Abstract
We have analyzed the importance of specific amino acids in the cytoplasmic tail of the glycoprotein GN for packaging of ribonucleoproteins (RNPs) into virus-like particles (VLPs) of Uukuniemi virus (UUK virus), a member of the Bunyaviridae family. In order to study packaging, we added the GN/GC glycoprotein precursor (p110) to a polymerase I-driven minigenome rescue system to generate VLPs that are released into the supernatant. These particles can infect new cells, and reporter gene expression can be detected. To determine the role of UUK virus glycoproteins in RNP packaging, we performed an alanine scan of the glycoprotein GN cytoplasmic tail (amino acids 1 to 81). First, we discovered three regions in the tail (amino acids 21 to 25, 46 to 50, and 71 to 81) which are important for minigenome transfer by VLPs. Further mutational analysis identified four amino acids that were important for RNP packaging. These amino acids are essential for the binding of nucleoproteins and RNPs to the glycoprotein without affecting the morphology of the particles. No segment-specific interactions between the RNA and the cytoplasmic tail could be observed. We propose that VLP systems are useful tools for analyzing protein-protein interactions important for packaging of viral genome segments, assembly, and budding of other members of the Bunyaviridae family.
Uukuniemi virus (UUK virus) belongs to the Phlebovirus genus within the Bunyaviridae family and has been a model virus for the family for over 35 years. Bunyaviruses are negative-sense RNA viruses with three genome segments, namely, L, M, and S, with the L segment encoding the RNA-dependent RNA polymerase (L). The M segment of UUK virus encodes the glycoprotein precursor p110, which is cotranslationally processed to GN (70 kDa) and GC (65 kDa) (17). After GN and GC have formed heterodimers in the endoplasmic reticulum (ER), they are transported to and accumulate in the Golgi apparatus due to a retention signal present in the GN cytoplasmic tail (2). The S segment encodes the nucleoprotein (N) and a nonstructural protein (NSs), utilizing an ambisense coding strategy. The nucleoprotein (N) is a cytoplasmic protein that binds viral RNA (vRNA) and cRNA, thereby generating a functional template, the ribonucleoprotein (RNP), which is important for efficient polymerase-mediated transcription and replication. The RNPs consist of the three single-stranded RNA segments, the N protein (25 kDa), and a few copies of the L protein (about 200 kDa) (33). Together, the glycoproteins and the RNPs accumulate in the Golgi complex, the site where budding of the viral particles occurs. Mature viruses are then transported in large vesicles to the plasma membrane, where they are released after fusion of the vesicles with the plasma membrane (13, 18-20).
Some viruses (e.g., rhabdo-, orthomyxo-, paramyxo-, and retroviruses) also possess a matrix protein that is present in the virus particles and interacts with both the envelope glycoproteins and the RNPs. These matrix proteins have been shown to be important for packaging, structure formation, and budding of particles (37, 46). Remarkably, such a matrix protein is missing in other viruses (e.g., alpha-, flavi-, rubella-, bunya-, corona-, and arenaviruses). The Bunyaviridae family members contain only four structural proteins, and no matrix protein, which may assist the interaction between the glycoproteins and the RNPs, is present. Instead, it has been suggested that the cytoplasmic tails of the glycoproteins can interact directly with the nucleoproteins, thereby facilitating the packaging of the RNPs into particles in order to prevent the release of empty particles. Such an interaction between the GN/GC and N proteins was indeed demonstrated by coimmunoprecipitation studies (17).
The cytoplasmic tail of UUK virus GN is 98 amino acids (aa) long and includes the signal sequence for GC (17 aa) (Fig. 1A). The region encompassing residues 1 to 81 contains a Golgi targeting/retention signal between aa 10 and 40 that is responsible for the transport to and localization in the Golgi apparatus of the GN/GC dimers (2). Also, two palmitylation sites, at positions 25 and 28, were identified and shown to be involved in the anchoring of the GN cytoplasmic tail to the Golgi membrane (1). It is unknown whether the GC signal sequence remains buried in the membrane or is exposed to the cytoplasm. The carboxy terminus of Sindbis virus E2 protein is initially buried in the ER membrane and becomes exposed to the cytoplasm during or after viral protein export from the ER, a process critical for the interaction with the nucleocapsids (23, 24). Since the GC cytoplasmic tail of UUK virus is only 5 aa long, with an as yet unknown function, it may very well be possible that the GN tail is responsible for the interaction with the nucleoproteins during the budding process (1).
FIG. 1.
Transfer of reporter gene activity of VLPs generated by glycoprotein mutants. (A) Schematic representation of the glycoprotein precursor p110, with both glycoproteins GN and GC indicated. The amino acid composition of the cytoplasmic tail of GN is shown. (B) Transfer of reporter gene activity by VLPs obtained from wt and mutant glycoprotein-transfected cells. Cells were transfected with the minigenome M-CAT and expression plasmids pUUK-L and pUUK-N (lane 1) or with M-CAT, pUUK-L, pUUK-N, and wt pUUK-GN/GC (lane 2) or mutant pUUK-GN/GC plasmids where segments of 5 aa at a time were exchanged with alanines (lanes 3 to 18), and CAT activity was determined in the cell lysate (upper panel). The corresponding supernatants were collected and used to infect new cells previously transfected with pUUK-L and pUUK-N, and reporter gene activity was measured by the CAT assay (lower panel). The asterisk indicates the cellular background. The data are representative of three independent experiments.
