Abstract
Poliovirus 3CD is a multifunctional protein that serves as a precursor to the protease 3Cpro and the viral polymerase 3Dpol and also plays a role in the control of viral replication. Although 3CD is a fully functional protease, it lacks polymerase activity. We have solved the crystal structures of 3CD at a 3.4-Å resolution and the G64S fidelity mutant of 3Dpol at a 3.0-Å resolution. In the 3CD structure, the 3C and 3D domains are joined by a poorly ordered polypeptide linker, possibly to facilitate its cleavage, in an arrangement that precludes intramolecular proteolysis. The polymerase active site is intact in both the 3CD and the 3Dpol G64S structures, despite the disruption of a network proposed to position key residues in the active site. Therefore, changes in molecular flexibility may be responsible for the differences in fidelity and polymerase activities. Extensive packing contacts between symmetry-related 3CD molecules and the approach of the 3C domain's N terminus to the VPg binding site suggest how 3Dpol makes biologically relevant interactions with the 3C, 3CD, and 3BCD proteins that control the uridylylation of VPg during the initiation of viral replication. Indeed, mutations designed to disrupt these interfaces have pronounced effects on the uridylylation reaction in vitro.
Poliovirus (PV), a member of the Picornaviridae family of RNA viruses, must simultaneously perform many tasks inside a host cell for efficient and successful viral replication, and only a small number of gene products are responsible for these processes. The virus produces a single polyprotein that is cleaved by virally encoded proteases. Many of the viral proteins, including precursor proteins, play multiple roles in viral replication.
The viral protein 3CD is a multifunctional precursor to the poliovirus protease 3Cpro and the RNA-dependent RNA polymerase 3Dpol. The 3CD molecule retains its ability to function as a protease but lacks polymerase activity (20). Its proteolytic functions include cleavages, resulting in the production of structural proteins VP0, VP1, and VP3 and nonstructural proteins 3AB, 3CD, 3Cpro, and 3Dpol. In many of these functions, 3CD serves as a better protease than 3Cpro, suggesting that the 3D region of 3CD contributes to that activity (34).
3CD is also a crucial component of the viral replication complex. The 5′-terminal region of the poliovirus genome adopts a cloverleaf structure to which 3CD can bind in the presence of the viral protein 3AB or the cellular protein PCBP2 (2, 35, 49). Both forms of this ribonucleoprotein complex appear to play important roles in inducing RNA synthesis (49). The initiation of RNA synthesis is primed by the addition of two uridines to the 22-amino-acid protein primer VPg (3B) at the side-chain hydroxyl of Tyr3. Positive-strand synthesis is thought to use an RNA hairpin structure as its template within the PV genome (37, 42). One model suggests that this cis-acting replication signal in the 2C-noncoding region of the PV genome, cre(2C), binds to 3CD and forms a multicomponent complex, with 3Dpol bound to VPg (37). Uridylylated VPg is then transferred to the 3′ end of viral RNA where it is extended by the polymerase to produce VPg-linked RNA. A recently reported crystal structure of 3Dpol from foot-and-mouth-disease virus (FMDV) in complex with VPg shows how the N terminus of VPg interacts with 3Dpol (12). Alternatively, in negative-strand synthesis, poly(A) may serve as a template for uridylylation, though a poly(A) template does not provide much specificity and does not require 3CD for the uridylylation of VPg (38). Uridylylation of VPg is essential for viral replication and, having no counterpart in host cell metabolism, represents an attractive therapeutic target.
Cleavage of 3CD at its Gln-Gly pair, either by 3C or by a second molecule of 3CD, releases 3Cpro and 3Dpol. Crystal structures of 3Cpro and 3Dpol have been determined (18, 29, 48). 3Cpro, a cysteine protease, is comprised of two antiparallel, six-stranded, β-barrel domains, four helices, and a flexible polypeptide strand linking the two barrel domains. The shallow active site cavity utilizes Cys147 as its nucleophile, with His40 and Glu71 serving as the acid/base catalysts (44).
The initial crystal structure of poliovirus 3Dpol showed that the protein contains a fingers-palm-thumb arrangement common in DNA and RNA polymerases (18). However, much of the fingers region was disordered due to steric clashes among the fingers regions of neighboring polymerase molecules. The wild-type crystal-packing arrangement was stabilized by a strong intermolecular interaction named interface I. Interface I involves the insertion of Leu446 from the thumb of one molecule into a hydrophobic pocket on the palm of another molecule, which is further stabilized by intermolecular salt bridges. More recently, a more accurate structure of the poliovirus polymerase with the crystal-packing artifacts removed was solved by mutating Leu446 and a residue involved in salt bridge formation, Arg455, to prevent the formation of interface I (48). In the crystal form that results from eliminating interface I interactions, the finger region of 3Dpol is well ordered and wraps around to touch the thumb, forming a closed circle that is likely to be important for processive RNA synthesis. The latter structure is most likely the biologically relevant one, as it closely resembles the structures of other known viral RNA-dependent RNA polymerases (1, 6, 8, 13, 25, 32).
The complete structure of 3Dpol shows the N terminus buried in a pocket at the base of the fingers region. The N terminus participates in a network of hydrogen bonds that was proposed to help in positioning the 3D Asp238 residue in the active site (48). Asp238 selects for the 2′-OH group of incoming ribonucleoside triphosphates (rNTPs), and mutations made to this residue abolish polymerase activity (16). The lack of polymerase activity in poliovirus 3CD protein is thought to be associated with the removal of the N terminus of 3Dpol from its binding pocket, as modifications made to the N terminus of 3Dpol disrupt its polymerase activity. The deletion of the first 6 residues of 3Dpol or the transfer of 11 residues from the C terminus of 3Cpro to the 3Dpol N terminus leads to a complete loss of activity, while the G1A mutation of 3Dpol shows a twofold reduction in polymerase activity (21, 43, 48).
For many years, 3CD has been a high-priority target for crystallographic studies, but the tendency of the protein to aggregate at high concentrations has hampered these efforts. We have produced a construct with mutations made to interface I of 3Dpol, to the crystallographic dimer interface of 3Cpro, and to the 3Cpro active site and have used this construct to solve the structure of 3CD to a 3.4-Å resolution. We have also solved the structure of the 3Dpol G64S mutant to a 3.0-Å resolution. The arrangement of residues in the polymerase active site of our construct is very similar to that in 3CD, 3Dpol, and the 3Dpol G64S fidelity mutant, with the exception of a hydrogen bond network involving the N terminus of 3D. Since the N-terminal residues of 3CD's 3D domain are part of the covalent linker with the 3C domain, the network is absent in 3CD, but it appears to be modified relative to that of the wild type in the 3Dpol G64S structure. These findings suggest that changes in molecular flexibility rather than large structural rearrangements may be important determinants of polymerase activity and fidelity, as is sometimes observed in other polymerases (19, 22, 23).
In the 3CD crystal structure, the 3D domain makes extensive contacts with the 3C and 3D domains of neighboring molecules, and the N terminus of 3C lies close to the VPg binding site. This arrangement is consistent with a possible biological role for these contacts in forming and regulating the VPg uridylylation complex. Indeed, several mutations designed to disrupt these proposed interfaces have pronounced effects on the uridylylation reaction, helping to explain how 3CD plays an essential regulatory role in the complex.
MATERIALS AND METHODS
Expression and purification of 3CD proteins.
