Abstract
Unlike activated T cells, quiescent CD4+ T cells have shown resistance to human immunodeficiency virus (HIV) infection due to a block in the early events of the viral life cycle. To further investigate the nature of this block, we infected quiescent CD4+ T cells with HIV-1NL4-3 and immediately stimulated them. Compared to activated (prestimulated) cells, these poststimulated cells showed slightly decreased viral entry and delays in the completion of reverse transcription. However, the relative efficiency of integration was similar to that of prestimulated cells. Together, this resulted in decreased expression of tat/rev mRNA and synthesis of viral protein. Furthermore, based on cell cycle staining and BrdU incorporation, poststimulated cells expressing viral protein failed to initiate a second round of their cell cycle, independently of Vpr-mediated arrest. Together, these data demonstrate that the early stages of the HIV life cycle are inefficient in these poststimulated cells and that efficient replication cannot be induced by subsequent activation.
Unlike their stimulated counterparts, quiescent CD4+ T cells have demonstrated resistance to human immunodeficiency virus (HIV) infection. Our earlier studies showed that quiescent CD4+ T cells, when infected with HIV, were unable to support productive viral replication, characterized by incompletely reverse transcribed viral DNA (47, 48). However, the permissiveness of other quiescent cell types, such as macrophages (12, 28, 35, 36, 39), raised further questions regarding the exact nature of the block of HIV infection in quiescent CD4+ T cells. Cells in the G0/1a stage of the cell cycle are truly quiescent, while cells in G1b are characterized by high levels of RNA synthesis in the absence of DNA synthesis (10, 45). Further studies demonstrated that T cells at the G0/1a stage of the cell cycle are truly resistant to viral infection, whereas cells in the G1b phase are permissive (21). Thus, T cells need not be actively dividing to support a productive HIV infection, a phenomenon also seen in macrophages (36). Treatment of G0/1a cells with nucleotides resulted in completion of reverse transcription (RT) but was insufficient to rescue productive viral infection (22). Therefore, the block in HIV replication was not merely a lack of raw materials but possibly of crucial cellular factors or the presence of inhibitors. This hypothesis was supported by subsequent studies demonstrating that reverse transcription of viral vectors was slow in quiescent cells, requiring 2 to 3 days, and that the half-life of the full-length viral genome was approximately 1 day (31, 50). Alternative methods of infection, such as spinoculation, while increasing the amount of viral entry, do not overcome the block in HIV infection (30, 43, 44).
A number of proteins have been suggested to inhibit HIV-1 replication in CD4+ T cells, such as Murr1 and APOBEC3G. Decreased Murr1 expression rendered quiescent CD4+ T cells permissive to HIV infection (13). APOBEC3G, a cytidine deaminase, has been shown to cause high levels of hypermutation of the HIV genome (15, 24, 49). The effects of APOBEC3G are countered by a viral protein, Vif, which has been shown to degrade APOBEC3G and prevent it from being packaged in the virion (9, 20, 26, 27, 37, 42, 46). Recently, Chiu et al. showed that APOBEC3G exists in an active enzymatic form at the G0/1a stage of the cell cycle, which inhibits HIV infection at the level of reverse transcription, although not by cytidine deaminase activity (5).
To further characterize the block of HIV infection in quiescent CD4+ T cells, we infected highly purified cells with the CXCR4-tropic HIV-1NL4-3 and stimulated (17, 19) these cells before (prestimulated) or 2 h after (poststimulated) infection. Quiescent cells displayed slightly lower levels of viral entry than stimulated cells. We also analyzed infected cells for initiation and completion of reverse transcription, integration, and expression of viral RNA and protein. We found strikingly inefficient rescue of virus production with stimulation immediately after infection. Furthermore, bromodeoxyuridine (BrdU) incorporation was used to determine whether infected cells could complete one cell cycle and reenter the G0/1a phase. Interestingly, those few cells that did express virus following activation failed to enter a second round of the cell cycle, and this phenomenon was independent of Vpr-mediated cell cycle arrest (1, 8, 11, 16, 18, 32, 33, 41). Together, these results suggest that infection remains inefficient for a period of time following stimulation of quiescent CD4+ T cells.
MATERIALS AND METHODS
Cell lines, primary cells, and virus stocks.
CEM is a human T4-lymphoblastoid cell line (ATCC no. CCL-119). Human peripheral blood mononuclear cells were obtained from healthy HIV-seronegative donors, separated over a Ficoll-Hypaque gradient, and cultured in RPMI 1640 medium supplemented with 10% fetal calf serum (Gibco-Invitrogen, Carlsbad, CA), 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM l-glutamine (RPMI 10). The HIV molecular clone NL4-3 (HIVNL4-3) and a Vpr-mutated virus (HIVNL4-3/Vpr−) (1, 14) were used in these studies. Virus stocks were generated from 24-h harvests of supernatants from CEM cells, electroporated with full-length infectious cloned viral DNA, and further amplified on CEM cells. The supernatants collected were cleared by centrifugation and treated with DNase I (1 μg/ml) in the presence of 10 mM MgCl2 for 30 min at room temperature. Subsequently, the supernatants were concentrated 10- to 20-fold using Amicon Ultra-15 Centrifugal Units (minimum molecular mass retention, 100 kDa; Millipore, Billerica, MA) according to the manufacturer's instructions. Viral stocks were titered by limiting-dilution assays using CEM cells.
Isolation of quiescent CD4+ T cells.
