the antivirally acting transcription factor interferon-regulatory factor 3 (27), suggesting a function in the defense against viral infection. While we were able to confirm such an activity in influenza virus-infected cells, we could also demonstrate that the virus exploits the pathway for a virus-supportive function, namely, to regulate the process of viral entry (12). However, despite this function, which would match the very early activation peak observed, a much stronger activation in later stages of the replication cycle could also be detected. Inhibitor studies revealed that in addition to entry, PI3K further regulates a virus-supportive function during later stages of replication (12).
It was interesting to note that a virus mutant lacking the viral NS1 protein (delNS1) failed to activate the PI3K/Akt pathway (12). Although a surprising multitude of activities had been originally assigned to the RNA binding NS1 protein, recent analyses have shown that a major in vivo function is to antagonize the antiviral type I interferon (IFN) system (15). Comparisons of wild-type and delNS1 viruses revealed that NS1 inhibits the activation of the IFN-β gene, which is controlled by the transcriptional activators NF-κB, interferon-regulatory factor 3, and ATF-2/c-Jun (20, 28, 31). Mechanistically, it was shown that the NS1 protein interferes with the intracellular signaling events that trigger activation of these dsRNA-responsive transcription factors (15). Significantly, this activity involves the targeting of the RNA helicase RIG-I, a cellular sensor for viral RNA (24, 34). In clear contrast to the dsRNA-activated IFN-β-inducing signaling pathways, the PI3K/Akt pathway, although activated by dsRNA, remained silent rather than being activated upon infection by delNS1 (12). This prompted us to analyze whether NS1 is involved in the activation of PI3K and which virus-supportive function the pathway may fulfill in later stages of the replication cycle.
MATERIALS AND METHODS
Viruses, cells, and viral infections.
Avian influenza virus A/FPV/Bratislava/79 (H7N7) (FPV), human influenza virus A/Puerto Rico/8/34 (H1N1) (PR8), the corresponding mutant delta NS1 (delNS1) (16), the human influenza virus strain A/Victoria/3/75 (H3N2) (Victoria), and the reassortant virus WSN-HK (H1N1) were propagated and used as described earlier (11, 20, 26, 30, 33). The human influenza virus isolate of a highly pathogenic avian virus strain, A/Thailand/KAN-1/2004 (H5N1), was a kind of gift from P. Puthavathana (Mahidol, University, Bangkok, Thailand) and has been passaged on Madin-Darby canine kidney (MDCK) cells. The recombinant PR8 virus and the corresponding truncated mutants comprising amino acids 1 to 80, 1 to 125, and 1 to 126 were propagated and used as described earlier (10, 31). For infection, cells were washed with phosphate-buffered saline (PBS) and incubated with virus at the indicated multiplicities of infection (MOI) diluted in PBS containing 0.2% bovine serum albumin (BSA), 1 mM MgCl2, 0.9 mM CaCl2, 100 U/ml penicillin, and 0.1 mg/ml streptomycin for 30 min at 37°C. The inoculum was aspirated, and cells were incubated with either minimal essential medium or Dulbecco modified Eagle medium (DMEM) (Invitrogen) containing 0.2% BSA and antibiotics. MDCK and African green monkey kidney (Vero) cells were grown in minimal essential medium. HEK 293 cells and the human lung epithelial cell line A549 were grown in DMEM. All media were supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen).
Plasmids, reagents, and inhibitors.
Expression constructs for wild-type NS1 of the influenza virus strain A/Puerto Rico/8/34 (H1N1) (PR8) and the corresponding mutant NS-IAmut1, which carries amino acid substitution at position 181 to 185 (LIGGL→KQRRS), were described earlier (20). Wild-type NS1 of the A/WSN/33 strain and the corresponding NS1 mutant with amino acid substitutions R38A and K41A, which is no longer able to bind to dsRNA, were described previously (20). Plasmids coding for the truncated PR8 NS1 mutant forms comprising amino acids 1 to 125 and 1 to 126 were originally constructed by inserting the corresponding cDNAs into pcDNA3. The latter mutant additionally codes for three nonviral amino acids and thus is slightly shifted in polyacrylamide gel electrophoresis gels (see Fig. 1D and F). Additional mutations affecting the dsRNA binding activity of the encoded NS1 proteins were introduced by using the QuikChange mutagenesis kit (Stratagene). The PR8 NS1 mutant proteins were inserted in a pTracer-CMV vector (Invitrogen) containing a hemagglutinin (HA) tag. Expression constructs for a wild-type form of Akt in pCMV5 were kindly provided by Jakob Troppmair, Daniel Swarovski Research Laboratory, University of Innsbruck, Austria, and have been described before (29).
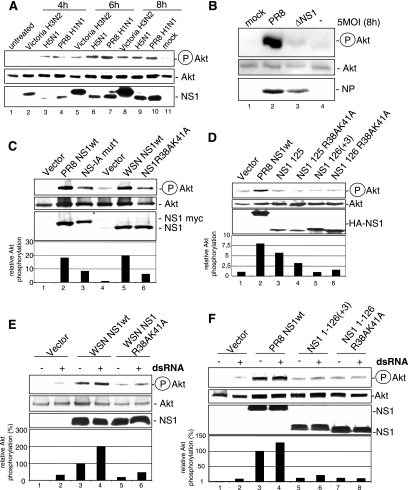
FIG. 1.
