Abstract
Human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T cells in early infection are associated with the dramatic decline of peak viremia, whereas their antiviral activity in chronic infection is less apparent. The functional properties accounting for the antiviral activity of HIV-1-specific CD8+ T cells during early infection are unclear. Using cytokine secretion and tetramer decay assays, we demonstrated in intraindividual comparisons that the functional avidity of HIV-1-specific CD8+ T cells was consistently higher in early infection than in chronic infection in the presence of high-level viral replication. This change of HIV-1-specific CD8+ T-cell avidity between early and chronic infections was linked to a substantial switch in the clonotypic composition of epitope-specific CD8+ T cells, resulting from the preferential loss of high-avidity CD8+ T-cell clones. In contrast, the maintenance of the initially recruited clonotypic pattern of HIV-1-specific CD8+ T cells was associated with low-level set point HIV-1 viremia. These data suggest that high-avidity HIV-1-specific CD8+ T-cell clones are recruited during early infection but are subsequently lost in the presence of persistent high-level viral replication.
Early human immunodeficiency virus type 1 (HIV-1) infection is a transient symptomatic illness that is characterized by extremely high viral loads (27). This high-level viremia typically declines dramatically once a virus-specific CD8+ T-cell-mediated immune response is mounted. Evidence for a strong antiviral activity of HIV-1-specific CD8+ T cells during early infection is based on at least three distinct observations: (i) the temporal coincidence between the first emergence of HIV-1-specific CD8+ T cells in the peripheral blood and the resolution of the acute retroviral syndrome (10, 32), (ii) the rapid selection of viral escape mutations in targeted CD8+ T-cell epitopes (25, 42, 47), and (iii) the dramatically accelerated simian immunodeficiency virus (SIV) disease progression that is noted to occur in rhesus macaques following the artificial depletion of CD8+ cells (51). Early HIV-1 infection therefore offers a unique opportunity to analyze correlates of protective immunity mediated by HIV-1-specific CD8+ T cells.
In previous studies, it was shown that the total magnitude of HIV-1-specific CD8+ T cells in acute infection, as determined by gamma interferon (IFN-γ) secretion assays or tetramer binding studies, is relatively low and directed against a narrow repertoire of viral epitopes (6, 17, 58), which are typically structured in a clear hierarchical order (60). In contrast, progressive viremia in chronic infection usually occurs in the presence of strong and broadly diversified IFN-γ-secreting HIV-1-specific CD8+ T cells (1, 8, 21, 22), which are only infrequently associated with viral sequence diversification (31), suggesting limited immune pressure mediated by these responses. These data imply that HIV-1-specific CD8+ T cells in acute and chronic infections differ in their abilities to contain viral replication, the reasons for which still remain largely unclear.
Recent studies have shown that HIV-1-specific CD8+ T cells in early infection have strong ex vivo proliferative activities, while this effector function appears to be selectively lost during the subsequent disease process in the presence of high-level viremia (36). This loss of ex vivo proliferative activity appeared to be at least partially related to the simultaneous loss of interleukin-2 (IL-2)-producing HIV-1-specific CD4+ T helper cells (24, 59) but might not necessarily reflect an intrinsic functional deficiency of HIV-1-specific CD8+ T cells themselves. Understanding intrinsic differences between HIV-1-specific CD8+ T cells in acute and chronic HIV-1 infections therefore remains crucial for the ultimate definition of CD8+ T-cell-mediated protective immunity against HIV-1.
In the present study, we comparatively analyzed HIV-1-specific CD8+ T cells in early and chronic infections, using serial dilutions of antigenic peptides for peripheral blood mononuclear cell (PBMC) stimulation and four different functional readouts to quantify responding CD8+ T cells. Our data show that HIV-1-specific CD8+ T cells in early infection have a higher functional avidity than those in chronic infection and a clonotypic composition that is different from those in chronic HIV-1 infection. The selective depletion of high-avidity HIV-1-specific CD8+ T cells during the transition to chronic progressive HIV-1 infection may contribute to the ultimate inability to immunologically contain viral replication.
MATERIALS AND METHODS
Subjects studied.
PBMC samples from 30 HIV-1-infected individuals were obtained and investigated in this study. Ten individuals with early HIV-1 infection, defined as HIV-1 seroconversion within 6 months prior to study enrollment, were compared to 10 individuals with chronic HIV-1 infection in a cross-sectional analysis. Individuals with chronic infection had been infected with HIV-1 for at least 3 years. In addition, in another 10 individuals identified during early infection, the first detectable HIV-1-specific CD8+ T-cell response was analyzed and longitudinally followed over the subsequent disease process. Five of these were treated with highly active antiretroviral therapy during early infection and subsequently exposed to structured treatment interruptions (STIs) (28, 49), and the other five individuals remained completely antiretroviral therapy naïve. Study subjects were recruited from Massachusetts General Hospital (MGH) and the Fenway Community Health Center in Boston, MA. Relevant clinical and demographic data of all study subjects are summarized in Tables 1 and 2. The study was approved by the respective institutional review boards, and all subjects gave written informed consent to participate.
TABLE 1.
Cross-sectional analysis of demographic and clinical characteristics of the study individuals
| Study cohort | Median age (yr) (range) | Sex (male:female ratio) | Ethnicities | Median CD4+ T-cell count/μl (range) | Median HIV-1 RNA copies/ml (range) |
|---|---|---|---|---|---|
| Individuals with early infection (n = 10) | 36 (27-49) | 10:0 | Caucasian (n = 9) and Hispanic (n = 1) | 454 (237-784) | 151,500 (2,500-750,000) |
| Individuals with chronic infection (n = 10) | 37 (29-51) | 9:1 | Caucasian (n = 8) and African-American (n = 2) | 547.5 (68-1,106) | 27,350 (1,743-175,000) |
TABLE 2.
Longitudinal analysis of demographic and clinical characteristics of the study individuals
| Patient IDa no. | Patient age (yr) | Patient sexb | Patient ethnicity | HLA class I types | Antiretroviral treatment and subsequent STIsc | Initial presentation
|
Day p.p.d of T-cell analysis in chronic infection (no. of days off therapy) | Time of analysis of chronic infection
|
Epitope studied (sequence) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD4+ T-cell count (cells/μl) | HIV-1 RNA (copies/ml) | CD4+ T-cell count (cells/μl) | HIV-1 RNA (copies/ml) | ||||||||
| AC-15 | 40 | M | Caucasian | A1,3; B7,8; Cw7 | Yes | 413 | 27,000 | 1752 (765) | 542 | 12,200 | B8-FL8 (FLKEKGGL) |
| AC-14 | 44 | M | Caucasian | A2,3; B8,62; Cw7,10 | Yes | 981 | 951,000 | 686 (186) | 969 | 10,860 | B8-FL8 (FLKEKGGL) |
| AC-46 | 49 | M | Caucasian | A1,26; B8,51; Cw7,15 | Yes | ND | 123,000 | 1040 (547) | 562 | 20,300 | B8-FL8 (FLKEKGGL) |
| AC-02 | 42 | M | Caucasian | A11,29; B8,44; Cw4 | Yes | ND | 4.85 × 106 | 941 (98) | 625 | 18,400 | B8-FL8 (FLKEKGGL) |
| AC-09 | 35 | M | Hispanic | A2,31; B61; Cw10 | Yes | 365 | 750,000 | 904 (270) | 361 | 30,277 | A2-IV9 (ILKEPVHGV) |
| AC-131 | 32 | M | Caucasian | A1,3; B8,15; Cw7 | No | 475 | 750,000 | 477 | NDe | 175,000 | B8-EI8 (EIYKRWII) |
| AC-177 | 44 | M | Caucasian | A1,2; B8; Cw7 | No | 363 | 26,800 | 162 | 217 | 427,000 | B8-EI8 (EIYKRWII) |
| AC-132 | 30 | M | Caucasian | A3,68; B14,44; Cw8,16 | No | 815 | 5.6 × 106 | 27 | 790 | 115,000 | A3-QK10 (QVPLRPMTYK) |
| AC-160 | 46 | M | Caucasian | A1,2; B7,27; Cw1,7 | No | ND | 468,000 | 205 | 874 | 2,830 | B27-KK10 (KRWIILGLNK) |
| AC-121 | 37 | M | Hispanic | A1,24; B35,57; Cw7,12 | No | 858 | 12,600 | 562 | ND | 5,940 | B57-KF11 (KAFSPEVIPMF) |
ID, identification.
M, male.
For details on the length of antiretroviral treatment, the length of STIs, and the evolution of HIV-1 RNA and CD4+ T-cell counts, see reference 28.
Day p.p., day postpresentation.
ND, not determined.
HLA class I typing.
HLA class I molecular typing was performed at the MGH tissue typing laboratory or at a commercial laboratory (Dynal Biotech, Oxford, United Kingdom) by use of sequence-specific PCRs according to standard operational protocols.
Synthetic HIV-1 peptides.
Four hundred ten peptides, overlapping by 10 amino acids, which were 13 to 18 amino acids in length and spanned the entire expressed HIV-1 clade B 2001 consensus sequence (Gag, Pol, Vif, Vpr, Vpu, Rev, Tat, Env, and Nef), were synthesized at the MGH Peptide Core Facility on an automated peptide synthesizer (MBS 396; Advanced Chemtech, Louisville, KY) using 9-fluorenylmethoxy carbonyl chemistry. Peptides corresponding to previously defined HIV-1-specific optimal CD8+ T-cell epitopes (11) were also synthesized according to this procedure.
Intracellular cytokine staining and multiparameter flow cytometry.
