Abstract
Mouse mammary tumor virus (MMTV) is a milk-transmitted betaretrovirus that causes mammary tumors in mice. Although mammary epithelial cells are the ultimate targets of MMTV, the virus utilizes components of the host immune system to establish infection. Previous studies indicated that dendritic cells play a role in MMTV infection. Here we show that dendritic cells are the first cells to be infected by MMTV in vivo and that they are capable of producing infectious virus that can be transmitted to other cell types. Moreover, upon contact with the virus, dendritic cells became more mature and migrated in response to the chemokine macrophage inflammatory protein 3β. Finally, we demonstrate that targeted ablation of dendritic cells in vivo dramatically attenuated MMTV infection. These data indicate that MMTV infection of dendritic cells is critical to initial propagation of the virus in vivo.
Dendritic cells (DCs) play key roles in the immune system as initiators and regulators of antigen-specific T-cell responses. Initial studies of virus-DC interactions focused predominantly on the outcomes of antiviral immune responses and the control of infection (36). More recently, however, it has been demonstrated that DCs play multiple roles in the pathogenesis induced by a variety of viruses, including human immunodeficiency virus (HIV), cytomegalovirus, measles virus, herpesvirus, influenza virus, and respiratory syncytial virus (3, 28). In particular, DC participation in the immune response to the murine retrovirus mouse mammary tumor virus (MMTV) has been demonstrated.
MMTV is a betaretrovirus transmitted through milk that causes mammary tumors in mice (25). Although mammary epithelial cells are the ultimate targets of MMTV, the virus initially uses lymphoid cells to establish infection; with the exception of its association with milk, the virus is cell associated in vivo (31). Previous studies indicated that milk-borne MMTV first infects B cells in neonatal Peyer's patches (1, 7, 14). These infected B cells were thought to present the virus-encoded superantigen (Sag) to T cells expressing Sag-specific T-cell receptor (TCR) Vβ chains, resulting in their stimulation. T-cell stimulation results in the subsequent amplification of infection, through inducing the proliferation of infected B cells and the recruitment of additional infection-competent target cells. B cells are clearly required to establish high-level infection, since immunoglobulin μ knockout mice that lack B cells do not support long-term MMTV infection (7). However, although B cells present antigen to experienced T cells, they are inefficient at inducing immune responses by naïve T cells (9, 10), as would occur when cognate T cells first encounter Sag during MMTV infection. Moreover, mice with targeted mutation of the β7 integrin chain that have almost no B or T cells in their Peyer's patches are infected with milk-borne MMTV at wild-type levels (11).
Thus, several groups have tested whether DCs, which are professional antigen-presenting cells, participate in MMTV Sag presentation to cognate T cells and thereby initiate virus infection. Indeed, MMTV has been shown to interact with DCs. Both experimental subcutaneous inoculation with MMTV and milk-borne infection produce dramatic increases in the numbers of DCs in the draining lymph nodes and Peyer's patches, respectively (8, 24). In addition, DCs can be infected by MMTV and can present Sag to T cells, suggesting their involvement in the early phase of infection (5, 24, 38). Finally, it has been demonstrated that MMTV can induce DC maturation and up-regulation of surface expression of the virus entry receptor transferrin receptor 1 (TfR1) via interaction with Toll-like receptor 4 (TLR4) (8). However, whether DCs produced infectious MMTV or were required to initiate or sustain infection has not been demonstrated.
Here we show that DCs are the first cells to be infected with MMTV in vivo and that they are capable of producing infectious virus that can be transmitted to other cell types in vitro and in vivo. Upon contact with the virus, these cells became more mature and migrated in response to the chemokine macrophage inflammatory protein 3β (MIP-3β), and this effect depended on functional TLR4. Finally, we demonstrate that MMTV infection is attenuated in a transgenic mouse model with transient DC ablation and that functional DCs are required throughout the early stages of virus infection in vivo.
MATERIALS AND METHODS
Mice.
C3H/HeN, C3H/HeJ, and BALB/c mice were obtained from the Animal Program of the National Cancer Institute (Frederick, MD). Transgenic mice bearing the HYB PRO construct in the C3H/HeN background were previously described and were maintained by backcrossing to C3H/HeN mice (15). CD11c-diphtheria toxin receptor (CD11c-DTR) transgenic mice [C.FVB-Tg (Itgax-DTR/EGFP) 57Lan/J] were originally obtained from Dan Littman and were maintained by crosses with BALB/c mice; offspring were genotyped from tail DNAs, using published primer sequences (22). Nontransgenic littermates of between 8 and 12 weeks of age were used as controls. For systemic DC depletion, CD11c-DTR transgenic mice were injected intraperitoneally with 4 ng/g body weight diphtheria toxin (DT) (Sigma Chemical Co., St. Louis, MO). All mice were housed according to the policies of the Institutional Animal Care and Use Committee of the University of Pennsylvania.
Virus preparation.
MMTV(LA) and MMTV(FM) were isolated from milk, and MMTV(HP) was isolated from DC culture supernatants as previously described (18, 19, 43). Dilutions of purified virus were tested for B-cell- and Sag-mediated T-cell activation in vivo, as previously described (29). For each virus preparation, the maximum virus dilution giving significant activation in the draining versus nondraining lymph node was used in all subsequent experiments.
Pseudovirus preparation.
An MMTV Env-pseudotyped recombinant murine leukemia virus was made by transient cotransfection of 293T cells with the mouse stem cell virus-based vector MIGR1 (carrying the green fluorescent protein [GFP] gene), pcap (carrying the gag/pol genes under the control of a murine leukemia virus promoter), and pR739 (a modified version of the pEnv plasmid which contains MMTV env with the cytoplasmic tail from Moloney murine leukemia virus [a gift from Lorraine Albritton]) (17, 27). Virus was concentrated from cell supernatants by centrifugation at 105,000 × g for 1 h on a 30% sucrose cushion in phosphate-buffered saline (PBS). The pellet was resuspended in endotoxin-free PBS.
