Abstract
The plus-strand RNA genome of flavivirus contains a 5′ terminal cap 1 structure (m7GpppAmG). The flaviviruses encode one methyltransferase, located at the N-terminal portion of the NS5 protein, to catalyze both guanine N-7 and ribose 2′-OH methylations during viral cap formation. Representative flavivirus methyltransferases from dengue, yellow fever, and West Nile virus (WNV) sequentially generate GpppA → m7GpppA → m7GpppAm. The 2′-O methylation can be uncoupled from the N-7 methylation, since m7GpppA-RNA can be readily methylated to m7GpppAm-RNA. Despite exhibiting two distinct methylation activities, the crystal structure of WNV methyltransferase at 2.8 Å resolution showed a single binding site for S-adenosyl-l-methionine (SAM), the methyl donor. Therefore, substrate GpppA-RNA should be repositioned to accept the N-7 and 2′-O methyl groups from SAM during the sequential reactions. Electrostatic analysis of the WNV methyltransferase structure showed that, adjacent to the SAM-binding pocket, is a highly positively charged surface that could serve as an RNA binding site during cap methylations. Biochemical and mutagenesis analyses show that the N-7 and 2′-O cap methylations require distinct buffer conditions and different side chains within the K61-D146-K182-E218 motif, suggesting that the two reactions use different mechanisms. In the context of complete virus, defects in both methylations are lethal to WNV; however, viruses defective solely in 2′-O methylation are attenuated and can protect mice from later wild-type WNV challenge. The results demonstrate that the N-7 methylation activity is essential for the WNV life cycle and, thus, methyltransferase represents a novel target for flavivirus therapy.
Eukaryotic mRNAs possess a 5′ cap structure that is cotranscriptionally formed in the nucleus. mRNA capping is essential for mRNA stability and efficient translation (13, 39). Most animal viruses that replicate in cytoplasm encode their own capping machinery to produce capped RNAs. RNA capping generally consists of three steps in which the 5′ triphosphate end of nascent RNA transcript is first hydrolyzed to a 5′ diphosphate by an RNA triphosphatase, then capped with GMP by an RNA guanylyltransferase, and finally methylated at the N-7 position of guanine by an RNA guanine-methyltransferase (N-7 MTase) (15). Additionally, the first and second nucleotides of many cellular and viral mRNAs are further methylated at the ribose 2′-OH position by a nucleoside 2′-O MTase, to form cap 1 (m7GpppNm) and cap 2 (m7GpppNmNm) structures, respectively (13). Both N-7 and 2′-O MTases use S-adenosyl-l-methionine (SAM) as a methyl donor and generate S-adenosyl-l-homocysteine (SAH) as a by-product. The order of capping and methylation varies among cellular and viral RNAs (13).
The genus Flavivirus comprises approximately 70 viruses, many of which are important human pathogens, including four serotypes of dengue virus (DENV), yellow fever virus (YFV), St. Louis encephalitis virus, and West Nile virus (WNV) (23). The flavivirus genome is a single-stranded RNA of positive (i.e., mRNA sense) polarity. The 5′ end of the genome contains a type 1 cap followed by a conserved dinucleotide sequence 5′-AG-3′ (7, 41). The single open reading frame of the flavivirus genome encodes a polyprotein, which is processed by viral and cellular proteases into three structural proteins and seven nonstructural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5) (23). Of the four enzymes required for synthesis of flavivirus m7GpppAm cap structure, the RNA triphosphatase and 2′-O MTase have been, respectively, mapped to NS3 (20, 42) and NS5 (9). We recently showed that WNV NS5 carries both guanine N-7 and ribose 2′-O MTase activities (34). The guanylyltransferase for flavivirus capping remains elusive.
Flavivirus NS5 consists of an N-terminal MTase and a C-terminal RNA-dependent-RNA polymerase (RdRp) domain (1, 16, 28). The structure of DENV-2 MTase suggests that flavivirus NS5 MTase belongs to a family of SAM-dependent MTases (9). Most of the MTases within this family, including both N-7 and 2′-O RNA MTases such as Encephalitozoon cuniculi (Ecm1) N-7 MTase and vaccinia virus 2′-O MTase VP39 (10, 18), share a common core structure referred to as a “SAM-dependent MTase fold,” composed of an open α/β/α sandwich structure (11, 24). Structure and sequence comparisons of the 2′-O MTases suggest that a conserved K-D-K-E tetrad forms the active site for the 2′-O methyl transfer reaction (9). Using Ala substitution, we recently showed that all residues within the K61-D146-K182-E218 tetrad of the WNV MTase are essential for 2′-O methylation activity, whereas D146 is more critical than the other three residues for N-7 methylation. In addition, we found that methylations of guanine N-7 and ribose 2′-O of the WNV cap structure are sequential, with N-7 preceding 2′-O methylation (34). The WNV MTase represents a unique system to study how a single enzyme catalyzes two distinct cap methylations.
Here we report that, similar to the WNV, MTases from other flaviviruses also sequentially methylate viral RNA cap at guanine N-7 and ribose 2′-O positions, indicating that it is a general mechanism for flaviviruses to encode the NS5 MTase with dual methylation activities for an efficient synthesis of the viral RNA cap. By contrast, the crystal structure of the WNV MTase in complex with SAH shows only a single SAM-binding site. Thus, the 5′ cap of flavivirus RNA must evidently be repositioned to accept two methyl groups from SAM during methylations. Biochemical and mutagenesis analyses suggest that the WNV MTase methylates the N-7 and 2′-O positions using two distinct mechanisms. In the context of full-length WNV, a mutation (D146A) defective in both the N-7 and 2′-O methylations is lethal to the virus. Mutant viruses inactive for 2′-O but not N-7 methylation (K61A, K182A, or E218A) are attenuated in cell culture and in mice and can be used to protect mice from challenge with wild-type WNV.
MATERIALS AND METHODS
Cloning, expression, and purification of WNV, DENV-1, and YFV MTases.
The WNV MTase domain containing the N-terminal 300 amino acids of NS5 was prepared for crystallization and enzyme assays (34). A QuikChange II XL site-directed mutagenesis kit (Stratagene) was used to engineer mutations into the K61-D146-K182-E218 motif of the WNV MTase. DNA fragments representing the N-terminal 262 and 264 amino acids of DENV-1 and YFV NS5, respectively, were PCR-amplified from a DENV-1 replicon cDNA (33) and a YFV infectious cDNA clone (3) and cloned into plasmid pET26b(+) (Novagen) at NdeI and XhoI sites. The DENV-1 and YFV MTases containing a C-terminal His6 tag were expressed and purified through a Ni-nitrilotriacetic acid column followed by a gel filtration 16/60 Superdex column (Amersham). Briefly, Escherichia coli strain Rosetta 2(DE3)pLysS (Novagen) bearing the expression plasmid was grown at 37°C to 0.8 absorbance optical density at 600 nm (OD600), induced with 0.5-mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 15°C for 12 h, and harvested by centrifugation. Cell pellets were resuspended and sonicated in a lysis buffer containing 20 mM Tris-HCl, pH 8.0, 0.5 M NaCl, and 5 mM β-mercaptoethanol. After centrifugation, the lysate supernatant was applied to a Ni-nitrilotriacetic acid column, washed with the lysis buffer supplemented with 20 mM imidazole, and eluted with lysis buffer containing 300 mM imidazole. The proteins were then concentrated and subjected to size exclusion chromatography by a gel filtration 16/60 Superdex column (Amersham). For crystallization, the purified WNV MTase was concentrated to 5 to 10 mg/ml in a buffer composed of 10 mM HEPES, pH 7.5, 0.5 M NaCl, and 5 mM dithiothreitol (DTT).
Crystallization, X-ray data collection, and structure determination and refinement.
Crystals of the WNV MTase domain were grown at room temperature in hanging drops, by mixing 2 μl of protein solution with an equal volume of reservoir solution containing 13 to 15% polyethylene glycol 4000, 5% isopropanol, and 0.1 M sodium citrate, pH 5.6. Crystals appeared within a week and grew to maximal size in a month.
The crystals belong to space group P1. Prior to data collection, all crystals were transferred to a reservoir solution containing 25% glycerol, and then flashed-cooled under a nitrogen stream at 100 K, and stored in liquid nitrogen. Diffraction data were collected at 100 K at a beam line X4A of the national synchrotron light source (Brookhaven National Laboratory). All of the data were processed and scaled using HKL2000 (30).
With the structure of the DENV MTase (PDB code 1L9K) used as a search model, clear solutions were found using AmoRe (26). Structural refinement was carried out using Crystallography & NMR System (5). Noncrystallographic symmetry restraints were applied to the main chain atoms throughout the refinement. At 2.8-Å resolution, the final Rcryst is 25.8%, with an Rfree of 33.3% (Table 1).
