Abstract
Dysfunctional CD8+ T cells present in chronic virus infections can express programmed death 1 (PD-1) molecules, and the inhibition of the engagement of PD-1 with its ligand (PD-L1) has been reported to enhance the antiviral function of these T cells. We took advantage of the wide fluctuations in levels of viremia which are typical of chronic hepatitis B virus (HBV) infection to comprehensively analyze the impact of prolonged exposure to different virus quantities on virus-specific T-cell dysfunction and on its reversibility through the blocking of the PD-1/PD-L1 pathway. We confirm that chronic HBV infection has a profound effect on the HBV-specific T-cell repertoire. Despite the use of a comprehensive panel of peptides covering all HBV proteins, HBV-specific T cells were rarely observed directly ex vivo in samples from patients with chronic infection, in contrast to those from patients with acute HBV infection. In chronic HBV infection, virus-specific T cells were detected mainly in patients with lower levels of viremia. These HBV-specific CD8+ T cells expressed PD-1, and their function was improved by the blocking of PD-1/PD-L1 engagement. Thus, a broad spectrum of anti-HBV immunity is expressed by patients with chronic HBV infection and this spectrum is proportional to HBV replication levels and can be improved by blocking the PD-1/PD-L1 pathway. This information may be useful for the design of immunotherapeutic strategies to complement and optimize available antiviral therapies.
Quantitative and qualitative impairments of virus-specific T cells are present in different chronic viral infections, in both humans and mice. The persistent exposure to viral antigens can lead to virus-specific T-cell deletion or to progressive functional impairment. Recent data show that these exhausted T cells hyperexpress the programmed death 1 (PD-1) molecule (8, 14, 19, 23) and that blocking the engagement of PD-1 with its ligand (PD-L1) leads to an enhancement of the antiviral functions of these T cells (1, 7, 23, 30, 32). The characterization of the functional defects of virus-specific T cells in patients with chronic hepatitis B virus (HBV) infection, a condition that affects 350 million people worldwide, is still largely incomplete and often based on an oversimplified dichotomy between patients who control acute infection and subjects with established chronicity. While it is well documented that patients who succeed in resolving HBV infection express a vigorous and functionally efficient HBV-specific T-cell response (9, 12, 13, 15, 21, 29), the degree of HBV-specific T-cell impairments which affect chronically infected patients has so far been only partially analyzed with small groups of patients by using a limited set of well-characterized HLA-A2-restricted epitopes (3, 18, 21, 25, 27, 28, 34) or by focusing mainly on HLA class II-restricted responses targeting HBV structural antigens (9, 11-13, 17, 26, 31).
To overcome these limitations, we performed a longitudinal study of HBV-specific T-cell responses by using pools of overlapping peptides covering the overall protein sequence of HBV genotype D. Well-characterized groups of patients with anti-HBV e antigen (anti-HBe)-positive chronic hepatitis B or with acute HBV infection were studied. Since anti-HBe-positive patients are often characterized by periodic flare-ups in alanine aminotransferase (ALT) and HBV DNA levels (6, 10), the longitudinal study of these patients allowed for the detailed evaluation of the impact of fluctuations in HBV DNA levels and disease activity on the profile of the global HBV-specific T-cell repertoire. In addition, using the tetramer technology coupled to intracellular cytokine staining (ICS), we analyzed the potential role of the PD-1/PD-L1 pathway in the modulation of the HBV-specific T-cell function.
MATERIALS AND METHODS
Patients.
A total of 27 patients were enrolled at the Unit of Infectious Diseases and Hepatology of the Azienda Ospedaliero-Universitaria of Parma, Parma, Italy. Twenty patients displayed clinical, biochemical, and virological evidence of anti-HBe-positive chronic hepatitis B (HBsAg positive, anti-HBc positive, HBe negative, anti-HBe positive), with fluctuating levels of ALT (ranging from 22 to 1,372 U/liters) and HBV DNA (ranging from 0.049 ×105 to 1,220 ×105 copies/ml). Nine patients were monitored for at least 3 years; all other patients were monitored for at least 12 months. Patients C8 to C20 were HLA-A2 positive. Screening for HLA-A2 was performed by staining peripheral blood mononuclear cells (PBMC) with a fluorescent anti-HLA-A2.01 antibody (Serotec).
Seven patients had clinical, biochemical, and virological evidence of acute HBV infection (aminotransferase levels at least 10 times the upper limit of the normal range of levels and HBsAg and immunoglobulin M anti-HBc antibodies detected in the serum). All patients were negative for anti-hepatitis C virus, delta virus, human immunodeficiency virus type 1 (HIV-1), and HIV-2 antibodies and for other markers of viral or autoimmune hepatitis. The study was approved by the Ethical Committee of the Azienda Ospedaliero-Universitaria of Parma, and all subjects gave written, informed consent.
Virological assessment.
The presence or absence of HBsAg, anti-HBsAg, total and immunoglobulin M anti-HBc, HBe, anti-HBe, anti-hepatitis D virus, anti-hepatitis C virus, and anti-human immunodeficiency virus types 1 and 2 was determined by using commercial enzyme immunoassay kits (Abbott Labs, IL; Ortho-Clinical Diagnostic, Johnson & Johnson, Raritan, NJ; DiaSorin, Vercelli, Italy). Levels of HBV DNA in serum samples were quantified by PCR (Cobas Amplicor test; Roche Diagnostics, Basel, Switzerland). HBV genotyping was performed by detecting type-specific sequences in the HBV pol gene domains B and C (INNO-LIPA HBV genotyping line probe assay; Innogenetics, Alpharetta, GA).
Synthetic peptides, peptide-HLA class I tetramers, and antibodies.
A panel of 315 15-mer peptides overlapping by 10 residues and covering the overall protein sequence of HBV genotype D were purchased from Chiron Mimotopes (Victoria, Australia). 15-mer peptides were pooled in 16 mixtures as indicated in Table 1. Synthetic peptides and the corresponding phycoerythrin (PE)-labeled tetrameric peptide-HLA class I complexes representing the HLA-A2-restricted epitopes on the core peptide spanning amino acids 18 to 27 (FLPSDFFPSV), the envelope peptides spanning amino acids 183 to 191 (FLLTRILTI), 335 to 343 (WLSLLVPFV), and 348 to 357 (GLSPTVWLSV), and the polymerase peptides spanning amino acids 575 to 583 (FLLSLGIHL) and 816 to 824 (SLYADSPSV) of HBV genotype D; the HLA-A2 restricted epitope cytomegalovirus (CMV) pp65 (NLVPMVATV); and the influenza virus epitope matrix (GILGFVFTL) were purchased from Proimmune (Oxford, United Kingdom) and from Beckman-Coulter, Inc. (Fullerton, CA). Anti-CD8 (peridinin chlorophyll protein [PerCP]- or allophycocyanin [APC]-conjugated), anti-CD3 (PerCP-conjugated), anti-CD4 (PE-conjugated), and anti-interleukin-2 (anti-IL-2; APC-conjugated) antibodies were purchased from Becton Dickinson Immunocytometry Systems (San Jose, CA). Anti-gamma interferon (anti-IFN-γ)-fluorescein isothiocyanate (FITC) was purchased from Sigma-Aldrich (St. Louis, MO). Anti-perforin (FITC-conjugated), anti-PD-1 (FITC-conjugated), and anti-CD127 antibodies and goat anti-mouse antibody conjugated with FITC or APC were purchased from BD Biosciences-Pharmingen (San Jose, CA). Anti-CCR7 FITC-conjugated antibody was purchased from R&D Systems (Minneapolis, MN).