Virus injection.
Fifty microliters of diluted MMTV(LA) or -(FM) virus stock was injected into the right hind footpad of each mouse; the left hind footpad served as the uninfected control. At different times postinfection, the mice were killed, and single-cell suspensions were prepared from the popliteal lymph nodes, stained, and studied by fluorescence-activated cell sorting (FACS) analysis. Cells were subjected to four-color flow cytometry on a FACSCalibur flow cytometer and analyzed using CellQuest 3.2.1f1 software (Becton Dickinson, Inc., San Jose, CA).
For virus inactivation studies, MMTV was boiled for 30 min, diluted to the same final concentration as untreated virus, and injected. Injections were also performed in the presence of 3 mg of azidothymidine (AZT; Sigma Co., Saint Louis, MO), which was administered intraperitoneally before injection as described previously (20).
Tissue culture cells.
NMuMG (normal mouse mammary gland epithelial) cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum and 10 μg of insulin per ml. 293T cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. TRH3 cells (293T cells stably expressing mouse TfR1 [44]) were grown in the same medium supplemented with G418 (100 μg/ml).
Primary cell cultures.
Total splenocytes were enriched for mononuclear cells by density gradient centrifugation over Ficoll (Sigma Chemical Co., St. Louis, MO). Naïve B cells were isolated using CD43 (Ly-48) microbeads according to the manufacturer's instructions (Miltenyi Biotech, Auburn, CA). The purity of the enriched populations always exceeded 98%, as determined by flow cytometry.
Bone marrow DCs (BMDCs) were generated according to the method of Lutz et al. (23). Cells were differentiated with recombinant murine granulocyte-macrophage colony-stimulating factor (20 ng/ml; Peprotech, Rocky Hill, NJ). Immature DCs were incubated with MMTV at the indicated dilution or with 1 μg/ml lipopolysaccharide (LPS) (Sigma Chemical Co., St. Louis, MO) for 48 h. Differentiation into immature or mature DCs was documented by flow cytometry detection of CD80, CD86, and major histocompatibility complex class II molecules.
In vitro infection by coculture.
Virus infection of naïve C3H/HeN B lymphocytes and DCs was done by coculturing these cells (104 cells/plate) with 105 HYB PRO DCs in 60-mm-diameter tissue culture plates for 5 days. To prevent cell-cell contact, coculturing was carried out with 0.4-μm-pore-size filter inserts.
In vitro infection of BMDCs for in vivo injection.
DCs were infected by the spinoculation method as previously described (32). Briefly, DCs were plated in a 96-well plate at a cell density of 105/100 μl. One microliter of undiluted MMTV(LA) was added to the cells, and the plate was centrifuged at 1,200 × g for 120 min at room temperature. After centrifugation, the supernatant was discarded and fresh medium was added to the cells. The cells were incubated overnight at 37°C and washed with PBS, and 4 × 106 cells/animal were injected intraperitoneally into naïve C3H/HeN mice. DNAs were also extracted from the DCs prior to injection into animals to monitor the initial infection levels.
Virus detection by PCR.
DNAs from 293T, TRH3, and NMuMG cells and in vitro-cultured DC or B- or T-cell pellets were isolated by proteinase K-sodium dodecyl sulfate digestion followed by phenol-chloroform extraction and ethanol precipitation. Viral DNA was detected by PCR, using primers specific to the MMTV(C3H) Sag hypervariable region (5′ AATTCGGAGAACTCGACCTTCC 3′ and 5′ CCCCCATGAGTATATTTGA 3′). The PCR products were analyzed by electrophoresis on 1.5% agarose gels.
RT-PCR.
Virus from 100 ml of HYB PRO BMDC supernatant was passed through a 0.22-μm filter and concentrated by centrifugation for 1 h at 24,000 × g. Total RNA was isolated using an RNeasy Mini kit (QIAGEN, Inc., Valencia, CA) according to the manufacturer's instructions. Reverse transcriptase PCR (RT-PCR) was performed using an Access RT-PCR system kit (Promega, Inc., Madison, WI) according to the manufacturer's instructions. In addition to the MMTV-specific primers described above, we used mouse β-actin primers to control for RNA integrity (5′ TCATGAAGTGTGACGTTGACATC 3′ and 5′ CCTAGAAGCATTTGCGGTGCAACGATG 3′). The RT-PCR products were analyzed in 1.5% agarose gels.
Flow cytometry.
Cells were stained with fluorochrome-conjugated monoclonal antibodies against CD11c (HL3), CD80 (16-10A1), CD86 (GL1), B220 (RAE-6B2), CD4 (L3T4), CD71 (C2), CD69 (H1.2F3), Vβ2 TCR (B20.6), Vβ14 TCR (14-2), Vβ8 TCR (MR5-2), Vβ6 TCR (RR4-7) (BD-Pharmingen, Inc., San Diego, CA), CCR6 (140706) (R&D Systems, Inc., Minneapolis, MN), and CCR7 (4B12) (eBioscience, Inc., San Diego, CA) and subjected to FACS analysis.
Chemotaxis assay.
Migration of murine DCs towards MIP-3β (100 ng/ml; R&D Systems, Minneapolis, MN) was assessed in 96-well chemotaxis chambers, using an 8-μm-pore-size nitrocellulose membrane (Neuroprobe, Gaithersburg, MD). Pyrogen-free RPMI 1640 containing 1% bovine serum albumin was used as the chemotactic medium. Results are presented as chemotactic indexes, defined as x-fold increases in cell migration in the presence of chemotactic factors compared to that in chemotactic medium alone. Each determination was performed in triplicate.
Real-time quantitative PCR.