Human peripheral blood mononuclear cells were incubated with a cocktail of mouse monoclonal antibodies against human lymphocyte markers to remove activated CD4+ T cells (CD25, CD38, CD69, and HLA-DR [BD Biosciences, San Jose, CA]) and unwanted cell lineages, such as CD8 T cells (CD8 [BDBiosciences]), macrophages (CD14 and CD16 [BDBiosciences]), B cells (CD19 [BDBiosciences]), granulocytes (CD123 [Beckman Coulter, Fullerton, CA]), and NK cells (CD56 [BD Biosciences]). Cells stained with the above-mentioned antibodies were removed after incubation with magnetic beads coated with goat antibodies against mouse immunoglobulin G (Miltenyi Biotech, Auburn, CA) and separated using an AutoMACS (Miltenyi Biotech) cell sorter.
Flow cytometric analysis for markers.
To assess the purity of the quiescent cell population, 5 × 104 cells were stained against the markers listed above using monoclonal antibodies conjugated to phycoerythrin or fluorescein isothiocyanate (FITC), as previously described (21). Ten thousand events were acquired on a FACSCalibur flow cytometer (BD Biosciences). Live cells were gated by using forward-versus-side-scatter dot plots. Data were analyzed by using the CellQuest program (BD Biosciences). Quiescent cells contained less than 1% contaminating cell populations. The protocol described above was used to determine the levels of CD25, CD69, and HLA-DR on stimulated cells.
Infection of quiescent CD4+ T cells followed by stimulation.
The quiescent cell population was infected by incubation at a multiplicity of infection (MOI) of 1 (unless otherwise specified) for 1 to 2 h with virus in the presence of Polybrene (10 μg/ml). The cells were washed to remove residual free virus and recultured under the appropriate conditions. Specifically, the cells were cultured in RPMI 10 and costimulated with 1 μg/ml of OKT3 (anti-CD3) monoclonal antibody immobilized on goat anti-mouse antibody (Beckman Coulter)-coated plates with the simultaneous addition of soluble anti-CD28 antibody (Beckman Coulter) at a concentration of 50 ng/ml. Some of the quiescent cells were treated with either a 3.5 mM concentration of the cell cycle inhibitor n-butyrate, a 5 μM concentration of aphidicolin, or 10 ng/ml nocodazole (Sigma-Aldrich, St. Louis, MO). Similarly, some cells were cultured in the presence of zidovudine (AZT) (Sigma-Aldrich) to serve as negative controls. We also included CD4+ T cells that were stimulated for 48 h (prestimulated positive controls) and unstimulated quiescent cells. To prevent virus spread and to achieve a single-cycle infection, the cells were treated with a 100 nM concentration of a protease inhibitor (indinavir; Merck, Rahway, NJ) following infection. In some experiments, cells were cultured in the presence of 100 μM BrdU (Sigma-Aldrich) to identify the completion of cell division.
Cell cycle and viral-protein expression analyses.
Approximately 0.5 × 106 to 1 × 106 cells from each condition were stained for DNA, RNA, and Gag expression. The cells were washed with phosphate-buffered saline (PBS) supplemented with 3% fetal calf serum and fixed with 2% paraformaldehyde for 30 min at 4°C. Then, the cells were incubated with 0.5 ml of PBS supplemented with 0.02% Tween 20 (PBS-T) (Sigma-Aldrich) for 20 min at room temperature. The cells were then stained with 0.02 mg/ml 7-aminoactinomycin D (7-AAD) (Sigma-Aldrich) and 5 μl FITC-conjugated KC57 (anti-Gag) antibody (Beckman Coulter), followed by 10 min of incubation. The cells were then washed, resuspended in PBS-T containing 5 μM Pyronin Y (Sigma-Aldrich), and analyzed by flow cytometry. For the BrdU incorporation experiments, cells were fixed and then permeabilized. Subsequently, they were washed and resuspended in 0.1 ml PBS containing 0.3 mg/ml DNase (Gibco-Invitrogen) and incubated for 1 h at room temperature. The cells were then stained with 2 μl Alexa 647-conjugated anti-BrdU (Molecular Probes, Carlsbad, CA) and FITC-conjugated KC57 for 30 min at room temperature, followed by the addition of 7-AAD. Finally, samples were resuspended in PBS-T containing Pyronin Y and analyzed by flow cytometry.
Viral-DNA analysis.
Approximately 0.3 × 106 cells from each condition were used for reverse transcription and integration assays. Quantitative real-time DNA PCR was performed as previously described (21) using a primer-probe pair that amplifies cellular β-globin sequences and one that amplifies full-length HIV reverse transcripts (the long terminal repeat-gag junction), formed near the completion of the reverse transcription process. All amplifications were performed on the ABI7700 (Applied Biosystems, Foster City, CA) in parallel with a set of known quantitative standards. The standard curve used to determine HIV DNA levels ranged from 10 to 20,000 copies of cloned HIV DNA. The standard curve used to determine levels of beta-globin gene sequences consisted of DNA derived from 10 to 100,000 normal human peripheral blood lymphocytes. Quantitation of HIV-1 sequences was achieved by extrapolation from these standard curves. Detection and quantitation of integrated viral DNA was assessed by Alu PCR as previously described (29). Briefly, we performed two nested PCR amplification steps. In the first preamplification step, we used primers to Alu and gag. This was followed by real-time PCR using internal primers and probes to the long terminal repeat-gag junction. To control for any nonintegrated viral DNA, we performed the preamplification step with only the gag primer (linear preamplification of DNA) or without any primers (no preamplification of DNA). A standard curve representing integrated HIV sequences was generated from cells infected with a nonspreading HIV-based reporter vector. In control experiments, there was no background from nonintegrated viral DNA, and values for integrated DNA copies varied less than 20% within triplicates. The beta-globin standard was used to determine the approximate number of proviruses per cell.
