Abstract
Herpes simplex virus type 1 (HSV-1) envelope proteins are posttranslationally modified by the addition of sialic acids to the termini of the glycan side chains. Although gC, gD, and gH are sialylated, it is not known whether sialic acids on these envelope proteins are functionally important. Digestion of sucrose gradient purified virions for 4 h with neuraminidases that remove both α2,3 and α2,6 linked sialic acids reduced titers by 1,000-fold. Digestion with a α2,3-specific neuraminidase had no effect, suggesting that α2,6-linked sialic acids are required for infection. Lectins specific for either α2,3 or α2,6 linkages blocked attachment and infection to the same extent. In addition, the mobility of gH, gB, and gD in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels was altered by digestion with either α2,3 specific neuraminidase or nonspecific neuraminidases, indicating the presence of both linkages on these proteins. The infectivity of a gC-1-null virus, ΔgC2-3, was reduced to the same extent as wild-type virus after neuraminidase digestion, and attachment was not altered. Neuraminidase digestion of virions resulted in reduced VP16 translocation to the nucleus, suggesting that the block occurred between attachment and entry. These results show for the first time that sialic acids on HSV-1 virions play an important role in infection and suggest that targeting virion sialic acids may be a valid antiviral drug development strategy.
Herpes simplex virus type 1 (HSV-1) is a widespread human pathogen with 70 to 90% of the adults in the United States testing seropositive for the virus (92). The most common manifestation is mucous membrane infection resulting in ulcerative lesions that are usually self-limiting in immunocompetent individuals. However, serious illnesses, including lethal neonatal HSV, encephalitis, and blinding keratitis, can occur (43, 49, 83, 92). Primary and recurrent infections in the immunocompromised, such as transplant recipients, those on chemotherapy, or those infected with human immunodeficiency virus (HIV) can be life-threatening (64, 80, 94). A number of antivirals are approved for HSV-1 treatment (21, 39, 60), but they are not completely effective. One significant problem in dealing with HSV infections is the ability of the virus to persist in the host as a latent infection (65). None of the currently available antivirals can eliminate a latent infection. Preventing the establishment of a persistent infection, which could be accomplished either by blocking infection or the establishment of latency, would be an ideal strategy for dealing with this virus.
The development of agents to block HSV infection requires a greater understanding of HSV entry. Infection is initiated by the binding of viral glycoprotein C (gC) or gB to cell surface heparan sulfate proteoglycans (37, 73, 74). After attachment, gD can bind to any of several cellular receptors including herpes virus entry mediator, nectin-1, nectin-2, or 3-O-sulfated heparan sulfate (17, 30, 54, 70, 84), triggering a conformational change in gD (18, 28, 48). The conformational change in gD is thought to be required for the assembly of the entry-fusion complex which consists of gD, gB, and the gH-gL heterodimer. Recent evidence suggests that gB is recruited to the complex first, followed by gH-gL (32, 62). The gB protein functions as a trimer and appears to undergo a conformational change during entry but lacks features characteristic of a number of viral fusion proteins (35). The gH protein contains sequences similar to known fusion proteins, including a peptide fusion loop and two heptad repeats, suggesting that gH may be the actual fusion protein (31). HSV-1 gB reportedly binds to cell surface receptors, but the identity of these receptors is unknown, and their significance for fusion and entry is not clear (10). HSV-1 gH has also been reported to bind to a cell surface receptor, αvβ3 integrin, but the significance of this binding is unknown (59, 67). It is clear that more needs to be learned about the function of gD, gB, and gH in fusion and entry.
Viral envelope glycoproteins are synthesized and processed through the cellular exocytic pathway and modified through glycosylation by host cell enzymes. These modifications include the addition of sialic acid residues in the trans-Golgi compartment. The predominant terminal carbohydrate on glycans in mammalian cells are α2,3- or α2,6-linked sialic acids. Sialic acids have a stabilizing effect on glycoproteins and enzymatic desialylation often results in significant changes in the structure and function of these proteins (13, 27, 40, 46, 47, 58, 82). Previous studies have shown that gC, gD, and gH are sialylated but the specific linkages and possible functions of sialic acids on these proteins have not been determined (11, 22, 25, 51, 61, 68). The goal of the present study was to determine whether sialylation of HSV-1 envelope proteins is important for infectivity. The data suggest that α2,6-linked sialic acids on one or more HSV entry proteins are required for viral entry into cells.
MATERIALS AND METHODS
Cell culture and viruses.
All studies were carried out in Vero cells (ATCC CLL-81) or Hep-2 cells (ATCC CCL-23) cultured in Dulbecco modified Eagle medium supplemented with 5% calf serum and 5% fetal bovine serum (34). Experiments requiring prolonged incubations at 4°C were performed with cells on poly-l-lysine (P4707; Sigma-Aldrich, Inc., St. Louis, MO)-coated plates. Microscopic examination was used to confirm the presence of stable cell layers throughout each experiment. For some experiments the growth medium was buffered with 25 mM HEPES (pH 7.3) in place of carbonate.
HSV-1 KOS and a β-galactosidase-expressing variant, hrR3, were used for the majority of the studies (33, 34). For studies involving the role of gC, ΔgC2-3, a mutant virus expressing β-galactosidase in place of gC, and ΔgC2-3rev, a rescued virus, were used (38, 87, 88). High-titer viral stocks were produced in Vero or Hep-2 cells as described previously (34). Purification of virions was carried out with sucrose gradients as we described previously (87) with minor modifications (50). The titers of viral stocks were determined by plaque assay on Vero cells.
Enzymatic digestion of virions and cells.
The carbohydrate-digesting enzymes used in these studies and their linkage specificities are shown in Table 1. The α2,3 specific neuraminidase (NEB 2-3) has a 260-fold preference for α2,3 compared to α2,6 linkages (42). The Vibrio cholerae and Arthrobacter ureafaciens enzymes digest both linkages with perhaps a slight preference for α2,6 linkages (1, 85, 86). For digestion, high-titer viral stocks were diluted into identical final volumes in 1× enzyme buffer as specified by the manufacturer. The amount of neuraminidase used was 0.01 to 0.04 U for V. cholerae, 0.1 to 0.4 U for A. ureafaciens, and 500 to 2,000 U for NEB 2-3 as determined by the suppliers. Each digestion contained 2 × 106 to 2 × 108 PFU in 200 to 600 μl of buffer. The virions were then incubated at 37°C for either 1 h or 4 h as noted in the text and then diluted 1,000-fold prior to determining the titers on Vero or Hep-2 cells. Controls included mock-treated virus or enzymes added just prior to the assay to minimize digestion. For cell treatments, confluent Vero cell monolayers in six-well plates were exposed to 0.2 U of A. ureafaciens, 0.02 U of V. cholerae, or 1,000 U of NEB 2-3 for 1 h at 37°C in Dulbecco modified Eagle medium with 2% serum. The cells were rinsed once with medium and then infected with virus.
