Abstract
Previous studies have attempted to clarify the roles of the pre-S1 and pre-S2 domains of the large envelope protein of hepatitis B virus (HBV) in attachment and entry into susceptible cells. Difficulties arise in that these domains contain regions involved in the nucleocapsid assembly of HBV and overlapping with the coding regions of the viral polymerase and RNA sequences required for reverse transcription. Such difficulties can be circumvented with hepatitis delta virus (HDV), which needs the HBV large envelope protein only for infectivity. Thus, mutated HBV envelope proteins were examined for their effects on HDV infectivity. Changing the C-terminal region of pre-S1 critical for HBV assembly allowed the envelopment of HDV and had no effect on infectivity in primary human hepatocytes. Similarly, a deletion of the 12 amino acids of a putative translocation motif (TLM) in pre-S2 had no effect. Thus, these two regions are not necessary for HDV infectivity and, by inference, are not needed for HBV attachment and entry into susceptible cells.
Hepatitis B virus (HBV) is an important human pathogen, causing acute and chronic hepatitis and hepatocellular carcinoma, and yet we have only a very partial understanding of how it uses its envelope proteins to attach and enter susceptible cells (12). Here we point out some important similarities between the major envelope protein of HBV and that of its distant relative, duck hepatitis B virus (DHBV). Also we make use of hepatitis delta virus (HDV), a subviral agent that uses the envelope proteins of HBV, to address two controversial issues regarding the requirements for HBV attachment and entry.
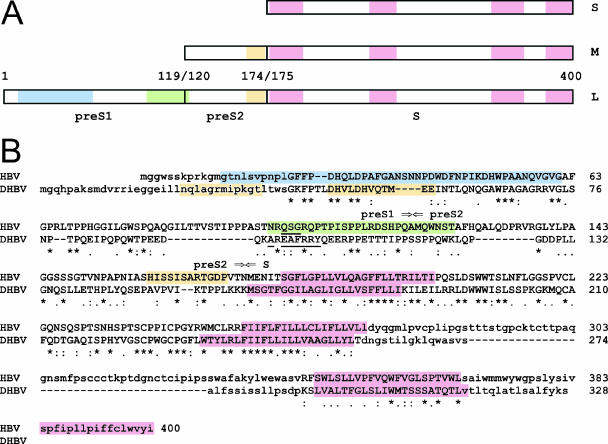
The Hepadnaviridae family is divided into two genera, the ortho- and avihepadnaviruses. HBV is the prototype of the orthohepadnaviruses. As represented in Fig. 1A, HBV encodes three envelope proteins, large (L), middle (M), and small (S), that have a common C terminus. Pre-S1 is the N terminus of L, which is unique relative to M. Similarly, pre-S2 is the N terminus of M, which is unique relative to S. DHBV is the prototype of the avihepadnaviruses. It has only two envelope proteins, L and S (12). We used alignment programs to compare the L proteins of representative HBV and DHBV, with results as summarized in Fig. 1B. Amidst many amino acid differences and several deletions in DHBV relative to HBV, some conserved regions were revealed. Some of these conservations might be due to the fact that the open reading frame for L overlaps with that of the viral polymerase (12). However, other conservations might reflect features of L that are needed for virus assembly and/or infectivity. As indicated, HBV and DHBV share three predicted transmembrane domains in S (7). Only HBV has a fourth domain (12). Beyond this, the folding of the hepadnavirus L proteins is complicated by the fact that the pre-S region is considered to exist in two topologies, inside or outside, relative to the host endoplasmic reticulum during assembly and/or to the viral envelope after release (5, 12, 27). These two conformations may lead to different protein binding partners, even for the same region. For both viruses, a glycine penultimate to the N terminus is myristylated (28). This modification, while not needed for assembly, is essential for infectivity (4, 15). For both viruses, domains of about 50 amino acids near the N termini are required for infectivity (2, 13, 14, 21, 25, 36). Presumably some of these domains are exposed, although which host components they interact with remains unclear. Antibodies to these domains block infectivity (24, 25, 30). Also, peptides corresponding to these regions are potent inhibitors, especially if myristylated (2, 9, 14, 36). A region near the C terminus of HBV pre-S1 (Fig. 1) and the corresponding region of DHBV L, when exposed intracellularly on the cytosolic side of the endoplasmic reticulum, bind to the nucleocapsid to facilitate assembly and has been referred to as a matrix-like domain (3, 34, 37). It can also be found bound to a heat shock protein, Hsc70 (20, 23). Conversely, the pre-S1 domain, when expressed intracellularly on the luminal side, has been found bound to another heat shock protein, BiP (6, 20).