RNA analysis.
Real-time multiplex reverse transcription and PCR amplification were performed simultaneously for viral full-length and tat/rev, as well as cellular β2m, RNAs with the QIAGEN One Step RT-PCR kit (QIAGEN, Chatsworth, CA), using the manufacturer's protocol. HIV full-length, tat/rev, and β2m primers have been described (3). All RT-PCR amplifications were performed on the ABI7700 (Applied Biosystems).
Viral-entry assay.
A total of 107 cells were washed and resuspended in an HIV suspension containing 300 ng of p24 for 30 min at 4°C under gentle agitation. After successive washes, the cells were treated with pronase immediately or incubated at 37°C for 1 h before pronase treatment. Some cells were not treated with pronase to assess the amount of virus binding on the cell surface. These cells were lysed immediately after incubation. For pronase treatment, cells were resuspended in a solution containing 1 ml of RPMI, 20 mM HEPES, and 0.1 mg/ml of pronase for 5 min at 4°C. The cells were washed successively to eliminate pronase and subsequently lysed in 1% Triton X-100. The lysates were spun at 14,000 rpm for 10 min to remove cellular debris. The supernatants were used to quantitate p24 levels by enzyme-linked immunosorbent assay.
RESULTS
Kinetics of quiescent CD4+ T-cell proliferation following stimulation.
We carefully examined the kinetics of HIV infection within the context of the cell cycle, utilizing flow cytometry. First, we determined the kinetics of cell division in quiescent T cells stimulated with anti-CD3/anti-CD28. By 18 h poststimulation, the cells began to enter the G1b phase, and by 36 h, the cells initiated DNA synthesis, indicative of entry into S phase (Fig. 1). Approximately 20% of the cells completed their cycle by 48 h, and this increased to 60% by 72 h. In addition, we looked at the expression of T-cell activation markers. Expression of CD69 appeared 12 h after stimulation, CD25 48 h after stimulation, and HLA-DR at 96 h poststimulation (data not shown). Based on the above-mentioned results, cell cycle progression of stimulated quiescent CD4+ T cells is relatively synchronous.
FIG. 1.
Quiescent CD4+ T-cell proliferation following stimulation. Quiescent CD4 T cells were purified and stimulated as described in Materials and Methods. Cells were then harvested and stained with 7-AAD (y axis; linear fluorescence) (DNA) and Pyronin Y (x axis; linear fluorescence) (RNA) and analyzed by flow cytometry. The quadrants were set based on n-butyrate (G1a arrest) and aphidicolin (G1b arrest) treatment of the cells.
Levels of HIV entry in quiescent and prestimulated cells.
We examined the levels of p24 bound and internalized in quiescent and prestimulated cells to determine the levels of viral entry. Quiescent and prestimulated CD4+ T cells were exposed to the same amount of virus at 4°C for 30 min to facilitate viral binding. Subsequently, the cells were washed and a portion were incubated at 37°C to allow internalization. Following incubation, the cells were treated with pronase to remove any noninternalized virus and lysed. Control cells were treated with pronase immediately after the 4°C incubation and lysed. The supernatants were assayed for levels of cytosolic p24, which is a reliable indicator of viral entry (25). While both nonstimulated and prestimulated cells bound the same amount of virus (data not shown), quiescent cells contained approximately fourfold-lower levels of cytosolic p24 than did prestimulated cells (Fig. 2). Therefore, HIV enters prestimulated cells slightly more efficiently than it enters quiescent cells.
FIG. 2.
Viral entry in quiescent and prestimulated CD4+ T cells. Quiescent CD4 T cells were purified, and some were stimulated as described in Materials and Methods. Equal numbers of cells were incubated with equal amounts of virus (based on p24 values), followed by pronase treatment to remove noninternalized virus. The cells were lysed and assayed for cytosolic p24 by enzyme-linked immunosorbent assay. The data are an average of six independent experiments and indicate the levels of p24 in 1 ml of lysate. Background values of cells incubated with virus at 4°C and immediately treated with pronase were subtracted. The error bars indicate standard deviations.
Kinetics of viral reverse transcription and integration.
We next determined the kinetics of reverse transcription and integration of full-length HIV DNA in cells stimulated at different times. Prestimulated cells initiated reverse transcription at the 2-hour time point, and complete reverse transcripts appeared at the 4-hour time point (Fig. 3). Reverse transcription peaked at 18 h poststimulation, followed by a rapid decrease of full-length viral DNA transcripts, suggesting death of infected cells. However, in cells stimulated 2 h following infection (poststimulated cells), the kinetics of viral DNA synthesis were considerably slower, although with similar trends (Fig. 3). Completion of reverse transcription occurred at 18 to 24 h. The levels of viral DNA in poststimulated cells were 30-fold lower than in the prestimulated group. Interestingly, nonstimulated cells (Fig. 3) showed levels of reverse transcription comparable to those of poststimulated cells. However, the levels of complete viral transcripts in nonstimulated cells plateaued, suggesting that these cells did not die following infection.
FIG. 3.
Immediate activation of HIV-infected quiescent CD4+ T cells does not rescue reverse transcription and integration. Quiescent cells infected with HIV and immediately stimulated (poststimulated) or not were harvested at different times following stimulation or infection (nonstimulated groups). Prestimulated controls were also included. DNA was isolated and used as a template in a real-time PCR for the detection of initiated (⧫) and complete (▪) reverse transcripts, as well as integrated (▴) viral DNA. The levels of viral DNA in cells treated with AZT were below detection levels. For reverse transcription, 10−2% cells was the limit of sensitivity, and for the integration assays, the limit was less than 10−5%.