To study the packaging of RNPs into particles and, more specifically, the interaction between the glycoprotein cytoplasmic tails and the nucleoproteins, we made use of the recently developed virus-like particle (VLP) system for UUK virus (30). The expression of structural proteins of many nonenveloped and enveloped viruses leads to the formation of VLPs (28). Such VLPs frequently exhibit morphologies very similar to those of wild-type (wt) viruses (14, 30). We have shown that the structural proteins of UUK virus expressed from cloned cDNAs are able to incorporate UUK virus minigenomes containing reporter genes and to form infectious VLPs (30). Since VLPs have a tropism similar to that of the wt virus and show comparable cellular uptake and intracellular trafficking, the formation of VLPs can be used to study various aspects of the virus life cycle, such as assembly, morphogenesis, budding processes, genome packaging, receptor binding, and virus entry (4, 14, 21, 22, 38, 39).
In the present study, we have used the VLP system developed for UUK virus to generate infectious VLPs containing artificial, virus-like RNA segments. An alanine scan of the 81 aa in the cytoplasmic tail of GN was performed in order to study the packaging interactions between the GN cytoplasmic tail and the UUK virus RNPs. We demonstrate that only 4 aa residues in the GN cytoplasmic tail are important for RNP packaging and that these residues do not affect budding or the morphology of the released particles.
MATERIALS AND METHODS
Plasmids.
pUUK-GN/GC, pUUK-L, and pUUK-N are cytomegalovirus-driven plasmids expressing the Uukuniemi viral glycoprotein precursor, RNA-dependent RNA polymerase, and nucleoprotein, respectively (7). M-CAT, L-CAT, and S-CAT are polymerase I (PolI)-driven UUK virus minigenome plasmids containing the noncoding regions (NCRs) of the M, L, and S segments, respectively, flanking a reporter gene encoding chloramphenicol acetyltransferase (CAT) (6). pRF7 and pRF202 are PolI-driven plasmids expressing the full-length vRNA M and S segments, respectively (7). The construction of all alanine mutant plasmids used in the alanine scan was performed by standard PCR cloning methods, such as overlap PCR and two- and three-fragment ligation. KOD HiFi polymerase, KOD hot start polymerase (Novagen), restriction enzymes, and T4 DNA ligase (New England Biolabs) were used according to the manufacturers' recommendations. A BsmBI restriction site was inserted directly after the GN cytoplasmic tail, at bp 1489 in pUUK-GN/GC (7), and this restriction site was subsequently removed in the sequential cloning steps. All derivatives of pUUK-GN/GC were sequenced to verify the correct introduction of mutations in the absence of undesirable mutations. Primer sequences are available upon request.
Cell culture and transfection.
BHK-21 cells (American Type Culture Collection) were grown in plastic dishes in minimum essential medium with Earle's salts supplemented with 5% fetal calf serum, 5% tryptose phosphate broth, 2 mM l-glutamine, 50 IU penicillin/ml, and 50 μg streptomycin/ml (Invitrogen). For the VLP reporter gene system, BHK-21 cells were transfected with pUUK-L, pUUK-N, pUUK-GN/GC, and M-CAT, using the Lipofectamine 2000 reagent (Invitrogen). Transfections were performed as described previously (30). Briefly, the transfection medium was removed at 6 h posttransfection before fresh medium (minimum essential medium with 2% fetal calf serum and 5% tryptose phosphate broth) was added. Cells were analyzed for reporter gene expression at 24 h posttransfection, and the corresponding supernatants were used for VLP infection. Twenty-four hours prior to passage of the supernatant, BHK-21 cells were transfected with L and N expression plasmids in order to support minigenome replication, transcription, and detection. After a 3-h incubation period, fresh medium was added to the VLP-infected cells, and cells were incubated for 24 h before reporter gene analysis.
CAT assays.
Cells were resuspended in 50 μl of 0.25 M Tris-HCl (pH 7.4) and lysed by two freeze-thaw cycles. The cell lysates were centrifuged for 10 min at 9,000 × g, and CAT activity was determined using a commercially available Fast Cat kit (Invitrogen) as described previously (7). The reaction products were visualized by UV illumination and documented by photography.
Harvesting and purification of UUK VLPs.
The harvesting and purification of UUK VLPs were done as previously described (30). Briefly, supernatants from VLP-expressing cells were collected and clarified by centrifugation (4,000 × g, 10 min, 4°C). The particles were concentrated through a 20% (wt/vol) sucrose cushion dissolved in TN buffer (0.05 M Tris-HCl, pH 7.4, and 0.1 M NaCl) by centrifugation at 100,000 × g for 1 h at 4°C. The pellet was dried for 10 min before resuspension in nonreducing Laemmli sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer for subsequent analysis.
Western blotting.
Precast 10% polyacrylamide gels (Bio-Rad) were used according to the manufacturer's recommendations and run under nonreducing conditions unless indicated otherwise. Rabbit polyclonal antibodies (Abs) recognizing both GN and GC were used to detect GN and GC, and polyclonal Abs recognizing the UUK virus N protein were used to detect the N protein (19).
Immunofluorescence microscopy.
BHK-21 cells were grown on coverslips, transfected with mutant or wt glycoprotein, and fixed at 24 h posttransfection with 3% paraformaldehyde. After being quenched with 10 mM glycine, cells were permeabilized with 0.1% Triton X-100, and the UUK virus glycoproteins and Golgi marker protein were detected using a mix of polyclonal UUK virus GN/GC Abs and GM130 monoclonal Ab (BD Biosciences), followed by Alexa Fluor 488-conjugated goat anti-rabbit immunoglobulin G and Alexa Fluor 594-conjugated goat anti-mouse immunoglobulin G (Molecular Probes). Images were collected using a Zeiss microscope equipped with a charge-coupled device camera.
TEM.