3CD protein was expressed using the ubiquitin fusion system developed and reported by Gohara et al. (17). BL21(DE3)/pCG1 cells were transformed with pET26Ub fusion plasmids containing the 3CD gene and plated on NZCYM agar enriched with kanamycin (25 μg/ml), chloramphenicol (20 μg/ml), and 0.4% dextrose. The resulting colonies were then used to inoculate 100 ml of NZCYM medium and grown overnight at 30°C. This culture was used to seed 1 liter of NZCYM medium, inoculated to a beginning optical density at 600 nm (OD600) of 0.03, and grown at 37°C until they reached an OD600 of 1. The cells were chilled to 25°C and induced with isopropyl-β-d-thiogalactopyranoside (IPTG) to a final concentration of 500 μM. Cells were grown for an additional 4 h at 25°C, harvested, and pelleted. The cell pellets were stored at −80°C. Frozen cell pellets were thawed on ice and resuspended in lysis buffer (100 mM potassium phosphate [pH 8.0], 20% glycerol, 1 mM dithiothreitol [DTT], 0.5 mM EDTA, 60 μM ZnCl2, and protease inhibitor tablets [Roche]) at a concentration of 5 ml lysis buffer per gram of cell pellet. Cells were homogenized and passed through a cell disruptor (Avestin). Polyethylenimine was added to the cell lysate at a concentration of 0.25% (vol/vol) to precipitate nucleic acid. The lysate was stirred at 4°C for 30 min and then centrifuged for 30 min at 17,500 rpm at 4°C. Granular ammonium sulfate was added slowly to a 40% saturation to the decanted supernatant. The solution was centrifuged for 30 min at 17,500 rpm at 4°C. The resulting ammonium sulfate pellets were resuspended in buffer A (50 mM Tris [pH 8.0], 20% glycerol, 1 mM DTT, 0.1% NP-40, and 60 μM ZnCl2) and diluted to a final salt concentration of 50 mM. The diluted sample was loaded onto a phosphocellulose column (approximately 1 ml bed volume/20 mg total protein) at a flow rate of 1 ml/min. The column was washed to baseline with buffer A containing 25 mM NaCl and eluted with a linear gradient from 25 to 350 mM NaCl in buffer A. Fractions containing 3CD were pooled and again diluted to a salt concentration of 50 mM. The diluted protein was loaded onto a Q-Sepharose column (approximately 1 ml bed volume/120 mg total protein). The column was washed to baseline with 50 mM NaCl in buffer A and eluted with a linear gradient from 50 to 500 mM NaCl in buffer A. Fractions containing 3CD were pooled and concentrated. The protein was loaded onto a HiLoad 26/60 Superdex 200 column equilibrated and eluted with buffer A containing 350 mM NaCl in the absence of the detergent NP-40. The purified protein was concentrated to 10 mg/ml, aliquoted, and stored at −80°C.
The 3CD construct utilized for crystallization contains a series of mutations designed to abrogate the activity of the protease, inhibit the formation of a 3Cpro dimer, and disrupt interface I of 3Dpol. The wild-type protease is inactivated in this construct by mutating the nucleophilic Cys147 to an alanine residue. In order to disrupt interface I of 3Dpol, the 3Dpol residues Leu446 (3CD residue Leu629) and Arg455 (3CD residue Arg638) were mutated to aspartic acid residues (L446D and R455D, respectively). The interface mutations resulted in encoding a protein that could be concentrated to ∼15 mg/ml and was less prone to precipitation than the wild-type 3CD protein. This 3CD protein was crystallized, apparently, in the same space group as the structure we report here. However, both the appearance and the diffraction of these crystals were poor. 3Cpro forms a dimer in the 3Cpro crystal structure that is stabilized by a number of intermolecular contacts between the two molecules, though there is no evidence that 3Cpro forms a dimer in vivo (29). To prevent any tendency of 3Cpro to dimerize, residues Glu55, Asp58, and Glu63 were mutated to alanines, whereupon the highest quality crystals were obtained.
Expression and purification of the 3Dpol G64S mutant.
The 3Dpol G64S construct utilized for crystallization contains the same interface I mutations made in the 3CD construct (L446D and R455D). Gly64 within the fingers domain was mutated to a serine residue. 3Dpol protein was expressed in the same manner as 3CD and purified similarly to 3CD, with some notable differences. Ammonium sulfate was added to a 60% saturation following polyethylenimine extraction. Phosphocellulose and Q-Sepharose steps followed. The protein was then loaded onto a HiLoad 26/60 Superdex 200 column equilibrated and eluted with buffer containing 200 mM NaCl, 5 mM Tris (pH 7.5), 0.1 mM EDTA, and 2 mM DTT. The purified protein was concentrated to 10 mg/ml.
Crystallization and structure solution.
Crystals were grown using hanging drop vapor diffusion at 20°C, mixed 1:1 with a well solution. Crystals of 3CD grew over 2 to 3 days over a well solution containing 2 M ammonium sulfate, 0.1 M HEPES (pH 7.0), and 0.3% Jeffamine M600 (pH 7.0). These crystals were transferred to a corresponding precipitant solution containing 20% glycerol (vol/vol) and soaked in this solution overnight prior to flash freezing in a nitrogen stream. Crystals of 3Dpol with G64S grew overnight over a well solution containing 2 M sodium acetate and 0.1 M sodium cacodylate (pH 6.8), similar to crystallization conditions used to solve the complete structure of 3Dpol (48). These crystals were transferred to a cryoprotectant containing the crystallization solution with 20% glycerol and were flash frozen in a nitrogen stream. In both cases, diffraction data were collected at the 19-ID beamline at the Advanced Photon Source (Argonne, IL). Data were integrated, merged, and scaled using DENZO and SCALEPACK (33) programs. Structures were solved by molecular replacement using PHASER (28). The search models were the structures of PV-3Cpro (PDB code 1LIN) and PV-3Dpol (PDB code 1RA6) with solvent and key residues omitted. The model of 3CD was built using Coot (11) and refined with REFMAC5 (30).
VPg uridylylation assay.
Full-length cre (bases 3702 to 3763 of the human poliovirus 1 Mahoney strain, EMBL accession ID V01149.1) was synthesized using a MEGAshortscript kit (Ambion) and then gel purified, extracted with phenol-chloroform, lyophilized, and resuspended in water prior to use. VPg peptide (poliovirus protein 3B, Swiss-Prot accession ID P03300) was synthesized by Alpha Diagnostics. 3Dpol and 3CD proteins were purified as described above, except that purification was stopped after the Q-Sepharose step and Zn2+ was omitted from buffers used in that purification step. 3Dpol and 3CD mutants were constructed using wild-type and inactive protease (C147G) plasmid backgrounds, respectively, obtained from the Cameron laboratory. Mutagenesis was carried out using a QuikChange II kit (Stratagene), and mutants were verified by DNA sequencing.
Uridylylation of VPg, using cre as a template (with or without 3CD as a stimulatory factor), was assayed as previously described (36), except that the concentration of 3Dpol was increased to 2 μM and reactions were run from 1 to 4 h at either 30 or 37°C. Wild-type and mutant proteins 3Dpol and 3CD were used in various combinations to see if the mutations affected the quantity of uridylylated VPg that was produced. Reaction products were analyzed by Tris-tricine gel electrophoresis. The gels were dried and subsequently analyzed by phosphorimager, quantifying free and VPg-bound radiolabel by using Quantity One software (Bio-Rad).
Mathematical treatment of the uridylylation data.
The first objective was to measure and normalize the amount of uridylylation activity in each sample. The amount of labeled VPg produced in each experiment was first normalized to correct for the decay of the label by dividing the signal of the VPg-bound band by the total of the bound and unbound radioactivity in the lane, then corrected for exposure time, and finally averaged over as many replicates as were available. To correct for substrate depletion (which was usually insignificant), the fraction bound was replaced by the negative of the natural logarithm of the unbound fraction. To measure the unstimulated controls accurately, they were determined in triplicate, with bound radioactivity exposed 12 times longer than the usual period.
The final objective was to determine the degree of stimulation, specifically how much more uridylylation is produced by a 3Dpol mutant (or wild-type 3Dpol) and a 3CD mutant (or wild-type 3CD) together, relative to the level produced by that particular 3Dpol mutant (or wild-type 3Dpol) alone. Thus, values were normalized to correct for possible variations among 3Dpol preparations by dividing by the level of the corresponding “unstimulated” control that lacked 3CD. Each value listed in Table 3, therefore, represents a degree of stimulation.
TABLE 3.
Effect of the 3CD-3D interface on the ratio between 3CD-stimulated cre-dependent uridylylation of VPg and unstimulated uridylylationa
| 3CD WT or mutants | 3D WT | 3Dpol mutant proteins (residue no.)d
|
||||
|---|---|---|---|---|---|---|
| R7A (190) | D260A (443) | D263A (446) | D260A D263A | D319A (502)c | ||
| 3CD WT | 36.3 | 9.8 | 5.9 | 6.9 | 6.1 | 5.0 |
| T154Ac | 47.3 | 16.6 | 11.9 | 9.7 | 7.1 | 7.4 |
| K156Ab,c | 2.2 | 0.8 | 1.1 | 1.5 | 0.5 | 0.5 |
| K156A T154Ab,c | 1.4 | 2.7 | 3.5 | 2.5 | 2.1 | 2.4 |
| R190Ad | 148.1 | 78.6 | 29.8 | 37.4 | 24.7 | 11.2 |
| D446Ad | 153.8 | 77.6 | 41.1 | 46.4 | 26.1 | 18.4 |
| D443A D446Ad | 138.0 | 82.7 | 51.7 | 50.2 | 50.8 | 25.0 |
Each value represents the ratio of 3CD-stimulated uridylylation to unstimulated uridylylation (see Materials and Methods). WT, wild type.
Bands too weak to measure reliably were observed in the K156A mutant and in the 3CD K156A T154A double mutant.