FIG. 1.
Features of hepadnavirus large envelope proteins. (A) A representation for HBV of the three envelope proteins, L, M, and S. The domains pre-S1, pre-S2, and S are indicated. The numbers refer to the amino acid positions for genotype A, serotype adw2 (NCBI protein database accession number AAK58874.1). Coloring is as described in the legend for panel B. (B) Alignment of the amino acid sequences for the large envelope proteins of HBV and DHBV (NCBI protein database accession number AAA62820.1). The alignment was obtained after three iterations of PSI-BLAST. Aligned regions, indicated in uppercase letters, span residue 23 of the human sequence (beginning with GFFP) to residue 271 (ending with LLVL). In lowercase letters are the regions before and after these positions that could not be meaningfully aligned. Transmembrane helices predicted using the program TMHMM (18) are shown in pink. The predicted third transmembrane helices of the two sequences were then manually aligned. Note that HBV has a fourth transmembrane domain. Previous reports have made somewhat different predictions for the number of such domains (7, 11, 12). Identical amino acids are marked under the alignment with asterisks, highly similar residues with colons (e.g., R/K or V/I pairs), and similar residues with periods (e.g., two hydrophobic residues or two hydrophilic residues). The amino acids of the predicted RBDs of HBV and DHBV are underlined. For DHBV, this region is overlapped by what is considered to be the binding site for CPD and may include an alpha-helical region (37). Indicated in yellow are the putative TLMs: two for DHBV and one for HBV (29). Indicated in blue is the region of HBV pre-S1 that has been implicated in attachment (14). The corresponding region of DHBV would be amino acids 2 to 41 (ending GKFP) (36). Indicated in green is the so-called matrix domain of HBV (positions 98 to 124) (3). The limits of HBV pre-S1, pre-S2, and S are indicated by arrows.
We analyzed the entire HBV and DHBV L proteins with a receptor binding domain (RBD) finder program (10). This program is known to be an efficient first approach for localizing putative interaction sites from protein sequences in the absence of structural data, although it cannot distinguish a receptor binding site from a site that interacts with another protein, whether viral or cellular. This program predicted one RBD for HBV L protein and one for DHBV L protein. These RBDs are indicated in Fig. 1. Note that they are at the same location on the alignments of the HBV and DHBV proteins. Also, they are within what was referred to above as the matrix-like domain. Furthermore, for DHBV, the predicted RBD overlaps with what has been predicted to fold into an alpha-helical structure (37), which is part of a sequence that binds to carboxypeptidase D (CPD). For some time, CPD has been asserted to be a putative receptor for DHBV entry (19, 37). While an interaction with CPD may be necessary for DHBV infection, the expression of CPD is not sufficient to confer susceptibility to resistant cells (19, 37). Nevertheless, such findings have been sufficient to open the possibility that the comparable region on HBV L might be directly relevant to infectivity. As described subsequently, we have exploited the advantages of HDV to resolve the putative requirements for attachment and entry away from the matrix-like function needed for HBV assembly.
Another issue relating to the role of the L protein in hepadnavirus entry has come from studies of what is called a translocation motif (TLM), a short sequence (12 amino acids) with the ability to form an amphipathic helix and possibly the ability to cross cellular membranes (26). A recent study asserted that two such sequences in the pre-S region of DHBV L (Fig. 1B) are essential for infectivity (29). A comparable domain was predicted to be within the pre-S2 domain of HBV (as indicated), but data for an essential role in virus infectivity were not presented (29). In contrast, a separate study reported that small deletions of HBV sequences partially overlapping this TLM domain did not interfere with HBV infectivity (22). In this paper, we address this disagreement as to whether HBV needs the putative TLM for infectivity and we test the previously mentioned question of whether the C-terminal region of HBV pre-S1 is needed for virus attachment and entry. Our approach, as described below, was to use measurements of the infectivity of HDV assembled with mutated HBV envelope proteins.
