Abstract
Recombinant adenoviral vectors have been widely used for gene therapy applications and as vaccine vehicles for treating infectious diseases such as human immunodeficiency virus disease. The innate immune response to adenoviruses represents the most significant hurdle in clinical application of adenoviral vectors for gene therapy, but it is an attractive feature for vaccine development. How adenovirus activates innate immunity remains largely unknown. Here we showed that adenovirus elicited innate immune response through the induction of high levels of type I interferons (IFNs) by both plasmacytoid dendritic cells (pDCs) and non-pDCs such as conventional DCs and macrophages. The innate immune recognition of adenovirus by pDCs was mediated by Toll-like receptor 9 (TLR9) and was dependent on MyD88, whereas that by non-pDCs was TLR independent through cytosolic sensing of adenoviral DNA. Furthermore, type I IFNs were pivotal in innate and adaptive immune responses to adenovirus in vivo, and type I IFN blockade diminished immune responses, resulting in more stable transgene expression and reduction of inflammation. These findings indicate that adenovirus activates innate immunity by its DNA through TLR-dependent and -independent pathways in a cell type-specific fashion, and they highlight a critical role for type I IFNs in innate and adaptive immune responses to adenoviral vectors. Our results that suggest strategies to interfere with type I IFN pathway may improve the outcome of adenovirus-mediated gene therapy, whereas approaches to activate the type I IFN pathway may enhance vaccine potency.
Adenoviridae are nonenveloped, double-stranded DNA (dsDNA) viruses with a genome of 35 to 40 kb. Replication-defective recombinant adenoviruses have been studied extensively and developed as vehicles for gene therapy applications. This is in great part due to the high efficiency with which they transfer genes into a wide spectrum of nondividing cells in vivo (53). However, the enthusiasm for use of adenoviral vectors in gene therapy has been tempered by significant problems of attendant host immune responses that limit their safety and efficacy in vivo (53). The experience with first-generation E1-deleted adenoviral vectors in various animal models and in human clinical trials has consistently demonstrated that transgene expression from adenoviral vectors in vivo usually is extinguished within 2 to 3 weeks, concurrent with the development of inflammation (9, 30, 60). This is caused by the rapid activation of potent CD8+ and CD4+ T-cell responses against both the viral antigens and the transgene (9, 56, 59). In addition, activation of B cells by viral capsid proteins, leading to the production of neutralizing antibodies, limits effective readministration of the vector (9, 57). Interestingly, the inherent immunogenicity of recombinant adenoviruses has led to their development as vaccine vehicles for infectious diseases, such as human immunodeficiency virus disease, and cancer (4, 50).
Adenoviral vectors can also effectively elicit the innate immune response immediately after infection, leading to the secretion of proinflammatory cytokines and chemokines in mice, humans, and nonhuman primates (45, 48, 61). Activation of innate immunity is associated with a reduction in efficacy of gene transfer (54, 61) but also in profound damage to healthy tissue and significant morbidity in transduced hosts (45, 48). Newer generations of helper-dependent, gutted adenoviral vectors, which are deleted of almost all viral coding sequences (44), have diminished the adaptive immune responses to these vectors and improved the duration of gene transfer (42). However, acute toxicity and diminished vector persistence provoked by the innate immune response remains the most significant barrier associated with clinical application of this otherwise promising technology (6, 42). Therefore, to improve the safety, efficacy, and duration of gene transfer by adenoviral vectors, it is necessary to understand the mechanism(s) by which adenovirus triggers innate immune response. On the other hand, a clear understanding of how adenovirus activates the innate immune response will help us design effective vaccines.
The innate immune system is phylogenetically conserved and is present in almost all multicellular organisms (20). It is the first line of defense against invading pathogens through recognition of conserved microbial structures or products known as pathogen-associated molecular patterns (PAMPs) by a set of receptors called pattern recognition receptors (2). The best-studied family of pattern recognition receptors is the Toll-like receptors (TLRs) that are expressed on various immune cells, including macrophages and dendritic cells (DCs). So far, 13 TLRs have been identified in mammals, and each TLR appears to recognize a unique set of PAMPs that are distinct in chemical structure, such as peptidoglycan (PGN) (TLR2 ligand), dsRNA (TLR3 ligand), lipopolysaccharide (LPS) (TLR4 ligand), and CpG DNA (TLR9 ligand) (2). Upon recognition of PAMPs, TLRs trigger a series of signaling cascades leading to induction of antimicrobial genes and inflammatory cytokines, which results in direct killing of the invading pathogens (2) as well as promoting the initiation of adaptive immune responses (24). In addition, emerging evidence supports the existence of TLR-independent innate immune recognition of pathogens (28).
In this study, we describe a molecular mechanism by which adenovirus elicits innate immune response. We found that adenovirus induced the production of high levels of type I interferons (IFNs) both in vitro and in vivo. The production of type I IFNs by plasmacytoid DCs (pDCs) was mediated by TLR9 in a MyD88-dependent manner, whereas that by non-pDCs such as conventional DCs (cDCs) and macrophages was TLR independent through cytosolic detection of adenoviral DNA. More importantly, we demonstrated that adenovirus-induced type I IFNs were critical for both innate and adaptive immune responses against adenoviral vectors in vivo. Functional blockade of type I IFNs in mice diminished both innate and adaptive immune responses to adenovirus, which resulted in more stable transgene expression and reduction of inflammation in vivo.
MATERIALS AND METHODS
Mice.
C57BL/6 mice were purchased from the Jackson Laboratory. TLR9−/− (18), MyD88−/− (1), and TRIF−/− (55) mice on the C57BL/6 background were kindly provided by Shizuo Akira (Osaka University, Osaka, Japan). IFN-αβR−/− mice (41) on the 129/Sv background and their normal control 129/Sv mice were obtained from B & K Universal. Groups of 6- to 8-week-old mice were selected for this study. All experiments involving the use of mice were done in accordance with protocols approved by the Animal Care and Use Committee of Duke University.
DC culture.
Granulocyte-macrophage colony-stimulating factor (GM-CSF) DCs were generated as described previously (58). Briefly, bone marrow cells were harvested from femurs and tibiae of mice and cultured in the presence of murine GM-CSF (1,000 U/ml) and IL-4 (500 U/ml) (R & D Systems). On day 5, DCs were stimulated with various agents, and at different time points after stimulation, culture supernatants were measured for cytokine secretion by enzyme-linked immunosorbent assay (ELISA). For generation of Flt-3 ligand (Flt-3L) pDCs, bone marrow cells were cultured in the presence of 200 ng/ml of Flt-3L (R & D Systems) for 9 days as described previously (13).
Adenoviruses.
Recombinant E1- and E3-deleted adenovirus encoding LacZ (Ad-lacZ) or green fluorescent protein (Ad-GFP) under the control of the cytomegalovirus promoter was generated by homologous recombination in Escherichia coli as described previously (16). Virus was grown in 293 cells, purified by two rounds of CsCl density centrifugation, and desalted by gel filtration through a Sephadex G-25 column (PD-10 column; Amersham Bioscience) as described previously (56). The titer of virus was determined as PFU by plaque formation assay on 293 cells (14). The viral particle-to-PFU ratio was about 50:1. UV-inactivated adenoviruses were prepared as described previously (57). Briefly, purified virus was resuspended in 0.33 mg/ml 8-methoxypsoralen (Sigma) and exposed to a 365-nm long-UV lamp (model UVGL-25; UVP, Inc) on ice for 1 h. The virus sample was then passed over a Sephadex G-25 column and stored at −70°C until use. Limiting-dilution transduction assays on inactivated viral stocks showed less than one functional virus per 108 particles of inactivated virus. Endotoxin concentrations in all viral preparations were below the detection levels of a Limulus amebocyte lysate QCL-1000 kit (Cambrex Bioscience) that detects up to 0.1 endotoxin unit/ml.