VLPs and UUK virus used for negative staining for transmission electron microscopy (TEM) were fixed with 0.5% glutaraldehyde in phosphate buffer at 4°C before concentration through a sucrose cushion, as described above. The pellet was resuspended in water, and Formvar-carbon-coated copper grids were floated on the drops of the virus or VLP suspensions and, after blotting, were stained with 2% aqueous uranyl acetate.
Immunoprecipitation.
BHK-21 cells transfected with plasmids L-CAT, pRF7, pRF202, pUUK-L, pUUK-N, and pUUK GN/GC (wt or mutants) were washed with phosphate-buffered saline at 48 h posttransfection and cross-linked with 0.5 mM Lomant's reagent (DSP; Sigma) for 20 min. The reaction was stopped by quenching with 0.1 M glycine before cell lysis (1% Triton X-100, 50 mM Tris-HCl, pH 8, 150 mM NaCl, and protease inhibitor). The nucleoprotein-GN/GC protein complex was immunoprecipitated with a mixture of two monoclonal Abs recognizing the GN protein (6G9 and 11C8) (32). Protein A-Sepharose beads (Amersham Biosciences) were used to collect the Ab-protein complex, and the proteins were resuspended in reducing Laemmli SDS-PAGE sample buffer before Western blot analysis.
RESULTS
To determine the functional role of the GN cytoplasmic tail in UUK virus budding and in packaging of UUK virus RNPs, we made use of the recently developed VLP system for UUK virus (30). By cotransfecting the UUK virus glycoprotein precursor expression plasmid with a minigenome system (6), infectious VLPs are generated and released into the medium. These particles are a useful tool for studying different functions in the virus life cycle. For this study, the role of the cytoplasmic tail of GN in the packaging of the minigenomes into VLPs was analyzed, using an alanine scan in which 5 aa at a time in the entire cytoplasmic tail of GN (1 to 81 aa) were replaced by alanines (Fig. 1A and B). The mutants were tested in the VLP reporter gene system for the ability to incorporate the RNPs into VLPs, as monitored by the transfer of CAT reporter activity to newly VLP-infected cells. BHK-21 cells were transfected with M-CAT, pUUK-N, pUUK-L, and either the wt or a mutated pUUK-GN/GC expression plasmid and analyzed for CAT activity at 24 h posttransfection (Fig. 1B, upper panel). All mutants showed strong CAT reporter activity comparable to that of the wt GN/GC-transfected cells, indicating efficient amplification of the UUK virus minigenome RNA segments. The supernatants from these transfected cells were collected and passaged in fresh BHK-21 cells pretransfected with only the N and L protein expression plasmids to support amplification of the UUK virus RNA segments, thereby allowing expression of the reporter protein. These cells were harvested 24 h after infection with VLPs and analyzed for CAT activity (Fig. 1B, lower panel). No CAT activity was detected in the negative control, where the supernatant was taken from cells transfected with all plasmids except the glycoprotein expression plasmid (Fig. 1B, lower panel, lane 1), and maximum activity was observed in the positive control, containing wt GN/GC (Fig. 1B, lower panel, lane 2). Only 4 of the 16 different glycoprotein mutants, corresponding to regions 21 to 25, 46 to 50, 71 to 75, and 76 to 81, were deficient in VLP-mediated transfer of reporter protein expression to fresh cells (Fig. 1B, lower panel, lanes 7, 12, 17, and 18). To exclude any possible segment-specific interaction of the GN cytoplasmic tail with the M segment, all mutants were also analyzed for the ability to package the L segment-based minigenome instead of the M segment-based minigenome. No difference could be detected between the M and L segment-based minigenomes regarding the transfer of CAT activity to new cells for all 16 mutants (data not shown). To verify protein expression of the wt and mutant glycoproteins, the four glycoprotein mutants unable to transfer CAT activity were analyzed by Western blotting (Fig. 2, upper panels). No difference in expression levels of both the GN/GC and N proteins could be seen in the cell lysates of the transfected cells. Next, the protein composition of the VLPs present in the supernatant harvested from the transfected cells was analyzed (Fig. 2, lower panels). The supernatants from transfected BHK-21 cells were collected at 24 h posttransfection, concentrated by ultracentrifugation, and analyzed by Western blotting. No UUK virus-specific proteins (neither GN/GC nor N) could be detected in the supernatant when the glycoprotein expression plasmid was omitted (Fig. 2, lower panels, lane 1), and all three proteins were present in the positive control containing the wt GN/GC plasmid (Fig. 2, lower panels, lane 2), as also previously demonstrated (30). No UUK virus-specific proteins were detected in the supernatant when the GN cytoplasmic tail was mutated at positions 21 to 25 and 46 to 50 (Fig. 2, lower panels, lanes 3 and 4), although both mutant glycoproteins were expressed at the same levels as the wt glycoproteins, as observed in the cell lysates (upper panels), indicating that these cells do not release VLPs. This implies a defect in budding of particles rather than in RNP packaging and will be addressed in another report. The two glycoprotein mutants with alanine substitutions at positions 71 to 75 and 76 to 81 showed no significant difference in glycoprotein release into the supernatant compared to the wt GN/GC-transfected cells (Fig. 2, lower panels, compare lanes 5 and 6 to lane 2). This indicates that although particles were formed, as indicated by the presence of GN/GC, they did not contain any ribonuclear protein (N).
FIG. 2.
Protein analysis of VLPs. Western blot analysis was performed with transfected cells (upper panels) and the corresponding supernatants after concentration through a sucrose cushion (lower panels). Blots were analyzed with antibodies recognizing the glycoproteins (GN and GC) and the nucleoprotein (N). Control cells were transfected with M-CAT, pUUK-L, and pUUK-N (lane 1), and cells transfected in addition with the wt glycoproteins (wt GN/GC) served as a positive control (lane 2). Only the four glycoprotein mutants that did not transfer reporter gene activity to new cells, shown in Fig. 1B, were analyzed for glycoprotein and nucleoprotein expression in the lysates and supernatants from transfected cells (lanes 3 to 6). The data are representative of three independent experiments.
