Abstract
The beetle Hemisphaerota cyanea (Chrysomelidae; Cassidinae) responds to disturbance by activating a tarsal adhesion mechanism by which it secures a hold on the substrate. Its tarsi are oversized and collectively bear some 60,000 adhesive bristles, each with two terminal pads. While walking, the beetle commits but a small fraction of the bristles to contact with the substrate. But when assaulted, it presses its tarsi flatly down, thereby touching ground with all or nearly all of the bristles. Once so adhered, it can withstand pulling forces of up to 0.8 g (≈60 times its body mass) for 2 min, and of higher magnitudes, up to >3 g, for shorter periods. Adhesion is secured by a liquid, most probably an oil. By adhering, the beetle is able to thwart attacking ants, given that it is able to cling more persistently than the ant persists in its assault. One predator, the reduviid Arilus cristatus, is able to feed on the beetle, possibly because by injecting venom it prevents the beetle from maintaining its tarsal hold.
Keywords: Chrysomelidae: Cassidinae, tarsal bristle, predation, Formicidae, Reduviidae
Hemisphaerota cyanea is a small blue beetle (Chrysomelidae; Cassidinae) found on palmetto plants in the southeastern United States. Anyone who has attempted to collect this insect knows that it is able to cling tenaciously to the frond when one attempts to pick it off. Ordinarily the beetle walks or rests with a loose hold, but if it is disturbed, it clamps down with such vigor that considerable force is required to pry it loose. Years ago we reported briefly on this behavior and on its possible defensive role (1). Here we provide data on the magnitude of force that the beetle is able to withstand, and on the mechanism by which it effects its defensive “anchorage.”
Materials and Methods
The Beetle.
We obtained the beetles mostly at the Archbold Biological Station (Lake Placid, Highlands County, FL), where they occur primarily on two palmetto plants, Sabal etonia and Serenoa repens. The beetle survives well in captivity if given fresh pieces of palmetto frond, on which it feeds by carving out long narrow trenches with the mouthparts.
Tests with Ants.
These were done with Camponotus floridanus, an ant native to Florida, and with Formica exsectoides, from Ithaca, NY. With the former, the tests (n = 10) were done in Petri dishes and involved presenting one beetle to one ant. With the latter, the tests (n = 10) were done in a closed foraging arena (10 × 10 cm; containing 6–10 ants) attached to a laboratory colony of the ant. With both ants, tests were of 10-min duration.
Microscopy.
Examination of living tarsi, in contact with a glass surface, was done with epi-illumination lenses, in polarized and nonpolarized light. Epi-illumination, in combination with Nomarski interference contrast microscopy, was used for observation of the oily footprints. For scanning electron microscopy, specimens were gold-coated, after (in some cases) cleaning by sonic vibration in methanol/chloroform.
Tarsal Observation.
Individual beetles were placed on a glass slide, which was inverted and set down as a cover over a small chamber. The chamber was positioned on the stage of a compound microscope for examination by epi-illumination. The chamber was of such dimensions that the beetle could not right itself in it and could only walk in a tight circle while on the underside of the slide.
To subject such confined beetles to simulated attack, an electromagnet was placed directly beneath their chamber, and they themselves were outfitted with a small piece of ferromagnetic iron attached with wax to their elytra. The effect that application of an electromagnetic pull had on the tarsal “grip” was recorded photographically.
Adhesive Strength and Adhesive Endurance.
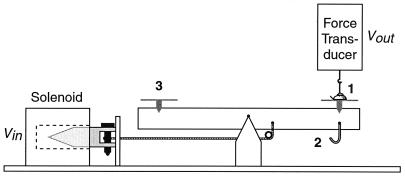
The apparatus used for measuring these parameters (Fig. 1) consisted of a platform on which the beetle was positioned, and which could be subjected to a downward force, either electronically with a solenoid, or by hanging weights beneath it. The beetle was connected, by way of a hook attached to its elytra, to a force transducer positioned directly above it. The arrangement was such that when the downward pull was applied to the platform, the force was sensed by the transducer and relayed electronically for visual display on an oscilloscope (Fig. 2 H and I).