TABLE 1.
Neuraminidases and lectins used in this study
| Enzyme or lectin | Description | Specificity | Manufacturer | Catalog no. |
|---|---|---|---|---|
| V. cholerae neuraminidase | α2,6/α2,3 | Roche | 11 080 725 001 | |
| A. ureafaciens neuraminidase | α2,6/α2,3 | Roche | 10 269 611 001 | |
| NEB 2-3 | S. enterica serovar Typhimurium LT2 neuraminidase | α2,3 | New England Biolabs | P0728L |
| ELD | Sialic acid binding lectin | α2,6 | Vector Laboratories | B-1305 |
| MAL1 | Sialic acid binding lectin | α2,3 | Vector Laboratories | B-1315 |
Viral attachment.
Viral attachment to cells was measured by using a cell-based enzyme-linked immunosorbent assay (CELISA). Vero cells were plated in poly-l-lysine-coated 96-well culture plates at a density of 104 cells per well. Three days later, the cells were incubated with control virions, digested virions, or virions incubated with either Maackia amurensis lectin (MAL1) or Elderberry bark lectin (ELD) (Table 1) at 4°C for 1 h. The MAL1 lectin has a 40-fold preference for α2,3-linked sialic acid over α2,6 linkages (89). The ELD lectin has a 50- to 125-fold preference for α2,6 over α2,3 linkages (69). The cells were then rinsed with ice-cold phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde in PBS for 10 min at room temperature, and rinsed three times with PBS. The wells were blocked with 1% bovine serum albumin (catalog no. 160069; ICN Biochemicals, Cleveland, OH) in PBS and incubated with rabbit HSV-1 specific polyclonal antiserum (B 0114; Dakocytomation, Glostrup, Denmark) for 1 h at 22°C, followed by a rinse with PBS. The cells were incubated with an alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (A3687; Sigma-Aldrich, St. Louis, MO) for 1 h and rinsed three times with PBS. The alkaline phosphatase substrate para-nitrophenyl phosphate (H1007; Sigma-Aldrich) was then added, and the absorbance at 405 nm in the linear range of the assay was determined by using an ELX-800 plate reader (BioTek Instruments, Winoski, VT). To control for the possibility that the desialylation of virions altered antibody binding in the CELISA, equal amounts of mock- or neuraminidase-digested virions were serially diluted and adsorbed to 96-well plates, and the amount of antibody binding was determined as described above. To control for the possibility of altered binding of virions to the plates, mock- and neuraminidase-digested virions were adsorbed to plates and then subjected to BCA protein determination (Pierce, Rockford, IL). The amount of binding in the CELISA was corrected for the differences. The polyclonal rabbit anti-HSV antiserum had a 10% greater binding preference to virions digested with the NEB 2-3 enzyme and was 40% less effective in binding to V. cholerae- or A. ureafaciens-digested samples (data not shown). All CELISA values reported were corrected for the difference in binding of the antibody to mock- and enzyme-treated virions.
VP16 translocation to the nucleus.
Vero cells were exposed to mock- and enzyme-treated virions at a multiplicity of infection of 2. The cells were incubated for 3 h at 37°C, harvested by centrifugation, and resuspended in Laemmli buffer. Nuclear fractions were isolated by using the NucBuster extraction kit (71183-3; Novagen, Inc., San Diego, CA). Whole-cell and nuclear fractions were then sonicated with 10 pulses at a 30% duty cycle using a Branson cell disruptor 200 (Branson Ultrasonics, Danbury, CT), and the amount of protein was determined by using the BCA assay (Pierce). Nuclear fractions and whole-cell samples were normalized for protein content and then electrophoresed in 10% denaturing polyacrylamide gels and transferred to nitrocellulose. Immunoblotting was carried out as previously described (75, 87). The blots were probed with primary mouse monoclonal anti-VP16 antibody (V4388; Sigma-Aldrich) and developed by using goat alkaline phosphatase-conjugated anti-mouse immunoglobulin G (A3562; Sigma-Aldrich) and alkaline phosphatase substrate (B5655; Sigma-Aldrich).
RESULTS
Neuraminidase digestion reduces infectivity of HSV-1.
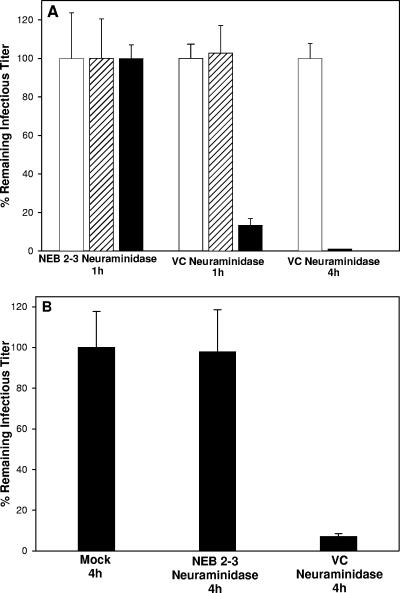
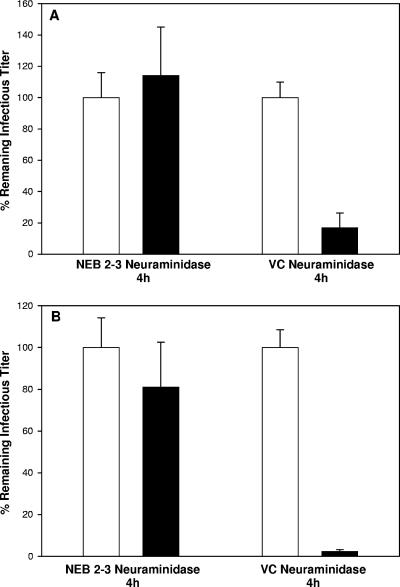
To test the hypothesis that sialylation of viral envelope glycoproteins was important for maintaining infectivity, gradient-purified virions were digested with V. cholerae, A. ureafaciens, or NEB 2-3 neuraminidases at 37°C for either 1 h or 4 h, and the titer of infectious virus was determined by plaque assay. Controls included virions incubated in buffer only, addition of enzyme just before the titer was determined, and virions digested with Endo H (endo-β-N-acetylglucosaminidase H), which cleaves high mannose side chains that are not sialylated. Digestion with NEB 2-3 for 1 h (Fig. 1A) or 4 h (data not shown) had no effect on viral infectivity. This was not due to a lack of these linkages on the HSV envelope proteins (Fig. 2) or a failure of the enzyme to digest the samples (Fig. 3 and 6). In contrast, digestion of virions with V. cholerae or A. ureafaciens neuraminidases for 1 h reduced infectivity 10-fold, and digestion for 4 h reduced infectivity by 1,000-fold (Fig. 1A). Immunoblotting for gC, gB, gD, and gH indicated that contamination of the neuraminidases with protease did not explain the loss in infectivity (Fig. 3 and 6). There was no reduction in titer for either mock-treated virions or samples when enzyme was added just prior to the titer being determined. Incubation of cells for 1 h with a concentration of neuraminidases 1,000-fold higher than what they were exposed to in the titer reduction assays did not alter infectivity with virions (data not shown). These results indicate that removal of α2,3-linked sialic acid on virions has no effect on infectivity, implying that α2,6-linked sialic acids on viral envelope proteins are critical for efficient infection by HSV-1.