TABLE 1.
HBV amino acid coordinates of 15-mer peptide pools
| Peptide mixture | HBV regiona | Peptides | Amino acid position |
|---|---|---|---|
| 1 | HBV x | 1-15 | 1-85 |
| 2 | HBV x | 16-30 | 76-154 |
| 3 | HBV core | 31-51 | 1-115 |
| 4 | HBV core | 52-72 | 106-214 |
| 5 | HBV ENV | 73-92 | 1-110 |
| 6 | HBV ENV | 93-111 | 101-205 |
| 7 | HBV ENV | 112-130 | 196-300 |
| 8 | HBV ENV | 131-149 | 291-389 |
| 9 | HBV POL | 150-170 | 1-115 |
| 10 | HBV POL | 171-191 | 106-220 |
| 11 | HBV POL | 192-212 | 211-325 |
| 12 | HBV POL | 213-233 | 316-430 |
| 13 | HBV POL | 234-254 | 421-535 |
| 14 | HBV POL | 255-274 | 526-635 |
| 15 | HBV POL | 275-294 | 626-735 |
| 16 | HBV POL | 295-315 | 726-832 |
ENV, envelope; POL, polymerase.
Isolation of PBMC and in vitro expansion of HBV-specific CD8 cell population.
PBMC were isolated from fresh heparinized blood by Ficoll-Hypaque density gradient centrifugation and resuspended in RPMI 1640 supplemented with 25 mM HEPES, 2 mM l-glutamine, 50 μg/ml of gentamicin, and 8% human serum (complete medium). For CD8 expansion, PBMC were resuspended in complete medium supplemented with IL-7 (5 ng/ml) and IL-12 (100 pg/ml) at a concentration of 2 × 106/ml, seeded at 200 μl/well in 96-well plates, and stimulated with HLA-A2-restricted HBV peptides at a 1 μM final concentration. Recombinant IL-2 (50 IU/ml) was added on day 4 of culture, and the immunological assays were performed on day 10. For PBMC stimulation with overlapping 15-mer peptides covering the overall HBV protein sequence, only IL-2 was added on day 4.
Cell surface and intracellular staining. (i) Staining with tetramers and other surface markers.
A total of 1 × 106 to 4 × 106 PBMC, either freshly isolated or obtained following in vitro expansion of CD8 cell populations for 10 days, were incubated for 30 min at room temperature with PE-labeled tetramers in RPMI 1640 and 8% human serum. After a wash with phosphate-buffered saline-0.1% fetal calf serum, staining was performed for 15 min in the dark by using FITC-, PerCP-, or APC-conjugated antibodies. The cells were then washed and analyzed immediately on a BD Biosciences flow cytometer (FACSCalibur) by using the CellQuest software. Tetramer-positive responses are reported as the percentage of tetramer-positive T cells among the total CD8 population. Frequencies of tetramer-positive cells exceeding 0.02% of CD8+ cells were considered to indicate positive responses. The expansion (n-fold) was calculated as the ratio between the percentage of tetramer-positive T cells after 10 days of in vitro stimulation and the percentage of tetramer-positive T cells detected ex vivo.
(ii) IFN-γ staining.
Tetramer-stained cells were incubated in medium alone (control) or with viral peptides (1 μM) for 1 h; brefeldin A (10 μg/ml) was added for an additional 18 h of incubation. After washing, the cells were stained with anti-CD8 PerCP-conjugated monocolonal antibody for 20 min at 4°C and then fixed and permeabilized as described above. Cells were finally stained with FITC-conjugated anti-IFN-γ for 15 min at room temperature, washed again, and analyzed by flow cytometry.
ELISPOT assays.
Enzyme-linked immunospot (ELISPOT) assays were performed using the panel of 315 15-mer peptides pooled in 16 mixtures as described above. HBV-specific T-cell responses were analyzed after overnight incubation with individual peptide mixtures of either PBMC (ex vivo analysis) or short-term polyclonal T-cell lines previously expanded in vitro by 10 days of stimulation with the same peptide mixtures used for the ELISPOT assays (in vitro analysis). Briefly, 96-well plates (Multiscreen-IP; Millipore S.A.S., Malshelm, France) were coated overnight at 4°C as recommended by the manufacturer with 5 μg/ml capture mouse anti-human IFN-γ monoclonal antibody (1DIK; Mabtech, Sweden). Plates were then washed seven times with phosphate-buffered saline-0.05% Tween 20 and blocked with RPMI 1640-10% fetal calf serum for 2 h at 37°C. PBMC (2 × 105/well) or T cells from short-term polyclonal T-cell lines (5 × 104/ well) were seeded in duplicate for each individual peptide mixture. Plates were incubated for 18 h at 37°C in the presence or absence of peptides. After a wash with phosphate-buffered saline-0.05% Tween 20, 50 μl of 1 μg/ml biotinylated secondary mouse anti-human IFN-γ monoclonal antibody (7B6-1; Mabtech, Sweden) was added. After 3 h of incubation at room temperature, plates were washed four times, 100 μl of goat alkaline phosphatase anti-biotin antibody (Vector Laboratories Inc., Burlingame, CA) was added to the wells, and the plates were incubated for a further 2 h at room temperature. Plates were then washed four times, and 75 μl of alkaline phosphatase-conjugated substrate (5-bromo-4-chloro-3-indolyl phosphate; Bio-Rad Laboratories, Hercules, CA) was added. After 4 to 7 min, the colorimetric reaction was stopped by washing with distilled water. Plates were air dried, and spots were counted using an automated ELISPOT reader (AID ELISPOT reader system; Autoimmune Diagnostika). Numbers of IFN-γ-secreting cells were expressed as the numbers of spot-forming cells per 2 × 105 cells. The number of specific IFN-γ-secreting cells was calculated by subtracting the value for the unstimulated control from the value for the stimulated sample. While unstimulated controls for ex vivo analysis were the same for all stimulated PBMC samples, distinct controls were set up for each individual polyclonal T-cell line derived from 10 days of PBMC stimulation with individual peptide mixtures. Positive controls consisted of PBMC stimulated with phytohemagglutinin. Wells were considered positive if they had values at least two times the background value and the number of spots was more than 10. To avoid the possibility of missing weakly positive wells, all in vitro experimental sample sets with a ratio of spot-forming cells in peptide-stimulated and unstimulated wells of more than 1.5 were retested by ICS. ICS was also applied to every positive sample to determine the T-cell subset (CD8+ or CD4+) responsible for IFN-γ production.
PD-1/PD-L1 blockage.