Residue located at interface D-C.
Residue located at interface D-D.
Coordinate accession numbers.
The refined coordinates and data for 3CD and 3Dpol are available from the Protein Data Bank (PDB entries 2IJD and 2IJF, respectively).
RESULTS AND DISCUSSION
Structure determination and refinement of 3CD and 3Dpol G64S mutants.
3CD crystallizes in space group C2221, with two molecules per asymmetric unit and a solvent content of 80.5%. X-ray diffraction data were collected to 3.4-Å resolution (Table 1), and a molecular replacement solution was found using known 3Cpro and 3Dpol crystal structures as input models. Each molecule of 3CD includes all 644 amino acid residues and 10 proposed ion-binding sites. At the beginning of structure determination, the structure was built to fit global omit maps, and noise was routinely added to the atomic coordinates at the beginning of each refinement cycle to prevent overfitting of the data. During refinement, missing and weakly ordered parts of the model were repeatedly rebuilt into omit maps (30). Twofold noncrystallographic symmetry (NCS) restraints were applied. Initially, strong NCS restraints were applied to the main-chain atoms and moderate restraints to the side chains. Later in the refinement, only weak NCS restraints were applied, as this optimized the Rfree value. It should be noted that the twofold noncrystallographic symmetry roughly doubles the observation-to-parameter ratio and provides an internal check of the accuracy of the result. Translation-libration-screw (TLS) parameters were imposed, treating the two 3C and two 3D domains in the asymmetric unit as independent rigid bodies. In the final stages of refinement, individual temperature factors were included, giving R and Rfree values of 20.0 and 23.1, respectively, with good geometry (Table 1).
TABLE 1.
3CD and 3Dpol data collection and refinement statistics
| Data set | 3CD | 3Dpol G64S mutant |
|---|---|---|
| Space group | C2221 | P65 |
| Unit cell | a = 208.5, b = 230.4, c = 151.0 | a = b = 126.3, c = 113.5 |
| Resolution limits (Å) | 20-3.4 (3.58-3.40)a | 20-3.0 (3.11-3.00)a |
| Total observations | 249,335 | 110,896 |
| Unique reflections | 49,539 | 20,317 |
| Redundancy | 5.0 (4.8)a | 6.0 (4.7)a |
| Completeness (%) | 98 (98)a | 99 (98)a |
| Rmerge (%)b | 7.3 (38.7)a | 8.5 (32.4)a |
| Rcrystc | 20.0 | 21.1 |
| Rfreec | 23.1 | 24.4 |
| No. of protein atoms | 10,086 | 3,695 |
| No. of ions | 10 | 0 |
| No. of waters | 8 | 0 |
| rmsd bond length (Å) | 0.011 | 0.010 |
| rmsd bond angle (°) | 1.88 | 1.55 |
Values in parentheses are those shown for the highest resolution shell.
Rmerge=∑Ih − <Ih>/∑Ih over all h, where Ih is the intensity of reflection h.
Rcryst and Rfree=∑∥Fo − Fc∥/∑Fo, where Fo and Fc are observed and calculated amplitudes, respectively. Rfree was calculated using 5% of data that was excluded from the refinement.
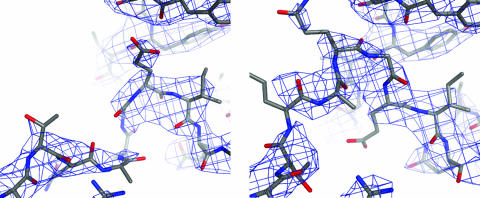
The 3Dpol G64S construct crystallizes in space group P65. The G64S structure was solved to 3.0-Å resolution by molecular replacement, using the known 3Dpol crystal structure as an input model. There is one molecule of 3Dpol in the asymmetric unit containing all 461 amino acid residues of the polymerase. The mutated residues and several surrounding residues were initially excluded from the refinement of the molecular replacement solution to eliminate bias. TLS parameters were included, and restrained individual temperature factors were included only during the final stages of refinement. The final R and Rfree values for 3Dpol G64S were 21.1 and 24.4, respectively, with good geometry (Table 1). Omit electron density maps of each structure were of high quality and were readily interpretable (Fig. 1b and 2).
FIG. 1.
The poliovirus 3CD structure. (a) There are two molecules of 3CD in the asymmetric unit. In this stereo view, one molecule is red and one is blue. The 3C and 3D domains are tethered together by a polypeptide linker region (upper black arrow). Within each molecule of 3CD, there is no direct contact between the 3C and 3D domains, except through the linker. The length of the polypeptide linker and the position of the protease site within the 3C domain (lower purple arrow) preclude intramolecular cleavage of 3CD. Regions of greatest variability between 3CD and the 3Cpro and 3Dpol structures are shown in green. (b) Representative electron density from an omit-phased 2Fo − Fc map contoured at 1.4σ is shown in stereo. This view shows density surrounding a proposed Zn2+ site at an interface between the 3D domains of two symmetry-related copies of 3CD, in gray and light blue. The Zn2+ ion appears to be tetrahedrally coordinated by Asp446, His453, His455, and Cys464. Density for the uppermost 3D domain comes from an α helix, while the density in the lower 3D domain includes β strands.
FIG. 2.
Hydrogen-bonding interactions around the N terminus of 3Dpol G64S, shown in stereo. An omit-phased 2Fo − Fc map contoured at 1.4σ shows density including the G64S mutation. Ser64 forms a hydrogen bond with Glu2, which in turn interacts with Gln4 of the N terminus. Gly1 maintains contacts with Ala239. Additional hydrogen bonding interactions are formed between the backbone of the N terminus and the backbone of a neighboring β strand.
Structural overview of 3CD.
The 3CD molecule consists of two domains, the 3C domain and the 3D domain (Fig. 1a). These two domains do not make any direct contact with one another but are instead separated by a seven-residue polypeptide linker (residues 180 to 186). The crystal lattice is formed by a network of noncrystallographic- and crystallographic-symmetry contacts.
The 3C and 3D component domains are similar in structure to 3Cpro and 3Dpol, respectively. Like 3Cpro, the 3C domain of 3CD contains two β-barrel domains and a number of helices and loop regions. The only significant structural differences observed between 3Cpro and 3CD were associated with crystal packing interactions or were located in the flexible N termini and loop regions (Fig. 1a). Main-chain atoms in the two NCS-related 3C domains (residues 1 to 180) can be superimposed with a root mean square displacement (rmsd) of 0.29 Å, and 3Cpro (ordered in residues 4 to 180) shows a 0.60-Å difference with either copy.
The 3D domain of 3CD adopts the same fingers-palm-thumb structure shared by other RNA-dependent RNA polymerases. The two NCS-related 3D domains (residues 184 to 644) are very similar to each other, having an rms difference of only 0.37 Å along the main chain, and to the 3Dpol domain (with differences of 0.49-Å and 0.67-Å rms, respectively). This level of difference between the coordinates provides an estimate for the uncertainty in atomic position, combined with the amount of local structural variability. Though the estimate of error that comes from molecular replacement may be an underestimate, it does provide a way to distinguish between better ordered or structurally conserved parts of the molecule. Not unexpectedly, the N-terminal residues of the 3D domain that comprise part of the polypeptide linker differ in position and conformation from the corresponding residues of 3Dpol, where the cleaved N terminus is inserted into a binding pocket and extensively hydrogen bonded. Surprisingly, only the first three residues of the 3D domain are arranged in a qualitatively different way. Smaller but statistically significant changes also involve residues 190 to 198 of 3CD (3Dpol residues 7 to 15), possibly due to the D-D′ dimerization of 3D that is seen in the 3CD structure, wherein the Arg7 side chain appears to pull its main-chain strand out of position while binding to Asp260 in the opposite monomer. Additional positional differences occur within the fingers domain of the polymerase (3CD residues 226 to 251; 3Dpol residues 43 to 68), where several side chains, including Phe242 and Tyr245 (3Dpol residues Phe59 and Tyr62), have shifted by more than 2 Å. A third area of variability lies within the pinky domain of 3Dpol (3CD residues 312 to 319; 3Dpol residues 129 to 136). The maximum differences in these regions range from 1.6 to 2.0 Å.