PBMCs were separated from whole blood by Ficoll-Hypaque (Sigma, St. Louis, MO) density gradient centrifugation. Cells (0.5 to 1 million) were stimulated with five pools of overlapping peptides (final concentration, 4 μg/ml) spanning all expressed HIV-1 gene products (Gag, Nef, Pol, Env, and a combined pool of Vpr, Vpu, Vif, Tat, and Rev) or with peptides corresponding to optimal CD8+ T-cell epitopes in serial 10-fold dilutions (0.0001 μg/ml to 10 μg/ml). PBMCs were incubated with anti-CD28 and anti-CD49d antibodies (1 μg/ml each; BD Biosciences, San Jose, CA) for 6 h at 37°C and 5% CO2 in the presence of fluorescein isothiocyanate (FITC)-labeled CD107a/b antibodies (10 μl/ml; BD Biosciences). Brefeldin A (10 μg/ml; Sigma) and monensin (6 μg/ml; BD Biosciences) were added after the initial hour of incubation. Afterwards, cells were washed with phosphate-buffered saline (PBS)-1% fetal calf serum and stained with surface antibodies (CD8-peridinin chlorophyll protein-Cy5.5 and CD3-allophycocyanin [APC]-Cy7; all antibodies from BD Biosciences). Cells were then washed again, fixed and permeabilized using a Caltag fixation/permeabilization kit (Caltag, Burlingame, CA), and stained for intracellular expression of cytokines (macrophage inflammatory protein 1β [MIP-1β]-phycoerythrin [PE], tumor necrosis factor alpha [TNF-α]-APC, and IFN-γ-PE-Cy7 [BD Biosciences]). For phenotyping experiments, cells were initially stained with APC-labeled major histocompatibility complex (MHC) class I tetramers (Beckman Coulter, Fullerton, CA) and surface antibodies (CD8-Alexa 405 [Caltag], CD38-peridinin chlorophyll protein-Cy5.5 [BD Biosciences], CD127-APC-Cy5.5 [R&D Systems, Minneapolis, MN], and T-cell receptor [TCR] Vβ 20, 9, 27, 28, or 10.3-PE [Immunotech, Marseille, France] antibodies). Following fixation and permeabilization, cells were labeled with Ki-67-FITC antibodies (BD Biosciences). Cells were acquired on an LSRII flow cytometer (BD Biosciences), using fluorescence-activated cell sorter DiVa software. Electronic compensation was performed with antibody capture beads (BD Biosciences) stained separately with individual antibodies used in the test samples. Flow data were analyzed with the FlowJo software package (Treestar, Ashland, OR). The total magnitude of HIV-1-specific CD8+ T-cell responses was calculated by summing up the responses to the individual peptide pools following background subtraction.
Tetramer dissociation assays.
Overall avidity of epitope-specific CD8+ T cells was analyzed using tetramer dissociation assays according to a recently published protocol (57). PBMCs suspended in staining buffer (PBS containing 2% bovine serum albumin and 0.2% sodium azide) were stained with PE- or APC-labeled tetramers (Beckman Coulter) refolded with epitopic HIV-1 peptides and FITC-labeled CD8 antibodies (BD Biosciences) for 45 min at room temperature and then washed three times with staining buffer. To observe the dissociation of the tetramer, cells were resuspended in staining buffer with unlabeled tetramer at a 100-fold surplus concentration. At selected time points, ∼0.5 × 106 cells were withdrawn and fixed in 150 μl 2% paraformaldehyde-PBS. Cells were then analyzed on a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). The MHC class I tetramer dissociation analysis was based on the total fluorescence of the tetramer-positive population (50). Briefly, the total fluorescence of the gated CD8+ MHC class I tetramer-positive population was calculated and normalized per gated lymphocyte. This total antigen-specific fluorescence of the CD8+ tetramer-positive cell population was then normalized to the total fluorescence at the initial time point, and the graphs were plotted on a logarithmic scale.
Sorting of tetramer-positive HIV-1-specific CD8+ T-cell populations.
Fresh or frozen PBMC samples were stained with APC- or PE-labeled MHC class I tetramers refolded with epitopic HIV-1 peptides (Beckman Coulter) and fluorophore-labeled CD8+ antibodies, followed by decontamination with fixation solution A (1:100 dilution; Caltag). Tetramer-positive CD8+ cells were then sorted on a fluorescence-activated Aria cell-sorting instrument (BD Biosciences) at 70 lb/in2. Electronic compensation was performed with antibody capture beads (BD Biosciences) stained separately with individual antibodies used in the test samples. The purity of sorted cell populations was consistently higher than 98%.
TCR β chain sequencing.
mRNA was extracted from at least 4,000 tetramer-specific CD8+ T cells by use of an RNeasy mini kit (QIAGEN, Valencia, CA). Anchored reverse transcriptase PCR (RT-PCR) was then performed using a modified version of the SMART (switching mechanism at 5′ end of RNA transcript) procedure and a TCR β chain constant region 3′ primer to obtain PCR products containing the Vβ chain in addition to the CDR3 region, the Jβ region, and the beginning of the Cβ region (20). Briefly, reverse transcription was carried out at 42°C for 90 min with primers provided for the 5′ rapid amplification of cDNA end reaction in a SMART-RACE PCR kit (BD Biosciences). First- and second-round PCRs were then performed using universal 5′-end primers (BD Biosciences) and nested gene-specific 3′-end primers annealing to the constant region of the TCR β chain (Cβ outer, 5′-TGTGGCCAGGCACACCAGTGTGGCC-3′; Cβ inner, 5′-GGTGTGGGAGATCTCTGCTTCTGA-3′). PCR conditions were as follows: for the first run, 95°C for 30 s and 72°C for 2 min (for five cycles), 95°C for 30 s, 70°C for 30 s, and 72°C for 2 min (for five cycles), and 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min (for 25 cycles); for the second run, 95°C for 30 s, 60°C for 30 s, and 72°C for 1 min (for 30 cycles). The PCR product was ligated into the TOPO TA cloning vector (Invitrogen, Carlsbad, CA) and used to transform Escherichia coli (Mach 1; Invitrogen). At least 40 colonies were selected, amplified by PCR with M13 primers, and sequenced by T7 or T3 primers on an ABI 3100 PRISM automated sequencer. Sequences were edited and aligned using Sequencher (Gene Codes Corp., Ann Arbor, MI) and Se-Al (University of Oxford, Oxford, United Kingdom) and compared to sequences in the human TCR gene database (http://imgt.cines.fr:8104/home.html). The TCR clonotype composition remained consistent in repeated RT-PCR procedures using the same mRNA sample. The TCR Vβ chain classification system of the international ImMunoGeneTics database (IMGT) (35) is used throughout the entire paper.
Sequencing of autologous virus.
Nested PCR for gag, RT, and nef on proviral DNA or plasma viral RNA was performed as previously described (5). PCR fragments were population sequenced to identify regions of sequence variation. All fragments were sequenced bidirectionally on an ABI 3100 PRISM automated sequencer. If the height of the secondary peak at a given residue in the chromatogram was reproducibly more than 25% of the dominant peak, a mixed base was considered present at that position. Sequencher (Gene Codes Corp., Ann Arbor, MI) and MacVector 4.1 (Oxford Molecular) were used to edit and align sequences.
Statistics.
Data are expressed as means and standard deviations or medians and ranges, respectively. Generation of dose-response curves and tetramer dissociation curves, including calculations of 50% effective concentration levels and dissociation rate constants (Koff), was performed using the Prism software package (version 2.1; GraphPad Software Corporation, San Diego, CA). Data sets were tested for normality distribution using the Shapiro-Wilks W test. In case of normal distributions, statistical hypotheses were tested using paired or unpaired Student t tests; otherwise, the Wilcoxon matched-pair test or the Mann-Whitney U test was used. A P value of <0.05 was considered significant.
RESULTS
Total magnitude of cytokine-secreting and degranulating HIV-1-specific CD8+ T cells in early and chronic HIV-1 infections.
In prior studies, the magnitude of HIV-1-specific CD8+ T cells in early and chronic infections has been analyzed using IFN-γ enzyme-linked immunospot assays (6, 13, 33, 37) or more recently by carboxyfluoroscein succinimidyl ester-based proliferation assays (36). Yet, HIV-1-specific CD8+ T cells can also exert antiviral activities by secreting a variety of additional cytokines or by releasing cytotoxic enzymes (7, 9). Here, we compared the total magnitudes of HIV-1-specific CD8+ T cells in early and chronic HIV-1 infections, using four different functional readouts, including the antigen-specific secretion of IFN-γ, TNF-α, and MIP-1β as well as the antigen-dependent degranulation, measured by surface expression of CD107a/b. These different effector functions were analyzed by multiparameter flow cytometry following stimulation of PBMC samples with excess concentrations of overlapping HIV-1 peptides corresponding to all expressed HIV-1 gene products.
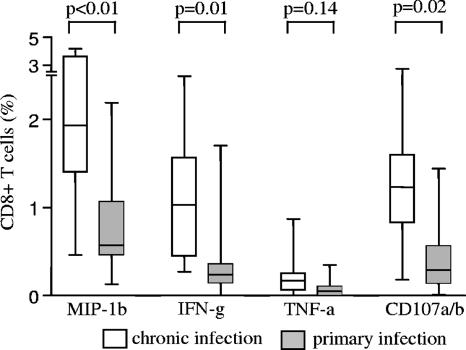
In line with previous results (6, 17, 33, 37), the total magnitude of IFN-γ-secreting HIV-1-specific CD8+ T cells was substantially weaker during early HIV-1 infection than during chronic infection (Fig. 1). Importantly, a smaller magnitude of HIV-1-specific CD8+ T cells was also observed in early infection, when HIV-1-specific CD8+ T cells were quantified by flow cytometry according to secretion of TNF-α or MIP-1β or surface expression of CD107a/b. In addition, the hierarchies of these different effector functions were similar in early and chronic infections (MIP-1β > CD107 > IFN-γ > TNF-α). Overall, these data show that for a variety of different functional readouts, the total magnitude of HIV-1-specific CD8+ T cells is consistently lower during early HIV-1 infection than during chronic HIV-1 infection.
FIG. 1.
Lower total magnitude of cytokine-secreting and degranulating HIV-1-specific CD8+ T cells during early HIV-1 infection than during chronic infection. Data reflect the total magnitude of CD8+ T cells secreting IFN-γ, TNF-α, or MIP-1β or expressing CD107a/b on the cell surface following stimulation with overlapping peptides corresponding to the entire HIV-1 proteome in individuals with early (n = 10) and chronic (n = 10) HIV-1 infection. Results are presented as box-whisker plots, indicating the median and the 10th, 25th, 75th, and 90th percentiles.
Higher functional avidity of HIV-1-specific CD8+ T cells during early HIV-1 infection.
In the above-described experiments, HIV-1-specific CD8+ T cells were analyzed following stimulation with excess concentrations of antigenic peptides; however, this approach did not allow us to discern differences between the functional avidities of these cells, defined as the antigen concentration eliciting 50% of the maximal functional response. To address this, we focused on 10 individuals who were identified as having early HIV-1 infection and then longitudinally monitored them over the ensuing disease course. Five of these study persons were treated with highly active antiretroviral therapy during early infection and subsequently exposed to a number of STIs (28). The other five study individuals had never received antiretroviral therapy since early infection and exhibited either high-level viremia (>100,000 copies/ml in three persons) or low-level viremia (<6,000 HIV-1 copies/ml in two persons). The demographic, immunogenetic, and clinical characteristics of the study subjects are summarized in Table 2. In these study persons, the functional avidity of the earliest detectable immunodominant HIV-1-specific CD8+ T-cell response was analyzed by stimulating PBMCs with the epitopic peptide in serial, 10-fold dilutions, again using the above-mentioned four different functional readouts in a multiparameter flow cytometric assay. In addition, we longitudinally assessed the functional avidity of these responses during the subsequent disease process (median, 237.5 days off therapy; range, 27 to 765 days off therapy following the baseline assessment). Importantly, none of the targeted epitopes exhibited evidence for viral sequence changes during the time of observation, as determined by population sequencing of the autologous viruses (Table 4).