TLR ligands.
LPS (Escherichia coli O26:B6) was purchased from Sigma and used at 100 ng/ml for DC stimulation in vitro and at 30 μg for in vivo infusion, PGN (Staphylococcus aureus) was also obtained from Sigma and used at 10 μg/ml for DC stimulation, and phosphorothioate-stabilized CpG oligodeoxynucleotide (ODN) (5′-TCCATGACGTTCCTGATGCT-3′) (Integrated DNA Technologies) was used at 1 nM.
Antibodies, flow cytometry, and ELISA.
Fluorescein isothiocyanate (FITC)-conjugated B220, biotinylated anti-CD11, and streptavidin-Cychrome were purchased from BD Biosciences, FITC-conjugated F4/80 was obtained from Serotec, and phycoerythrin-conjugated anti-mPDCA-1 was purchased from Miltenyi Biotec. FACScan (BD Biosciences) was used for flow cytometry event collection, and events were analyzed using CELLQuest software (BD Biosciences). Interleukin-1β (IL-1β), IL-6, IL-12p70, monocyte chemoattractant protein 1, and tumor necrosis factor alpha ELISA kits were purchased from Endogen Pierce. A mouse macrophage inflammatory protein 2 kit was purchased from R&D. IFN-α and IFN-β kits were obtained from PBL Biomedical Laboratories.
Isolation of splenic DCs, peritoneal macrophages, and hepatic Kupffer cells.
Splenic DC isolation was performed as described previously (58). After perfusion with Liberase CI (Roche Biochemicals), single-cell suspensions were subjected to a 30% bovine serum albumin gradient, and the interface DC fraction was collected and stained with anti-B220-FITC and anti-CD11c-biotin followed by streptavidin-microbeads (Miltenyi Biotec). CD11c+ DCs were purified by positive selection by microbeads and subjected to fluorescence-activated cell sorting (FACS) into pDCs (CD11c+ B220+) and cDCs (CD11c+ B220−) with the FACS StarPLUS system (BD Biosciences). Macrophages were isolated from the peritoneal cavities of mice 3 days after intraperitoneal injection of 2.5 ml of 3% thioglycolate as described previously (39). Kupffer cells were isolated from mouse livers as described previously (35). After perfusion in situ via the portal vein with collagenase, single-cell suspensions were subjected to gradient centrifugation with 11.5% OptiPrep solution. Kupffer cell fraction was collected from the interface and purified by FACS sorting for F4/80-positive cells.
Adenoviral DNA transfection.
Adenoviral genomic DNA was extracted from purified adenovirus as described previously (60) and was free of endotoxin. For DC transfection, day 5 GM-CSF DCs were incubated for 18 h with 0, 0.2, 1, or 5 μg/ml of adenoviral DNA mixed with Lipofectamine 2000 (Invitrogen) prepared according to the manufacturer's instructions.
In vivo delivery of recombinant adenovirus and antibody blocking.
Ad-lacZ at 2 × 109 PFU in 0.1 ml of phosphate-buffered saline was injected into mice intravenously as described previously (56). Mice were sacrificed at the indicated time points for histological and immunological assays. All animals that received recombinant virus survived to the time of necropsy. For in vivo blocking of type I IFNs, mice were intraperitoneally administered neutralizing antibodies to mouse IFN-α and IFN-β (10,000 IU each; PBL Biomedical Laboratories) 6 h prior to infusion of Ad-lacZ and on day 5 after infection. The neutralizing activity of these antibodies was verified in a cytopathic effect inhibition assay with elicitation by IFN-α or IFN-β as described previously (46).
Adenoviral DNA quantitative real-time PCR.
Total genomic DNA was isolated from the liver tissue as described previously (60). Real-time quantitative PCR was used to measure the amount of adenoviral genomic DNA in liver by using primers and a probe (Integrated DNA Technologies) located in the fiber gene. The sequences of the forward and reverse primers were 5′-CCACCGATAGCAGTACCCTT-3′ and 5′-GACCAGTTGCTACGGTCAAA-3′, respectively. The sequence of the probe was 5′-6-carboxyfluorescein-TGCCCAAGCTACCAGTGGCAGT-6-carboxytetramethylrhodamine-3′.
Proliferation assay.
Splenocytes (2 × 105) were cultured in graded doses (multiplicity of infection [MOI] of 1, 10, or 100) of Ad-lacZ for 72 h in 200 μl of RPMI 1640 medium supplemented with 10% fetal bovine serum, 2 mM l-glutamine, and 50 μM β-mercaptoethanol in 96-well U-bottom tissue culture plates in triplicates. Cultures were pulsed with 1 μCi per well of [3H]thymidine. At 16 to 20 h after [3H]thymidine pulsing, the plates were harvested using a 96-well cell harvester and the [3H]thymidine incorporation was counted using a 1205 Betaplate liquid scintillation counter (Wallac, Gaithersburg, MD).
CTL assay.
Cytotoxic T-lymphocyte (CTL) assay was performed as described previously (56). In brief, splenocytes were restimulated in vitro with Ad-lacZ at an MOI of 1 for 5 days, and a standard 6-h chromium-51 (51Cr) release assay was performed using different ratios of effector to target cells (C57SV, H-2b; provided by Hildegund Ertl). The percentage of specific 51Cr release was calculated as 100 × [(cpm of sample − cpm of spontaneous release)/(cpm of maximal release − cpm of spontaneous release)]. Spontaneous release was determined by culturing target cells in medium only, and maximal release was established by culturing target cells in 1% sodium dodecyl sulfate.
X-Gal histochemistry and histopathology.
Sections of fresh-frozen tissue (5 μm) were stained for LacZ expression by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining as described previously (56). Paraffin sections (5 μm) were stained with hematoxylin and eosin (H&E). Random sections were evaluated in a blinded fashion for histopathology.
Neutralizing antibody assay.
The neutralizing antibody titer in the serum was measured as described previously (57). Briefly, serum samples were incubated at 56°C for 30 min and then diluted in Dulbecco modified Eagle medium in twofold steps starting from 1:20 or 1:50. Each serum dilution (100 μl) was mixed with Ad-lacZ (2 × 106 PFU in 20 μl), incubated for 1 h at 37°C, and applied to 80% confluent HeLa cells in 96-well plates (2 × 104 cells per well). After 60 min of incubation at 37°C, 100 μl of Dulbecco modified Eagle medium containing 20% fetal bovine serum was added to each well. Cells were fixed and analyzed for LacZ expression by X-Gal staining on the following day as described previously (57). All of the cells stained blue in the absence of serum samples. The titer of neutralizing antibody for each sample was reported as the highest dilution with which fewer than 50% of cells stained blue.
Statistical analysis.
Results are expressed as means ± standard deviations (SD). Differences between groups were examined for statistical significance using the Student t test.
RESULTS
Adenovirus induces high levels of type I IFNs in vitro and in vivo.