Integration of viral DNA could be detected by 4 h postinfection and peaked at 12 h in prestimulated cells (Fig. 3). Poststimulated and nonstimulated cells showed similar slower kinetics of integration, with a peak at 36 h. However, the relative ratios of full-length and integrated viral DNAs were similar in all cell types. In prestimulated cells, integrated DNA decreased to an undetectable level by the 120-hour time point, suggesting cell death. However, nonstimulated cells showed a plateau of integrated DNA, which suggests survival of the cells. Together, these results demonstrate delayed kinetics in the early steps of HIV infection in cells not stimulated prior to infection.
Viral-RNA expression.
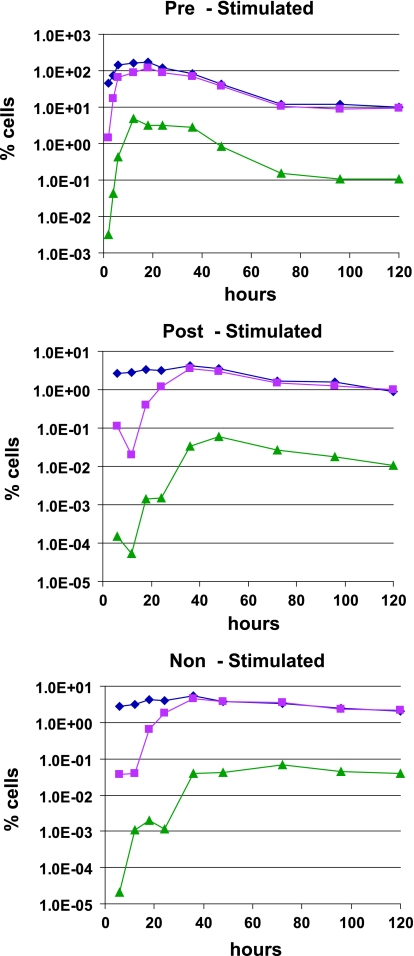
We also examined the kinetics of viral-RNA expression. Total RNA was isolated from pre-, post-, and nonstimulated cells and assayed for the presence of multiply spliced tat/rev transcripts to avoid complications from virion-associated RNA. In prestimulated cells, tat/rev transcripts are first detectable at the 12-hour time point, with a peak at 18 h (Fig. 4). The poststimulated group showed 30-fold-lower levels of viral RNA and slower kinetics, consistent with our previous data (2). In this group, transcription began at 24 h poststimulation, peaked at 72 h (Fig. 4), and then showed a gradual decrease consistent with the viral-DNA analysis. Similar kinetics were observed in the nonstimulated group, although tat/rev transcript levels were 10-fold lower at their peak than in poststimulated cells (Fig. 4). Thus, consistent with poor reverse transcription, we observed lower viral-RNA expression in poststimulated cells than in prestimulated cells.
FIG. 4.
Viral-RNA expression is diminished in poststimulated cells. Total cellular RNA was isolated from prestimulated (diamonds), poststimulated (triangles), and nonstimulated (squares) groups and used to detect the amount of multiply spliced (tat/rev) viral RNA.
Expression of viral protein in the context of the cell cycle.
We then determined the kinetics of viral protein (Gag) expression in the infected cells and correlated this with cell cycle progression. Consistent with the RNA kinetics, Gag protein expression was seen within 24 h of infection in prestimulated cells but not until 72 h in poststimulated cells (Fig. 5). Expression in poststimulated cells persisted for up to 96 h (Fig. 5) and was absent by 120 h (data not shown). In contrast, viral-protein expression in prestimulated cells infected in parallel started earlier (24 h) and reached 4% of intracellular Gag staining (data not shown). A similar attrition in the number of Gag-expressing cells over time was seen in the prestimulated group (data not shown). Despite levels of reverse transcription and integration similar to those of poststimulated cells (Fig. 3 and 4), nonstimulated cells did not show any viral-protein expression (data not shown).
FIG. 5.
HIV gag expression is significantly decreased in poststimulated cells. Quiescent CD4+ T cells were infected with HIV at an MOI of 1 and immediately stimulated. Cells were then harvested at various times and stained with FITC-conjugated anti-Kc57 (log10 fluorescence), a Gag p24-specific antibody. No viral-protein expression was observed before 36 h poststimulation.
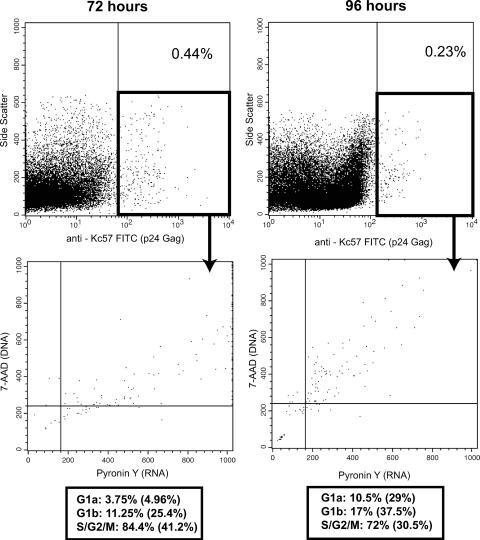
In the poststimulated cells at 72 h, the majority of the Gag-positive cells were in the S/G2/M phases of the cell cycle (approximately 80%), with smaller numbers in the G0/G1a (approximately 5%) and G1b (approximately 15%) phases (Fig. 6). These distributions were the same at the 96-hour time point, with the majority of cells in the later stages of the cell cycle. Based on these data, Gag-positive cells may not complete the entire cell cycle but rather die at the S/G2/M phases. However, a number of cells in the G1a stage were positive for Gag. Thus, it was of interest to determine if these cells had been infected at this stage or had progressed toward a second round of division.