FIG. 1.
Digestion of HSV-1 with neuraminidases reduces infectious titer. (A) HSV-1 KOS produced in Vero cells was mock digested (□) or digested with the indicated neuraminidases (▪) for either 1 or 4 h, and the remaining infectious virus was determined by plaque assay in Vero cells. Mock-digested virus was also mixed with the indicated enzyme immediately prior to the plaque assay to minimize the time for digestion (▒). (B) HSV-1 KOS produced in Hep-2 cells was mock digested or digested with NEB 2-3 neuraminidase or V. cholerae neuraminidase for 4 h before the titers were determined in Hep-2 cells. Identical results were obtained with A. ureafaciens neuraminidase (data not shown). The data presented represent the means and standard deviations of three independent assays. All values are reported as percentages with the “mock” value defined as 100%. NEB 2-3, Salmonella enterica serovar Typhimurium LT2 neuraminidase; VC, V. cholerae neuraminidase.
FIG. 2.
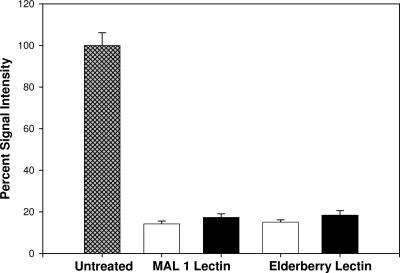
Exposure of HSV-1 to sialic acid-binding lectins reduces viral attachment. HSV-1 hrR3 grown in Vero cells was exposed to MAL1 or ELD for 45 min prior to exposure to cells. Attachment was then measured by using a CELISA, with results given as the percentage of the signal intensity with the untreated samples defined as 100% (▩). The open bars represent background signal with lectins only and no virus. The solid bars indicate binding of virions in the presence of the lectins. The data represent the means and standard deviations of three independent assays.
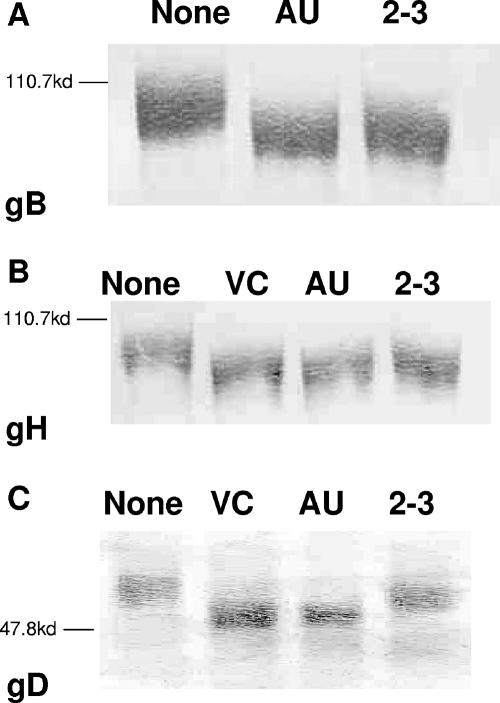
FIG. 3.
Neuraminidase digestion of HSV-1 virions alters the mobility of HSV-1 glycoproteins B, H, and D in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (PAGE). HSV-1 stocks were digested for 4 h at 37°C with the indicated enzymes. The digested virions were mixed with Laemmli buffer, boiled for 5 min, and subjected to PAGE. After transfer to nitrocellulose, the blots were developed with antibodies specific for gB (A), gH (B), and gD (C) (Virusys, Sykesville, MD; Advanced Biotechnologies, Columbia, MD). The blots shown are representative examples of multiple independent assays. NEB 2-3, S. enterica serovar Typhimurium LT2 neuraminidase; VC, V. cholerae neuraminidase; AU, A. ureafaciens neuraminidase. The positions of the molecular weight markers are denoted on the left.
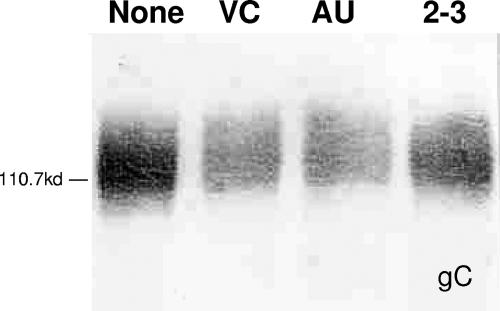
FIG. 6.
Neuraminidase digestion of HSV-1 virions decreases the mobility of gC in sodium dodecyl sulfate-PAGE. HSV-1 stocks were digested as indicated with the indicated enzymes for 4 h at 37°C. The digested virions were mixed with Laemmli buffer, boiled for 5 min, and subjected to PAGE. After transfer to nitrocellulose, the blot was developed with polyclonal anti-gC antibody. The blot shown is a representative example of multiple independent blots. NEB 2-3, S. enterica serovar Typhimurium LT2 neuraminidase; VC, V. cholerae neuraminidase; AU, A. ureafaciens neuraminidase.
Reduced infectivity is not specific for Vero cells.