As DCs play a pivotal role in detecting pathogens and activating innate immune responses (28), we first examined cytokines secreted by DCs upon adenoviral infection in vitro. DCs were generated from bone marrow cells in the presence of GM-CSF (GM-CSF DCs) and stimulated with recombinant adenoviral vector encoding LacZ (Ad-lacZ) at an MOI of 250. The dose chosen was based on our dose-response studies showing high levels of cytokine secretion without toxicity (data not shown). At 1, 6, 12, or 18 h later, supernatants were analyzed for secretion of proinflammatory cytokines and chemokines, i.e., IL-6, IL-12, IL-1, TNF-α, macrophage inflammatory protein 2, and monocyte chemoattractant protein 1. Because viral infection often induces type I IFNs, we also measured secretion of IFN-α and IFN-β. We found that the levels of these cytokines and chemokines increased over time and appeared to level off at around 18 h after infection (data not shown). We then compared the profile of cytokines elicited by adenovirus at 18 h after infection with those elicited by different TLR ligands. DCs infected with Ad-lacZ produced much higher levels of type I IFNs, including IFN-α and IFN-β, and lower levels of proinflammatory cytokines such as IL-6 and IL-12 than those stimulated with PGN (TLR2 ligand), LPS (TLR4 ligand), or CpG (TLR9 ligand) (Fig. 1A). The induction of cytokine production by Ad-lacZ appeared to be independent of newly synthesized viral gene products after infection, as similar levels of cytokines were induced by UV-inactivated Ad-lacZ (Fig. 1A). These results indicated that adenovirus induced GM-CSF DCs to secrete high levels of type I IFNs compared to TLR ligands.
FIG. 1.
Induction of mainly type I IFNs upon adenoviral infection. (A) GM-CSF DCs were generated from C57BL/6 bone marrow cells in the presence of GM-CSF and IL-4 for 5 days. On day 5, DCs (1 × 106 cells/ml) were either unstimulated (medium) or stimulated with PGN, LPS, CpG ODN (CpG), Ad-lacZ (Ad) or UV-inactivated Ad-lacZ (UV-Ad) for 18 h. Culture supernatants were analyzed by ELISA for secretion of IL-6, IL-12, IFN-α, and IFN-β. (B) Mice were administered intravenously with Ad-lacZ (Ad) or LPS. Serum samples were harvested 6 h later and analyzed for secretion of IL-6 and IFN-α.
We next examined whether adenovirus also induced high levels of type I IFNs in vivo upon adenoviral infection. Mice were injected with 5 × 109 PFU (based on in vivo dose-response studies [data not shown]) of Ad-lacZ intravenously, and at 1, 6, 12, or 18 h later, sera were harvested and assayed for IL-6 and IFN-α. In contrast to the in vitro cytokine kinetics, the serum levels of IL-6 peaked at 6 h, whereas those of IFN-α peaked at 12 h after infection (data not shown). This difference in cytokine kinetics could be due to a different cytokine clearance in vivo. Mice were then injected with Ad-lacZ or LPS intravenously, and serum IL-6 and IFN-α were measured at 6 h (the peak level for IL-6) after infection. Consistent with the in vitro data, Ad-lacZ also induced high levels of type I IFNs in vivo in comparison with LPS (Fig. 1B).
Endogenous pDCs produce higher levels of type I IFNs than non-pDCs.
We next tested which cell type(s) was responsible for the production of type I IFNs upon adenoviral infection in vivo. Endogenous DCs are roughly divided into two subsets, pDCs and cDCs, which are identified as CD11c+ B220+ and CD11c+ B220−, respectively (7, 51). Studies have shown that pDCs produce large amounts of type I IFNs in response to various viral infections in vivo (7, 51). Also, cDCs, macrophages, and Kupffer cells have been implicated in the production of cytokines upon adenoviral infection in vivo (36, 61). We thus compared the capacities of endogenous pDCs, cDCs, macrophages, and Kupffer cells to secrete type I IFNs upon adenoviral infection. Splenic pDCs and cDCs were purified by FACS to purities of 94% and 98%, respectively (Fig. 2A). The properties of these DCs were further confirmed by staining with a pDC-specific antibody, mPDCA-1 (7), which identified pDCs as mPDCA-1+ and cDCs as mPDCA-1− (Fig. 2A). The Kupffer cells (F4/80+) were isolated from the liver with a purity of 96% (Fig. 2B). Purified pDCs, cDCs, Kupffer cells, and peritoneal macrophages were stimulated with Ad-lacZ and assayed for the secretion of IFN-α and IFN-β. Our data indicated that freshly isolated, endogenous pDCs produced much higher levels of type I IFNs than non-pDCs upon adenoviral infection (Fig. 2C). To investigate if the higher levels of type I IFNs produced by pDCs than by cDCs were due to a more efficient transduction of pDCs by adenovirus, we infected pDCs or cDCs with Ad-GFP or Ad-lacZ at an MOI of 250 and evaluated the expression of GFP 24 h later by FACS. Our results showed that efficiency of transduction by adenovirus was much higher in cDCs than in pDCs (Fig. 2D), suggesting that the unique ability of pDCs to produce type I IFNs was not a result of higher efficiency of transduction upon adenoviral infection.
FIG. 2.
Production of type I IFNs by pDCs versus non-pDCs in response to adenoviral infection. (A) Isolation of splenic pDCs and cDCs. Splenocytes from C57BL/6 mice were enriched for CD11c+ DCs by a bovine serum albumin gradient and microbeads, and the CD11c+ DC-enriched fraction was then stained with anti-CD11c and anti-B220 and sorted by FACS into pDCs and cDCs. Aliquots of sorted cells (postsort) were analyzed by FACS to determine the purity of pDCs (CD11c+ B220+ mPDCA-1+) and cDCs (CD11c+ B220− mPDCA-1−). (B) Isolation of hepatic Kupffer cells. Kupffer cells were enriched from mouse livers after collagenase perfusion and gradient centrifugation. The Kupffer cell-enriched fraction was then stained with antibody to F4/80, a Kupffer cell marker, and subjected to FACS sorting. The FACS plot indicates the purity of Kupffer cells after sorting. (C) Splenic pDCs, cDCs, hepatic Kupffer cells (KC), or peritoneal macrophages (MΦ) at 2.5 × 105 cells/ml were either unstimulated (medium) or stimulated with Ad-lacZ (Ad) at an MOI of 250 for 18 h, and the supernatants were measured for IFN-α and IFN-β. (D) Transduction of pDCs and cDCs by adenovirus. Purified pDCs and cDCs were infected with Ad-GFP or Ad-lacZ at an MOI of 250, and 24 h later cells were analyzed for GFP expression by FACS. The percentage of GFP+ cells is indicated.
pDC recognition of adenovirus is mediated by TLR9 and dependent on MyD88.
We next examined if TLRs were involved in induction of high levels of type I IFNs by pDCs in response to adenovirus. Since all TLR signaling is mediated by MyD88 and/or TRIF (28), freshly isolated pDCs from mice deficient for MyD88 (MyD88−/−) or TRIF (TRIF−/−) were tested for their ability to produce IFN-α upon adenoviral infection. IFN-α production by MyD88−/− pDCs was abolished, whereas that by TRIF−/− pDCs was not affected (Fig. 3A). This result indicated that type I IFN production by pDCs in response to adenovirus was TLR mediated and dependent on MyD88.
FIG. 3.