TABLE 1.
X-ray data collection and structure refinement statistics
| Parameter | Result |
|---|---|
| Data collection | |
| Space group | P1 |
| Cell dimensions | |
| a (Å) | 39.2 |
| b (Å) | 66.0 |
| c (Å) | 78.8 |
| α (°) | 112.5 |
| β (°) | 102.9 |
| γ (°) | 89.8 |
| Resolution (Å) | 39.1-2.8 |
| Redundancy | 1.7 (1.6) |
| Completeness (%) | 91.5 (91.7) |
| Average I/σ (I) | 4.3 (2.5) |
| Rsym (%) | 11.2 (21.4) |
| Refinement | |
| Resolution limits (Å) | 39.1-2.8 |
| No. of reflections | 15,957 |
| Rwork (%) | 25.8 |
| Rfree (%) | 33.3 |
| No. of non-H atoms | |
| Protein | 4,208 |
| Water | 256 |
| AdoHcy | 2 |
| Average B (Å2) | 26.5 |
| Geometry | |
| rmsd bond length (Å) | 0.008 |
| rmsd bond angle (°) | 1.4 |
| Ramachandran plot | |
| Most favored (%) | 85.7 |
| Additionally allowed (%) | 13.6 |
| Generously allowed (%) | 0.7 |
| Disallowed (%) | 0.0 |
Atomic coordinates have been deposited in the Protein Data Bank as entry 2OY0.
RNA cap methylation assays.
Vaccinia virus capping enzyme (Ambion) was used to prepare 32P-labeled RNA substrates, G*pppA- and m7G*pppA-RNA (representing the 5′-terminal 190 nucleotides of the WNV genome; an asterisk indicates that the following phosphate is 32P labeled) for the N-7 and 2′-O methylation assays, respectively. Since the optimal pH values for the N-7 and 2′-O methylation are at 7 and 10 (see details in Fig. 3), respectively, the N-7 methylation was performed in 50 mM Tris, pH 7.0, 50 mM NaCl, and 2 mM DTT at 22°C for 5 or 30 min as indicated; the 2′-O methylation was incubated in 50 mM glycine, pH 10, and 2 mM DTT at 22°C for 1 h. The other components of the reaction mixtures were identical to those previously described (34). Time course experiments for YFV and DENV-1 MTase (see Fig. 2E) were performed in the 2′-O methylation buffer because this buffer could support both N-7 and 2′-O methylations, whereas the N-7 methylation buffer could barely support the 2′-O methylation. Control m7G*pppAm-RNA was prepared by incubating the vaccinia virus VP39 protein in a 20-μl reaction mixture (50 mM Tris-HCl pH 8.0, 5 mM DTT, 10 μM SAM, 15,000 cpm of m7G*pppA-RNA, and 30 pmol of recombinant VP39) for 1 h at 37°C. The methylation reaction mixtures were digested with nuclease P1 or tobacco acid pyrophosphatase (TAP) and analyzed on polyethyleneimine cellulose thin-layer chromatograph (TLC) plates (JT Baker) (34).
FIG. 3.
Optimization of conditions for the WNV N-7 and 2′-O cap methylations. Reactions for N-7 (•) and 2′-O methylation (○) contained substrate G*pppA-RNA and m7G*pppA-RNA and were incubated for 5 and 60 min, respectively. The optimal conditions were determined by individually titrating pH (A), temperature (B), MgCl2 (C), and NaCl (D), while keeping the other three parameters constant at the optimal levels. The reaction mixtures were then treated with nuclease P1, analyzed on TLC plates, and quantified by PhosphorImager analysis. For each parameter, relative activities were presented using the optimal level as 100%. Average results from two independent experiments are shown.
FIG. 2.
Flavivirus NS5 sequentially methylates guanine N-7 and ribose 2′-OH of the viral RNA cap. (A) Sequence alignment of flavivirus MTases. The MTase sequences of WNV, DENV-1, DENV-2, and YFV are derived from GenBank accession numbers AF404756, DVU88535 U87411 and YFU17-66, respectively. The alignment was performed using GCG software (Genetics Computer Group). Identical amino acids among all MTases are shaded. The conserved K61-D146-K182-E218 residues mutated in this study are indicated by an asterisk. The exact sequences of the recombinant DENV-1 and YFV MTases (not including the C-terminal His tag) are shown. (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of recombinant MTase proteins of YFV, DENV-1, and WNV. (C) Guanine N-7 methylation activity. Substrate G*pppA-RNA was methylated with the indicated MTases or no enzyme (Mock) in the presence of cold SAM in the N-7 assay buffer for 30 min. The reaction mixtures were then digested by TAP and analyzed on a TLC plate followed by autoradiography. (D) Ribose 2′-O methylation activity. m7G*pppA-RNA was methylated by the indicated MTases and digested by nuclease P1. The nuclease P1-resistant cap structures were then analyzed on a TLC plate. (E) Time course analyses of the DENV-1 and YFV MTase activities. 32P-labeled G*pppA-RNA was methylated by DENV-1 (left panel) and YFV MTase (right panel) in the 2′-O methylation buffer for the indicated times, digested with nuclease P1, and analyzed by TLC and autoradiography. The 2′-O methylation buffer is optimal for 2′-O methylation and also supports N-7 methylation. The positions of the origin and the migrations of G*p, m7G*p, G*pppA, m7G*pppA, and m7G*pppAm molecules are indicated on the left.
Construction of mutant WNV cDNA.
Mutant cDNA plasmids of full-length WNV were constructed using a modified infectious cDNA clone pFLWNV (38) and a shuttle vector. The shuttle vector was created by digestion of the pFLWNV plasmid with restriction enzymes KpnI and XbaI, followed by ligation of the resulting 3.2-kb fragment (representing nucleotide position 7762 to the 3′ end of the genome; GenBank no. AF404756) into the predigested pcDNA3.1 (+) vector. For each K-D-K-E mutant, the specific mutation was first introduced into the shuttle vector using an overlapping PCR-mediated mutagenesis. The mutations in the shuttle vectors were confirmed by DNA sequencing. The mutated DNA fragments from the shuttle vectors were then swapped into the pFLWNV through unique restriction enzymes SpeI and XbaI (representing nucleotide position 8022 to the 3′ end of the genome). The mutant pFLWNV was again verified by DNA sequencing.
In vitro transcription, RNA transfection, IFA, and real-time RT-PCR.
Genome-length RNAs were in vitro transcribed and transfected into BHK cells (37). After transfection, viral protein synthesis was monitored by immunofluorescence assay (IFA) using the WNV-immune mouse ascites fluid (American Type Culture Collection) and goat anti-mouse immunoglobulin G conjugated with Texas Red as the respective primary and secondary antibodies (38). For viral RNA quantification, the transfected cells were thoroughly washed with phosphate-buffered saline twice. Total cellular RNA was extracted using RNeasy kits (QIAGEN) and quantified with a NanoDrop spectrophotometer. Equal amounts of extracted RNA (100 ng) were measured for viral RNA by real-time reverse transcription (RT)-PCR (7500 RT-PCR system; Applied Biosystems) using a primer/probe set targeting the NS5 gene (36). Full-length RNA transcribed from an infectious cDNA clone of WNV (38) was used as a reference for quantification of the real-time RT-PCR.
Specific infectivity assay and virus growth kinetics.
For specific infectivity assays, BHK cells were electroporated with wild-type and mutant genome-length RNAs. The transfected cells were adjusted to 1.0 × 107 cells per ml of culture medium. One milliliter each of a series of 1:10 dilutions of the transfected cells was seeded onto nearly confluent Vero cells (6 × 105 cells per well were seeded in a six-well plate 3 days in advance). The seeded cells were allowed to attach to the plates for 7 to 10 h before the first layer of agar was added (31). A second layer containing neutral red was added after incubation of the plates for 2 days. Plaques were counted at 12 to 18 h after addition of the second layer of agar. The specific infectivity was calculated as the number of PFU per microgram of transfected RNA. For viral growth curves, Vero and C6/36 cells grown in 12-well plates were infected with WNV (0.1 multiplicity of infection [MOI]). After 1 h incubation, the virus inocula were removed. The cells were washed twice with phosphate-buffered saline and cultured with 1 ml of fresh medium per well. The culture medium was collected at indicated time points, stored at −80°C, and then assayed for virus titers using standard plaque assays on Vero cells (33).
In vivo virulence analysis in mice.