Adherent mononuclear cells isolated from PBMC of patients with chronic HBV infection were incubated for 45 min at 37°C with anti-PD-L1 (10 μg/ml) or isotype control antibody (e-Bioscience, Boston, MA). Cells were then washed and coincubated for 10 days with nonadherent cells and specific peptides (the HBV core peptide spanning amino acids 18 to 27 or pools of overlapping 15-mer peptides covering the overall HBV protein sequence) at the final concentration of 1 μM (IL-2 was added on day 4). On day 10, cells were stained with surface antibodies, tetramers, anti-IFN-γ, and anti-IL-2 and analyzed by flow cytometry.
Statistical analysis.
Levels of IFN-γ production in groups with different viremia levels were compared by using the Mann-Whitney test. The correlation between HBV DNA log10 values and frequencies of HBV-specific T cells was evaluated by using linear regression analysis and Spearman's correlation coefficient. The percentages of CD127+ cells and PD-1-positive cells among tetramer-positive CD8 lymphocytes in patients with acute and chronic HBV infections were compared by using the Mann-Whitney test. Frequencies of tetramer-positive CD8 cells and IFN-γ- and IL-2-producing CD8 T cells in samples from chronic patients upon peptide stimulation in the presence or absence of anti-PD-L1 antibody were compared by using the Wilcoxon paired test.
RESULTS
Longitudinal analysis of HBV-specific T-cell responses.
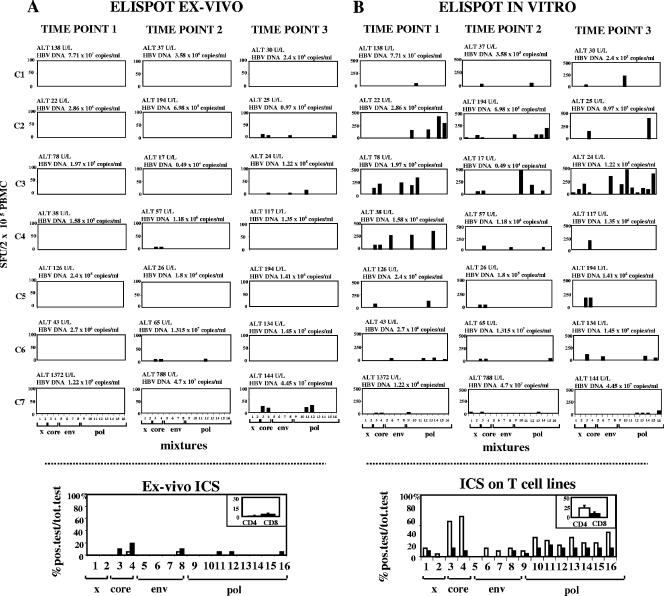
HBV-specific T-cell responses in seven anti-HBe-positive chronic hepatitis B patients (infected with HBV genotype D) with periodic disease flare-ups were tested longitudinally by using ELISPOT assays of samples ex vivo and after in vitro stimulation using a panel of overlapping 15-mer peptides covering all HBV proteins. Data from three time points characterized by different levels of HBV DNA and ALT in serum samples were analyzed for each patient (Fig. 1). For comparison, the T-cell responses in six patients with acute self-limited HBV infection were analyzed ex vivo at the time of clinical presentation and those in three of these patients were also analyzed sequentially from the time of acute illness. Despite the use of the comprehensive panel of HBV peptides, HBV-specific T-cell responses were very rarely observed ex vivo in samples from chronic HBV patients and, when detectable, they were always very weak (from 3 to 27 spots per 200,000 PBMC). IFN-γ-producing T cells were detectable at much higher frequencies ex vivo in samples from acute patients (12 to 350 spot-forming cells per 200,000 PBMC) (data not shown).
FIG. 1.
Results from ex vivo and in vitro ELISPOT assays for IFN-γ and the identification of the responding T-cell subsets in samples from patients with chronic HBV infection. (A) Ex vivo IFN-γ ELISPOT analysis was performed with samples from seven chronic hepatitis B patients (C1 through C7) studied longitudinally at three time points throughout a 12-month period by using 16 pools of 15-mer peptides covering the overall protein sequence of HBV genotype D. ALT and HBV DNA levels are expressed at the top of each histogram. (B) ELISPOT analysis was also performed after 10 days of in vitro stimulation with the same pools of peptides in the presence of IL-2. Samples from seven chronic hepatitis B patients were analyzed at the same time points as those assessed ex vivo. Levels of IFN-γ-producing cells are represented as numbers of spot-forming units (SFU) per 200,000 PBMC. The number of specific IFN-γ-secreting cells was calculated by subtracting the value for the unstimulated control from the value for the stimulated sample. The positive control consisted of PBMC stimulated with phytohemagglutinin. Each bar represents the response to an individual peptide mixture. The graph at the bottom of each panel indicates the responding T-cell subsets (CD4 and CD8) in the samples from the group of seven chronic hepatitis B patients. After the identification of the immunogenic peptide mixtures by ELISPOT assays, the responding T-cell subsets were defined by intracellular cytokine staining with individual peptide pools either ex vivo (A) or after 10 days of in vitro peptide stimulation (B). Chronic hepatitis B patients were studied at three time points throughout the study period. Each bar represents the percentage of total positive (pos.) tests with the indicated peptide pools. Bars in the insets represent the mean percentages (± standard errors) of positive responses expressed by CD4 and CD8 cells relative to the total (tot.) number of tests. x, x antigen peptide pools; env, envelope peptide pools; pol, polymerase peptide pools.
IFN-γ production was detected in samples from all anti-HBe-positive chronic patients after the in vitro expansion of T-cell populations, with the exception of patient C7, who developed a severe ALT flare-up with a particularly elevated and persistent increase of HBV viremia (more than 1 × 107 DNA copies/ml) (Fig. 1B). Virus-specific T-cell responses were generally directed against more than a single peptide pool and were more vigorous in samples from patients who showed levels of HBV DNA constantly below 1 × 106 to 1.5 × 106 copies/ml (patients C2, C3, and C4) than in those from patients with higher levels of HBV DNA (patients C1, C6, and C7) (Fig. 1B). Both CD8+ and CD4+ T cells contributed to the overall HBV-specific T-cell responses observed in samples from chronic patients. By using intracellular cytokine staining experiments, CD8+ T cells could be detected, even though at a very low frequency, directly ex vivo (Fig. 1A, bottom) while CD4+ T cells were detected only after in vitro population expansion (Fig. 1B, bottom).
Positive IFN-γ responses were directed mostly against HBV core and polymerase peptides, with maximal percentages of positive tests corresponding to CD4 T cells directed to core mixtures 3 and 4 (65% and 70%, respectively) (Fig. 1B, bottom).