TABLE 4.
Sequences of the CD8+ T-cell target epitopes in the autologous virus of the study patients
| Patient IDa no. | Time point of viral sequencing (day p.p.b) | Epitope sequence in autologous virus |
|---|---|---|
| AC-15 | 43 | FLKEKGGL |
| 1332 | FLKEKGGL | |
| 1823 | FLKEKGGL | |
| AC-14 | 81 | FLKEKGGL |
| 672 | FLKEKGGL | |
| AC-46 | 76 | FLKEKGGL |
| 1040 | FLKEKGGL | |
| AC-02 | 28 | FLKEKGGL |
| 934 | FLKEKGGL | |
| AC-09 | 1 | ILKEPVHGV |
| 904 | ILKEPVHGV | |
| AC-131 | 94 | EIYKRWII |
| 477 | EIYKRWII | |
| AC-177 | 27 | EIYKRWII |
| 189 | EIYKRWII | |
| AC-132 | 6 | QVPLRPMTYK |
| 27 | QVPLRPMTYK | |
| AC-160 | 33 | KRWIILGLNK |
| 205 | KRWIILGLNK | |
| AC-121 | 63 | KAFSPEVIPMF |
| 476 | KAFSPEVIPMF | |
| 562 | KAFSPEVIPMF |
ID, identification.
Day p.p., day postpresentation.
Experimental results for subject AC-15 are depicted in Fig. 2A and B. In this intraindividual analysis, the B8-FL8 peptide concentration eliciting 50% of the maximal IFN-γ response was 0.004 μg/ml during the initial assessment and 0.026 μg/ml during chronic infection, thus indicating a substantial avidity change between the initially mounted CD8+ T-cell response and the same epitope-specific response in the same study individual during chronic infection. A similar difference between the functional avidities of IFN-γ-secreting HIV-1-specific CD8+ T cells during early and chronic infections was observed in the other study subjects undergoing STIs (Fig. 2B) or experiencing high-level viremia in the absence of antiretroviral therapy (Fig. 2C). Interestingly, the difference between the functional avidities of IFN-γ-secreting HIV-1-specific CD8+ T cells during early and chronic infections was lowest in the two study individuals who, in the background of the protective HLA class I alleles HLA-B57 or -B27, spontaneously controlled HIV-1 viremia after early infection to levels below 6,000 copies/ml (Fig. 2D). Overall, in the 10 subjects who were studied, the functional avidity of HIV-1-specific CD8+ T cells for secretion of IFN-γ and MIP-1β and antigen-specific degranulation (CD107a/b expression) was significantly higher at the time of early infection than at the time of chronic infection (Fig. 2E). There was also a trend for a higher functional avidity of TNF-α-secreting CD8+ T cells during early infection than during chronic infection (Fig. 2E); however, antigen-specific TNF-α secretion below the level of detection by flow cytometry precluded this analysis in study persons AC-131, AC-177, AC-132, AC-160, and AC-121. Importantly, for all CD8+ T-cell effector functions tested, the smallest difference between the functional avidities during early and chronic infections was observed in the two study individuals spontaneously achieving low-level viremia after early infection (Fig. 2E). Taken together, these results show in intraindividual comparisons that HIV-1-specific CD8+ T cells emerging during early infection have a higher functional avidity than those CD8+ T cells targeting the same viral epitope in progressive chronic infection.
FIG. 2.
Higher functional avidity of HIV-1-specific CD8+ T cells in early infection than in chronic HIV-1 infection. (A) Dot plots reflecting the proportion of IFN-γ-secreting CD8+ T cells following stimulation with the HIV-1 epitopic peptide B8-FL8 in serial 10-fold dilutions in study individual AC-15 during early and chronic infections. Percentages indicate the proportions of gated CD8+ IFN-γ-positive lymphocytes. (B to D) Intraindividual analysis of the functional avidities of IFN-γ-secreting HIV-1-specific CD8+ T cells recognizing the same viral epitope in early and chronic infections. Dose-response curves indicate the proportions of CD8+ T cells secreting IFN-γ, following peptide stimulation in serial 10-fold dilutions. Data were normalized (maximum = 1). Dashed lines and triangles correspond to chronic infection; solid lines and squares reflect data obtained during early infection. (B) Data from individuals started on antiretroviral therapy during early infection and subsequently undergoing STIs. (C and D) Data from study persons who remained completely untreated after early infection in the presence of high-level (C) or low-level (D) viremia. (E) Epitopic peptide concentrations eliciting 50% of the maximum of the indicated functional activity (EC50) in the 10 study individuals during early and chronic infections. Antigen-specific TNF-α secretion was below the threshold of detection by flow cytometry for five study individuals.
Slower tetramer dissociation rate of HIV-1-specific CD8+ T cells in early infection than in chronic infection.
The higher functional avidity of HIV-1-specific CD8+ T cells in early HIV-1 infection might reflect a higher overall avidity of the TCR/peptide-MHC class I interaction in early infection, which can be analyzed using a tetramer drop-off assay (50). Figure 3. shows the tetramer dissociation curves for all 10 study individuals. In study subjects undergoing STIs (Fig. 3A) or maintaining high-level viremia in the absence of antiretroviral therapy (Fig. 3B), we consistently observed a slower dissociation of the tetramer (lower Koff) in early infection than in chronic infection. Corresponding to the functional avidity data described above, the difference between tetramer dissociation rates in early and chronic infections was least pronounced in the two HLA-B57- or -B27-expressing individuals achieving spontaneous low-level viral loads following early infection (Fig. 3C). Overall, the difference between the tetramer dissociation rate constants during early and chronic infections in the 10 study individuals was statistically significant (Fig. 3D). Thus, in intraindividual comparisons, these results indicate a significantly higher avidity of the TCR/peptide-MHC interaction of HIV-1-specific CD8+ T cells recruited in early infection than that for chronic progressive infection, while a substantially smaller degree of avidity changes was observed for persons achieving control of viral replication at low levels following early infection.
FIG. 3.
Slower tetramer dissociation of HIV-1-specific CD8+ T cells in early infection than in chronic infection. Tetramer dissociation curves of HIV-1-specific CD8+ T cells in early (squares) and chronic (triangles) HIV-1 infection in study persons treated during early infection and subsequently undergoing STIs (A) or remaining completely untreated after early infection in the presence of high-level (B) or low-level (C) viremia. (D) Koff of HIV-1-specific CD8+ T cells during early and chronic infections in the 10 study persons described in the text.
Evolution of the TCR clonotype repertoire between early and chronic HIV-1 infections.
To determine if the observed avidity differences between HIV-1-specific CD8+ T cells in early and chronic infections corresponded to changes in their clonal compositions, we next conducted a longitudinal analysis of the clonotypic repertoire of the respective HIV-1-specific CD8+ T-cell populations. In order to overcome the limitations of previously described (40) Vβ chain-specific immunostaining for the clonotypic assessment of HIV-1-specific CD8+ T cells, we determined the clonotypic composition by TCR β chain sequencing using a PCR amplification technique without bias for selected TCR Vβ chains to ensure that epitope-specific clonotypes were represented in the PCR product with a relative frequency reflecting that in the originally sorted cell population (47). Figure 4 and Table 3 summarize the clonotypic TCR repertoires of the epitope-specific CD8+ T-cell populations analyzed during early and chronic HIV-1 infections in the 10 study subjects. In the study subjects exposed to STIs (Fig. 4A), the dominant CD8+ T-cell clonotypes recruited during early infection were subsequently either deleted and replaced by alternative clonotypes (AC-02, AC-14, and AC-09) or preserved but substantially reduced in their overall contribution to the clonotypic repertoire (AC-15). In study individual AC-46, one codominant clonotype detected during early infection persisted until chronic infection, while the other codominant clonotypes were eliminated after early infection. In addition, we observed with these individuals that clonotypes which were rare or hardly detectable during early infection became dominant during chronic infection (AC-46 and AC-09). In untreated study individuals with continuous high-level viremia (Fig. 4B), either the original clonotypic composition entirely changed (AC-131) or the dominance of initially recruited clonotypes was subsequently reduced due to the expansion of subdominant clonotypes that were completely (AC-177) or almost completely (AC-132) undetectable during the first assessment. Moreover, in study subjects AC-177 and AC-132, clonotype preservation after early infection was not related to clonal persistence but resulted from a considerable underlying switch in the composition of nucleotypes coding for identical CDR3 amino acid sequences (Table 3), as previously observed in the context of TCRs specific for an immunodominant influenza virus epitope (30). Finally, in line with our previous data on CD8+ T-cell avidity in the two HLA-B57- or -B27-positive study individuals achieving spontaneous control of viremia at low levels, the initially recruited clonotypic repertoire remained fairly consistent after early infection in these two study persons, with a preservation of three (AC-160) or four (AC-121) clonotypes (Fig. 4C), on the level of both the amino acid and the nucleic acid CDR3 sequences (Table 3). Overall, these data indicate a substantial switch of the TCR clonotype repertoire after early HIV-1 infection in individuals experiencing high viral set points, while control of viral replication at low levels after early infection was associated with a largely conserved pattern of the originally recruited clonotypic TCR repertoire.
FIG. 4.
Clonotypic compositions of HIV-1-specific CD8+ T-cell populations during early and chronic HIV-1 infections (A) in individuals with STIs and (B and C) in five study persons who remained treatment naïve after early infection in the presence of high-level (B) or low-level (C) viremia. Each fraction corresponds to one Vβ clonotype detected by TCR sequencing. Clonotypes shown in black or gray were detected during both early and chronic infection, while clonotypes shown in white were detected exclusively during early or chronic infection.
TABLE 3.
