Abstract
Following genital herpes simplex virus type 2 (HSV-2) exposure, NK cells and T cells are mobilized to sites of infection to control viral replication and spread. The present investigation sought to determine the role of the chemokine receptor CCR5 in this process. Mice deficient in CCR5 (CCR5−/−) displayed a significant reduction in cumulative survival following infection in comparison to wild-type, HSV-2-infected controls. Associated with decreased resistance to viral infection, CCR5−/− mice yielded significantly more virus and expressed higher levels of tumor necrosis factor alpha, CXCL1, CCL2, CCL3, and CCL5 in the vagina, spinal cord, and/or brain stem than did wild-type mice. Whereas there was no difference in absolute number of leukocytes (CD45high), CD4 T cells, or CD8 T cells residing in the draining lymph nodes, spleen, spinal cord, or brain stem comparing HSV-2-infected wild-type to CCR5−/− mice prior to or after infection, there were significantly more NK cells (NK1.1+ CD3−) residing in the brain stem and spleen of infected wild-type mice. Functionally, NK activity from cells isolated from the brain stem of HSV-2-infected wild-type mice was greater than that from HSV-2-infected CCR5−/− mice. In addition, antibody-mediated depletion of NK cells resulted in an increase in HSV-2 levels in the vaginal, spinal cord, and brain stem tissue of wild-type but not CCR5−/− mice. Collectively, the absence of CCR5 expression significantly impacts the ability of the host to control genital HSV-2 infection, inflammation, and spread associated with a specific reduction in NK cell expansion, infiltration, and activity in the nervous system.
With greater than 1.6 million Americans infected annually (4), herpes simplex virus type 2 (HSV-2) is a sexually transmitted pathogen with a significant impact on public health. Typically, infection results in a lifelong latent infection of the host (15, 52). Causes for the success of HSV-2 in the human population include asymptomatic shedding of the virus even in the presence of CD8+ cytotoxic T lymphocytes and the production of a viral glycoprotein that indirectly elicits NK cell death (8, 44, 51). There is also evidence to suggest that genital HSV-2 infection increases the susceptibility of individuals to other sexually transmitted diseases, including human immunodeficiency virus (6, 20). Consequently, it is imperative to characterize the host response to infection and identify key components that contribute to virus resistance to devise a strategy that will significantly reduce viral prevalence.
The development of a mouse model of genital HSV-2 infection (7, 40) has contributed to our understanding of the innate and adaptive immune response to infection. Specifically, the application of progesterone to mice prior to infection enhances susceptibility through the direct immunosuppressive action of the hormone and expression of a herpesvirus entry receptor, nectin 1 (20, 25, 34). In this rodent model, investigators have demonstrated the importance of the innate immune response, including neutrophils, NK cells, NKT cells, and the production of alpha/beta interferon (IFN-α/β), interleukin-12 (IL-12), IL-15, and IL-18 in suppressing HSV-2 replication and reducing virus-mediated mortality (5, 13, 16, 30, 36). Relative to the adaptive immune response, T and B cells are involved in controlling virus infection, although T cells appear to be more critical in this process (12, 28, 29, 31, 35, 41). The contribution of IFN-γ produced by CD4+ T cells in reducing HSV-2 pathogenesis has been demonstrated using CD4-deficient mice and CD4 T cell, CD8 T cell, and IFN-γ depletion studies (17, 32, 42). Such results are consistent with a supportive role in the development of a Th1 response (55) and a detrimental role in the development of a Th2 response (38) in the host following HSV-2 infection.
Mobilization of T and B cells between the vaginal epithelium and the draining (iliac) lymph nodes during genital HSV-2 infection have been described previously (22). However, the role of chemokines in regulating this process is relatively unknown. CCR5 and CCR1 have been implicated in resistance to intraperitoneal infection, with HSV-2 impacting NK cell recruitment and activity at the site of infection (3, 49). Using a more conventional site of infection, RANTES/CCL5 (regulated on activation, normal T-cell expressed, and secreted) was found to reduce HSV-2-induced morbidity and mortality associated with an increase in IL-2 and IFN-γ production in vaccinated mice when administered in the form of a DNA vaccine adjuvant (48). Since it has been reported that CCR5-deficient mice show a decrease in Th1 responses as measured by a decrease in IFN-γ and IL-12 production in response to infection or inflammation (1, 2), we predicted that mice deficient in CCR5 would respond poorly to genital HSV-2 infection as a result of an inability to mobilize effector cells (i.e., NK and T cells) to the site of infection. To test this hypothesis, mice deficient in CCR5 were evaluated for resistance to HSV-2 infection, focusing on the inflammatory response and recruitment of cells within the nervous system as it relates to virus titer.
MATERIALS AND METHODS
Virus and cells.
HSV-2 (clinical isolate obtained from Charity Hospital, New Orleans, LA) was propagated in Vero cells. Stock virus was stored at −80°C at a concentration of 1.5 × 107 PFU/ml and diluted in RPMI 1640 medium containing 10% fetal bovine serum (FBS) immediately before use. African green monkey kidney fibroblasts (Vero cells, ATCC CCL-81; American Type Culture Collection, Manassas, VA) were propagated in RPMI 1640 medium containing 10% FBS, gentamicin, and antimycotic-antibiotic solution (complete medium; Invitrogen, Carlsbad, CA) at 37°C, 5% CO2, and 95% humidity.
Mice.
Female (6 to 8 weeks of age) C57BL/6 wild-type (WT) mice (The Jackson Laboratory, Bar Harbor, ME) and CCR5-deficient (CCR5−/−) mice, backcrossed to the C57BL/6 genetic background for 10 generations (24), were used in these experiments. Five days following a subcutaneous injection of 2.0 mg DepoProvera (Pharmacia and Upjohn Co., Kalamazoo, MI), mice were intravaginally challenged with HSV-2 (2,000 PFU in 20 μl). Starting at day 1 postinfection (p.i.) and continuing through day 7 p.i., vaginal lavages using 20 μl of complete medium were performed and fluids were collected and assayed for viral content by plaque assay. Mice were euthanized at times p.i. to determine virus titer, perform phenotypic analysis of leukocytes within the spinal cord, brain stem, spleen, and inguinal/iliac lymph nodes, and measure cytokine/chemokine content within infected tissues. Alternatively, mice were monitored for survival over 30 days p.i. All procedures were approved by The University of Oklahoma Health Sciences Center and Dean A. McGee Eye Institute animal care and use committee.