In order to further identify the residues responsible for the packaging interaction between the GN tail and the RNPs, segments of 2 aa were replaced with alanine within the region of positions 71 to 81 of the GN cytoplasmic tail. Again, these mutants were analyzed for the ability to transfer CAT expression through VLPs to new cells (Fig. 3A). The CAT activity was reduced to background levels when residues 76 and 77 were mutated, while mutation of residues 78 and 79 or residues 80 and 81 severely compromised the transfer of CAT activity (Fig. 3A, lower panel, compare lanes 6 to 8 with lane 1). No significant difference compared to the wt was observed when residues 70 and 71, 72 and 73, or 74 and 75 were mutated to alanine, demonstrating that these residues did not affect the transfer of CAT activity (Fig. 3A, lower panel, compare lane 2 with lanes 3 to 5). To confirm that these glycoprotein mutants were correctly targeted to the Golgi apparatus, the site of VLP formation (19), their intracellular localization was determined by immunofluorescence microscopy. BHK-21 cells transfected with the wt pUUK-GN/GC plasmid or the glycoprotein mutant plasmids were fixed at 24 h posttransfection and costained for UUK virus GN/GC proteins and the Golgi marker GM130 (Fig. 3B). No significant difference in intracellular localization was observed between the wt and the glycoprotein mutants 76-77, 78-79, and 80-81 (Fig. 3B), and they all colocalized with the Golgi marker. Also, the mutants 70-71, 72-73, and 74-75 were correctly targeted to the Golgi apparatus, the site where budding occurs (data not shown). This demonstrated that the lack of CAT activity transfer caused by these alanine mutants was not caused by a defect in intracellular targeting.
FIG. 3.
Reporter gene transfer and intracellular localization of double-alanine-mutant glycoproteins. (A) Two amino acids at a time were exchanged with alanine, and CAT activities in transfected cells (upper panel) and VLP-infected cells (lower panel) were determined. (B) Glycoprotein precursor plasmids (wt or mutant) were transfected into BHK-21 cells, and the cellular glycoprotein location was determined by immunofluorescence microscopy. Cells were costained for the glycoproteins (green) and the Golgi marker GM130 (red).
Finally, single-amino-acid mutants were generated to identify the individual amino acid residues in the GN cytoplasmic tail which are critical for RNP packaging into VLPs. All single-amino-acid mutants with substitutions in positions 76 to 81 of GN colocalized with the Golgi marker GM130 (data not shown), confirming their proper localization to the Golgi apparatus. These single-amino-acid mutants were also analyzed for the ability to generate VLPs, package RNPs into the particles, and infect new cells (Fig. 4). Four residues were identified to be important for transfer of CAT reporter activity to new cells, namely, Met at position 76, Leu at position 79, Thr at position 80, and Arg at position 81 (Fig. 4A, lanes 3, 6, 7, and 8), while in the cell lysates from plasmid-transfected cells no difference in CAT activity was observed (data not shown). Analysis of the protein content in the released particles revealed no significant reduction in the amount of glycoproteins (Fig. 4B, upper panel), demonstrating that these specific residues do not influence the generation of particles. However, no nucleoprotein was detected in the VLPs generated in the presence of the M76A, L79A, and T80A mutants (Fig. 4B, lanes 3, 6, and 7), demonstrating that the mutated residues are essential for the incorporation of nucleoprotein into VLPs and the subsequent transfer of reporter activity. Interestingly, only small amounts of nucleoprotein were detected in the supernatant when Phe at position 77 was mutated (Fig. 4B, lane 4), although the transfer of CAT activity by the mutant VLPs was as efficient as that for wt glycoprotein VLPs. In contrast, VLPs generated in the presence of the R81A mutation were unable to transfer significant CAT activity, while similar amounts of nucleoprotein to those generated by the F77A mutant were present in these VLPs (Fig. 4B, lane 8). The transfected cells were also evaluated for their nucleoprotein and glycoprotein amounts to verify the expression of all three proteins for all single-amino-acid mutants, and no difference could be detected (Fig. 4C, lanes 1 to 8, and data not shown).
FIG. 4.
Reporter gene transfer and protein composition of VLPs generated with single-amino-acid glycoprotein mutants. (A) CAT activities in cells infected with VLPs collected from cells transfected with single-amino-acid glycoprotein mutants. (B) Western blot analysis of the supernatants used for infection in panel A. Blots were analyzed with antibodies recognizing the glycoproteins (GN and GC, upper panel) and the nucleoprotein (N, lower panel). (C) Lysates from wt and mutant glycoprotein-transfected cells were analyzed for the presence of nucleoprotein (N). (D) Single-amino-acid glycoprotein mutants were tested for the ability to transfer the L and S segment-based minigenomes (upper and lower panels, respectively). Asterisks indicate the cellular background.
To analyze whether the glycoprotein cytoplasmic tail-RNP interaction is RNA segment specific or only UUK virus N protein specific, we also tested the transfer and packaging of the other two minigenomes (L-CAT and S-CAT) into VLPs. The transfer of activity of both minigenomes was analyzed using the single-amino-acid glycoprotein mutants with substitutions in the region between residues 76 and 81 (Fig. 4D). No significant difference in the ability of the glycoprotein mutants to transfer reporter gene activity could be detected between the different minigenome segments (compare Fig. 4A and D), indicating that the NCRs from the different segments did not interact specifically with the GN cytoplasmic tail.