We and others have realized that HDV offers some unique advantages as part of a strategy for understanding HBV attachment and entry (2, 9, 16, 31, 33). Unlike HBV assembly, HDV assembly does not require a pre-S1 or pre-S2 domain. HDV RNP can be enveloped with just the S protein, making use of binding to a small cytosolic loop near its C terminus (17, 31). Nevertheless, for HDV to be infectious, the L protein must also be incorporated into the virion (31, 32). Thus, as described here, we have been able to address certain requirements for infectivity without compromising assembly. The strategy is based on studies described in detail elsewhere (16).
Briefly, to assemble HDV RNA into particles, we transfected Huh7 cells with a cDNA construct that initiated the replication of HDV, together with another that expressed HBV L, M, and S. Some of these HBV constructs had specific mutations in the pre-S regions of L. Particles containing the HDV genome were harvested from days 6 to 9 after transfection. Subsequently, aliquots of these clarified media, in the presence of 5% polyethylene glycol, were used to infect monolayer cultures of primary human hepatocytes. After 6 days, total RNA was extracted and both this RNA and that which was extracted from the input HDV particles were assayed using quantitative real-time PCR relative to an HDV RNA standard. From these data, we could determine the initial virus titer (genome equivalents [GE]/ml of medium), the input multiplicity of infection (MOI) (GE per cell, with 200,000 hepatocytes per well of a collagen-coated, 48-well plate), and the HDV infectivity, which is the accumulation of HDV RNA (GE per cell) following infection. All infections were carried out at a multiplicity of <300 GE/cell, that is, in what has been previously determined to be the linear response range (16). Finally, we determined the ratio of the output of GE per cell accumulated in the infected hepatocytes to the input multiplicity of GE per cell. We refer to this ratio as the specific infectivity. For example, a specific infectivity of 200 means that 200 GE were accumulated for every GE used for infection.
To address the role of the C-terminal region of HBV pre-S1, we used HBV envelope protein-expressing vectors bearing previously characterized double-alanine substitutions (3). These mutations, designated A1 to A7, are summarized in Table 1. By using the endogenous DNA polymerase reaction of released HBV particles, it was previously shown that all mutations, with the exception of A4, resulted in the abolishment of HBV nucleocapsid envelopment (3). However, by using quantitative real-time PCR assays, we were able to demonstrate the successful assembly of HDV RNA with each of the mutated envelopes (Table 1). This result was expected because HDV assembly requires RNP binding to a tryptophan-rich region located at the C terminus of S (17, 31). For all mutants except A1, the efficiency of HDV assembly was comparable to that of the wild-type (wt) envelope protein. The A1 mutation lowered virus yield to 3.5%. One possible explanation for this is that the altered L protein might have interfered with assembly. However, we favor a second explanation, that the mutation weakened the strength of the promoter for the M and S mRNAs. Certainly, the deleted region overlaps with important sequence features of the S promoter (8). Furthermore, as previously documented (3) for this A1 mutant, there was a specific reduction in the intracellular expression of S but not of L.
TABLE 1.
Role of sequences in the vicinity of the C terminus of HBV pre-S1 in HDV assembly and infectivitya
| Protein | Sequence | HBV assemblyb | HDV assembly (107 GE/ml)c | HDV MOI (GE/cell)d | HDV infectivity (GE/cell)e | HDV specific infectivityf |
|---|---|---|---|---|---|---|
| pre-S1 ⇒ ⇐ pre-S2 | ||||||
| wt | 91-IPPPASTNRQSGRQPTPISPPLRDSHPQA MQWNSTAFH-128 | + | 4.21 | 10.5 | 2,400 | 230 |
| A1 | .......AA.................... ......... | − | 0.15 | 0.38 | 48 | 127 |
| A2 | ...........AA................ ......... | − | 4.00 | 10.0 | 980 | 98 |
| A3 | ..............AA............. ......... | − | 5.47 | 13.7 | 770 | 56 |
| A4 | .................AA.......... ......... | + | 5.92 | 14.8 | 1,440 | 97 |
| A5 | ......................AA..... ......... | − | 6.66 | 16.6 | 940 | 57 |
| A6 | .........................AA.. ......... | − | 5.65 | 14.1 | 830 | 59 |
| A7 | ............................. ..AA..... | − | 2.54 | 6.36 | 830 | 131 |
| S only | − | 3.90 | 9.74 | 9 | 0.97 |
The indicated wt sequence is as shown in Fig. 1. The A1-to-A7 series of mutants and their effects on HBV assembly are as previously described (3). As a negative control for infectivity, we used particles assembled with HBV S protein only (1, 32).
The qualitative assessment of HBV assembly was as previously reported (3).