Influenza A virus NS1 induces activation of the PI3K/Akt signaling pathway. (A) A549 cells were left untreated (lane 1), were mock infected (lane 11), or were infected with influenza A virus strain Victoria/3/75 (H3N2) (lanes 2, 5, and 8), Thailand/KAN-1/2004 (H5N1) (lanes 3, 6, and 9), or PR8 (H1N1) (lane 4, 7, and 10) at a MOI of 5 for the indicated times. (B) A549 cells were left untreated (lane 4), mock infected (lane 1), or infected with influenza A virus strain PR8 (lane 2) or delNS1 (ΔNS1) (lane 3) at a MOI of 5 for 8 h. (C) A549 cells were transfected with empty vectors (lanes 1 and 4) or expression constructs for wild-type PR8 NS1 (PR8 NS1wt) (lane 2), NS-IAmut1 expressing a NS1 protein of wild-type length with five amino acid replacements at positions 181 to 185 (LIGGL to KQRRS) (lane 3), wild-type WSN NS1 (WSN NS1wt) (lane 5), and the corresponding NS1 with a R38AK41A mutation in the dsRNA binding site (lane 6). (D) A549 cells were transfected with constructs expressing NS1wt (lane 2) or truncated NS1 consisting of the N-terminal amino acids 1 to 125 (lane 3) or 1 to 126(+3) (lane 5), as well as their corresponding dsRNA binding site mutants bearing an R38AK41A mutation (lanes 4 and 6). (E) A549 cells were transfected with an empty vector or expression constructs for WSN NS1wt (lanes 3 and 4) and the corresponding NS1 with a R38AK41A mutation (lanes 5 and 6). Cells were left untreated (lanes 1, 3, and 5) or were transfected with the dsRNA analog poly(IC) (5 μg/ml) in the presence of DOTAP for 45 min and subsequently harvested (lanes 2, 4, and 6). (F) A549 cells were transfected with constructs expressing NS1wt (lanes 3 and 4) or truncated NS1 consisting of the N-terminal amino acids 1 to 126(+3) (lanes 5 and 6) or the corresponding dsRNA binding site mutant bearing an R38AK41A mutation (lanes 7 and 8). Note that cells in panels C to F were cotransfected with a plasmid expressing wild-type Akt. In all assays phosphorylated Akt (Ser473) was detected by Western blotting. Equal protein loading of the kinase was verified in Akt Western blots. Ongoing viral replication was demonstrated by accumulation of the viral NP in panel B. Equal NS1 production from plasmids was monitored in Western blots detecting NS1 (panel A, lanes 2 to 10; panel C, lanes 2, 3, 5, and 6; and panel E, lanes 3 to 6) or HA-tagged NS1 (panel D, lanes 2 to 6, and panel F, lanes 3 to 8). Relative Akt phosphorylation was normalized to Akt and NS1 content, upon quantification with the Lumi-Analyst program (Boehringer Mannheim). Akt phosphorylation levels of vector-transfected cells (panel C, lane 4, and panel E, lane 1) were arbitrarily set at 1, while phosphorylation of Akt in untreated WSN NSwt-expressing cells (panel D, lane 3) or untreated PR8 NS1wt-expressing cells (panel F, lane 1) was arbitrarily set at 100%.
An expression construct for a wild-type form of p85α C-terminally tagged with the yellow fluorescent protein (YFP) in pCDNA3 was kindly provided by Bernd Nürnberg, Institute of Biochemistry and Molecular Biology, Heinrich-Heine-University of Düsseldorf, and was previously described (4). The dsRNA analog poly(IC) was purchased from Amersham Biosciences and transfected at a concentration of 5 μg/ml with DOTAP (Roth) for 45 min according to the manufacturer's protocol. The specific PI3K inhibitor LY294002 (Promega) was dissolved in dimethyl sulfoxide (DMSO) and was added directly after infection as indicated. The apoptosis inducer staurosporine dissolved in DMSO (Sigma) was added directly to the medium at the indicated concentration.
Transient transfections.
A549 cells were transfected with Lipofectamine 2000 (Life Technologies) according to a protocol described previously (2). HEK 293 cells were transfected with polyethylenimine as described previously (13).
Immunoprecipitations and Western blotting.
For immunoprecipitations, cells were lysed on ice with Triton lysis buffer (20 mM Tris-HCl, pH 7.4; 137 mM NaCl; 10% glycerol; 1% Triton X-100; 2 mM EDTA; 50 mM sodium glycerophosphate, 20 mM sodium pyrophosphate; 5 μg ml−1 aprotinin; 5 μg ml−1 leupeptin; 1 mM sodium vanadate; and 5 mM benzamidine) for 30 min. For Western blotting, cells were lysed on ice with radioimmunoprecipitation assay lysis buffer (25 mM Tris, pH 8.0; 137 mM NaCl; 10% glycerol; 0.1% sodium dodecyl sulfate; 0.5% natrium-deoxycholate; 1% NP-40; 2 mM EDTA, pH 8.0; 5 μg ml−1 aprotinin; 5 μg ml−1 leupeptin; 1 mM sodium vanadate; and 5 mM benzamidine) for 30 min. Cell lysates were cleared by centrifugation, and the protein concentration was determined by the Bradford method. Cell lysates were used for immunoprecipitation with antibodies or antisera against green fluorescent protein (GFP) (B-2) or p85α (B-9) (Santa Cruz Biotechnologies), p85β (Serotec), or NS1 (polyclonal antiserum) coupled to protein A or G agarose (Roche). Mouse or rabbit sera were used for control purposes. Alternatively, lysates were directly subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subsequent blotting. The phosphorylated active form of Akt (Ser473) was detected in crude cell lysates by a phosphospecific Akt (Ser473) rabbit antiserum (Biosource). Different influenza A virus proteins were visualized using either a nucleoprotein (NP)-specific mouse antiserum (Serotec), a PB1-specific (vK-20) goat antiserum (Santa Cruz Biotechnologies), or NS1-specific rabbit antisera that were kind gifts of Ilkka Julkunen, National Public Health Institute, Finland, and Juan Ortin, CNB-CSIC, Spain. HA-tagged PR8 NS1 expression constructs were detected with an anti-HA monoclonal antibody (3F10; Roche). Different apoptosis-specific markers were visualized with the following specific antisera: a phospho-specific glycogen synthase-kinase 3 β (GSK-3β) (Ser9) rabbit antibody (Cell Signaling Technologies), a caspase 9 (Asp330)-specific rabbit antiserum (Cell Signaling Technologies), or a poly(ADP-ribose) polymerase (PARP)-specific mouse antibody (BD Transduction Laboratories). In some of the assays, loading controls were performed with a pan-ERK2 antiserum (Santa Cruz Biotechnologies) or a pan-Akt antiserum (Cell Signaling Technologies). Protein bands were visualized in a standard enhanced chemiluminescence reaction.
Indirect immunofluorescence microscopy.