Figure 1.
Apparatus for application of pulling forces to beetle. 1, beetle; 2, hook for suspension of weights; 3, pan for placement of balancing weights.
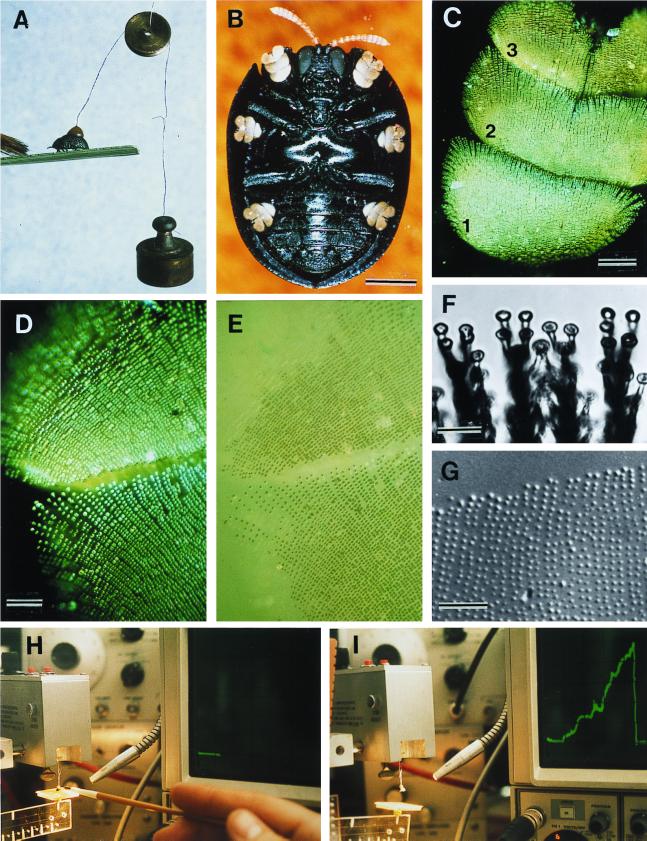
Figure 2.
(A) Beetle withstanding a 2-g pull; brush strokes are causing the beetle to adhere with its tarsi. (B) Ventral view of beetle, showing yellow tarsi. (C) Tarsus (numbers refer to tarsomeres). (D) Tarsus in contact with glass (polarized epi-illumination). (E) Same as preceding, in nonpolarized light; contact points of the bristles are seen to be wet. (F) Bristle pads, in contact with glass. (G) Droplets left on glass as part of a tarsal “footprint.” (H and I) Apparatus diagrammed in Fig. 1. In H, beetle is on platform, before lift is applied (horizontal trace on oscilloscope); in I, the lift has been applied (ascending green trace) to point where beetle has detached (return of trace to baseline). [Bars = 1 mm (B), 100 μm (C), 50 μm (D), 10 μm (F), and 50 μm (G).]
The solenoid permitted application of a linearly increasing force (100 mg/sec) to the platform; electronic feedback from the force transducer insured that the output of the solenoid remained linear. Such force application was used for measurement of the beetle's adhesive strength, defined as the force in g at which the beetle became detached from the platform. Fixed forces in the form of weights hung beneath the platform were used to determine the beetle's adhesive endurance, that is, the length of time that it withstood pulls of different magnitude without detaching.
A standard procedure was adopted for testing. A given substrate (Serenoa frond, glass, Parafilm, aluminum foil) was first fastened to the platform, and the platform was then brought into horizontal equilibrium by addition of counterweights to the opposite side of the balance beam. The beetle was then hung by its hook from the sensing element of the force transducer and lowered until its feet contacted the substrate. To ensure that it clamped down, light stroking motions were administered to its front end with a fine brush. This stimulus was discontinued when the pull was initiated.