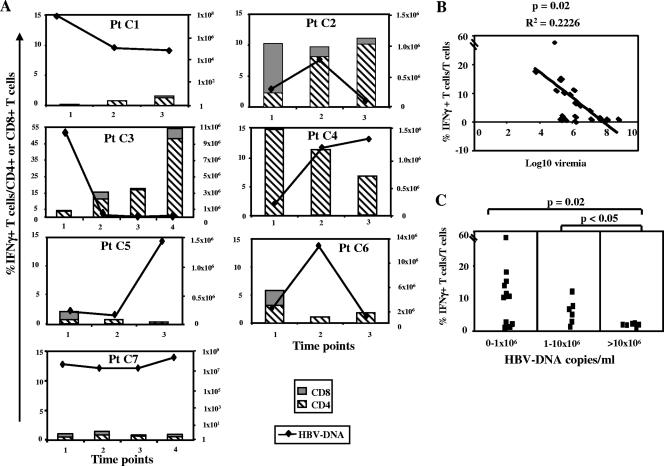
HBV-specific CD4+- or CD8+-T-cell responses in relation to the different virological profiles.
Levels of CD8+- and CD4+-T-cell responses in relation to HBV DNA values were analyzed by ICS (Fig. 2A). An inverse correlation between levels of T-cell responses and levels of viremia was observed by using linear regression (P = 0.02; R2 = 0.2226) (Fig. 2B) and Spearman's correlation (P = 0.0014) analyses (Fig. 2B). The strongest responses were detected in samples from patients C2, C3, and C4 when HBV DNA levels were <1 × 106 to 1.5 ×106 copies/ml. Also, in patients C1 and C5, responses were generally greater when viremia levels were lower, while this type of correlation was less clear in patients C6 and C7, who showed persistently elevated levels of HBV DNA throughout the follow-up period. HBV-specific CD4+- or CD8+-T-cell responses were weak or totally undetectable when HBV DNA levels were greater than 10 × 106 copies/ml, confirming previous data obtained by studying selected HBV-specific CD8 epitopes (34) (Fig. 2C).
FIG. 2.
Longitudinal analysis of HBV-specific T-cell responses in relation to the different virological profiles for anti-HBe-positive chronic patients. (A) IFN-γ production by CD4 and CD8 T cells from seven chronic hepatitis B patients (Pt C1 through Pt C7) was tested by ICS after 10 days of in vitro peptide stimulation; levels of HBV DNA were determined at the same time points. Each bar represents the total frequency of IFN-γ-positive cells among the overall CD4+ (striped bars) or CD8+ (gray bars) population at each individual time point as determined by summing the responses to each individual peptide pool as detected by ICS. Black lines indicate HBV DNA levels. Panel B illustrates the inverse relationship between IFN-γ production and the level of HBV viremia, as shown by linear regression analysis. Each square in panel C illustrates the percentage of IFN-γ-positive T cells among total T cells detected at different levels of viremia (HBV DNA, <1 × 106 copies/ml, 1 × 106 to 10 × 106 copies/ml, and >10 × 106 copies/ml). A statistically significant difference between the levels of IFN-γ production in the first (HBV DNA, <1 × 106 copies/ml) and third (HBV DNA, >10 × 106 copies/ml) groups and between the second (HBV DNA, 1 × 106 to 10 × 106 copies/ml) and third groups of HBV DNA profiles as determined by the Mann-Whitney test was observed.
Phenotypic and functional profile of HBV-specific CD8+ T cells.
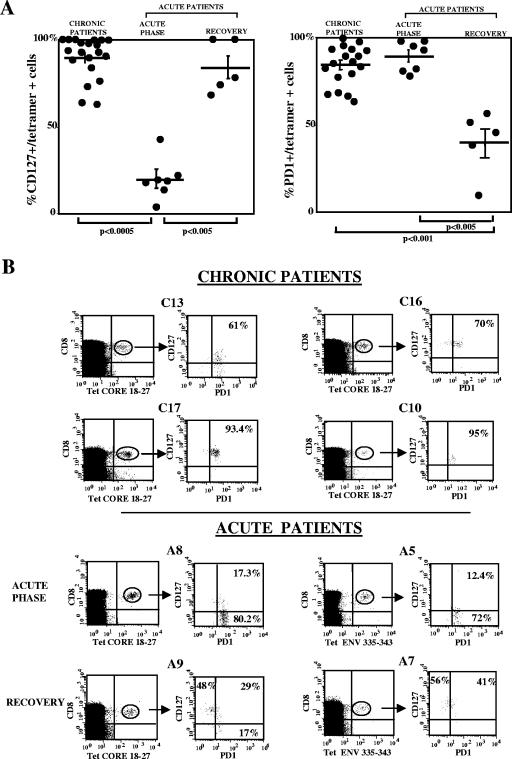
Phenotypes and functions of HBV-specific CD8+ cells from 12 anti-HBe-positive chronic hepatitis B patients and from seven patients with acute resolved hepatitis B were analyzed. All patients were HLA-A2 positive. Although six different HLA-A2 tetramers (the core peptide spanning amino acids 18 to 27, the envelope peptides spanning amino acids 183 to 191, 335 to 343, and 348 to 357, and the polymerase peptides spanning amino acids 575 to 583 and 816 to 824) were used, only low frequencies of CD8 cells specific for the core peptide spanning amino acids 18 to 27 were detected in samples from the chronic patients (0.02 to 0.08% of circulating CD8 lymphocytes). HBV core-specific CD8 cells were predominantly CD127 positive (63% to 100%; CD127 mean fluorescence intensity [MFI] expression, 11 to 103), with various levels of expression of CCR7 (proportions of cells with the CD127+, CCR7-positive pattern ranged from 24 to 90%), and were mostly PD-1 positive (64% to 100%; PD-1 MFI expression, 24.4 to 66) (Fig. 3A).
FIG. 3.
Phenotypic and functional profiles of tetramer-positive CD8 cells from patients with acute and chronic HBV infections. (A) Percentages of CD127+ cells and PD-1-positive cells among HBV tetramer-positive CD8 lymphocytes from 12 patients with anti-HBe-positive chronic hepatitis (three of them were tested at different time points) and from seven patients with acute HBV infection tested at two different time points, one corresponding to the acute phase of infection and the other at a later period (during the 2 to 15 months of follow-up after the clinical presentation). Percentages of CD127+, tetramer-positive cells in samples from chronic patients were significantly higher than those in samples from patients in the acute stage of infection (P, <0.0005 by Mann-Whitney), and those in samples from patients in the recovery phase of acute hepatitis were significantly higher than those in samples from patients in the acute phase of infection (P, <0.005 by Mann-Whitney); percentages of PD-1-positive, tetramer-positive cells in samples from chronic patients were significantly higher than those in samples from patients in the recovery phase of acute infection (P, <0.001 by Mann-Whitney), and those in samples from patients in the acute phase of infection were significantly higher than those in samples from patients in the recovery phase (P, <0.005 by Mann-Whitney). (B) Representative dot plots for HBV tetramer (Tet)-positive cells from four patients with chronic HBV infection (top) and from two patients in the acute phase of hepatitis and at the time of recovery (bottom) stained with anti-CD8, anti-PD-1, and anti-CD127 monoclonal antibodies. Because of the very low frequency of tetramer-positive cells in chronic HBV patients, a large number of events (>1 × 106) was analyzed by flow cytometry to allow for a reliable phenotypic analysis. Core 18-27, core peptide spanning amino acids 18 to 27; ENV 335-343, envelope peptide spanning amino acids 335 to 343.
