Abstract
Characterization of the neutralizing interaction between antibody and virus is hindered by the nonsynchronized progression of infection in cell cultures. Discrete steps of the viral entry sequence cannot be discerned, and thus, the mode of antibody-mediated interference with virus infectivity remains undefined. Here, we magnetically synchronize the motion and cell attachment of human immunodeficiency virus type 1 (HIV-1) to monitor the progression of neutralization, both in solution and following virus attachment to the cell. By simultaneous transfer of all viral particles from reaction solution with antibody to the cell-bound state, the precise rate of neutralization of cell-free virus could be determined for each antibody. HIV-1 neutralization by both monoclonal and polyclonal antibody preparations followed distinct pseudo-first-order kinetics. For all antibodies, cell types, and HIV-1 strains examined, postattachment interference served a major role in the neutralizing effect. To monitor the progression of postattachment interference, we synchronized the entry process at initiation and measured the escape of cell-bound virus from antibody. We found that different antibodies neutralized the virus over different time frames during the entry phase. Virus was observed to progress through a sequence of shifting sensitivities to different antibodies during entry, suggested here to correlate with the exposure time of the target epitope on receptor-activated viral envelope proteins. Thus, by monitoring the progression of HIV-1 entry under synchronized conditions, we identify a new and significant determinant of antibody neutralization capacity, namely, the time frames for neutralization during the course of the viral entry phase.
It is commonly accepted that antibodies (Abs) neutralize viruses by binding to the virion surface (22, 34). Indeed, good correlation exists between neutralizing capacity and binding affinity of the Ab to the target epitope (35, 39). However, the mode of Ab-mediated interference with virus infectivity remains undefined, largely due to the limited ability to monitor the neutralizing interaction between virus and Ab, in solution or following virus attachment to the cell surface. The diffusion-limited nature of the virus-cell interaction is central to this shortcoming of current in vitro systems.
Viruses in solution behave as charged colloidal masses. Their motion is controlled by diffusion (1, 32), and their attachment to cells is primarily determined by electrostatic interactions with the charged cell surface (12). It is these coupled stochastic processes of cell encounter and attachment that constitute the rate-limiting steps to infection of cells in culture (1, 20). Cell attachment progresses continuously, and thus, infection is initiated asynchronously, precluding step-by-step monitoring of viral events that precede or follow the attachment step.
The lack of synchrony has challenged attempts both to characterize the dynamics of the viral entry sequence and to determine the mechanism and precise stage of infection that is inhibited by Ab binding. While several studies have shown that specific Abs may prevent virus attachment to certain cell types (5, 43), others have demonstrated that neutralization may be effected by interference with a postattachment step of infection (28, 33, 40). Indeed, several Abs have shown the capacity to neutralize virus that has already attached to the cell surface but has not yet entered the cytoplasm (2, 23). However, the progression of postattachment neutralization and the specific stage of entry inhibited by each Ab could not be defined.
Several approaches have been employed to increase synchronicity of infection in cell cultures in order to follow early steps of the infection sequence. Most commonly, viruses are adsorbed to target cells at low temperatures (nonpermissive for entry), followed by removal of unbound virus and elevation to physiologic temperature in order to initiate entry (18, 36). However, for a large number of viruses, including human immunodeficiency virus (HIV), the temperature-dependent step occurs at a late stage of the entry process (14, 25). The sequence of events that precedes this step therefore remains nonsynchronized. Similarly, the use of chemically triggered forms of the HIV envelope protein allows arrest only at a late stage of entry, after engagement of receptor and coreceptor (6, 11). Cell-to-cell-fusion assays are also widely used to study both viral entry and neutralization (17). However, the capacity of envelope-mediated cell-to-cell fusion to reflect the dynamics of the interaction between intact virus and cells is not clear.
To surmount limitations imposed by the diffusion-dependent cell association step, we previously described a method for magnetically controlling viral motion and cell attachment (20). Viruses are synchronously transferred to the stable cell-bound state at physiologic temperature and simultaneously initiate the infection sequence. Here, we apply this technology to monitor the Ab-virus interaction in solution and on the cell surface. By controlling cell attachment, the Ab-virus interaction in solution is quenched, allowing precise kinetic measurements of cell-free virus neutralization. By synchronously initiating infection, the progression of cell-bound virus escape from different Abs could be monitored. Using these tools, we analyze here the dynamics of Ab-mediated neutralization of HIV-1.
MATERIALS AND METHODS
Cells.
CD4-positive HeLa cell line clone 1022 was obtained through the AIDS Research and Reference Reagent Program (ARRRP; contributed by B. Chesebro). Canine thymocyte Cf2Th cells, which stably express CD4 and high levels of CCR5 (4), were kindly provided by J. Sodroski. HeLa-CD4, wild-type HeLa, 293T, and Cf2Th-CD4/CCR5 cells were cultured in Dulbecco's modified Eagle's medium supplemented with 2 mM l-glutamine, 100 U/ml penicillin G, 0.1 mg/ml streptomycin, and 10% fetal calf serum (DMEM-10% FCS) and maintained at 37°C and 5% CO2.
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats obtained from healthy blood donors. Following density gradient centrifugation using Ficollpaque Plus lymphocyte separation medium (Amersham), cells were incubated for 2 h at 37°C to remove adherent cells. Remaining cells were then stimulated with interleukin 2 (IL-2; 250 units/ml; Chiron Corp.) for 6 days prior to infection. PBMCs were maintained throughout in RPMI medium supplemented with 20% FCS, 2 mM glutamine, 10 mM HEPES (pH 7.3), and the above-mentioned antibiotics.
Antibodies.
Anti-CD4-binding-site monoclonal Ab (MAb) immunoglobulin G1 (IgG1) b12 was kindly provided by D. Burton. The following Abs were obtained through the ARRRP: MAbs 2G12 and 2F5 (both contributed by H. Katinger), MAb 12G5 (contributed by J. Hoxie), and polyclonal IgG isolated from HIV-1-positive blood donors (HIVIG). Normal human serum IgG (IVIG) from HIV-1-seronegative blood donors was manufactured from pooled-source plasma (Omrix Biopharmaceuticals).
Preparation of viruses.
Recombinant HIV-1 was generated using a three-plasmid packaging system, which consisted of the pCMVΔR8.2 packaging vector (31), a transfer vector, and an envelope vector. Transfer vectors included pHR′hPGK.nlsLacZ and pHR′CMV.Luc (31, 49). The HIV-1 gp160 envelope glycoprotein of the T-cell-line-adapted HIV-1IIIB strain, the dualtropic primary isolate HIV-189.6, and the R5-tropic primary HIV-1JRFL strain were expressed by the pRSV-IIIB, pRSV-89.6, and pE7-JRFL vectors, respectively.
Viral stocks were generated by transient cotransfection of 293T cells with the three plasmids, using the calcium phosphate precipitation method. Briefly, 293T cells (3.5 × 106) were seeded in 10-cm culture dishes and transfected the following day with 10 μg of pCMVΔR8.2, 10 μg of pHR′ transfer vector, and 2.5 μg of envelope vector. Sixteen hours posttransfection, the medium was changed to DMEM-10% FCS and 12 h later replaced with serum-free DMEM. Supernatants were collected 12 h later, cleared of cell debris by low-speed centrifugation, and passed through 0.2-μm filters (Sartorius). Preparations were subsequently loaded into dialysis tubing (molecular mass cutoff, 12 to 14 kDa) and dialyzed against HS buffer (140 mM NaCl, 10 mM HEPES, pH 7.3) for 24 h at 4°C. The infectivities of the preparations were unaltered by the dialysis procedure, as determined by dilution-based titration on HeLa-CD4 cells.