The ABI Prism 7900 sequence detection system and a SYBR green I PCR kit (both from Applied Biosystems, Foster City, CA) were used for real-time PCR following the manufacturer's directions. The following primers specific to the Sag hypervariable regions were used to detect virus (see reference 19 for descriptions of the viruses): for MMTV(LA) (see Fig. 2), 5′ CGTGAAAGACTCGCCAGAGCTA 3′ and 5′ GAAGATCTCCCCCATGAGTATATTTCA 3′; for BALB2 (see Fig. 4), 5′ AACTCAAGGGCAATGCCTTAATACTATCT 3′ and 5′ CGTTTATCGAGGGACAGGAATGA 3′; and for the mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene (control), 5′ CCTGCACCACCAACTGCTTA 3′ and 5′ CATGAGTCCTTCCACGATACCA 3′). Data are expressed as numbers of MMTV molecules per 106 GAPDH molecules. Samples were considered virus positive if more than five copies of MMTV/106 GAPDH molecules were detected.
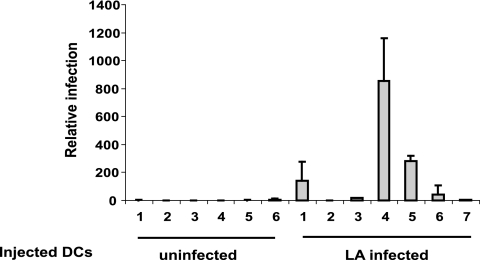
FIG. 2.
MMTV-infected DCs transmit virus in vivo. C3H/HeN mice injected with MMTV(LA)-infected BMDCs were tested 8 months after inoculation. DNAs from peripheral blood lymphocytes were prepared, and viral DNA was quantified by real-time PCR with virus-specific primers. Data were normalized to the GAPDH level of each sample. These data are representative of three experiments with similar results. The error bars indicate standard deviations (SD).
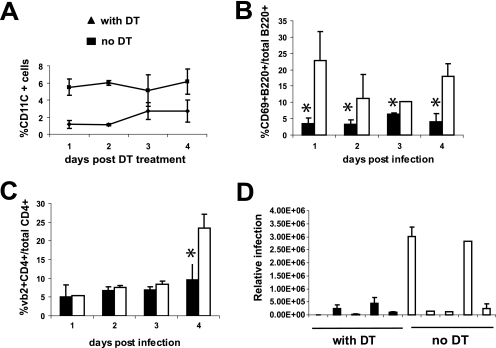
FIG. 4.
Effects of CD11c+ cell ablation on MMTV-dependent T- and B-cell activation and infection. CD11c-DTR transgenic mice were treated with 4 ng of DT/g of body weight, and 2 h later, they were injected subcutaneously with MMTV(LA). At different times (1 to 4 days postinfection), lymphocytes from the draining popliteal lymph nodes were analyzed by FACS and PCR. (A) The efficacy of DT treatment was checked by analyzing the percentage of CD11c+ cells in the spleen at each time point of the experiment. (B) B-cell activation was analyzed by determining the percentage of B220+ CD69+ cells. (C) Stimulation of MMTV(LA) Sag-reactive T cells was analyzed by determining the percentage of CD4+ Vβ2+ cells. Lymphocytes isolated from the draining lymph nodes of mice that did not receive DT were used as positive controls; mice that received no MMTV showed no activation of T or B cells (not shown; see Fig. 5B and C). (D) DNAs from the draining lymph node lymphocytes of five DT-treated and five control mice were prepared, and viral DNA was quantified by real-time quantitative PCR. Each bar represents an individual mouse, and the error bars indicate SD. These data are representative of three experiments with similar results. *, P ≤ 0.05. Open boxes, mice treated with DT; filled boxes, control mice.
Statistics.
The statistical significance of differences between groups was tested using the unpaired Student t test.
RESULTS
DCs produce infectious MMTV.
Previous studies showed that DCs could be infected by MMTV in vitro (8) and that a small percentage of DCs with newly integrated proviral DNA could be isolated from the lymph nodes of acutely infected adult mice (24, 38). However, these studies did not demonstrate that infected DCs produce infectious MMTV. To determine if MMTV-expressing DCs actively shed transmissible virus, we used BMDCs isolated from HYB PRO transgenic mice. HYB PRO mice express a molecular clone of MMTV in lymphoid tissue and mammary glands and shed infectious MMTV into milk (16, 34). The HYB PRO DCs provided us with a larger number of MMTV-expressing cells than that found in infected mice, thus allowing us to detect virus production and infection. First, we collected supernatants from HYB PRO BMDC cultures and purified the virions, from which we extracted total RNA. We then used RT-PCR with MMTV-specific primers to detect the virus. As shown in Fig. 1A, the supernatants from HYB PRO BMDCs contained virus, while no virus was found in the supernatants from control uninfected C3H/HeN BMDCs.
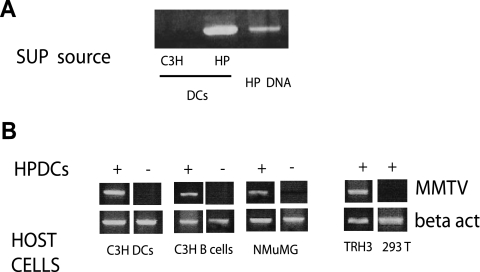
FIG. 1.
HYB PRO BMDCs shed virus and transmit infection to other cell types. (A) Viral RNAs isolated from the supernatants (SUP) of HYB PRO (HP) BMDC cultures were analyzed by RT-PCR for MMTV sequences. The supernatants of C3H/HeN BMDCs and HYB PRO genomic DNA were used as negative and positive controls, respectively. (B) HYB PRO BMDCs were cocultured for 3 days with uninfected C3H/HeN BMDCs, C3H/HeN primary B cells, and NMuMG mammary gland cells in the presence of a 0.4-μm barrier filter. +, coculture with HYB PRO BMDCs; −, coculture with C3H/HeN BMDCs. DNAs from the recipient cells were prepared, and viral DNA was detected by PCR with MMTV-specific primers. TRH3 and 293T cells were used as positive and negative controls for infection, respectively. The data are representative of three experiments with similar results.