Virus plaque assay.
Tissues (vagina, spinal cord, and brain stem) obtained from HSV-2-infected mice were placed in complete medium and homogenized using a tissue homogenizer (Fisher Scientific, Pittsburgh, PA). Supernatants were clarified (10,000 × g, 1 min) and subsequently assessed for virus content by plaque assay (18). Results are reported in log PFU/g tissue or ml (in case of tear film). If no virus was detected in the sample, the sample was assigned a value of 0.0001 log PFU.
Enzyme-linked immunosorbent assay.
Detection of CCL2, CCL3, CCL5, CXCL1, IL-1β, IL-6, tumor necrosis factor alpha (TNF-α), and IFN-γ were performed using a suspension array system (Bio-Rad, Hercules, CA) with a sensitivity of 1 to 2 pg/tissue for each targeted analyte. Samples were analyzed in duplicate along with known amounts of cytokine/chemokine, which were used to generate standard curves for each cytokine/chemokine. The amount of cytokine/chemokine measured was normalized to the wet weight of tissue.
Lymph node cells and spleen cells.
Inguinal and iliac lymph nodes (I/ILN) and spleens from infected and uninfected perfused mice were collected and passed through a 70-μm cell strainer (BD Biosciences, Bedford, MA). The strainer was flushed with 5.0 ml of RPMI 1640 containing 10% FBS to generate single-cell suspensions. The cells were counted, following trypan blue staining, and subsequently analyzed by flow cytometry or NK activity.
Vaginal tissue, spinal cord, and brain stem cell suspensions.
Mice were anesthetized and perfused at day 7 p.i., and the spinal cord and brain stem were removed and subjected to homogenization on ice using a Dounce homogenizer (Fisher Scientific). Following homogenization, samples were passed through a 70-μm cell strainer (BD Biosciences), flushed with 5 ml of RPMI 1640, centrifuged (300 × g, 5 min), and resuspended in 4.0 ml phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA). Vaginas were removed and placed in 1 ml PBS containing 1.0 mg collagenase type I (Sigma Chemical Co.). The tissue was triturated every 10 min for 30 min and then passed through a 10-μm cell strainer (BD Biosciences). Samples were subsequently prepared for flow cytometry. For samples that were assessed for NK cytolytic activity, CD45+ cells were enriched from brain stem single-cell suspensions using anti-CD45-conjugated microbeads (Miltenyi Biotec, Auburn, CA).
Flow cytometry.
Single-cell suspensions of I/ILN and spleen cells (1 × 106 cells) were transferred into 5-ml polystyrene round-bottom tubes (Becton Dickinson, Franklin Lakes, NJ) in 100 μl of PBS containing 1% BSA. Cells were incubated with 2 μl of anti-mouse CD16/32 (Fcγ III/II receptor) (2.4G2; BD Pharmingen, San Diego, CA) for 20 min on ice. Following the incubation, cells were washed with 1.0 ml of ice-cold PBS containing 1% BSA (300 × g, 5 min at 4°C) and labeled with 1 to 2 μg of antibodies obtained from BD Pharmingen, phycoerythrin (PE)-conjugated anti-CD3 (clone 17A2) with either fluorescein isothiocyanate (FITC)-conjugated anti-CD4 (clone RM4-5) or anti-CD8α (clone 53-6.7) or a combination of FITC-conjugated anti-CD3 and PE-conjugated anti-NK1.1 (clone PK136), in 50 μl of PBS containing 1% BSA. Following a 30-min incubation in the dark on ice, the cells were washed twice with 1 ml PBS containing 1% BSA (300 × g, 5 min at 4°C) and resuspended in 1% paraformaldehyde. After a 20-min incubation at room temperature, the cells were centrifuged (300 × g, 5 min) and resuspended in 1 ml of PBS containing 1% BSA for analysis by flow cytometry. For measuring vaginal, spinal cord, and brain stem T and NK cell content, single-cell suspensions were triple labeled with PE-conjugated anti-CD3, FITC-conjugated anti-CD4 or -CD8, and PE-Cy5-conjugated anti-CD45 (clone 30-F11; BD Pharmingen) or FITC-conjugated anti-CD3, PE-conjugated anti-NK1.1, and PE-Cy5-conjugated anti-CD45. Following a 30-min incubation in the dark on ice, the cells were washed three times with 1 ml PBS containing 1% BSA (1,000 × g, 5 min at 4°C) and resuspended in 1% paraformaldehyde. After a 60-min incubation period at room temperature, the cells were centrifuged (300 × g, 5 min) and resuspended in 3 ml of PBS containing 1% BSA for analysis by flow cytometry. Twenty microliters of Countbright (Invitrogen, Eugene, OR) beads (20,800 beads) was added to each spinal cord and brain stem sample, followed by gentle mixing immediately before analysis by flow cytometry. Cells from the I/ILN, spleen, vaginal tissue, spinal cord, and brain stem were analyzed on a Coulter Epics XL flow cytometer (Beckman Coulter, Miami, FL), and the data were analyzed using EXPO 32 ADC software (Beckman Coulter). Spinal cord and brain stem samples were gated on CD45-expressing cells, and the percentages of CD4 and CD8 T cells as well as NK cells were determined under this gate setting. A second gate was used to count the number of beads that passed thru during the sampling time. Samples were analyzed for 1,100 s; the absolute number of leukocytes (CD45high) in the tissue was determined by multiplying the ratio of number of input beads/number collected per sample × the number of CD45high events × the dilution factor. The absolute number of lymphocytes was then determined based on the absolute number of CD45high cells and the percentage of each lymphocyte population per sample. Isotypic control antibodies were included in the analysis to establish background fluorescence levels. Likewise, samples from uninfected mice were also analyzed to determine the degree of contamination from incomplete perfusion.
NK cell cytolytic assay using CFSE-labeled Yac-1 cells.