MDCK or A549 cells were grown onto 15-mm coverslips, and 24 h later cells were left uninfected or were infected with the influenza A virus strain FPV (MOI = 0.01) or PR8 (MOI = 20), respectively. LY294002 (50 μM) or the same volume of the solvent (DMSO) was added directly upon infection. To detect the viral nucleoprotein, cells were stained with an anti-NP antibody as described earlier (12). To visualize cell nuclei in these assays, cells were additionally stained with 1 μM DAPI (4′,6′-diamidino-2-phenylindole) (Molecular Probes). For detection of apoptotic cell nuclei at 6 h postinfection (p.i.), cells were incubated with DMEM containing 2 μg/ml of propidium iodide (PI) for 15 min and fixed with 3.7% paraformaldehyde at 4°C for 20 min, as described previously (21). Cells were washed with PBS and incubated with 0.1% BSA for 5 min and phalloidin-Alexa 488 (Molecular Probes) for 30 min to detect the actin cytoskeleton. Cells were washed three times with PBS and once with water before being covered with fluorescent mounting medium (DAKO). Fluorescence was visualized using a Leitz DMRB fluorescence microscope.
Nicoletti assay.
Transfected or untransfected MDCK cells were infected with FPV (MOI = 0.005) for 18 h. Cells were treated with LY294002 (50 μM) or with the same volume of the solvent (DMSO) directly after infection. For determination of late stages of apoptotic cell death, apoptotic hypodiploid nuclei were detected by fluorescence-activated cell sorter analysis (22).
RESULTS
Expression of NS1 results in activation of the PI3K/Akt signaling pathway.
Activation of the PI3K/Akt pathway was observed upon infection with a variety of influenza A virus strains, including laboratory strains of the H1N1 subtype such as A/Puerto-Rico/8/34 (PR8), the human H3N2 isolate Victoria/3/75, and a human H5N1 isolate of the highly pathogenic avian influenza strains of the Asia type (Fig. 1A). Since infection of cells with the delNS1 virus mutant results in an almost complete abrogation of PI3K activation in A549 cells (Fig. 1B) or MDCK cells (12), we analyzed whether NS1 itself induces PI3K and Akt activation. Full-length NS1 or different mutants of the protein were expressed, and activation of the PI3K/Akt pathway was determined using a phosphospecific Akt antibody that detects active Akt when phosphorylated at S473. This phosphorylation/activation event has been shown to occur in a strictly PI3K-dependent manner (1, 12). Expression of full-length NS1 derived from the virus strain PR8 or WSN readily resulted in Akt phosphorylation and thereby activation of the kinase (Fig. 1C, lanes 2 and 5). Similar results were also obtained with NS1 proteins from other virus isolates, including those of highly pathogenic avian H7N1 and H5N1 strains or contemporary human virus isolates, such as A/New Caledonia/2007/99 (H1N1) (data not shown). Interestingly, a mutant of the NS1 protein that carries amino acid exchanges in the RNA binding site did not activate Akt as efficiently as the wild type (Fig. 1C, lane 6) although it was expressed at similar levels. Reduced Akt phosphorylation compared to that induced by wild-type NS1 was also observed with a mutant that carries amino acid replacements at positions 181 to 185 (LIGGL to KORRS) (Fig. 1C, lane 3). These mutations abrogate the binding to the NS1 binding protein NS1-BP (32) and cause a predominant cytoplasmic localization of the protein (20). Expression of truncated NS1 proteins with deletions of the C terminus of NS1 up to amino acid 125 or 126 resulted in an even weaker activation of PI3K/Akt (Fig. 1D, lanes 3 and 5), which was in some cases further reduced if the RNA binding domains of these fragments were mutated (Fig. 1D, lane 4). This suggests that an intact C terminus and the integrity of amino acids in the RNA binding domain as well as amino acids 181 to 185 are required for the full capacity of NS1 to activate the PI3K/Akt pathway. Since it has been shown previously that dsRNA can activate PI3K (27), the capacities of NS1 and the mutants to activate PI3K/Akt in the presence of the dsRNA analog poly(IC) was analyzed. Wild-type NS1- and mutant-expressing cells were transfected with poly(IC) and subsequently analyzed for Akt phosphorylation. As expected from earlier studies (12, 27), poly(IC) treatment resulted in a weak activation of the PI3K/Akt pathway also in the absence of NS1 (Fig. 1E and F, lanes 2). Strikingly higher levels of activation were detected in untreated cells that express wild-type NS1 (Fig. 1E and F, lanes 3). dsRNA treatment of wild-type NS1-expressing cells resulted in an enhanced PI3K/Akt activity (Fig. 1E and F, lanes 4). While this may indicate a synergism of dsRNA and NS1, an almost similar additive effect was also observed with the mutant lacking the RNA binding domain, although the overall activation of the PI3K/Akt pathway was much weaker in this case (Fig. 1E, lane 6, and F, lane 8). These findings indicate that dsRNA binding of NS1 is not a prerequisite for the capacity of the protein to activate the PI3K pathway and that both activation pathways might act independently of each other. Nevertheless, the integrity of the NS1 RNA binding domain appears to be required for full activation.
Infection of cells with mutant influenza viruses expressing truncated NS1 proteins results in reduced PI3K activity.
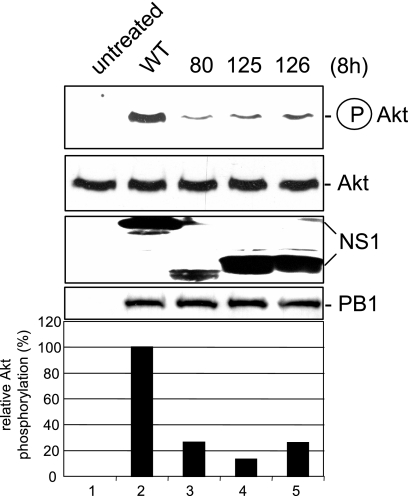
Singular expression of a viral protein may yield misleading results, since this scenario does not fully reflect the situation during virus propagation. Thus, besides the delNS1 virus, we also employed isogenic PR8 mutant viruses with truncated NS1 proteins to infect cells and assess PI3K/Akt activation. Consistent with the results obtained by transfection of NS1, we observed a reduction of PI3K/Akt activation with decreasing length of the protein (Fig. 2). This further supports the assumption that the C terminus of NS1 is required for full activation of the signaling pathway and confirms the previous findings in the context of a genuine virus infection.
FIG. 2.