FIG. 6.
Gag-positive cells are mainly located in the later phases of the cell cycle. Quiescent CD4+ T cells were infected with HIV at an MOI of 1 and immediately stimulated. Cells were then harvested at various times and stained with FITC-conjugated anti-Kc57, a Gag p24-specific antibody; 7-AAD (DNA); and Pyronin Y (RNA). The distributions of the Gag-positive cells are indicated below the cell cycle plots. The percentages in parentheses refer to the total cell distribution.
To examine this question, we infected quiescent CD4+ T cells and stimulated them in the presence of BrdU. Based on the amount of BrdU incorporation during S phase, we could determine whether the cells had completed mitosis. As with the previous experiments, we included prestimulated cells, nonstimulated cells, and AZT-treated controls for each condition. At 72 h poststimulation, the majority of the Gag-positive cells were BrdU positive (data not shown). The Gag+ BrdU+ cells were mostly localized in the S/G2/M phases of the cell cycle. In contrast, the Gag+ BrdU− cells were mainly in the G0/1a and G1b stages, with significant numbers in the S/G2/M stages, most likely beginning entry into the S phase. At the 96-hour time point (Fig. 7), almost all of the Gag-positive cells were BrdU+ and mainly in the S/G2/M phases with essentially no cells in G0/1a. In contrast, in prestimulated cells, we observed some Gag+ BrdU+ cells in the G1a phase (data not shown). This was expected, since the cells were asynchronous and could have been infected at different stages of the cell cycle. Therefore, in this single-round infection protocol, poststimulated cells infected with HIV failed to complete their life cycle. This may be due to cell death prior to entry into a second round of DNA synthesis or arrest in G2. The presence of G0/1a cells that expressed viral protein suggests that these cells had become partially permissive under these culture conditions.
FIG. 7.
Infected cells fail to complete their cell cycle. Quiescent CD4+ T cells cultured as in previous experiments in the presence of BrdU were harvested at 96 h poststimulation; stained for Gag (anti-Kc57 FITC), BrdU (anti-BrdU Alexa 647; log10 fluorescence), DNA (7-AAD), and RNA (Pyronin Y); and analyzed by flow cytometry.
Previous studies have suggested that the viral accessory protein Vpr arrests cells in the G2 phase of the cell cycle, promoting production of viral proteins and contributing to apoptosis (1, 8, 11, 16, 18, 32, 33, 41). We reasoned that Vpr may be responsible for the inability of the infected cells to complete the cell cycle. In the absence of Vpr, however, infected cells might pass the G2 block and cycle back to G1a. To examine the role of Vpr in our system, we infected quiescent CD4+ T cells with either HIVNL4-3 or HIVNL4-3/Vpr− and cultured these cells under the same conditions. No significant differences in reverse transcription, integration, or Gag production between the two virus strains were observed in poststimulated cells (data not shown). The cell cycle distribution and subsequent loss of Gag-positive cells were also similar in the two strains (Table 1). Based on the above observations, Vpr is not responsible for the failure of infected cells to initiate a second cell cycle.
TABLE 1.
Absence of Vpr does not improve the survival of HIV-infected cellsa
| Time (h) and infection status | % BrdU+ Gag cells | % BrdU− Gag cells | Cell cycle distribution of BrdU+ Gag+
|
Cell cycle distribution of BrdU− Gag+
|
|||
|---|---|---|---|---|---|---|---|
| Phase | % | Phase | % | ||||
| 48 | |||||||
| NL4-3-infected | 74 | 26 | G0/1a | 3 | G0/1a | 8 | |
| G1b | 11 | G1b | 57 | ||||
| S/G2/M | 86 | S/G2/M | 35 | ||||
| NL4-3Vpr−-infected | 70 | 30 | G0/1a | 6 | G0/1a | 4 | |
| G1b | 10 | G1b | 55 | ||||
| S/G2/M | 84 | S/G2/M | 41 | ||||
| 72 |
|
||||||
| NL4-3-infected | 64 | 22 | G0/1a | 8 | G0/1a | 68 | |
| G1b | 15 | G1b | 9 | ||||
| S/G2/M | 74 | S/G2/M | 23 | ||||
| NL4-3Vpr−-infected | 49 | 30 | G0/1a | 10 | G0/1a | 56 | |
| G1b | 38 | G1b | 38 | ||||
| S/G2/M | 52 | S/G2/M | 6 | ||||
| 96 |
|
||||||
| NL4-3-infected | 100 | 0 | G0/1a | 9 | |||
| G1b | 29 | ||||||
| S/G2/M | 60 | ||||||
| NL4-3Vpr−-infected | 85 | 0 | G0/1a | 25 | |||
| G1b | 25 | ||||||
| S/G2/M | 50 | ||||||
Comparative protein expression kinetics and BrdU incorporation between wild-type HIVNL4-3 and the Vpr mutant. The data shown are representative of four independent experiments.
DISCUSSION
Infection of quiescent cells by HIV has been the subject of much debate. Although there were early conflicting reports (4, 6, 7, 23, 34, 38, 40, 47, 48), it has now been conclusively shown that quiescent CD4+ T cells are resistant to infection (21, 31, 43, 44, 50). This important observation was determined through a detailed analysis of the cell cycle, as well as through the finding that even partial cell activation is sufficient to support HIV infection.