NK cell cytolytic assay was performed using CellTrace carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen)-labeled Yac-1 cells as target cells. Specifically, 5 mM CellTrace CFSE dye stock solution was prepared in 18 μl of the dimethyl sulfoxide. The stock solution was diluted in PBS to the desired working concentration of 0.25 μM. The desired number of Yac-1 cells was resuspended in prewarmed (37°C) PBS containing CFSE (0.25 μM) and incubated for 15 min at 37°C. After the incubation, cells were washed with PBS and resuspended in fresh prewarmed RPMI 1640 containing 10% FBS. CD45-enriched cells from brain stems of WT or CCR5−/− mice were added to the CFSE-labeled YAC-1 target cells in 96-well microtiter plate wells in a volume of 200 μl of RPMI 1640 containing 10% FBS at an effector-to-target cell ratio of 25:1 for brain stem cell samples. After a 4-h incubation at 37°C in 5% CO2-95% air, the cells were transferred to 5-ml polystyrene round-bottom tubes (Becton Dickinson, Franklin Lakes, NJ), and fresh medium was added to make a total volume of 1 ml. To each tube, 0.5 μl of propidium iodide (0.5 μg) was added and incubated for 15 min at 37°C. After the incubation, cells were washed twice with 1× PBS and resuspended in 1× PBS. Cells were immediately processed on a Coulter Epics XL flow cytometer (Beckman Coulter, Miami, FL), and the data were analyzed using EXPO 32 ADC software (Beckman Coulter). The gate was set for CFSEhigh-expressing cells. The percent cytotoxicity was calculated using the following formula: % cytotoxicity = number of propidium iodide-labeled CFSE-expressing cells/total number of CFSE-expressing cells × 100.
Real time reverse transcription-PCR.
Total RNA was isolated from infected CCR5−/− and WT mouse brain stems 7 days following infection with HSV-2 in Ultraspect RNA isolation reagent (Biotecx Inc., Houston, TX) by following the manufacturer's protocol. First-strand cDNA was synthesized using avian myeloblastosis virus reverse transcriptase (Promega) and an oligo(dT) primer (Promega). Real-time PCR was carried out in 200-μl flat-cap PCR tubes (Bio-Rad) using a Bio-Rad icycler. Real-time PCR conditions for all primers included an initial denaturation step for 3 min at 95°C, followed by 40 cycles at 95°C for 30 s and annealing/elongation at 60°C for 50 s. Each reaction mixture contained 5 μl of cDNA and 45 μl of iQ Supermix (Bio-Rad) supplemented with the primer sequences. The PCR results were analyzed on the iCycler Software (version 3.0), and threshold cycles were determined as follows: after subtraction of the background fluorescence for each sample, the threshold fluorescence for each gene was determined at that point where the relative light units reached a level above 10 standard deviations over the baseline relative light units. The semiquantitative comparison between samples was calculated using the ΔCT method as previously described (19). Oligonucleotide sequences for the targeted genes include the following: B-actin, 5′-GCTGTATTCCCCTCCATCGTG-3′ and 5′-CACGGTTGGCCTTAGGGTTCA-3′; granzyme A, 5′-CACGGTTGTTCCTCACTCAA-3′ and 5′-CAGCAGTCAACACCCAGTTC-3′; granzyme B, 5′-CTCGACCCTACATGGCCTTA-3′ and 5′-GAGCAGCAGTCAGCACAAAG-3′; perforin, 5′-GAATGCAAGCAGAAGCACAA-3′ and 5′-TGTGTGTTCACTGGGAAGGA-3′.
NK cell depletion.
Five days following subcutaneous injection with 2.0 mg DepoProvera (Pharmacia and Upjohn Co.), mice were anesthetized, administered 100 μg of rat anti-mouse NK1.1 (Leinco Technologies, Inc., St. Louis, MO) or rat isotypic control (immunoglobulin G2a) (Southern Biotech, Birmingham, AL) retro-orbitally, and infected with 2,000 PFU HSV-2/vagina. At 3 days p.i., the mice were again treated with 100 μg of rat anti-mouse NK1.1 or the rat isotope control. Seven days following infection, the mice were anesthetized and perfused with 20 ml PBS, and vaginal tissue, spinal cord, and brain stem were removed and processed as described above for viral content or flow cytometry.
Statistics.
One-way analysis of variance and Tukey's post hoc t test were used to determine significant (P < 0.05) differences between WT and CCR5−/− mice for each parameter measured. For survival studies, the Mann-Whitney rank order test was performed for each infectious dose at each time point from day 1 p.i. to day 30 p.i. All statistical analyses were carried out using the GBSTAT program (Dynamic Microsystems, Silver Spring, MD).
RESULTS
CCR5−/− mice are highly susceptible to genital HSV-2 infection.
The sensitivity of WT and CCR5−/− mice to genital HSV-2 infection was initially evaluated measuring virus-mediated mortality. Using three different virus inoculums, including 20,000, 2,000, and 500 PFU HSV-2/vagina, WT mice were found to show greater resistance to virus infection, as determined by a delay in mortality and number of survivors at each infectious dose of virus, than CCR5−/− mice (Fig. 1). Consistent with this finding, CCR5−/− mice were found to shed more virus in the vaginal lumen from day 3 to day 7 p.i. than WT control animals (Fig. 2a). Likewise, vaginal tissue, spinal cord, and brain stem from the CCR5−/− mice harbored more infectious virus than WT mice at day 7 p.i. (Fig. 2b). No significant differences of infectious virus were found in the vaginal tissue and spinal cord at an earlier time point (day 3 p.i.) comparing the two groups of mice (Fig. 2b). In addition, HSV-2 was not found in the brain stem at day 3 p.i. in either the CCR5−/− or WT mice.
FIG. 1.
CCR5-deficient mice are highly susceptible to genital HSV-2 infection. WT and CCR5-deficient (CCR5 KO) mice (11 to 23/group per infectious inoculum) were vaginally infected with HSV-2 at 20,000 PFU (a), 2,000 PFU (b), or 500 PFU (c) and monitored and recorded for survival. *, P < 0.05 comparing the WT and CCR5 KO mice for each time point.