The electron density of the polypeptide linker between the 3C and 3D domains of 3CD is weak and differs significantly between the two NCS-related copies of the linker (Fig. 3). The presence of a visible electron density along the main chain implies that the linker is not entirely disordered. Nevertheless, the existence of at least two distinctly different conformations suggests that they are likely to be similar in energy and that the barrier to their interconversions is likely to be low. The flexibility of the linker and its accessibility to the protease active site may be relevant to the timing of 3CD cleavage (see below). Although the residues comprising this region were modeled into the density as accurately as possible, it was not possible to find an entirely satisfactory low-energy conformation for either copy of the linker. This, together with the lack of strong density in this region, suggests that the linker is not well ordered. Indeed, the presence of a flexible linker may be necessary for the proteolytic cleavage of 3CD into 3Cpro and 3Dpol, since a stable association of the linker with either domain might make the cleavage site inaccessible. Contrary to a previous suggestion, the linker seen in the 3CD structure is not long enough for the cleavage of 3CD at its intramolecular Gln-Gly site to be autocatalytic (7). After cleavage has taken place, the N-terminal residues of 3Dpol have a stable, well-characterized binding site, but the final three residues at the C terminus of 3Cpro become disordered (29).
FIG. 3.
Structure of the polypeptide linkers between the 3C and 3D domains of the two molecules of 3CD in the asymmetric unit. An omit map contoured at 1.4σ shows weak density in the linker regions, though the overall path of the backbone is clear. The linker regions of the two 3CD molecules in the asymmetric unit differ in structure, confirming that the linker is only weakly ordered. The two linkers are shown in similar orientations, with the 3C domain on the left and the 3D domain on the right.
Formation of interfaces between 3CD molecules.
The symmetry-related copies of 3CD form a number of crystal-packing contacts, some of them quite extensive. One notable interface is formed between the 3C domain of one 3CD molecule and the 3D domain of a twofold symmetry-related 3CD molecule (interface D-C) (Fig. 4a and b). This interface, involving the back of the 3D palm, appears to be stabilized by interactions involving several 3CD residues (Table 2 and Fig. 4b), with Lys438, Lys156, and Thr154 playing key roles. A second interface, D-D, is formed between the twofold symmetry-related 3D domains, mostly involving the back of the fingers region (Table 2 and Fig. 4c), including interactions involving Arg190 and Asn334. Interface D-D is further stabilized by the binding of a cation, possibly Zn2+. There is a strong peak in the electron density, with four tetrahedrally arranged ligands surrounding this peak (Fig. 1b). This potential Zn2+ atom coordinates His453 (3Dpol residue His270), His455 (3Dpol residue His272), and Cys464 (3Dpol residue Cys281) from one 3CD molecule and Asp446 (3Dpol residue Asp263) from the other 3CD molecule. Interestingly, the residues that appear to be involved in the stabilization of these interfaces are conserved among picornaviruses, and a role for Zn2+ in the modulation of cooperative polymerase activity has been noted (21).
FIG. 4.
Key interfaces formed by 3CD-3CD interactions. (a) The three labeled interfaces are formed between symmetry-related molecules of 3CD. Interfaces D-C and D-C′ are each formed between the 3C domain of one molecule and the 3D domain of another. Interface D-D is formed between two 3D domains that are related by twofold crystallographic symmetry. Green, yellow, and blue ribbons come from three different 3CD molecules. The thumb, palm, and fingers region of the green molecule are labeled. The N terminus of the yellow 3C domain is indicated. (b) Interface D-C is shown in stereo. Key residues and hydrogen bonds are indicated. (c) A portion of interface D-D is shown in stereo, with some key residues and hydrogen bonds indicated. Two symmetry-related copies of the putative Zn2+ coordination site are present, with Zn2+ ions indicated as gray spheres. Pro88 from interface D-C is included in both panel b and panel c as an aid to orientation.
TABLE 2.
Residues forming interfaces between symmetry-related 3CD molecules
| Molecule (residue no.), chain location (side or main)
|
Notes and distance (Å) | ||
|---|---|---|---|
| C | D | D′a | |
| His89, sidec | Lys438 (255), mainb | 2.8 | |
| Pro88, maind | Lys438 (255), sideb | 2.4 | |
| Gly155b | Lys438 (255), sideb | 3.5 | |
| Lys156c | Glu259 (76), sidec | 3.3 | |
| Lys156c | Asp262 (79), sidec | Potential | |
| Thr154c | Asp502 (319), sideb | 2.7 | |
| Thr154c | His263 (80), sideb | Potential | |
| Gln277 (94)c | Arg190 (7)d | 3.1 | |
| Asp443 (260)c | Arg190 (7)d | 3.5 | |
| His453 (270)c | Asp446 (263)c | Putative Zn2+ | |
| His455 (272)c | Asp446 (263)c | Putative Zn2+ | |
| Cys464 (281)c | Asp446 (263)c | Putative Zn2+ | |
| Asn334 (151)c | Asn334 (151)c | 3.2 | |
Note that molecules D and D′ are related by a crystallographic twofold axis so that each of the listed interactions occurs twice in the D-D interface.
Conserved among polioviruses, enteroviruses, coxsackieviruses, echoviruses, and certain rhinoviruses (A. Palmenberg, personal communication).
Conserved among polioviruses.
Not conserved.
A possible biological role for these interfaces (discussed below) is consistent with their size, which is large relative to that of most ordinary crystal-packing contacts. Each of the D-C and D-D contacts buries about 500 Å2 of solvent-accessible surface, for a total of about 1,000 Å2. However, their shape complementarity statistics are only 0.51 and 0.43, respectively (24). These low values reflect the possibility that most of the binding across these interfaces is mediated by salt bridges and polar interactions between flexible residues (Fig. 4b and c) and is consistent with the contacts being formed transiently.
Structural overview of 3Dpol G64S.
A mutation of 3Dpol Gly64 to a serine (G64S) in the fingers domain results in a polymerase with increased fidelity, allowing the polymerase to discriminate against ribavirin, a nucleoside analog (9, 39). Though the G64S mutant supports a lower overall rate of spontaneous mutation in the virus, this comes at the expense of making 3Dpol threefold less efficient in nucleotide incorporation (4). In the structure of 3Dpol, Gly1 forms two hydrogen bonds with the main-chain N and O of Gly64 (48). At the same time, the alpha-amino group of Gly1 hydrogen bonds to Ala239 and Leu241, helping to position these residues for proper nucleotide selection. The overall crystal structure of the G64S mutant is similar to that of the wild-type 3Dpol, with an rmsd of only 0.28 Å along the main chain. The arrangement of residues involved in the Gly1 hydrogen-bonding network, particularly in the vicinity of the G64S mutation, is also similar, except that the Ser64 side chain is able to form an additional hydrogen bond to the side chain of the carboxylate group of Glu2 and thereby promotes the simultaneous hydrogen bonding of Glu2 to Gln4 (Fig. 2). Because no large positional shifts are evident in the apoenzyme, it is possible that the mutation affects fidelity and efficiency primarily by helping to lock the 3Dpol N terminus and its surroundings into a more stable arrangement.
Implications of the 3CD and 3Dpol G64S structures for polymerase activity.
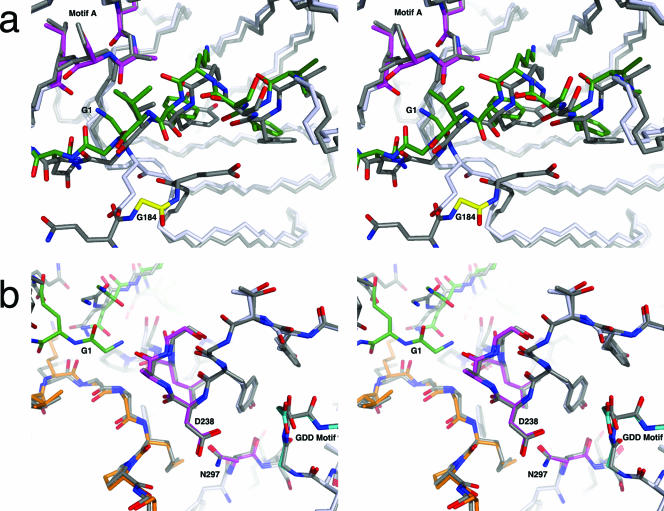
The 3C and 3D domains of the 3CD molecule are joined by a flexible, solvent-exposed polypeptide linker that covalently links the N terminus of the 3D domain to the C terminus of the 3C domain. A comparison of the 3CD and 3Dpol structures reveals that only the first three residues that comprise the N terminus of 3Dpol are significantly rearranged by formation of the linker in 3CD. These three residues (numbered 184 to 186 in 3CD) are rotated out of the pocket, using Gln187 as a pivot (Fig. 5a). In contrast, these three residues insert into a surface pocket in 3Dpol, where Gly1 of 3Dpol participates in forming a network of hydrogen bonds linking Gly1 to Gly64 and anchoring residues 238 to 241 (48). In other viral polymerases, analogous hydrogen-bonding networks are present that serve to connect the fingers domain with residues 238 to 241 (Fig. 6) (4).