Next, to show that HYB PRO BMDCs produced infectious virus, we cocultured them with splenic B cells and BMDCs from uninfected C3H/HeN mice, an uninfected mammary gland tissue culture cell line (NMuMG), and the human cell lines 293T and TRH3 (293T cells stably expressing the MMTV entry receptor, mouse TfR1); all coculturing was done in the presence of a 0.4-μm barrier filter. After coculturing the cells for 3 days, we removed the DCs, prepared DNAs from the recipient cells, and tested for the presence of viral DNA by PCR, using primers specific for the HYB PRO long terminal repeat. All MMTV-susceptible cells were infected after coculture with HYB PRO BMDCs, indicating that the DCs can produce virus that is transmitted to other cell types (Fig. 1B). Importantly, this infection was dependent on the presence of the MMTV entry receptor, mouse TfR1, since 293T cells lacking this receptor did not become infected.
MMTV-producing DCs transmit virus in vivo.
Next, to determine whether DCs could transmit virus in vivo, 107 naïve BMDCs or BMDCs infected in vitro with MMTV(LA) were subcutaneously injected into naïve C3H HeN mice. The injected mice were bled, and genomic DNAs were isolated from peripheral blood lymphocytes. Real-time quantitative PCR specific for MMTV(LA) was used to examine infection. As shown in Fig. 2, four of seven mice injected with infected DCs were positive for virus sequences. Thus, infected DCs could transmit virus in vivo, albeit with low efficiency.
DCs are the initial targets of MMTV infection in vivo.
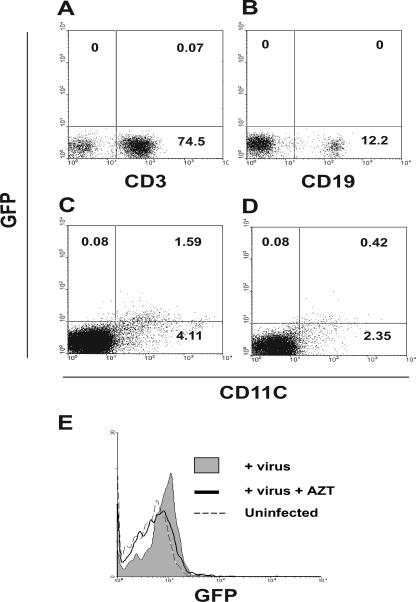
The data presented thus far indicated that MMTV-producing DCs were capable of transmitting virus to uninfected cells. Although previous work had indicated that DCs were infected in vivo, several studies indicated that B cells were the initial targets of infection (1, 2, 7, 24, 38). Moreover, it is well established that replication-competent MMTV spreads to the entire lymphoid compartment during in vivo infection (13, 41). To determine which cells (B cells, T cells, or DCs) are the initial MMTV target(s), we constructed MMTV Env-bearing pseudovirions tagged with a GFP-encoding genome and used these one-hit viruses for in vivo infection. Naïve mice received subcutaneous footpad injections of the pseudovirus, and 24 h later, lymphocytes from the draining lymph nodes were collected and analyzed by FACS. As shown in Fig. 3A and B, no GFP was associated with T (CD3+) or B (CD19+) cells. In contrast, 28% of the DCs (CD11c+) were GFP positive (Fig. 3C). Treatment of the mice with the antiretroviral drug AZT, which is known to inhibit MMTV infection, significantly diminished the percentage of GFP+ CD11c+ cells (Fig. 3D and E) (20, 29). These data indicated that DCs are the primary in vivo targets of MMTV.
FIG. 3.
In vivo infection with a GFP-tagged MMTV pseudovirus. GFP-tagged MMTV Env-pseudotyped recombinant murine leukemia virus (MMTV/GFP) was injected into the right hind footpads of mice; the left hind footpads served as uninfected controls. Mice were killed 24 h later, and single-cell suspensions were prepared from the draining and nondraining popliteal lymph nodes. Cells were stained with anti-CD3 (A), anti-CD19 (B), or anti-CD11c (C), and GFP association with each cell type was analyzed by FACS. The same experiment was performed after AZT treatment (D and E). These data are representative of three experiments with similar results.
Efficient MMTV infection in vivo requires DCs.
We next tested whether MMTV infection could proceed efficiently in vivo in the absence of DCs. We used CD11c-DTR transgenic mice, in which DCs can be transiently ablated by DT treatment (22). CD11c-DTR transgenic mice received subcutaneous footpad injections of MMTV(LA) in the presence or absence of DT treatment. At different times postinfection (1 to 4 days), lymphocytes from the draining popliteal lymph nodes were analyzed by FACS for B-cell activation (CD69+ B220+) and the percentage of Sag-reactive T cells (Vβ2+ CD4+ and Vβ6+ CD4+) and by real-time quantitative PCR to determine viral DNA levels. Lymphocytes isolated from the contralateral nondraining lymph nodes served as controls.
As previously reported, the DT-mediated depletion of DCs in transgenic mice was transient, and by day 3, the levels of CD11c+ cells in the spleens of treated animals had partially recovered (Fig. 4A) (22). However, DT treatment abolished the characteristic response to MMTV, in both the infection- and Sag-independent activation of B cells (<24 h) and the Sag/infection-dependent activation that occurs on day 4 after virus injection (Fig. 4B) (2); transgenic mice that were not treated with DT showed the characteristic response to MMTV at both early and late times after virus injection. Likewise, DT treatment completely ablated the characteristic increase in Sag-reactive Vβ2+ CD4+ cells seen on day 4 in the control group (Fig. 4C). Similar results were observed for Vβ6+ CD4+ cells (data not shown). Although low levels of viral DNA were detected in the DT-treated mice, two of five animals in the control group showed 10-fold higher infection levels (Fig. 4D). We performed this experiment three times in total and found high levels of infection in 7/14 control mice; in contrast, 0/14 DT-treated mice showed significant levels of infection. Thus, the loss of DCs at early stages of MMTV infection had a profound effect on early virus spread.