Magnetically mediated viral adsorption.
Viral preparations suspended in HS buffer were supplemented with 1.5% FCS and preincubated for 2 min with magnetite nanoparticles (MNPs; 0.5 mg/ml; TransMAG-PD; Chemicell). The solution was then added dropwise to the culture medium of a confluent monolayer of target cells at a 1:13 (vol/vol) ratio. A magnetic field was applied by positioning a permanent Nd-Fe-B magnet (Magma Magnets) under the culture plate (magnetic flux density of approximately 1 T). Following incubation with cells (for 2 min, unless indicated otherwise), the magnet was removed, and cells were washed three times with culture medium and further incubated at 37°C and 5% CO2. At 12 h, cells were detached by trypsinization, reseeded at 50% confluence, and further cultured for 4 days for infectivity assays.
We note that modifications to previously reported conditions of viral preparation and MNP association were introduced to minimize the formation of biologically inactive virus-MNP aggregates (20). As a result, virus-MNP complexes retained both diffusion dependence and infective stability in the cell culture medium and could be effectively transferred from the medium to the infective-cell-bound state by application of a magnetic field at least 2 h after addition of complexes to the cells (data not shown).
Viral particle adsorption assay.
Viral attachment to cells was determined by a capture enzyme-linked immunosorbent assay (ELISA) for HIV-1 p24 antigen. Following adsorption of virus, cell association was examined with or without washing of the cell culture. For cultures examined with no washing step, the medium was removed by gentle decantation while maintaining the application of a magnetic field. For cultures examined after a washing step, the magnetic field was first removed, followed by three washes with culture medium. Cells were then disrupted in ice-cold lysis buffer (1% Triton X-100 in culture medium) for 15 min at 4°C and assayed with an HIV-1 p24 antigen capture assay kit (SAIC Frederick, AIDS Vaccine Program).
To examine the fraction of p24 in virus preparations that is virion incorporated, we measured the proportion of pelletable p24. HIV-1IIIBnls.β-gal preparations were placed over a 20% sucrose cushion and centrifuged at 100,000 × g for 2 h at 4°C in a Beckman SW55Ti rotor. The amount of p24 in the pellet was then measured by ELISA and compared with that of the input virus. We found that 83% of the input p24 pelleted through the sucrose cushion, indicative of a virion-associated form.
Neutralization kinetics of cell-free HIV.
HIV preparations were preincubated with Ab at 37°C for different time periods and then adsorbed to the cells magnetically or by diffusion. Preparations were added at a 1:13 (vol/vol) ratio to the cell culture medium (final Ab dilution, 1:28). After a 2-min (magnetically adsorbed) or 12-h (diffusion adsorbed) incubation period, cells were washed twice with culture medium and further incubated for infectivity assays.
Kinetics of escape of cell-bound HIV from Abs.
Cell cultures were magnetically adsorbed with virus for 2 min at 37°C, washed twice with culture medium, and further incubated at 37°C. At different times postadsorption, culture medium containing Ab was added to the cells. For measurements indicating no interval prior to Ab addition, virus was magnetically adsorbed to cells overlaid with culture medium containing Ab. Cells were then further cultured for 4 days and examined for infectivity.
In kinetic measurements of HIV escape from protease-mediated inactivation, cells were treated with digestion buffer containing 2.5 mg/ml trypsin and 0.54 mM EDTA (Biological Industries). After 12 min incubation at 37°C, DMEM-10% FCS was added to halt protease activity. Parallel cultures were treated with culture medium containing 5 μg/ml MAb b12 and at 13 h postadsorption were similarly treated with trypsin-EDTA.
Infection of PBMCs.
IL-2-stimulated PBMCs were seeded in 12-well culture plates coated with poly-l-lysine (Sigma) at 6 × 106 cells per well. Cells were immobilized to the well surface by centrifuging plates at 500 × g for 5 min immediately before infection. HIV-1IIIBluc preparations were preincubated with Ab for 1 h and adsorbed to the cells magnetically or by diffusion. Twelve hours later, cells were resuspended, washed once with PBMC culture medium, and further incubated for 4 days in PBMC culture medium supplemented with 250 U/ml IL-2. Cells were then washed once with phosphate-buffered saline (PBS) and assayed for luciferase enzyme activity.
X-Gal staining.
Cultures infected with β-galactosidase-expressing viruses were stained using the 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) substrate (Inalco, Italy). Four days postinfection, cells were washed once with culture medium and then treated with fixative solution containing 2% formaldehyde, 0.2% glutaraldehyde, and 2 mM MgCl2 in PBS (pH 7.3) for 1 min at room temperature. Cultures were subsequently washed four times with PBS and treated with staining solution containing the X-Gal substrate at 0.8 μg/ml, 0.02% NP-40, and 2 mM MgCl2 in PBS. Following a 24-h incubation period at 37°C, β-galactosidase-positive cells were counted with an inverted-light microscope. To correct for cell division, each cluster of positive cells was considered one infection event.
Luciferase assay.
Infected cultures were washed once with culture medium and lysed with passive lysis buffer (Promega) for 10 min at room temperature. Preparations were then assayed for luciferase enzyme activity with a dual luciferase kit (Promega). Light emission from cell extracts was measured with a Mithras LB 940 luminometer (Berthold Technologies).
RESULTS
Synchronizing HIV attachment to cells.
To synchronize the first and rate-limiting step of infection, diffusion-dependent cell attachment, we rendered viruses magnetically reactive. To this end, virus was associated with negatively charged MNPs (50 nm in diameter) composed of an iron oxide core coated by a starch polymer modified with phosphate groups (20). The formation of stable complexes between MNPs and HIV particles, which also bear a negative surface charge (12), is facilitated by counterion-dependent depletion of electrostatic repulsive barriers (19). The rate of virus-MNP complex formation could thus be determined by the ionic composition of the solution, which was adjusted to produce maximal association after 1 min, as determined by the fraction of magnetically reactive viral particles (data not shown). The HIV-1 structural component p24 was used throughout this study as a quantitative viral particle marker since 83% of the p24 in viral preparations was found to be in pelletable virion-associated form (see Materials and Methods).
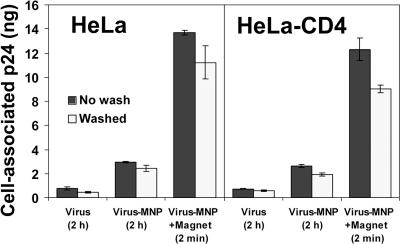
As shown in Fig. 1, addition of virus-MNP complexes to cell cultures applied with a magnetic field resulted in rapid transfer of the entire viral inoculum (14 ng p24 input) to the cell-bound state. Viruses stably attached to the cells, independent of the cellular expression of the HIV entry receptor CD4. Importantly, we observed that after initial magnetically induced attachment, cell surface retention of HIV-MNP complexes was unaffected by continued application of the magnetic field; complexes remained stably attached to the cells and mostly resistant to subsequent washes (Fig. 1). Moreover, no increase in medium concentrations of virus above the p24 assay threshold of detection was observed for 12 h after magnetically controlled adsorption (data not shown).
FIG. 1.
HIV attachment to CD4-positive and -negative HeLa cells. HIV-1IIIBnls.β-gal preparations (14 ng p24 per well) were adsorbed magnetically or by diffusion to wild-type or CD4-expressing HeLa cells for the indicated times at 37°C. Cell-associated virus was then quantified by ELISA for HIV-1 p24. Values represent means ± SEMs for three replicate samples.