FIG. 5.
Comparison of the 3CD and 3Dpol structures at the N terminus, the active site, and the fingers domain shown in stereo. (a) The structure of the active site of the polymerase remains mostly unchanged between 3CD (light gray) and 3Dpol (dark gray). Notable residues of the 3Dpol active site are colored: Asp238, Ala239, Leu241, and Asn297 are magenta, the GDD motif (Asp328) is cyan, the N-terminal fingers are green, and the middle finger is orange. (b) One of the largest positional differences between 3CD and 3Dpol occurs within the fingers region of the 3D domain (3CD residues 226 to 251; 3Dpol residues 43 to 68). 3CD is dark gray with Gly184 in yellow; 3Dpol is light gray and green with Gly1 also in green and motif A in magenta. Note that the N-terminal G184 of 3CD is removed from the binding cleft, whereas Gly1 of 3Dpol is buried. Only slight differences are seen in motif A (residues 238 to 241 of 3Dpol), magenta in 3Dpol.
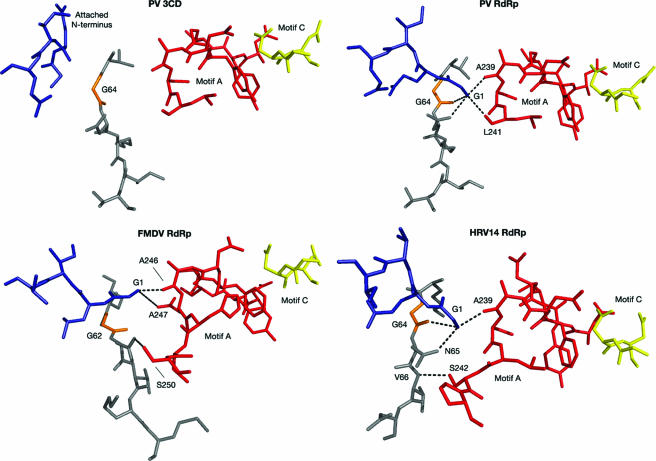
FIG. 6.
Hydrogen-bonded linkage between amino-terminal residues (blue), active site residues (red and yellow), and the fingers domain (gray) in several viral RNA-dependent RNA polymerases (RdRps). The RdRps of poliovirus, FMDV and HRV14, form hydrogen-bonding networks, connecting the fingers domain with the N terminus and active site residues. These interactions are absent in PV 3CD. Structural motif A is red; structural motif C is yellow (18).
In poliovirus, the importance of proper positioning of the N terminus for polymerase activity was suggested by the crystal structure of 3Dpol with the N-terminal glycine mutated to alanine (G1A). This 3Dpol G1A mutant was only partially active, and the N-terminal hydrogen-bonding network was partly disrupted, leading to the proposal that it might be critical for the positioning of Asp238 (48). However, contrary to this hypothesis, the absence of the N-terminal glycine residue in 3CD has no effect on the positioning of the active site residues or on those involved in the hydrogen-bonding network in the structure of the 3CD apoenzyme (Fig. 5b). Thus, the structure of 3CD would suggest that the canonical active site of 3Dpol is mostly preformed and does not require the N terminus to be buried in order to achieve the correct conformation for initial NTP binding. Although it has been speculated that the postcleavage insertion of the 3Dpol N terminus into its binding cleft might shift active site residues from nonproductive to productive positions, we cannot rule out the possibility that such a shift occurs in the elongation complex when 3Dpol binds its substrates. However, in our current snapshot of the apoenzyme structure, no such structural difference is evident. Indeed, the observation that 3CD of FMDV shows some polymerase activity suggests that the specific contacts made by Gly1 are unlikely to be essential for catalysis (31). In addition, a glutathione S-transferase tag can be added to the N terminus of human rhinovirus 2 polymerase without significantly affecting its activity (14, 15).
Because the structure of the active site seems to retain a functional conformation, we looked for structural variability in other regions of the polymerase to see what differences might be associated with the lack of polymerase activity in 3CD. Aside from the polypeptide linker connecting the 3C and 3D domains of 3CD, the largest differences in the 3D domain occurred in three areas of the molecule that are remote from the active site. One difference involved a main-chain shift at the D-D interface, possibly to accommodate the salt linkage of Arg7 and Asp260 between the two domains. A second main-chain shift occurred in the pinky region of the polymerase, which was reported to have a relatively poor electron density in the 3Dpol crystal structure (48). A structural alignment of the 3Dpol active site with the active site of bacteriophage T7 RNA polymerase indicated that this region of the pinky domain (residues 124 to 149) is analogous to the T7 specificity loop (48). The T7 specificity loop, which includes several conserved arginines and lysines in both T7 and 3Dpol, specifically recognizes the promoter and also interacts with the nascent transcript, possibly stabilizing the elongation complex (47). After clustered charged-to-alanine mutation of the corresponding residues in poliovirus, transfection into HeLa cells failed to yield viable virus (10).
A possibly more relevant shift occurs in a stretch of residues in the fingers domain of 3D (3CD residues 226 to 251; 3Dpol residues 43 to 68). This area displays significant variability between the 3CD and the 3Dpol structures, with the main-chain atoms of 3CD shifting up to ∼1.7 Å compared to those of the structure of 3Dpol (Fig. 5a). In 3Dpol, the functions of several residues in this region are well characterized, and their mutations have detrimental effects on polymerase activity. The 3Dpol residue Lys61 appears to be critical, as its mutation eliminates the binding of GTP and polymerase activity (41). Simultaneous charged-to-alanine mutation of 3Dpol residue Lys51-Asp53 or Asp53-Glu55-Glu56 prevents the accumulation of viral RNA (40). Removal of the first 68 amino acid residues of 3Dpol also inactivates the polymerase (48). Conceivably, residues 43 to 68 of 3Dpol may serve as an allosteric switch for polymerase activity that is controlled by the presence or absence of the 3Dpol N terminus.
In a number of viral polymerases, the N terminus and the active site are stabilized via hydrogen bonding networks involving the fingers domain, a conservation suggesting that this binding scheme could be relevant for polymerase activity (Fig. 6) (3, 4, 13). It may also be possible that insertion of the 3D N terminus in its binding pocket serves to modulate activity by regulating molecular flexibility. Indeed, there is precedent by other polymerases for fidelity mutations to occur distantly from the active site, which operate by making their polymerases less flexible (23). We can certainly envision an activity spectrum wherein the molecule that is too inflexible (e.g., G64S) is less efficient than that in the wild type; the molecule that is more flexible (e.g., G1A, due to an imperfect fit) is poorer than that in the wild type; and the molecule that is much too flexible (i.e., 3CD or 3D missing its N-terminal residues) is incapable of supporting viral replication.
Implications for the uridylylation of VPg.
Both 3CD and 3Dpol contribute to the initiation of poliovirus RNA replication. This requires the uridylylation of VPg, wherein two copies of UMP are linked in succession to the hydroxyl group of Tyr3 via phosphodiester bonds (38). Uridylylation is catalyzed by 3Dpol using an RNA template, either from the 3′-poly(A) tail or from cre(2C). Although uridylylation requires only 3D, VPg, and Mg2+, the uridylylation templated by cre(2C) is stimulated 20-fold by the addition of 3CD or 10-fold by the addition of 3Cpro (36, 37) (Fig. 7). Furthermore, the uncleaved precursor 3BC is a ninefold better substrate for uridylylation than 3B alone. The uncleaved precursor 3BCD is also a substrate for uridylylation, albeit a poorer one than 3B or 3BC. Collectively, these observations suggest that domains 3B, 3C, and 3D each have distinct, nonoverlapping binding sites in the biologically relevant uridylylation complex. They also imply that the binding sites must be separated by distances short enough for the available linkers to connect them all together.
FIG. 7.

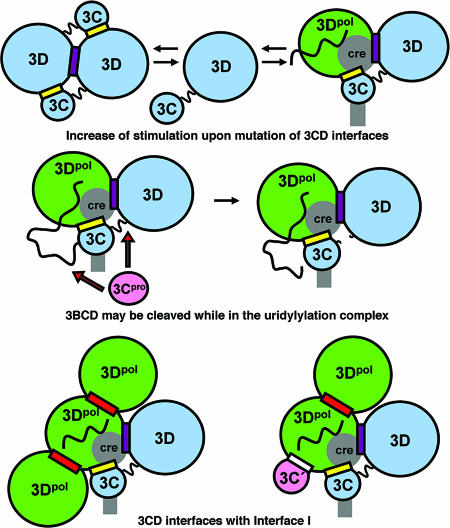
3BCD and its cleavage products: substrates and stimulatory factors for uridylylation. Large ovals represent the viral protein domains; wavy lines represent polypeptide chains. The cre RNA is predicted to have a stem-loop structure and is shown in gray. The purple and yellow rectangles represent the D-D and D-C interfaces, respectively, as they are seen in the 3CD crystal structure (shown in Fig. 5). The binding of additional copies of the 3C and/or 3D domains and the involvement of additional interfaces cannot be ruled out.