To determine adhesive strength, 10 beetles were tested per each of the four substrates used. To determine adhesive endurance, 10 beetles (on glass) each were subjected to 14 loads, ranging from 0.0 to 3.4 g, tested in ascending sequence (at least 2 min intervened between consecutive tests with the same beetle).
Statistics.
Except where otherwise indicated, values are given as mean ± SE.
Results and Conclusions
Observations in the field indicated that the beetle ordinarily is not strongly fastened to the substrate. If it was abruptly stroked with a brush it was usually swept from its frond. It clamped down only if first stimulated by gentle strokings with the brush. The beetle was evidently able to secure its hold on demand, in response to disturbance. A simple technique, by which weights were directly attached to the beetle, showed the animal to be able to withstand a pull upward of 2 g (Fig. 2A). The body mass of the beetle was found to be 13.5 ± 0.4 mg (range 8.6–19.5 mg; n = 78).
Tests with Ants.
Six of the 10 C. floridanus attacked the beetles, which were offered to them on pieces of palmetto frond. They palpated the beetles with antennae and mouthparts and attempted to seize them with the mandibles, but without success. The beetles, which clamped down when assaulted, survived uninjured.
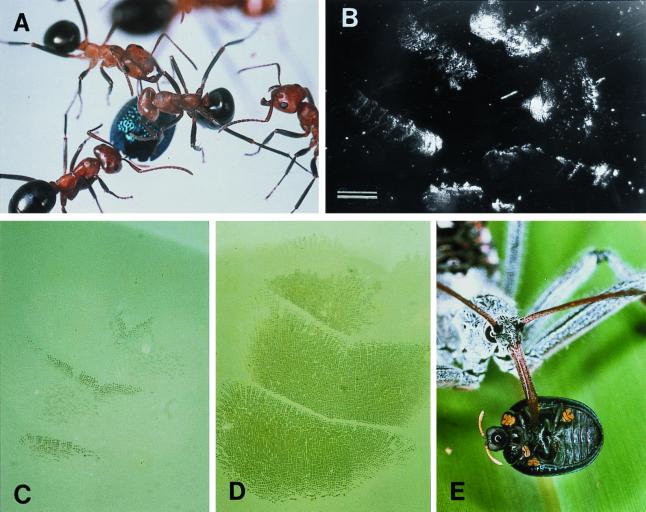
Fourteen individual ant attacks (Fig. 3A) occurred in the 10 tests with F. exsectoides. The substrate in these tests was glass. In two of the attacks, the beetle was pried loose (in each case after 11 sec of assault) and carried along for a time in the ant's mandibles, but in both cases the beetles eventually were released by the ants. In the remaining 12 attacks, the ants were unsuccessful. They tried for a protracted period [22.8 ± 6.4 sec (SD); n = 12] to pry the beetle loose, but the beetle, motionless, and with its appendages retracted, resisted the efforts. None of the beetles, including the two initially seized, suffered injury.
Figure 3.
(A) F. exsectoides attacking beetle, on glass surface. (B) Footprints left by a beetle attacked as in A (dark-field illumination). (C) Tarsal contact during ordinary walking: only a few bristles touch the glass. (D) Tarsus pressed down flatly, in defensive response (beetle subjected to electromagnetic pull). (E) A. cristatus feeding on beetle. [Bar = 1 mm (B).]
The Tarsi.
Examination of the beetle in ventral view (Fig. 2B) showed the animal to have unusually large tarsi. The “sole” of the beetle's foot (that is, the part of the tarsus that is brought into contact with the substrate) is made up of tarsomeres (tarsal segments) 1, 2, and 3 (Fig. 2C). These three tarsomeres are densely beset with bristles, which expand terminally into contact pads (Fig. 2F). As is apparent by examination of a tarsus that is in contact with a glass substrate, the pads are wetted by what appears to be an oily liquid (Fig. 2 D and E). Examination of the portion of a glass surface on which a tarsus had previously tread reveals a footprint of tiny droplets (Fig. 2G). These do not go into solution when the surface is flooded with water.