Clonotypic compositions of epitope-specific CD8+ T cells during early and chronic HIV-1 infections
| Study person; epitope | Primary HIV-1 infection
|
Chronic HIV-1 infection
|
||||||
|---|---|---|---|---|---|---|---|---|
| Vβ clonotype | CDR3 sequencea | Jβ clonotype | Frequency | Vβ clonotype | CDR3 sequencea | Jβ clonotype | Frequency | |
| AC-46; B8-FL8 | 29.1 | CSV, WGTGKTYEQY | FG-2.7 | 19/75 | ||||
| 29.1 | CSV, WGEGRSYEQY | FG-2.7 | 5/75 | |||||
| 20.1 | CSA, TILAGVPYGEQY | FG-2.7 | 17/75 | 20.1 | CSA, TILAGVPYGEQY | FG-2.7 | 24/76 | |
| ACGATCCTAGCGGGAGTTCCCTATGGGGAGCAGTAC | 17/17 | ACGATCCTAGCGGGAGTTCCCTATGGGGAGCAGTAC | 23/24 | |||||
| ACGATCCTAGCGGGAGTCCCCTATGGGGAGCAGTAC | 1/24 | |||||||
| 20.1 | CSA, TILAGVPYGGQH | FG-2.7 | 1/75 | |||||
| 20.1 | CSA, SEGTSSYEQY | FG-2.7 | 1/75 | |||||
| 7.3 | CASS, FDREVTGELF | FG-2.2 | 7/75 | |||||
| 7.3 | CASS, PDGGNTEAF | FG-1.2 | 2/75 | |||||
| 19 | CASS, VGAGTEAF | FG-1.1 | 5/75 | |||||
| 10.3 | CAI, SESGYGGPPGANVLT | FG-2.6 | 1/75 | |||||
| 10.3 | CTI, SESGYRGPPGANVLT | FG-2.6 | 1/75 | |||||
| 10.3 | CAI, SEPGYRGPPGANVLT | FG-2.6 | 1/75 | |||||
| 10.3 | CAI, SESGYRGPPGANVLT | FG-2.6 | 1/75 | |||||
| 28 | CAS, RPTDRNTGELF | FG-2.2 | 3/75 | |||||
| 28 | CASS, LAAGGPYEQY | FG-2.7 | 1/75 | |||||
| 7.9 | CASS, PPSGSYEQY | FG-2.7 | 1/75 | |||||
| 7.9 | CASS, LESGQRPYEQY | FG-2.7 | 1/75 | |||||
| 19 | CASS, IGPLEGNEQF | FG-2.1 | 2/75 | 19 | CASS, IGPLEGNEQF | FG-2.1 | 1/76 | |
| 27 | CASS, WGQGOLSYEQY | FG-2.7 | 2/75 | 27 | CASS, WGQGQLSYEQY | FG-2.7 | 35/76 | |
| 5.4 | CASS, YRGQGNYGYT | FG-1.2 | 1/75 | |||||
| 6.3 | CASS, YERGMNTEAF | FG-1.1 | 1/75 | |||||
| 6.5 | CASS, MGQGATEAF | FG-1.1 | 1/75 | |||||
| 6.6 | CASS, YPMGANEKLF | FG-1.4 | 1/75 | |||||
| 27 | CAS, RIGQGTVGELF | FG-2.2 | 3/76 | |||||
| 29.1 | CSV, DNSYEQY | FG-2.7 | 3/76 | |||||
| 29.1 | CSV, VGLESSYEQY | FG-2.7 | 2/76 | |||||
| 29.1 | CSV, GENTEAF | FG-1.1 | 1/76 | |||||
| 7.2 | CASS, IFGSPFNQPQH | FG-1.5 | 4/76 | |||||
| 19 | CAT, LREGPTTGELF | FG-2.2 | 3/76 | |||||
| AC-14; B8-FL8 | 27 | CASS, LGQGLANYGYT | FG-1.2 | 17/40 | ||||
| 20.1 | CSA, RPMAASGLTYEQY | FG-2.7 | 6/40 | |||||
| 6.1 | CASS, EYGAAVYEQY | FG-2.7 | 8/40 | |||||
| 7.3 | CASS, LNLRGSSGNTIY | FG-1.3 | 5/40 | |||||
| 28 | CASS, SNPGTSGGYSYEQY | FG-2.7 | 3/40 | 28 | CASS, SNPGTSGGYSYEQY | FG-2.7 | 3/51 | |
| 7.9 | CASS, GSPGLAGEQT | FG-2.1 | 1/40 | |||||
| 6.3 | CASS, LLGQYNEQF | FG-2.1 | 22/51 | |||||
| 6.3 | CASS, LLGQCNEQF | FG-2.1 | 1/51 | |||||
| 6.3 | CASS, FHPGQGASYSNQPQH | FG-1.5 | 11/51 | |||||
| 6.6 | CASS, YERGGLPKNIQY | FG-2.4 | 10/51 | |||||
| 27 | CASS, LGQGRSLGHEQY | FG-2.7 | 3/51 | |||||
| 28 | CASS, PRDRVIEDTQY | FG-2.1 | 1/51 | |||||
| AC-09; A2-IV9 | 20.1 | CSA, RQYRTDMNTEAF | FG-1.1 | 15/39 | ||||
| 29.1 | CSV, EVGAGETQY | FG-2.5 | 7/39 | |||||
| 29.1 | CSV, EDLGNEQF | FG-2.3 | 1/39 | |||||
| 29.1 | CSA, ETSDSYEQY | FG-2.7 | 1/39 | |||||
| 6.6 | CASS, YEWGGLPKNIQY | FG-2.4 | 7/39 | |||||
| 27 | CAS, RLVGIGNSPLH | FG-1.6 | 5/39 | |||||
| 10.3 | CAI, SEDGGETQY | FG-2.5 | 3/39 | 10.3 | CAI, SEDGGETQY | FG-2.5 | 29/47 | |
| 10.3 | CAI, SGDGGETQY | FG-2.5 | 1/47 | |||||
| 10.3 | CTI, SEWGGETQY | FG-2.5 | 1/47 | |||||
| 28 | CASS, FGGDEQH | FG-1.5 | 4/47 | |||||
| 28 | CASS, LGGDTQY | FG-2.1 | 3/47 | |||||
| 28 | CASS, LGGDEQY | FG-2.7 | 1/47 | |||||
| 28 | CASS, FGGDTQY | FG-2.1 | 1/47 | |||||
| 28 | CASS, LRDEQY | FG-2.7 | 1/47 | |||||
| 29.1 | CSV, EDSGNEQF | FG-2.1 | 2/47 | |||||
| 29.1 | CSV, EDRANEQY | FG-2.7 | 1/47 | |||||
| 29.1 | CSV, RAPPMTSPTDEQY | FG-2.7 | 1/47 | |||||
| 6.2 | CASS, LDGGETQY | FG-2.5 | 1/47 | |||||
| 6.2 | CASS, QDGGETQY | FG-2.5 | 1/47 | |||||
| AC-02; B8-FL8 | 7.2 | CASS, EPALAEQYYEQY | FG-2.7 | 39/46 | ||||
| 7.2 | CASS, FTPGTGAPYSNQPQH | FG-1.5 | 4/46 | |||||
| 29.1 | CSV, EGGSAYEQY | FG-2.7 | 3/46 | |||||
| 7.3 | CASS, FDREVTGELF | FG-2.2 | 32/45 | |||||
| 7.3 | CASS, LDREVTGELF | FG-2.2 | 1/45 | |||||
| 20.1 | CSA, HLYRAYGYT | FG-1.2 | 5/45 | |||||
| 19 | CASS, MGQHSNQPQH | FG-1.5 | 4/45 | |||||
| 7.2 | CASS, LAWGRAESSYNEQF | FG-2.1 | 2/45 | |||||
| 29.1 | CSV, WGTGKTYEQY | FG-2.7 | 1/45 | |||||
| AC-15; B8-FL8 | 6.1 | CAS, KWDPGQGSHYSNQPQH | FG-1.5 | 17/37 | 6.1 | CAS, KWDPGQGSHYSNQPQH | FG-1.5 | 2/41 |
| 29.1 | CSV, EPSGRARTYNEQF | FG-2.1 | 10/37 | |||||
| 30 | CAW, DVKDRRIGNEQF | FG-2.1 | 5/37 | |||||
| 20.1 | CSA, RDSGRGIENYEQY | FG-2.7 | 2/37 | |||||
| 20.1 | CSA, SSQRGGIYEQY | FG-2.7 | 2/37 | |||||
| 7.9 | CASS, LVGVGTSDEQF | FG-2.1 | 1/37 | |||||
| 7.3 | CASS, FDREVTGELF | FG-2.2 | 18/41 | |||||
| 27 | CASS, LGQGLANYGYT | FG-2.1 | 11/41 | |||||
| 27 | CASS, LSPGTSGSRANEQF | FG-2.1 | 1/41 | |||||
| 28 | CASS, LELAGEDYEQY | FG-2.7 | 4/41 | |||||
| 28 | CASS, LSSNEQF | FG-2.1 | 2/41 | |||||
| 9 | CASS, TLTSGGARDEQF | FG-2.7 | 3/41 | |||||
| AC-131; B8-EI8 | 29.1 | CSV, RDGYEQY | FG-2.7 | 23/46 | ||||
| 7.2 | CASS, PVRGRTEAF | FG-1.1 | 4/46 | |||||
| 7.2 | CASS, LVGPGGREKLF | FG-1.4 | 3/46 | |||||
| 9 | CASS, VLTGGRETQY | FG-2.5 | 7/46 | |||||
| 19 | CASS, IIKGNQPQH | FG-1.5 | 4/46 | |||||
| 19 | CASS, SGLPTNEKLF | FG-1.4 | 3/46 | |||||
| 6.1 | CAS, PYMDARSEAF | FG-1.1 | 2/46 | |||||
| 9 | CASS, VVGDFRETQY | FG-2.5 | 48/48 | |||||
| AC-177; B8-EI8 | 6.6 | CASS, YGGTEAF | FG-1.1 | 23/49 | 6.6 | CASS, YGGTEAF | FG-1.1 | 18/46 |
| TACGGAGGCACTGAAGCTTTC | 14/23 | |||||||
| TACGGGGGCACTGAAGCATTC | 1/23 | |||||||
| TACGGGGGCACTGAAGCTCTC | 1/23 | |||||||
| TACGGAGGGACTGAAGCTTTC | 4/23 | |||||||
| TACGGAGGAACTGAAGCTTTC | 1/23 | TACGGAGGAACTGAAGCTTTC | 10/18 | |||||
| TATGGGGGGACTGAAGCTTTC | 2/23 | |||||||
| TACGGGGGCACTGAAGCTTTC | 5/18 | |||||||
| TACGGGGGGACTGAAGCTTTC | 3/18 | |||||||
| 7.2 | CASS, LPGQGRTPLH | FG-1.6 | 4/49 | 7.2 | CASS, LPGQGRTPLH | FG-1.6 | 7/46 | |
| TTACCTGGACAGGGAAGGACACCCCTCCAC | 4/4 | TTACCTGGACAGGGAAGGACACCCCTCCAC | 6/7 | |||||
| TTACCTGGACAGGGAAGGACGCCCCTCCAC | 1/7 | |||||||
| 7.2 | CASS, LETRNSPLH | FG-1.6 | 1/49 | |||||
| 7.2 | CASS, FLPKNEQF | FG-2.1 | 4/49 | |||||
| 7.2 | CASS, LVRDDRIEQY | FG-2.7 | 2/49 | |||||
| 7.2 | CASS, FPGQGRTEAF | FG-1.1 | 2/49 | |||||
| 20.1 | CSA, RLSYEQY | FG-2.7 | 7/49 | |||||
| 20.1 | CSA, RDNYEQY | FG-2.7 | 2/49 | |||||
| 20.1 | CSA, RDSYEQY | FG-2.7 | 1/49 | |||||
| 20.1 | CSA, RLDYEQY | FG-2.7 | 1/49 | |||||
| 20.1 | CSA, RYDYEQY | FG-2.7 | 1/49 | |||||
| 7.3 | CASS, LIPSGGRNEQF | FG-2.1 | 1/49 | |||||
| 9 | CASS, AITSGGARDEQF | FG-2.1 | 11/46 | |||||
| 9 | CASS, VGGDHREEQH | FG-2.7 | 3/46 | |||||
| 9 | CASS, AGDIQY | FG-2.4 | 2/46 | |||||
| 9 | CASS, AGGIQY | FG-2.4 | 1/46 | |||||
| 10.3 | CAT, IRTGFSSYEQY | FG-2.7 | 4/46 | |||||
| AC-132; A3-OK10 | 6.6 | CASS, YSRGSGNTIY | FG-1.3 | 19/43 | 6.6 | CASS, YSRGSGNTIY | FG-1.3 | 7/42 |
| TACTCTAGGGGATCTGGAAACACCATATAT | 6/19 | TACTCTAGGGGATCTGGAAACACCATATAT | 5/7 | |||||
| TACTCTAGGGGCTCTGGAAACACCATATAT | 13/19 | TACTCTAGGGGCTCTGGAAACACCATATAT | 2/7 | |||||