The indicated values of HDV assembly, infectivity, and specific infectivity were based on the average of independent, duplicate, quantitative real-time PCR assays of GE, as previously described (16).
Values were deduced from using 50 μl of assembled HDV per 200,000 hepatocytes.
At day 6, total RNA was extracted and assayed for GE. Using an RNA concentration measurement and the assumption of 25 pg RNA per cell, we deduced the indicated number of GE per cell (16).
The specific infectivity is the ratio of HDV infectivity to HDV MOI. Based on this and a separate experiment, we consider the range of values for the wt and the A1 to A7 mutants to be not significantly different.
Equal volumes of HDV-containing media were used to inoculate primary human hepatocytes. The actual MOI was deduced in each case (Table 1). These values are each <300 GE/cell, and thus, based on previous studies (16), we know that the amount of replication subsequent to infection is linearly proportional to the input MOI. After 6 days, the total RNA was extracted and assayed by quantitative real-time PCR assays. We found that each mutant was infectious (Table 1). Infectivity values ranged from 48 to 2,400 GE per average hepatocyte. Furthermore, when specific infectivity values were deduced, it became clear that all mutants were similar to the wt within a factor of 4 (Table 1). Given the above results of the A1 to A7 mutations being without significant effect on the HDV-specific infectivity, we constructed an actual deletion of amino acids 98 through 124, spanning the entire matrix-like domain. HDV particle assembly was achieved with this construct, and following infection of primary hepatocytes, we observed no statistically significant difference in specific infectivity relative to that obtained for the virus with unmodified envelope protein (data not shown). Taken together, these data demonstrate that the C-terminal region of pre-S1 is dispensable for HDV assembly and infectivity.
The second question addressed in this study was the requirement of the TLM for infectivity. We generated an HBV envelope protein mutant bearing a deletion of the entire predicted TLM (Table 2). We found that HDV was assembled with the mutated envelope protein as efficiently as with the wt. Furthermore, assembled virus particles possessed no defects in infectivity or specific infectivity. Thus, the putative TLM of HBV is dispensable for HDV assembly and infectivity. Our results thus support and extend earlier observations by Le Seyec et al. (22) and Tai et al. (35).
TABLE 2.
Role of the putative TLM in the HBV pre-S2 in HDV assembly and infectivitya
| Protein or motif | Sequence | HDV assembly (107 GE/ml) | HDV MOI (GE/cell) | HDV infectivity (GE/cell) | HDV specific infectivity |
|---|---|---|---|---|---|
| wt | 156-PNIASHISSISARTGDPVTNME-177 | 6.9 | 10.4 | 2,690 | 260 |
| TLM− | PNIAS------------VTNME | 11.9 | 17.8 | 2,660 | 156 |
| S only | 13.4 | 20.1 | 2.84 | 0.14 |
The wt sequence shown is as in shown Fig. 1 and represents that part of the HBV pre-S2 that spans the TLM proposed by Stoeckl et al. (29). The mutation (TLM−) was made using a QuikChange kit (Stratagene). Particles assembled with only HBV S protein were used as a negative control for infectivity. The assembly, infectivity, and specific infectivity of HDV were assayed as described in Table 1 and as previously described (16), and all are based on the average of independent, duplicate, quantitative real-time PCR assays of GE. Based on this and a separate experiment, we consider the range of specific infectivity values for wt and TLM− to be not significantly different.
Overall, this study demonstrates the value of the HDV assembly and infection system in solving critical nontrivial issues of HBV attachment and entry. Our findings using HDV, if they can be extrapolated to HBV, indicate that maybe only one domain in pre-S1, that near the N terminus, is directly involved in virus attachment. This would leave open the question of why DHBV has the additional requirement of a CPD-binding domain. In addition, a critical role for the pre-S2 domain in the HBV life cycle could not yet be defined. How additional domains located in S, such as the major antigenic loop, might influence attachment and entry also remains to be understood (31).
Acknowledgments
J.T. was supported by grants AI-058269 and CA-06927 from the NIH and by an appropriation from the Commonwealth of Pennsylvania.
Constructive comments on the manuscript were given by Glenn Rall, William Mason, Christoph Seeger, Richard Katz, and Jinhong Chang. We thank Emmanuelle Nicolas and the Fox Chase Biochemistry and Biotechnology Facility for the real-time PCR assays.
Footnotes
Published ahead of print on 24 January 2007.