Six- to seven-week-old, female C3H/HeN (C3H) mice (Taconic) were inoculated subcutaneously (s.c.) in the left rear footpad with a 10-μl inoculum, using a 30-gauge needle and 100-μl glass syringe (Hamilton, Reno, NV). Four mice were inoculated with diluent alone, and eight mice per group were inoculated with 10, 103, or 105 PFU of wild-type, mutant K61A, or mutant K182A WNV. All mice were observed for clinical disease daily for the entire study and weighed daily for at least 14 days postinoculation and then three times weekly for the duration of the study. Clinical signs included ruffled fur, ataxia, weakness, and weight loss. Morbidity was defined as clinical signs for at least 2 days and/or greater than 9% weight loss. Mice that exhibited severe disease were euthanized. On day 28 postinoculation, surviving mice were bled, and sera were tested for WNV-specific antibodies by enzyme-linked immunosorbent assay (32). On day 34 after the initial inoculation, surviving mice were challenged by intraperitoneal inoculation with 106 PFU of wild-type WNV in 100 μl, using a 25-gauge needle and 1-ml syringe. Mice were monitored as described above for 28 days postchallenge. All studies were approved by the Institutional Animal Care and Use Committee of the Wadsworth Center and followed criteria established by the National Institutes of Health. Survival curves were analyzed with a Logrank test using GraphPad Prism (GraphPad Software, Inc.). A chi-squared test (Microsoft Office Excel) was used to compare morbidity data.
RESULTS
Crystal structure of the WNV MTase.
We recently showed that the WNV MTase carries both guanine N-7 and ribose 2′-O MTase activities (34). However, no N-7 MTase activity was detected for the DENV-2 MTase in a previous study (9). To exclude the possibility that the discrepancy results from structural differences between the two flavivirus MTases, we have crystallized the WNV NS5 MTase domain (amino acids [aa] 1 to 300). The crystal structure was determined to 2.8-Å resolution using the molecular replacement method (Fig. 1A and Table 1). The electron density maps were of good quality (Fig. 1B). The WNV NS5 MTase forms homodimers in the crystals. In the final model, each chain of the homodimer contains 262 residues (aa 6 to 267) and one SAH molecule. The SAH molecule could have originated from E. coli and copurified with the WNV MTase, since no SAH was added during any step of the protein purification or crystallization. Similar observations have been reported for many SAM-dependent MTases, including the DENV-2 MTase (9). In addition to the SAH, 256 water molecules were included in the final model. No density was observed for the N-terminal residues 1 to 5, the C-terminal residues 268 to 300, and the His tag (fused to the N terminus of the MTase), presumably due to disorder. Similar disorder has also been found in the crystal structure of the DENV-2 MTase, in which the C-terminal 29 residues were missing from the structure (9).
FIG. 1.
Crystal structure of the WNV MTase and comparison with the DENV-2 MTase. (A) Ribbon representation of the crystal structure of the WNV MTase. The MTase structure is colored as follows: N-terminal domain, red; MTase core, green; C-terminal domain, cyan. The bound SAH is shown in ball-and-stick representation with atom colors as follows: carbon, yellow; oxygen, red; nitrogen, blue; sulfur, green. (B) A representative omit (Fo-Fc) electron density map (magenta) showing the bound SAH and its interactions with the MTase residues. Hydrogen bonds are shown as orange dashed lines. (C) Superposition of the crystal structures of the DENV-2 (2) (pink) and WNV (cyan and red) MTases. Ribavirin (occupying the putative GTP cap-binding site for the 2′-O methylation) (see Fig. 8) and SAH are shown in ball-and-stick representation. The loops of the WNV structure that show significant differences relative to the DENV-2 structure are colored in red. (D and E) Solvent-accessible molecular GRASP (27) surface representation of the electrostatic potential of the WNV (D) and DENV-2 (E) MTases, showing the putative RNA substrate binding site. The surface is colored blue for positive (15 kT), red for negative (−15 kT) and white for neutral, where k is the Boltzmann constant and T is the temperature (27). In panels D and E, ribavirin and SAH are in stick representation with atom colors as follows: oxygen, red; nitrogen, blue; carbon, white; sulfur, green.
Structure comparison of the WNV and DENV-2 MTases.
The core of the WNV MTase domain displays a structural fold shared by nearly all SAM-dependent MTases (Fig. 1A). The WNV MTase contains 8 α-helices and 12 β-strands that are organized into three domains: the N-terminal domain, the core domain, and the C-terminal domain. The overall structure of the WNV MTase is similar to that of the DENV-2 MTase (9), with a root-mean-squared deviation (rmsd) of 0.62 Å (Fig. 1C). In addition, SAH, the by-product of the methyl transfer reaction, binds into similar pockets of the two enzymes, indicating that the two enzymes have similar SAM-binding sites (Fig. 1C to E).
Between the two structures, four regions, residues 36 to 51, 107 to 110, 173 to 177, and 245 to 252, showed the greatest structural differences, with rmsd values of 1.8 Å, 1.2 Å, 2.1 Å, and 1.9 Å, respectively (Fig. 1C). Three of the four structure differences are, at least in part, due to a one-residue insertion at each of positions 51, 173, and 251 for the WNV MTase, compared to the DENV-2 MTase (see sequence alignment in Fig. 2A). In contrast, all residues are identical at loop 107 to 110 in the two proteins. Loop 173 to 177 is located at the surface opposite to that of the SAM-binding site, while loop 245 to 252 is far from the SAM donor site and is structurally hindered from access to the SAM site by the helix α2 (Fig. 1C). Therefore, the structural differences in these two regions may not be of significance to the biological functions of the MTases.
In contrast to the loops formed by aa 173 to 177 and 245 to 252, residues 36 to 51 form the helix α3 and a short loop (Fig. 1C). These residues do not contact the SAH. However, they could participate in binding of the RNA substrate, since they are at the edge of the potential RNA binding pocket (see below). Loop 107 to 110 is lined at one side of the adenosine base of the bound SAH. As a consequence, the ND1 atom of His-110 of the WNV MTase forms a hydrogen bond with the ribose 2′-OH of the SAH molecule (Fig. 1B), whereas the His-110 in the DENV-2 MTase structure does not. Interestingly, although the SAH-contacting residues are essentially identical in the WNV and DENV-2 MTases, the SAH binds to the two enzymes in slightly different conformations. Indeed, with only a 0.2-Å difference at the Cα positions of the SAH molecules in the two structures,the rmsd for the adenosine bases in the two structures is about 0.83 Å. The adenosine base of the SAH molecule in the WNV MTase structure does not bind as deeply as does that in the DENV-2 structure. Nevertheless, structure differences at the SAH-binding site lead to different surface appearance for the two proteins. We noted that the WNV MTase has an enclosed SAM-binding site, whereas the SAM-binding site in the DENV-2 MTase is much more open (Fig. 1D and E). The structure differences at this region between the two MTases are, at least in part, due to the conformation differences of residues H110 and E149 that are lined at opposite sites along the bound SAH. Alternatively, conformational differences at loop 107 to 110 may contribute as well. The open and closed configurations in the two MTase structures could represent two distinct states for SAM binding, with the closed state to ensure a tight SAM binding, whereas the open state facilitates the release of SAH, the reaction by-product.
The DENV-2 MTase structure contains a novel GTP-binding site (occupied by ribavirin in Fig. 1C and E), which was suggested to be a cap-binding site (9). Surface comparison showed that the putative cap recognition site in WNV is much more open than that in DENV-2 (Fig. 1D and E). Despite the structural differences, a common feature of the two enzymes is a highly positively charged surface adjacent to the SAM and GTP binding sites but not in other regions (Fig. 1D and E). This positively changed region is likely the site for binding of the capped RNA substrates. Structure superposition of the VP39 2′-O and Ecm1 N-7 MTases in complex with their RNA substrates (10, 18) onto the WNV MTase indicated that the RNA substrates are lined along with the positively charged region (data not shown).
Sequential methylations at the N-7 and 2′-O positions of the RNA cap by the DENV-1 and YFV MTases.
To demonstrate that NS5 from other flaviviruses also possesses N-7 and 2′-O MTase activities, we prepared recombinant MTase domains of YFV and DENV-1 (Fig. 2A and 2B). For detection of N-7 methylation, we incubated capped RNA substrate, G*pppA-RNA (the asterisk indicates that the following phosphate is 32P labeled), representing the 5′-terminal 190 nucleotides of the WNV genome, with each of the YFV, DENV-1, and WNV MTases in the presence of SAM. The reaction products were digested by TAP to release m7G*p (product) and G*p (substrate). TLC analysis of the reactions showed that the WNV, DENV-1, and YFV MTases could convert G*pppA-RNA to m7G*pppA-RNA (Fig. 2C). For detection of 2′-O methylation, substrate m7G*pppA-RNA was methylated by the recombinant flavivirus MTases and cleaved by nuclease P1 to release m7G*pppAm (product) and m7G*pppA (substrate). The results showed that, similar to VP39 (a known guanine N-7-dependent 2′-O MTase as a positive control) (18), YFV, DENV-1, and WNV MTases could methylate m7G*pppA-RNA to m7G*pppAm-RNA (Fig. 2D).