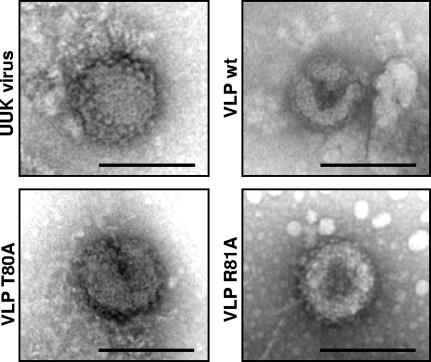
The morphologies of the VLPs that were generated in the presence of the T80A and R81A mutants were analyzed by negative-staining electron microscopy. The supernatants of cells infected with UUK virus or transfected with wt and mutant glycoproteins were collected and fixed with 0.5% glutaraldehyde, and the particles were concentrated by centrifugation through a sucrose cushion. The morphology of the particles was analyzed by TEM. VLPs formed by the mutant glycoproteins did not show any significant difference in size and morphology compared to VLPs generated with wt glycoproteins or UUK virus particles (Fig. 5), even though they did not contain significant amounts of nucleoprotein.
FIG. 5.
Particle morphology of VLPs and UUK virus by transmission electron microscopy. Supernatants containing UUK virus and VLPs generated in the presence of wt and T80A and R81A mutant glycoproteins were collected and concentrated through a sucrose cushion, and particle morphology was determined by negative-staining TEM. Bars, 100 nm.
Previously, it was demonstrated that in UUK virus-infected cells, the glycoproteins GN/GC can be coimmunoprecipitated together with the nucleoprotein, indicating that these proteins interact with each other (17). To examine the possible interaction between the GN cytoplasmic tail and the N protein, coimmunoprecipitation studies were performed on plasmid-transfected BHK-21 cells. Cells were transfected, cross-linked at 48 h posttransfection, and lysed, and the glycoprotein-RNP complexes were immunoprecipitated with monoclonal antibodies recognizing the GN protein. The immunoprecipitated proteins were analyzed by Western blotting (Fig. 6). No nucleoprotein or glycoprotein was detected in the immunoprecipitates from nontransfected cells (Fig. 6, lane 1) or cells transfected with all plasmids except for the glycoprotein plasmid (lane 2). In cells transfected with the complete set of plasmids, including the glycoprotein and nucleoprotein plasmids, the nucleoprotein could be coprecipitated with the glycoproteins (lane 3). As a positive control, the immunoprecipitate from UUK virus-infected cells is shown (lane 10). As expected, the N78A mutant, which was shown to produce infectious VLPs (Fig. 4A), was also able to coimmunoprecipitate with the nucleoprotein. However, such an interaction could not be observed when cells were transfected with the M76A, L79A, T80A, and R81A glycoprotein mutants previously shown to be unable to produce VLPs with significant CAT reporter activity (Fig. 4A). Despite the fact that the F77A mutant was able to generate infectious VLPs that contained nucleoprotein, no nucleoprotein could be coimmunoprecipitated with this mutant.
FIG. 6.
Interaction of the GN glycoprotein cytoplasmic tail with the nucleoprotein. Cells were transfected with L-CAT and two PolI-driven plasmids expressing the full-length vRNA M and S segments, together with the nucleoprotein and polymerase expression plasmid (lane 2) and, in addition, the wt (lane 3) and single-amino-acid-mutant glycoproteins (lanes 4 to 9). Cells were cross-linked and lysed, and proteins were immunoprecipitated with GN monoclonal antibodies. Immunoprecipitated protein complexes were separated by SDS-PAGE under reducing conditions and analyzed by Western blotting using polyclonal antibodies to detect nucleoprotein (N) and glycoprotein (GN). As controls, immunoprecipitates obtained with nontransfected cells (lane 1) and UUK virus-infected cells (lane 10) are shown.
DISCUSSION
By using our recently developed VLP system for UUK virus, we demonstrated that the packaging of RNPs into particles is mediated through a specific interaction between the cytoplasmic tail of the GN protein and the nucleoprotein. Moreover, we identified, by systematic mutagenesis, four residues in the tail that are essential for this interaction, namely, M76, L79, T80, and R81.
In some enveloped viruses, an interaction between the viral RNP complex and envelope proteins drives and facilitates the budding of virus particles, as shown, e.g., for Semliki Forest virus (40), simian virus 5 (38), murine leukemia virus (27), and Ebola virus (22). For other enveloped viruses, the RNPs are dispensable for viral envelope formation and production of virus particles, as demonstrated, e.g., for coronavirus (43), Marburg virus (41), influenza virus (10), vesicular stomatitis virus (15), and human parainfluenza virus type 1 (5). It was also reported in the 1990s that for Hantaan virus, in the Bunyaviridae family, the nucleoprotein is essential for generating VLPs and for the initiation of budding (3). We recently demonstrated that for UUK virus, the glycoproteins alone are sufficient for VLP formation, thus excluding a role for the nucleoprotein in the budding process (30), which was also confirmed in the present study. It was previously suggested that the glycoproteins are responsible for the structural stability of the virus, since spikeless particles were found to be highly deformed (44). We similarly suggest that the GN/GC proteins by themselves can generate particles and bud into the Golgi complex, and also the ER-Golgi intermediate compartment (13), when a critical glycoprotein concentration is reached. It is therefore imperative that the RNPs are present close to the budding sites around the Golgi complex in virus-infected cells in order to optimize RNP incorporation and to minimize the number of empty particles released. The fact that RNPs themselves do not initiate budding was further confirmed by the observation that mutation of the GN cytoplasmic tail had no effect on particle formation and release into the supernatant compared to those for wt GN, even though the mutations resulted in particles lacking the nucleoprotein.