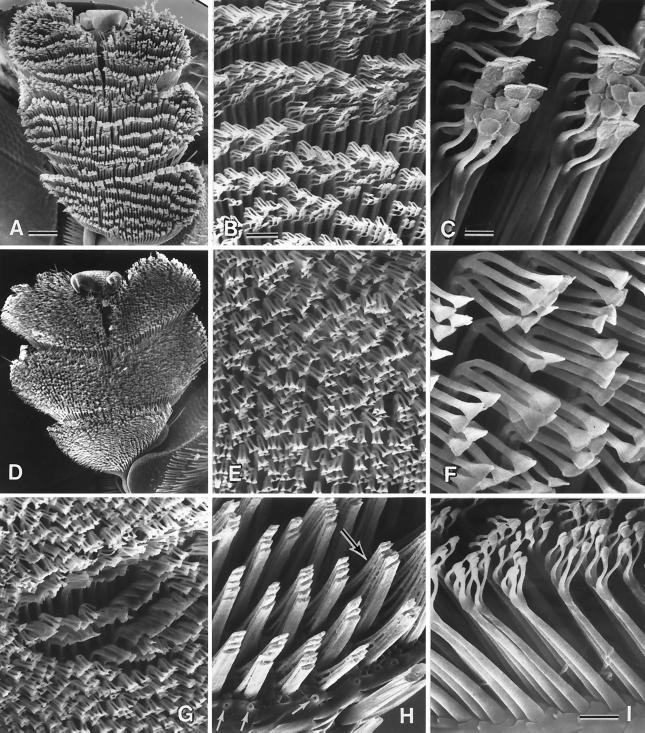
Scanning electron microscopy revealed each bristle to be forked near the tip, so that each has, in fact, two contact pads (Fig. 4 F and I). A count made of bristles (from photographs such as the ones in Figs. 2D and 4D) gave a total of ≈10,000 bristles per tarsus, or 60,000 per beetle. We presume the oil to act as a thin-film adhesive that provides for the attachment of each individual bristle, by its two pads, to the substrate.
Figure 4.
(A–C) Normal tarsus, and details thereof; the pads are stuck together in clusters (C), which are arranged in rows (A). (D–F) Comparable with preceding, but of a tarsus cleaned of oil by treatment with methanol/chloroform solution. (G) Comparable with E but with some of the bristles clustered where a droplet of oil has been applied. (H) Portion of tarsus where tips of bristles have been cut off, showing how bristle shafts are stuck together in groups; a substance, presumed to be oil, is seen between the bases of the bristles (upper arrow). Lower arrows point to pores from which tarsal oil is presumed to be secreted. (I) Bristles, in profile view, showing the component parts (shaft, bifurcated tip, pads) and oil pores between their bases. [Bars = 100 μm (A), 20 μm (B), 5 μm (C), 10 μm (I).]
Scanning electron microscopy also revealed the presence of small circular pores (Fig. 4 H and I), irregularly distributed amidst the base of the bristles. We take these pores to be the glandular openings from which the tarsal oil is secreted.
The Wetting of the Tarsal Bristles.
In the normal tarsus, the bristles are terminally clumped into clusters, which tend to be arranged in rows (Fig. 4 A–C). The number of bristles per cluster is in the range of 4 to 8. The clusters apparently are splayed apart when the tarsus touches down, as evidenced by the fact that the bristles are distinctly free from one another when in contact with the substrate (Fig. 2F). The clusters also are broken up when the tarsi are sonically cleaned in methanol/chloroform solvent (Fig. 4 D–F). We conclude from this that the bristles in the clumps ordinarily are held together by tarsal oil. We assume that the oil, on emergence from the pores, seeps by capillarity into the narrow clefts between the flattened shafts of the bristles, and onward to the bristle tips, causing these to stick together in clusters. Cutting off the bristle tips shows the shafts themselves to be stuck together in groups, by a material that is sometimes visible and that we presume to be tarsal oil (Fig. 4H). The spreading of oil onto the bristle tips may be facilitated by the terminal bifurcation of the shafts. In fact, the bristle endings, by being bunched, could act in the manner of a physical sponge that draws the oil and ensures that the bristles are terminally wetted. Addition of a droplet of oil (lubricant oil, from a vacuum diffusion pump) to a tarsus that had been cleaned beforehand with solvent caused the wetted bristles to become clumped again (Fig. 4G). Another role of the bifurcation is that it bestows stability on the bristles when the tarsus bears down, preventing the bristles from being deflected.