Three models for uridylylation have recently been published. Two of the models, based on mutation and computation studies, assume that VPg and the VPg precursor 3AB bind to the same surface on 3Dpol and conclude that VPg enters the polymerase active site from the back of the polymerase molecule (45, 46). In contrast, structural evidence from the FMDV 3Dpol-VPg complex suggests an alternative arrangement, since the last ordered residues at the carboxyl end of VPg are seen to bind to the front face of 3Dpol. The crystal structure of 3CD supports the latter model. The 3CD structure appears to be relevant to the uridylylation reaction, as it suggests how the components of the 3CD-stimulated uridylylation reaction may be arranged in the complex.
VPg binding to FMDV 3Dpol is likely to be highly analogous to the binding in poliovirus 3Dpol, particularly because the FMDV enzyme can use poliovirus VPg as its substrate (31). Additionally, mutations made to PV 3Dpol residues Tyr326, Asp358, and Lys359, which correspond to FMDV residues actively involved in VPg binding, result in an almost complete loss of uridylylation activity (26, 27). In FMDV, the VPg primer appears to enter from the front of the molecule and occupies regions of the polymerase that bind to the template and primer RNA (13). The N terminus of VPg binds a portion of the primer's binding site; the C-terminal end of VPg looks as if it would follow along the path of the exiting RNA duplex product, though only the first 15 residues of VPg are actually visible (12). Conserved residues in the fingers and thumb domains of the polymerase were identified as being responsible for stabilizing VPg in its binding cavity (Fig. 8a).
FIG. 8.
Proposed model of the uridylylation complex. (a) Several poliovirus 3Dpol residues appear to stabilize VPg (red) in its putative binding pocket. Lys172 and Arg179 (green) are critical for stabilizing Tyr3 of VPg in the active site. Residues in blue and red correspond to residues of FMDV that position VPg in its binding pocket. (b) The D-D and D-C interfaces may be responsible for stabilizing interactions between 3BC(D) (yellow and red) and a 3D polymerase molecule (green). VPg (red) has been modeled into the 3D domain using the FMDV-VPg structure as a template (12). These binding interactions might be relevant to the stimulation of VPg uridylylation by either 3C or 3CD or to the binding of uncleaved 3BC or 3BCD as substrates for uridylylation.
In the 3CD crystal structure, each 3D domain forms extensive interfaces with the 3C and 3D domains of a symmetry-related 3CD molecule (Fig. 4a). Several residues, some conserved, appear to be stabilizing these interfaces (Table 2 and Fig. 4b and c). Notably, the N terminus of one 3C domain is located close to the VPg binding site of an adjacent 3D domain of another 3CD molecule, as VPg was seen in the FMDV polymerase complex (12). Assuming that these interfaces are relevant to the 3CD-stimulated uridylylation reaction, it is only possible to connect the VPg binding site to the N terminus of 3C by linking across the front face of 3Dpol (Fig. 8b). As in the FMDV structure, VPg would exit the polymerase on the front of 3Dpol, and there would be no way for a connection on the back face to reach any 3C N terminus.
Insofar as 3BC and 3BCD are both known to be substrates for uridylylation, we looked to see whether the FMDV model for VPg mapped onto the poliovirus 3Dpol structure could plausibly be connected to the N terminus of the 3C domain, and we produced a simple model of such an interaction (Fig. 8). In our model, the VPg portion of 3BCD could bind to the polymerase, where it is then uridylylated prior to or possibly after the cleavage of 3CD or even 3D from 3BC (Fig. 9, middle panel). The 3BCD molecule has been shown to be inefficiently uridylylated, though 3BC is a much better substrate for uridylylation than VPg alone. These biochemical results may suggest that the 3D region is preferentially cleaved prior to uridylylation to accommodate other components of the uridylylation reaction. Additionally, the observation that 3B yields a faster turnover of uridylylated product than 3BCD can possibly be explained by the difficulty that uridylylated 3BCD may have in dissociating from the uridylylation complex. Although we have noticed that an alternative pathway could connect VPg to the N terminus of a different 3CD molecule via the D-C′ interface (Fig. 4a), we do not believe that this 3CD molecule is relevant to the uridylylation reaction: its 3C domain makes less extensive contacts than the D-C interface, and its 3D domain makes no contacts at all.
FIG. 9.
How the structure of the uridylylation complex might affect biological function. Large ovals represent the viral protein domains; wavy lines represent polypeptide chains. The cre RNA is predicted to have a stem-loop structure and is shown in gray. The purple and yellow rectangles represent the D-D and D-C interfaces, respectively, as they are seen in the 3CD crystal structure. Aggregation of 3CD may compete with the formation of the uridylylation complex (top panel). 3CD might dissociate either intact or after it has been cleaved (middle panel). Interfaces D-D and D-C can coexist with interface I (red rectangle, bottom panel, left); however, the formation of interface D-C′ (white rectangle) would be in steric conflict with interface I (bottom panel, right). Interface II (not shown) would conflict with interface D-C.
Importantly, the 3BCD model showing how 3BCD might interact with 3Dpol makes testable predictions about the nature of the enzyme-substrate complexes (3D-3B, 3D-3BC, and 3D-3BCD) and about the complexes wherein uridylylation is stimulated by 3C or 3CD binding (3D-3B-3C and 3D-3B-3CD). Therefore, we have used the 3CD structure and the 3BCD model to identify residues involved in binding at the interface regions (Table 2 and Fig. 4), and mutations have been made to several of these residues. Uridylylation reactions using these 3CD and 3Dpol mutant proteins were used to assess how well the uridylylation reaction could be stimulated in vitro (Table 3). In general, interface mutations made to 3Dpol decreased 3CD-stimulated uridylylation of VPg, whereas mutations made to 3CD led to an increase in stimulation. The finding that uridylylation is consistently affected lends support to our hypothesis that the 3D-3CD contacts identified in the crystal structure are biologically relevant ones. When 3Dpol is mutated, stimulation is reduced, as would be expected if the mutations reduced the extent of the contacts with 3CD. On the other hand, attempts to mutate the interfaces from the 3CD side were less predictable, since equilibria involving the interactions of 3CD molecules with one another would also be affected, perhaps even more strongly (Fig. 9, top panel). The most dramatic effect of a tested mutation was the complete inability of the 3CD K156A mutant at interface D-C to stimulate uridylylation (Table 3). A double mutant that includes K156A showed the same behavior. Previously, it was also found that a 3CD K156E mutant was incapable of forming another regulatory ribonucleoprotein complex that included the 5′ cloverleaf RNA of poliovirus and that mutations made to other residues surrounding K156 in the 3CD structure abolished RNA replication in vivo (2).
Roles of other protein-protein interfaces in the uridylylation complex.
The 3CD protein is poorly behaved at high concentrations. It was difficult to crystallize 3CD due partly to multiple interactions between its component domains. More than one set of these interactions is likely to be biologically significant, affecting 3CD's roles in uridylylation, RNA elongation, and the regulation of transcription and RNA polymerization. Additionally, interactions may vary, depending on the context of higher-order complexes. To further understand what the various multicomponent complexes may look like, it may be fruitful to ask which of the interfaces seen in this and other studies can physically coexist with one another without producing steric clashes.
Previously, much attention has been focused on the head-to-tail stacking of 3Dpol molecules (interface I) that was discovered by Hansen et al. (18) in the 3Dpol crystal structure and which is seen by electron microscopy (26) at high concentrations. Here, residues from the back of the thumb of one 3Dpol molecule (residues Leu446, Pro448, Thr452, Arg455, and Arg456) interact with residues from the back of the palm of a second 3Dpol molecule (including residues Tyr313, Asp339, Ser341, Leu342, Gln345, and Asp349). Although this interaction is not conserved among picornaviruses (which argues that its role may not be crucial), the obvious strength of the interaction does suggest a possible biological role, as interface I has previously been proposed to be involved in elongation (21) and in uridylylation (5, 36). In this report, we have provided additional pieces to the puzzle, showing that the mutations of conserved residues in the D-D and D-C interfaces affect the stimulation of uridylylation and, in particular, that mutations on the 3Dpol side reduce this stimulation (Table 3).