| 6.6 | CASS, PYRGPNTEAF | FG-1.1 | 1/43 | 6.6 | CASS, PYRGPNTEAF | FG-1.1 | 1/42 | |
| 10.3 | CAI, SAGASFVTRSTDTQY | FG-2.1 | 6/43 | 10.3 | CAI, SAGASFVTRSTDTQY | FG-2.1 | 1/42 | |
| 10.3 | CAI, RSTDTQY | FG-2.1 | 1/43 | |||||
| 6.1 | CAS, RQQGFVFEAKNIQY | FG-2.4 | 3/43 | |||||
| 6.1 | CASS, EEVEAF | FG-1.1 | 1/43 | |||||
| 20.1 | CSA, PTSGSAAF | FG-1.1 | 3/43 | |||||
| 20.1 | CSA, RDSIQFSSNQPQH | FG-1.5 | 1/43 | |||||
| 6.2 | CASS, YSMTSGSFSDLGAKNIQY | FG-2.4 | 1/43 | |||||
| 6.2 | CAS, RPGPVKNTGELF | FG-2.2 | 1/43 | 6.2 | CAS, RPGPVKNTGELF | FG-2.2 | 7/42 | |
| 9 | CASS, LYHNTGELF | FG-2.2 | 1/43 | |||||
| 9 | CASS, GGAHFSKIPLAGYNEQF | FG-2.1 | 1/43 | 9 | CASS, GGAHFSKIPLAGYNEQF | FG-2.1 | 3/42 | |
| 5.4 | CASS, RTDFTAGELF | FG-2.2 | 1/43 | |||||
| 27 | CASS, LTGHPYEQY | FG-2.7 | 1/43 | 27 | CASS, LTGHPYEQY | FG-2.7 | 9/42 | |
| 28 | CASS, PGEKYEQY | FG-2.1 | 1/43 | |||||
| 29.1 | CSV, EDRHYEQY | FG-2.7 | 1/43 | 29.1 | CSV, EDRHYEQY | FG-2.7 | 5/42 | |
| 6.6 | CASS, YSRGAGNTIY | FG-1.3 | 7/42 | |||||
| 6.6 | CAS, TRSGGFRDEQY | FG-2.7 | 1/42 | |||||
| 7.9 | CASS, RRDHQETQY | FG-2.5 | 1/42 | |||||
| AC-160; B27-KK10 | 5.4 | CASS, LTAPDTEAF | FG-1.1 | 30/45 | 5.4 | CASS, LTAPDTEAF | FG-1.1 | 8/53 |
| 5.4 | CASS, GTAPAAEAF | FG-1.1 | 1/45 | |||||
| 5.4 | CASS, STAPDTEAF | FG-1.1 | 1/45 | |||||
| 5.4 | CASS, TAPGTEAF | FG-1.1 | 1/45 | |||||
| 27 | CASS, RSTGELF | FG-2.2 | 10/45 | 27 | CASS, RSTGELF | FG-2.2 | 27/53 | |
| 20.1 | CSA, RDQRDYQETQY | FG-2.5 | 2/45 | 20.1 | CSA, RDQRDYQETQY | FG-2.5 | 1/53 | |
| 27 | CASS, VRTGELF | FG-2.2 | 14/53 | |||||
| 27 | CASS, PRTGELF | FG-2.2 | 3/53 | |||||
| AC-121; B57-KF11 | 19 | CASS, GQGYGYT | FG-1.2 | 24/46 | 19 | CASS, GQGYGYT | FG-1.2 | 25/45 |
| GGACAGGGGTATGGCTACACG | 28/24 | GGACAGGGGTATGGCTACACC | 22/25 | |||||
| GGGCAGGGGTATGGCTACACC | 1/24 | |||||||
| GGACAGGGATATGGCTACACC | 3/25 | |||||||
| 19 | CAS, TGGGYGYT | FG-1.2 | 8/46 | |||||
| 19 | CASS, GQDYGYT | FG-1.2 | 5/46 | 19 | CASS, GQDYGYT | FG-1.2 | 10/45 | |
| 19 | CASS, GQGYGYA | FG-1.2 | 1/46 | |||||
| 19 | CAS, TGSGYGYT | FG-1.2 | 1/46 | |||||
| 19 | CASS, GQEYGYT | FG-1.2 | 1/46 | 19 | CASS, GQDYGYT | FG-1.2 | 1/45 | |
| 6.1 | CAS, TDSYGYT | FG-1.2 | 6/46 | 6.1 | CAS, TDSYGYT | FG-1.2 | 5/45 | |
| ACTGACAGCTATGGCTACACC | ACTGACAGCTATGGCTACACC | 4/5 | ||||||
| ACTGACAGCTATGGCTATACC | 1/5 | |||||||
| 19 | CASS, GGSYGYT | FG-12 | 4/45 | |||||
Nucleotide data are provided when multiple nucleotypes coding for identical CDR3 amino acid sequences were detected in clonotypes persisting between early and chronic infections. TCR clonotypes detected in both primary and secondary infection are shown in boldface type.
Selective loss of high-avidity HIV-1-specific CD8+ T cells after early infection.
We next explored the potential mechanisms accounting for the selective loss or persistence of specific CD8+ T-cell clones after early infection. In three of our study subjects, we had the opportunity for a direct flow cytometric comparison of the avidity of HIV-1-specific CD8+ T-cell clonotypes persisting or disappearing after early infection, using tetramer dissociation assays in conjunction with TCR Vβ chain-specific antibodies. In study subject AC-46, we compared the B8-FL8-specific CD8+ T-cell clonotypes using Vβ9 (6.5% of all clonotypes in early infection and 0% of clonotypes in chronic infection) or Vβ20.1 (25% of all clonotypes in early infection and 31.6% of clonotypes in chronic infection); in study individual AC-09, we compared the A2-IV9-specific CD8+ T-cell clonotypes using Vβ20.1 (38% of clonotypes in early infection and 0% of clonotypes in chronic infection) or Vβ10.3 (7.6% of clonotypes in early infection and 62% in chronic infection); and in study person AC-14, we analyzed the B8-FL8-specific clonotypes using Vβ27 (42.5% of all clonotypes in early infection and 0% in chronic infection) or Vβ28 (7.9% of all clonotypes in early infection and 5.8% in chronic infection) (Table 3). The individual contributions of these clonotypes to the overall magnitude of epitope-specific CD8+ T cells during early infection, as determined by TCR sequencing, closely matched the proportions of tetramer-positive CD8+ T cells determined by using the corresponding TCR Vβ chains, as measured by flow cytometry in cell samples collected from the same time point (Fig. 5A). Using tetramer dissociation assays, we found that in each of these three study persons the above-mentioned clonotypes persisting after early infection had a lower avidity than did the corresponding clonotypes being deleted after early infection (Fig. 5B). These results demonstrate that high-avidity CD8+ T-cell clonotypes generated during early infection were preferentially lost in chronic infection, while CD8+ T-cell clonotypes with intermediate or lower TCR avidity persisted.
FIG. 5.
Tetramer dissociation and phenotypic characteristics of HIV-1-specific CD8+ T-cell clonotypes persisting or disappearing after early HIV-1 infection. (A) Dot plots indicating the proportions of tetramer-positive CD8+ T cells with specific TCR Vβ chain usage during early infection in the three indicated study persons. Gating was performed according to forward scatter/side scatter characteristics of the lymphocyte population and CD8 expression. (B) Intraindividual comparison of tetramer dissociation rates of HIV-1-specific CD8+ T-cell clonotypes persisting or disappearing after early infection. (C) Histograms (presented on a log scale) indicating the expression of CD127, CD38, and Ki-67 during early HIV-1 infection in HIV-1-specific clonotypes persisting or disappearing after early infection. Dotted lines correspond to HIV-1-specific CD8+ T-cell clones eliminated after early infection, and solid lines correspond to CD8+ T-cell clones persisting after acute HIV-1 infection. Scattered lines indicated background staining intensity (no antibodies). In study subject AC-46, B8-FL8-specific CD8+ T cells were analyzed using Vβ9 (6.5% of all clonotypes in early infection and 0% of clonotypes in chronic infection) and compared to those analyzed using Vβ20.1 (25% of all clonotypes in early infection and 31.6% of clonotypes in chronic infection). In study individual AC-09, A2-IV9-specific CD8+ T-cell clonotypes were analyzed using Vβ20.1 (38% of clonotypes in early infection and 0% of clonotypes in chronic infection) and compared to those analyzed using Vβ10.3 (7.6% of clonotypes in early infection and 62% in chronic infection). In study person AC-14, we analyzed the B8-FL8-specific clonotypes using Vβ27 (42.5% of all clonotypes in early infection and 0% in chronic infection) or Vβ28 (7.9% of all clonotypes in early infection and 5.8% in chronic infection) (Table 3). MFI, mean fluorescence intensity.