Innate immune recognition of adenovirus by pDCs is mediated by TLR9 and dependent on MyD88. (A) Splenic pDCs purified from WT, MyD88−/−, or TRIF−/− C57BL/6 mice were stimulated with Ad-lacZ at an MOI of 250 for 18 h at 2.5 × 105 cells/ml and assayed for the secretion of IFN-α. (B) Splenic pDCs purified from WT or TLR9−/− C57BL/6 mice were stimulated with Ad-lacZ at an MOI of 250 or CpG ODN (CpG) for 18 h at 2.5 × 105 cells/ml and assayed for the secretion of IFN-α. (C) pDCs (5 × 105 cells/ml) generated from bone marrow cells of WT, MyD88−/−, TRIF−/−, or TLR9−/− C57BL/6 mice in the presence of Flt-3L were stimulated with Ad-lacZ at an MOI of 250 for 18 h and assayed for the secretion of IFN-α. (D) WT, MyD88−/−, or TLR9−/− mice were administered Ad-lacZ intravenously. Serum samples were harvested from these mice 12 h later and analyzed for secretion of IFN-α. Serum from uninfected naïve mice was used as a control.
We then investigated which TLR mediated type I IFN production by pDCs. Among all TLRs characterized to date, only TLR7, TLR8, and TLR9 are known to mediate MyD88-dependent production of type I IFNs (28). Since the known ligand for TLR7 and TLR8 is single-stranded RNA and adenovirus is a dsDNA virus, we hypothesized that a likely candidate to mediate induction of type I IFNs by pDCs was TLR9. To test this, we examined whether pDCs lacking TLR9 (TLR9−/−) secreted type I IFNs upon adenoviral infection. Similar to pDCs stimulated with TLR9 ligand, CpG (Fig. 3B), IFN-α production by TLR9−/− pDCs stimulated with adenovirus was abolished (Fig. 3B). These observations indicated that adenovirus-induced type I IFN production by pDCs was mediated through TLR9.
We further confirmed the above observations using pDCs generated from bone marrow precursors in the presence of Flt-3L (13). Similar to the observations with freshly isolated splenic pDCs, IFN-α induction by adenovirus was also abolished in MyD88−/− or TLR9−/− bone marrow pDCs compared to the wild-type (WT) control (Fig. 3C). Taking the observations together, we conclude that innate immune recognition of adenovirus by pDCs is mediated by TLR9 and dependent on MyD88.
We next sought to study the in vivo relevance of TLR9- and MyD88-dependent secretion of type I IFNs by pDCs in response to adenoviral infection. Mice were injected with 2 × 109 PFU of Ad-lacZ intravenously, and 12 h later, sera were harvested and assayed for IFN-α. Compared to that in the WT mice, the secretion of IFN-α was reduced, but not abolished, in MyD88−/− or TLR9−/− mice (Fig. 3D). This result suggested that TLR9- and MyD88-dependent recognition of adenovirus by pDCs only partially contributed to the production of type I IFNs upon adenoviral infection in vivo.
Non-pDC recognition of adenovirus is TLR independent.
Only partial reduction of IFN-α in MyD88−/− or TLR9−/− mice in vivo (Fig. 3D) suggested that innate recognition of adenovirus by non-pDCs might be mediated through a different pathway. To address this question, we first examined whether TLRs were involved in recognition of adenovirus by cDCs. In contrast to IFN-α production by pDCs, IFN-α production by MyD88−/− or TLR9−/− cDCs stimulated with Ad-lacZ was not significantly different from that by WT or TRIF−/− cDCs (Fig. 4A). Similarly, we found that IFN-α secretion by MyD88−/−, TLR9−/−, or TRIF−/− hepatic Kupffer cells (Fig. 4B), peritoneal macrophages (Fig. 4C), and GM-CSF DCs (Fig. 4D) was not significantly different from that by their respective WT controls. These results showed that non-pDC recognition of adenovirus is TLR independent.
FIG. 4.
Innate immune recognition of adenovirus by non-pDCs is TLR independent. (A to C) Splenic cDCs (A), hepatic Kupffer cells (B), and peritoneal macrophages (C) purified from WT, MyD88−/−, TRIF−/−, or TLR9−/− mice were stimulated with Ad-lacZ at an MOI of 250 for 18 h at 2.5 × 105 cells/ml and assayed for secretion of IFN-α. (D) GM-CSF DCs generated from bone marrow cells of WT, MyD88−/−, TRIF−/−, or TLR9−/− mice in the presence of GM-CSF and IL-4 were stimulated with Ad-lacZ at an MOI of 250 for 18 h at 1 × 106 cells/ml and assayed for secretion of IFN-α.
The TLR-independent pathway is triggered by cytosolic recognition of adenoviral DNA.
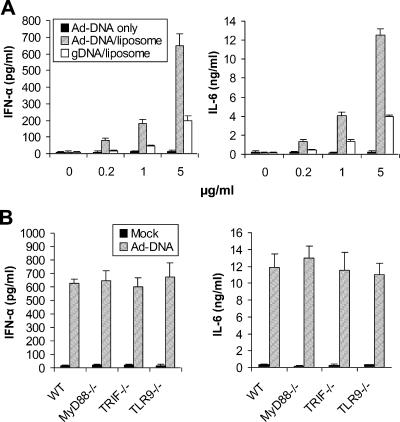
What, then, triggered TLR-independent recognition of adenovirus? It has been recently shown that dsRNA and dsDNA viruses can activate innate immunity through a cytosolic recognition system independent of TLRs, leading to production of type I IFNs (23, 29). Although dsRNA can potentially be generated during adenoviral infection, our observation that UV-inactivated adenovirus induced similar levels of cytokines in GM-CSF DCs (Fig. 1A) makes dsRNA transcripts a less likely candidate. We thus hypothesized that adenoviral DNA could also activate a TLR-independent pathway. To test this, we examined whether delivery of adenoviral DNA to cytosol of GM-CSF DCs by liposome-mediated transfection elicited IFN-α and IL-6. Indeed, both IFN-α and IL-6 were induced in a dose-dependent manner upon transfection of adenoviral DNA (Fig. 5A). Consistent with the previous observations (23, 43), both IFN-α and IL-6 could also be induced by murine genomic DNA, albeit at significantly (P < 0.01) reduced levels compared to adenoviral DNA (Fig. 5A). No significant production of IFN-α or IL-6 over background was detected when GM-CSF DCs were stimulated with purified “naked” adenoviral DNA alone (Fig. 5A), suggesting that DNA delivery to the cytosol is critical for cytokine induction. Furthermore, induction of cytokines by cytosolic recognition of adenoviral DNA was TLR independent, as MyD88−/−, TRIF−/−, or TLR9−/− GM-CSF DCs produced amounts of IFN-α and IL-6 similar to those produced by WT controls (Fig. 5B).
FIG. 5.
Cytosolic recognition of adenoviral DNA triggers a TLR-independent pathway. (A) GM-CSF DCs at 1 × 106 cells/ml were incubated with “naked” adenoviral DNA (Ad-DNA only), an adenoviral DNA-liposome mix (Ad-DNA/liposome), or a mouse genomic DNA-liposome mix (gDNA/liposome) at a final DNA concentration of 0, 0.2, 1, or 5 μg/ml for 18 h. The supernatants were measured for IFN-α and IL-6 by ELISA. (B) GM-CSF DCs generated from WT, MyD88−/−, TRIF−/−, or TLR9−/− mice were either mock transfected or transfected with 5 μg/ml of adenoviral DNA/liposome mix (Ad DNA) for 18 h and assayed for IFN-α and IL-6.