Reduced Akt phosphorylation upon infection with virus mutants bearing C-terminal deletions in the NS1 protein. A549 cells were infected with a recombinant PR8 strain (wild type [WT]) (lane 2) or corresponding virus mutants expressing truncated NS1 proteins of amino acids 1 to 80 (80, lane 3), 1 to 125 (125, lane 4), or 1 to 126 (126, lane 5) at a MOI of 5 for 8 h. Phosphorylated Akt (Ser473) was detected by Western blotting. Equal protein loading of the kinase was verified in Akt Western blots. Equal viral replication status was demonstrated by accumulation of the viral polymerase protein PB1. Expression levels of NS1 and mutants were monitored in NS1 Western blots. Relative Akt phosphorylation was normalized to Akt and NS1 content, upon quantification with the Lumi-Analyst program (Boehringer Mannheim), Akt phosphorylation levels in cells infected with the recombinant PR8 strain (lane 2) were arbitrarily set at 100%.
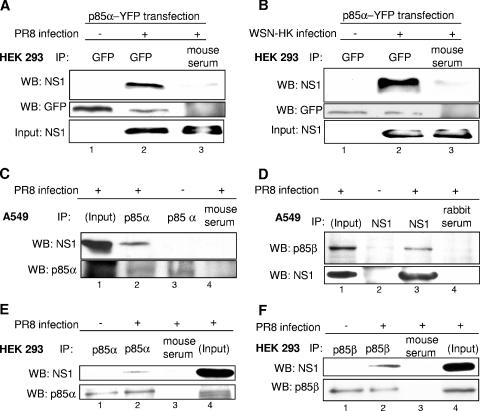
The NS1 protein coimmunoprecipitates with the p85α and -β regulatory subunits of PI3K.
A remaining question concerns the mechanism by which NS1 expression leads to PI3K activation. Most strikingly this may occur by direct interaction of NS1 with PI3K. Thus, we initially analyzed whether the α form of the regulatory subunit of PI3K, p85α, would precipitate with NS1 in infected cells. Indeed, we were able to coimmunoprecipitate the NS1 of either the PR8 or the WSN-HK strain with either a transfected p85α-YFP fusion protein (Fig. 3A and B) or the endogenous p85α protein (Fig. 3C and E). Furthermore we were also able to coimmunoprecipitate endogenous p85β protein with NS1 of the PR8 strain (Fig. 3D) and vice versa (Fig. 3F), confirming recent findings by Hale et al. (17). Interestingly, this coimmunoprecipitation was observed only in infected cells and not in cells where both proteins were expressed from plasmids (data not shown).
FIG. 3.
NS1 coimmunopreciptates with p85α and -β, two isoforms of the regulatory subunit of PI3K. (A and B) HEK 293 cells were transfected with a plasmid expressing a YFP-tagged version of p85α (p85α-YFP). At 24 h posttransfection cells were left untreated or infected with the influenza A virus strain PR8 (A) or the reassortant WSN-HK (B) (MOI = 5) for 4 h and subsequently harvested. Cells were subjected to immunoprecipitation (IP) with an anti-GFP antibody directed against the YFP tag (lanes 1 and 2) or a serum control (lanes 3). Coimmunoprecipitated NS1 was detected by Western blotting (WB). Equal protein loads of p85α-YFP in the immunoprecipitates were verified using the anti-GFP antibody detecting the YFP tag. The viral NS1 protein input of crude cell lysates served as control (lower panels). (C to F) A549 cells (C and D) or HEK 293 cells (E and F) were left untreated or infected with the influenza A virus strain PR8 (MOI = 5) for 4 h and subsequently harvested. Cells were subjected to immunoprecipitation of endogenous p85α with an anti-p85α antibody (panel C, lanes 2 and 3, and panel E, lanes 1 and 2), an anti-NS1 antiserum (panel D, lanes 2 and 3), an anti-p85β (panel F, lanes 1 and 2), or serum as a control (panels C and D, lanes 4, and panels E and F, lanes 3). Coimmunoprecipitated NS1 (C, E, and F) or coimmunoprecipitated p85β (D) was detected by Western blotting. Equal protein loads in the immunoprecipitates were verified in p85α (C and E), p85β (F), or NS1 (D) Western blots. The viral NS1 protein and endogenous p85α or -β input of crude cell lysate served as a control.
Viral PI3K activation is required to suppress apoptotic signaling responses.
The fact that the viral NS1 protein, which so far was known only to suppress antiviral signaling responses, now was found to specifically activate a signaling pathway points to a virus-supportive rather than a virus-antagonizing function of PI3K. In support of this consideration, inhibition of the kinase by wortmannin, a specific inhibitor of PI3K, still led to reduced progeny virus titers even if added as late as 2 h postinfection, suggesting a requirement of PI3K in intermediate or late stages of the infection cycle (12).
Besides its capacity to suppress activation of dsRNA-induced transcription factors, NS1 has also been shown to inhibit viral apoptosis induction (35). This effect of the protein was thought to occur primarily indirectly through the suppression of type I interferons, which are known initiators of programmed cell death. However, the finding that delNS1 virus infection resulted in a hyperinduction of apoptosis also in Vero cells that are devoid of functional IFN-α/β genes indicates that there are other, type I IFN-independent modes of NS1 action to suppress induction of cell death (35).
Among the various functions of PI3K, the protein is also known to mediate cell survival via activation of Akt (8). Akt directly phosphorylates caspase 9 and thereby inhibits activation of this apoptotic protease (6). Furthermore, among other activities, Akt phosphorylates and inactivates GSK-3β (25). This protein is involved in glycogen metabolism and also triggers proapoptotic metabolic events. Thus, phosphorylation by Akt silences the apoptosis-promoting activity of the kinase.