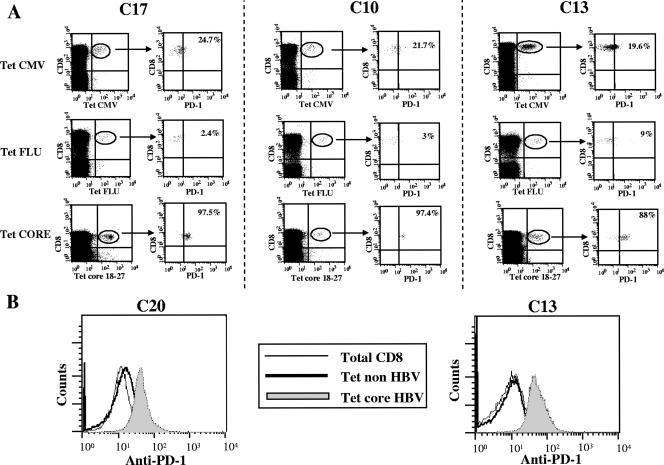
PD-1 expression tended to be higher when HBV DNA levels were lower, but the inverse relationship was not statistically significant (data not shown). By staining CD8 cells simultaneously with antibodies to CD127 and PD-1 molecules, a high frequency of HBV-specific CD8 cells was shown to coexpress both molecules, with a CD127+, PD-1-positive pattern among 55% to 98% of tetramer-positive CD8 cells (Fig. 3B). PD-1 up-regulation in chronic HBV patients was selective for HBV-specific CD8 cells since the frequencies of PD-1-positive cells (Fig. 4A) and the intensities of PD-1 expression (Fig. 4B) were significantly lower among CMV- and influenza virus-specific CD8+ cells as well as among total CD8 cells derived from these patients. The frequency of PD-1-positive CD8 cells among influenza virus- and CMV-specific CD8 cells ranged from 2.4 to 29%. For the frequency of PD-1-positive, HBV-specific cells versus that of PD-1-positive, non-HBV-specific CD8 cells, the P value was <0.0001. PD-1 MFI expression on influenza virus- and CMV-specific CD8 cells ranged from 10 to 17 and from 14.4 to 19.8, respectively; for the PD-1 MFI expression among HBV-specific CD8 cells versus that among non-HBV-specific CD8 cells, the P value was <0.0001.
FIG. 4.
PD-1 expression on HBV-, CMV-, and influenza virus-specific CD8+ T cells. (A) Dot plots representative of PD-1 expression on CMV-, influenza virus (FLU)-, and HBV-specific tetramer (Tet)-positive cells are shown for three representative patients with chronic HBV infection. (B) The PD-1 fluorescence intensities expressed by total CD8+ cells and non-HBV-specific (CMV-specific on the left and influenza virus-specific on the right) and HBV-specific tetramer-positive CD8 cells are illustrated for two representative chronic HBV patients. Core 18-27, core peptide spanning amino acids 18 to 27.
In samples from patients with acute hepatitis, HBV-specific CD8 cells were PD-1 positive at frequencies comparable to those observed in samples from chronic patients, ranging from 78 to 98% (Fig. 3A) during the acute stage of disease. However, the percentages of PD-1-positive HBV-specific CD8 cells declined after the resolution of the infection to levels ranging from 8 to 55% (Fig. 3A). On the other hand, the level of expression of CD127 on HBV-specific T cells was markedly low at the time of the ALT peak (4% to 43% of the tetramer-positive CD8 population) (Fig. 3A). A progressive increase in the level of CD127 expression on tetramer-positive CD8 cells was then observed from the time of the ALT peak (mean frequency of CD127-positive cells, 23%) to later times of disease resolution, with values ranging from 68% to 100% (mean frequency, 85.5%) (Fig. 3A).
Blocking of PD-1/PD-L1 engagement on HBV-specific CD8+ T cells.
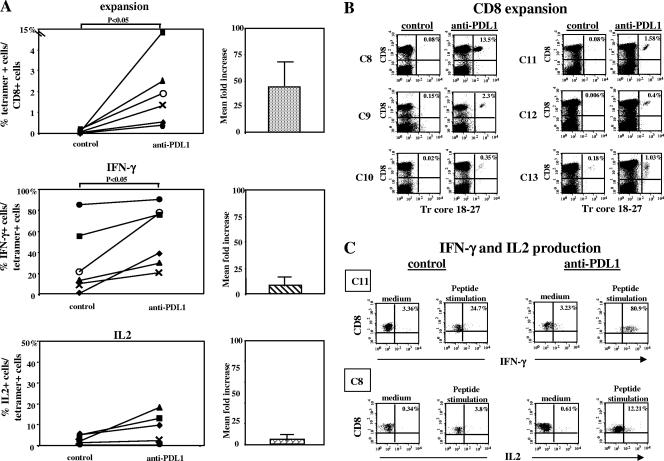
The high level of PD-1 expression on HBV-specific CD8 cells suggests that exhaustion by persistent exposure to high antigen concentrations may play an important role in the pathogenesis of the HBV-specific CD8 dysfunction. To further investigate this hypothesis, HBV-specific CD8 population expansion and cytokine production were tested after 10 days of in vitro peptide stimulation in the presence or absence of anti-PD-L1 antibody to block PD-1/PD-L1 engagement. As shown in Fig. 5A and B, PD-1/PD-L1 blockage significantly enhanced the capacity for expansion of populations of CD8 cells specific for the core peptide spanning amino acids 18 to 27, and this functional recovery was observed in samples from all six chronic patients tested. The increases in the frequencies of tetramer-positive cells in the presence of anti-PD-L1 antibodies ranged from 5.7- to 168-fold compared to the levels detected in control cultures with irrelevant antibodies (mean increase, 44.3-fold; frequency difference, P < 0.05 by Wilcoxon paired test). PD-1/PD-L1 blockage also enhanced IL-2 and IFN-γ production by HBV-specific CD8 cells. Various increases in the frequencies of tetramer-positive CD8 cells able to produce IFN-γ in the presence of anti-PD-L1 antibodies were observed in samples from the six patients studied (1.05- to 37-fold; mean, 7.6-fold; difference between cultures with anti-PD-L1 and those with control antibody, P < 0.05 by Wilcoxon paired test) (Fig. 5A and C). Although an improvement in IFN-γ production was detectable in samples from all patients, this CD8 function was less affected by PD-1/PD-L1 blockage than was the capacity for expansion (Fig. 5A). Finally, only in samples from patients C8 and C9 was an improvement in IL-2 production, of 9.1- and 3.3-fold, induced by PD-1/PD-L1 blockage, while no effect in samples from the other patients was observed (Fig. 5A and C). No significant correlation between levels of PD-1 expression and levels of improvement of T-cell responses induced by anti-PD-L1 antibodies was found, although functional restoration tended to be better when the level of PD-1 expression on T cells was lower.
FIG. 5.