Type I IFNs are critical for innate and adaptive immune responses to adenoviral vectors.
Induction of high levels of type I IFNs through TLR-dependent and -independent pathways suggested that type I IFNs may play an important role in innate immunity to adenoviral vectors in vivo. To address this, we first examined whether production of proinflammatory cytokines such as IL-6 and IL-12 was dependent on type I IFNs. Ad-lacZ was injected intravenously into WT mice or mice deficient for the IFN-α and IFN-β receptor (IFN-αβR−/−), and 6 h later, sera were measured for IL-6 and IL-12. Our results showed that the production of IL-6 and IL-12 was significantly (P < 0.001) diminished in IFN-αβR−/− mice (Fig. 6A), suggesting that type I IFNs were required for efficient induction of proinflammatory cytokines in vivo. We next investigated the role of type I IFNs in the innate immune defense against adenovirus. Previous studies in a variety of animal models have shown that liver was the primary target for gene transfer mediated by adenoviral vectors (53). We therefore evaluated the copies of adenoviral genome in the liver by real-time quantitative PCR at 2 h and 3 days after infusion. Comparable copy numbers of adenoviral DNA were found in WT and IFN-αβR−/− mice at 2 h after infusion, suggesting that a similar level of adenoviral transduction was achieved in vivo initially (Fig. 6B). By contrast, significantly (P < 0.001) more copies of adenoviral DNA were detected in IFN-αβR−/− mice than in WT mice 3 days later (Fig. 6B), suggesting that type I IFNs were critical in the innate immune defense against adenoviral infection.
FIG. 6.
Critical role of type I IFNs in innate immune responses to adenoviral vectors. Ad-lacZ was injected intravenously into WT or IFN-αβR−/− (IFN-αβR−/−) 129/Sv mice. (A) Six hours later, sera were measured for IL-6 and IL-12. (B) Two or 72 h later, total genomic DNA was isolated from the liver and analyzed for adenoviral genome copies by quantitative real-time PCR. Data represent adenoviral copies per μg of total genomic DNA.
We next sought to study whether type I IFNs also contributed to the adaptive immune response to adenoviral vectors in vivo. Ad-lacZ was injected intravenously into WT or IFN-αβR−/− mice, and 10 days later, splenocytes were analyzed for virus-specific T-cell activation using proliferation and CTL assays. In WT mice, adenoviral infection resulted in robust T-cell activation (Fig. 7A) and cytolytic function (Fig. 7B), whereas in IFN-αβR−/− mice, T-cell responses were severely compromised. In WT mice, activation of virus-specific T cells led to rapid clearance of LacZ-expressing hepatocytes by day 10 concurrent with the development of inflammation (Fig. 7C). In contrast, stable transgene expression and little inflammation were observed in the livers of IFN-αβR−/− mice (Fig. 7C). Furthermore, even at day 21, a significant portion of hepatocytes still expressed LacZ in IFN-αβR−/− mice (Fig. 7C). We also examined whether adenovirus-induced type I IFNs influenced adaptive B-cell response to adenovirus. Serum samples were harvested 21 days after viral infection and assayed for the presence of neutralizing antibody to adenoviral vectors. Sera from WT mice infected with Ad-lacZ were found to contain high titers of neutralizing antibody compared to those from uninfected WT mice (Fig. 7D). However, neutralizing antibody titers were significantly (P < 0.001) diminished in Ad-lacZ-infected IFN-αβR−/− mice (Fig. 7D). These results indicated that type I IFNs are also critical for adaptive immunity to adenoviral vectors.
FIG. 7.
Critical role of type I IFNs in adaptive immune responses to adenoviral vectors. Ad-lacZ was injected intravenously into WT or IFN-αβR−/− 129/Sv mice. (A) Splenocytes from WT or IFN-αβR−/− mice at day 10, along with uninfected WT splenocytes (Control) were restimulated with Ad-lacZ at an MOI of 1, 10, or 100 for 72 h. Proliferation of virus-specific T cells was analyzed by [3H]thymidine incorporation. Data reflect the mean ± SD of the stimulation index, calculated by dividing 3H counts in cpm in the presence of viral stimulation by those in the absence of stimulation, as a function of different virus doses. (B) Splenocytes from infected WT or IFN-αβR−/− mice at day 10, or uninfected splenocytes, were restimulated in vitro for 5 days and assayed for virus-specific CTL using a standard 51Cr release method. Data represent the percentage of specific lysis as a function of different effector-to-target ratios. (C) At 3, 10, or 21 days after infusion of virus, liver tissues were harvested and cryosections were stained for LacZ expression by X-Gal histochemistry. On day 10, paraffin sections were also prepared and stained with hematoxylin and eosin for histopathology. (D) Serum samples were harvested from WT or IFN-αβR−/− mice 21 days after infection (Ad), or from uninfected mice (Control), for the measurement of neutralizing antibody (Ab) titers to adenoviral vectors.
Blockade of type I IFNs diminishes innate and adaptive immune responses to adenovirus.
The critical role of type I IFNs in innate and adaptive immune responses to adenoviral vectors suggested that blockade of type I IFNs may improve the outcome of adenovirus-mediated gene therapy in vivo. To test this strategy, mice were treated with either a control antibody or antibodies to IFN-α and IFN-β 6 h prior to infusion of Ad-lacZ. Compared with the control antibody-treated mice, mice treated with antibodies to IFN-α and IFN-β showed a significant (P < 0.001) increase in viral DNA copies in the liver 3 days later (Fig. 8A). On day 10, mice treated with antibodies to IFN-α and IFN-β showed a diminished virus-specific T-cell response (Fig. 8B) accompanied by more stable transgene expression and reduction of inflammation in the liver (Fig. 8C). These results indicated that neutralizing antibodies to IFN-α and IFN-β were effective in blocking innate and adaptive immune responses to the adenoviral vector, leading to improved transgene expression and reduction of inflammation in vivo.
FIG. 8.

Type I IFN blockade diminishes innate and adaptive immune responses to adenovirus. Ad-lacZ (2 × 109 PFU) was injected intravenously into C57BL/6 mice that had been treated with neutralizing antibodies to IFN-α and IFN-β (IFN-αβ Ab) or a control antibody (Control Ab). (A) At 3 days after administration of virus, liver tissues were harvested, and total genomic DNA was isolated and analyzed for adenoviral genome copies by quantitative real-time PCR. Data represent adenoviral copies per μg of total murine liver genomic DNA. (B) Ten days later, splenocytes were restimulated with Ad-lacZ at an MOI of 1, 10, or 100. Proliferation of virus-specific T cells was analyzed by [3H]thymidine incorporation. Data reflect the mean ± SD of the stimulation index as described in legends to Fig. 7. (C) Ten days after infusion of virus, liver tissues were harvested. Cryosections were stained for LacZ expression by X-Gal histochemistry, and paraffin sections were stained with H&E for histopathology.
DISCUSSION
In this study, we have presented evidence that adenovirus activates the innate immune response through the induction of high levels of type I IFNs by both pDCs and non-pDCs. The production of type I IFNs by pDCs is mediated by TLR9 and dependent on MyD88, whereas that by non-pDCs is mediated by a TLR-independent pathway. We have further demonstrated that adenovirus-induced type I IFNs are critical for both innate and adaptive immune responses to adenoviral vectors in vivo and that neutralization of type I IFNs in mice diminishes innate and adaptive immunity to adenovirus, resulting in a reduction of inflammation and more stable transgene expression in vivo.