MMTV infection induces DC migration.
DCs arise from progenitors in the bone marrow, enter the blood, and traffic into secondary lymphoid organs and peripheral tissues, such as skin or the gut, where they contribute to the front line of defense against pathogens. When DCs encounter inflammatory stimuli, they undergo a switch in chemokine receptor expression from CCR6 to CCR7, enabling their egress into lymphatic vessels and transport to draining lymph nodes (33, 35). Thus, if MMTV-infected DCs are responsible for trafficking from the site of infection in the periphery to the lymph node where virus amplification occurs, they should undergo this receptor switch in response to virus.
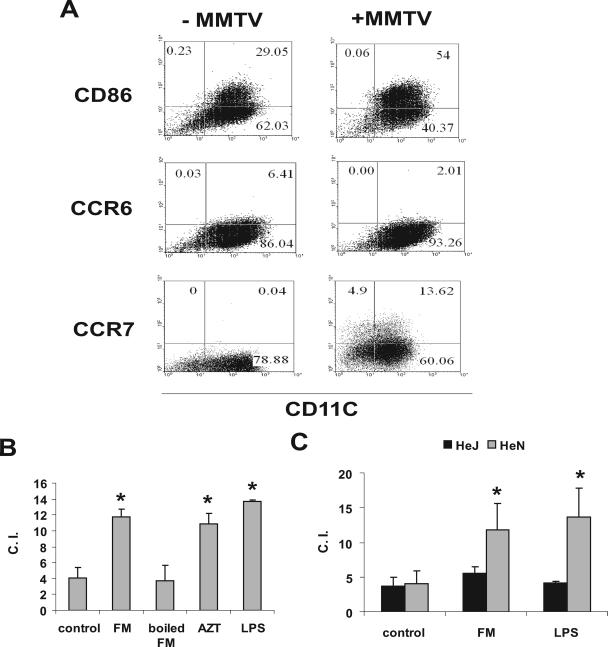
To determine if MMTV altered the migratory properties of DCs, BMDCs were produced and incubated with MMTV. Two days later, the cells were harvested, their phenotype was analyzed by FACS, and their migratory properties were tested in a chemotaxis chamber. As previously shown, MMTV induced DC maturation (8); for example, the expression of costimulatory molecules, such as CD86, increased (Fig. 5A). At the same time, there was a diminution in the percentage of CCR6+ cells and an increase in the percentage of CCR7+ cells (Fig. 5A).
FIG. 5.
MMTV induces TLR4-dependent migration of CD11c+ BMDCs toward MIP-3β. (A) C3H/HeN BMDCs were infected with MMTV. Twenty-four hours later, cells were harvested, and expression of CD86, CCR6, and CCR7 was checked by FACS. (B) Chemotaxis assays with MIP-3β were performed on the lymphocytes used for panel A. LPS, boiled virus, and AZT pretreatment of the cells were used as controls. (C) C3H/HeN and C3H/HeJ BMDCs were subjected to infection as described for panel A, and chemotaxis assays with MIP-3β were performed. These data are representative of three experiments with similar results. The error bars indicate SD. *, P ≤ 0.05. C.I., chemotactic index.
Incubation with MMTV also caused the BMDCs to migrate towards the chemokine MIP-3β, at a level similar to that induced by LPS (Fig. 5B). When the virus was inactivated by boiling, the migratory capacity was reduced to the level for untreated DCs, indicating that this effect required intact virus and ruling out endotoxin contamination as being responsible for cell movement. In contrast, treatment with AZT had no effect, indicating that MMTV-induced migration was independent of infection. Previously, we showed that MMTV activates DCs via TLR4 and that this activation is also infection independent (8). Thus, we tested DCs from C3H/HeJ mice, which harbor a nonsignaling, defective Tlr4 allele, for the ability to migrate in response to MMTV. No changes in the migratory capacity of C3H/HeJ DCs were observed after incubation with MMTV, indicating that this effect depended on a functional TLR4 receptor (Fig. 5C).
DCs are required for efficient virus amplification in the lymph nodes.
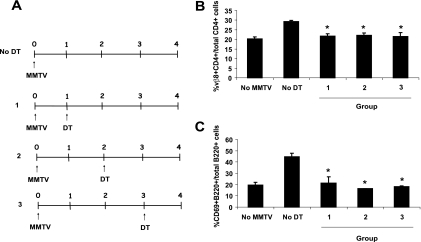
HIV type 1 (HIV-1)-infected DCs have been shown to traffic from the periphery to lymph nodes, where they transmit virus to lymphocytes (40, 42). To determine if DCs were required only at this initial step in MMTV infection or if they were important for additional steps in the pathway, such as Sag presentation to T cells in the lymph node, we infected CD11c-DTR transgenic mice with MMTV(FM) and treated the animals with DT at different times after infection (Fig. 6A). The depletion of DCs on all days significantly impaired the Sag-mediated T- and B-cell activation on day 4, indicating not only that DCs are involved in transporting virus from the site of infection to the lymph node but also that they play a modulatory role that helps establish the immune response associated with chronic MMTV infection (Fig. 6).
FIG. 6.
Effects of CD11c+ cell ablation on MMTV-dependent T- and B-cell activation at different times after infection. MMTV(FM)-inoculated mice were treated at different times postinfection with 4 ng DT/g body weight. The levels of activation of B and T cells on day 4 after infection were checked by FACS analysis of cell suspensions from the draining lymph nodes. Lymphocytes isolated from draining lymph nodes of untreated mice were used as positive controls. (A) Scheme of DT treatment. (Β) Sag-mediated T-cell activation (Vβ8+ CD4+ cells/total CD4+ cells). (C) Sag-mediated B-cell activation (CD69+ B220+ cells/total B220+ cells). Error bars indicate SD. *, P ≤ 0.05.