Similar to the redistribution of the viral particle content (measured by the p24 assay), the infectious content of the magnetically controlled preparations was rapidly and completely transferred from the medium to the cell-bound state. HIV-1IIIBluc preparations (with a mean infectivity measured at 5.5 × 106 relative light units per well, corresponding to approximately 180 infectious units as determined by dilution-based titration) were magnetically adsorbed to cells for 2 min. The medium was then removed from the infected cell culture and assayed for residual infectivity. We found that only 0.2% (± 0.09% standard error of the mean [SEM]) of the input infectivity remained in the medium after the 2-min adsorption period.
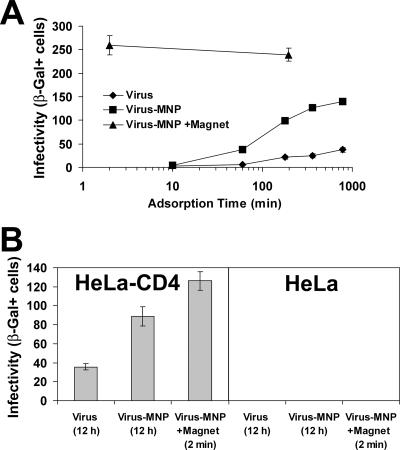
Given synchronized attachment, the productive adsorption step (i.e., cell attachment that results in a measured infection event) was completed within 1 min by magnetically controlled virus (Fig. 2A). By contrast, diffusion-controlled systems reached maximal productive adsorption after approximately 12 h. At the 12-h time point, we examined the medium of cultures infected with virus alone for both residual infectivity and viral particle content (by a p24 antigen assay). While only negligible infectivity was detected (less than 2%), more than 50% of the viral particles initially added were still present in the medium after 12 h (data not shown). Given a half-life of 5.5 h for virus infectivity in the culture medium (data not shown), we attribute this decrease in the infectious- to total-particle ratio to spontaneous viral decay (24). Therefore, the higher infectivities of magnetically controlled preparations (Fig. 2A) resulted from both complete and immediate transfer of viruses to the cell-bound state (Fig. 1).
FIG. 2.
Magnetically controlled infection. (A) Productive adsorption kinetics. HIV-1IIIBnls.β-gal preparations were adsorbed to HeLa-CD4 cultures magnetically or by diffusion. At the indicated times, the infection medium was removed, cells were washed three times with culture medium and further incubated for 4 days, and infectivity was measured. Data points represent means ± SEMs for triplicate wells. (B) Infection of CD4-positive and -negative HeLa cells. HIV-1IIIBnls.β-gal preparations were adsorbed to the cells for the indicated times. Cultures were then washed twice and further cultured for 4 days. Values represent means ± SEMs for three replicate samples. β-Gal+, β-galactosidase positive.
Without application of a magnetic field, the attachment of virus-MNP complexes to cells was increased relative to that for virus alone (Fig. 1). Higher attachment efficiency correlated with increased productive-adsorption rate and infectivity (Fig. 2A and B). These differences also correlated with the higher rate of cell attachment of diffusion-dependent MNPs alone (measured by atomic absorption spectrophotometry for cell-associated iron) than that of virus alone (measured by the p24 assay; data not shown). Hence, since virus-MNP complexes retained diffusion-dependent motion in the culture medium (see Materials and Methods), their higher cell attachment and infection rates were taken to reflect increased efficiency of MNP attachment to cells. While in this work we focus on the use of magnetic field-controlled virus-MNP complexes, these results demonstrated the high stability of MNP-complexed virus in culture medium, unaffected by inactivation by complex-complex aggregation (20).
Whereas cell attachment of magnetically adsorbed virus (measured by the p24 assay) was not affected by CD4 expression (Fig. 1), infectivity remained strictly receptor dependent and was limited to CD4-expressing cells (Fig. 2B). For viral inocula of up to 1,500 infectious units magnetically adsorbed to the CD4-negative HeLa cells, no infection events were detected (data not shown). In accordance, blockage of the coreceptor CXCR4 with the MAb 12G5 (16) reduced the infectivity of magnetically adsorbed HIV-1IIIB in a concentration-dependent manner (data not shown).
Cell attachment and infectivity of Ab-bound HIV.
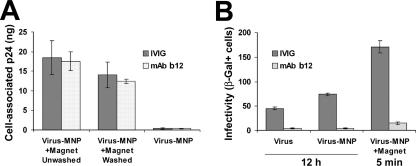
To determine whether HIV that is bound by Abs can be magnetically transferred to the cell-bound state and presented for infection, similar to Ab-free virus, we preincubated virus with either IVIG as a control or the neutralizing MAb b12 (8) and magnetically adsorbed preparations to cells. The MAb b12 was chosen since it recognizes the CD4-binding domain of the envelope protein and thus interferes with the first step of entry. We observed that the viral content of both preparations was efficiently transferred to the cell-bound state, irrespective of Ab (Fig. 3A). Therefore, binding of MAb b12 to HIV does not interfere with subsequent MNP association, magnetic field-mediated mobilization of complexes, or stable attachment to cells.
FIG. 3.
Magnetically controlled adsorption of Ab-bound virus. HIV-1IIIBnls.β-gal preparations (18 ng p24 per well) were preincubated with 5 μg/ml of MAb b12 or IVIG for 1 h at 37°C and then adsorbed magnetically or by diffusion to HeLa-CD4 cultures. (A) Following a 10-min incubation period, cell-associated virus was quantified by ELISA for HIV-1 p24. (B) Following the indicated incubation times, cells were washed and further cultured for 4 days, and infectivity was determined. Values represent means ± SEMs for three replicate samples. β-Gal+, β-galactosidase positive.
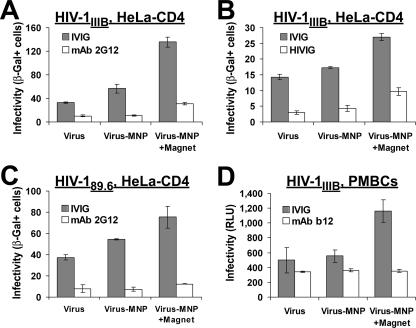
Despite stable, magnetically controlled attachment of Ab-bound HIV to the cells, the virus was still efficiently neutralized as the diffusion-adsorbed virus (Fig. 3B). Similar retention of neutralizing effect after magnetically induced attachment was observed for (i) MAb 2G12, which binds to an envelope protein epitope that does not overlap the CD4 or coreceptor binding sites (9), (ii) polyclonal IgG isolated from HIV-1-positive donors (HIVIG), (iii) the dualtropic HIV-189.6 primary clinical isolate, and (iv) PBMCs as target cells (Fig. 4). Therefore, while the participation of an attachment block cannot be ruled out, these findings demonstrate that binding of Ab to the cell-free virus interferes with a step of infection that is subsequent to cell attachment.
FIG. 4.
Effect of Ab binding on infectivity of HIV adsorbed magnetically or by diffusion. (A to C) The indicated viruses expressing β-galactosidase were preincubated with Ab (or IVIG at the corresponding concentration) for 1 h at 37°C and adsorbed to HeLa-CD4 cultures by diffusion (12 h) or magnetically (2 min). Cells were subsequently washed, further cultured for 4 days, and examined for infectivity. Values represent means ± SEMs for three or four replicate samples. The experiments described for panels A and B were performed with different source virus preparations. (D) Infection of PBMCs by HIV-1IIIBluc that was preincubated with MAb b12 or IVIG for 1 h at 37°C. Four days later, cultures were assayed for luciferase activity. Results are presented as mean relative light unit (RLU) measurements ± SEMs for three replicate samples. β-Gal+, β-galactosidase positive.