Effect of anti-PD-L1 antibody on the function of HBV-specific tetramer-positive CD8+ T cells. (A) Frequencies of tetramer-positive CD8 cells in samples from six patients with chronic HBV infection were analyzed after 10 days of peptide stimulation in the presence of anti-PD-L1 or control antibodies (P, <0.05 by the Wilcoxon paired test) (top). The mean increase (n-fold) in the frequency of tetramer-positive cells among cells cultured with anti-PD-L1 antibody with respect to the frequency of tetramer-positive cells among cells cultured with the control antibody is shown in the graph at the right. The middle and bottom parts of panel A illustrate the percentages of HBV-specific CD8 T cells able to produce IFN-γ and IL-2 as detected by intracellular cytokine staining upon the restimulation of T-cell cultures derived from 10 days of HBV peptide stimulation in the presence of anti-PD-L1 or control antibodies (difference for IFN-γ by the Wilcoxon paired test, P < 0.05). Pooled data are expressed as mean increases (n-fold) in the percentages of IFN-γ- and IL-2-producing CD8 T cells derived from cultures performed in the presence of anti-PD-L1 antibody with respect to those derived from cultures performed in the presence of the control antibody (graphs at the right). (B) Dot plots representative of frequencies of CD8 cells positive for the tetramer core peptide spanning amino acids 18 to 27 (Tr core 18-27) after 10 days of HBV peptide stimulation in the presence of anti-PD-L1 or control antibodies. (C) Dot plots representative of IFN-γ and IL-2 production by T-cell lines induced by 10 days of HBV peptide stimulation in the presence of anti-PD-L1 or control antibodies in samples from two representative chronic hepatitis B patients (C11 and C8).
Effects of PD-1/PD-L1 blockage on the global HBV-specific T-cell reactivity.
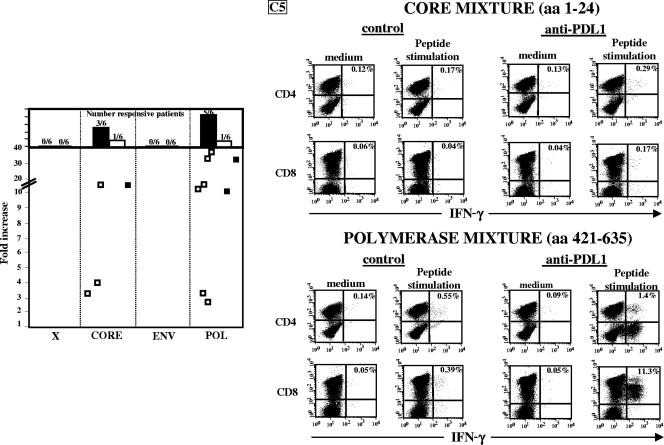
We then tested whether PD-1/PD-L1 blockage can affect the function of HBV-specific T cells of different specificities. PBMC from chronic patients were stimulated with the peptide pools covering the overall HBV sequence in the presence or absence of anti-PD-L1 antibodies, and the frequencies of IFN-γ peptide-specific T cells were assessed after 10 days of culture. Not only CD8 but also CD4 responses improved upon anti-PD-L1 incubation. Differential increases in IFN-γ production were detected for different HBV T-cell specificities. Responses to only some HBV core and polymerase pools showed a significant improvement (3.2- to 15-fold and 2.8- to 37-fold for core and polymerase pools, respectively) (Fig. 6), while in none of the samples from six chronic patients did anti-PD-L1 antibodies improve CD4 or CD8 responses directed against HBV x and envelope peptides (Fig. 6).
FIG. 6.
Effect of anti-PD-L1 antibody on the HBV-specific T-cell function analyzed with peptides spanning the entire HBV protein sequence. We determined the effect of anti-PD-L1 antibody on IFN-γ production by CD4 and CD8 T cells after the expansion of T-cell populations by 10 days of in vitro stimulation with individual peptide pools covering the overall HBV sequence in the presence of anti-PD-L1 or control antibodies. Each individual culture obtained after 10 days of stimulation with each peptide pool was incubated for 18 h in the presence of the corresponding peptide pool or medium alone, and cytokine production was tested by ICS as described in Materials and Methods. Each dot or point represents the increase (n-fold) in IFN-γ production induced by anti-PD-L1 antibody, calculated as the ratio between the percentages of cytokine-producing T cells in cultures expanded with peptide pools in the presence of anti-PD-L1 and those expanded in the presence of control antibodies. For these experiments, polymerase (POL) peptides were pooled in four mixtures, envelope (ENV) peptides were pooled in two mixtures, and core and x peptides were pooled in individual mixtures. Only responses to peptide pools that were increased at least two times by incubation with anti-PD-L1 antibodies are illustrated. □, CD4 cells; ▪, CD8 cells. The panels on the right show dot plots representative of IFN-γ-producing CD4 and CD8 T cells induced by the indicated peptide pools in the presence of anti-PD-L1 or control antibodies. aa, amino acids.
DISCUSSION
Available data indicate that HBV-specific T-cell responses in the chronic stage of HBV infection are functionally impaired and much weaker than those detectable in acute self-limited infection (4, 9, 12, 13, 21, 29). However, studies of the CD8-mediated response have been carried out with a limited number of peptides (3, 16, 18, 21, 25, 27, 28, 34) while CD4 analysis using recombinant proteins has been focused on structural HBV proteins (9, 12, 13, 26, 31) and limited information is available about CD4-mediated responses to polymerase (17) and HB x antigen (11) proteins.
Here, we used a panel of overlapping 15-mer peptides covering the overall protein sequence of HBV genotype D, which allowed us to detect both CD4- and CD8-mediated responses directed against all structural and nonstructural HBV regions. We longitudinally studied anti-HBe-positive patients chronically infected with genotype D HBV who experienced periodic reactivations of disease and HBV replication in order to correlate the intensity and breadth of the HBV-specific T-cell repertoire with levels of virus replication and disease activity.
The first finding of our work is that despite the use of a comprehensive panel of HBV peptides, peripheral blood CD8- and CD4-mediated T-cell responses were weaker in samples from chronic patients than in those from patients with acute hepatitis who resolved the infection. This finding was also true when responses were studied in connection with disease flare-ups at the times of ALT elevations. In general, HBV-specific T cells were rarely detected directly ex vivo in samples from chronic patients in contrast to those from patients with acute HBV infection. HBV-specific T cells from chronic patients were demonstrated mainly after in vitro expansion, and the strengths of HBV-specific T-cell responses correlated with the levels of HBV viremia. Thus, in keeping with the results in a previous report by Webster et al. (34), our comprehensive analysis showed an inverse correlation between the intensities of peripheral blood HBV-specific T-cell responses and levels of viremia as well as a lack of association between disease flare-ups and an increase in peripheral blood T-cell responses. Having demonstrated that the viral load can directly influence the HBV-specific T-cell repertoire, we then analyzed whether persistent antigenic exposure had an influence on the phenotypes and functions of HBV-specific CD8+ T cells. Recent studies indicate that exhausted T cells chronically exposed to high antigen loads express PD-1 (8, 14, 19, 23) and that the blocking of PD-1/PD-L1 engagement allows for the recovery of T-cell functions (1, 7, 23, 30, 32). In line with these observations derived from work with other viral infections, we found that circulating HBV-specific CD8 cells in chronic HBV patients were mainly PD-1 positive. Most of these cells coexpressed the CD127 molecule, and this phenotype appeared to be stably expressed without detectable changes in relation to fluctuations in levels of viremia. While this high level of CD127 expression contrasts with data from other persistent human viral infections (8, 20, 30, 33), a similarly high level of CD127 expression on hepatitis C virus-specific CD8 cells in chronic hepatitis C has recently been reported (2, 22, 24), suggesting similar mechanisms of virus-specific CD8 cell failure in HBV and hepatitis C virus infections. This profile of PD-1-CD127 coexpression differed from the phenotypes of influenza virus-specific CD127+, CD8+ memory cells derived from the same patients with chronic HBV infection, which were virtually all PD-1 negative. Thus, PD-1 expression on HBV-specific CD8+ cells was likely due to the chronically high level of HBV antigenic stimulation, since PD-1 was highly expressed only on HBV-specific CD8+ T cells but not on CMV- and influenza virus-specific CD8+ T cells. Moreover, low levels of PD-1 expression were detected in the resolution phase of infection in association with the control of HBV replication and a progressive improvement of the capacity of CD8 cell populations to expand in vitro following peptide stimulation, similar to the results recently reported by Boettler et al. (5).