To ascertain whether the requirement for sialic acid was specific for Vero cells, we measured the infectivity of desialylated virions in Hep-2 cells. We also compared viral stocks prepared in either Vero or Hep-2 cells to determine whether the cell line used for preparation of viral stocks was important. As shown in Fig. 1B, virus grown in Hep-2 cells showed the same loss of infectivity when digested with V. cholerae neuraminidase seen with virus prepared in Vero cells. Identical results were obtained with A. ureafaciens neuraminidase (data not shown). Digestion with NEB 2-3 neuraminidase had no effect. Virions prepared in either Vero or Hep-2 cells and whose titers were determined on the other cell type after digestion with V. cholerae or A. ureafaciens also showed the same pattern of infectivity loss (data not shown). These results indicate that the loss of infectivity after desialylation is not specific for Vero cells and that potential cell-specific differences in sialylation patterns between Vero and Hep-2 cells are inconsequential.
HSV-1 virions contain both α2,6- and α2,3-linked sialic acids.
One possible explanation for the observation that digestion with NEB 2-3 neuraminidase did not reduce infectivity is that HSV-1 envelope proteins lack α2,3-linked sialic acid. To determine whether α2,3- and α2,6-linked sialic acids were present on virions, a CELISA measuring virion attachment to cells was carried out in the presence or absence of MAL1 or ELB lectins that are specific for α2,3- and α2,6-linked sialic acids, respectively (Fig. 2). Incubation of virions with either lectin reduced viral attachment to background levels. Incubation of virions with either lectin also reduced the infection of cells by 100- to 1,000-fold (data not shown). These results suggest that HSV-1 virions contain both α2,3- and α2,6-linked sialic acids on envelope glycoproteins.
α2,6- and α2,3-linked sialic acids are present on gB, gD, and gH.
Having shown that both types of linkages were present on virions, we sought to determine whether individual glycoproteins involved in entry contained both types of linkages. Virions were enzymatically digested for 4 h with NEB 2-3, V. cholerae, or A. ureafaciens neuraminidase, electrophoresed, and immunoblotted with antiserum specific for gB, gH, or gD (Fig. 3). Mock-digested virions were included as controls. Digestion with any of the enzymes shifted the mobility of gB and gH to the same extent (Fig. 3A and B). Digestion with any of the enzymes also altered the mobility of gD (Fig. 3C), but the shift was greater for the V. cholerae and A. ureafaciens enzymes compared to NEB 2-3-digested gD. These results confirm the lectin-binding results and show that gB, gD, and gH contain both α2,6- and α2,3-linked sialic acids.
Neuraminidase digestion does not reduce viral attachment.
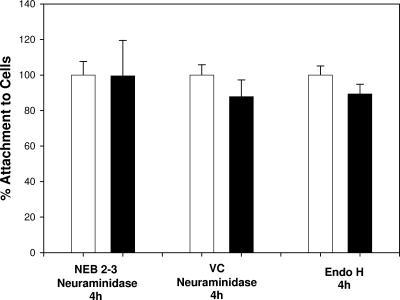
To determine whether the loss of infectivity was due to reduced attachment of virions to cells, the binding of mock-treated and desialylated virions to cells was measured by using the CELISA-based attachment assay. As shown in Fig. 4, the amount of virus attached to cells was not significantly different between the controls and the digested virions, suggesting that viral attachment was not affected by desialylation.
FIG. 4.
Attachment of HSV-1 hrR3 is not affected by digestion with neuraminidase or Endo H. HSV-1 hrR3 grown in Vero cells was digested with NEB 2-3, V. cholerae neuraminidase, or Endo H (▪) for 4 h at 37°C. For each enzyme condition the virus was mock digested in the same buffer (□). After digestion, attachment was measured by using a CELISA, with the results given as percentages with mock-digested controls defined as 100% for each sample pair. Digestion with A. ureafaciens neuraminidase gave the same results as for V. cholerae neuraminidase (data not shown). The data represent the means and standard deviations of triplicate independent assays. NEB 2-3, S. enterica serovar Typhimurium LT2 neuraminidase; VC, V. cholerae neuraminidase.
Glycoprotein C is not involved in the loss of infectivity.
The loss of infectivity after V. cholerae or A. ureafaciens digestion was not due to a defect in attachment, which is mediated primarily by gC. To rule out a role for gC in the loss of infectivity after neuraminidase digestion, we repeated these studies with ΔgC2-3, a gC-null virus, and ΔgC2-3rev, in which the gC gene was reinserted. Digestion with NEB 2-3 neuraminidase did not reduce the infectivity of either the gC-null virus (Fig. 5A) or the revertant virus (Fig. 5B). In contrast, digestion with V. cholerae or A. ureafaciens enzymes reduced the infectivity of both ΔgC2-3 and ΔgC2-3rev to a level similar to that seen with the wild-type virus, indicating that gC is not involved in the reduction of infectivity (Fig. 5A and B). As shown in Fig. 6, digestion of gC with each of the neuraminidases resulted in a slight decrease in mobility. The decreased mobility of neuraminidase-digested gC has been seen previously (G. Cohen, unpublished data), but the reason for the apparent increase in molecular weight is not clear. These results suggest that gC contains sialic acids with both linkages and confirms reports that gC is sialylated, although the types of linkages present had not been determined previously (51, 55).
FIG. 5.
The absence of gC does not affect the titer reduction seen after neuraminidase digestion. HSV-1 ΔgC2-3 (A) or ΔgC2-3rev (B) was mock digested (□) or digested with the indicated neuraminidase (▪) for 4 h before the titer was determined by plaque assay. Values are reported as percentages, with “mock” defined as 100%. The data represent the means and standard deviations of triplicate independent assays. Digestion with A. ureafaciens neuraminidase gave results identical to those for the V. cholerae enzyme (data not shown). NEB 2-3, S. enterica serovar Typhimurium LT2 neuraminidase; VC, V. cholerae neuraminidase.
Sialic acid is required for efficient viral entry.