Previous studies on innate immune responses to adenovirus have largely focused on secretion of proinflammatory cytokines and chemokines and the associated toxicity in mice, humans, and nonhuman primates (19, 45, 48, 54, 61). It has also been shown that adenovirus can activate NK cells, which contribute to tissue damage in vivo (37, 47). Furthermore, a recent report has suggested that a functional complement system is required for effective innate immune responses to adenoviral vectors (31). Our study showed that in addition to proinflammatory cytokines and chemokines, adenovirus also induced high levels of type I IFNs. This is in line with a recent observation by Huarte et al. (22). However, in contrast to their study, we found that adenovirus-induced type I IFNs played a critical role in the control of innate immunity against adenoviral vectors in vivo. This discrepancy could be due to the fact that their conclusion that IFN does not interfere with gene transduction by adenovirus was based mainly on in vitro experiments using HeLa cells. In addition, they employed a pretreatment procedure to address the role of type I IFNs in vivo rather than the IFN-αβR−/− mice or WT mice depleted of type I IFNs with neutralizing antibodies that we utilized.
Our observation that type I IFN blockade diminishes innate and adaptive immune responses to adenovirus in vivo suggests a feasible and appealing strategy to improve the outcome of adenovirus-mediated gene therapy in vivo. Inhibition of innate and adaptive T-cell immunity to adenovirus by neutralizing type I IFNs activity could lead to reduction of inflammation and more stable transgene expression in vivo. In addition, suppression of neutralizing antibody production by type I IFN blockade could allow repeated administration of the vector. A potential concern for the application of this strategy is that type I IFNs might not be produced at high levels upon adenoviral infection in humans and nonhuman primates in vivo. However, our data that the pattern of cytokines secreted by DCs in vitro coincides with that produced in vivo in mice and that human DCs also produce mainly type I IFNs upon adenoviral infection (data not shown) should alleviate this concern. Ultimately, the feasibility and effectiveness of type I IFN blockade need to be tested in nonhuman primates prior to human clinical trials.
Among many cell types that can produce type I IFNs in response to various types of viral infection, pDCs are identified as the major source of type I IFNs (7, 51). However, recent studies have shown that non-pDCs such as cDCs and macrophages can also become efficient producers of type I IFNs upon certain viral infections (11, 26). In addition, nonprofessional immune cells such as fibroblasts also produce type I IFNs in response to some viruses (23, 27). Our data that, albeit at lower levels than pDCs, non-pDCs can also produce type I IFNs in response to adenoviral vectors are in line with these observations. It is not entirely clear why pDCs produce higher levels of type I IFNs. We have shown that it is not due to higher transduction efficiency in pDCs than cDCs by adenovirus. However, higher levels of type I IFN production in pDCs might be related to a high constitutive expression of interferon regulatory factor-7, a key transcriptional factor for type I IFN synthesis, compared with other cell types (25).
Recent studies have demonstrated a crucial role of TLRs in innate recognition of viruses, such as TLR4 in respiratory syncytial virus infection (32), TLR3 in dsRNA viral infection (3), TLR7 and TLR8 in single-stranded RNA virus infection (10, 17), and TLR9 in herpes simplex virus infection (38). A more recent report has also suggested a functional role of the TLR pathway in the innate immune response to adenovirus in vitro (15). Our data indicate that TLR9 is involved in innate immune recognition of adenovirus by pDCs, which is consistent with a recent observation (5), in a MyD88-dependent fashion. However, non-pDC recognition of adenovirus is TLR independent. The mechanism underlying this cell type-specific involvement of the TLR9/MyD88 pathway in the innate immune response to adenovirus remains unclear, but it may be related to that fact that TLR9 is highly expressed in pDCs (28). In addition, a recent study has suggested that CpG DNAs are retained longer in the endosome (where TLR9 sensing of CpG DNAs occurs) in pDCs but are rapidly transferred to the lysosome for degradation in non-pDCs, thus facilitating interaction between TLR9 and the CpG DNA in pDCs (21). As endosomal escape is a necessary step during adenoviral infection, retention of DNA in the endosome by pDCs could also explain the much lower transduction efficiency in pDCs by adenovirus.
Besides TLR-dependent innate recognition of viruses, emerging evidence has indicated the existence of TLR-independent mechanisms of virus sensing (28). It has been shown that TLR-independent sensing of DNA in the cytosol, which has been introduced into the host cells through bacterial or viral infection, can trigger type I IFN production (23, 49). A recent report suggests that mammalian genomic DNA that escapes from apoptotic degradation can also elicit TLR-independent production of type I IFNs (43). We demonstrated here that adenoviral DNA can also be recognized in the cytosol of non-pDCs in a TLR-independent manner. It is not known why adenoviral DNA is more efficient in triggering the TLR-independent pathway than genomic DNA. It might be related to their differential recognition by a yet-identified cytosolic receptor(s). Thus, future studies are required to identify the cytosolic innate sensor(s) for adenoviral DNA, which may shed light on the mechanism of viral DNA recognition in the cytoplasm in general but also on the design of strategies to interfere with adenoviral DNA-triggered innate immunity. Recent studies have also shown that viral dsRNA can activate innate immune cells through a TLR3-independent intracellular sensor, RIG-I, leading to production of type I IFNs (29). It is not clear if adenoviral RNA is encapsidated during viral packaging. If so, it is possible that viral RNA might also contribute to the activation of the TLR-independent innate immune response.
Although the function of type I IFNs has been closely associated with innate antiviral responses (34), their role in innate immune defense against adenovirus remains unknown. Our study clearly demonstrates that type I IFNs play a key role not only in innate control of adenoviral infection but also in induction of proinflammatory cytokines such as IL-6 and IL-12 in vivo. How type I IFNs contribute to the innate immune defense against adenovirus remains to be defined. Previous studies on dsRNA viruses have indicated that type I IFNs have direct antiviral functions through suppression of viral replication and induction of cell death in infected cells, mediated by pathways involving RNA-dependent protein kinase and 2′-5′ oligoadenylate synthetase, both of which are activated by viral dsRNA (12). However, it is unclear how type I IFNs regulate the innate immune defense against dsDNA viruses such as adenovirus. Delineation of mechanisms underlying type I IFN-mediated innate immune control of adenoviral infection will help us design effective strategies to improve the outcome of adenovirus-mediated gene therapy in vivo.
In addition to their critical role in the activation of innate immunity to adenovirus, type I IFNs also play a pivotal role in adaptive T-cell responses to adenoviral infection in vivo. Besides promoting DC maturation by upregulating costimulatory molecules such as CD86 (data not shown), how type I IFNs contribute to the activation of adaptive T-cell immunity remains unclear. Previous studies with other models have shown that type I IFNs can promote cross-priming of virus-specific CD8+ T cells (33) and enhance the survival of activated T cells directly or indirectly through induction of IL-15 (40, 52). Moreover, type I IFN signaling also upregulates IFN-γ production by DCs and T cells and thereby favors the induction and maintenance of Th1 responses (8). Thus, future studies should focus on defining the mechanisms by which type I IFNs promote adaptive immune responses to adenoviral vectors.