FIG. 2.
HSV-2 titers are elevated in CCR5−/− mice. (a) Vaginal lavages using 20 μl of complete medium were performed and fluids were obtained from HSV-2-infected (2,000 PFU/vagina) WT and CCR5-deficient (CCR5 KO) mice (n = 12 to 20/group/time point) at the indicated time p.i. and assayed for viral content by plaque assay. Each point represents the mean ± standard error of the mean. *, P < 0.05 comparing WT to CCR5 KO mice. (b) Vaginal tissue (V), spinal cord (SC), and brain stem (BS) were obtained from infected WT and CCR5 KO mice (n = 8 to 15/group/time point) at day 3 or day 7 p.i. Following homogenization of each sample, the supernatant was clarified and assessed for viral content by plaque assay. Bars represent means ± standard errors of the means. *, P < 0.05 comparing the WT and CCR5 KO groups.
CCR5−/− mice express higher levels of TNF-α and selective chemokines in infected tissue.
CCR5 is the receptor for CCL3, CCL4, and CCL5, which are chemokines that facilitate the recruitment of effector cells known to regulate virus infection, including NK cells and activated T cells (56). To determine if any of these chemokines were expressed locally, vaginal tissue was assessed for chemokine content prior to and following HSV-2 infection. Although CCL4 was not measured, both CCL3 and CCL5 protein levels were up-regulated within the first 24 h p.i. (Fig. 3a). In addition, CXCL1 and CCL2 levels were increased in the infected vaginal tissue compared to uninfected controls at 24 h p.i. (Fig. 3a). Proinflammatory cytokine levels, including IL-1β and TNF-α, were not found to be significantly different from uninfected samples when measured 24 h p.i. (Fig. 3a). Based on this outcome, WT and CCR5−/− mice were compared for cytokine/chemokine levels at an early (day 3 p.i.) and later (day 7 p.i.) time point during acute genital HSV-2 infection. There was no significant difference in the cytokine/chemokine levels in the infected tissues, vagina, spinal cord, or brain stem, at day 3 p.i. between the WT and CCR5−/− mice (data not shown). By day 7 p.i., vaginal tissue from CCR5−/− mice contained significantly more TNF-α and CXCL1 than did WT samples (Fig. 4a). Likewise, TNF-α, CXCL1, CCL2, CCL3, and CCL5 were elevated in the spinal cord (Fig. 4b) and/or brain stem (Fig. 4c) of the CCR5−/− mice compared to infected WT mice. Similar to what was found in the vaginal samples, all levels of cytokines and chemokines measured within the spinal cord and brain stem of infected mice far exceeded what was found in the uninfected control mice (Fig. 3b), suggesting the expression is driven by HSV-2 infection.
FIG. 3.
Chemokines are up-regulated in HSV-2-infected vaginal tissue. (a) Vaginal tissue (n = 4) from HSV-2-infected (2,000 PFU/vagina) mice was collected 24 h p.i. and surveyed for cytokine/chemokine content using a suspension array system. Uninfected tissue was assayed to determine basal measurements in the vagina (a), spinal cord (b), and brain stem (b) (n = 3/group). Bars represent means ± standard errors of the means. *, P < 0.05 comparing infected to uninfected vaginal tissue.
FIG. 4.
TNF-α and selective chemokine levels are elevated in HSV-2-infected CCR5-deficient mice. WT and CCR5-deficient (CCR5 KO) mice (n = 8/group) were infected with HSV-2 (2,000 PFU/vagina). Seven days following infection, the mice were euthanized and perfused, and the vagina (a), spinal cord (b), and brain stem (c) were collected and assayed for cytokine/chemokine content using a suspension array system. Bars represent means ± standard errors of the means. *, P < 0.05 comparing the WT and CCR5 KO mice for each analyte/tissue.
NK cell but not T cell infiltration into the nervous system is reduced in CCR5−/− mice following genital HSV-2 infection.
To further evaluate the impact of CCR5 expression on the host response to genital HSV-2 infection, NK and T cell infiltration was measured in spinal cord and brain stem samples from WT and CCR5−/− mice at times post infection. We found no significant differences in the absolute number of CD45high, NKT, CD4+ T, CD8+ T, or total CD3+ T cell populations infiltrating the spinal cord or brain stem samples between the WT and CCR5−/− mice in response to genital HSV-2 infection (data not shown). However, there was a noticeable difference in the number of NK cells recruited to the spinal cord and brain stem following virus infection. Specifically, NK cell numbers were elevated in the spinal cord of WT mice compared to CCR5−/− mice by day 7 p.i. The difference became much more apparent in the brain stem, with a significant reduction in the infiltration of NK cells in the CCR5−/− mice at day 5 and day 7 p.i. (Fig. 5). Surprisingly, only 15 to 20% of the NK cells infiltrating the nervous system express CCR5 (data not shown), suggesting factors independent of CCR5 influence the migration of these effector cells to the nervous system during genital HSV-2 infection.
FIG. 5.
Mobilization of NK cells in CCR5-deficient mice is modified following HSV-2 infection. WT and CCR5-deficient (CCR5−/−) mice (n = 6 to 9/group) were infected with HSV-2 (2,000 PFU/vagina). Five or seven days p.i., the mice were euthanized and perfused, and the spleen (A), I/ILN (B), spinal cord (C), and brain stem (D) were processed and analyzed for NK cell (NK1.1+ CD3−) content by flow cytometry. The day zero time point represents uninfected controls. Bars represent means ± standard errors of the means. **, P < 0.01; *, P < 0.05 comparing the WT and CCR5−/− groups.
NK cell infiltration was also investigated in the vaginal tissue during the initial period of infection (day 3 p.i.). Although there was no difference in the absolute number of NK cells residing in the vagina of HSV-2-infected WT (2,052 ± 730 NK cells) versus CCR5−/− (2,240 ± 496 NK cells) mice at 3 days p.i., there was a modest increase (P < 0.05) in the total number of infiltrating leukocytes (defined as CD45high) in the vaginal tissue of CCR5−/− mice (17,713 ± 2,142 cells) compared to WT controls (10,519 ± 486 cells).