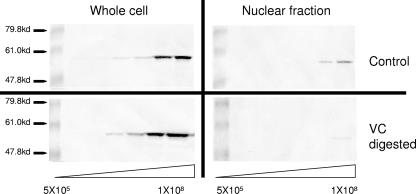
We next sought to determine whether the removal of sialic acids affected viral entry into cells by quantifying the amount of VP16 translocated to the nucleus. VP16 is a tegument protein that traffics to the nucleus shortly after entry and is an accepted marker for viral entry (16). Vero cell monolayers were cooled to 4°C and then exposed to either mock- or V. cholerae neuraminidase-digested virions for 2 h at 4°C. The cells were exposed to increasing concentrations of virus ranging from 5 × 105 to 1 × 108 PFU per well increasing in half-log steps. After attachment, the cultures were shifted to 37°C for 3 h. The cells were then harvested, and the amount of VP16 was measured by immunoblotting. For one set of samples, nuclear fractions were isolated and analyzed. A duplicate set of samples was lysed and electrophoresed without fractionation to measure total VP16. As shown in Fig. 7, in the whole-cell lysates VP16 was first detected when 5 × 106 virions were loaded for either mock- or V. cholerae-digested samples (left panels). In the example shown, there appeared to be slightly more VP16 signal in the V. cholerae-digested whole-cell samples (twofold). These results confirm that attachment of the virus to cells was not significantly altered by V. cholerae digestion. When the amount of VP16 in the nuclear fractions was compared, we found that VP16 was first detected in the lane loaded with 5 × 107 virions. There was a faint VP16 signal in the nuclear fractions from cells exposed to V. cholerae-treated neuraminidase in the lane loaded with 108 virions, suggesting a 5- to 10-fold decrease in entry compared to the mock-digested samples (right panels).
FIG. 7.
Digestion of HSV-1 with V. cholerae neuraminidase reduces nuclear localization of VP-16 without reducing viral attachment. In replicate experiments HSV-1 hrR3 was either mock digested (top panels) or digested with V. cholerae neuraminidase (bottom panels). After the treatment, Vero cells in confluent six-well plates were infected in increasing in half-log steps with 5 × 105 to 1 × 108 PFU/well. The amount of virions added was based on the starting titer before digestion. After 3 h at 37°C, the cells were processed in two ways. To measure the total VP16, whole-cell samples were centrifuged, mixed with Laemmli buffer, subjected to BCA protein determination to ensure equal loading, and subjected to PAGE (left panels). To determine the amount of nuclear VP16, the cells were fractionated, and the nuclear fractions were subjected to BCA protein concentration to ensure equal loading and subjected to PAGE (right panels). All blots were developed with an antibody specific for VP16. VC, V. cholerae neuraminidase.
DISCUSSION
The HSV-1 glycoproteins involved in entry are posttranslationally modified in the exocytic pathway by glycosylation, with the terminal step being the addition of sialic acid in the trans-Golgi compartment. For most mammalian cell types, sialic acids can be added either in α2,3 or α2,6 linkages. It is common to find both types of linkages on an individual glycoprotein. Although the HSV-1 proteins involved in attachment and entry have been extensively characterized, the significance of sialylation has not been studied. Our data provide the first evidence that sialic acids on one or more viral glycoproteins are critical for maintaining the infectivity of the virions and that gB, gD, and gH contain both α2,3 and α2,6 linkages. In addition, we have shown that the maintenance of infectivity appears to be specific for α2,6-linked sialic acids. The data also show that the reduction in infectivity was not due to reduced attachment or to an effect on gC but instead was due to inefficient entry of virions into cells.
Digestion of virions with NEB 2-3, which has a 260-fold preference for α2,3-linked sialic acid (41), had no effect on infectivity, whereas digestion with V. cholerae or A. ureafaciens neuraminidases reduced infectivity. These results suggest that α2,6-linked sialic acids are critical for virion infectivity, but this conclusion is tentative because neuraminidases with the required degree of specificity for α2,6 linkages are not currently available. The apparent requirement for α2,6 linkages is not due to selective α2,6 sialylation of gB, gD, or gH since digestion with each of the neuraminidases resulted in a mobility shift. These observations are consistent with previous studies showing that gD and gH are sialylated (61, 68). One potential explanation for the specificity is that α2,6-linked sialic acids are located at critical positions in gB, gD, or gH. This implies that domain-specific sialylation may be occurring and will require further studies to determine the location of α2,3- and α2,6-linked sialic acids on the viral glycoproteins.
The electrophoretic mobility of gB and gH after neuraminidase digestion was similar whether V. cholerae, A. ureafaciens, or NEB 2-3 neuraminidase was used. In contrast, digestion of gD with NEB 2-3 resulted in only a slight shift compared to gD from virions digested with V. cholerae or A. ureafaciens neuraminidases. It is possible that some of the α2,3-linked sialic acids on gD may not be accessible for digestion. Alternatively, gD may contain a higher ratio of α2,6- to α2,3-linked sialic acid. If gD were to contain more α2,6-linked sialic acid, removal could significantly affect gD-mediated entry functions. HSV-1 gD contains three N-linked and two O-linked glycosylation sites that are modified (19, 20, 44, 71). Mutation of the three N-linked sites in gD results in a conformationally altered but functional protein, suggesting that glycan side chains play a role in maintaining gD structure (72). The removal of sialic acids from the O-linked side chains could alter the structure and therefore the function of gD. Confirmation that desialylation of gD is involved in the reduced infectivity and whether desialylation alters the interaction of gD with cellular receptors or affects assembly of the fusion complex will require further work.
The HSV-1 gC is heavily glycosylated with at least eight N linkages and numerous O linkages, and up to 80% of these side chains are sialylated (11, 22, 44, 50, 56, 57, 66), so it was surprising that the reduction in infectivity was not due to reduced attachment. Digestion of gC with each of the enzymes resulted in a similar increase in the apparent molecular weight for reasons that are not clear. It is possible that gC is resistant to enzymatic desialylation, which would explain the lack of effect on attachment. However, the migration of gC did shift after neuraminidase digestion, suggesting that the protein was altered by exposure to the enzymes. Further studies on the effect of desialylation on gC structure and function will be needed to explain the apparent increase in molecular weight.
Sialic acids on cells are known to serve as receptors for a number of viruses, including influenza virus; respiratory syncytial virus; adeno-associated virus types 1, 4, 5, and 6; adenovirus type 37; polyomaviruses; minute virus of mice; feline calicivirus; and avian infectious bronchitis virus (2, 3, 8, 15, 24, 26, 29, 45, 76, 77, 93, 95). For influenza virus, polyomaviruses, and adenovirus type 37, the crystal structures of the sialic acid binding sites have been determined, and the structural features important for the stereoselectivity of binding to specific sialic acids are known (14, 15, 26, 77, 78). These differences in receptor specificity play an important role in pathogenesis for several viruses (55, 76). Notably, for influenza virus, acquisition of the ability to bind α2,6-linked sialic acid is required for infection of humans with avian strains (29, 79). When we incubated cells for 1 h with 1,000 times the amount of neuraminidase they would have seen in the titer assays, we saw no reduction in infection (data not shown), suggesting that sialic acids on cells do not play a role in infection. This is consistent with the fact that sialic acid has not been reported to function as a receptor for HSV-1.