Neutralization kinetics of cell-free HIV.
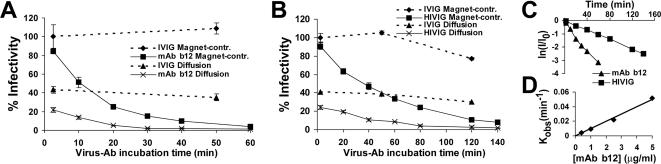
Since magnetically controlled viruses are rapidly transferred from the Ab-virus reaction solution to the cell-associated state, independent of Ab binding status, this system provided the means to accurately measure the kinetics of cell-free HIV neutralization. The MAb b12 and polyclonal HIVIG were examined in this work (Fig. 5A and B). For both Ab types, significant differences were observed between the kinetic profiles obtained by the magnetically controlled and diffusion-controlled methods. (i) The maximal infectivity of diffusion-adsorbed virus was considerably reduced relative to that of virus adsorbed magnetically; the measured infectivity in the diffusion-based assay therefore reflected only a fraction of infectious viruses added to the cells. (ii) There was an initial sharp drop in the infectivity of diffusion-adsorbed virus, from maximal infectivity to the first time point examined after Ab addition (2 min). This decline was inconsistent with the subsequent rate of neutralization and was thus suspected to result from continued binding of Ab to the virus after inoculation of the cells. Indeed, separate addition of MAb b12 and virus (diffusion adsorbed) to the cells demonstrated a 39% reduction of infectivity relative to cultures treated with virus and IVIG control Ab. Therefore, continued Ab-virus binding in the infection medium significantly affected measurements in the diffusion-based assay. The magnetically controlled neutralization assay, in contrast, was able to quench the interaction by rapid transfer of viruses from the reaction solution to the cell-bound state and immediate clearance of unbound Ab.
FIG. 5.
Kinetics of cell-free HIV-1IIIBnls.β-gal neutralization by 5 μg/ml MAb b12 (A) and 2.5 mg/ml HIVIG (B). In both experiments, IVIG at the corresponding concentration was examined as a negative control. Data represent means ± SEMs for four replicate samples and are expressed as percentages of the mean infectivity measured in preparations incubated with IVIG for 2 min (201 and 97 β-galactosidase-positive cells for panels A and B, respectively). (C) Measurements of magnetically controlled neutralization assays plotted as the natural logarithm of the fraction of residual infectivity (I/I0) at each time point. (D) The neutralization rate constant (Kobs) for the indicated MAb b12 concentrations was calculated by dln(I/I0)/dt and plotted as a function of Ab concentration.
Following removal of diffusion-associated effects, neutralization by both monoclonal and polyclonal Abs demonstrated precise pseudo-first-order kinetics, as indicated by the log linearity of the residual infectivity versus the time plot (Fig. 5C) and the linear increase in the neutralization rate constant, directly proportional to the increase in Ab concentration (Fig. 5D). Furthermore, we noted the absence of a delay (lag phase) between the time of MAb b12 addition to virus and the decrease in infectivity. Viruses removed from the reaction solution with Ab after 1, 2.5, and 3 min (by magnetically controlled transfer to the cell surface) were neutralized 6, 15.5, and 18%, respectively (data not shown). Therefore, at the 1-min resolution, cell-free virus neutralization was initiated immediately following Ab exposure and progressed at a constant rate, further supporting the pseudo-first-order nature of the neutralization reaction.
We note that the neutralizing effect of Abs was measured under nonequilibrium conditions (i.e., unbound Ab was removed from the medium after cell attachment). Under these conditions, no deviation from first-order kinetics was observed with the different Ab concentrations during the time periods examined (producing up to 96 and 92% neutralization by MAb b12 and HIVIG, respectively). Since reversibility of neutralization by Ab dissociation is expected to decrease the measured rate of decline (22, 34, 42), the consistency of the elimination rate implied the functional irreversibility of the neutralizing interaction, as previously suggested (26).
Escape of HIV from Abs by cell entry.
The capacity to synchronously initiate infection in the cell culture enabled us to monitor the escape of cell-bound virus, engaged in entry, from Ab-mediated neutralization. Since magnetically adsorbed virus is associated with MNPs, we initially examined whether MNP association status affects virus accessibility to Ab. For this purpose, HIV preparations were preincubated with MNPs for 2 min and then Ab was added for 45 min (either MAb b12 or IVIG as a control, both at 5 μg/ml). The reverse order of incubation was also examined, by first preincubating virus with Ab for 45 min and then adding MNPs for 2 min. Preparations were then magnetically adsorbed to HeLa-CD4 cells, and infectivity was assayed 4 days later. We found that virus was similarly neutralized, whether MNPs were added before or after incubation with the MAb (data not shown). Likewise, virus treated with negative-control IVIG was equally infectious in both cases. We thus conclude that MNP-associated virus and free virus are similarly accessible to neutralization.
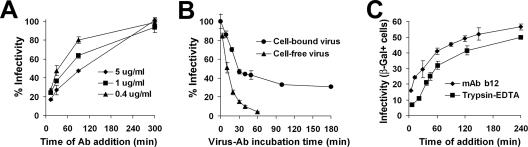
To monitor the progression of HIV escape from neutralization at physiologic temperature, we synchronously initiated infection and then added Ab at different times postattachment. Virus sensitivity to neutralization gradually decreased with time (Fig. 6A). Initially, the capacity to escape was dependent on Ab concentration; as the concentration was decreased (i.e., neutralization progressed at a lower rate), a larger fraction was able to infect the cells. Subsequently, with the decrease in the number of viruses accessible to neutralization, no significant differences were observed between Ab concentrations.
FIG. 6.
Neutralization and escape of cell-bound HIV. (A) Kinetics of escape of magnetically adsorbed HIV-1IIIBnls.β-gal from neutralization by MAb b12. (B) Neutralization kinetics of cell-bound HIV-1IIIBnls.β-gal. Virus was magnetically adsorbed at 37°C to HeLa-CD4 cells cultured in medium containing 5 μg/ml MAb b12. At different times postadsorption, cells were washed twice with culture medium containing no Ab and further incubated for 4 days. Data are presented as percentages of the mean infectivity measured in cultures incubated with IVIG control Ab. Neutralization kinetics of cell-free HIV-1IIIB by 5 μg/ml MAb b12 are plotted for comparison. (C) Kinetics of escape of cell-bound HIV from protease-mediated inactivation and MAb b12-mediated neutralization. Data points represent means ± SEMs for three or four replicate samples. β-Gal+, β-galactosidase positive.
Cell-bound virus escape from Abs can thus be regarded as a competition between two processes that progress simultaneously on the cell surface: entry and neutralization. This was further illustrated by comparing the neutralization kinetics of cell-bound and cell-free virus (Fig. 6B). As expected, infectivity of the cell-bound virus steadily declined as a function of MAb exposure time; however, neutralization leveled off at 70%. The remaining 30% infectivity, representing the Ab-resistant fraction, could be further reduced by increasing Ab concentrations; a fourfold increase to 20 μg/ml MAb b12 resulted in a 39% (± 2.1% SEM) decrease in the resistant fraction (Fig. 6B and data not shown). The Ab-resistant fraction therefore represents viruses that had escaped neutralization by progressing beyond the point of sensitivity to Ab.
Escape of cell-bound HIV from protease-mediated inactivation.