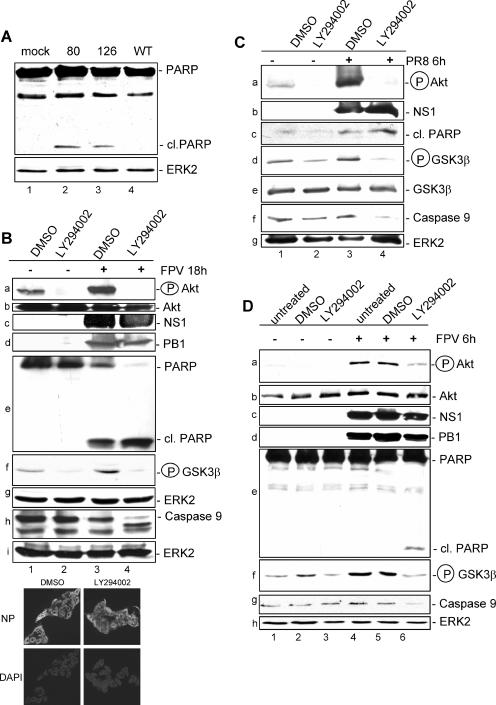
Besides the existing data regarding the delNS1 virus, the results shown in Fig. 4A further indicated that the capacity of influenza viruses to activate the PI3K/Akt pathway inversely correlates with viral induction of apoptosis. Here, virus mutant strains with truncated NS1 proteins were used that, according to the results shown in Fig. 2, only inefficiently activate Akt. While infection of cells with wild-type PR8 virus for 8 h did not lead to apoptosis induction, as measured by cleavage of PARP, a prominent substrate of apoptotic caspases, a cleaved PARP band is clearly visible upon infection with the truncated mutants (Fig. 4A). To further test whether NS1-mediated PI3K activation is involved in suppression of apoptosis, we treated uninfected and infected cells with the specific PI3K inhibitor LY294002. At 18 h postinfection (MOI = 0.01), cells were harvested and cell lysates were examined for Akt phosphorylation (Fig. 4B, panel a) and PARP cleavage (Fig. 4B, panel e). Furthermore, the activating cleavage of caspase 9 (Fig. 4B, panel h) and the phosphorylation of GSK-3β (Fig. 4B, panel f) as direct downstream effector functions of PI3K/Akt were analyzed. In uninfected cells the PI3K inhibitor was able to reduce basal Akt phosphorylation (Fig. 4B, panel a, lane 2). Neither PARP cleavage nor activating cleavage of caspase 9 was observed in these uninfected cells. Infection of solvent-treated cells showed induction of PARP cleavage (Fig. 4B, panel e, lane 3), consistent with earlier findings that influenza virus infection of cells results in caspase activation (33). However, in the same sample we also found enhanced levels of phosphorylated Akt (Fig. 4B, panel a, lane 3), which is thought to confer an antiapoptotic signal. As a consequence, in these cells, GSK-3β is phosphorylated and cleavage of caspase 9 is barely visible, most likely due to inactivation by active Akt (Fig. 4B, panels f and h, lane 3). Once PI3K/Akt activity is suppressed by LY294002, a strong increase of PARP cleavage concomitant with an activating cleavage of caspase 9 is observed (Fig. 4B, panels e and h, lane 4). This also coincides with a reduced phosphorylation of GSK-3β, an event that keeps this proapoptotic kinase active (Fig. 4B, panel f, lane 4). In addition, enhanced cleavage of caspase 3 and caspase 7 was also detectable in LY294002-treated and infected cells, indicating a massive onset of an apoptotic response in the absence of PI3K activation (data not shown). The detected effects were not due to an impact of PI3K inhibition on viral entry (12), as immunofluorescence analysis of the viral NP showed that the same number of cells were infected at 18 h p.i. in LY294002 and solvent-treated cells (Fig. 4B, bottom panels).
FIG. 4.
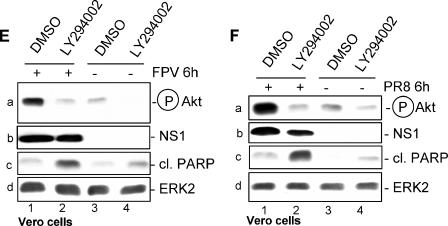
An active PI3K/Akt pathway suppresses viral apoptosis induction via inactivation of the Akt effectors GSK-3β and caspase 9. (A) MDCK cells were infected with a recombinant PR8 strain (wild type [WT]) (lane 4) or corresponding virus mutants expressing truncated NS1 proteins of amino acids 1 to 80 (80) (lane 2) and 1 to 126 (126) (lane 3) at a MOI of 1 for 8 h. (B, C, and D) MDCK cells were treated with the specific PI3K inhibitor LY294002 (50 μM) or equal volumes of the solvent (DMSO) right after infection with influenza A virus strain PR8 at a MOI of 20 (C) or with FPV at a MOI of 0.01 (B) or 1 (D) for 6 h (C and D) or 18 h (B). Thereafter cells were lysed and phosphorylated Akt (Ser473) was detected by Western blotting. Induction of apoptosis-regulating markers, such as cleavage of PARP and caspase 9 or phosphorylation of GSK-3β, was detected with specific antibodies against PARP (cleaved [cl.] or uncleaved), phosphorylated GSK-3β, and caspase 9. Note that in panels B and D the caspase 9 antibody detected only the full-length form of the caspase. Equal protein loads were verified with Akt or ERK2 blots. Ongoing viral replication was demonstrated by accumulation of the viral NS1 or PB1 protein in Western blots. Additionally, ongoing viral replication was verified via NP staining of MDCK cells which were infected with FPV and/or treated with LY294002 (50 μM) under the same conditions as described for the experiments shown in panel B. (E and F) Vero cells were treated with the specific PI3K inhibitor LY294002 (50 μM) or equal volumes of the solvent (DMSO) right after infection with the influenza A virus strain FPV (MOI = 1) (E) or PR8 (MOI = 7) for 6 h. Phosphorylated Akt (Ser473) and induction of PARP cleavage were detected as described above. Equal protein loads were verified with ERK2 blots. Ongoing viral replication was demonstrated by accumulation of the viral NS1 protein.
However, to further rule out that the observed effects are due to secondary autocrine or paracrine events that may have occurred during the 18-h infection period, we also analyzed lysates of cells that were infected for only 6 h with different influenza A virus strains (Fig. 4C and D), a time point within the primary replication cycle postinfection. At this earlier time point, viral activation/phosphorylation of Akt in untreated or solvent-treated cells coincided with a complete suppression of PARP cleavage, as well as enhanced phosphorylation and thereby inactivation of GSK-3β (Fig. 4C, lane 3, and D, lanes 4 and 5). Inhibition of PI3K by LY294002 in infected cells resulted in reduced phosphorylation of Akt and GSK-3β as well as cleavage of full-length caspase 9 and onset of PARP cleavage (Fig. 4C, lane 4, and D, lane 6). Finally, an indirect impact of IFN-α/β could be ruled out, since enhanced PARP cleavage was also detected in LY294002-treated Vero cells (Fig. 4E and F).