REFERENCES
- 1.Barrera, A., B. Guerra, H. Lee, and R. E. Lanford. 2004. Analysis of host range phenotypes of primate hepadnaviruses by in vitro infections of hepatitis D virus pseudotypes. J. Virol. 78:5233-5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrera, A., B. Guerra, L. Notvall, and R. E. Lanford. 2005. Mapping of the hepatitis B virus pre-S1 domain involved in receptor recognition. J. Virol. 79:9786-9798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruss, V. 1997. A short linear sequence in the pre-S domain of the large hepatitis B virus envelope protein required for virion formation. J. Virol. 71:9350-9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bruss, V., J. Hagelstein, E. Gerhardt, and P. R. Galle. 1996. Myristylation of the large surface protein is required for hepatitis B virus in vitro infectivity. Virology 218:396-399. [DOI] [PubMed] [Google Scholar]
- 5.Bruss, V., X. Lu, R. Thomssen, and W. H. Gerlich. 1994. Post-translational alterations in transmembrane topology of the hepatitis B virus large envelope protein. EMBO J. 13:2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cho, D. Y., G. H. Yang, C. J. Ryu, and H. J. Hong. 2003. Molecular chaperone GRP78/BiP interacts with the large surface protein of hepatitis B virus in vitro and in vivo. J. Virol. 77:2784-2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chojnacki, J., D. A. Anderson, and E. V. Grgacic. 2005. A hydrophobic domain in the large envelope protein is essential for fusion of duck hepatitis B virus at the late endosome. J. Virol. 79:14945-14955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De-Medina, T., O. Faktor, and Y. Shaul. 1988. The S promoter of hepatitis B virus is regulated by positive and negative elements. Mol. Cell. Biol. 8:2449-2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engelke, M., K. Mills, S. Seitz, P. Simon, P. Gripon, M. Schnolzer, and S. Urban. 2006. Characterization of a hepatitis B and hepatitis delta virus receptor binding site. Hepatology 43:750-760. [DOI] [PubMed] [Google Scholar]
- 10.Gallet, X., B. Charloteaux, A. Thomas, and R. Brasseur. 2000. A fast method to predict protein interaction sites from sequences. J. Mol. Biol. 302:917-926. [DOI] [PubMed] [Google Scholar]
- 11.Ganem, D., and R. J. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-2969. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 12.Gerlich, W. H., and M. Kann. 2005. Hepatitis B, p. 1226-1268. In B. W. J. Mahy and V. ter Meulen (ed.), Topley and Wilson's microbiology and microbial infections, vol. 2. ASM Press, Washington, DC. [Google Scholar]
- 13.Glebe, D., M. Aliakbari, P. Krass, E. V. Knoop, K. P. Valerius, and W. H. Gerlich. 2003. Pre-S1 antigen-dependent infection of Tupaia hepatocyte cultures with human hepatitis B virus. J. Virol. 77:9511-9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gripon, P., I. Cannie, and S. Urban. 2005. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J. Virol. 79:1613-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gripon, P., J. Le Seyec, S. Rumin, and C. Guguen-Guillouzo. 1995. Myristylation of the hepatitis B virus large surface protein is essential for viral infectivity. Virology 213:292-299. [DOI] [PubMed] [Google Scholar]
- 16.Gudima, S., J. He, A. Meier, J. Chang, R. Chen, M. Jarnik, E. Nicolas, V. Bruss, and J. Taylor. 2007. Assembly of hepatitis delta virus: particle characterization including the ability to infect primary human hepatocytes. J. Virol. 81:3608-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Komla-Soukha, I., and C. Sureau. 2006. A tryptophan-rich motif in the carboxyl terminus of the small envelope protein of hepatitis B virus is central to the assembly of hepatitis delta virus particles. J. Virol. 80:4648-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krogh, A., B. Larsson, G. von Heijne, and E. L. Sonnhammer. 2001. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305:567-580. [DOI] [PubMed] [Google Scholar]
- 19.Kuroki, K., F. Eng, T. Ishikawa, C. Turck, F. Harada, and D. Ganem. 1995. gp180, a host cell glycoprotein that binds duck hepatitis B virus particles, is encoded by a member of the carboxypeptidase gene family. J. Biol. Chem. 270:15022-15028. [DOI] [PubMed] [Google Scholar]