Our results show that four residues in the GN cytoplasmic tail, namely, M76, L79, T80, and R81, are crucial for RNP packaging. VLPs generated in the presence of these glycoprotein mutants did not incorporate RNPs and were practically empty, since no reporter protein activity could be transferred to new cells. Lack of RNP incorporation was not caused by mislocalization of the mutant glycoproteins, since the Ala mutants were correctly localized to the Golgi apparatus, the site of particle formation. In addition, as shown by TEM, the morphology of the particles appeared to be unaffected. The results from the coimmunoprecipitation experiments indicate that the glycoprotein specifically interacts with the nucleoprotein. Moreover, this interaction could only be observed after chemical cross-linking, suggesting a weak interaction. The wt glycoprotein as well as the N78A mutant, which produced infectious VLPs containing nucleoprotein, was shown to interact with the nucleoprotein. The F77A mutant, however, displayed wt-like activity and produced VLPs containing little nucleoprotein compared to the wt and the N78A mutant. We were unable to convincingly show an interaction between this mutant glycoprotein and the nucleoprotein. The strong reporter gene activity, together with the small amount of nucleoprotein present in these particles, as seen for the F77A mutant, might be caused by the incorporation of free RNA into particles. However, this is not likely, since free RNA would also be packaged into particles generated with the M76A, L79A, and T80A mutants, and no significant reporter protein activity was observed with these mutants. The M76A, L79A, and T80A glycoprotein mutants were unable to interact with the nucleoprotein, and as a consequence, VLPs were devoid of nucleoprotein and RNPs, resulting in very little or no transfer of CAT activity. The particles produced in the presence of the R81A mutant contained only small amounts of detectable nucleoprotein, comparable to that with the active F77A mutant, but were not able to efficiently transfer CAT activity. This indicates that free nucleoprotein could be incorporated into these particles, since they lack the RNA segments responsible for the transfer of activity. Free nucleoprotein has been shown to be incorporated into UUK VLPs when particles are generated in the absence of a minigenome (30). Our initial results using glycoprotein mutants where segments of 5 aa residues at a time were mutated to alanines suggest that the secondary structure of the GN tail is important for RNP packaging into viral particles. This is illustrated by the lack of nucleoprotein incorporation and transfer of CAT activity for the glycoprotein mutant 71-75. However, when only two residues at a time were mutated in this region, no effect on transfer of reporter activity was observed compared to that of the wt glycoprotein.
Surprisingly, no segment-specific interaction between the NCRs in the RNPs and the GN cytoplasmic tail was detected. It was previously shown for UUK virus and Bunyamwera virus that L segment-based minigenomes have the strongest packaging efficiency compared to the M and S segments (6, 16). Defective interfering particles for the members of the Bunyaviridae family have revealed major deletions in the L segment leaving the terminal nucleotides intact. Such deletions have not been found in the M or S segment (12, 25, 31, 36). This suggested that the L segments in bunyaviruses contain unique sequences in the terminal nucleotides which are important for replication and packaging. We have no indication that these unique packaging sequences in the UUK virus L segment directly interact with the GN cytoplasmic tail, since no differences in packaging were observed between the M and L segment-based minigenomes. These unique RNA sequences in the L segment might instead interact with the other segments or the RNA polymerase. It has been shown that reassortment is a nonrandom process (34, 35, 42; L. Perrone, personal communication), and the L and S segments from the same strain readily reassort together for the Orthobunyavirus genus (35) and the phlebovirus serogroup (Perrone, personal communication). Together, these findings indicate that the packaging mechanism for the bunyaviruses resembles that of influenza virus. In influenza A virus, all eight segments are packaged in an equimolar ratio clustered together in the viral particle, with seven segments surrounding one in the middle (29). All segments contain sequences important for packaging in both the NCR and the open reading frame (ORF) (8, 9, 26, 45). These ORF sequences might serve as linking sites enhancing and facilitating the interaction with other vRNA segments, since it has been shown that one segment can affect the incorporation of other segments and that the packaging efficiency is dependent on all segments present (26). An analogous linking site between the genomes is found in all retroviruses, where the positive RNA genome forms dimers in mature virions and the linking between the two genomes occurs in the dimer linking site, which overlaps with the packaging signal (11). We therefore suggest that the three RNA segments in bunyaviruses form a complex together with the nucleoprotein and RNA polymerase and that these RNPs are packaged into progeny particles via an interaction between the nucleoprotein and the GN cytoplasmic tail. The L segment might have an important structural function in this complex by binding the M and S segments.
The VLP system for UUK virus was recently developed, enabling us to study molecular mechanisms involved in packaging and budding of a bunyavirus. This system also brings us one step closer to rescuing the UUK virus. Our focus in this paper has been to evaluate the VLP system and its usefulness for studying packaging mechanisms. Our results clearly demonstrate that only a few aa residues in the GN cytoplasmic tail are important for the RNP-GN interaction during the packaging event. Also, we show that there are no segment-specific interactions between the RNA and the GN tail. These findings give further clues about the packaging mechanism for UUK virus, and it remains to be investigated whether the same packaging mechanism is applicable for other members of the Bunyaviridae family, such as the clinically important phlebo-, hanta-, and nairoviruses.