Importantly, the blocking of the PD-1/PD-L1 interaction by anti-PD-L1 antibodies enhanced, with various levels of efficiency, the capacity of HBV-specific T-cell populations to expand and to produce cytokines in samples from all chronic HBV patients tested. Thus, in addition to those to lymphocyte choriomeningitis virus (1), HIV (8, 23, 30), and hepatitis C virus (32) infections, the blocking of PD-1/PD-L1 is able to improve, at least in vitro, the efficiency of the antiviral immune response to chronic HBV infection, confirming the importance of the PD-1/PD-L1 pathway during chronic viral infections.
We also observed that T cells of different antigen specificities were differentially affected by HBV persistence. The HBV-specific T-cell responses detectable in patients with chronic HBV infection were primarily those directed against core and polymerase peptides, with an almost complete lack of reactivity against envelope sequences. This finding is in contrast with the common detection of envelope-specific T cells in patients with acute hepatitis B. The existence of this hierarchy of exhaustion among T cells specific for different HBV antigens was also confirmed by PD-1/PD-L1 blocking experiments. A functional restoration of the HBV-specific T-cell response by anti-PD-L1 antibodies primarily affected core- and polymerase-specific T cells, without a detectable effect of anti-PD-L1 antibodies on envelope specificities. These results may reflect a deeper level of exhaustion or deletion of HBV envelope-specific T cells due to the extremely high envelope antigen concentration constantly present in the chronic stage of HBV infection.
Taken together, our data show a hierarchical loss of the HBV-specific T-cell repertoire, which is likely influenced by the levels of HBV replication. The existence of this gradient of exhaustion among T cells specific for different HBV antigens will need to be confirmed with larger groups of patients with chronic HBV infection and a diverse HLA genetic background. Different factors, like T-cell receptor avidity and the quantity of HBV epitopes generated by antigen processing and presented by different HLA molecules, might affect the results obtained. This hierarchy of T-cell exhaustion in chronic HBV patients can have important implications for the development of rational immunological strategies to treat chronic HBV infection.
Acknowledgments
The study was supported by grant RBNE013PMJ_006 from FIRB (Fondo per gli Investimenti della Ricerca di Base)-MIUR (Ministry of Education, University and Research), by grant no. 120 (Progetto di Ricerca Finalizzato) from the Ministry of Health, Italy, by the VIRGIL EC grant QLK2-CT-2002-00700, by Schering-Plough S.p.A., Milan, Italy, and by Fondazione Cassa Risparmio di Parma, Parma, Italy.
Footnotes
Published ahead of print on 7 February 2007.
REFERENCES
- 1.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439:682-687. [DOI] [PubMed] [Google Scholar]
- 2.Bengsch, B., H. C. Spangenberg, N. Kersting, C. Neumann-Haefelin, E. Panther, F. von Weizsacker, H. E. Blum, H. Pircher, and R. Thimme. 2007. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J. Virol. 81:945-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertoletti, A., C. Ferrari, F. Fiaccadori, A. Penna, R. Margolskee, H. J. Schlicht, P. Fowler, S. Guilhot, and F. V. Chisari. 1991. HLA class I-restricted human cytotoxic T cells recognize endogenously synthesized hepatitis B virus nucleocapsid antigen. Proc. Natl. Acad. Sci. USA 88:10445-10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertoletti, A., A. Costanzo, F. V. Chisari, M. Levrero, A. Artini, A. Sette, A. Penna, T. Giuberti, F. Fiaccadori, and C. Ferrari. 1994. Cytotoxic T lymphocyte response to a wild type hepatitis B virus epitope in patients chronically infected by variant viruses carrying substitutions within the epitope. J. Exp. Med. 180:933-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boettler, T., E. Panther, B. Bengsch, N. Nazarova, H. C. Spangenberg, H. E. Blum, and R. Thimme. 2006. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J. Virol. 80:3532-3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunetto, M. R., F. Oliveri, B. Coco, G. Leandro, P. Colombatto, J. Monti Gorin, and F. Bonino. 2002. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J. Hepatol. 36:263-270. [DOI] [PubMed] [Google Scholar]
- 7.Cai, G., A. Karni, E. M. Oliveira, H. L. Weiner, D. A. Hafler, and G. J. Freeman. 2004. PD-1 ligands, negative regulators for activation of naïve, memory, and recently activated human CD4+ T cells. Cell. Immunol. 230:89-98. [DOI] [PubMed] [Google Scholar]
- 8.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. R. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443:350-354. [DOI] [PubMed] [Google Scholar]
- 9.Ferrari, C., A. Penna, A. Bertoletti, A. Valli, A. Degli Antoni, T. Giuberti, A. Cavalli, M.-A. Petit, and F. Fiaccadori. 1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 145:3442-3449. [PubMed] [Google Scholar]
- 10.Hadziyannis, S. J., and D. Vassilopoulos. 2001. Hepatitis B e antigen-negative chronic hepatitis B. Hepatology 34:617-621. [DOI] [PubMed] [Google Scholar]
- 11.Jung, M. C., M. Stemler, T. Weimer, U. Spengler, J. Dohrmann, R. Hoffmann, D. Eichenlaub, J. Eisenburg, G. Paumgartner, G. Riethmuller, et al. 1991. Immune response of peripheral blood mononuclear cells to HBx-antigen of hepatitis B virus. Hepatology 13:637-643. [PubMed] [Google Scholar]
- 12.Jung, M.-L., B. Hartmann, J.-T. Gerlach, H. Diepolder, R. Gruber, W. Schraut, N. Gruner, R. Zachoval, R. Hoffmann, T. Santantonio, M. Wachter, and G. R. Pape. 1999. Virus-specific lymphokine production differs quantitatively but not qualitatively in acute and chronic hepatitis B infection. Virology 261:165-172. [DOI] [PubMed] [Google Scholar]
- 13.Jung, M.-L., U. Spengler, W. Schraut, R. Hoffmann, R. Zachoval, J. Eisemburg, D. Eichenlaub, G. Riethmuller, G. Paumgartner, H. W. L. Ziegler-Heitbrock, H. Will, and G. R. Pape. 1991. Hepatitis B virus antigen-specific T cell activation in patients with acute and chronic hepatitis B. J. Hepatol. 13:310-317. [DOI] [PubMed] [Google Scholar]