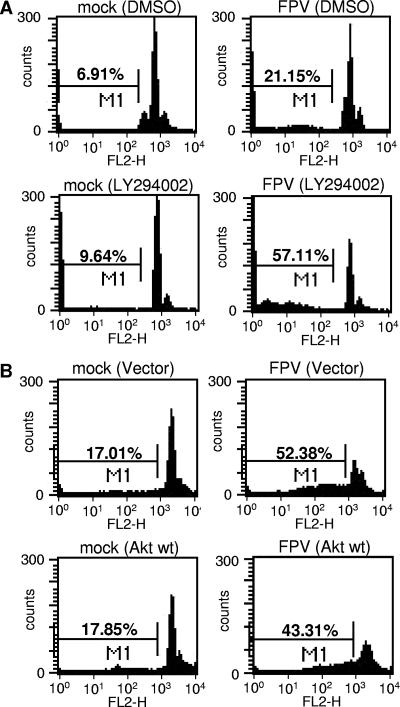
The apoptosis-suppressing effect of PI3K could further be detected when later events of apoptosis induction were analyzed, e.g., the onset of DNA fragmentation as measured by accumulation of cells with hypodiploid DNA content in Nicoletti assays (Fig. 5A and B). Virus-induced DNA fragmentation was strongly enhanced in infected cells treated with LY294002 (Fig. 5A, lower panels), while the number of cells with hypodiploid DNA content was reduced in infected cells that overexpress a wild-type form of Akt (Fig. 5B, lower panels).
FIG. 5.
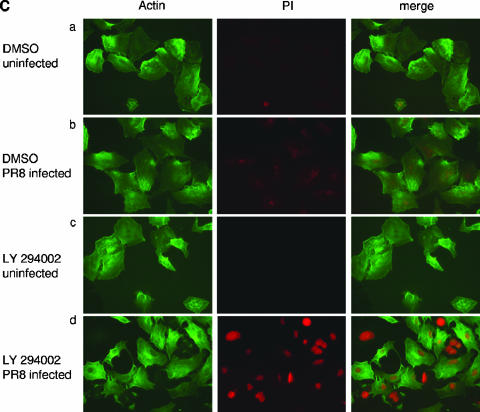
Inhibition of PI3K enhances virus-induced apoptosis, while overexpression of Akt results in reduced cell death. (A) MDCK cells were treated with LY294002 (50 μM) or equivalent volumes of the solvent (DMSO) right after infection with FPV (MOI = 0.005). (B) MDCK cells were transfected with plasmids expressing empty vector or wild-type Akt. At 48 h posttransfection cells were infected with the influenza A virus strain FPV (MOI = 0.005). (A and B) Apoptotic hypodiploid nuclei were measured in Nicoletti assays at 16 h p.i. as described in Materials and Methods. Note that basal apoptotic rates in transfected cells in panel B were higher than those in panel A due to the transfection procedure. (C) A549 cells were treated with the specific PI3K inhibitor LY294002 (50 μM) or equal volumes of the solvent (DMSO) right after infection with PR8 (MOI = 20). At 6 h postinfection, apoptotic leakage of the nuclear membrane was determined via nuclear PI inclusion. Cell bodies were visualized via actin staining.
Another hallmark of apoptosis induction is disintegration of the nuclear membrane, a process that allows DNA-staining compounds such as PI to easily enter the apoptotic cell nucleus (21). While a weak nuclear PI staining could be detected upon influenza virus infection (Fig. 5C, panels b), this was dramatically enhanced in infected cells that were treated with LY294002 (Fig. 5C, panels d). The compound alone had no effect (Fig. 5C, panels c).
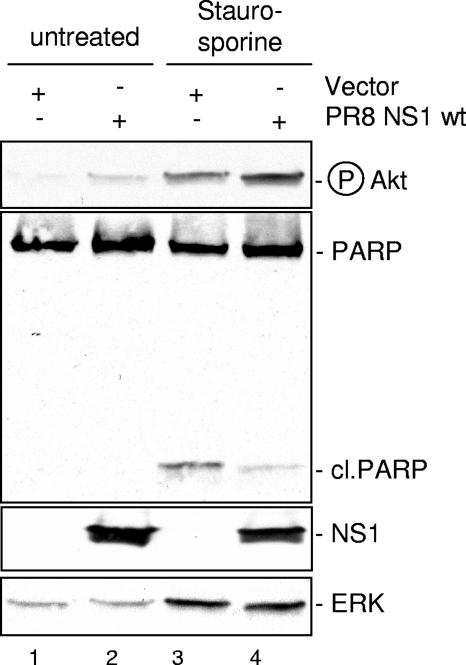
Finally, Fig. 6 shows that the ability of NS1 to enhance Akt phosphorylation in cells treated with the nonviral apoptosis stimulus staurosporine correlated with its capacity to reduce staurosporine-induced PARP cleavage (lanes 3 and 4).
FIG. 6.
NS1 expression results in reduced PARP cleavage induced by the nonviral apoptosis stimulus staurosporine. MDCK cells were transfected with plasmids expressing empty vectors or the PR8 NS1 wild-type protein (PR8 NS1 wt). At 24 h posttransfection cells were left untreated or treated with 1 μM staurosporine for 5 h. Phosphorylated Akt (Ser473) was detected by Western blotting. Induction of PARP cleavage as a hallmark of apoptosis was detected with specific antibodies against PARP (cleaved [cl.] or uncleaved). Protein loads were controlled with an ERK2 blot. Equal viral protein expression was verified in NS1 Western blots.
Taken together, these data clearly indicate that NS1-mediated activation of the PI3K/Akt pathway suppresses the onset of premature virus-induced caspase activation and apoptosis.
DISCUSSION
Influenza virus infection leads to the activation of a variety of signaling pathways, including the PI3K/Akt signaling cascade. The kinetics of activation were previously shown to be biphasic, peaking at early and late phases of infection (12). While activation of the early phase most likely is mediated by binding of the virus to the cell surface, which leads to signals that promote a very early step in virus uptake (12), it remained enigmatic for which virus-supportive event the later activation phase of PI3K may be required. Here we show that late activation of PI3K confers an antiapoptotic signal via activation of Akt and the Akt effectors GSK-3β and caspase 9. The influenza virus NS1 protein plays a major role in influenza virus activation of PI3K, presumably by association with the regulatory subunits p85α and -β. Thus, NS1, via activation of PI3K and Akt, appears to prevent premature apoptosis induction to ensure efficient replication.