To better understand what structure(s) of the uridylylation complex is possible and as a preliminary step to modeling the complex (A. B. Wass et al., manuscript in preparation), we assessed whether interfaces I, D-D, and D-C can coexist simultaneously within a single molecular complex. Indeed, they can (Fig. 9, bottom panel, left). When a vertical column of head-to-tail 3Dpol domains is modeled, corresponding to the crystal structure (PDB entry 1RDR) described by Hansen et al. (18), and a 3D domain (here representing 3Dpol) from the 3CD crystal structure is superimposed by a least-squares fit, the 3C and 3D domains of the twofold-related 3CD molecule would project laterally from the side of the column. Thus, a central 3Dpol molecule can be decorated simultaneously by two copies of 3Dpol (each bound across interface I) and one copy of 3CD (bound to interfaces D-D and D-C) without any of these additional molecules clashing with one another. The closest approach between the decorations is 9 Å, measured main chain to main chain, which would preclude steric hindrance, though one favorable side-chain interaction can be modeled.
A role should also be considered for the D-C′ interface (Fig. 4a). Here, a second copy of 3C is bound whose N terminus is close enough to the neighboring 3Dpol active site for 3BC's role as a substrate to be modeled. However, we argue against the likelihood of its participation in the uridylylation complex because its 3D domain is not located close enough to participate in a 3BCD complex with 3Dpol. Furthermore, its Lys156 and Thr154 residues are located too distant from 3Dpol to account for their dramatic effect on uridylylation (Table 3). The existence of interface I may also be relevant because its formation would inhibit 3C binding at the D-C′ interface and vice versa, though the other copy of interface I (involving the thumb of 3Dpol) would be unaffected (Fig. 9, bottom panel, right). Regardless of its biological relevance, the ability of 3C to interact with residues from the back of the palm (3Dpol residues Tyr313 and Lys314) might complicate the interpretation of previous mutation studies of interface I (5, 10). Nothing in the present crystal structure supports the prior hypothesis that the back of the thumb is involved in 3C binding at some point (36), but this interaction cannot be ruled out.
Finally, the formation of interface II between 3Dpol molecules, as described by Hansen et al. (18), is sterically incompatible with the formation of interface D-C, though the key binding interactions do not involve identical residues. Central to interface II is Asp89 (3CD residue 272), which is involved in divalent cation binding, while Lys255 (3CD residue 438) lies peripherally in the interface and does not bind directly. In contrast, in the 3CD structure, Asp89 (residue 272) makes no obvious contacts, while Lys255 (residue 438) is central to forming the D-C interface (Fig. 4b). Indeed, it forms a hydrogen bond to the backbone oxygen of Gly155, and mutations in that vicinity have a pronounced effect on uridylylation in vitro (Table 3). Mutation studies of charged residues in 3Dpol (10) have confirmed the likely importance of Lys255 (residue 438) for virus viability (at least in combination with Glu254) and the likely unimportance of Asp89 (residue 272) and interface II. The conservation of Lys255 (residue 438) among the polioviruses, enteroviruses, echoviruses, coxsackieviruses, and certain rhinoviruses is probably relevant.
The poliovirus polymerase facilitates many critical functions of the virus. However, its activity must be regulated. Regulation is provided in the form of 3CD, a precursor with no polymerase activity. The crystal structure of 3CD reveals how the two domains of the protein are tethered together, helps to define the structural requirements for an active polymerase, and provides a model for the uridylylation of VPg. Uridylylation and subsequent replication require the binding of a host of macromolecular components to form large replication complexes. As the structures of the individual components of the replication complex become known, our incomplete picture of catalysis and regulation steadily improves. Our structure of 3CD provides a more complete understanding of how 3CD and 3Dpol function in viral replication and provides testable models for future experiments.
Acknowledgments
We thank Piotr Sliz for assistance with data collection and members of the Hogle and Cameron laboratories for useful advice and critical reading of the manuscript. We thank the staff members of Argonne Advance Photon Source and of the Brookhaven National Synchrotron Light Source for their assistance in data collection. We also thank Todd Parsley and Bert Semler for their previous work with 3CD and generous contribution of reagents and protocols over the years.
This work was supported by NIH grant AI20566 (to J.M.H.) and NIH/NIAID grant AI053531 (to C.E.C.).
Footnotes
Published ahead of print on 24 January 2007.
REFERENCES
- 1.Ago, H., T. Adachi, A. Yoshida, M. Yamamoto, N. Habuka, K. Yatsunami, and M. Miyano. 1999. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Structure 7:1417-1426. [DOI] [PubMed] [Google Scholar]
- 2.Andino, R., G. E. Rieckhof, P. L. Achacoso, and D. Baltimore. 1993. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. EMBO J. 12:3587-3598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appleby, T. C., H. Luecke, J. H. Shim, J. Z. Wu, I. W. Cheney, W. Zhong, L. Vogeley, Z. Hong, and N. Yao. 2005. Crystal structure of complete rhinovirus RNA polymerase suggests front loading of protein primer. J. Virol. 79:277-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold, J. J., M. Vignuzzi, J. K. Stone, R. Andino, and C. E. Cameron. 2005. Remote site control of an active site fidelity checkpoint in a viral RNA-dependent RNA polymerase. J. Biol. Chem. 280:25706-25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boerner, J. E., J. M. Lyle, S. Daijogo, B. L. Semler, S. C. Schultz, K. Kirkegaard, and O. C. Richards. 2005. Allosteric effects of ligands and mutations on poliovirus RNA-dependent RNA polymerase. J. Virol. 79:7803-7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bressanelli, S., L. Tomei, F. A. Rey, and R. De Francesco. 2002. Structural analysis of the hepatitis C virus RNA polymerase in complex with ribonucleotides. J. Virol. 76:3482-3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burns, C. C., M. A. Lawson, B. L. Semler, and E. Ehrenfeld. 1989. Effects of mutations in poliovirus 3Dpol on RNA polymerase activity and on polyprotein cleavage. J. Virol. 63:4866-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi, K. H., J. M. Groarke, D. C. Young, R. J. Kuhn, J. L. Smith, D. C. Pevear, and M. G. Rossmann. 2004. The structure of the RNA-dependent RNA polymerase from bovine viral diarrhea virus establishes the role of GTP in de novo initiation. Proc. Natl. Acad. Sci. USA 101:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crotty, S., M. C. Saleh, L. Gitlin, O. Beske, and R. Andino. 2004. The poliovirus replication machinery can escape inhibition by an antiviral drug that targets a host cell protein. J. Virol. 78:3378-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diamond, S. E., and K. Kirkegaard. 1994. Clustered charged-to-alanine mutagenesis of poliovirus RNA-dependent RNA polymerase yields multiple temperature-sensitive mutants defective in RNA synthesis. J. Virol. 68:863-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Emsley, P., and K. Cowtan. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr. Sect. D 60:2126-2132. [DOI] [PubMed] [Google Scholar]
- 12.Ferrer-Orta, C., A. Arias, R. Agudo, R. Perez-Luque, C. Escarmis, E. Domingo, and N. Verdaguer. 2006. The structure of a protein primer-polymerase complex in the initiation of genome replication. EMBO J. 25:880-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferrer-Orta, C., A. Arias, R. Perez-Luque, C. Escarmis, E. Domingo, and N. Verdaguer. 2004. Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J. Biol. Chem. 279:47212-47221. [DOI] [PubMed] [Google Scholar]