The resistance of virus to protease inactivation is commonly used as a measure of cell entry (27). We thus compared the escape of magnetically adsorbed virus from MAb b12-mediated neutralization with escape from trypsin-EDTA activity. The MAb b12 was added at a concentration eliciting half-maximal neutralization of the cell-free virus after 10 min, as determined by the magnetically controlled assay. We found that at each time point, cell-bound HIV was more sensitive to inactivation by trypsin-EDTA, resulting in a relative shift of the linear phase of the escape curve by approximately 30 min (Fig. 6C).
A direct comparison between these two escape profiles cannot be made, due to the different modes of inactivation and since the critical step of sensitivity (beyond which the virus is no longer accessible for inactivation) is yet unknown for both reagents. Nevertheless, given a half-maximal neutralization rate of 10 min for MAb b12, the existence of a 30-min shift between escape curves suggested that the MAb b12 effect persisted over a shorter time frame during the entry phase than the trypsin-EDTA effect. The consistency of the shift throughout the course of the experiment suggested that the stage of virus insensitivity to MAb b12 occurred at an earlier time point of the entry sequence than the stage of virus insensitivity to trypsin-EDTA.
Escape of cell-bound HIV from Abs directed against different envelope protein epitopes.
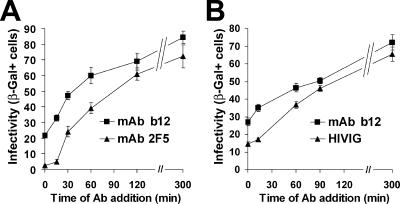
Is neutralization escape of cell-bound HIV affected only by Ab association rate or is it also determined by target epitope? To examine this issue, we compared two MAbs directed against well-characterized epitopes on the viral envelope protein that are exposed to Ab binding over different time frames during the entry sequence: (i) MAb b12, which binds to the CD4-binding site of gp120 only until CD4 receptor engagement (30, 37), representing the first step of viral entry, and (ii) MAb 2F5, which binds to both native and intermediate conformations of the envelope protein until a late stage of entry (13). The MAbs were used at concentrations that elicit identical neutralization rates (half-maximal neutralization of free virus after 10 min, as determined by the magnetically controlled neutralization assay). We found that, at each time point, cell-bound virus was more effectively neutralized by MAb 2F5 than by MAb b12 (Fig. 7A). Differential neutralization efficiency resulted in a significant right shift of the MAb 2F5 escape curve by approximately 30 min.
FIG. 7.
Kinetics of escape of cell-bound HIV-1IIIB from neutralization by 5 μg/ml MAb b12 and 70 μg/ml MAb 2F5 (A) or 2 μg/ml MAb b12 and 2.5 mg/ml HIVIG (B). Data points represent means ± SEMs for four replicate samples. Results are representative of two separate experiments performed for each pair of Ab preparations. β-Gal+, β-galactosidase positive.
We note that the requirement for higher concentrations of MAb 2F5 than of MAb b12 (i.e., lower functional-association rate) correlated with the decreased binding rate of this Ab, as measured by surface plasmon resonance (48). The exceptionally low and comparable dissociation rates of these Abs suggested that association is functionally irreversible under virus-Ab equilibrium conditions (10, 35). In effect, our measurements of cell-free virus neutralization kinetics implied the functional irreversibility of neutralization even under nonequilibrium conditions, as noted above.
Therefore, given identical neutralization rates and functional irreversibility of neutralization, the existence of a time lag between the escape of virus from the two MAbs suggested that sensitivity to neutralization was determined by the functional-association time of the Ab during the course of the entry phase.
Escape of cell-bound HIV from human polyclonal anti-HIV-1 IgG.
The polyclonal Ab response that is mounted against HIV following infection includes a diversity of Abs that target different viral epitopes, most commonly the CD4-binding site of the envelope protein (29) and the V1/V2 and V3 loops (21, 44, 46). We thus compared the neutralization scope of MAb b12 to that of HIVIG preparations. Abs were used at concentrations that elicit identical neutralization rates (half-maximal neutralization of free virus at 40 min). Surprisingly, we found that for each time point examined, cell-bound virus was more effectively neutralized by HIVIG than by MAb b12 (Fig. 7B), shifting the HIVIG escape curve by approximately 30 min. We note that similar differences were observed with two different pooled-source HIVIG preparations examined in separate experiments. The neutralizing effect of HIVIG was thus sustained over extended time frames during the viral entry phase, significantly beyond the stage of CD4 engagement.
Effect of coreceptor antagonist AMD3100 on HIV escape from Abs.
The HIV envelope protein sequentially engages the CD4 receptor and coreceptor (CXCR4 or CCR5). Cell surface density of coreceptor has been shown to determine engagement rate, thus affecting viral entry kinetics (36, 38). We therefore hypothesized that by extending the time required for the CD4-bound envelope protein to engage the coreceptor, susceptibility time for MAb 2F5 would increase, while susceptibility to MAb b12 would be less significantly affected.
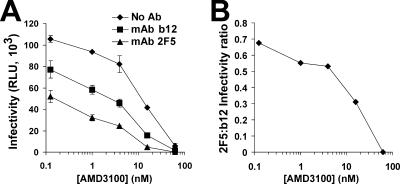
To examine this hypothesis, we used CXCR4 antagonist AMD3100 to reduce the density of functional cell surface coreceptors (15). As expected, AMD3100 potently inhibited HIV-1IIIB infectivity in a concentration-dependent manner (Fig. 8A). For each level of AMD3100-mediated inhibition, we measured the neutralization efficiencies of MAbs 2F5 and b12, defined as the reductions (n-fold) of infectivity relative to infectivity with AMD3100 but no Ab. The relative potency of the Abs at each inhibition level is presented as the infectivity ratio in Fig. 8B, which demonstrates that with increasing AMD3100 concentrations, the MAb 2F5 effect was considerably enhanced relative to the MAb b12 effect. Therefore, by decreasing the rate of coreceptor engagement, neutralization by MAb 2F5 was preferentially increased. This result further supported the concept that HIV neutralization sensitivity is determined by the time frames for binding of Ab to the receptor-activated envelope proteins during the course of entry.
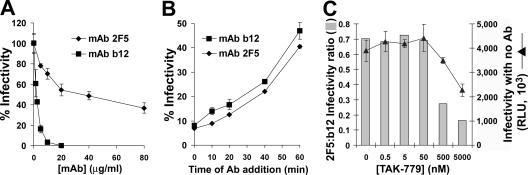
FIG. 8.
Escape of cell-bound HIV-1IIIB from Abs in the presence of AMD3100. (A) HeLa-CD4 cells were preincubated with the indicated concentrations of AMD3100 suspended in culture medium for 30 min at 37°C and then magnetically adsorbed with HIV-1IIIBluc. Cells were further incubated for 40 min and then treated with culture medium containing 5 μg/ml MAb b12, 70 μg/ml MAb 2F5, or no Ab. Four days later, cultures were assayed for luciferase activity. Results are presented as mean relative light unit (RLU) measurements ± SEMs for three replicate samples. (B) Data are presented as ratios of the mean infectivity measured in cultures treated with MAb 2F5 to that measured in cultures treated with MAb b12.
Neutralization and escape of the primary strain HIV-1JRFL.
To verify that the above-described principles of neutralization and escape are not limited to the T-cell-line-adapted, X4-tropic HIV-1IIIB strain, we examined the behavior of the R5-tropic primary strain HIV-1JRFL. Canine thymocyte Cf2Th cells, which stably express both CD4 and high levels of the CCR5 coreceptor, were used as target cells (4).
The efficiencies of MAbs 2F5 and b12 in neutralizing cell-free virus were determined by measuring the concentration of Ab required to reduce infectivity by 50% following a 12-min exposure to the virus (Fig. 9A). Similar to the neutralization of the HIV-1IIIB strain, the functional on-rates of the two MAbs differed significantly, with concentrations of 2 and 32 μg/ml for MAbs b12 and 2F5, respectively, required to achieve half-maximal neutralization of the cell-free virus after 12 min.