This reveals an additional function of the NS1 protein, which apparently not only suppresses dsRNA-induced pathways (15) but is shown here also to activate a dsRNA-dependent signaling process. The precise contribution of the NS1 dsRNA binding domain remains to be fully understood, since poly(IC) treatment resulted in the same relative enhancement of PI3K activity in the presence of NS1, regardless of the integrity of its RNA binding domain. Nevertheless, the finding that a dsRNA binding-deficient NS1 mutant shows a weaker PI3K activation than the corresponding wild-type protein points to a functional involvement of this protein domain. Other structural constraints of the NS1 protein that appear to be required for optimal PI3K activation are an intact C terminus downstream of position 125 and a domain at amino acids 181 to 185. Currently it is unclear whether the respective domains are directly involved in the PI3K-NS1 interaction or whether mutations of these sites alter the secondary structure of the protein, which indirectly may prevent full PI3K activation.
Interestingly, interaction of p85α and -β with NS1 could be detected only in infected cells and not in transfected cells. Consistent with that, preliminary results from yeast two-hybrid assays showed only a very weak interaction of NS1 with p85α, indicating that an important cofactor for efficient binding is not present in yeast. This factor is most likely not dsRNA, since transfection of poly(IC) could not rescue coimmunoprecipitation of transfected p85 and NS1 proteins, again arguing against an essential role of dsRNA in NS1-mediated PI3K activation.
While the present paper was in preparation, another publication demonstrated direct binding of NS1 to the β isoform but not the α isoform of p85, which resulted in activation of PI3K (17). In our experiments we also observed an interaction with the α isoform of p85, although at a somewhat lower level, which may point to a slightly higher affinity of the NS1 to the β isoform of p85. In the study by Hale et al. (17), the authors describe amino acid 89 in the NS1 protein as being critical for binding to p85. This is consistent with our observation that a mutant virus expressing a NS1 protein of amino acids 1 to 80 induces a weaker Akt phosphorylation than the wild type. However, since both a virus bearing a NS1 protein of 125 N-terminal amino acids and a virus with a NS1 protein with mutations of amino acids 181 to 185 are also weak PI3K/Akt inducers, additional sites beside amino acid 89 in the protein appear to be required. Thus, our findings confirm the observations by Hale et al. (17) and expand the data by identification of the functional consequences of NS1-mediated PI3K activity, namely, suppression of viral apoptosis induction.
It might appear puzzling that influenza virus expresses a protein that suppresses apoptosis via PI3K while another viral protein, PB1-F2, even enhances apoptosis in infected cells (7). Furthermore, while the observations described here point to a predominant antiviral role of apoptosis induction, there are also data showing that apoptosis-inducing factors or early caspase activation is beneficial for virus replication (23, 33). However, these data are not contradictory if one takes into account that the virus not only might have evolved strategies to keep the overinduction of an apoptotic response at a tolerable limit but also may have acquired the capability to exploit the remaining activities to support its replication. This would also explain why NS1 does not completely suppress apoptotic responses or induction of other antiviral activities. Thus, influenza virus has evolved strategies to adjust the delicate balance of pro- and antiviral activities for an optimum of replication efficiency.
Acknowledgments
We thank Gudrun Heins, Sandra Neumann, Andrea Stadtbäumer, Carmen Theseling, and Ludmilla Wixler for excellent technical assistance. We also thank Ilkka Julkunen and Juan Ortin for generously providing antibodies and Andrej Egorov for recombinant viruses.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (DFG), the DFG Graduate Schools (GRK1045 and GRK1409), and the fund “Innovative Medical Research” as well as the IZKF of the University of Muenster Medical School. This work is also part of the activities of the European Union STREP EUROFLU and VIRGIL European Network of Excellence on Antiviral Drug Resistance, supported by a grant (LSHMCT-2004-503359) from the Priority 1 “Life Sciences, Genomics and Biotechnology for Health” program in the 6th Framework Programme of the European Union.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 2.Basler, C. F., X. Wang, E. Muhlberger, V. Volchkov, J. Paragas, H. D. Klenk, A. Garcia-Sastre, and P. Palese. 2000. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl. Acad. Sci. USA 97:12289-12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brazil, D. P., Z. Z. Yang, and B. A. Hemmings. 2004. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem. Sci. 29:233-242. [DOI] [PubMed] [Google Scholar]
- 4.Brock, C., M. Schaefer, H. P. Reusch, C. Czupalla, M. Michalke, K. Spicher, G. Schultz, and B. Nurnberg. 2003. Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J. Cell Biol. 160:89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cantley, L. C. 2002. The phosphoinositide 3-kinase pathway. Science 296:1655-1657. [DOI] [PubMed] [Google Scholar]
- 6.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase-9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 7.Chen, W., P. A. Calvo, D. Malide, J. Gibbs, U. Schubert, I. Bacik, S. Basta, R. O'Neill, J. Schickli, P. Palese, P. Henklein, J. R. Bennink, and J. W. Yewdell. 2001. A novel influenza A virus mitochondrial protein that induces cell death. Nat. Med. 7:1306-1312. [DOI] [PubMed] [Google Scholar]
- 8.Cooray, S. 2004. The pivotal role of phosphatidylinositol 3-kinase-Akt signal transduction in virus survival. J. Gen. Virol. 85:1065-1076. [DOI] [PubMed] [Google Scholar]