We subsequently assessed whether these high-avidity epitope-specific CD8+ T-cell clones differed from the persisting lower-avidity clones in activation status and expression of the receptor for IL-7, a cytokine that has recently been identified to be responsible for the maintenance of CD8+ T cells and their transformation into memory cells (26). Interestingly, in these three study subjects, the epitope-specific CD8+ T-cell clonotypes that were lost in chronic infection had a lower surface expression of the IL-7 receptor α chain (CD127), a higher expression of the activation marker CD38 (16), and a higher intracellular expression of the proliferation-associated antigen Ki-67 (Fig. 5C) during early infection. Taken together, these data show that during early HIV-1 infection, clonal subsets of HIV-1-specific CD8+ T cells with higher avidity are more strongly activated and less likely to persist during the subsequent disease process.
DISCUSSION
A striking feature of the natural HIV-1 disease process is the dramatic decline of HIV-1 viremia during early HIV-1 infection. A number of observations suggest that this decrease of viral replication is mediated by HIV-1-specific CD8+ T cells; however, the precise characteristics of these cells accounting for their apparent antiviral activities are still unknown. Here, we conducted a detailed analysis of the functional properties of HIV-1-specific CD8+ T-cell responses during early and chronic HIV-1 infections, using both cross-sectional and longitudinal study designs as well as assays to evaluate the avidities and the clonotypic compositions of HIV-1-specific CD8+ T-cell populations.
Our results indicate that HIV-1-specific CD8+ T cells in early infection, despite being lower in magnitude than those in chronic infection, have a higher avidity by multiple functional readouts, which was associated with a slower tetramer dissociation rate. In addition, the reduced avidity of CD8+ T cells in chronic infection was linked to a substantial switch in the clonotypic composition of epitope-specific CD8+ T cells compared to those in early infection. Interestingly, the avidity and clonotypes of HIV-1-specific CD8+ T cells were largely preserved after early infection in individuals who spontaneously controlled HIV-1 replication at low-level viral set points, suggesting an association between the conservation of the originally recruited pattern of HIV-1-specific CD8+ T cell clonotypes and the degree of spontaneous viral control. Finally, our data indicate on the level of the CD8+ T-cell clonotype that high-avidity epitope-specific CD8+ T-cell populations lost after early infection have higher degrees of activation than lower-avidity CD8+ T-cell clonotypes specific for the same epitope that persist after early infection. These data suggest that high-avidity HIV-1-specific CD8+ T-cell clones recruited during early infection are more prone to clonal deletion, thus contributing to a defective memory response in chronic infection.
Previous studies have mainly relied on IFN-γ secretion to characterize and enumerate HIV-1-specific CD8+ T cells during early HIV-1 infection (6, 13, 14, 17, 43). In addition to the identification of preferential viral targets (13, 37), these studies indicated a substantially smaller magnitude and breadth of HIV-1-specific CD8+ T cells in early infection than in chronic infection but did not allow for quantification of subsets of HIV-1-specific CD8+ T cells with alternative functional activities. Assessing a combination of different functional readouts, our data indicate that in addition to IFN-γ-secreting HIV-1-specific CD8+ T cells, the total magnitude of HIV-1-specific CD8+ T cells with antigen-specific secretion of TNF-α or MIP-1β or expression of CD107a/b is also lower in early infection than in chronic infection. Moreover, the hierarchy of effector functions during early infection was similar to that seen in chronic infection and closely resembled the functional pattern of CD8+ T cells observed during chronic HIV-1 infection by other investigators (9a). Overall, these data indicate that quantitative characteristics of HIV-1-specific CD8+ T cells and the specificities of their cytokine secretion profiles are unlikely to account for the significant decline of HIV-1 viremia during early HIV-1 infection.
In this study, we observed a preferential recruitment of HIV-1-specific CD8+ T cells with high functional avidities and slow tetramer dissociation rates during early infection. The dominance of high-avidity CD8+ T-cell responses in early HIV-1 infection may be related to the fact that these CD8+ T cells can sense their HIV-1 cognate epitopes at the first stage of systemic antigenemia, providing these high-avidity clones with a kinetic selection advantage over CD8+ T-cell clones with lower avidity (29). Furthermore, data from in vivo studies with animal models suggested that the functional avidity of CD8+ T cells is linked to their antiviral activity (19, 52, 54). For instance, the adoptive transfer of high-avidity lymphocytic choriomeningitis virus-specific CD8+ T cells resulted in complete clearance of the infection, while viral persistence was noticed following transfer of low-avidity CD8+ T cells (3, 23). In addition, high-avidity SIV-specific CD8+ T cells preferentially selected for viral escape mutations during early SIV infection in epitopes targeted by high-avidity SIV-specific CD8+ T cells (42, 56). The fact that no viral epitope diversification within tested cytotoxic-T-lymphocyte epitopes occurred in our study subjects most likely reflects structural constraints that make it difficult for the virus to escape rapidly at these positions. However, in many of our study persons, viral sequence diversification consistent with escape mutations was detected within the respective epitopes at a later time point (after the time points chosen for our analysis) (data not shown). Overall, these data suggest that high-avidity HIV-1-specific CD8+ T cells recruited during early infection might significantly contribute to the dramatic decline of HIV-1 viremia during early infection.
In order to analyze the fate of these high-avidity HIV-1-specific CD8+ T-cell responses primed during early infection, we performed a longitudinal analysis of the clonotypic compositions of epitope-specific CD8+ T-cell populations in early and chronic infections. These studies indicated a considerable switch of the clonotypic repertoire of HIV-1-specific CD8+ T cells between early and chronic infections, although the persistence of individual clonotypes was observed in 8 out of 10 study subjects analyzed, and we cannot exclude low-frequency persistence of some of the initially recruited clonotypes below the level of detection. Thus, our data suggest that the observed alterations of epitope-specific CD8+ T-cell avidity between early and chronic infections were at least partially due to the elimination of high-avidity CD8+ T-cell clones initially recruited during early infection and their subsequent replacement by CD8+ T-cell clones with lower avidity, although changes in the TCR signaling cascade between early and chronic HIV-1 infections might also have contributed (53). In the presence of high viral loads, this loss of high-avidity CD8+ T cells can apparently occur rapidly after early infection, as in study subject AC-132, in whom a substantial decrease of HIV-1-specific CD8+ T-cell avidity, together with a prominent alteration of the TCR clonotype pattern, was observed within 27 days after the initial assessment. Moreover, in the study subjects undergoing STIs, brief exposures to high viral loads during off-therapy periods seemed to be sufficient to lead to a considerably lower HIV-1-specific CD8+ T-cell avidity. Thus, our data indicate that the previously described fluctuations of the clonotypic repertoire of HIV-1-specific CD8+ T cells (40) can be prominently shaped by their antigenic avidity, as was recently suggested for the clonotypic repertoire of Epstein-Barr virus-specific CD8+ T cells (18, 46). Moreover, our results show that the previously reported loss of certain HIV-1-specific (45, 55) or SIV-specific (47) CD8+ T-cell clones after early infection can be associated with significant changes in functional avidity. However, our experiments were performed only with HIV-1-specific CD8+ T-cell populations recognizing immunodominant epitopes, and it is quite possible that differences in avidity and clonotype composition between early and chronic infections are less pronounced in subdominant HIV-1-specific CD8+ T-cell populations.
What are the mechanisms that govern the elimination or persistence of HIV-1-specific CD8+ T cells after early infection? Our data demonstrate that high-avidity CD8+ T-cell clones that were no longer detectable after early infection exhibited higher degrees of activation, while the persisting clones with lower avidity had a lower degree of activation, measured by CD38 and Ki-67 expression ex vivo. Thus, persistence or elimination of initially recruited CD8+ T cells, at least under conditions of chronic antigenic stimulation, appears to be linked to the degree of cellular activation, which seems to depend on functional avidity of the lymphocyte. This view is supported by previous studies with animal models of lymphocytic choriomeningitis virus-infected mice. In these studies, it was shown that high-dose viral infection not only led to an almost complete switch in the clonotypic repertoire of epitope-specific T cells between acute and chronic infections (38) but also resulted in the emergence of dominating T cells with intermediate- to low-avidity TCRs in chronic infection (48). One mechanism potentially accounting for the preferential deletion of high-avidity CD8+ T cells in the presence of high-level antigenemia could be their increased sensitivity to induction of apoptosis by high levels of peptide-MHC complexes, through a mechanism involving induction of TNF-α and TNF-α receptor II (2, 4). In contrast, in different animal models of chronic viral or bacterial infections, low-dose antigenic challenge was associated with a more stable clonotypic TCR pattern and the ultimate evolution of dominant T cells with high-avidity TCRs (12, 50). Our data support a similar model in which extremely high HIV-1 loads during early HIV-1 infection lead to the overactivation and subsequent depletion of high-avidity HIV-1-specific CD8+ T cells. This appears to result in a domination of intermediate- to low-avidity CD8+ T cells in chronic infection, because T cells, unlike B lymphocytes, cannot take advantage of the mechanism of somatic mutation to create new cells with higher avidity receptors during the longitudinal course of an immune response. In contrast, in the two study subjects who spontaneously achieved a low-level set point viremia in the absence of STI, we observed an almost undetectable difference in receptor avidity between early and chronic infections, together with substantial persistence (yet differential contributions) of recruited CD8+ T-cell clonotypes, suggesting that the persistence of HIV-1-specific CD8+ T-cell clones after early infection critically depends on the levels of viral control that these CD8+ T cells and the other components of the innate and adaptive immune systems are able to achieve.
When comparing short-lived high-avidity HIV-1 epitope-specific CD8+ T-cell clones with long-term-persisting low-avidity clones specific for the same epitope in the same individual during early infection, we observed that these subsets of CD8+ T cells strikingly differ in surface expression of the IL-7 receptor α chain (CD127). IL-7 has recently been identified as a cytokine responsible for the maintenance of antigen-specific CD8+ T cells (26, 34) and their transformation into functional antigen-specific memory responses, a process that appears to be selectively disturbed in chronic HIV-1 infection (44). Our study suggests that the characteristic reduction of CD127 expression (15, 39, 41, 44) on HIV-1-specific CD8+ T cells originates early in early HIV-1 infection and that the early loss of CD127 expression on the highly activated high-avidity CD8+ T-cell clones might codetermine the inability of these clones to persist during the ensuing disease process. Since this analysis was performed with only three study persons, the investigation of factors contributing to the impairment of an effective antigen-specific memory response in chronic HIV-1 infection needs further elucidation in future studies.