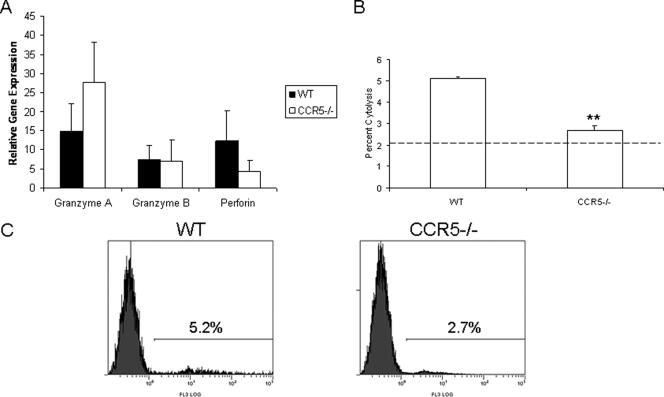
Since the number of NK cells infiltrating the brain stem was elevated in WT mice, the level of perforin, granzyme A, and granzyme B expression was measured by real-time PCR (Fig. 6a). The results revealed no significant differences in the expression of these effector molecules in brain stem tissue. Nevertheless, an increase in NK cytolytic activity by cells obtained from the brain stem of WT mice was observed in comparison to brain stem cells from CCR5−/− mice (Fig. 6b and c).
FIG. 6.
NK activity is reduced in the brain stem leukocyte population from HSV-2-infected CCR5−/− mice. (A) Brain stem samples from C57BL/6 WT and CCR5-deficient (CCR5−/−) mice (n = 3/group) were collected at day 7 following infection with HSV-2 (2,000 PFU/vagina). Total RNA was extracted from the samples, and 1-μg samples were used for first-strand cDNA synthesis. The first-strand cDNA along with primers specific for granzyme A, granzyme B, and perforin were subjected to real-time PCR. The mean relative value ± standard error of the mean for each targeted gene was determined using the ΔCT method (20). (B) CD45-enriched cell from the brain stems of C57BL/6 WT and CCR5-deficient (CCR5−/−) mice were assayed for NK activity using CFSE-labeled YAC-1 cells as targets and measuring propidium iodide uptake in the YAC-1 targets at an effector-to-target cell ratio of 25:1. The data are displayed as mean cytolytic activity + standard error of the mean. The background (absence of effector cells) level is shown by the dotted line. (C) A representative profile of propidium iodide uptake in YAC-1 cells incubated with WT CD45-enriched brain stem cells (left panel) and CCR5−/− CD45-enriched brain stem cells (right panel) is shown.
In addition to differences in NK cells recruited to the nervous system comparing WT to CCR5−/− mice, there was also a significant increase in the number of NK cells residing in the spleen but not draining I/ILN of WT mice following HSV-2 infection (Fig. 5). The increase in the absolute number of splenic NK cells in WT mice matched the increase in NK activity in comparison to splenic cells obtained from CCR5−/− mice (data not shown).
Depletion of NK cells results in an increase in HSV-2 in the vagina, spinal cord, and brain stem.
Since the above findings suggest that NK cells are involved in controlling HSV-2 replication following genital infection, mice depleted of NK cells were evaluated for virus titer in infected tissues. NK cell levels in the vaginal tissue, spinal cord, and brain stem of mice treated with anti-NK1.1 antibody were significantly reduced (>97%) in comparison to isotype-treated controls (Fig. 7a to c). In comparison, there was no significant difference in the CD4 or CD8 T cells residing in the infected tissue of anti-NK1.1- versus isotype-treated controls (Fig. 7a to c). Conversely, there was a rise in HSV-2 recovered in the infected tissue of NK cell-depleted WT mice in comparison to control-treated mice (Fig. 7d). There was no additional increase in the viral titer of NK-depleted CCR5−/− mice in comparison to the isotypic control-treated CCR5−/− animals (Fig. 7d).
FIG. 7.
NK cell depletion of WT mice increases HSV-2 titers. C57BL/6 mice (n = 5/group) were treated with anti-NK1.1 antibody or isotypic control at the time of infection and 3 days p.i. At 7 days p.i., the mice were euthanized and perfused, and the vaginal tissue (a), spinal cord (b), and brain stem (c) were processed for flow cytometric analysis for detection of NK, CD4 T, and CD8 T cells. (d) Alternatively, processed tissue from NK cell-depleted or control (isotype) WT or CCR5−/− mice (n = 3/group) were assessed for HSV-2 levels by plaque assay. This experiment is representative of results from two experiments. Bars represent the mean log PFU ± standard errors of the means.
DISCUSSION
The present study reports a heightened sensitivity to genital HSV-2 infection in mice deficient in CCR5 expression. While it was predicted that the lack of CCR5 would diminish the capacity of T cells to infiltrate into the site of infection, we found no difference in the percentage or absolute number of T cells recruited to the central nervous system (CNS) following genital HSV-2 infection. Our findings are consistent with those previously reported showing no apparent change in T-cell recruitment into the CNS following an intracranial infection with lymphocytic choriomeningitis virus (11), suggesting redundancy in the recruitment of T cells into the nervous system following virus infection. Although there were no differences in the total leukocyte (CD45high) or T-cell infiltration into the CNS of HSV-2-infected mice, a reduction in NK cell mobilization and NK activity was readily apparent in the brain stem of CCR5-deficient mice in comparison to WT animals at day 5 and 7 p.i. The importance of NK cells in the control of HSV-2 replication and spread was underscored in a depletion study in which the near absence (>97% depletion) of NK cells led to a rise in HSV-2 recovered in the infected tissue of WT mice but no change in virus levels found in NK-depleted CCR5−/− mice. Interestingly, we found that only 15 to 20% of the NK cells residing in the brain stem of WT mice expressed CCR5. The lack of uniform, high-level expression may be the result of elevated levels of the natural ligands for this receptor, including CCL3 and CCL5, which has previously been shown to interfere with the detection of CCR5 on NK cells (26). The significant impact of CCR5 mobilization of NK cells in response to HSV-2 has also been described for an unrelated pathogen, Toxoplasma gondii (21). Taken together, it would appear that CCR5 contributes significantly to NK cell trafficking and is critical for control of genital HSV-2 infection via these effector cells.