To systematically analyze the kinetics of HIV infection in quiescent cells, we isolated highly purified CD4+ T cells by negative selection and infected them with a CXCR4-tropic strain of HIV. Some of these cells were stimulated immediately after infection, while some were left unstimulated. In addition, we performed a series of viral-entry experiments. Quiescent and prestimulated cells bound comparable levels of virus. However, there was a fourfold decrease in the amount of cytosolic Gag, indicating somewhat impaired entry into quiescent cells. This could contribute to less efficient productive infection with subsequent stimulation. Furthermore, poststimulated cells displayed delayed completion of reverse transcription by approximately 12 to 16 h compared to the prestimulated group. There was also a 30-fold decrease in both the initiation and completion of reverse transcription compared to prestimulated cells. However, those DNA copies that initiated reverse transcription eventually completed the process. Interestingly, the efficiencies of integration of fully reverse-transcribed DNA were similar in poststimulated and prestimulated cells, a difference of approximately 2.5-fold less than those for the prestimulated group. Based on our cell cycle data, quiescent CD4+ T cells enter the G1b phase (which has been shown to support productive HIV infection) 24 h following activation. It appears that even with immediate activation this delayed transition into a permissive stage can compromise the productivity of infection. These observations are strengthened by the fact that the nonstimulated group showed the same reverse transcription kinetics and integration kinetics as the poststimulated group. Prestimulated cells were somewhat more efficient in allowing viral entry, initiated reverse transcription more efficiently, and completed reverse transcription more rapidly than quiescent and poststimulated cells. Thus, a series of deficiencies contribute to less efficient virus rescue in poststimulated cells. Consistent with this, previous studies have shown that the reverse transcription block has been relieved by nucleoside treatment but without viral rescue (21, 31, 43, 44, 50), suggesting the presence of multiple barriers to HIV infection. Our studies suggest that the largest quantitative defect in these cells is postentry, at or prior to the initiation of reverse transcription. Therefore, based on our data, immediate activation following infection does not greatly increase the cell's ability to support the early stages of the viral life cycle.
Both pre- and poststimulated groups displayed a rise and a subsequent rapid decrease in viral RNA transcripts. This phenomenon was not seen in the nonstimulated group, suggesting that the cells remain alive, with full-length and a very small number of integrated viral transcripts. The kinetics of viral-protein expression confirmed these trends. The prestimulated cells expressed viral protein earlier and at higher levels and died by 72 to 96 h postinfection. The poststimulated group, on the other hand, expressed protein at 48 to 72 h poststimulation, and by the 120-hour time point, no Gag-positive cells remained. Although there were low but persistent levels of full-length and integrated viral DNA, nonstimulated cells did not express viral protein.
Cells that stained positive for Gag were analyzed for their cell cycle status using DNA (7-AAD) and RNA (Pyronin Y) stains. The majority of cells resided in the later phases of the cell cycle (S, G2, and M), with a significant number of cells in the G1b phase. This observation was expected, as cells can be infected in G1b and move through the cell cycle. However, a number of cells in the G1a phase expressed viral protein. These cells could have completed one round of cell division, or they may have been activated later under our culture conditions and thus were permissive to infection.
To address whether G1a cells had already completed one round of cell division, we treated cells with BrdU, which is incorporated into a cell's genome once the cell enters the S phase. Our results indicated that essentially no G1a cells had incorporated BrdU and expressed viral protein, suggesting that once the cells were infected and produced virus, they either arrested in G2 or died. A similar result was observed when we infected cells with a Vpr-mutated HIVNL4-3. Thus, Vpr-mediated G2 arrest was not responsible for this phenotype in our system.
Our findings have important implications for HIV pathogenesis. The apparently irreversible inhibition presented by quiescent CD4+ T cells suggests that there are cellular factors required to support or simply inhibit multiple stages of HIV infection. These could be additional targets for future therapies. Furthermore, this knowledge will provide tools to optimize lentivirus-based gene delivery into nondividing or quiescent hematopoietic stem cells.
Acknowledgments
We thank Helen J. Brown for critical reading of the manuscript. We acknowledge the support of the UCLA Center for AIDS Research Virology/BSL3 Core Laboratory in providing p24 assays.
This work was supported by National Institutes of Health grant AI36059 and by the University of California, Los Angeles Center for AIDS Research (AI28697).