- 14.Gerber, K., E. Wimmer, and A. V. Paul. 2001. Biochemical and genetic studies of the initiation of human rhinovirus 2 RNA replication: identification of a cis-replicating element in the coding sequence of 2Apro. J. Virol. 75:10979-10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerber, K., E. Wimmer, and A. V. Paul. 2001. Biochemical and genetic studies of the initiation of human rhinovirus 2 RNA replication: purification and enzymatic analysis of the RNA-dependent RNA polymerase 3Dpol. J. Virol. 75:10969-10978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gohara, D. W., S. Crotty, J. J. Arnold, J. D. Yoder, R. Andino, and C. E. Cameron. 2000. Poliovirus RNA-dependent RNA polymerase (3Dpol): structural, biochemical, and biological analysis of conserved structural motifs A and B. J. Biol. Chem. 275:25523-25532. [DOI] [PubMed] [Google Scholar]
- 17.Gohara, D. W., C. S. Ha, S. Kumar, B. Ghosh, J. J. Arnold, T. J. Wisniewski, and C. E. Cameron. 1999. Production of “authentic” poliovirus RNA-dependent RNA polymerase (3D(pol)) by ubiquitin-protease-mediated cleavage in Escherichia coli. Protein Expr. Purif. 17:128-138. [DOI] [PubMed] [Google Scholar]
- 18.Hansen, J. L., A. M. Long, and S. C. Schultz. 1997. Structure of the RNA-dependent RNA polymerase of poliovirus. Structure 5:1109-1122. [DOI] [PubMed] [Google Scholar]
- 19.Harris, D., N. Kaushik, P. K. Pandey, P. N. Yadav, and V. N. Pandey. 1998. Functional analysis of amino acid residues constituting the dNTP binding pocket of HIV-1 reverse transcriptase. J. Biol. Chem. 273:33624-33634. [DOI] [PubMed] [Google Scholar]
- 20.Harris, K. S., S. R. Reddigari, M. J. Nicklin, T. Hammerle, and E. Wimmer. 1992. Purification and characterization of poliovirus polypeptide 3CD, a proteinase and a precursor for RNA polymerase. J. Virol. 66:7481-7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hobson, S. D., E. S. Rosenblum, O. C. Richards, K. Richmond, K. Kirkegaard, and S. C. Schultz. 2001. Oligomeric structures of poliovirus polymerase are important for function. EMBO J. 20:1153-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim, T. W., L. G. Brieba, T. Ellenberger, and E. T. Kool. 2006. Functional evidence for a small and rigid active site in a high fidelity DNA polymerase: probing T7 DNA polymerase with variably sized base pairs. J. Biol. Chem. 281:2289-2295. [DOI] [PubMed] [Google Scholar]
- 23.Kool, E. T. 2002. Active site tightness and substrate fit in DNA replication. Annu. Rev. Biochem. 71:191-219. [DOI] [PubMed] [Google Scholar]
- 24.Lawrence, M. C., and P. M. Colman. 1993. Shape complementarity at protein/protein interfaces. J. Mol. Biol. 234:946-950. [DOI] [PubMed] [Google Scholar]
- 25.Love, R. A., K. A. Maegley, X. Yu, R. A. Ferre, L. K. Lingardo, W. Diehl, H. E. Parge, P. S. Dragovich, and S. A. Fuhrman. 2004. The crystal structure of the RNA-dependent RNA polymerase from human rhinovirus: a dual function target for common cold antiviral therapy. Structure 12:1533-1544. [DOI] [PubMed] [Google Scholar]
- 26.Lyle, J. M., E. Bullitt, K. Bienz, and K. Kirkegaard. 2002. Visualization and functional analysis of RNA-dependent RNA polymerase lattices. Science 296:2218-2222. [DOI] [PubMed] [Google Scholar]
- 27.Lyle, J. M., A. Clewell, K. Richmond, O. C. Richards, D. A. Hope, S. C. Schultz, and K. Kirkegaard. 2002. Similar structural basis for membrane localization and protein priming by an RNA-dependent RNA polymerase. J. Biol. Chem. 277:16324-16331. [DOI] [PubMed] [Google Scholar]
- 28.McCoy, A. J., R. W. Grosse-Kunstleve, L. C. Storoni, and R. J. Read. 2005. Likelihood-enhanced fast translation functions. Acta Crystallogr. Sect. D 61:458-464. [DOI] [PubMed] [Google Scholar]
- 29.Mosimann, S. C., M. M. Cherney, S. Sia, S. Plotch, and M. N. James. 1997. Refined X-ray crystallographic structure of the poliovirus 3C gene product. J. Mol. Biol. 273:1032-1047. [DOI] [PubMed] [Google Scholar]
- 30.Murshudov, G. N., A. A. Vagin, and E. J. Dodson. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. Sect. D 53:240-255. [DOI] [PubMed] [Google Scholar]
- 31.Nayak, A., I. G. Goodfellow, and G. J. Belsham. 2005. Factors required for the uridylylation of the foot-and-mouth disease virus 3B1, 3B2, and 3B3 peptides by the RNA-dependent RNA polymerase (3Dpol) in vitro. J. Virol. 79:7698-7706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ng, K. K., M. M. Cherney, A. L. Vazquez, A. Machin, J. M. Alonso, F. Parra, and M. N. James. 2002. Crystal structures of active and inactive conformations of a caliciviral RNA-dependent RNA polymerase. J. Biol. Chem. 277:1381-1387. [DOI] [PubMed] [Google Scholar]
- 33.Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode, p. 307-326. In J. C. W. Carter and R. M. Sweet (ed.), Methods in enzymology, vol. 276. Academic Press, New York, NY. [DOI] [PubMed] [Google Scholar]
- 34.Parsley, T. B., C. T. Cornell, and B. L. Semler. 1999. Modulation of the RNA binding and protein processing activities of poliovirus polypeptide 3CD by the viral RNA polymerase domain. J. Biol. Chem. 274:12867-12876. [DOI] [PubMed] [Google Scholar]
- 35.Parsley, T. B., J. S. Towner, L. B. Blyn, E. Ehrenfeld, and B. L. Semler. 1997. Poly (rC) binding protein 2 forms a ternary complex with the 5′-terminal sequences of poliovirus RNA and the viral 3CD proteinase. RNA 3:1124-1134. [PMC free article] [PubMed] [Google Scholar]
- 36.Pathak, H. B., S. K. Ghosh, A. W. Roberts, S. D. Sharma, J. D. Yoder, J. J. Arnold, D. W. Gohara, D. J. Barton, A. V. Paul, and C. E. Cameron. 2002. Structure-function relationships of the RNA-dependent RNA polymerase from poliovirus (3Dpol). A surface of the primary oligomerization domain functions in capsid precursor processing and VPg uridylylation. J. Biol. Chem. 277:31551-31562. [DOI] [PubMed] [Google Scholar]
- 37.Paul, A. V., E. Rieder, D. W. Kim, J. H. van Boom, and E. Wimmer. 2000. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. J. Virol. 74:10359-10370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paul, A. V., J. H. van Boom, D. Filippov, and E. Wimmer. 1998. Protein-primed RNA synthesis by purified poliovirus RNA polymerase. Nature 393:280-284. [DOI] [PubMed] [Google Scholar]
- 39.Pfeiffer, J. K., and K. Kirkegaard. 2003. A single mutation in poliovirus RNA-dependent RNA polymerase confers resistance to mutagenic nucleotide analogs via increased fidelity. Proc. Natl. Acad. Sci. USA 100:7289-7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Plotch, S. J., O. Palant, and Y. Gluzman. 1989. Purification and properties of poliovirus RNA polymerase expressed in Escherichia coli. J. Virol. 63:216-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richards, O. C., S. Baker, and E. Ehrenfeld. 1996. Mutation of lysine residues in the nucleotide binding segments of the poliovirus RNA-dependent RNA polymerase. J. Virol. 70:8564-8570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rieder, E., A. V. Paul, D. W. Kim, J. H. van Boom, and E. Wimmer. 2000. Genetic and biochemical studies of poliovirus cis-acting replication element cre in relation to VPg uridylylation. J. Virol. 74:10371-10380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rothstein, M. A., O. C. Richards, C. Amin, and E. Ehrenfeld. 1988. Enzymatic activity of poliovirus RNA polymerase synthesized in Escherichia coli from viral cDNA. Virology 164:301-308. [DOI] [PubMed] [Google Scholar]
- 44.Sarkany, Z., and L. Polgar. 2003. The unusual catalytic triad of poliovirus protease 3C. Biochemistry 42:516-522. [DOI] [PubMed] [Google Scholar]
- 45.Schein, C. H., N. Oezguen, D. E. Volk, R. Garimella, A. Paul, and W. Braun. 2006. NMR structure of the viral peptide linked to the genome (VPg) of poliovirus. Peptides 27:1676-1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tellez, A. B., S. Crowder, J. F. Spagnolo, A. A. Thompson, O. B. Peersen, D. L. Brutlag, and K. Kirkegaard. 2006. Nucleotide channel of RNA-dependent RNA polymerase used for intermolecular uridylylation of protein primer. J. Mol. Biol. 357:665-675. [DOI] [PubMed] [Google Scholar]
- 47.Temiakov, D., P. E. Mentesana, K. Ma, A. Mustaev, S. Borukhov, and W. T. McAllister. 2000. The specificity loop of T7 RNA polymerase interacts first with the promoter and then with the elongating transcript, suggesting a mechanism for promoter clearance. Proc. Natl. Acad. Sci. USA 97:14109-14114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson, A. A., and O. B. Peersen. 2004. Structural basis for proteolysis-dependent activation of the poliovirus RNA-dependent RNA polymerase. EMBO J. 23:3462-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiang, W., K. S. Harris, L. Alexander, and E. Wimmer. 1995. Interaction between the 5′-terminal cloverleaf and 3AB/3CDpro of poliovirus is essential for RNA replication. J. Virol. 69:3658-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]