Changes in NK cell mobilization to the brain stem did not correlate with the level of chemokines expressed within the infected tissue. Rather, an increase in virus titer was associated with an increase in CXCL1, CCL2, CCL3, and CCL5 levels found in the spinal cord or brain stem of CCR5−/− mice. Previous results suggest that the severity of CNS inflammation and demyelination, as a result of macrophage recruitment in response to mouse hepatitis virus infection, is related to the local expression of CCL5 (14). Likewise, there is evidence to suggest that CNS levels of CCL2 are highly correlative with HSV encephalitis (23, 37). However, we found that levels of CCL2 or CCL5 within the CNS do not correspond to virus-mediated mortality as a result of ocular HSV-1 infection (10, 53). Of the remaining two chemokines we found elevated in the spinal cord or brain stem of HSV-2-infected CCR5−/− mice, CXCL1 has been found to activate ERK1/2 and phosphatidylinositol 3-kinase pathways and induce tau phosphorylation in cortical neurons (54), whereas CCL3 expression has been linked to a number of CNS inflammatory infections including dengue virus (45), progressive multifocal leukoencephalopathy associated with AIDS (9), and Listeria meningoencephalitis (47). It is equally likely that the increased expression of these chemokines in the CCR5−/− mice is not selective but reflects the inability of the host to control virus replication and spread, consistent with what has been described in a simian immunodeficiency virus encephalitic model (46).
In the present study, TNF-α was the sole cytokine found to be consistently up-regulated in HSV-2-infected CCR5−/− mouse tissues in comparison to WT infected mice. HSV-2 has previously been reported to induce TNF-α in vitro in dendritic cells and macrophages (27, 43). However, the relevance of this cytokine as an antiviral mediator in the mouse model is questionable, since neutralization of TNF-α has no effect on virus resolution following genital HSV-2 infection (33). However, elevated expression of TNF-α has been associated with other CNS infections, including rabies and trypanosomiasis, ultimately resulting in encephalitis and meningoencephalitis, respectively (39, 50). TNF-α is also a neurodegenerative cytokine due to its augmentation of glutamate neurotoxicity (57). Taken together, it seems likely that TNF-α may also contribute to the enhanced sensitivity of HSV-2-infected CCR5−/− mice as a result of its elevated expression in the CNS.
Collectively, this study indicates that the absence of CCR5 diminishes the capacity of the host to control genital HSV-2 infection, especially within the nervous system. The absence of CCR5 had no significant impact on T-cell mobilization or recruitment to sites of infection. Rather, NK cell infiltration was diminished, and a reduction in NK cell levels was associated with a rise in tissue-associated HSV-2 levels. It seems apparent that one or more CCR5 ligands are essential for an efficient immune response that ultimately clears the infection. Consequently, even though redundancy is often found within chemokine studies, CCR5 expression appears to be a necessary component within the host's immune repertoire to control genital HSV-2 infection in mice.
Acknowledgments
We are grateful to Lisa Tomanek and Todd Wuest for their excellent technical help.
This work was supported by USPHS grant AI053108 and NEI core grant EY12190.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Aliberti, J., C. R. Sousa, M. Schito, S. Hieny, T. Wells, G. B. Huffnagle, and A. Sher. 2000. CCR5 provides a signal for microbial induced production of IL-12 by CD8α+ dendritic cells. Nat. Immunol. 1:83-87. [DOI] [PubMed] [Google Scholar]
- 2.Andres, P. G., P. L. Beck, E. Mizoguchi, A. Mizoguchi, A. K. Bhan, T. Dawson, W. A. Kuziel, N. Maeda, R. P. MacDermott, D. K. Podolsky, and H. C. Reinecker. 2000. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J. Immunol. 164:6303-6312. [DOI] [PubMed] [Google Scholar]
- 3.Ank, N., K. Peterson, L. Malmgaard, S. C. Mogensen, and S. R. Paludan. 2005. Age-dependent role for CCR5 in antiviral host defense against herpes simplex virus type 2. J. Virol. 79:9831-9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armstrong, G. L., J. Schillinger, L. Markowitz, A. J. Nahmias, R. E. Johnson, G. M. McQuillan, and M. E. St. Louis. 2001. Incidence of herpes simplex virus type 2 infection in the United States. Am. J. Epidemiol. 153:912-920. [DOI] [PubMed] [Google Scholar]
- 5.Ashkar, A. A., and K. L. Rosenthal. 2003. Interleukin-15 and natural killer and NK T cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auvert, B., R. Ballard, C. Campbell, M. Carael, M. Carton, G. Fehler, E. Gouws, C. MacPhail, D. Taljaard, J. Van Dam, and B. Williams. 2001. HIV infection among youth in a South African mining town is associated with herpes simplex virus-2 seropositivity and sexual behavior. AIDS 15:885-889. [DOI] [PubMed] [Google Scholar]
- 7.Baker, D. A., and S. A. Plotkin. 1978. Enhancement of vaginal infection in mice by herpes simplex virus type II with progesterone. Proc. Soc. Exp. Biol. Med. 158:131-134. [DOI] [PubMed] [Google Scholar]
- 8.Bellner, L., F. Thoren, E. Nygren, J.-A. Liljeqvist, A. Karlsson, and K. Eriksson. 2005. A proinflammatory peptide from herpes simplex virus type 2 glycoprotein G affects neutrophil, monocyte, and NK cell functions. J. Immunol. 174:2235-2241. [DOI] [PubMed] [Google Scholar]
- 9.Bonwetsch, R., S. Croul, M. W. Richardson, C. Lorenzana, L. D. Valle, A. E. Sverstiuk, S. Amini, S. Morgello, K. Khalili, and J. Rappaport. 1999. Role of HIV-1 Tat and CC chemokine MIP-1alpha in the pathogenesis of HIV associated central nervous system disorders. J. Neurovirol. 5:685-694. [DOI] [PubMed] [Google Scholar]