- 20.Lambert, C., and R. Prange. 2003. Chaperone action in the posttranslational topological reorientation of the hepatitis B virus large envelope protein: implications for translocational regulation. Proc. Natl. Acad. Sci. USA 100:5199-5204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le Seyec, J., P. Chouteau, I. Cannie, C. Guguen-Guillouzo, and P. Gripon. 1999. Infection process of the hepatitis B virus depends on the presence of a defined sequence in the pre-S1 domain. J. Virol. 73:2052-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Le Seyec, J., P. Chouteau, I. Cannie, C. Guguen-Guillouzo, and P. Gripon. 1998. Role of the pre-S2 domain of the large envelope protein in hepatitis B virus assembly and infectivity. J. Virol. 72:5573-5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Löffler-Mary, H., M. Werr, and R. Prange. 1997. Sequence-specific repression of cotranslational translocation of the hepatitis B virus envelope proteins coincides with binding of heat shock protein Hsc70. Virology 235:144-152. [DOI] [PubMed] [Google Scholar]
- 24.Maeng, C. Y., C. J. Ryu, P. Gripon, C. Guguen-Guillouzo, and H. J. Hong. 2000. Fine mapping of virus-neutralizing epitopes on hepatitis B virus preS1. Virology 270:9-16. [DOI] [PubMed] [Google Scholar]
- 25.Neurath, A. R., S. B. H. Kent, N. Strick, and K. Parker. 1986. Identification and chemical synthesis of a host cell receptor binding site on hepatitis B virus. Cell 46:429-436. [DOI] [PubMed] [Google Scholar]
- 26.Oess, S., and E. Hildt. 2000. Novel cell permeable motif derived from the preS2-domain of hepatitis-B virus surface antigens. Gene Ther. 7:750-758. [DOI] [PubMed] [Google Scholar]
- 27.Ostapchuk, P., P. Hearing, and D. Ganem. 1994. A dramatic shift in the transmembrane topology of a viral envelope glycoprotein accompanies hepatitis B viral morphogenesis. EMBO J. 13:1048-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persing, D. H., H. E. Varmus, and D. Ganem. 1987. The preS1 protein of hepatitis B virus is acylated at its amino terminus with myristic acid. J. Virol. 61:1672-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stoeckl, L., A. Funk, A. Kopitzki, B. Brandenburg, S. Oess, H. Will, H. Sirma, and E. Hildt. 2006. Identification of a structural motif crucial for infectivity of hepatitis B viruses. Proc. Natl. Acad. Sci. USA 103:6730-6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sunyach, C., C. Rollier, M. Robaczewska, C. Borel, L. Barraud, A. Kay, C. Trepo, H. Will, and L. Cova. 1999. Residues critical for duck hepatitis B virus neutralization are involved in host cell interaction. J. Virol. 73:2569-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sureau, C. 2006. The role of the HBV envelope proteins in the HDV replication cycle. Curr. Top. Microbiol. Immunol. 307:113-131. [DOI] [PubMed] [Google Scholar]
- 32.Sureau, C., B. Guerra, and R. E. Lanford. 1993. Role of the large hepatitis B virus envelope protein in infectivity of the hepatitis delta virion. J. Virol. 67:366-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sureau, C., A. M. Moriarty, G. B. Thornton, and R. E. Lanford. 1992. Production of infectious hepatitis delta virus in vitro and neutralization with antibodies directed against hepatitis B virus pre-S antigens. J. Virol. 66:1241-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Swameye, I., and H. Schaller. 1997. Dual topology of the large envelope protein of duck hepatitis B virus: determinants preventing pre-S translocation and glycosylation. J. Virol. 71:9434-9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tai, P. C., F. M. Suk, W. H. Gerlich, A. R. Neurath, and C. Shih. 2002. Hypermodification and immune escape of an internally deleted middle-envelope (M) protein of frequent and predominant hepatitis B virus variants. Virology 292:44-58. [DOI] [PubMed] [Google Scholar]
- 36.Urban, S., and P. Gripon. 2002. Inhibition of duck hepatitis B virus infection by a myristoylated pre-S peptide of the large viral surface protein. J. Virol. 76:1986-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Urban, S., C. Schwarz, U. C. Marx, H. Zentgraf, H. Schaller, and G. Multhaup. 2000. Receptor recognition by a hepatitis B virus reveals a novel mode of high affinity virus-receptor interaction. EMBO J. 19:1217-1227. [DOI] [PMC free article] [PubMed] [Google Scholar]