DISCUSSION
DCs are well-known antigen-presenting cells. These cells are derived from bone marrow progenitors that home to peripheral mucosal sites, where they differentiate into immature DCs. After contact with an antigen and with the help of maturation signals elicited by infection or inflammation, immature DCs undergo a complex transformation process. In vivo, this process culminates in DC migration to the lymphoid organs, where the mature cells can efficiently present processed antigenic peptides to T cells (3, 4).
Although DCs play an essential role in immune defense, there is increasing evidence that DCs also participate in disease pathogenesis, particularly with regard to viral infections (30). The presence of DCs at sites of virus entry can be exploited by the virus to gain access to the host. For example, it is believed that DCs found at the sites of HIV infection, such as the rectal and vaginal mucosa, are critical for transporting virus to the sites of transmission to lymphocytes and virus replication, such as the lymph nodes (37, 40). Additionally, measles virus, which is known to infect DCs, enters the body through the tracheal epithelium, where these cells are very abundant; DCs may therefore be responsible for measles virus transport to lymphoid organs and subsequent transmission to T cells, similar to the role these cells are believed to play in HIV infection (12). In both situations, the virus-DC interaction is important for both virus dissemination and modulation of antiviral T-cell responses.
We show here that DCs likely play a critical role in MMTV infection as well. Previous studies had argued that B cells were the initial cells infected by MMTV in vivo, based on the observations that infection could not be sustained in B-cell-deficient mice (7, 14) and that B cells, but not T cells, could be infected ex vivo (2, 39). However, it was clear that cells in addition to B cells could present the MMTV Sag, since B-cell-deficient mice still showed deletion of endogenous MMTV Sag-cognate T cells (7). More recently, several groups showed that DCs could be infected by MMTV in vitro and that infected DCs could be isolated from lymph nodes of mice soon after virus injection (5, 24, 38). Additionally, both the number and the percentage of DCs increased in the draining lymph nodes after subcutaneous injection of MMTV and in the Peyer's patches after milk-borne infection (8, 24).
Here we definitively show that DCs are the first cells targeted by the virus and that this infection is required for establishment of infection in vivo. Using a one-hit, GFP-tagged pseudovirus, we found that virus infection of DCs occurred in the absence of infection of any other MMTV targets, such as T or B cells. We also demonstrated that BMDCs can shed infectious virus that can be transmitted to other cell types. Finally, depletion of the CD11c+ population had a dramatic effect on infection and resulted in significant inhibition of the characteristic T- and B-cell activation mediated by MMTV. Although we saw low levels of infection in the CD11c-depleted mice, this may be the result of incomplete and transient loss of DCs in this model.
Interestingly, DC depletion not only inhibited the infection-dependent Sag-mediated stimulation of T and B cells but also inhibited the early, Sag/infection-independent activation of B cells. Previous studies had argued that MMTV directly interacted with B cells, and we showed that this B-cell activation required a functional TLR4 allele (2, 29, 39). However, we demonstrate here that in the absence of DCs, no early B-cell activation occurred in the draining lymph node. This was true even in mice in which DC ablation occurred after MMTV injection (Fig. 6), where there was clearly time for infected DCs to traffic from the site of infection to the lymph nodes and to transmit virus to other cell types, such as B cells. Thus, these data argue that TLR4-dependent B-cell activation is not due to direct interaction with the virus, but instead results from signals emanating from the MMTV-infected DCs. Several studies have also argued that MMTV-infected B cells present Sag to T cells during the early stages of virus infection. However, we saw no Sag-mediated T-cell activation, even when DCs were ablated 3 days after the injection of virus, when there was ample time for B-cell infection. These data therefore also argue either that DCs are the primary presenters of Sag or that infected B cells require some signal from DCs to effectively present this viral protein to naïve T cells.
In the case of HIV-1, DCs serve as virus reservoirs after the virus enters through CD4 and chemokine receptors (30). In addition, DCs harbor HIV-1 that enters cells through binding to C-type lectins, such as DC-SIGN; in this case, HIV-1 is stored in a cytoplasmic compartment and then transferred to CD4+ T cells in the lymph nodes (30). In both cases, DC trafficking to the lymph nodes is required for virus spread. We therefore examined whether MMTV interaction with DCs would modify their migratory properties. Our experiments clearly demonstrated that upon contact with the virus, BMDCs became more mature, up-regulated CCR7 on the surface, and migrated in response to the CCR7 ligand MIP-3β. This maturation process did not require virus replication, because AZT treatment did not affect the migratory properties of the cells. Moreover, this change in migration capacity depended on functional TLR4, since migration was not observed with BMDCs from C3H/HeJ mice, which harbor a defective Tlr4 gene. This agrees with our previous results showing increased numbers of CD11c+ DCs in the Peyer's patches of mice with wild-type but not mutant Tlr4 genes (8).
We propose the following model for MMTV infection. During milk-borne transmission, the virus encounters immature DCs resident in either the Peyer's patch or the intestinal epithelial layer; the latter class of DCs is known to sample the intestinal luminal contents (26). These DCs first interact with the virus through TLR4, which induces their maturation and the expression of higher levels of the virus entry receptor TfR1. As a consequence, DCs become susceptible to MMTV infection and, in addition, migrate to the draining lymph nodes, where they release chemokines, present Sag to T cells, and serve as a reservoir of virus. Notably, while DC activation can result from direct interaction of MMTV with TLR4, it is also likely that activation by TLR ligands found on other commensal pathogens could accomplish this step, a prediction borne out by the observation that while milk-borne MMTV infection is somewhat delayed in C3H/HeJ mice bearing a mutant TLR4 allele, it nonetheless occurs (21; our unpublished observations). Additionally, our data show that functional DCs are required for efficient infection even after their migration to the lymph nodes. This indicates that MMTV-infected DCs secrete cytokines that stimulate T- or B-cell proliferation, thereby facilitating the transfer of virus to other cell types in which replication is more efficient.