The mechanism by which we presume the tarsal bristles to be wetted with oil is illustrated in Fig. 5. When the tarsus is lifted, the bristles clump together and oil flows to the clustered tips, wetting the bristle pads. When the tarsus then touches down, the bristles are splayed, and the prewetted pads make contact with the substrate; because of the splaying, additional oil is prevented from seeping to the bristle tips and spilling onto the substrate. When the tarsus is then lifted up again, the bristles clump together and the pads are rewetted.
Figure 5.
Postulated mechanism by which tarsal bristle pads adhere and become prewetted for adherence. Details in Results and Conclusions.
An additional observation was made on tarsi that were submerged in water. First, it was noted that the contact surface of the tarsi was water repellant. But in places in which water did seep into the spaces between clustered bristles, the clusters were seen to be wetted by a fluid that did not mix with water. Again, we presume that fluid to be tarsal oil.
We have evidence that what we call tarsal oil is indeed an oil. Chemical extracts of tarsi, or of glass surfaces to which H. cyanea had clung, yielded mixtures of saturated and unsaturated linear hydrocarbons, of C20 to C28 chain length, with (Z)-9-pentacosene as the principal component (A. Attygalle and T.E., unpublished data).
Oil Relinquished in Walking and Defense.
Observation of individual beetles confined to the chamber above the electromagnet showed that, for as long as the beetle remained undisturbed (magnet off), it tread lightly, committing to contact only a fraction of the bristles of each tarsus (specifically, the anterior-most rows of bristles of each tarsomere) (Fig. 3C). When the magnet was turned on and the pull exerted on the beetle, the animal responded immediately by pressing its six tarsi down flatly, so that virtually all of its bristles were brought into contact (Fig. 3D). The results were consistent with each of 15 beetles so tested. We conclude that the beetle is adapted to relinquish a minimum of oil during locomotion and to put its full complement of bristles to use only in the context of defense.
We predicted that at sites of attack there should be evidence of substantial oil loss on the part of the beetle. Examination of glass surfaces on which encounters between beetles and ants (F. exsectoides) had taken place revealed the entire area of engagement to be beset by droplets and streaks of oil (Fig. 3B).
We calculated from footprint photos that the volume of the droplet of oil relinquished by a bristle pad is 1.5 μm3 [diameter of circular contact area of droplet = 1.8 ± 0.05 μm (n = 100); droplet is taken to be a hemisphere]. The total amount of oil lost by a beetle as a consequence of committal of its entire bristle complement to contact would then be 0.00018 mm3, or (if oil density is taken to be 1 mg per mm3) ≈0.001% of body mass. That amount of oil can be taken to represent the approximate minimum lost by the beetle in conflict with an ant. In actuality, the beetle probably expends more than the minimum, because during the attack it sometimes repositions its tarsi. (Note how in Fig. 3B the individual feet left multiple prints.)
Adhesive Strength.