Kinetic analyses were performed on substrate G*pppA-RNA to examine the order of cap methylations mediated by the DENV-1 (Fig. 2E, left panel) and YFV (Fig. 2E, right panel) MTases. For both enzymes, m7G*pppA was first detected at 1 min, reached a maximum at 5 to 15 min, and gradually declined at later time points. Concurrently, the double-methylated m7G*pppAm was first detected at 15 min and increased until 60 min. The kinetics of the methylation pattern derived from the DENV-1 and YFV MTases was similar to that derived from the WNV MTase (34). These results suggest that flavivirus NS5 functions similarly to methylate both N-7 and 2′-O positions of the cap structure, in the order of GpppA → m7GpppA → m7GpppAm.
Distinct conditions required for flavivirus N-7 and 2′-O MTase activities.
Using the WNV MTase as a model, we determined the optimal conditions for the N-7 and 2′-O cap methylations (Fig. 3). Both activities reached maximums when performed at 22°C, whereas the optimal pH and concentrations of MgCl2 and NaCl differed. The N-7 methylation required neutral pH 7.0 and 50 to 100 mM NaCl; MgCl2 inhibited the activity. In contrast, the 2′-O methylation required high pH 10 and 5 to 10 mM MgCl2; NaCl inhibited the activity. Similar results were obtained for the DENV-1 and YFV MTase (data not shown). These data demonstrate that optimal N-7 and 2′-O methylations of flavivirus cap require distinct biochemical conditions.
Differential roles of the K-D-K-E motif in N-7 and 2′-O MTase activities.
A K-D-K-E motif conserved among various 2′-O MTases was suggested to catalyze an SN2-reaction-mediated 2′-O methyl transfer (17, 18). Structural alignment of the WNV MTase with the VP39 tertiary complex (MTase, short RNA with cap, and SAH) (18) showed that the K-D-K-E tetrad is nearly superimposable between the two enzymes (Fig. 4A). We mutated the K61-D146-K182-E218 motif of the WNV MTase to determine the elements required for the N-7 and 2′-O methylations. Each amino acid was mutated to a residue having an electronic charge either similar to or different from that of the wild type. Analysis of the mutant MTases showed that all substitution within the K61-D146-K182-E218 motif abolished the 2′-O methylation (Fig. 4B). An increase in enzyme amount or incubation time did not improve the 2′-O activity (data not shown). In contrast, mutations of the tetrad residues exhibited different effects on N-7 methylation (Fig. 4C). In 5-min reactions, mutations of D146 showed the most dramatic effects on N-7 activity: all three mutants (D146A, D146N, and D146K) were inert in N-7 methylation (Fig. 4C). Mutations of K182 had less severe effects on N-7 methylation: K182E, K182N, and K182A showed 67%, 57%, and 42% of the wild-type activity level, respectively. For residue K61, no N-7 methylation was detected for mutant K61E, whereas the N-7 activities of mutants K61R and K61A were reduced to 87% and 70% of the wild-type, respectively. For amino acid E218, mutants E218K, E218D, and E218A contained 5%, 55%, and 76% of the wild-type N-7 activity, respectively. For mutants that were defective in N-7 methylation, a longer incubation time, up to 30 min, resulted in 4% and 22% of wild-type activity for K61E and D146A, respectively, but not for D146N and D146K (Fig. 4C, bottom panel). Overall, the results demonstrate that the exact K-D-K-E motif is required for the 2′-O methylation, but not for the N-7 MTase activity.
FIG. 4.
Methylation activities of the WNV K-D-K-E mutant MTases. (A) Superposition of the active site residues K-D-K-E of the VP39 and WNV MTases. SAH and K-D-K-E residues of the WNV MTase are in ball-and-stick representation with carbon colored yellow. The carbon atoms of Gppp-RNA and K-D-K-E residues from VP39 are colored in pink. Colors for other atoms are as follows: phosphate, cyan; sulfur, green; oxygen, red; nitrogen, blue. (B and C) Mutant MTases (1 μg) containing the indicated substitutions were assayed for 2′-O (B) and N-7 (C) methylation activities. The experimental details are described in Materials and Methods. 32P-labeled markers, m7G*pppA and G*pppA, are indicated on top (B). The relative conversions for 2′-O methylation (m7G*pppA to m7G*pppAm in panel B) and for N-7 methylation (G*pppA to m7G*pppA in panel C) were calculated by comparing the products generated from the mutant MTases with that produced from the wild-type protein (set at 100%). For N-7 methylation (C), the reaction mixtures were incubated for 5 min (top panel) or 30 min (TLC data not shown); the relative activities between the mutants and wild type for both incubation times are summarized (bottom panel). Average results from two to three experiments are shown.
RNA replication, protein synthesis, and virus production in cells transfected with genome-length RNAs with K-D-K-E mutations.
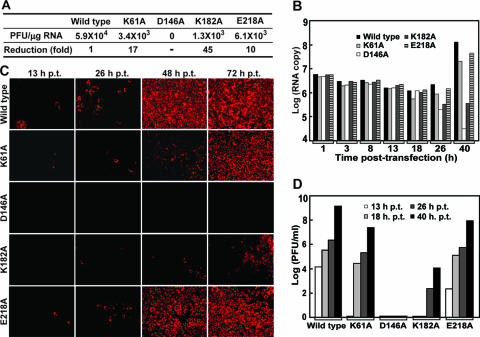
To examine the biological relevance of the above mutagenesis results, we performed Ala substitutions of the MTase K61-D146-K182-E218 tetrad in an infectious cDNA clone of WNV (38). A number of parameters were used to compare the replication efficiency between the wild-type and mutant RNAs. Initially, we measured the specific infectivity of the genome-length RNAs. After transfection into BHK cells (day 3 to 4 posttransfection [p.t.]), mutant K61A, K182A, E218A, and wild-type RNAs showed specific infectivity values of 3.4 × 103, 1.3 × 103, 6.1 × 103, and 5.9 × 104 PFU per microgram of transfected RNA, respectively (Fig. 5A). The specific infectivity values for mutant K61A, K182A, and E218A RNA were 17-, 45-, and 10-fold lower than that of the wild-type RNA, respectively. No PFU was detected in the specific infectivity assay for mutant D146A RNA.
FIG. 5.
Comparison of specific infectivity, RNA replication, protein synthesis, and virus production in cells transfected with genome-length RNA containing K-D-K-E mutations. Equal amounts of wild-type and mutant genome-length RNAs were transfected into BHK cells and assayed for specific infectivity (A). At indicated time points, viral RNA replication, protein expression, and virus production were monitored by real-time RT-PCR (B), IFA (C), and plaque assay (D), respectively. For specific infectivity, an average result of four independent experiments is shown (A). One representative result of two independent experiments is shown for real-time RT-PCR (B), IFA (C), and viral yield production (D). Viral RNA synthesis (B) is presented as RNA copy number per 100 ng of total cellular RNA, using full-length WNV RNA transcribed from an infectious cDNA clone (38) as a reference. Virus production (D) in the supernatants of transfected cells was quantified by plaque assays on Vero cells.
RNA and protein syntheses, as well as virion production, were compared for cells transfected with the wild-type and mutant RNAs. After transfection of BHK cells with equal amounts of RNA, similar levels of viral RNA were detected up to 18 h p.t. using real-time RT-PCR. From 26 to 40 h p.t., the amounts of wild-type and mutant K61A and E218A RNAs increased substantially and the level of mutant K182A RNA remained low, whereas the level of D146 RNA decreased dramatically (Fig. 5B). For viral protein synthesis, IFAs showed that IFA-positive cells transfected with the wild-type RNA appeared earlier than did those transfected with the mutant RNAs, with the following order of appearance: wild-type, E218A, K61A, and K182A (Fig. 5C). No IFA-positive cells were detected in cells transfected with the D146 RNA up to 72 h p.t., the latest time point tested (Fig. 5C). Concurrently, cells transfected with wild-type and mutant RNAs began to secrete viruses at different time points p.t.: 13 h for wild type and E218A (no virus was detected at 8 h p.t.), 18 h for K61A, and 26 h for K182A (Fig. 5D). No infectious virus was detected from the D146A RNA-transfected cells, up to 40 h p.t. (Fig. 5D). The results demonstrate that full-length RNAs containing the K61-D146-K182-E218 mutations are attenuated or defective in production of infectious viruses.
Characterization of K-D-K-E mutant WNV in cell culture.