The role of sialic acids on virions is less well understood, but two general effects have been reported. Neuraminidase digestion of some viruses, including lentiviruses, vesicular stomatitis virus, respiratory syncytial virus, and influenza virus, results in enhanced infectivity (8, 42, 53, 63, 80). For HIV-1, sialic acids may sterically hinder attachment and entry of the virus (43, 81). In contrast, neuraminidase digestion of porcine reproductive and respiratory syndrome virus inhibits infection of porcine alveolar macrophages by reducing attachment to cells (23). Based on our studies, HSV-1 can be added to the list of viruses that require sialylation of envelope proteins for efficient infection and is the first example of sialic acids on virion glycoproteins specifically affecting entry into cells.
Our observation that sialic acid is required for efficient entry of HSV-1 into cells raises the possibility that sialic acid-binding agents could be effective antivirals; thus, we have identified a new target for the development of drugs to prevent HSV-1 infection. Recently, we described a novel peptide, TAT-C, that blocks HSV-1 entry (13a), and preliminary studies suggest that TAT-C binds to sialic acid on virions (unpublished data). Thus, TAT-C may be inhibiting entry by interfering with sialic acid-mediated entry functions. Other carbohydrate-binding agents have been shown to have antiviral activity. A modified theta defensin, RC-2, blocks the attachment and entry of HSV-1 (96) and HIV-1 (91) and has been shown to act as a minilectin (90). Lactoferrin, which blocks HSV-1 attachment, binds to cellular glycosaminoglycans (52). Cyanovirin-N binds to carbohydrates and inhibits the infection of several viruses, including HIV-1 and hepatitis C virus and is currently in clinical trials as a microbicide to block sexually transmitted viral infection (9, 12, 36). Mannose-binding proteins from several plants inhibit HIV-1 infection and can select for HIV-1 with mutations in glycosylation sites within gp120 (4-7). These results clearly indicate that carbohydrates, and sialic acid in particular for HSV-1, are valid targets for antiviral drug development.
In summary, we report for the first time that sialic acids on one or more HSV-1 envelope proteins are required for efficient infection of cells. The observation that infectivity was reduced by digestion with V. cholerae or A. ureafaciens neuraminidase, but not NEB 2-3 neuraminidase, suggests that α2,6-linked sialic acids are involved. The effect of neuraminidase digestion is not specific for Vero cells, nor did it depend on the cell line used for viral propagation. We have also shown that enzymatic desialylation does not affect attachment to cells and that gC is not involved in the reduced infectivity. The reduced infectivity of neuraminidase-digested virions is due to inefficient entry of the virus into cells, suggesting that the fusion proteins, gB, gD, or gH are involved. Our results also suggest that sialic acids on HSV-1 envelope proteins may be valid targets for antiviral drug development. Further studies on the role of sialic acid in HSV-1 entry will likely provide novel insights into the function of gB, gD, and gH in entry.
Acknowledgments
We thank Sharon Altmann, Aaron Kolb, Gilbert Jose, Radeekorn Akkarawongsa, Hermann Bultmann, Stacey Schulz-Cherry, and Donna Peters for helpful comments on these studies and the manuscript. We also thank Elizabeth Froelich for administrative assistance.
This study was supported in part by NIH grants PO1-AI52089, RO1-EY07336, and P30 EY016665 to C.R.B.; the Consortium for Functional Glycomics (GM-62116); and by an unrestricted grant from Research to Prevent Blindness to the Department of Ophthalmology and Visual Sciences.
Footnotes
Published ahead of print on 17 January 2007.
REFERENCES
- 1.Ada, G. L., E. L. French, and P. E. Lind. 1961. Purification and properties of neuraminidase from Vibrio cholerae. J. Gen. Microbiol. 24:409-421. [DOI] [PubMed] [Google Scholar]
- 2.Amanda, D., T. Stuart, and D. K. Brown. 2007. α2,6-linked sialic acid acts as a receptor for feline calicivirus. J. Gen. Virol. 88:177-186. [DOI] [PubMed] [Google Scholar]
- 3.Arnberg, N., P. Pring-Akerblom, and G. Wadell. 2002. Adenovirus type 37 uses sialic acid as a cellular receptor on Chang C cells. J. Virol. 76:8834-8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balzarini, J., D. Schols, J. Nyets, E. Van Damme, W. Peumans, and E. De Clereq. 1991. Alpha-(1-3)- and alpha-(1-6)-d-mannose-specific plant lectins inhibitory to human immunodeficiency virus and cytomegalovirus infections in vitro. Antimicrob. Agents Chemother. 35:410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balzarini, J., K. V. Van Laethem, S. Hatse, K. Vermeire, W. Peumans, E. Van Damme, A.-M. Vandamme, and A. D. Schols. 2004. Profile of resistance of human immunodeficiency virus to specific plant lectins. J. Virol. 78:10617-10627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Balzarini, J., K. Van Laethem, S. Hatse, M. Froeyen, W. Peumans, E. Van Damme, and D. Schols. 2005. Carbohydrate-binding agents cause deletions of highly conserved glycosylation sites in HIV gp120: a new therapeutic concept to hit the Achilles heel of HIV. J. Biol. Chem. 280:41005-41014. [DOI] [PubMed] [Google Scholar]
- 7.Balzarini, J., K. V. Laethem, S. Hatse, M. Froeyen, E. Van Damme, A. Bolmstedt, W. Peumans, E. De Clereq, and D. Schols. 2005. Marked depletion of glycosylation sites in HIV-1 gp120 under selection pressure by the mannose-specific plant lectins of Hippeastrum hybrid and Galanthus nivalis. Mol. Pharmacol. 67:1556-1565. [DOI] [PubMed] [Google Scholar]
- 8.Barretto, N., L. K. Hallak, and M. E. Peeples. 2003. Neuraminidase treatment of respiratory syncytial virus-infected cells or virions, but not target cells, enhances cell-cell fusion and infection. Virology 313:33-43. [DOI] [PubMed] [Google Scholar]
- 9.Barrientos, L. G., and A. M. Gronenborn. 2005. The highly specific carbohydrate-binding protein cyanovirin-N: structure, anti-HIV/Ebola activity and possibilities for therapy. Mini Rev. Med. Chem. 5:21-31. [DOI] [PubMed] [Google Scholar]
- 10.Bender, F. C., J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2005. Herpes simplex virus glycoprotein B binds to cell surfaces independently of heparan sulfate and blocks virus entry. J. Virol. 79:11588-11597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biller, M., K. Mardberg, H. Hassan, H. Clausen, A. Bolmstedt, T. Bergstrom, and S. Olofsson. 2000. Early steps in O-linked glycosylation and clustered O-linked glycans of herpes simplex virus type 1 glycoprotein C: effects on glycoprotein properties. Glycobiology 10:1259-1269. [DOI] [PubMed] [Google Scholar]
- 12.Boyd, M. R., K. R. Gustafson, J. B. McMahon, R. H. Shoemaker, B. R. O'Keefe, T. Mori, R. J. Gulakowski, L. Wu, M. I. Rivera, C. M. Laurencot, M. J. Currens, J. H. Gardellina II, R. W. Buckheit, Jr., P. L. Nara, L. K. Pannell, R. C. Sowder II, and L. E. Henderson. 1997. Discovery of cyanovirin-N, a novel human immunodeficiency virus inactivating protein that binds to viral surface envelope glycoprotein: potential applications to microbicide development. Antimicrob. Agents Chemother. 41:1521-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brooks, S. A., M. V. Dwek, and U. Schumacher. 2002. Functional and molecular glycobiology. Bios Scientific Publishers, Ltd., Abingdon, Oxfordshire, England.