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Aldrovandi, G. M., and J. A. Zack. 1996. Replication and pathogenicity of human immunodeficiency virus type 1 accessory gene mutants in SCID-hu mice. J. Virol. 70:1505-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arlen, P. A., D. G. Brooks, L. Y. Gao, D. Vatakis, H. J. Brown, and J. A. Zack. 2006. Rapid expression of human immunodeficiency virus following activation of latently infected cells. J. Virol. 80:1599-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks, D. G., D. H. Hamer, P. A. Arlen, L. Gao, G. Bristol, C. M. Kitchen, E. A. Berger, and J. A. Zack. 2003. Molecular characterization, reactivation, and depletion of latent HIV. Immunity 19:413-423. [DOI] [PubMed] [Google Scholar]
- 4.Bukrinsky, M. I., T. L. Stanwick, M. P. Dempsey, and M. Stevenson. 1991. Quiescent T lymphocytes as an inducible virus reservoir in HIV-1 infection. Science 254:423-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiu, Y. L., V. B. Soros, J. F. Kreisberg, K. Stopak, W. Yonemoto, and W. C. Greene. 2005. Cellular APOBEC3G restricts HIV-1 infection in resting CD4+ T cells. Nature 435:108-114. [DOI] [PubMed] [Google Scholar]
- 6.Chou, C. S., O. Ramilo, and E. S. Vitetta. 1997. Highly purified CD25− resting T cells cannot be infected de novo with HIV-1. Proc. Natl. Acad. Sci. USA 94:1361-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun, T. W., K. Chadwick, J. Margolick, and R. F. Siliciano. 1997. Differential susceptibility of naive and memory CD4+ T cells to the cytopathic effects of infection with human immunodeficiency virus type 1 strain LAI. J. Virol. 71:4436-4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 9.Conticello, S. G., R. S. Harris, and M. S. Neuberger. 2003. The Vif protein of HIV triggers degradation of the human antiretroviral DNA deaminase APOBEC3G. Curr. Biol. 13:2009-2013. [DOI] [PubMed] [Google Scholar]
- 10.Darzynkiewicz, Z., T. Sharpless, L. Staiano-Coico, and M. R. Melamed. 1980. Subcompartments of the G1 phase of cell cycle detected by flow cytometry. Proc. Natl. Acad. Sci. USA 77:6696-6699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Marzio, P., S. Choe, M. Ebright, R. Knoblauch, and N. R. Landau. 1995. Mutational analysis of cell cycle arrest, nuclear localization and virion packaging of human immunodeficiency virus type 1 Vpr. J. Virol. 69:7909-7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Embretson, J., M. Zupancic, J. L. Ribas, A. Burke, P. Racz, K. Tenner-Racz, and A. T. Haase. 1993. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362:359-362. [DOI] [PubMed] [Google Scholar]
- 13.Ganesh, L., E. Burstein, A. Guha-Niyogi, M. K. Louder, J. R. Mascola, L. W. Klomp, C. Wijmenga, C. S. Duckett, and G. J. Nabel. 2003. The gene product Murr1 restricts HIV-1 replication in resting CD4+ lymphocytes. Nature 426:853-857. [DOI] [PubMed] [Google Scholar]
- 14.Gibbs, J. S., A. A. Lackner, S. M. Lang, M. A. Simon, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1995. Progression to AIDS in the absence of a gene for Vpr or Vpx. J. Virol. 69:2378-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris, R. S., K. N. Bishop, A. M. Sheehy, H. M. Craig, S. K. Petersen-Mahrt, I. N. Watt, M. S. Neuberger, and M. H. Malim. 2003. DNA deamination mediates innate immunity to retroviral infection. Cell 113:803-809. [DOI] [PubMed] [Google Scholar]
- 16.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenkins, M. K., P. S. Taylor, S. D. Norton, and K. B. Urdahl. 1991. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J. Immunol. 147:2461-2466. [PubMed] [Google Scholar]
- 18.Jowett, J. B., V. Planelles, B. Poon, N. P. Shah, M. L. Chen, and I. S. Chen. 1995. The human immunodeficiency virus type 1 vpr gene arrests infected T cells in the G2 + M phase of the cell cycle. J. Virol. 69:6304-6313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.June, C. H., J. A. Ledbetter, P. S. Linsley, and C. B. Thompson. 1990. Role of the CD28 receptor in T-cell activation. Immunol. Today 11:211-216. [DOI] [PubMed] [Google Scholar]
- 20.Kao, S., M. A. Khan, E. Miyagi, R. Plishka, A. Buckler-White, and K. Strebel. 2003. The human immunodeficiency virus type 1 Vif protein reduces intracellular expression and inhibits packaging of APOBEC3G (CEM15), a cellular inhibitor of virus infectivity. J. Virol. 77:11398-11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Korin, Y., and J. A. Zack. 1998. Progression to the G1b phase of the cell cycle is required for completion of human immunodeficiency virus type 1 reverse transcription in T cells. J. Virol. 72:3161-3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Korin, Y. D., and J. A. Zack. 1999. Nonproductive human immunodeficiency virus type 1 infection in nucleoside-treated G0 lymphocytes. J. Virol. 73:6526-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, G., M. Simm, M. J. Potash, and D. J. Volsky. 1993. Human immunodeficiency virus type 1 DNA synthesis, integration, and efficient viral replication in growth-arrested T cells. J. Virol. 67:3969-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mangeat, B., P. Turelli, G. Caron, M. Friedli, L. Perrin, and D. Trono. 2003. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature 424:99-103. [DOI] [PubMed] [Google Scholar]
- 25.Marechal, V., F. Clavel, J. M. Heard, and O. Schwartz. 1998. Cytosolic Gag p24 as an index of productive entry of human immunodeficiency virus type 1. J. Virol. 72:2208-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mariani, R., D. Chen, B. Schrofelbauer, F. Navarro, R. Konig, B. Bollman, C. Munk, H. Nymark-McMahon, and N. R. Landau. 2003. Species-specific exclusion of APOBEC3G from HIV-1 virions by Vif. Cell 114:21-31. [DOI] [PubMed] [Google Scholar]