Mutant viruses recovered from the transfected cells exhibited plaque sizes smaller than that of the wild-type WNV (Fig. 6A, upper panel). The relative plaque size among the wild-type and mutant viruses correlated well with the reduction in specific infectivity (compare Fig. 6A and 5A). Continuous culturing of the D146A RNA-transfected cells began to produce detectable virus on day 8 p.t.; the recovered viruses displayed a plaque size similar to that of the wild-type virus (Fig. 6A). Sequencing of the recovered viruses showed a reversion of the mutated D146A to the wild-type D residue, whereas mutant K61A, K182A, and E218A viruses retained the engineered changes (Fig. 6A, lower panels) and the small-plaque morphology, even after four passages of the viruses in Vero cells for over 12 days (data not shown). The results demonstrate that K61A, K182A, and E218A mutant viruses are stable, whereas the virus recovered from the D146A-transfected cells contained a reversion of the mutation to the wild-type sequence.
FIG. 6.
Plaque morphology and growth kinetics of the wild-type and K-D-K-E mutant viruses. (A) Plaque morphologies of wild-type and mutant viruses on Vero cells. A single- or double-nucleotide change was engineered into the genome-length RNA to introduce an Ala substitution within the K-D-K-E motif. Wild-type and K61A (AAA to GCA; mutated nucleotide underlined), K182A (AAG to GCG), and E218A (GAG to GCG) mutant viruses were harvested on day 5 p.t. “Mutant” D146A (GAC to GCC) virus was recovered starting on day 8 p.t. Plaques were developed at 96 h p.i. Sequences of the mutated regions, derived from the harvested viruses, are presented below the plaque assay plates. (B and C) Growth kinetics of the wild-type, K61A, K182A, and E218A viruses were compared in Vero cells (B) and C6/36 cells (C) by infecting cells with an MOI of 0.1, followed by quantification of viral titers using plaque assay on Vero cells.
Growth kinetics of the wild-type and mutant viruses were compared in mammalian Vero and mosquito C6/36 cells. After infection of Vero cells at an MOI of 0.1, mutant viruses generated lower viral titers than did the wild-type virus during the initial 36 h postinfection (p.i.) (Fig. 6B). At 48 h p.i. and later, titers of the mutant viruses were similar to or slightly higher than that of the wild-type virus. In C3/36 cells, mutant viruses produced lower viral titers than did the wild-type virus at all tested time points (Fig. 6C). Since the D146A RNA-derived virus had reverted to the wild type, the growth curve of the revertant virus has not been included in Fig. 6B and 6C. Overall, the results demonstrate that within the K-D-K-E motif, residues K61, K182, and E218 are important, but not essential, for the WNV reproduction, whereas residue D146 is essential for the viral life cycle in cell culture.
A D146E mutation of the K-D-K-E motif supports the WNV replication and the N-7 methylation.
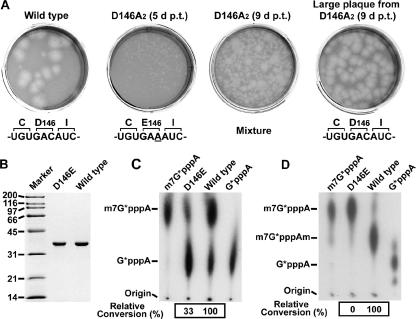
The essential role of D146 of the WNV MTase in the viral life cycle prompted us to ask whether viral isolates containing an amino acid other than D at position 146 of the MTase could be selected in cell culture. We reasoned that the quick reversion of the mutated D146A to its wild-type D146 described above was due to a single nucleotide change from GAC (Asp) to GCC (mutated nucleotide underlined; Ala). To minimize such reversion, we prepared another genome-length RNA, D146A2, which contained a two-nucleotide change from GAC (Asp) to GCA (Ala). Transfection of the two-nucleotide mutant D146A2 RNA into BHK cells yielded viruses. Viruses collected on day 5 p.t. showed homogenous small plaques (Fig. 7A, upper panel). On day 9 p.t., the viral population exhibited a mixed-plaque phenotype: large plaques, similar to the plaques of wild-type virus, emerged from the background of small plaques. Sequencing of the viruses showed that the engineered GCA (Ala) had been changed to GAA (Glu) in the small-plaque virus, and the wild-type GAC (Asp) was recovered in the large-plaque virus (Fig. 7A). No other mutations were found in the complete NS5 coding sequence (data not shown). These results showed a stepwise reversion of A→E→D at position 146 of the NS5 gene after the D146A2 RNA was transfected into cells.
FIG. 7.
A D146E mutation of the K-D-K-E motif could support the WNV replication and the N-7 MTase activity. (A) A genome-length RNA containing a double-nucleotide mutation D146A2 (GAC to GCA) was transfected into BHK cells. Viruses in culture fluids were harvested on day 5 and 9 p.t. and assayed for plaque morphologies on Vero cells. The large plaques derived from day 9 p.t. were amplified and are shown. For each virus, the sequences of the mutated NS5 regions were presented below the plaque morphology. (B) Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of the D146E mutant. (C and D) The D146E MTase was assayed for the N-7 (C) and 2′-O methylation activities (D) using substrate G*pppA-RNA and m7G*pppA-RNA, respectively. The relative activities between the wild-type (set as 100%) and mutant D146E are presented below the TLC images in panels C and D. The N-7 reactions in panel C were incubated in N-7 methylation buffer for 5 min. The positions of the origin and the G*pppA, m7G*pppA, and m7G*pppAm molecules are indicated on the left of the TLC plates.
The stepwise reversion of MTase A146→E146→D146 in virus indicated that amino acid E could functionally substitute for the essential D of the K-D-K-E motif. To test this hypothesis, we prepared recombinant D146E MTase (Fig. 7B) and assayed for the N-7 (Fig. 7C) and 2′-O methylation activities (Fig. 7D). Compared with the wild-type enzyme, the D146E mutant retained 33% of the N-7 activity (in a 5-min reaction) but completely lost the 2′-O methylation activity. The results suggest that amino acid E could replace D146 to maintain the N-7 MTase activity and, consequently, support virus replication.
The K-D-K-E mutant viruses were attenuated and can protect mice from later challenge with wild-type WNV.
We compared the virulence of the wild-type and MTase mutant WNV in an adult C3H mouse model, by inoculating 10, 103, or 105 PFU s.c. We chose K61A and K182A mutant viruses in the mouse study because K61A and E218A mutant viruses replicated to a similar level, whereas the replication level of K182A mutant virus was more attenuated in cell culture (Fig. 5). Table 2 shows the morbidity, mortality, average survival time, and infection rate. For the mutant viruses, no mortality was observed at any dose; morbidity was only observed for mice inoculated with mutant K61A WNV at 105 PFU (38%). In contrast, 88 to 100% morbidity and 50 to 100% mortality were observed for mice inoculated with the wild-type WNV. The differences in survival of mice inoculated with the mutant and wild-type viruses were statistically significant (survival analysis, P value < 0.05). All mice that were inoculated with wild-type or mutant viruses at 103 or 105 PFU were productively infected with WNV, as demonstrated by seroconversion. At the low dose of 10 PFU, the infection rate was similar for both wild-type and K182A mutant viruses (63 to 75%), but it was decreased for the K61A mutant (13%), suggesting that the K61A mutation is more detrimental to replication in vivo.
TABLE 2.
K-D-K-E mutant viruses are attenuated in mice compared to wild-type WNVa
| Inoculum | Dose (PFU) | Morbidity (no. sick/total) | Mortality (no. dead/ total) | AST (SD)b | Infection rate (no. infected/ total)c |
|---|---|---|---|---|---|
| Diluent | NA | 0/4 | 0/4 | NA | 0/4 |
| WNV NS5 K61A | 10 | 0/8 | 0/8 | NA | 1/8 |
| WNV NS5 K61A | 103 | 0/8 | 0/8 | NA | 8/8 |
| WNV NS5 K61A | 105 | 3/8 | 0/8 | NA | 8/8 |
| WNV NS5 K182A | 10 | 0/8 | 0/8 | NA | 6/8 |
| WNV NS5 K182A | 103 | 0/8 | 0/8 | NA | 8/8 |
| WNV NS5 K182A | 105 | 0/8 | 0/8 | NA | 8/8 |
| WNV wild type | 10 | 7/8 | 5/8 | 10.6 (1.9) | 5/8 |
| WNV wild type | 103 | 8/8 | 4/8 | 10.3 (1.3) | 8/8 |
| WNV wild type | 105 | 8/8 | 7/8 | 9.0 (1.2) | 8/8 |
Six-week old C3H female mice were inoculated s.c. in the left rear footpad and monitored for weight loss and clinical signs. NA, not applicable.
AST, average survival time (in days) calculated for mice that died only.
Surviving mice were bled on day 28 postinoculation and tested for WNV-specific antibody by enzyme-linked immunosorbent assay. Positive WNV infection was defined as either seropositivity or death consistent with WNV disease.