In summary, taken together with previous studies, our results indicate that DCs play a pivotal role in MMTV infection. They capture virus at the site of infection, carry it to the lymph node, support virus replication, and facilitate the infection of susceptible cells. Given the known involvement of DCs in shaping adaptive immune responses (4, 6), it is likely that the MMTV-DC interaction will also affect the host immune response to this virus.
Acknowledgments
We thank Dan Littman for the CD11c-DTR transgenic mice, Lorraine Albritton for plasmid pR739, and Fabian Benencia for helpful discussions and advice.
This work was supported by PHS grants R01CA45954, R21AI059625, and R03TW01103.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Ardavin, C., I. Ferrero, I. Azcoitia, F. Anjuere, H. Diggelman, F. Luthi, S. Luther, and H. Acha-Orbea. 1999. B cell response after MMTV infection: extrafollicular plasmablasts represent the main infected population and can transmit viral infection. J. Immunol. 162:2538-2545. [PubMed] [Google Scholar]
- 2.Ardavin, C., F. Luthi, M. Andersson, L. Scarpellino, P. Martin, H. Diggelmann, and H. Acha-Orbea. 1997. Retrovirus-induced target cell activation in the early phases of infection: the mouse mammary tumor virus model. J. Virol. 71:7295-7299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y.-J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Baribaud, F., I. Maillard, S. Vacheron, T. Brocker, H. Diggelmann, and H. Acha-Orbea. 1999. Role of dendritic cells in the immune response induced by mouse mammary tumor virus superantigen. J. Virol. 73:8403-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barton, G. M., and R. Medzhitov. 2002. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 14:380-383. [DOI] [PubMed] [Google Scholar]
- 7.Beutner, U., E. Draus, D. Kitamura, K. Rajewsky, and B. T. Huber. 1994. B cells are essential for murine mammary tumor virus transmission, but not for presentation of endogenous superantigens. J. Exp. Med. 179:1457-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burzyn, D., J. C. Rassa, D. Kim, I. Nepomnaschy, S. R. Ross, and I. Piazzon. 2004. Toll-like receptor 4-dependent activation of dendritic cells by a retrovirus. J. Virol. 78:576-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassell, D. J., and R. H. Schwartz. 1994. A quantitative analysis of antigen-presenting cell function: activated B cells stimulate naive CD4 T cells but are inferior to dendritic cells in providing costimulation. J. Exp. Med. 180:1829-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesnut, R. W., and H. M. Grey. 1981. Studies on the capacity of B cells to serve as antigen-presenting cells. J. Immunol. 126:1075-1079. [PubMed] [Google Scholar]
- 11.Czarneski, J., P. Berguer, P. Bechinschtein, I. Nepomnaschy, I. Piazzon, N. Wagner, and S. R. Ross. 2002. Neonatal infection with mouse mammary tumor virus is not dependent on b7 integrin- or L-selectin-expressing lymphocytes. Eur. J. Immunol. 32:945-956. [DOI] [PubMed] [Google Scholar]
- 12.de Witte, L., M. Abt, S. Schneider-Schaulies, Y. van Kooyk, and T. B. Geijtenbeek. 2006. Measles virus targets DC-SIGN to enhance dendritic cell infection. J. Virol. 80:3477-3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dzuris, J. L., T. V. Golovkina, and S. R. Ross. 1997. Both T and B cells shed infectious MMTV. J. Virol. 71:6044-6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golovkina, T., M. Schlomchik, L. Hannum, and A. Chervonsky. 1999. Organogenic role of B lymphocytes in mucosal immunity. Science 286:1965-1968. [DOI] [PubMed] [Google Scholar]
- 15.Golovkina, T. V., A. Chervonsky, J. A. Prescott, C. A. Janeway, and S. R. Ross. 1994. The mouse mammary tumor virus envelope gene product is required for superantigen presentation to T cells. J. Exp. Med. 179:439-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Golovkina, T. V., J. P. Dudley, A. Jaffe, and S. R. Ross. 1995. Mouse mammary tumor viruses with functional superantigen genes are selected during in vivo infection. Proc. Natl. Acad. Sci. USA 92:4828-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golovkina, T. V., J. L. Dzuris, B. van den Hoogen, A. B. Jaffe, P. C. Wright, S. M. Cofer, and S. R. Ross. 1998. A novel membrane protein is a mouse mammary tumor virus receptor. J. Virol. 72:3066-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golovkina, T. V., A. B. Jaffe, and S. R. Ross. 1994. Coexpression of exogenous and endogenous mouse mammary tumor virus RNA in vivo results in viral recombination and broadens the virus host range. J. Virol. 68:5019-5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Golovkina, T. V., I. Piazzon, I. Nepomnaschy, V. Buggiano, M. de Olano Vela, and S. R. Ross. 1997. Generation of a tumorigenic milk-borne mouse mammary tumor virus by recombination between endogenous and exogenous viruses. J. Virol. 71:3895-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Held, W., G. A. Waanders, H. Acha-Orbea, and H. R. MacDonald. 1994. Reverse transcriptase-dependent and -independent phases of infection with mouse mammary tumor virus: implications for superantigen function. J. Exp. Med. 180:2347-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jude, B. A., Y. Pobezinskaya, J. Bishop, S. Parke, R. M. Medzhitov, A. V. Chervonsky, and T. V. Golovkina. 2003. Subversion of the innate immune system by a retrovirus. Nat. Immunol. 4:573-578. [DOI] [PubMed] [Google Scholar]