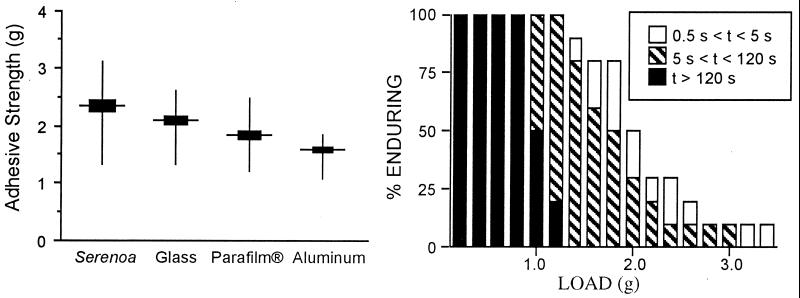
The beetle bonded most strongly to Serenoa palmetto frond, its natural substrate (Fig. 6). It bonded somewhat less strongly to glass, indicating that our predation tests with F. exsectoides, which were done on glass, were actually carried out under conditions of slightly increased risk to the beetle. The beetle adhered even less strongly to the other two unnatural substrates, Parafilm and aluminum foil. The fact that the beetle adhered with different strengths to different substrates is in itself indicative that it clings by adhesion. If the contact pads operated as suction cups, one might have expected the beetle to cling with equal strength to the various solids (2).
Figure 6.
(Left) Pull in g needed to detach beetle from four different substrates (n = 10 beetles per substrate). The four mean values differ significantly from one another (P < 0.01 for all comparisons). (Right) Length of time that a fraction of beetles endured detachment pulls (load in g) of given magnitudes. Ten beetles each were subjected to the full range of loads, beginning with the lightest and proceeding incrementally to the heaviest.
Adhesive Endurance.
It is clear from Fig. 6 that the beetle's clinging endurance is a function of the force applied. We chose 2 min as the arbitrary cutoff point for our timings, because the value exceeds by a substantial margin the mean time (22.8 sec) that F. exsectoides persist in their assault. The beetle was evidently able to resist a pull of up to 0.8 g, or nearly 60 times its body mass, for the full 2-min period. Its endurance gradually shortened with increasing pull, to the point at which at values of >3 g it tended to hold on for no more than seconds.
The fact that endurance decreases with load could be taken to indicate that the defensive response of the beetle (that is, the pressing down of its tarsi) requires sustained muscular action, such as would be subject to fatigue. Indeed, we suggest that the prime trigger of the tarsal response is the flexion of the legs at the level of the knee joints, and that the maintenance of defensive tarsal contact requires the sustained contraction of the muscles that effect that flexion. However, other leg muscles could be involved as well.
Vulnerability to Predation.
At a site near Fargo, Clinch County, GA, we noted a reduviid predator, the so-called wheel bug (Arilus cristatus), feeding on an H. cyanea. It had impaled the beetle on its proboscis and was in the process of imbibing its contents. We collected eight such bugs at the site, and fed each an H. cyanea, with consistent results. The bug approached the beetle and straddled it, to which the beetle responded by clamping down. The bug then proceeded to probe the beetle until it found a membranous site for beak insertion. Within seconds after being pierced, the beetle went limp, and as it did, the bug simply lifted it up and pulled it off its hold (Fig. 3E). With its legs gone flaccid, the beetle seemed to detach readily. The beetles were thoroughly sucked out (mass of carcass = 5.5 ± 0.3 mg; n = 8).
Discussion
Tarsal bristles are commonplace in beetles and other insects, and their role in locomotion has been documented (3–9). Evidence also has been presented that tarsal bristles secure their hold by adhesion, and that the adhesive fluid may in some cases be an oil (3–7). The phenomenon of surface adhesion has itself been the subject of review, both from a physical (2) and a comparative biological (3) point of view.
There can be no question that the extraordinary clinging ability of H. cyanea is defensive, certainly against ants. The beetle is more persistent in its clinging than the ant is in its attack, and it can maintain its hold against forces exceeding by many times its body mass. Whether ants can, in fact, exert forces >1 g or so that the beetle seems safely able to sustain remains open to question. It should be noted that the beetle, because of its smooth hemispherical shape, is intrinsically difficult to grasp for an ant. By pressing itself down, the beetle provides yet another measure of protection, because the edge of its body is then no longer easily graspable and its legs are inaccessibly tucked away. The maneuver is not, however, failsafe. Two of the 14 F. exsectoides that attacked H. cyanea managed to prod the beetle loose. Interestingly, both ants eventually released the beetles. Is H. cyanea perhaps chemically protected? Evidence that we have from orb-weaving spiders, which we found to reject the beetles, suggest that they might indeed be unpalatable to some predators.