- 13a.Bultman, H., J. Teuton, and C. R. Brandt. Addition of a C-terminal cysteine improves the anti-herpes simplex virus activity of a peptide containing the human immunodeficiency virus type 1 TAT protein transduction domain. Antimicrob. Agents Chemother., in press. [DOI] [PMC free article] [PubMed]
- 14.Burmeister, W. P., D. Guilligay, S. Cusack, G. Wadell, and N. Arnberg. 2004. Crystal structures of species D adenovirus fiber knobs and their sialic acid binding sites. J. Virol. 78:7727-7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cahan, L. D., R. Singh, and J. C. Paulson. 1983. Sialyloligosaccharide receptors of binding variants of polyomavirus. Virology 130:281-289. [DOI] [PubMed] [Google Scholar]
- 16.Chesenko, N., B. Del Rosario, C. Woda, D. Marcellino, L. M. Satlin, and B. C. Herold. 2003. Herpes simplex virus triggers activation of calcium signaling pathways. J. Cell Biol. 163:283-293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomains of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocchi, F., D. Fusco, L. Menotti, T. Gianni, R. J. Eisenberg, G. H. Cohen, and G. Campadelli-Fiume. 2004. The soluble ectodomains of herpes simplex virus gD contains a membrane proximal pro-fusion domain and suffices to mediate virus entry. Proc. Natl. Acad. Sci. USA 101:7445-7450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen, G. H., M. Katze, C. Hydrean-Stern, and R. J. Eisenberg. 1978. Type common CP-1 antigen of herpes simplex virus is associated with a 59,000-molecular weight envelope glycoprotein. J. Virol. 27:172-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen, G. H., D. Long, J. T. Matthews, M. May, and R. Eisenberg. 1983. Glycopeptides of the type-common glycoprotein gD of herpes simplex virus types 1 and 2. 46:679-689. [DOI] [PMC free article] [PubMed]
- 21.Corey, L., A. Wald, R. Patel, S. L. Sacks, S. K. Tyring, T. Warren, J. M. J. Douglas, J. Paavonen, R. A. Morrow, K. R. Beutner, L. S. Stratchounsky, G. Mertz, O. N. Keene, H. A. Watson, D. Tait, M. Vagas-Cortes, and V. H. T. S. Group. 2004. Once-daily valacyclovir to reduce the risk of transmission of genital herpes. N. Engl. J. Med. 350:11-20. [DOI] [PubMed] [Google Scholar]
- 22.Dall'Olio, F., N. Malagolini, V. Speziali, G. Campadelli-Fiume, and F. Serafini-Cessi. 1985. Sialylated oligosaccharides O-glycosidically linked to glycoprotein C from herpes simplex virus type 1. J. Virol. 56:127-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delputte, P. L., and H. J. Nauwynck. 2004. Porcine arterivirus infection of alveolar macrophages is mediated by sialic acid on the virus. J. Virol. 78:8094-8101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dugan, A. S., S. Eash, and W. J. Atwood. 2005. An N-linked glycoprotein with α(2,3)-linked sialic acid is a receptor for BK virus. J. Virol. 79:14442-14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foa-Tomasi, L., E. Avitabile, A. Boscaro, R. Brandmarti, R. Gualandri, R. Manservigi, F. Dall'Olio, F. Serafini-Cessi, and G. Campadelli-Fiume. 1991. Herpes simplex virus (HSV) glycoprotein H is partially processed in a cell line that expresses the glycoprotein and fully processed in cells infected with deletion or ts mutants in the known HSV glycoproteins. Virology 180:474-482. [DOI] [PubMed] [Google Scholar]
- 26.Fried, H., L. D. Cahan, and J. C. Paulson. 1981. Polyoma virus recognizes specific sialyloligosaccharide receptors on host cells. Virology 109:188-192. [DOI] [PubMed] [Google Scholar]
- 27.Fukuda, M. 1996. Possible roles of tumor-associated carbohydrate antigens. Cancer Res. 56:2237-2244. [PubMed] [Google Scholar]
- 28.Fusco, D., C. Forghieri, and G. Campadelli-Fiume. 2005. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc. Natl. Acad. Sci. USA 102:9323-9328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gamblin, S. J., L. F. Haire, R. J. Russel, D. J. Stevens, B. Xiao, Y. Ha, N. Vasisht, D. A. Steinhauer, R. S. Daniels, A. Elliot, D. C. Wiley, and J. J. Skehel. 2004. The structure and receptor binding properties of the 1918 influenza hemagglutinin. Science 303:1838-1842. [DOI] [PubMed] [Google Scholar]
- 30.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 31.Gianni, T., P. L. Martelli, R. Casadio, and G. Campadelli-Fiume. 2005. The ectodomain of herpes simplex virus glycoprotein H contains a membrane alpha-helix with attributes of an internal fusion peptide, positionally conserved in the herpesviridae family. J. Virol. 79:2931-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gianni, T., C. Forghieri, and G. Campadelli-Fiume. 2006. The herpesvirus glycoproteins B and H-L are sequentially recruited to the receptor-bound gD to effect membrane fusion at virus entry. Proc. Natl. Acad. Sci. USA 103:14572-14577. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Goldstein, D. J., and S. K. Weller. 1988. Factors present in herpes simplex virus type-1-infected cells can compensate for the loss of the large subunit of the viral ribonucleotide reductase: characterization of an ICP6 deletion mutant. Virology 166:41-51. [DOI] [PubMed] [Google Scholar]
- 34.Grau, D. R., R. J. Visalli, and C. R. Brandt. 1989. Herpes simplex virus stromal keratitis is not titer-dependent and does not correlate with neurovirulence. Investig. Ophthalmol. Vis. Sci. 30:2474-2480. [PubMed] [Google Scholar]