- 27.Marin, M., K. M. Rose, S. L. Kozak, and D. Kabat. 2003. HIV-1 Vif protein binds the editing enzyme APOBEC3G and induces its degradation. Nat. Med. 9:1398-1403. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien, W. A., A. Namazi, H. Kalhor, S. H. Mao, J. A. Zack, and I. S. Chen. 1994. Kinetics of human immunodeficiency virus type 1 reverse transcription in blood mononuclear phagocytes are slowed by limitations of nucleotide precursors. J. Virol. 68:1258-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Doherty, U., W. J. Swiggard, D. Jeyakumar, D. McGain, and M. H. Malim. 2002. A sensitive, quantitative assay for human immunodeficiency virus type 1 integration. J. Virol. 76:10942-10950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pierson, T. C., Y. Zhou, T. L. Kieffer, C. T. Ruff, C. Buck, and R. F. Siliciano. 2002. Molecular characterization of preintegration latency in human immunodeficiency virus type 1 infection. J. Virol. 76:8518-8531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Planelles, V., F. Bachelerie, J. B. Jowett, A. Haislip, Y. Xie, P. Banooni, T. Masuda, and I. S. Chen. 1995. Fate of the human immunodeficiency virus type 1 provirus in infected cells: a role for vpr. J. Virol. 69:5883-5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Planelles, V., J. B. Jowett, Q. X. Li, Y. Xie, B. Hahn, and I. S. Chen. 1996. Vpr-induced cell cycle arrest is conserved among primate lentiviruses. J. Virol. 70:2516-2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramilo, O., K. D. Bell, J. W. Uhr, and E. S. Vitetta. 1993. Role of CD25+ and CD25− T cells in acute HIV infection in vitro. J. Immunol. 150:5202-5208. [PubMed] [Google Scholar]
- 35.Rich, E. A., I. S. Chen, J. A. Zack, M. L. Leonard, and W. A. O'Brien. 1992. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J. Clin. Investig. 89:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schuitemaker, H., N. A. Kootstra, R. A. Fouchier, B. Hooibrink, and F. Miedema. 1994. Productive HIV-1 infection of macrophages restricted to the cell fraction with proliferative capacity. EMBO J. 13:5929-5936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheehy, A. M., N. C. Gaddis, and M. H. Malim. 2003. The antiretroviral enzyme APOBEC3G is degraded by the proteasome in response to HIV-1 Vif. Nat. Med. 9:1404-1407. [DOI] [PubMed] [Google Scholar]
- 38.Spina, C. A., J. C. Guatelli, and D. D. Richman. 1995. Establishment of a stable, inducible form of human immunodeficiency virus type 1 DNA in quiescent CD4 lymphocytes in vitro. J. Virol. 69:2977-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevenson, M., B. Brichacek, N. Heinzinger, S. Swindells, S. Pirruccello, E. Janoff, and M. Emerman. 1995. Molecular basis of cell cycle dependent HIV-1 replication. Implications for control of virus burden. Adv. Exp. Med. Biol. 374:33-45. [DOI] [PubMed] [Google Scholar]
- 40.Stevenson, M., T. L. Stanwick, M. P. Dempsey, and C. A. Lamonica. 1990. HIV-1 replication is controlled at the level of T cell activation and proviral integration. EMBO J. 9:1551-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart, S. A., B. Poon, J. B. Jowett, and I. S. Chen. 1997. Human immunodeficiency virus type 1 Vpr induces apoptosis following cell cycle arrest. 71:5579-5592. [DOI] [PMC free article] [PubMed]
- 42.Stopak, K., C. de Noronha, W. Yonemoto, and W. C. Greene. 2003. HIV-1 Vif blocks the antiviral activity of APOBEC3G by impairing both its translation and intracellular stability. Mol. Cell 12:591-601. [DOI] [PubMed] [Google Scholar]
- 43.Swiggard, W. J., C. Baytop, J. J. Yu, J. Dai, C. Li, R. Schretzenmair, T. Theodosopoulos, and U. O'Doherty. 2005. Human immunodeficiency virus type 1 can establish latent infection in resting CD4+ T cells in the absence of activating stimuli. J. Virol. 79:14179-14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swiggard, W. J., U. O'Doherty, D. McGain, D. Jeyakumar, and M. H. Malim. 2004. Long HIV type 1 reverse transcripts can accumulate stably within resting CD4+ T cells while short ones are degraded. AIDS Res. Hum. Retrovir. 20:285-295. [DOI] [PubMed] [Google Scholar]
- 45.Toba, K., E. F. Winton, T. Koike, and A. Shibata. 1995. Simultaneous three-color analysis of the surface phenotype and DNA-RNA quantitation using 7-amino-actinomycin D and pyronin Y. J. Immunol. Methods 182:193-207. [DOI] [PubMed] [Google Scholar]
- 46.Yu, X., Y. Yu, B. Liu, K. Luo, W. Kong, P. Mao, and X. F. Yu. 2003. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science 302:1056-1060. [DOI] [PubMed] [Google Scholar]
- 47.Zack, J. A., S. J. Arrigo, S. R. Weitsman, A. S. Go, A. Haislip, and I. S. Chen. 1990. HIV-1 entry into quiescent primary lymphocytes: molecular analysis reveals a labile, latent viral structure. Cell 61:213-222. [DOI] [PubMed] [Google Scholar]
- 48.Zack, J. A., A. M. Haislip, P. Krogstad, and I. S. Chen. 1992. Incompletely reverse-transcribed human immunodeficiency virus type 1 genomes in quiescent cells can function as intermediates in the retroviral life cycle. J. Virol. 66:1717-1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang, H., B. Yang, R. J. Pomerantz, C. Zhang, S. C. Arunachalam, and L. Gao. 2003. The cytidine deaminase CEM15 induces hypermutation in newly synthesized HIV-1 DNA. Nature 424:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou, Y., H. Zhang, J. D. Siliciano, and R. F. Siliciano. 2005. Kinetics of human immunodeficiency virus type 1 decay following entry into resting CD4+ T cells. J. Virol. 79:2199-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]