Surviving mice were challenged with 106 PFU of the wild-type WNV intraperitoneally. Remarkably, all mice that were seropositive after the initial inoculation exhibited no morbidity. In contrast, the control group (mice that were previously inoculated with diluent only) showed 100% morbidity and 50% mortality after challenge. Statistical analysis revealed that mice that were inoculated initially with 103 or 105 PFU of virus were significantly protected from the WNV-associated disease compared to the control group (chi-squared test, P value < 0.005). These results demonstrate that the K61A and K182A viruses are attenuated and that both mutant viruses protect mice from subsequent challenge with the wild-type WNV.
DISCUSSION
We previously found that the NS5 protein of the flavivirus WNV carries both guanine N-7 and ribose 2′-O MTase activities (34). To investigate whether the N-7 and 2′-O activities are unique to the WNV NS5, we extended our studies to additional representative flaviviruses. We have demonstrated that the MTases of other representative flaviviruses, DENV-1 and YFV, similar to that of WNV, sequentially catalyze the guanine N-7 and ribose 2′-O methylations involved in formation of the viral RNA cap (Fig. 2). Therefore, it is highly likely that flaviviruses encode the NS5 MTase as a general mechanism for dual methylations of the viral RNA cap.
The MTase domain from DENV-2 was previously reported to have only 2′-O, but not N-7, methylation activity (9). The discrepancy was due to the use of different RNA substrates in the two studies; a nonviral GpppA(C)5 substrate was used in the DENV-2 study. We recently found that distinct RNA elements are required for flavivirus RNA cap methylation events (7a); the cross activities of cap methylation between DENV-1 and YFV MTases and WNV RNA could result from the conserved 5′ terminal nucleotides and the RNA structure shared by the flaviviruses (4). The physical linkage of the MTase to the RdRp domain in a single NS5 protein, together with the selective recognition between the MTase domain and the 5′ terminus of viral RNA, could confer specificity for viral cap methylation during flavivirus RNA synthesis. Similarly, the L protein from viruses in the order Mononegavirales also contains functionally discrete cap-MTase and RdRp domains (8, 14, 29); capping of cellular mRNAs is achieved through direct binding of the cellular capping apparatus to the RNA polymerase II elongation complex (6, 25, 43).
Our biochemical analysis suggests that flavivirus MTase methylates the RNA cap at both the N-7 and 2′-O positions, in the order GpppA → m7GpppA → m7GpppAm (Fig. 2E). The chemistry of the ribose 2′-O methylation is distinct from that of the guanine N-7 methylation (10, 18). For N-7 methylation, the structure of Ecm1 N-7 MTase suggests an in-line mechanism: no direct contact is observed between the enzyme and either the attacking nucleophile N-7 atom of guanine, the methyl carbon of SAM, or the leaving group sulfur of SAH. Instead, the catalysis is achieved through the close proximity and geometry of the two substrates (10). In contrast, for 2′-O methylation, structural and mutagenesis studies of VP39 and RrmJ 2′-O MTase suggest an SN2 reaction of methyl transfer: the conserved K-D-K-E tetrad mediates deprotonation of the target 2′-OH, which nucleophilically attacks the methyl moiety of SAM to accomplish the methyl transfer (17, 18). In this study, we show that flavivirus WNV and DENV-2 MTases exhibit a similar structure, with a core fold shared by many SAM-dependent MTases (11). Although flavivirus MTase catalyzes two distinct methylation reactions, only a single SAH-binding pocket was found in the MTase structure (Fig. 1). In addition, one clustered and positively charged surface, which presumably accounts for the binding of the negatively charged phosphate backbone of the RNA substrates, was found to be adjacent to the single SAM-binding site (Fig. 1). These results suggest that SAM, located in the same binding pocket for SAH, donates methyl groups to both the N-7 and 2′-O positions during flavivirus RNA cap methylations. However, since the two reactions have different methyl acceptors that are located at different positions, the 5′ terminus of RNA must be repositioned during the two methylations. In line with the flavivirus MTases, the N-7 and 2′-O MTases of vesicular stomatitis virus were recently suggested to share a single SAM-binding site (22).
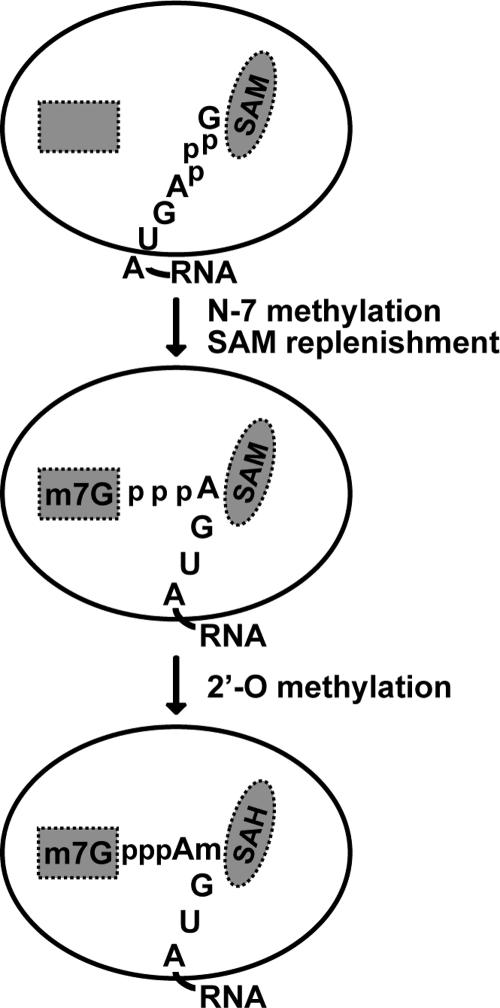
We hypothesize that the flavivirus MTases bind the RNA substrates in different positions to carry out two distinct methyl transfer reactions. During flavivirus cap methylation, the guanine N-7 would first be positioned in proximity to the methyl group of SAM, to generate m7GpppA-RNA (Fig. 8). Structure superposition of the WNV and DENV-2 MTases onto the Ecm1 N-7 MTase-substrate complex (10) did not show any steric hindrance between the guanosine cap analogue and the WNV and DENV-2 MTases (data not shown), providing structural feasibility. Our mutagenesis results indicate that K61, D146, and E218, but not K182, within the conserved K-D-K-E tetrad likely contribute to the binding of the cap structure, to modulate the N-7 methylation (Fig. 4C).
FIG. 8.
Model for the sequential guanine N-7 and ribose 2′-OH methylations of the RNA cap by flavivirus MTase. The SAM- and m7G-binding sites are shaded and depicted by an oval and a rectangle, respectively. The 5′-terminal nucleotides of the WNV genome, GpppAGUA-RNA, are shown.
Once the guanine N-7 has been methylated, the 5′ terminus of m7GpppA-RNA is repositioned. The m7Gppp moiety moves into a GTP-binding pocket (Fig. 8), as previously described for the DENV-2 MTase (9). Residues involved in binding of the m7Gppp moiety are superimposable between the DENV-2 and WNV MTase structures: the guanine base stacks against the aromatic ring of F24, the ribose 2′-OH forms hydrogen bonds with the side chains of K13 and N17, the ribose 3′-OH interacts with K13, and the α-phosphate forms hydrogen bonds with R28 and S150. The binding of the m7Gppp moiety precisely repositions the ribose 2′-OH of the first transcribed adenosine in close proximity to SAM. Our mutagenesis experiments showed that any alteration to the K61-D146-K182-E218 tetrad completely abolishes 2′-O methylation (Fig. 4B), suggesting that the tetrad forms the active site of 2′-O MTase through a SN2 methyl transfer mechanism. The latter conclusion is further supported by the observation that the optimal pH for 2′-O methylation is at pH 10 (Fig. 3A). Structural alignment with the VP39 2′-O MTase suggests that K182 within the WNV tetrad directly participates in the deprotonation of ribose 2′-OH (Fig. 4A). Since the pKa of Lys is at pH 10, a high pH would facilitate the deprotonation of K182, which would in turn favor the deprotonation of the 2′-OH, leading to efficient formation of the SN2-like transition state so as to accomplish the methyl transfer (17-19). The requirement of high pH for methylation has also been reported for DIM-5 (a histone H3 lysine 9 MTase) (44).
What is the biological relevance of the optimal pH of 10 for 2′-O methylation? It is not unreasonable to speculate that, during flavivirus infection, the replication complexes associated with the endoplasmic reticulum could lower the local pKa of the Lys residues within the K-D-K-E motif, allowing 2′-O methylation to occur at a physiological pH. The difference in optimal pH and concentrations of NaCl and MgCl2 for N-7 and 2-O methylation (Fig. 3) also supports the existence of distinct mechanisms for the two MTase activities. Finally, the 5′ cap translocation model (Fig. 8) is supported by our recent footprinting results: the 5′ termini of RNA substrates interact with NS5 and may undergo conformational change during the sequential methylation reactions (7a).