- 22.Jung, S., D. Unutmaz, P. Wong, G. Sano, K. De los Santos, T. Sparwasser, S. Wu, S. Vuthoori, K. Ko, F. Zavala, E. G. Pamer, D. R. Littman, and R. A. Lang. 2003. In vivo depletion of CD11c(+) dendritic cells abrogates priming of CD8(+) T cells by exogenous cell-associated antigens. Immunity 17:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lutz, M. B., N. Kukutsch, A. L. Ogilvie, S. Rossner, F. Koch, N. Romani, and G. Schuler. 1999. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J. Immunol. Methods 223:77-92. [DOI] [PubMed] [Google Scholar]
- 24.Martin, P., S. R. Ruiz, G. Martinez del Hoyo, F. Anjuere, H. H. Vargas, M. Lopez-Bravo, and C. Ardavin. 2002. Dramatic increase in lymph node dendritic cell numbers during infection by the mouse mammary tumor virus occurs by a CD62L-dependent blood-borne DC recruitment. Blood 99:1282-1288. [DOI] [PubMed] [Google Scholar]
- 25.Nandi, S., and C. M. McGrath. 1973. Mammary neoplasia in mice. Adv. Cancer Res. 17:353-414. [Google Scholar]
- 26.Niess, J. H., and H. C. Reinecker. 2006. Dendritic cells in the recognition of intestinal microbiota. Cell. Microbiol. 8:558-564. [DOI] [PubMed] [Google Scholar]
- 27.Pear, W. S., J. P. Miller, L. Xu, J. C. Pui, B. Soffer, R. C. Quackenbush, A. M. Pendergast, R. Bronson, J. C. Aster, M. L. Scott, and D. Baltimore. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92:3780-3792. [PubMed] [Google Scholar]
- 28.Pollara, G., A. Kwan, P. J. Newton, M. E. Handley, B. M. Chain, and D. R. Katz. 2005. Dendritic cells in viral pathogenesis: protective or defective? Int. J. Exp. Pathol. 86:187-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rassa, J. C., J. L. Meyers, Y. Zhang, R. Kudaravalli, and S. R. Ross. 2002. Murine retroviruses activate B cells via interaction with Toll-like receptor 4. Proc. Natl. Acad. Sci. USA 99:2281-2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rinaldo, C. R., Jr., and P. Piazza. 2004. Virus infection of dendritic cells: portal for host invasion and host defense. Trends Microbiol. 12:337-345. [DOI] [PubMed] [Google Scholar]
- 31.Ross, S. R. 2000. Using genetics to probe host-virus interactions: the mouse mammary tumor virus model. Microbes Infect. 2:1215-1223. [DOI] [PubMed] [Google Scholar]
- 32.Ross, S. R., J. J. Schofield, C. J. Farr, and M. Bucan. 2002. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl. Acad. Sci. USA 99:12386-12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sallusto, F., P. Schaerli, P. Loetscher, C. Schaniel, D. Lenig, C. R. Mackay, S. Qin, and A. Lanzavecchia. 1998. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur. J. Immunol. 28:2760-2769. [DOI] [PubMed] [Google Scholar]
- 34.Shackleford, G. M., and H. E. Varmus. 1988. Construction of a clonable, infectious, and tumorigenic mouse mammary tumor virus provirus and a derivative genetic vector. Proc. Natl. Acad. Sci. USA 85:9655-9659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sozzani, S., P. Allavena, G. D'Amico, W. Luini, G. Bianchi, M. Kataura, T. Imai, O. Oshie, R. Bonecchi, and A. A. Mantovani. 1998. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J. Immunol. 161:1083-1086. [PubMed] [Google Scholar]
- 36.Sprecher, E., and Y. Becker. 1989. Langerhans cell density and activity in mouse skin and lymph nodes affect herpes simplex type 1 (HSV-1) pathogenicity. Arch. Virol. 107:191-205. [DOI] [PubMed] [Google Scholar]
- 37.Steinman, R. M., A. Granelli-Piperno, M. Pope, C. Trumpfheller, R. Ignatius, G. Arrode, P. Racz, and K. Tenner-Racz. 2003. The interaction of immunodeficiency viruses with dendritic cells. Curr. Top. Microbiol. Immunol. 276:1-30. [DOI] [PubMed] [Google Scholar]
- 38.Vacheron, S., S. J. Luther, and H. Acha-Orbea. 2002. Preferential infection of immature dendritic cells and B cells by mouse mammary tumor virus. J. Immunol. 168:3470-3476. [DOI] [PubMed] [Google Scholar]
- 39.Vacheron, S., T. Renno, and H. Acha-Orbea. 1997. A highly sensitive in vitro infection assay to explore early stages of mouse mammary tumor virus infection. J. Virol. 71:7289-7294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 41.Waanders, G. A., A. N. Shakhov, W. Held, P. Karapetian, H. Acha-Orbea, and H. R. MacDonald. 1993. Peripheral T cell activation and deletion induced by transfer of lymphocyte subsets expressing endogenous or exogenous mouse mammary tumor virus. J. Exp. Med. 177:1359-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilflingseder, D., Z. Banki, M. P. Dierich, and H. Stoiber. 2005. Mechanisms promoting dendritic cell-mediated transmission of HIV. Mol. Immunol. 42:229-237. [DOI] [PubMed] [Google Scholar]
- 43.Yoshimoto, T., H. Nagase, H. Nakano, A. Matsuzawa, and H. Nariuchi. 1994. A Vβ8.2-specific superantigen from exogenous mouse mammary tumor virus carried by FM mice. Eur. J. Immunol. 24:1612-1619. [DOI] [PubMed] [Google Scholar]
- 44.Zhang, Y., J. C. Rassa, E. M. deObaldia, L. Albritton, and S. R. Ross. 2003. Identification of the mouse mammary tumor virus envelope receptor-binding domain. J. Virol. 77:10468-10478. [DOI] [PMC free article] [PubMed] [Google Scholar]