FIG. 9.
Neutralization and escape of HIV-1JRFL. (A) Cell-free virus neutralization. HIV-1JRFLluc preparations were preincubated with the indicated concentrations of Ab for 12 min at 37°C and then magnetically adsorbed to Cf2Th-CD4/CCR5 cells for 2 min. Cells were then washed, and infectivity was assayed 2 days later. Results are presented as percentages of the mean infectivity measured in the absence of Ab ± SEMs for three replicate samples. (B) Kinetics of escape of cell-bound HIV-1JRFL from neutralization by 2 μg/ml MAb b12 and 32 μg/ml MAb 2F5. Data are presented as percentages of the mean infectivity measured in cultures not treated with Ab ± SEMs for three replicate samples. Results are representative of two independent experiments performed separately. (C) Escape of cell-bound HIV-1JRFL from Abs in the presence of TAK-779. Cf2Th-CD4/CCR5 cells were preincubated with the indicated concentrations of TAK-779 suspended in culture medium for 30 min at 37°C and then magnetically adsorbed with HIV-1JRFLluc. Cells were further incubated for 15 min, and the medium was removed and replaced by culture medium containing 2 μg/ml MAb b12, 32 μg/ml MAb 2F5, or no Ab. Two days later, cultures were assayed for luciferase activity. Infectivities measured in cultures treated with no Ab are presented as mean relative light unit (RLU) measurements ± SEMs for three replicate samples (▴). Bars represent ratios of the mean infectivity measured in cultures treated with MAb 2F5 to that measured in cultures treated with MAb b12.
Using the concentrations that elicit identical neutralization rates for cell-free virus, we compared the escape profiles of cell-bound HIV-1JRFL from neutralization by the two MAbs (Fig. 9B). A time lag of approximately 10 min separated the escape curves. Thus, from the initiation of the entry process, MAb 2F5 neutralized the virus over longer time frames than MAb b12. This shift, while statistically significant and maintained throughout the time course of the experiment, was less pronounced than the 30-min time difference observed for the HIV-1IIIB virus infecting HeLa-CD4 cells (Fig. 7A).
To determine whether reducing the rate of fusion by limiting the density of the coreceptor would differentially affect the neutralization efficiencies of these MAbs, we used the CCR5 antagonist TAK-779 (3, 38). Virus was magnetically adsorbed to the cells in the presence of different TAK-779 concentrations which were then treated with the MAbs (or medium containing no Ab) at 15 min after synchronized initiation of infection. In the absence of Ab, HIV-1JRFL infectivity was modestly affected by TAK-779 (Fig. 9C). Comparison of the neutralization efficiencies of the Abs at each of the TAK-779 concentrations clearly demonstrated a preferential enhancement of the MAb 2F5 effect at higher TAK-779 inhibition levels. The resulting decrease of the 2F5/b12 infectivity ratio thus corresponded with the effect of the CXCR4 antagonist AMD-3100 (Fig. 8B).
DISCUSSION
In this study, we have synchronized the HIV infection sequence in cell cultures to allow monitoring of early events that immediately follow viral attachment to the cell. Our measurements indicate that virus sensitivity to neutralization by each Ab is not constant but rather changes during the course of the entry phase, thus indicating a new determinant of Ab neutralization efficiency.
Whether or not Ab-mediated inhibition of virus attachment to the cell plays a primary role in the neutralization of virus infectivity has been significantly debated. While several studies have shown that addition of Ab to virus preparations reduces binding of viral particles to target cells, the effect is limited to specific cell and Ab types (5, 7, 28, 33, 40, 43). The selective nature of the phenomenon may indeed derive from the differential permissiveness of cells for adsorption of virus-Ab complexes. Inasmuch, even contradictory findings reported for the same virus-Ab-cell system may be explained by the specific experimental conditions applied, and hence, by the experimental definition of stable attachment (5, 40). Nevertheless, since the large majority of virions in HIV preparations are noninfectious (ratios of infectious to physical particles range between 10−3 and 10−7), viral particle adsorption assays are limited in their capacities to reflect the behavior of the insignificant infection-relevant fraction (24, 45). Here, we remove such potential biases by simultaneous transfer of the entire viral inoculum to the stable cell-bound state. All virions in the sample, regardless of infectivity potential or Ab binding status, are anchored to the cell surface and presented for infection. Due to this large excess of inactive virions, we have focused our assays mostly on measurement of infectivity.
Under all conditions tested, Ab-bound HIV-1 that was stably anchored to the cell surface was noninfectious. Abs therefore inhibited a step of infection that is subsequent to cell attachment. Postattachment neutralization was observed with different cell types (immortalized cell lines and primary lymphocytes) and viral strains (T-cell-line-adapted HIV-1IIIB and primary isolates HIV-189.6 and HIV-1JRFL). Three of the major cross-clade-neutralizing MAbs (b12, 2F5, and 2G12) inhibited HIV infection postattachment, as did polyclonal IgG isolated from HIV-1-infected individuals. Thus, postattachment inhibition served a major and quantifiable role in virus elimination under all experimental conditions tested.
We emphasize that we do not exclude the possibility that a cell attachment block may contribute to the neutralizing effect of an Ab. Here, by magnetically inducing virus attachment to cells, we analyze only the effects of Ab binding on postattachment events. Hence, cell type-specific adsorption properties, which may affect the relationship between block of attachment and neutralization in diffusion-mediated infection, do not influence measurements in magnetically controlled infection.
To monitor the progression of postattachment interference, we synchronized the entry sequence by simultaneous attachment of viruses to cells and measured virus escape from neutralization. We observed that the neutralizing capacity of the Ab added at each time point depended on both the rate of Ab binding and the fraction of viruses that had not yet progressed beyond the step of insensitivity to the neutralizing effect.
The time frames for virus sensitivity to neutralization during entry were compared for two MAbs that target different envelope epitopes: (i) MAb b12, which binds only until CD4 engagement (30), and (ii) MAb 2F5, which can bind until a later fusion-intermediate stage (13, 17, 39). Therefore, from the initiation of the entry process, these Abs can bind to the envelope protein for different time spans. Under conditions of identical neutralization rates by the two Abs, we observed that HIV escape from MAb 2F5 was significantly delayed relative to escape from MAb b12. This lag was measured at 30 min for HIV-1IIIB infection of HeLa-CD4 cells and 10 min for HIV-1JRFL infection of Cf2Th-CD4/CCR5 cells. Given the functional irreversibility of Ab binding, the existence of a lag suggested that the time frame during which the Ab can bind to envelope proteins involved in entry determines the time frame for virus sensitivity to neutralization.
This proposed mechanism was further tested under infection conditions limited by the cell surface density of functional coreceptors, using CXCR4 and CCR5 antagonists. By decreasing coreceptor engagement rate, the CD4-activated envelope proteins are sustained over longer time frames in this fusion-intermediate state (36, 38), thus selectively extending the window of opportunity for those Abs that can bind to this envelope protein conformation. The observed preferential enhancement of MAb 2F5-mediated neutralization relative to the MAb b12 effect substantiated the concept that neutralization efficiency is determined by the time frames during which Ab can bind to the receptor-activated envelope proteins during the course of the entry phase. Moreover, these results demonstrated the differential effect of coreceptor density on the neutralization efficiencies of different Abs.