Acknowledgments
We thank Anita Bergström for excellent assistance with cell cultures and Kjell Hultenby for help with TEM. We are grateful to Rene Rijnbrand and Ulf Eriksson for help in critically revising the manuscript.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Andersson, A. M., L. Melin, R. Persson, E. Raschperger, L. Wikstrom, and R. F. Pettersson. 1997. Processing and membrane topology of the spike proteins G1 and G2 of Uukuniemi virus. J. Virol. 71:218-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersson, A. M., and R. F. Pettersson. 1998. Targeting of a short peptide derived from the cytoplasmic tail of the G1 membrane glycoprotein of Uukuniemi virus (Bunyaviridae) to the Golgi complex. J. Virol. 72:9585-9596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betenbaugh, M., M. Yu, K. Kuehl, J. White, D. Pennock, K. Spik, and C. Schmaljohn. 1995. Nucleocapsid- and virus-like particles assemble in cells infected with recombinant baculoviruses or vaccinia viruses expressing the M and the S segments of Hantaan virus. Virus Res. 38:111-124. [DOI] [PubMed] [Google Scholar]
- 4.Bos, E. C., W. Luytjes, and W. J. Spaan. 1997. The function of the spike protein of mouse hepatitis virus strain A59 can be studied on virus-like particles: cleavage is not required for infectivity. J. Virol. 71:9427-9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronel, E. C., K. G. Murti, T. Takimoto, and A. Portner. 1999. Human parainfluenza virus type 1 matrix and nucleoprotein genes transiently expressed in mammalian cells induce the release of virus-like particles containing nucleocapsid-like structures. J. Virol. 73:7035-7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flick, K., A. Katz, A. Overby, H. Feldmann, R. F. Pettersson, and R. Flick. 2004. Functional analysis of the noncoding regions of the Uukuniemi virus (Bunyaviridae) RNA segments. J. Virol. 78:11726-11738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flick, R., and R. F. Pettersson. 2001. Reverse genetics system for Uukuniemi virus (Bunyaviridae): RNA polymerase I-catalyzed expression of chimeric viral RNAs. J. Virol. 75:1643-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fujii, K., Y. Fujii, T. Noda, Y. Muramoto, T. Watanabe, A. Takada, H. Goto, T. Horimoto, and Y. Kawaoka. 2005. Importance of both the coding and the segment-specific noncoding regions of the influenza A virus NS segment for its efficient incorporation into virions. J. Virol. 79:3766-3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujii, Y., H. Goto, T. Watanabe, T. Yoshida, and Y. Kawaoka. 2003. Selective incorporation of influenza virus RNA segments into virions. Proc. Natl. Acad. Sci. USA 100:2002-2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Puertas, P., C. Albo, E. Perez-Pastrana, A. Vivo, and A. Portela. 2000. Influenza virus matrix protein is the major driving force in virus budding. J. Virol. 74:11538-11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greatorex, J., and A. Lever. 1998. Retroviral RNA dimer linkage. J. Gen. Virol. 79:2877-2882. [DOI] [PubMed] [Google Scholar]
- 12.Inoue-Nagata, A. K., R. Kormelink, J. Y. Sgro, T. Nagata, E. W. Kitajima, R. Goldbach, and D. Peters. 1998. Molecular characterization of tomato spotted wilt virus defective interfering RNAs and detection of truncated L proteins. Virology 248:342-356. [DOI] [PubMed] [Google Scholar]
- 13.Jantti, J., P. Hilden, H. Ronka, V. Makiranta, S. Keranen, and E. Kuismanen. 1997. Immunocytochemical analysis of Uukuniemi virus budding compartments: role of the intermediate compartment and the Golgi stack in virus maturation. J. Virol. 71:1162-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, J. E., and W. Chiu. 2000. Structures of virus and virus-like particles. Curr. Opin. Struct. Biol. 10:229-235. [DOI] [PubMed] [Google Scholar]
- 15.Justice, P. A., W. Sun, Y. Li, Z. Ye, P. R. Grigera, and R. R. Wagner. 1995. Membrane vesiculation function and exocytosis of wild-type and mutant matrix proteins of vesicular stomatitis virus. J. Virol. 69:3156-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohl, A., A. C. Lowen, V. H. Leonard, and R. M. Elliott. 2006. Genetic elements regulating packaging of the Bunyamwera orthobunyavirus genome. J. Gen. Virol. 87:177-187. [DOI] [PubMed] [Google Scholar]
- 17.Kuismanen, E. 1984. Posttranslational processing of Uukuniemi virus glycoproteins G1 and G2. J. Virol. 51:806-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuismanen, E., B. Bang, M. Hurme, and R. F. Pettersson. 1984. Uukuniemi virus maturation: immunofluorescence microscopy with monoclonal glycoprotein-specific antibodies. J. Virol. 51:137-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuismanen, E., K. Hedman, J. Saraste, and R. F. Pettersson. 1982. Uukuniemi virus maturation: accumulation of virus particles and viral antigens in the Golgi complex. Mol. Cell. Biol. 2:1444-1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuismanen, E., J. Saraste, and R. F. Pettersson. 1985. Effect of monensin on the assembly of Uukuniemi virus in the Golgi complex. J. Virol. 55:813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, P. P., A. Naknanishi, M. A. Tran, K. Ishizu, M. Kawano, M. Phillips, H. Handa, R. C. Liddington, and H. Kasamatsu. 2003. Importance of Vp1 calcium-binding residues in assembly, cell entry, and nuclear entry of simian virus 40. J. Virol. 77:7527-7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Licata, J. M., R. F. Johnson, Z. Han, and R. N. Harty. 2004. Contribution of Ebola virus glycoprotein, nucleoprotein, and VP24 to budding of VP40 virus-like particles. J. Virol. 78:7344-7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu, L. N., H. Lee, R. Hernandez, and D. T. Brown. 1996. Mutations in the endo domain of Sindbis virus glycoprotein E2 block phosphorylation, reorientation of the endo domain, and nucleocapsid binding. Virology 222:236-246. [DOI] [PubMed] [Google Scholar]