- 10.Carr, D. J. J., J. Ash, T. E. Lane, and W. A. Kuziel. 2006. Abnormal immune response of CCR5-deficient mice to ocular infection with herpes simplex virus type 1. J. Gen. Virol. 87:490-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Lemos, C., J. E. Christensen, A. Nansen, T. Moos, B. Lu, C. Gerard, J. P. Christensen, and A. R. Thomsen. 2005. Opposing effects of CXCR3 and CCR5 deficiency on CD8+ T cell-mediated inflammation in the central nervous system of virus-infected mice. J. Immunol. 175:1767-1775. [DOI] [PubMed] [Google Scholar]
- 12.Dudley, K. L., N. Bourne, and G. N. Milligan. 2000. Immune protection against HSV-2 in B-cell-deficient mice. Virology 270:454-463. [DOI] [PubMed] [Google Scholar]
- 13.Gill, N., R. L. Rosenthal, and A. A. Ashkar. 2005. NK and NKT cell-independent contribution of interleukin-15 to innate protection against mucosal viral infection. J. Virol. 79:4470-4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass, W. G., and T. E. Lane. 2003. Functional analysis of the CC chemokine receptor 5 (CCR5) on virus-specific CD8+ T cells following coronavirus infection of the central nervous system. Virology 312:407-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halioua, B., and J. E. Malkin. 1999. Epidemiology of genital herpes: recent advances. Eur. J. Dermatol. 9:177-184. [PubMed] [Google Scholar]
- 16.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Interleukin-12 (IL-12) and IL-18 are important in innate defense against genital herpes simplex virus type 2 infection in mice but are not required for the development of acquired gamma interferon-mediated protective immunity. J. Virol. 75:6705-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82:845-853. [DOI] [PubMed] [Google Scholar]
- 18.Härle, P., V. Cull, M.-P. Agbaga, R. Silverman, B. R. G. Williams, C. James, and D. J. J. Carr. 2002. Differential effect of murine alpha/beta interferon transgenes on antagonization of herpes simplex virus type 1 replication. J. Virol. 76:6558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hook, E., R. Cannon, A. J. Nahmias, F. F. Lee, C. H. Campbell, Jr., D. Glasser, and T. C. Quinn. 1992. Herpes simplex virus infection as a risk factor for human immunodeficiency virus infection in heterosexuals. J. Infect. Dis. 165:251-255. [DOI] [PubMed] [Google Scholar]
- 20.Kaushic, C., A. A. Ashkar, L. A. Reid, and K. L. Rosenthal. 2003. Progesterone increases susceptibility and decreases immune responses to genital herpes infection. J. Virol. 77:4558-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan, I. A., S. Y. Thomas, M. M. Moretto, F. S. Lee, S. A. Islam, C. Combe, J. D. Schwartzman, and A. D. Luster. 2006. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog. 2:e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King, N. J. C., E. L. Parr, and M. B. Parr. 1998. Migration of lymphoid cells from vaginal epithelium to iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J. Immunol. 160:1173-1180. [PubMed] [Google Scholar]
- 23.Kurt-Jones, E. A., M. Chan, S. Zhou, J. Wang, G. Reed, R. Bronson, M. M. Arnold, D. M. Knipe, and R. W. Finberg. 2004. Herpes simplex virus 1 interaction with toll-like receptor 2 contributes to lethal encephalitis. Proc. Natl. Acad. Sci. USA 101:1315-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuziel, W. A., T. C. Dawson, M. Quinones, E. Garavito, G. Chenaux, S. S. Ahuja, R. L. Reddick, and N. Maeda. 2003. CCR5 deficiency is not protective in the early stages of atherogenesis in apoE knockout mice. Atherosclerosis 167:25-32. [DOI] [PubMed] [Google Scholar]
- 25.Linehan, M. M., S. Richman, C. Krummenacher, R. J. Eisenberg, G. H. Cohen, and A. Iwasaki. 2004. In vivo role of nectin-1 in entry of herpes simplex virus type 1 (HSV-1) and HSV-2 through the vaginal mucosa. J. Virol. 78:2530-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mack, M., J. Cihak, C. Simonis, B. Luckow, A. E. I. Proudfoot, J. Plachy, H. Bruhl, M. Frink, H.-J. Anders, V. Vielhauer, J. Pfirstinger, M. Stangassinger, and D. Schlondorff. 2001. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J. Immunol. 166:4697-4704. [DOI] [PubMed] [Google Scholar]
- 27.Malmgaard, L., J. Melchjorsen, A. G. Bowie, S. C. Mogensen, and S. R. Paludan. 2004. Viral activation of macrophages through TLR-dependent and -independent pathways. J. Immunol. 173:6890-6898. [DOI] [PubMed] [Google Scholar]
- 28.McDermott, M. R., C. H. Goldsmith, K. L. Rosenthal, and L. J. Brais. 1989. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J. Infect. Dis. 159:460-466. [DOI] [PubMed] [Google Scholar]
- 29.McDermott, M. R., L. J. Brais, and M. J. Evelegh. 1990. Mucosal and systemic antiviral antibodies in mice inoculated intravaginally with herpes simplex virus type 2. J. Gen. Virol. 71:1497-1504. [DOI] [PubMed] [Google Scholar]
- 30.Milligan, G. N. 1999. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 73:6380-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milligan, G. N., and D. I. Bernstein. 1995. Generation of humoral immune responses against herpes simplex virus type 2 in the murine female genital tract. Virology 206:234-241. [DOI] [PubMed] [Google Scholar]
- 32.Milligan, G. N., and D. I. Bernstein. 1997. Interferon-γ enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology 229:259-268. [DOI] [PubMed] [Google Scholar]
- 33.Milligan, G. N., K. L. Dudley-McClain, C. G. Young, and C. F. Chu. 2004. T-cell-mediated mechanisms involved in resolution of genital herpes simplex virus type 2 (HSV-2) infection of mice. J. Reprod. Immunol. 61:115-127. [DOI] [PubMed] [Google Scholar]