In summary, our data suggest that high-avidity CD8+ T-cell clones are recruited early in HIV-1 infection but are subsequently deleted in the presence of high-level viremia, resulting in the persistence of intermediate- to low-avidity CD8+ T cells in chronic infection. Persistence of epitope-specific CD8+ T-cell clonotypes was associated with low viral set points, lower activation levels during early infection, and elevated expression of the IL-7 receptor α chain (CD127). These data provide insight into the mechanisms that govern the recruitment, maintenance, and elimination of HIV-1-specific CD8+ T cells and contribute to the understanding of events leading to the ultimate inability to immunologically control HIV-1 infection.
Acknowledgments
This study was supported by the National Institutes of Health (to M.A., X.G.Y., E.S.R., and B.D.W.), the Doris Duke Charitable Foundation (to X.G.Y., E.S.R., and B.D.W.), and the Howard Hughes Medical Institute (to B.D.W.). We declare no competing financial interests.
Footnotes
Published ahead of print on 7 February 2007.
REFERENCES
- 1.Addo, M. M., X. G. Yu, A. Rathod, D. Cohen, R. L. Eldridge, D. Strick, M. N. Johnston, C. Corcoran, A. G. Wurcel, C. A. Fitzpatrick, M. E. Feeney, W. R. Rodriguez, N. Basgoz, R. Draenert, D. R. Stone, C. Brander, P. J. Goulder, E. S. Rosenberg, M. Altfeld, and B. D. Walker. 2003. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J. Virol. 77:2081-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alexander-Miller, M. A., M. A. Derby, A. Sarin, P. A. Henkart, and J. A. Berzofsky. 1998. Supraoptimal peptide-major histocompatibility complex causes a decrease in bc1-2 levels and allows tumor necrosis factor alpha receptor II-mediated apoptosis of cytotoxic T lymphocytes. J. Exp. Med. 188:1391-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexander-Miller, M. A., G. R. Leggatt, and J. A. Berzofsky. 1996. Selective expansion of high- or low-avidity cytotoxic T lymphocytes and efficacy for adoptive immunotherapy. Proc. Natl. Acad. Sci. USA 93:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander-Miller, M. A., G. R. Leggatt, A. Sarin, and J. A. Berzofsky. 1996. Role of antigen, CD8, and cytotoxic T lymphocyte (CTL) avidity in high dose antigen induction of apoptosis of effector CTL. J. Exp. Med. 184:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allen, T. M., M. Altfeld, X. G. Yu, K. M. O'Sullivan, M. Lichterfeld, S. Le Gall, M. John, B. R. Mothe, P. K. Lee, E. T. Kalife, D. E. Cohen, K. A. Freedberg, D. A. Strick, M. N. Johnston, A. Sette, E. S. Rosenberg, S. A. Mallal, P. J. Goulder, C. Brander, and B. D. Walker. 2004. Selection, transmission, and reversion of an antigen-processing cytotoxic T-lymphocyte escape mutation in human immunodeficiency virus type 1 infection. J. Virol. 78:7069-7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Altfeld, M., E. S. Rosenberg, R. Shankarappa, J. S. Mukherjee, F. M. Hecht, R. L. Eldridge, M. M. Addo, S. H. Poon, M. N. Phillips, G. K. Robbins, P. E. Sax, S. Boswell, J. O. Kahn, C. Brander, P. J. Goulder, J. A. Levy, J. I. Mullins, and B. D. Walker. 2001. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J. Exp. Med. 193:169-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Appay, V., D. F. Nixon, S. M. Donahoe, G. M. Gillespie, T. Dong, A. King, G. S. Ogg, H. M. Spiegel, C. Conlon, C. A. Spina, D. V. Havlir, D. D. Richman, A. Waters, P. Easterbrook, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-specific CD8(+) T cells produce antiviral cytokines but are impaired in cytolytic function. J. Exp. Med. 192:63-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts, M. R., D. R. Ambrozak, D. C. Douek, S. Bonhoeffer, J. M. Brenchley, J. P. Casazza, R. A. Koup, and L. J. Picker. 2001. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J. Virol. 75:11983-11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Betts, M. R., D. A. Price, J. M. Brenchley, K. Lore, F. J. Guenaga, A. Smed-Sorensen, D. R. Ambrozak, S. A. Migueles, M. Connors, M. Roederer, D. C. Douek, and R. A. Koup. 2004. The functional profile of primary human antiviral CD8+ T cell effector activity is dictated by cognate peptide concentration. J. Immunol. 172:6407-6417. [DOI] [PubMed] [Google Scholar]
- 9a.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brander, C., and P. Goulder. 2000. The evolving field of HIV CRL epitope mapping: new approaches for the identification of novel epitopes. In B. T. M. Korber, C. Brander, B. D. Walker, R. A. Koup, J. Moore, B. Haynes, and G. Meyer (ed.), HIV molecular database. Los Alamos National Laboratory, Los Alamos, NM.
- 12.Busch, D. H., and E. G. Pamer. 1999. T cell affinity maturation by selective expansion during infection. J. Exp. Med. 189:701-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao, J., J. McNevin, S. Holte, L. Fink, L. Corey, and M. J. McElrath. 2003. Comprehensive analysis of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-secreting CD8+ T cells in primary HIV-1 infection. J. Virol. 77:6867-6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao, J., J. McNevin, U. Malhotra, and M. J. McElrath. 2003. Evolution of CD8+ T cell immunity and viral escape following acute HIV-1 infection. J. Immunol. 171:3837-3846. [DOI] [PubMed] [Google Scholar]
- 15.Carini, C., M. F. McLane, K. H. Mayer, and M. Essex. 1994. Dysregulation of interleukin-7 receptor may generate loss of cytotoxic T cell response in human immunodeficiency virus type 1 infection. Eur. J. Immunol. 24:2927-2934. [DOI] [PubMed] [Google Scholar]
- 16.Chun, T. W., J. S. Justement, C. Sanford, C. W. Hallahan, M. A. Planta, M. Loutfy, S. Kottilil, S. Moir, C. Kovacs, and A. S. Fauci. 2004. Relationship between the frequency of HIV-specific CD8+ T cells and the level of CD38+CD8+ T cells in untreated HIV-infected individuals. Proc. Natl. Acad. Sci. USA 101:2464-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalod, M., M. Dupuis, J. C. Deschemin, C. Goujard, C. Deveau, L. Meyer, N. Ngo, C. Rouzioux, J. G. Guillet, J. F. Delfraissy, M. Sinet, and A. Venet. 1999. Weak anti-HIV CD8(+) T-cell effector activity in HIV primary infection. J. Clin. Investig. 104:1431-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davenport, M. P., C. Fazou, A. J. McMichael, and M. F. Callan. 2002. Clonal selection, clonal senescence, and clonal succession: the evolution of the T cell response to infection with a persistent virus. J. Immunol. 168:3309-3317. [DOI] [PubMed] [Google Scholar]
- 19.Derby, M., M. Alexander-Miller, R. Tse, and J. Berzofsky. 2001. High-avidity CTL exploit two complementary mechanisms to provide better protection against viral infection than low-avidity CTL. J. Immunol. 166:1690-1697. [DOI] [PubMed] [Google Scholar]
- 20.Douek, D. C., M. R. Betts, J. M. Brenchley, B. J. Hill, D. R. Ambrozak, K. L. Ngai, N. J. Karandikar, J. P. Casazza, and R. A. Koup. 2002. A novel approach to the analysis of specificity, clonality, and frequency of HIV-specific T cell responses reveals a potential mechanism for control of viral escape. J. Immunol. 168:3099-3104. [DOI] [PubMed] [Google Scholar]
- 21.Draenert, R., C. L. Verrill, Y. Tang, T. M. Allen, A. G. Wurcel, M. Boczanowski, A. Lechner, A. Y. Kim, T. Suscovich, N. V. Brown, M. M. Addo, and B. D. Walker. 2004. Persistent recognition of autologous virus by high-avidity CD8 T cells in chronic, progressive human immunodeficiency virus type 1 infection. J. Virol. 78:630-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frahm, N., B. T. Korber, C. M. Adams, J. J. Szinger, R. Draenert, M. M. Addo, M. E. Feeney, K. Yusim, K. Sango, N. V. Brown, D. SenGupta, A. Piechocka-Trocha, T. Simonis, F. M. Marincola, A. G. Wurcel, D. R. Stone, C. J. Russell, P. Adolf, D. Cohen, T. Roach, A. StJohn, A. Khatri, K. Davis, J. Mullins, P. J. Goulder, B. D. Walker, and C. Brander. 2004. Consistent cytotoxic-T-lymphocyte targeting of immunodominant regions in human immunodeficiency virus across multiple ethnicities. J. Virol. 78:2187-2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallimore, A., T. Dumrese, H. Hengartner, R. M. Zinkernagel, and H. G. Rammensee. 1998. Protective immunity does not correlate with the hierarchy of virus-specific cytotoxic T cell responses to naturally processed peptides. J. Exp. Med. 187:1647-1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harari, A., S. Petitpierre, F. Vallelian, and G. Pantaleo. 2004. Skewed representation of functionally distinct populations of virus-specific CD4 T cells in HIV-1-infected subjects with progressive disease: changes after antiretroviral therapy. Blood 103:966-972. [DOI] [PubMed] [Google Scholar]
- 25.Jones, N. A., X. Wei, D. R. Flower, M. Wong, F. Michor, M. S. Saag, B. H. Hahn, M. A. Nowak, G. M. Shaw, and P. Borrow. 2004. Determinants of human immunodeficiency virus type 1 escape from the primary CD8+ cytotoxic T lymphocyte response. J. Exp. Med. 200:1243-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 27.Kahn, J. O., and B. D. Walker. 1998. Acute human immunodeficiency virus type 1 infection. N. Engl. J. Med. 339:33-39. [DOI] [PubMed] [Google Scholar]