In summary, we have shown that adenovirus activates innate immunity by its DNA through the TLR9-MyD88 pathway in pDCs and a TLR-independent pathway in non-pDCs, leading to production of mainly type I IFNs. We have further identified type I IFNs as key players in modulating both innate and adaptive immune responses to adenoviral vectors and have shown that blockade of type I IFNs diminishes innate and adaptive immunity, leading to more stable transgene expression and reduction of inflammation in vivo. These results suggest that strategies targeted to interfere with the type I IFN pathway will likely improve the safety and efficacy of adenoviral vectors for gene therapy and that strategies to activate the type I IFN pathway will likely enhance vaccine potency.
Acknowledgments
We thank Shizuo Akira for providing TLR9−/−, MyD88−/−, and TRIF−/− mice.
The study was supported by grants CA111807 and CA047741 from the National Institutes of Health and by an Investigator Award from the Alliance for Cancer Gene Therapy (to Y.Y.).
We have no conflicting financial interests.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Adachi, O., T. Kawai, K. Takeda, M. Matsumoto, H. Tsutsui, M. Sakagami, K. Nakanishi, and S. Akira. 1998. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 9:143-150. [DOI] [PubMed] [Google Scholar]
- 2.Akira, S., and K. Takeda. 2004. Toll-like receptor signalling. Nat. Rev. Immunol. 4:499-511. [DOI] [PubMed] [Google Scholar]
- 3.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 4.Barouch, D. H., and G. J. Nabel. 2005. Adenovirus vector-based vaccines for human immunodeficiency virus type 1. Hum. Gene Ther. 16:149-156. [DOI] [PubMed] [Google Scholar]
- 5.Basner-Tschakarjan, E., E. Gaffal, M. O'Keeffe, D. Tormo, A. Limmer, H. Wagner, H. Hochrein, and T. Tuting. 2006. Adenovirus efficiently transduces plasmacytoid dendritic cells resulting in TLR9-dependent maturation and IFN-alpha production. J. Gene Med. 8:1300-1306. [DOI] [PubMed] [Google Scholar]
- 6.Brunetti-Pierri, N., D. J. Palmer, A. L. Beaudet, K. D. Carey, M. Finegold, and P. Ng. 2004. Acute toxicity after high-dose systemic injection of helper-dependent adenoviral vectors into nonhuman primates. Hum. Gene Ther. 15:35-46. [DOI] [PubMed] [Google Scholar]
- 7.Colonna, M., G. Trinchieri, and Y. J. Liu. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219-1226. [DOI] [PubMed] [Google Scholar]
- 8.Cousens, L. P., R. Peterson, S. Hsu, A. Dorner, J. D. Altman, R. Ahmed, and C. A. Biron. 1999. Two roads diverged: interferon alpha/beta- and interleukin 12-mediated pathways in promoting T cell interferon gamma responses during viral infection. J. Exp. Med. 189:1315-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai, Y., E. M. Schwarz, D. Gu, W. W. Zhang, N. Sarvetnick, and I. M. Verma. 1995. Cellular and humoral immune responses to adenoviral vectors containing factor IX gene: tolerization of factor IX and vector antigens allows for long-term expression. Proc. Natl. Acad. Sci. USA 92:1401-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and C. Reis e Sousa. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 11.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. Reis e Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Sastre, A., and C. A. Biron. 2006. Type 1 interferons and the virus-host relationship: a lesson in detente. Science 312:879-882. [DOI] [PubMed] [Google Scholar]
- 13.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X. L. Xu, G. Trinchieri, A. O'Garra, and Y. J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham, F. L., J. Smiley, W. C. Russell, and R. Nairn. 1977. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J. Gen. Virol. 36:59-74. [DOI] [PubMed] [Google Scholar]
- 15.Hartman, Z. C., E. P. Black, and A. Amalfitano. 2007. Adenoviral infection induces a multi-faceted innate cellular immune response that is mediated by the toll-like receptor pathway in A549 cells. Virology 358:357-372. [DOI] [PubMed]
- 16.He, T. C., S. Zhou, L. T. da Costa, J. Yu, K. W. Kinzler, and B. Vogelstein. 1998. A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95:2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 18.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 19.Higginbotham, J. N., P. Seth, R. M. Blaese, and W. J. Ramsey. 2002. The release of inflammatory cytokines from human peripheral blood mononuclear cells in vitro following exposure to adenovirus variants and capsid. Hum. Gene Ther. 13:129-141. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann, J. A., F. C. Kafatos, C. A. Janeway, and R. A. Ezekowitz. 1999. Phylogenetic perspectives in innate immunity. Science 284:1313-1318. [DOI] [PubMed] [Google Scholar]
- 21.Honda, K., Y. Ohba, H. Yanai, H. Negishi, T. Mizutani, A. Takaoka, C. Taya, and T. Taniguchi. 2005. Spatiotemporal regulation of MyD88-IRF-7 signalling for robust type-I interferon induction. Nature 434:1035-1040. [DOI] [PubMed] [Google Scholar]
- 22.Huarte, E., E. Larrea, R. Hernandez-Alcoceba, C. Alfaro, O. Murillo, A. Arina, I. Tirapu, A. Azpilicueta, S. Hervas-Stubbs, S. Bortolanza, J. L. Perez-Gracia, M. P. Civeira, J. Prieto, J. I. Riezu-Boj, and I. Melero. 2006. Recombinant adenoviral vectors turn on the type I interferon system without inhibition of transgene expression and viral replication. Mol. Ther. 14:129-138. [DOI] [PubMed] [Google Scholar]
- 23.Ishii, K. J., C. Coban, H. Kato, K. Takahashi, Y. Torii, F. Takeshita, H. Ludwig, G. Sutter, K. Suzuki, H. Hemmi, S. Sato, M. Yamamoto, S. Uematsu, T. Kawai, O. Takeuchi, and S. Akira. 2006. A Toll-like receptor-independent antiviral response induced by double-stranded B-form DNA. Nat. Immunol. 7:40-48. [DOI] [PubMed] [Google Scholar]
- 24.Iwasaki, A., and R. Medzhitov. 2004. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 5:987-995. [DOI] [PubMed] [Google Scholar]
- 25.Izaguirre, A., B. J. Barnes, S. Amrute, W. S. Yeow, N. Megjugorac, J. Dai, D. Feng, E. Chung, P. M. Pitha, and P. Fitzgerald-Bocarsly. 2003. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74:1125-1138. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, Z., P. Georgel, X. Du, L. Shamel, S. Sovath, S. Mudd, M. Huber, C. Kalis, S. Keck, C. Galanos, M. Freudenberg, and B. Beutler. 2005. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6:565-570. [DOI] [PubMed] [Google Scholar]
- 27.Kato, H., S. Sato, M. Yoneyama, M. Yamamoto, S. Uematsu, K. Matsui, T. Tsujimura, K. Takeda, T. Fujita, O. Takeuchi, and S. Akira. 2005. Cell type-specific involvement of RIG-I in antiviral response. Immunity 23:19-28. [DOI] [PubMed] [Google Scholar]
- 28.Kawai, T., and S. Akira. 2006. Innate immune recognition of viral infection. Nat. Immunol. 7:131-137. [DOI] [PubMed] [Google Scholar]