The processivity of the two methylation events is confronted with a single SAM-binding site on the flavivirus MTase. Once the guanine N-7 is methylated, the SAH needs to be replaced with a fresh SAM before the ribose 2′-O methylation can occur. Alternatively, the m7GpppA-RNA needs to disassociate from the SAH-bound MTase and to reassociate with an SAM-bound enzyme for the 2′-O methylation. The latter scenario is reminiscent of the reovirus λ2 protein-mediated RNA capping, in which the N-7 and 2′-O methylations are sequentially executed by two separate MTase domains (35). In support of (but not distinguishing between) the above two possibilities, we found that an RNA substrate with preexisting N-7 methylation, m7GpppA-RNA, could readily be methylated to m7GpppAm-RNA (Fig. 2D). The results demonstrate that flavivirus 2′-O methylation can be uncoupled from the N-7 methylation in vitro. However, our current results do not prove that the 2′-O methylation is dependent on the prior N-7 methylation.
A panel of mutant WNV was prepared for examination of the requirement of MTase activities for flavivirus life cycle. Upon transfection into susceptible cells, genome-length RNAs containing Ala substitutions within the K-D-K-E motif showed decreases in levels of specific infectivity, viral protein synthesis, RNA replication, and virion production compared to the wild-type RNA (Fig. 5). In addition, the mutant viruses displayed smaller plaque morphology and slower growth kinetics in mammalian Vero and mosquito C6/36 cells (Fig. 6). Only mutant viruses containing the K61A, K182A, and E218A substitutions, which abolished the 2′-O but not the N-7 MTase activity, could be recovered from the RNA-transfected cells (Fig. 6). These results demonstrate that 2′-O MTase activity is important, but not essential, for the WNV reproduction. Consistent with the cell culture results, we showed that the K61A and K182A viruses were attenuated in vivo and can be used to protect mice from later challenge with wild-type WNV (Table 2). These findings underscore the feasibility of using MTase as a target for flavivirus vaccine development.
In contrast to mutations that affected 2′-O MTase, cells transfected with an RNA containing the D146A substitution, which abolished both the N-7 and 2′-O MTase activities (Fig. 4), did not yield any virus in our specific infectivity assay (day 3 to 4 p.t.) (Fig. 5). However, continuous culturing of the transfected cells produced viruses with a wild-type phenotype; sequencing of the recovered virus showed a reversion of the engineered mutation to the wild-type D146. The results suggest that the N-7 MTase activity is essential for the WNV reproduction. The conclusion was further supported by the stepwise reversion of A→E→D at position 146 of the MTase after a double-nucleotide mutant D146A2 RNA was transfected into cells (Fig. 7A). Mutant D146E virus was viable but exhibited small plaques. Remarkably, when the D146E mutation was engineered into recombinant protein, the D146E MTase was active in the N-7 methylation, but not the 2′-O methylation (Fig. 7C and D). Why is D146 more critical than other tetrad residues? It could be, at least in part, explained by the crystal structure of the MTase, in which D146 is the closest to SAM among the four K-D-K-E motif residues (Fig. 4A). D146 not only provides stabilization within the motif but also forms a hydrogen bond with the amine nitrogen N-2 of the bound SAH (Fig. 1B). Modeling suggests that an E146, but not other tested mutants, can also form hydrogen bond with the bound SAH (data not shown). Therefore, D146 may participate in binding of both SAM and RNA substrates in addition to its role in catalysis. Nevertheless, the essential role of the N-7 MTase in the viral life cycle, together with its specific methylation activity on viral RNA cap (7a), suggests that the flavivirus MTase is a novel target for antiviral therapy.
Why is the N-7 MTase activity essential in the flavivirus life cycle? We previously showed the critical role of N-7 MTase may function at the level of viral translation (34). Alternatively, flavivirus RNA cap formation could be coupled to viral RNA replication or other steps in the viral life cycle. In support of this possibility, RNA templates containing a 5′ cap were reported to enhance de novo RNA synthesis catalyzed by recombinant RdRp of WNV (1). The RdRp domain of DENV-2 was shown to recognize 5′ RNA elements so as to promote RNA synthesis (12). In vesicular stomatitis virus, cap formation was previously found to be required for nonabortive viral mRNA transcription (40); amino acid substitutions to the conserved K-D-K-E tetrad of the MTase were also shown to attenuate viral replication in cell culture (21). Further studies are required to define the intricate modulations between flavivirus RNA capping and RNA synthesis.
Acknowledgments
We are grateful to Kiong Ho for providing recombinant VP39 protein and for helpful discussions and to Corey Bennett and Aaloki Shah for technical assistance. We thank the Molecular Genetics Core, the Cell Culture Core, Mass Spectrometry and Proteomics Core, and the Macromolecular Crystallography Facility at the Wadsworth Center for DNA sequencing for maintenance of BHK and Vero cells, for verification of mutant MTases, and for crystal screening, respectively.
The work was partially supported by contract AI25490 and grants AI061193 and AI065562 from NIH. The BSL-3 animal facility at the Wadsworth Center was used, which is funded in part by the Northeast Biodefense Center's animal core (NIH/NIAID U54 AI05 7158). D.R. is supported by a postdoctoral fellowship from the National Sciences and Engineering Research Council of Canada. X-ray diffraction data for this study were measured at beamline X4A of the national synchrotron light source, which is supported by the Department of Energy, by grants from the NIH, and by the New York Structural Biology Center.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Ackermann, M., and R. Padmanabhan. 2001. De novo synthesis of RNA by the dengue virus RNA-dependent RNA polymerase exhibits temperature dependence at the initiation but not elongation phase. J. Biol. Chem. 276:39926-39937. [DOI] [PubMed] [Google Scholar]
- 2.Benarroch, D., M. P. Egloff, L. Mulard, C. Guerreiro, J. L. Romette, and B. Canard. 2004. A structural basis for the inhibition of the NS5 dengue virus mRNA 2′-O-methyltransferase domain by ribavirin 5′-triphosphate. J. Biol. Chem. 279:35638-35643. [DOI] [PubMed] [Google Scholar]
- 3.Bredenbeek, P. J., E. A. Kooi, B. Lindenbach, N. Huijkman, C. M. Rice, and W. J. Spaan. 2003. A stable full-length yellow fever virus cDNA clone and the role of conserved RNA elements in flavivirus replication. J. Gen. Virol. 84:1261-1268. [DOI] [PubMed] [Google Scholar]
- 4.Brinton, M. A., and J. H. Dispoto. 1988. Sequence and secondary structure analysis of the 5′-terminal region of flavivirus genome RNA. Virology 162:290-299. [DOI] [PubMed] [Google Scholar]
- 5.Brunger, A., P. Adams, G. Clore, W. DeLano, P. Gros, R. Grosse-Kunstleve, J. Jiang, J. Kuszewski, M. Nilges, N. Pannu, R. Read, L. Rice, T. Simonson, and G. Warren. 1998. Crystallography & NMR system: a new software suite for macromolecular structure determination. Acta. Crystallogr. D 54:905-921. [DOI] [PubMed] [Google Scholar]
- 6.Choi, K. H., J. M. Groarke, D. C. Young, R. J. Kuhn, J. L. Smith, D. C. Pevear, and M. G. Rossmann. 2004. The structure of the RNA-dependent RNA polymerase from bovine viral diarrhea virus establishes the role of GTP in de novo initiation. Proc. Natl. Acad. Sci. USA 101:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleaves, G. R., and D. T. Dubin. 1979. Methylation status of intracellular Dengue type 2 40S RNA. Virology 96:159-165. [DOI] [PubMed] [Google Scholar]
- 7a.Dong, H., D. Ray, S. Ren, B. Zhang, F. Puig-Basagoiti, Y. Takagi, C. K. Ho, H. Li, and P.-Y. Shi. Distinct RNA elements confer specificity to flavivirus RNA cap methylation events. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 8.Duprex, W. P., F. M. Collins, and B. K. Rima. 2002. Modulating the function of the measles virus RNA-dependent RNA polymerase by insertion of green fluorescent protein into the open reading frame. J. Virol. 76:7322-7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egloff, M. P., D. Benarroch, B. Selisko, J. L. Romette, and B. Canard. 2002. An RNA cap (nucleoside-2′-O-)-methyltransferase in the flavivirus RNA polymerase NS5: crystal structure and functional characterization. EMBO J. 21:2757-2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fabrega, C., S. Hausmann, V. Shen, S. Shuman, and C. D. Lima. 2004. Structure and mechanism of mRNA cap (guanine-N7) methyltransferase. Mol. Cell 13:77-89. [DOI] [PubMed] [Google Scholar]
- 11.Fauman, E., R. Blumenthal, and X. Cheng. 1999. Structure and evolution of AdoMet-dependent methyltransferase, p. 1-38. In X. Cheng and R. M. Blumenthal (ed.), S-Adenosylmethionine-dependent methyltransferase: structure and function. World Scientific Publishing, River Edge, Singapore.