- 14.Latchman, Y. E., S. C. Liang, Y. Wu, T. Chernova, R. A. Sobel, M. Klemm, V. K. Kuchroo, G. J. Freeman, and A. H. Sharpe. 2004. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissue negatively regulates T cells. Proc. Natl. Acad. Sci. USA 191:10691-10696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maini, M. K., C. Boni, G. S. Ogg, A. S. King, S. Reignat, C. K. Lee, J. R. Larrubia, G. J. M. Webster, A. J. McMichael, C. Ferrari, R. Williams, D. Vergani, and A. Bertoletti. 1999. Direct ex vivo analysis of hepatitis B virus-specific CD8+ T cells associated with the control of infection. Gastroenterology 117:1386-1396. [DOI] [PubMed] [Google Scholar]
- 16.Missale, G., A. Redeker, J. Person, P. Fowler, S. Guilhot, H. J. Schlicht, C. Ferrari, and F. V. Chisari. 1993. HLA-A31- and HLA-Aw68-restricted cytotoxic T cell responses to a single hepatitis B virus nucleocapsid epitope during acute viral hepatitis. J. Exp. Med. 177:751-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizukoshi, E., J. Sidney, B. Livingston, M. Ghany, J. H. Hoofnagle, A. Sette, and B. Rehermann. 2004. Cellular immune responses to the hepatitis B virus polymerase. J. Immunol. 173:5863-5871. [DOI] [PubMed] [Google Scholar]
- 18.Nayersina, R., P. Fowler, S. Guilhot, G. Missale, A. Cerny, H. J. Schlicht, A. Vitiello, R. Chesnut, J. L. Person, and A. G. Redeker. 1993. HLA A2 restricted cytotoxic T lymphocyte responses to multiple hepatitis B surface antigen epitopes during hepatitis B virus infection. J. Immunol. 150:4659-4671. [PubMed] [Google Scholar]
- 19.Okazaki, T., and T. Honjo. 2006. The PD-1-PD-L pathway in immunological tolerance. Trends Immunol. 27:195-201. [DOI] [PubMed] [Google Scholar]
- 20.Paiardini, M., B. Cervasi, H. Albrecht, A. Muthukumar, R. Dunham, S. Gordon, H. Radziewicz, G. Piedimonte, M. Magnani, M. Montroni, S. M. Kaech, A. Weintrob, J. D. Altman, D. L. Sodora, M. B. Feinberg, and G. Silvestri. 2005. Loss of CD127 expression of effector CD8+ T cells in HIV-infected individuals. J. Immunol. 174:2900-2909. [DOI] [PubMed] [Google Scholar]
- 21.Penna, A., F. V. Chisari, A. Bertoletti, G. Missale, P. Fowler, T. Giuberti, F. Fiaccadori, and C. Ferrari. 1991. Cytotoxic T lymphocytes recognize an HLA-A2-restricted epitope within the hepatitis B virus nucleocapsid antigen. J. Exp. Med. 174:1565-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Penna, A., M. Pilli, A. Zerbini, A. Orlandini, S. Mezzadri, L. Sacchelli, G. Missale, and C. Ferrari. 2007. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 45:588-601. [DOI] [PubMed]
- 23.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 203:2281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radziewicz, H., C. C. Ibegbu, M. L. Fernandez, K. A. Workowski, K. Obideen, M. Wehbi, H. L. Hanson, J. P. Steinberg, D. Masopust, E. J. Wherry, J. D. Altman, B. T. Rouse, G. J. Freeman, R. Ahmed, and A. Grakoui. 2006. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 81:2545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rehermann, B., P. Fowler, J. Sidney, J. Person, A. Redeker, M. Brown, B. Moss, A. Sette, and F. V. Chisari. 1995. The cytotoxic T lymphocyte response to multiple hepatitis B virus polymerase epitopes during and after acute viral hepatitis. J. Exp. Med. 181:1047-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossol, S., G. Marinos, P. Carucci, M. V. Singer, R. Williams, and N. V. Naoumov. 1997. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J. Clin. Investig. 99:3025-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sing, G. K., A. Ladhams, S. Arnold, H. Parmar, X. Chen, J. Cooper, L. Butterworth, K. Stuart, D. D'Arcy, and W. G. E. Cooksley. 2001. A longitudinal analysis of cytotoxic T lymphocyte precursor frequencies to the hepatitis B virus in chronically infected patients. J. Viral Hepat. 8:19-29. [DOI] [PubMed] [Google Scholar]
- 28.Sobao, Y., K. Sugi, H. Tomiyama, S. Saito, S. Fujiama, M. Morimoto, S. Hasuike, H. Tsubouchi, K. Tanaka, and M. Takiguchi. 2001. Identification of hepatitis B virus-specific CTL epitopes presented by HLA-A*2402, the most common HLA class I allele in East Asia. J. Hepatol. 34:922-929. [DOI] [PubMed] [Google Scholar]
- 29.Sobao, Y., H. Tomiyama, K. Sugi, M. Tokunaga, T. Ueno, S. Saito, S. Fujiama, M. Morimoto, K. Tanaka, and M. Takiguchi. 2002. The role of hepatitis B virus-specific memory CD8 T cells in the control of viral replication. J. Hepatol. 36:105-115. [DOI] [PubMed] [Google Scholar]
- 30.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimming, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 12:1198-1202. [DOI] [PubMed] [Google Scholar]
- 31.Tsai, S. L., P. J. Chen, M. Y. Yang, J. L. Sung, J. H. Huang, L. H. Hwang, T. H. Chang, and D. S. Chen. 1992. Acute exacerbations of chronic type B hepatitis are accompanied by increased T cell responses to hepatitis B core and e antigens. J. Clin. Investig. 89:87-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urbani, S., B. Amadei, D. Tola, M. Massari, S. Schivazzappa, G. Missale, and C. Ferrari. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 80:11398-11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Leeuwen, E. M. M., G. J. de Bree, E. B. M. Remmerswaal, S. L. Yong, K. Tesselaar, I. J. M. ten Berge, and R. A. W. van Lier. 2005. IL-7 receptor alpha chain expression distinguishes functional subsets of virus-specific human CD8+ T cells. Blood 106:2091-2098. [DOI] [PubMed] [Google Scholar]
- 34.Webster, G. J. M., S. Reignat, D. Brown, G. S. Ogg, L. Jones, S. L. Seneviratne, R. Williams, G. Dusheiko, and A. Bertoletti. 2004. Longitudinal analysis of CD8+ T cells specific for structural and nonstructural hepatitis B virus proteins in patients with chronic hepatitis B: implications for immunotherapy. J. Virol. 78:5707-5719. [DOI] [PMC free article] [PubMed] [Google Scholar]