- 24.Liu, N., and D. T. Brown. 1993. Phosphorylation and dephosphorylation events play critical roles in Sindbis virus maturation. Virology 196:703-711. [DOI] [PubMed] [Google Scholar]
- 25.Marchi, A., L. Nicoletti, L. Accardi, P. Di Bonito, and C. Giorgi. 1998. Characterization of Toscana virus-defective interfering particles generated in vivo. Virology 246:125-133. [DOI] [PubMed] [Google Scholar]
- 26.Muramoto, Y., A. Takada, K. Fujii, T. Noda, K. Iwatsuki-Horimoto, S. Watanabe, T. Horimoto, H. Kida, and Y. Kawaoka. 2006. Hierarchy among viral RNA (vRNA) segments in their role in vRNA incorporation into influenza A virions. J. Virol. 80:2318-2325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muriaux, D., S. Costes, K. Nagashima, J. Mirro, E. Cho, S. Lockett, and A. Rein. 2004. Role of murine leukemia virus nucleocapsid protein in virus assembly. J. Virol. 78:12378-12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noad, R., and P. Roy. 2003. Virus-like particles as immunogens. Trends Microbiol. 11:438-444. [DOI] [PubMed] [Google Scholar]
- 29.Noda, T., H. Sagara, A. Yen, A. Takada, H. Kida, R. H. Cheng, and Y. Kawaoka. 2006. Architecture of ribonucleoprotein complexes in influenza A virus particles. Nature 439:490-492. [DOI] [PubMed] [Google Scholar]
- 30.Overby, A. K., V. Popov, E. P. Neve, and R. F. Pettersson. 2006. Generation and analysis of infectious virus-like particles of Uukuniemi virus (Bunyaviridae), a useful system for studying bunyaviral packaging and budding. J. Virol. 80:10428-10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel, A. H., and R. M. Elliott. 1992. Characterization of Bunyamwera virus defective interfering particles. J. Gen. Virol. 73:389-396. [DOI] [PubMed] [Google Scholar]
- 32.Persson, R., and R. F. Pettersson. 1991. Formation and intracellular transport of a heterodimeric viral spike protein complex. J. Cell Biol. 112:257-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pettersson, R. F., M. J. Hewlett, D. Baltimore, and J. M. Coffin. 1977. The genome of Uukuniemi virus consists of three unique RNA segments. Cell 11:51-63. [DOI] [PubMed] [Google Scholar]
- 34.Pringle, C. R. 1996. Genetics and genome segment reassortment, p. 189-226. In R. M. Elliott (ed.), The Bunyaviridae. Plenum Press, New York, NY.
- 35.Pringle, C. R., J. F. Lees, W. Clark, and R. M. Elliott. 1984. Genome subunit reassortment among bunyaviruses analysed by dot hybridization using molecularly cloned complementary DNA probes. Virology 135:244-256. [DOI] [PubMed] [Google Scholar]
- 36.Resende Rde, O., P. de Haan, E. van de Vossen, A. C. de Avila, R. Goldbach, and D. Peters. 1992. Defective interfering L RNA segments of tomato spotted wilt virus retain both virus genome termini and have extensive internal deletions. J. Gen. Virol. 73:2509-2516. [DOI] [PubMed] [Google Scholar]
- 37.Rhee, S. S., and E. Hunter. 1991. Amino acid substitutions within the matrix protein of type D retroviruses affect assembly, transport and membrane association of a capsid. EMBO J. 10:535-546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schmitt, A. P., G. P. Leser, D. L. Waning, and R. A. Lamb. 2002. Requirements for budding of paramyxovirus simian virus 5 virus-like particles. J. Virol. 76:3952-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi, X., A. Kohl, V. H. Leonard, P. Li, A. McLees, and R. M. Elliott. 2006. Requirement of the N-terminal region of orthobunyavirus nonstructural protein NSm for virus assembly and morphogenesis. J. Virol. 80:8089-8099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suomalainen, M., P. Liljestrom, and H. Garoff. 1992. Spike protein-nucleocapsid interactions drive the budding of alphaviruses. J. Virol. 66:4737-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swenson, D. L., K. L. Warfield, K. Kuehl, T. Larsen, M. C. Hevey, A. Schmaljohn, S. Bavari, and M. J. Aman. 2004. Generation of Marburg virus-like particles by co-expression of glycoprotein and matrix protein. FEMS Immunol. Med. Microbiol. 40:27-31. [DOI] [PubMed] [Google Scholar]
- 42.Urquidi, V., and D. H. Bishop. 1992. Non-random reassortment between the tripartite RNA genomes of La Crosse and snowshoe hare viruses. J. Gen. Virol. 73:2255-2265. [DOI] [PubMed] [Google Scholar]
- 43.Vennema, H., G. J. Godeke, J. W. Rossen, W. F. Voorhout, M. C. Horzinek, D. J. Opstelten, and P. J. Rottier. 1996. Nucleocapsid-independent assembly of coronavirus-like particles by co-expression of viral envelope protein genes. EMBO J. 15:2020-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.von Bonsdorff, C. H., and R. Pettersson. 1975. Surface structure of Uukuniemi virus. J. Virol. 16:1296-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe, T., S. Watanabe, T. Noda, Y. Fujii, and Y. Kawaoka. 2003. Exploitation of nucleic acid packaging signals to generate a novel influenza virus-based vector stably expressing two foreign genes. J. Virol. 77:10575-10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye, Z., T. Liu, D. P. Offringa, J. McInnis, and R. A. Levandowski. 1999. Association of influenza virus matrix protein with ribonucleoproteins. J. Virol. 73:7467-7473. [DOI] [PMC free article] [PubMed] [Google Scholar]