- 12.Filomatori, C., M. Lodeiro, D. Alvarez, M. Samsa, L. Pietrasanta, and A. Gamarnik. 2006. A 5′ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes Dev. 20:2238-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furuichi, Y., and A. J. Shatkin. 2000. Viral and cellular mRNA capping: past and prospects. Adv. Virus Res. 55:135-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grdzelishvili, V. Z., S. Smallwood, D. Tower, R. L. Hall, D. M. Hunt, and S. A. Moyer. 2005. A single amino acid change in the l-polymerase protein of vesicular stomatitis virus completely abolishes viral mRNA cap methylation. J. Virol. 79:7327-7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu, M., and C. D. Lima. 2005. Processing the message: structural insights into capping and decapping mRNA. Curr. Opin. Struct. Biol. 15:99-106. [DOI] [PubMed] [Google Scholar]
- 16.Guyatt, K. J., E. G. Westaway, and A. A. Khromykh. 2001. Expression and purification of enzymatically active recombinant RNA-dependent RNA polymerase (NS5) of the flavivirus Kunjin. J. Virol. Methods 92:37-44. [DOI] [PubMed] [Google Scholar]
- 17.Hager, J., B. L. Staker, H. Bugl, and U. Jakob. 2002. Active site in RrmJ, a heat shock-induced methyltransferase. J. Biol. Chem. 277:41978-41986. [DOI] [PubMed] [Google Scholar]
- 18.Hodel, A. E., P. D. Gershon, and F. A. Quiocho. 1998. Structural basis for sequence-nonspecific recognition of 5′-capped mRNA by a cap-modifying enzyme. Mol. Cell 1:443-447. [DOI] [PubMed] [Google Scholar]
- 19.Hodel, A. E., P. D. Gershon, X. Shi, and F. A. Quiocho. 1996. The 1.85 A structure of vaccinia protein VP39: a bifunctional enzyme that participates in the modification of both mRNA ends. Cell 85:247-256. [DOI] [PubMed] [Google Scholar]
- 20.Li, H., S. Clum, S. You, K. E. Ebner, and R. Padmanabhan. 1999. The serine protease and RNA-stimulated nucleoside triphosphatase and RNA helicase functional domains of dengue virus type 2 NS3 converge within a region of 20 amino acids. J. Virol. 73:3108-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, J., E. C. Fontaine-Rodriguez, and S. P. Whelan. 2005. Amino acid residues within conserved domain VI of the vesicular stomatitis virus large polymerase protein essential for mRNA cap methyltransferase activity. J. Virol. 79:13373-13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li, J., J. Wang, and S. Whelan. 2006. A unique strategy for mRNA cap methylation used by vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 103:8493-8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindenbach, B. D., and C. M. Rice. 2001. Flaviviridae: the virus and their replication, 4th ed. Lippincott William & Wilkins, Philadelphia, PA.
- 24.Martin, J., and F. McMillan. 2002. SAM (dependent) I AM: the S-adenosylmethionine-dependent methyltransferase fold. Curr. Opin. Struct. Biol. 12:783-793. [DOI] [PubMed] [Google Scholar]
- 25.McCracken, S., N. Fong, E. Rosonina, K. Yankulov, G. Brothers, D. Siderovski, A. Hessel, S. Foster, S. Shuman, and D. L. Bentley. 1997. 5′-Capping enzymes are targeted to pre-mRNA by binding to the phosphorylated carboxy-terminal domain of RNA polymerase II. Genes Dev. 11:3306-3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Navaza, J. 1994. AmoRe: an automated package for molecular replacement. Acta Crystallogr. A 50:157-163. [Google Scholar]
- 27.Nicholls, A., K. Sharp, and B. Honig. 1991. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins 11:281-296. [DOI] [PubMed] [Google Scholar]
- 28.Nomaguchi, M., T. Teramoto, L. Yu, L. Markoff, and R. Padmanabhan. 2004. Requirements for West Nile virus (-)- and (+)-strand subgenomic RNA synthesis in vitro by the viral RNA-dependent RNA polymerase expressed in Escherichia coli. J. Biol. Chem. 279:12141-12151. [DOI] [PubMed] [Google Scholar]
- 29.Ogino, T., M. Kobayashi, M. Iwama, and K. Mizumoto. 2005. Sendai virus RNA-dependent RNA polymerase L protein catalyzes cap methylation of virus-specific mRNA. J. Biol. Chem. 280:4429-4435. [DOI] [PubMed] [Google Scholar]
- 30.Otwinowski, Z., and W. Minor. 1997. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276:307-325. [DOI] [PubMed] [Google Scholar]
- 31.Puig-Basagoiti, F., T. S. Deas, P. Ren, M. Tilgner, D. M. Ferguson, and P.-Y. Shi. 2005. High-throughput assays using luciferase-expressing replicon, virus-like particle, and full-length virus for West Nile virus drug discovery. Antimicrob. Agent. Chemother. 49:4980-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Puig-Basagoiti, F., M. Tilgner, C. Bennett, Y. Zhou, J. Munoz-Jordan, A. Garcia-Sastre, K. Bernard, and P. Shi. 2006. A mouse cell-adapted NS4B mutation attenuates West Nile virus RNA synthesis. Virology doi: 10.1016/j.virol.2006.11.012. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 33.Puig-Basagoiti, F., M. Tilgner, B. Forshey, S. Philpott, N. Espina, Wentworth, S. Goebel, P. S. Masters, B. Falgout, P. Ren, Ferguson, and P. Y. Shi. 2006. Triaryl pyrazoline compound inhibits flavivirus RNA replication. Antimicrob. Agents Chemother. 50:1320-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ray, D., A. Shah, M. Tilgner, Y. Guo, Y. Zhao, H. Dong, T. Deas, Y. Zhou, H. Li, and P. Shi. 2006. West Nile virus 5′-cap structure is formed by sequential guanine N-7 and ribose 2′-O methylations by nonstructural protein 5. J. Virol. 80:8362-8370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reinisch, K. M., M. L. Nibert, and S. C. Harrison. 2000. Structure of the reovirus core at 3.6 A resolution. Nature 404:960-967. [DOI] [PubMed] [Google Scholar]
- 36.Shi, P. Y., E. B. Kauffman, P. Ren, A. Felton, J. H. Tai, A. P. Dupuis II, S. A. Jones, K. A. Ngo, D. C. Nicholas, J. Maffei, G. D. Ebel, K. A. Bernard, and L. D. Kramer. 2001. High-throughput detection of West Nile virus RNA. J. Clin. Microbiol. 39:1264-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi, P. Y., M. Tilgner, and M. K. Lo. 2002. Construction and characterization of subgenomic replicons of New York strain of West Nile virus. Virology 296:219-233. [DOI] [PubMed] [Google Scholar]
- 38.Shi, P. Y., M. Tilgner, M. K. Lo, K. A. Kent, and K. A. Bernard. 2002. Infectious cDNA clone of the epidemic West Nile virus from New York City. J. Virol. 76:5847-5856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shuman, S. 2001. Structure, mechanism, and evolution of the mRNA capping apparatus. Prog. Nucleic Acid Res. Mol. Biol. 66:1-40. [DOI] [PubMed] [Google Scholar]
- 40.Stillman, E. A., and M. A. Whitt. 1999. Transcript initiation and 5′-end modifications are separable events during vesicular stomatitis virus transcription. J. Virol. 73:7199-7209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wengler, G. 1981. Terminal sequences of the genome and replicative-form RNA of the flavivirus West Nile virus: absence of poly(A) and possible role in RNA replication. Virology 113:544-555. [DOI] [PubMed] [Google Scholar]
- 42.Wengler, G., and G. Wengler. 1991. The carboxy-terminal part of the NS 3 protein of the West Nile flavivirus can be isolated as a soluble protein after proteolytic cleavage and represents an RNA-stimulated NTPase. Virology 184:707-715. [DOI] [PubMed] [Google Scholar]
- 43.Yue, Z., E. Maldonado, R. Pillutla, H. Cho, D. Reinberg, and A. J. Shatkin. 1997. Mammalian capping enzyme complements mutant Saccharomyces cerevisiae lacking mRNA guanylyltransferase and selectively binds the elongating form of RNA polymerase II. Proc. Natl. Acad. Sci. USA 94:12898-12903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang, X., H. Tamaru, S. I. Khan, J. R. Horton, L. J. Keefe, E. U. Selker, and X. Cheng. 2002. Structure of the Neurospora SET domain protein DIM-5, a histone H3 lysine methyltransferase. Cell 111:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]