- 29.Kawai, T., K. Takahashi, S. Sato, C. Coban, H. Kumar, H. Kato, K. J. Ishii, O. Takeuchi, and S. Akira. 2005. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 6:981-988. [DOI] [PubMed] [Google Scholar]
- 30.Kay, M. A., A. X. Holterman, L. Meuse, A. Gown, H. D. Ochs, P. S. Linsley, and C. B. Wilson. 1995. Long-term hepatic adenovirus-mediated gene expression in mice following CTLA4Ig administration. Nat. Genet. 11:191-197. [DOI] [PubMed] [Google Scholar]
- 31.Kiang, A., Z. C. Hartman, R. S. Everett, D. Serra, H. Jiang, M. M. Frank, and A. Amalfitano. 2006. Multiple innate inflammatory responses induced after systemic adenovirus vector delivery depend on a functional complement system. Mol. Ther. 14:588-598. [DOI] [PubMed] [Google Scholar]
- 32.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 33.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 34.Le Bon, A., and D. F. Tough. 2002. Links between innate and adaptive immunity via type I interferon. Curr. Opin. Immunol. 14:432-436. [DOI] [PubMed] [Google Scholar]
- 35.Lee, S. H., P. Crocker, and S. Gordon. 1986. Macrophage plasma membrane and secretory properties in murine malaria. Effects of Plasmodium yoelii blood-stage infection on macrophages in liver, spleen, and blood. J. Exp. Med. 163:54-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieber, A., C. Y. He, L. Meuse, D. Schowalter, I. Kirillova, B. Winther, and M. A. Kay. 1997. The role of Kupffer cell activation and viral gene expression in early liver toxicity after infusion of recombinant adenovirus vectors. J. Virol. 71:8798-8807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, Z. X., S. Govindarajan, S. Okamoto, and G. Dennert. 2000. NK cells cause liver injury and facilitate the induction of T cell-mediated immunity to a viral liver infection. J. Immunol. 164:6480-6486. [DOI] [PubMed] [Google Scholar]
- 38.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marrack, P., J. Kappler, and T. Mitchell. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Muller, U., U. Steinhoff, L. F. Reis, S. Hemmi, J. Pavlovic, R. M. Zinkernagel, and M. Aguet. 1994. Functional role of type I and type II interferons in antiviral defense. Science 264:1918-1921. [DOI] [PubMed] [Google Scholar]
- 42.Muruve, D. A., M. J. Cotter, A. K. Zaiss, L. R. White, Q. Liu, T. Chan, S. A. Clark, P. J. Ross, R. A. Meulenbroek, G. M. Maelandsmo, and R. J. Parks. 2004. Helper-dependent adenovirus vectors elicit intact innate but attenuated adaptive host immune responses in vivo. J. Virol. 78:5966-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okabe, Y., K. Kawane, S. Akira, T. Taniguchi, and S. Nagata. 2005. Toll-like receptor-independent gene induction program activated by mammalian DNA escaped from apoptotic DNA degradation. J Exp. Med. 202:1333-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parks, R. J., L. Chen, M. Anton, U. Sankar, M. A. Rudnicki, and F. L. Graham. 1996. A helper-dependent adenovirus vector system: removal of helper virus by Cre-mediated excision of the viral packaging signal. Proc. Natl. Acad. Sci. USA 93:13565-13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raper, S. E., N. Chirmule, F. S. Lee, N. A. Wivel, A. Bagg, G. P. Gao, J. M. Wilson, and M. L. Batshaw. 2003. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 80:148-158. [DOI] [PubMed] [Google Scholar]
- 46.Rubinstein, S., P. C. Familletti, and S. Pestka. 1981. Convenient assay for interferons. J. Virol. 37:755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruzek, M. C., B. F. Kavanagh, A. Scaria, S. M. Richards, and R. D. Garman. 2002. Adenoviral vectors stimulate murine natural killer cell responses and demonstrate antitumor activities in the absence of transgene expression. Mol. Ther. 5:115-124. [DOI] [PubMed] [Google Scholar]
- 48.Schnell, M. A., Y. Zhang, J. Tazelaar, G. P. Gao, Q. C. Yu, R. Qian, S. J. Chen, A. N. Varnavski, C. LeClair, S. E. Raper, and J. M. Wilson. 2001. Activation of innate immunity in nonhuman primates following intraportal administration of adenoviral vectors. Mol. Ther. 3:708-722. [DOI] [PubMed] [Google Scholar]
- 49.Stetson, D. B., and R. Medzhitov. 2006. Recognition of cytosolic DNA activates an IRF3-dependent innate immune response. Immunity 24:93-103. [DOI] [PubMed] [Google Scholar]
- 50.Tatsis, N., and H. C. Ertl. 2004. Adenoviruses as vaccine vectors. Mol. Ther. 10:616-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Theofilopoulos, A. N., R. Baccala, B. Beutler, and D. H. Kono. 2005. Type I interferons (alpha/beta) in immunity and autoimmunity. Annu. Rev. Immunol. 23:307-336. [DOI] [PubMed] [Google Scholar]
- 52.Tough, D. F., P. Borrow, and J. Sprent. 1996. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science 272:1947-1950. [DOI] [PubMed] [Google Scholar]
- 53.Wilson, J. M. 1996. Adenoviruses as gene-delivery vehicles. N. Engl. J. Med. 334:1185-1187. [DOI] [PubMed] [Google Scholar]
- 54.Worgall, S., G. Wolff, E. Falck-Pedersen, and R. G. Crystal. 1997. Innate immune mechanisms dominate elimination of adenoviral vectors following in vivo administration. Hum. Gene Ther. 8:37-44. [DOI] [PubMed] [Google Scholar]
- 55.Yamamoto, M., S. Sato, H. Hemmi, K. Hoshino, T. Kaisho, H. Sanjo, O. Takeuchi, M. Sugiyama, M. Okabe, K. Takeda, and S. Akira. 2003. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 301:640-643. [DOI] [PubMed] [Google Scholar]
- 56.Yang, Y., H. C. Ertl, and J. M. Wilson. 1994. MHC class I-restricted cytotoxic T lymphocytes to viral antigens destroy hepatocytes in mice infected with E1-deleted recombinant adenoviruses. Immunity 1:433-442. [DOI] [PubMed] [Google Scholar]
- 57.Yang, Y., K. Greenough, and J. M. Wilson. 1996. Transient immune blockade prevents formation of neutralizing antibody to recombinant adenovirus and allows repeated gene transfer to mouse liver. Gene Ther. 3:412-420. [PubMed] [Google Scholar]
- 58.Yang, Y., C. T. Huang, X. Huang, and D. M. Pardoll. 2004. Persistent Toll-like receptor signals are required for reversal of regulatory T cell-mediated CD8 tolerance. Nat. Immunol. 5:508-515. [DOI] [PubMed] [Google Scholar]
- 59.Yang, Y., K. U. Jooss, Q. Su, H. C. Ertl, and J. M. Wilson. 1996. Immune responses to viral antigens versus transgene product in the elimination of recombinant adenovirus-infected hepatocytes in vivo. Gene Ther. 3:137-144. [PubMed] [Google Scholar]
- 60.Yang, Y., F. A. Nunes, K. Berencsi, E. E. Furth, E. Gonczol, and J. M. Wilson. 1994. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc. Natl. Acad. Sci. USA 91:4407-4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, Y., N. Chirmule, G. P. Gao, R. Qian, M. Croyle, B. Joshi, J. Tazelaar, and J. M. Wilson. 2001. Acute cytokine response to systemic adenoviral vectors in mice is mediated by dendritic cells and macrophages. Mol. Ther. 3:697-707. [DOI] [PubMed] [Google Scholar]