Increased neutralization of cell-bound virus by MAb 2F5 relative to that by MAb b12 was indeed observed in previous studies (23, 43). However, since infection could not be synchronously initiated, cell-bound virus was mostly resistant to the effect of MAb b12 after the initial adsorption period. Differences in Ab resistance were thus taken to indicate that MAb b12 neutralizes by inhibiting cell attachment whereas MAb 2F5 inhibits a postattachment step. Here, by synchronizing the infection sequence at initiation, we show that both MAbs interfere with a postattachment step, but the time frames for virus sensitivity to neutralization differ.
We note that no attempt is made here to determine the stoichiometry of entry or neutralization. Measured kinetics of cell-free virus neutralization are employed as a basis for comparing the functional-association rates of different Abs and for examining the functional reversibility of Ab binding to the virus under nonequilibrium conditions (26). Whether single- or multiple-envelope proteins are required to mediate HIV entry (47), the changes that follow receptor activation define a sequence of shifting sensitivities of the virus to neutralization by different Abs.
In a recently published work, Steger and Root show that the 50% inhibitory concentration values of HIV-1 fusion inhibitors do not correlate with binding affinity but rather correlate inversely with the association rate constant of the inhibitor (41). The authors therefore suggest that inhibitor potency may be determined by temporal accessibility to the transiently exposed target. Here, we directly demonstrate the shifting sensitivities of virus to Ab-mediated neutralization during the course of the entry phase. We thus identify the existence of a new and significant determinant of Ab neutralization efficiency: the functional-association time frame for Ab during the viral entry process.
Neutralizing Abs generated following HIV infection have been shown to target mainly the CD4-binding site (29) and the V1/V2 and V3 variable loops of the envelope protein (21, 44, 46). Interestingly, we observed that pooled IgG obtained from HIV-1-positive blood donors neutralized the virus for a significant time span beyond the step of CD4 engagement and approximated the MAb 2F5 neutralization profile. The immune response to HIV infection is thus capable of eliciting the production of efficient neutralizing Abs with extended functional-association times. Correlation of specific envelope protein domains with this new measure of Ab neutralization efficiency may serve to better direct the choice of immunogens for the development of an AIDS vaccine.
Acknowledgments
We thank H. Falk for advice and technical support and A. Honigman for comments on the manuscript.
This work was supported by European Commission program no. 6, the Clinigene Consortium, and grants from the Philip Morris External Research Program, the Hadassah Medical Organization, the Israel Science Foundation, and the Ministry of Health, Chief Scientist, Israel.
Footnotes
Published ahead of print on 24 January 2007.
REFERENCES
- 1.Andreadis, S., T. Lavery, H. E. Davis, J. M. Le Doux, M. L. Yarmush, and J. R. Morgan. 2000. Toward a more accurate quantitation of the activity of recombinant retroviruses: alternatives to titer and multiplicity of infection. J. Virol. 74:3431-3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong, S. J., and N. J. Dimmock. 1996. Varying temperature-dependence of post-attachment neutralization of human immunodeficiency virus type 1 by monoclonal antibodies to gp 120: identification of a very early fusion-independent event as a neutralization target. J. Gen. Virol. 77:1397-1402. [DOI] [PubMed] [Google Scholar]
- 3.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannert, N., D. Schenten, S. Craig, and J. Sodroski. 2000. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J. Virol. 74:10984-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beirnaert, E., S. De Zutter, W. Janssens, and G. van der Groen. 2001. Potent broad cross-neutralizing sera inhibit attachment of primary HIV-1 isolates (groups M and O) to peripheral blood mononuclear cells. Virology 281:305-314. [DOI] [PubMed] [Google Scholar]
- 6.Binley, J. M., C. S. Cayanan, C. Wiley, N. Schulke, W. C. Olson, and D. R. Burton. 2003. Redox-triggered infection by disulfide-shackled human immunodeficiency virus type 1 pseudovirions. J. Virol. 77:5678-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burrer, R., S. Haessig-Einius, A. M. Aubertin, and C. Moog. 2003. Polyclonal immunoglobulin G from patients neutralizes human immunodeficiency virus type 1 primary isolates by binding free virions, but without interfering with an initial CD4-independent attachment of the virus to primary blood mononuclear cells. J. Virol. 77:11385-11397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. Parren, L. S. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, et al. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 9.Calarese, D. A., C. N. Scanlan, M. B. Zwick, S. Deechongkit, Y. Mimura, R. Kunert, P. Zhu, M. R. Wormald, R. L. Stanfield, K. H. Roux, J. W. Kelly, P. M. Rudd, R. A. Dwek, H. Katinger, D. R. Burton, and I. A. Wilson. 2003. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science 300:2065-2071. [DOI] [PubMed] [Google Scholar]
- 10.Conley, A. J., J. A. Kessler II, L. J. Boots, J. S. Tung, B. A. Arnold, P. M. Keller, A. R. Shaw, and E. A. Emini. 1994. Neutralization of divergent human immunodeficiency virus type 1 variants and primary isolates by IAM-41-2F5, an anti-gp41 human monoclonal antibody. Proc. Natl. Acad. Sci. USA 91:3348-3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Crooks, E. T., P. L. Moore, D. Richman, J. Robinson, J. A. Crooks, M. Franti, N. Schulke, and J. M. Binley. 2005. Characterizing anti-HIV monoclonal antibodies and immune sera by defining the mechanism of neutralization. Hum. Antib. 14:101-113. [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, H. E., M. Rosinski, J. R. Morgan, and M. L. Yarmush. 2004. Charged polymers modulate retrovirus transduction via membrane charge neutralization and virus aggregation. Biophys. J. 86:1234-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Rosny, E., R. Vassell, S. Jiang, R. Kunert, and C. D. Weiss. 2004. Binding of the 2F5 monoclonal antibody to native and fusion-intermediate forms of human immunodeficiency virus type 1 gp41: implications for fusion-inducing conformational changes. J. Virol. 78:2627-2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doms, R. W., and J. P. Moore. 2000. HIV-1 membrane fusion: targets of opportunity. J. Cell Biol. 151:F9-F14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donzella, G. A., D. Schols, S. W. Lin, J. A. Este, K. A. Nagashima, P. J. Maddon, G. P. Allaway, T. P. Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 16.Endres, M. J., P. R. Clapham, M. Marsh, M. Ahuja, J. D. Turner, A. McKnight, J. F. Thomas, B. Stoebenau-Haggarty, S. Choe, P. J. Vance, T. N. Wells, C. A. Power, S. S. Sutterwala, R. W. Doms, N. R. Landau, and J. A. Hoxie. 1996. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell 87:745-756. [DOI] [PubMed] [Google Scholar]
- 17.Finnegan, C. M., W. Berg, G. K. Lewis, and A. L. DeVico. 2002. Antigenic properties of the human immunodeficiency virus transmembrane glycoprotein during cell-cell fusion. J. Virol. 76:12123-12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golding, H., M. Zaitseva, E. de Rosny, L. R. King, J. Manischewitz, I. Sidorov, M. K. Gorny, S. Zolla-Pazner, D. S. Dimitrov, and C. D. Weiss. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J. Virol. 76:6780-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grosberg, A. Y., T. T. Nguyen, and B. I. Shklovskii. 2002. Colloquium: the physics of charge inversion in chemical and biological systems. Rev. Mod. Phys. 74:329-345. [Google Scholar]