- 34.Miyaura, H., and M. Iwata. 2002. Direct and indirect inhibition of Th1 development by progesterone and glucocorticoids. J. Immunol. 168:1087-1094. [DOI] [PubMed] [Google Scholar]
- 35.Morrison, L. A., L. Zhu, and L. G. Thebeau. 2001. Vaccine-induced serum immunoglobulin contributes to protection from herpes simplex virus type 2 genital infection in the presence of immune T cells. J. Virol. 75:1195-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy, J. A., R. J. Duerst, T. J. Smith, and L. A. Morrison. 2003. Herpes simplex virus type 2 virion host shutoff protein regulates alpha/beta interferon but not adaptive immune responses during primary infection in vivo. J. Virol. 77:9337-9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakajima, H., M. Kobayashi, R. B. Pollard, and F. Suzuki. 2000. A pathogenic role of Th2 responses on the severity of encephalomyelitis induced in mice by herpes simplex virus type 2 infection. J. Neuroimmunol. 110:106-113. [DOI] [PubMed] [Google Scholar]
- 38.Nakajima, H., M. Kobayashi, R. B. Pollard, and F. Suzuki. 2001. Monocyte chemoattractant protein-1 enhances HSV-induced encephalomyelitis by stimulating Th2 responses. J. Leukoc. Biol. 70:374-380. [PubMed] [Google Scholar]
- 39.Nuovo, G. J., D. L. Defaria, J. G. Chanona-Vilchi, and Y. Zhang. 2005. Molecular detection of rabies encephalitis and correlation with cytokine expression. Mod. Pathol. 18:62-67. [DOI] [PubMed] [Google Scholar]
- 40.Parr, M. B., L. Kepple, M. R. McDermott, M. D. Drew, J. J. Bozzola, and E. L. Parr. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. 70:369-380. [PubMed] [Google Scholar]
- 41.Parr, M. B., and E. L. Parr. 1998. Mucosal immunity to herpes simplex virus type 2 infection in the mouse vagina is impaired by in vivo depletion of T lymphocytes. J. Virol. 72:2677-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parr, M. B., and E. L. Parr. 1999. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology 258:282-294. [DOI] [PubMed] [Google Scholar]
- 43.Peretti, S., A. Shaw, J. Blanchard, R. Bohm, G. Morrow, J. D. Lifson, A. Gettie, and M. Pope. 2005. Immunomodulatory effects of HSV-2 infection on immature macaque dendritic cells modify innate and adaptive responses. Blood 106:1305-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Posavad, C. M., M. L. Huang, S. Barcy, D. M. Koelle, and L. Corey. 2000. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequent recurring genital herpes. J. Immunol. 165:1146-1152. [DOI] [PubMed] [Google Scholar]
- 45.Sanchez-Burgos, G., R. Hernandez-Pando, I. L. Campbell, J. Ramos-Castaneda, and C. Ramos. 2004. Cytokine production in brain of mice experimentally infected with dengue virus. Neuroreport 15:37-42. [DOI] [PubMed] [Google Scholar]
- 46.Sasseville, V. G., M. M. Smith, C. R. Mackay, D. R. Pauley, K. G. Mansfield, D. J. Ringler, and A. A. Lackner. 1996. Chemokine expression in simian immunodeficiency virus-induced AIDS encephalitis. Am. J. Pathol. 149:1459-1467. [PMC free article] [PubMed] [Google Scholar]
- 47.Seebach, J., D. Bartholdi, K. Frei, K. S. Spanaus, E. Ferrero, U. Widmer, S. Isenmann, R. M. Strieter, M. Schwab, H. Pfister, and A. Fontana. 1995. Experimental Listeria meningoencephalitis. Macrophage inflammatory protein-1 and -2 produced intrathecally and mediate chemotactic activity in cerebrospinal fluid of infected mice. J. Immunol. 155:4367-4375. [PubMed] [Google Scholar]
- 48.Sin, J.-I., J. J. Kim, C. Pachuk, C. Satishchandran, and D. B. Weiner. 2000. DNA vaccines encoding interleukin-8 and RANTES enhance antigen-specific Th1-type CD+ T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J. Virol. 74:11173-11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sorensen, L. N., and S. R. Paludan. 2004. Blocking CC chemokine receptor (CCR) 1 and CCR5 during herpes simplex virus type 2 infection in vivo impairs host defence and perturbs the cytokine response. Scand. J. Immunol. 59:321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sternberg, J. M., J. Rodgers, B. Bradley, L. Maclean, M. Murray, and P. G. Kennedy. 2005. Meningoencephalitic African trypanosomiasis: Brain IL-10 and IL-6 are associated with protection from neuron-inflammatory pathology. J. Neuroimmunol. 167:81-89. [DOI] [PubMed] [Google Scholar]
- 51.Wald, A., J. Zeh, S. Selke, T. Warren, A. J. Ryncarz, R. Ashley, J. N. Krieger, and L. Corey. 2000. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N. Engl. J. Med. 342:844-850. [DOI] [PubMed] [Google Scholar]
- 52.Whitley, R. J. 2002. Herpes simplex virus infection. Semin. Pediatr. Infect. Dis. 13:6-11. [DOI] [PubMed] [Google Scholar]
- 53.Wickham, S., B. Lu, J. Ash, and D. J. J. Carr. 2005. Chemokine receptor deficiency is associated with increased chemokine expression in the peripheral and central nervous systems and increased resistance to herpetic encephalitis. J. Neuroimmunol. 162:51-59. [DOI] [PubMed] [Google Scholar]
- 54.Xia, M., and B. T. Hyman. 2002. GROalpha/KC, a chemokine receptor CXCR2 ligand, can be a potent trigger for neuronal ERK1/2 and PI-3 kinase pathways and for tau hyperphosphorylation-a role in Alzheimer's disease? J. Neuroimmunol. 122:55-64. [DOI] [PubMed] [Google Scholar]
- 55.Zhao, X. Y., E. Deak, K. Soderberg, M. Linehan, D. Spezzano, J. Zhu, D. M. Knipe, and A. Iwasaki. 2003. Vaginal submucosal dendritic cells, but not Langerhans' cells, induce protective Th1 responses to herpes simplex virus-2. J. Exp. Med. 197:153-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]
- 57.Zou, J. Y., and F. T. Crews. 2005. TNF alpha potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NF kappa B inhibition. Brain Res. 1034:11-24. [DOI] [PubMed] [Google Scholar]