- 28.Kaufmann, D. E., M. Lichterfeld, M. Altfeld, M. M. Addo, M. N. Johnston, P. K. Lee, B. S. Wagner, E. T. Kalife, D. Strick, E. S. Rosenberg, and B. D. Walker. 2004. Limited durability of viral control following treated acute HIV infection. PLoS Med. 1:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kedl, R. M., J. W. Kappler, and P. Marrack. 2003. Epitope dominance, competition and T cell affinity maturation. Curr. Opin. Immunol. 15:120-127. [DOI] [PubMed] [Google Scholar]
- 30.Kedzierska, K., S. J. Turner, and P. C. Doherty. 2004. Conserved T cell receptor usage in primary and recall responses to an immunodominant influenza virus nucleoprotein epitope. Proc. Natl. Acad. Sci. USA 101:4942-4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koibuchi, T., T. M. Allen, M. Lichterfeld, S. K. Mui, K. M. O'Sullivan, A. Trocha, S. A. Kalams, R. P. Johnson, and B. D. Walker. 2005. Limited sequence evolution within persistently targeted CD8 epitopes in chronic human immunodeficiency virus type 1 infection. J. Virol. 79:8171-8181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lacabaratz-Porret, C., A. Urrutia, J. M. Doisne, C. Goujard, C. Deveau, M. Dalod, L. Meyer, C. Rouzioux, J. F. Delfraissy, A. Venet, and M. Sinet. 2003. Impact of antiretroviral therapy and changes in virus load on human immunodeficiency virus (HIV)-specific T cell responses in primary HIV infection. J. Infect. Dis. 187:748-757. [DOI] [PubMed] [Google Scholar]
- 34.Lang, K. S., M. Recher, A. A. Navarini, N. L. Harris, M. Lohning, T. Junt, H. C. Probst, H. Hengartner, and R. M. Zinkernagel. 2005. Inverse correlation between IL-7 receptor expression and CD8 T cell exhaustion during persistent antigen stimulation. Eur. J. Immunol. 35:738-745. [DOI] [PubMed] [Google Scholar]
- 35.Lefranc, M. P., C. Pommie, M. Ruiz, V. Giudicelli, E. Foulquier, L. Truong, V. Thouvenin-Contet, and G. Lefranc. 2003. IMGT unique numbering for immunoglobulin and T cell receptor variable domains and Ig superfamily V-like domains. Dev. Comp. Immunol. 27:55-77. [DOI] [PubMed] [Google Scholar]
- 36.Lichterfeld, M., D. E. Kaufmann, X. G. Yu, S. K. Mui, M. M. Addo, M. N. Johnston, D. Cohen, G. K. Robbins, E. Pae, G. Alter, A. Wurcel, D. Stone, E. S. Rosenberg, B. D. Walker, and M. Altfeld. 2004. Loss of HIV-1-specific CD8+ T cell proliferation after acute HIV-1 infection and restoration by vaccine-induced HIV-1-specific CD4+ T cells. J. Exp. Med. 200:701-712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichterfeld, M., X. G. Yu, D. Cohen, M. M. Addo, J. Malenfant, B. Perkins, E. Pae, M. N. Johnston, D. Strick, T. M. Allen, E. S. Rosenberg, B. Korber, B. D. Walker, and M. Altfeld. 2004. HIV-1 Nef is preferentially recognized by CD8 T cells in primary HIV-1 infection despite a relatively high degree of genetic diversity. AIDS 18:1383-1392. [DOI] [PubMed] [Google Scholar]
- 38.Lin, M. Y., and R. M. Welsh. 1998. Stability and diversity of T cell receptor repertoire usage during lymphocytic choriomeningitis virus infection of mice. J. Exp. Med. 188:1993-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacPherson, P. A., C. Fex, J. Sanchez-Dardon, N. Hawley-Foss, and J. B. Angel. 2001. Interleukin-7 receptor expression on CD8(+) T cells is reduced in HIV infection and partially restored with effective antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 28:454-457. [DOI] [PubMed] [Google Scholar]
- 40.Meyer-Olson, D., K. W. Brady, M. T. Bartman, K. M. O'Sullivan, B. C. Simons, J. A. Conrad, C. B. Duncan, S. Lorey, A. Siddique, R. A. Draenert, M. Addo, M. Altfeld, E. Rosenberg, T. M. Allen, B. D. Walker, and S. A. Kalams. 2006. Fluctuations of functionally distinct CD8+ T cell clonotypes demonstrate flexibility of the HIV-specific TCR repertoire. Blood 107:2373-2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reference deleted.
- 42.O'Connor, D. H., T. M. Allen, T. U. Vogel, P. Jing, I. P. DeSouza, E. Dodds, E. J. Dunphy, C. Melsaether, B. Mothe, H. Yamamoto, H. Horton, N. Wilson, A. L. Hughes, and D. I. Watkins. 2002. Acute phase cytotoxic T lymphocyte escape is a hallmark of simian immunodeficiency virus infection. Nat. Med. 8:493-499. [DOI] [PubMed] [Google Scholar]
- 43.Oxenius, A., D. A. Price, P. J. Easterbrook, C. A. O'Callaghan, A. D. Kelleher, J. A. Whelan, G. Sontag, A. K. Sewell, and R. E. Phillips. 2000. Early highly active antiretroviral therapy for acute HIV-1 infection preserves immune function of CD8+ and CD4+ T lymphocytes. Proc. Natl. Acad. Sci. USA 97:3382-3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paiardini, M., B. Cervasi, H. Albrecht, A. Muthukumar, R. Dunham, S. Gordon, H. Radziewicz, G. Piedimonte, M. Magnani, M. Montroni, S. M. Kaech, A. Weintrob, J. D. Altman, D. L. Sodora, M. B. Feinberg, and G. Silvestri. 2005. Loss of CD127 expression defines an expansion of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174:2900-2909. [DOI] [PubMed] [Google Scholar]
- 45.Pantaleo, G., H. Soudeyns, J. F. Demarest, M. Vaccarezza, C. Graziosi, S. Paolucci, M. Daucher, O. J. Cohen, F. Denis, W. E. Biddison, R. P. Sekaly, and A. S. Fauci. 1997. Evidence for rapid disappearance of initially expanded HIV-specific CD8+ T cell clones during primary HIV infection. Proc. Natl. Acad. Sci. USA 94:9848-9853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price, D. A., J. M. Brenchley, L. E. Ruff, M. R. Betts, B. J. Hill, M. Roederer, R. A. Koup, S. A. Migueles, E. Gostick, L. Wooldridge, A. K. Sewell, M. Connors, and D. C. Douek. 2005. Avidity for antigen shapes clonal dominance in CD8+ T cell populations specific for persistent DNA viruses. J. Exp. Med. 202:1349-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Price, D. A., S. M. West, M. R. Betts, L. E. Ruff, J. M. Brenchley, D. R. Ambrozak, Y. Edghill-Smith, M. J. Kuroda, D. Bogdan, K. Kunstman, N. L. Letvin, G. Franchini, S. M. Wolinsky, R. A. Koup, and D. C. Douek. 2004. T cell receptor recognition motifs govern immune escape patterns in acute SIV infection. Immunity 21:793-803. [DOI] [PubMed] [Google Scholar]
- 48.Rees, W., J. Bender, T. K. Teague, R. M. Kedl, F. Crawford, P. Marrack, and J. Kappler. 1999. An inverse relationship between T cell receptor affinity and antigen dose during CD4(+) T cell responses in vivo and in vitro. Proc. Natl. Acad. Sci. USA 96:9781-9786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 50.Savage, P. A., J. J. Boniface, and M. M. Davis. 1999. A kinetic basis for T cell receptor repertoire selection during an immune response. Immunity 10:485-492. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 52.Sedlik, C., G. Dadaglio, M. F. Saron, E. Deriaud, M. Rojas, S. I. Casal, and C. Leclerc. 2000. In vivo induction of a high-avidity, high-frequency cytotoxic T-lymphocyte response is associated with antiviral protective immunity. J. Virol. 74:5769-5775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slifka, M. K., and J. L. Whitton. 2001. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat. Immunol. 2:711-717. [DOI] [PubMed] [Google Scholar]
- 54.Snyder, J. T., M. A. Alexander-Miller, J. A. Berzofskyl, and I. M. Belyakov. 2003. Molecular mechanisms and biological significance of CTL avidity. Curr. HIV Res. 1:287-294. [DOI] [PubMed] [Google Scholar]
- 55.Soudeyns, H., G. Campi, G. P. Rizzardi, C. Lenge, J. F. Demarest, G. Tambussi, A. Lazzarin, D. Kaufmann, G. Casorati, L. Corey, and G. Pantaleo. 2000. Initiation of antiretroviral therapy during primary HIV-1 infection induces rapid stabilization of the T-cell receptor beta chain repertoire and reduces the level of T-cell oligoclonality. Blood 95:1743-1751. [PubMed] [Google Scholar]
- 56.Vogel, T. U., T. C. Friedrich, D. H. O'Connor, W. Rehrauer, E. J. Dodds, H. Hickman, W. Hildebrand, J. Sidney, A. Sette, A. Hughes, H. Horton, K. Vielhuber, R. Rudersdorf, I. P. De Souza, M. R. Reynolds, T. M. Allen, N. Wilson, and D. I. Watkins. 2002. Escape in one of two cytotoxic T-lymphocyte epitopes bound by a high-frequency major histocompatibility complex class I molecule, Mamu-A*02: a paradigm for virus evolution and persistence? J. Virol. 76:11623-11636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang, X. L., and J. D. Altman. 2003. Caveats in the design of MHC class I tetramer/antigen-specific T lymphocytes dissociation assays. J. Immunol. Methods 280:25-35. [DOI] [PubMed] [Google Scholar]
- 58.Wilson, J. D., G. S. Ogg, R. L. Allen, C. Davis, S. Shaunak, J. Downie, W. Dyer, C. Workman, S. Sullivan, A. J. McMichael, and S. L. Rowland-Jones. 2000. Direct visualization of HIV-1-specific cytotoxic T lymphocytes during primary infection. AIDS 14:225-233. [DOI] [PubMed] [Google Scholar]
- 59.Younes, S. A., B. Yassine-Diab, A. R. Dumont, M. R. Boulassel, Z. Grossman, J. P. Routy, and R. P. Sekaly. 2003. HIV-1 viremia prevents the establishment of interleukin 2-producing HIV-specific memory CD4+ T cells endowed with proliferative capacity. J. Exp. Med. 198:1909-1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu, X. G., M. M. Addo, E. S. Rosenberg, W. R. Rodriguez, P. K. Lee, C. A. Fitzpatrick, M. N. Johnston, D. Strick, P. J. Goulder, B. D. Walker, and M. Altfeld. 2002. Consistent patterns in the development and immunodominance of human immunodeficiency virus type 1 (HIV-1)-specific CD8+ T-cell responses following acute HIV-1 infection. J. Virol. 76:8690-8701. [DOI] [PMC free article] [PubMed] [Google Scholar]