- 20.Haim, H., I. Steiner, and A. Panet. 2005. Synchronized infection of cell cultures by magnetically controlled virus. J. Virol. 79:622-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, C. C., M. Tang, M. Y. Zhang, S. Majeed, E. Montabana, R. L. Stanfield, D. S. Dimitrov, B. Korber, J. Sodroski, I. A. Wilson, R. Wyatt, and P. D. Kwong. 2005. Structure of a V3-containing HIV-1 gp120 core. Science 310:1025-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klasse, P. J., and Q. J. Sattentau. 2002. Occupancy and mechanism in antibody-mediated neutralization of animal viruses. J. Gen. Virol. 83:2091-2108. [DOI] [PubMed] [Google Scholar]
- 23.Labrijn, A. F., P. Poignard, A. Raja, M. B. Zwick, K. Delgado, M. Franti, J. Binley, V. Vivona, C. Grundner, C. C. Huang, M. Venturi, C. J. Petropoulos, T. Wrin, D. S. Dimitrov, J. Robinson, P. D. Kwong, R. T. Wyatt, J. Sodroski, and D. R. Burton. 2003. Access of antibody molecules to the conserved coreceptor binding site on glycoprotein gp120 is sterically restricted on primary human immunodeficiency virus type 1. J. Virol. 77:10557-10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Layne, S. P., M. J. Merges, M. Dembo, J. L. Spouge, S. R. Conley, J. P. Moore, J. L. Raina, H. Renz, H. R. Gelderblom, and P. L. Nara. 1992. Factors underlying spontaneous inactivation and susceptibility to neutralization of human immunodeficiency virus. Virology 189:695-714. [DOI] [PubMed] [Google Scholar]
- 25.Markosyan, R. M., F. S. Cohen, and G. B. Melikyan. 2003. HIV-1 envelope proteins complete their folding into six-helix bundles immediately after fusion pore formation. Mol. Biol. Cell 14:926-938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McDougal, J. S., M. S. Kennedy, S. L. Orloff, J. K. Nicholson, and T. J. Spira. 1996. Mechanisms of human immunodeficiency virus type 1 (HIV-1) neutralization: irreversible inactivation of infectivity by anti-HIV-1 antibody. J. Virol. 70:5236-5245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McInerney, T. L., and N. J. Dimmock. 2001. Postattachment neutralization of a primary strain of HIV type 1 in peripheral blood mononuclear cells is mediated by CD4-specific antibodies but not by a glycoprotein 120-specific antibody that gives potent standard neutralization. AIDS Res. Hum. Retrovir. 17:1645-1654. [DOI] [PubMed] [Google Scholar]
- 28.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore, J. P., and D. D. Ho. 1993. Antibodies to discontinuous or conformationally sensitive epitopes on the gp120 glycoprotein of human immunodeficiency virus type 1 are highly prevalent in sera of infected humans. J. Virol. 67:863-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moulard, M., S. K. Phogat, Y. Shu, A. F. Labrijn, X. Xiao, J. M. Binley, M. Y. Zhang, I. A. Sidorov, C. C. Broder, J. Robinson, P. W. Parren, D. R. Burton, and D. S. Dimitrov. 2002. Broadly cross-reactive HIV-1-neutralizing human monoclonal Fab selected for binding to gp120-CD4-CCR5 complexes. Proc. Natl. Acad. Sci. USA 99:6913-6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naldini, L., U. Blomer, F. H. Gage, D. Trono, and I. M. Verma. 1996. Efficient transfer, integration, and sustained long-term expression of the transgene in adult rat brains injected with a lentiviral vector. Proc. Natl. Acad. Sci. USA 93:11382-11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olinger, G. G., M. Saifuddin, M. L. Hart, and G. T. Spear. 2002. Cellular factors influence the binding of HIV type 1 to cells. AIDS Res. Hum. Retrovir. 18:259-267. [DOI] [PubMed] [Google Scholar]
- 34.Parren, P. W., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77:195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parren, P. W., I. Mondor, D. Naniche, H. J. Ditzel, P. J. Klasse, D. R. Burton, and Q. J. Sattentau. 1998. Neutralization of human immunodeficiency virus type 1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J. Virol. 72:3512-3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Platt, E. J., J. P. Durnin, and D. Kabat. 2005. Kinetic factors control efficiencies of cell entry, efficacies of entry inhibitors, and mechanisms of adaptation of human immunodeficiency virus. J. Virol. 79:4347-4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purtscher, M., A. Trkola, G. Gruber, A. Buchacher, R. Predl, F. Steindl, C. Tauer, R. Berger, N. Barrett, A. Jungbauer, et al. 1994. A broadly neutralizing human monoclonal antibody against gp41 of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 10:1651-1658. [DOI] [PubMed] [Google Scholar]
- 38.Reeves, J. D., S. A. Gallo, N. Ahmad, J. L. Miamidian, P. E. Harvey, M. Sharron, S. Pohlmann, J. N. Sfakianos, C. A. Derdeyn, R. Blumenthal, E. Hunter, and R. W. Doms. 2002. Sensitivity of HIV-1 to entry inhibitors correlates with envelope/coreceptor affinity, receptor density, and fusion kinetics. Proc. Natl. Acad. Sci. USA 99:16249-16254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sattentau, Q. J., S. Zolla-Pazner, and P. Poignard. 1995. Epitope exposure on functional, oligomeric HIV-1 gp41 molecules. Virology 206:713-717. [DOI] [PubMed] [Google Scholar]
- 40.Spenlehauer, C., A. Kirn, A. M. Aubertin, and C. Moog. 2001. Antibody-mediated neutralization of primary human immunodeficiency virus type 1 isolates: investigation of the mechanism of inhibition. J. Virol. 75:2235-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steger, H. K., and M. J. Root. 2006. Kinetic dependence to HIV-1 entry inhibition. J. Biol. Chem. 281:25813-25821. [DOI] [PubMed] [Google Scholar]
- 42.Sune, C., G. Jimenez, I. Correa, M. J. Bullido, F. Gebauer, C. Smerdou, and L. Enjuanes. 1990. Mechanisms of transmissible gastroenteritis coronavirus neutralization. Virology 177:559-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ugolini, S., I. Mondor, P. W. Parren, D. R. Burton, S. A. Tilley, P. J. Klasse, and Q. J. Sattentau. 1997. Inhibition of virus attachment to CD4+ target cells is a major mechanism of T cell line-adapted HIV-1 neutralization. J Exp. Med. 186:1287-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei, X., J. M. Decker, S. Wang, H. Hui, J. C. Kappes, X. Wu, J. F. Salazar-Gonzalez, M. G. Salazar, J. M. Kilby, M. S. Saag, N. L. Komarova, M. A. Nowak, B. H. Hahn, P. D. Kwong, and G. M. Shaw. 2003. Antibody neutralization and escape by HIV-1. Nature 422:307-312. [DOI] [PubMed] [Google Scholar]
- 45.Welker, R., H. Hohenberg, U. Tessmer, C. Huckhagel, and H. G. Krausslich. 2000. Biochemical and structural analysis of isolated mature cores of human immunodeficiency virus type 1. J. Virol. 74:1168-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyatt, R., P. D. Kwong, E. Desjardins, R. W. Sweet, J. Robinson, W. A. Hendrickson, and J. G. Sodroski. 1998. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 47.Yang, X., S. Kurteva, X. Ren, S. Lee, and J. Sodroski. 2005. Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1. J. Virol. 79:12132-12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zeder-Lutz, G., J. Hoebeke, and M. H. Van Regenmortel. 2001. Differential recognition of epitopes present on monomeric and oligomeric forms of gp160 glycoprotein of human immunodeficiency virus type 1 by human monoclonal antibodies. Eur. J. Biochem. 268:2856-2866. [DOI] [PubMed] [Google Scholar]
- 49.Zufferey, R., D. Nagy, R. J. Mandel, L. Naldini, and D. Trono. 1997. Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15:871-875. [DOI] [PubMed] [Google Scholar]