- 9.Dhand, R., I. Hiles, G. Panayotou, S. Roche, M. J. Fry, I. Gout, N. F. Totty, O. Truong, P. Vicendo, K. Yonezawa, et al. 1994. PI 3-kinase is a dual specificity enzyme: autoregulation by an intrinsic protein-serine kinase activity. EMBO J 13:522-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Egorov, A., S. Brandt, S. Sereinig, J. Romanova, B. Ferko, D. Katinger, A. Grassauer, G. Alexandrova, H. Katinger, and T. Muster. 1998. Transfectant influenza A viruses with long deletions in the NS1 protein grow efficiently in Vero cells. J. Virol. 72:6437-6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ehrhardt, C., C. Kardinal, W. J. Wurzer, T. Wolff, C. von Eichel-Streiber, S. Pleschka, O. Planz, and S. Ludwig. 2004. Rac1 and PAK1 are upstream of IKK-epsilon and TBK-1 in the viral activation of interferon regulatory factor-3. FEBS Lett. 567:230-238. [DOI] [PubMed] [Google Scholar]
- 12.Ehrhardt, C., H. Marjuki, T. Wolff, B. Nurnberg, O. Planz, S. Pleschka, and S. Ludwig. 2006. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell Microbiol. 8:1336-1348. [DOI] [PubMed] [Google Scholar]
- 13.Ehrhardt, C., M. Schmolke, A. Matzke, A. Knoblauch, C. Will, V. Wixler, and S. Ludwig. 2006. Polyethylenimine, a cost-effective transfection reagent. Signal Transduction 6:179-184. [Google Scholar]
- 14.Franke, T. F., C. P. Hornik, L. Segev, G. A. Shostak, and C. Sugimoto. 2003. PI3K/Akt and apoptosis: size matters. Oncogene 22:8983-8998. [DOI] [PubMed] [Google Scholar]
- 15.Garcia-Sastre, A. 2004. Identification and characterization of viral antagonists of type I interferon in negative-strand RNA viruses. Curr. Top. Microbiol. Immunol. 283:249-280. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Sastre, A., A. Egorov, D. Matassov, S. Brandt, D. E. Levy, J. E. Durbin, P. Palese, and T. Muster. 1998. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 252:324-330. [DOI] [PubMed] [Google Scholar]
- 17.Hale, B. G., D. Jackson, Y. H. Chen, R. A. Lamb, and R. E. Randall. 2006. Influenza A virus NS1 protein binds p8beta and activates phosphatidylinositol-3-kinase signaling. Proc. Natl. Acad. Sci. USA 103:14194-14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig, S., O. Planz, S. Pleschka, and T. Wolff. 2003. Influenza-virus-induced signaling cascades: targets for antiviral therapy? Trends Mol. Med. 9:46-52. [DOI] [PubMed] [Google Scholar]
- 19.Ludwig, S., S. Pleschka, O. Planz, and T. Wolff. 2006. Ringing the alarm bells: signalling and apoptosis in influenza virus infected cells. Cell Microbiol. 8:375-386. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig, S., X. Wang, C. Ehrhardt, H. Zheng, N. Donelan, O. Planz, S. Pleschka, A. Garcia-Sastre, G. Heins, and T. Wolff. 2002. The influenza A virus NS1 protein inhibits activation of Jun N-terminal kinase and AP-1 transcription factors. J. Virol. 76:11166-11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGahon, A. J., S. J. Martin, R. P. Bissonnette, A. Mahboubi, Y. Shi, R. J. Mogil, W. K. Nishioka, and D. R. Green. 1995. The end of the (cell) line: methods for the study of apoptosis in vitro. Methods Cell Biol. 46:153-185. [DOI] [PubMed] [Google Scholar]
- 22.Nicoletti, I., G. Migliorati, M. C. Pagliacci, F. Grignani, and C. Riccardi. 1991. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J. Immunol. Methods 139:271-279. [DOI] [PubMed] [Google Scholar]
- 23.Olsen, C. W., J. C. Kehren, N. R. Dybdahl-Sissoko, and V. S. Hinshaw. 1996. bcl-2 alters influenza virus yield, spread, and hemagglutinin glycosylation. J. Virol. 70:663-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opitz, B., A. Rejaibi, B. Dauber, J. Eckhard, M. Vinzing, B. Schmeck, S. Hippenstiel, N. Suttorp, and T. Wolff. 28November2006. IFN-β induction by influenza A virus is mediated by RIG-I which is regulated by the viral NS1 protein. Cell Microbiol. [Epub ahead of print.] [DOI] [PubMed]
- 25.Pap, M., and G. M. Cooper. 1998. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J. Biol. Chem. 273:19929-19932. [DOI] [PubMed] [Google Scholar]
- 26.Pleschka, S., T. Wolff, C. Ehrhardt, G. Hobom, O. Planz, U. R. Rapp, and S. Ludwig. 2001. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 3:301-305. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar, S. N., K. L. Peters, C. P. Elco, S. Sakamoto, S. Pal, and G. C. Sen. 2004. Novel roles of TLR3 tyrosine phosphorylation and PI3 kinase in double-stranded RNA signaling. Nat. Struct. Mol. Biol. 11:1060-1067. [DOI] [PubMed] [Google Scholar]
- 28.Talon, J., M. Salvatore, R. E. O'Neill, Y. Nakaya, H. Zheng, T. Muster, A. Garcia-Sastre, and P. Palese. 2000. Influenza A and B viruses expressing altered NS1 proteins: a vaccine approach. Proc. Natl. Acad. Sci. USA 97:4309-4314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Gise, A., P. Lorenz, C. Wellbrock, B. Hemmings, F. Berberich-Siebelt, U. R. Rapp, and J. Troppmair. 2001. Apoptosis suppression by Raf-1 and MEK1 requires MEK- and phosphatidylinositol 3-kinase-dependent signals. Mol. Cell. Biol. 21:2324-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagner, R., T. Wolff, A. Herwig, S. Pleschka, and H. D. Klenk. 2000. Interdependence of hemagglutinin glycosylation and neuraminidase as regulators of influenza virus growth: a study by reverse genetics. J. Virol. 74:6316-6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, X., M. Li, H. Zheng, T. Muster, P. Palese, A. A. Beg, and A. Garcia-Sastre. 2000. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J. Virol. 74:11566-11573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolff, T., R. E. O'Neill, and P. Palese. 1998. NS1-binding protein: a novel human protein that interacts with the influenza A virus nonstructural NS1 protein is relocalized in the nuclei of infected cells. J. Virol. 72:7170-7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wurzer, W. J., O. Planz, C. Ehrhardt, M. Giner, T. Silberzahn, S. Pleschka, and S. Ludwig. 2003. Caspase 3 activation is essential for efficient influenza virus propagation. EMBO J. 22:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 35.Zhirnov, O. P., T. E. Konakova, T. Wolff, and H. D. Klenk. 2002. NS1 protein of influenza A virus down-regulates apoptosis. J. Virol. 76:1617-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]