The beetle loses oil in substantial quantity only when, under attack, it is forced to commit to contact the full complement of its bristles. During ordinary locomotion, it touches down with but a fraction of the bristles and loses little oil. But how “expensive” is the oil? The palmetto plants on which H. cyanea feeds are likely to be rich in waxes, as palm plants generally are (10). Deriving long-chain hydrocarbons from waxes could be, metabolically, a relatively undemanding process, given that wax molecules themselves incorporate long carbon chains. The tarsal oil therefore could come to the beetle relatively “cheaply.”
How does the beetle detach itself once it has clamped down? Perhaps it does so by rolling its feet off one tarsomere at a time (beginning with tarsomere 1 and proceeding to the tarsal tip) rather than by attempting to lift the entire tarsus at once. Rolling the tarsi off should pose no problem once the beetle has relaxed the leg muscles that were (presumably) tensed to press the tarsi down. It is probably by being able to relax these muscles with its injected venom that the wheel bug overpowers H. cyanea. Indeed, the bug seemed to have no difficulty lifting the beetle from the substrate once paralysis had set in.
The tarsi of many beetles, including that of cassidine chrysomelids, bear a terminal claw (9) by which the beetle can secure anchorage. Measurements that we made with one cassidine, Metriona bicolor, showed that by hooking their tarsi into the leaf of their foodplant, these beetles can withstand pulls upward of 4–5 g, amounting to hundreds of times their body mass. H. cyanea has tarsal claws, but these are atrophied, as well they might be, given that they would be inoperative on the hard surface of the palmetto frond. H. cyanea has evolutionarily compensated for the atrophy of its claws by expanding its tarsi and proliferating the bristles. M. bicolor, with its functional claws, has only ≈1,000 adhesive bristles per tarsus, in contrast to H. cyanea's 10,000.
Acknowledgments
We thank the staff at the Archbold Biological Station for much help received. Mark Deyrup and Jerrold Meinwald provided comments on the manuscript. We also thank Maria Eisner for help in the field and with the preparation of the manuscript and Susan Pulakis for the illustration in Fig. 5. The study was supported by Grant AI02908 from the National Institutes of Health. This is paper number 171 in the series “Defense Mechanisms of Arthropods.” Paper number 170 is ref. 11.
References
- 1.Eisner T. Verh Dtsch Zool Ges. 1972;65:123–137. [Google Scholar]
- 2.Baier R E, Shafrin E G, Zisman W A. Science. 1968;162:1360–1368. doi: 10.1126/science.162.3860.1360. [DOI] [PubMed] [Google Scholar]
- 3.Nachtigall W. Biological Mechanisms of Attachment. New York: Springer; 1974. [Google Scholar]
- 4.Walker G. Int J Adhes Adhes. 1992;13:3–7. [Google Scholar]
- 5.Walker G, Yule A B, Ratcliffe J. J Zool London. 1985;205:297–307. [Google Scholar]
- 6.Gorb S N. Proc R Soc London Ser B. 1998;265:747–752. [Google Scholar]
- 7.Ishii S. Appl Entomol Zool. 1987;22:222–228. [Google Scholar]
- 8.Stork N E. J Exp Biol. 1980;88:91–107. [Google Scholar]
- 9.Stork N E. Zool J Linn Soc. 1980;68:173–306. [Google Scholar]
- 10.Johnson D. Principes. 1971;15:127–130. [Google Scholar]
- 11.Eisner, T., Rossini, C. & Eisner, M. (2000) Chemoecology, in press.