- 35.Heldwein, E. E., H. Lou, F. C. Bender, G. H. Cohen, R. J. Eisenberg, and S. C. Harrison. 2006. Crystal structure of glycoprotein B from herpes simplex virus. Science 313:217-220. [DOI] [PubMed] [Google Scholar]
- 36.Helle, F., C. Wychowski, N. Vu-Dac, K. R. Gustafson, C. Voisset, and J. Dubuisson. 2006. Cyanovirin-N inhibits hepatitis C virus entry by binding to envelope protein glycans. J. Biol. Chem. 281:25177-25183. [DOI] [PubMed] [Google Scholar]
- 37.Herold, B. C., D. WuDunn, N. Soltus, and P. G. Spear. 1991. Glycoprotein C of herpes simplex virus type 1 plays a principle role in the adsorption of virus to cells and infectivity. J. Virol. 65:1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herold, B. C., R. J. Visalli, N. Susmarski, C. R. Brandt, and P. G. Spear. 1994. Glycoprotein-C independent binding of herpes simplex virus to cells requires cell surface heparan sulphate and glycoprotein B. J. Gen. Virol. 75:1211-1222. [DOI] [PubMed] [Google Scholar]
- 39.Herpetic Eye Disease Study Group. 2000. Oral acyclovir for herpes simplex virus eye disease. Arch. Ophthalmol. 118:1030-1036. [PubMed] [Google Scholar]
- 40.Hildebrandt, H., C. Becker, S. Gluer, H. Rosner, R. Garardy-Schahn, and H. Rahmann. 1998. Polysilalic acid on the neural cell adhesion molecule correlates with expression of polysialyltransferases and promotes neuroblastoma cell growth. Cancer Res. 58:779-784. [PubMed] [Google Scholar]
- 41.Hoyer, L. L., P. Roggentin, R. Schauer, and E. R. Vimr. 1991. Purification and properties of cloned Salmonella typhimurium LT2 sialidase with virus-typical kinetic preference for sialyl α2,3 linkages. J. Biochem. 110:462-467. [DOI] [PubMed] [Google Scholar]
- 42.Hu, H., T. Shioda, C. Moriya, X. Xin, M. K. Hasan, K. Miyake, T. Shimada, and Y. Nagai. 1996. Infectivities of human and other primate lentiviruses are activated by desialylation of the virion surface. J. Virol. 70:7462-7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyndiuk, R., and D. B. Glasser. 1986. Herpes simplex keratitis, p. 343-368. In K. Tabarra and R. A. Hyndiuk (ed.), Infections of the eye: diagnosis and management. Little-Brown, Boston, MA.
- 44.Johnson, D. C., and P. G. Spear. 1983. O-linked oligosaccharides are acquired by herpes simplex virus glycoproteins in the Golgi apparatus. Cell 32:987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kaludov, N., K. E. Brown, R. W. Walters, J. Zabner, and J. A. Chiorini. 2001. Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J. Virol. 75:6884-6893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaneko, Y., F. Nimmerjahn, and J. V. Ravetch. 2006. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313:670-673. [DOI] [PubMed] [Google Scholar]
- 47.Kelm, S., and R. Schauer. 1997. Sialic acids in molecular and cellular interactions. Int. Rev. Cytol. 175:137-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Krummenacher, C., V. M. Supekar, J. C. Whitbeck, E. Lazear, S. A. Connolly, R. J. Eisenberg, G. H. Cohen, D. C. Wiley, and A. Carfi. 2005. Structure of unliganded HSV gD reveals a mechanism for receptor-mediated activation of virus entry. EMBO J. 24:4244-4253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liesegang, T. J. 2001. Herpes simplex virus epidemiology and ocular importance. Cornea 20:1-13. [DOI] [PubMed] [Google Scholar]
- 50.Lim, F., D. Hartley, P. Starr, P. Lang, S. Song, L. Yu, Y. Wang, and A. I. Geller. 1996. Generation of high-titer defective HSV-1 vectors using an IE2 deletion mutant and quantitative study of expression in cultured cortical cells. BioTechniques 20:460-469. [DOI] [PubMed] [Google Scholar]
- 51.Lundstrom, M., S. Olofsson, S. Jeansson, E. Lycke, R. Datema, and J.-E. Mansson. 1987. Host cell-induced differences in O glycosylation of herpes simplex virus gC-1. Virology 161:385-394. [DOI] [PubMed] [Google Scholar]
- 52.Marchetti, M., E. Trybala, F. Superti, M. Johansson, and T. Bergstrom. 2004. Inhibition of herpes simplex virus infection by lactoferrin is dependent on interference with the virus binding to glycosaminoglycans. Virology 318:405-413. [DOI] [PubMed] [Google Scholar]
- 53.Means, R. E., and R. C. Desrosiers. 2000. Resistance of native, oligomeric envelope on simian immunodeficiency virus to digestion by glycosidases. J. Virol. 74:11181-11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 55.Nam, H. J., B. Gurda-Whitaker, W. Y. Gan, S. Ilaria, R. McKenna, P. Mehta, R. A. Alvarez, and M. Agbandje-McKenna. 2006. Identification of the sialic acid structures recognized by minute virus of mice and the role of binding affinity in virulence adaptation. J. Biol. Chem. 281:25670-25677. [DOI] [PubMed] [Google Scholar]
- 56.Olofsson, S., I. Sjoblom, M. Lundstrom, S. Jeansson, and E. Lycke. 1983. Glycoprotein C of herpes simplex virus: characterization of O-linked oligosaccharides. J. Gen. Virol. 64:2735-2747. [DOI] [PubMed] [Google Scholar]
- 57.Olofsson, S., A. Bolmstedt, M. Biller, K. Mardberg, J. Leckner, B. G. Malmstrom, E. Trybala, and T. Bergstrom. 1999. The role of a single N-linked glycosylation site for a functional epitope of herpes simplex virus type 1 envelope glycoprotein C. Glycobiology 9:73-81. [DOI] [PubMed] [Google Scholar]
- 58.Ong, E., J. Nakayama, K. Angata, L. Reyes, T. Katsuyama, Y. Arai, and M. Fukuda. 1998. Developmental regulation of polysialic acid synthesis in mouse directed by two polysialyltransferases, PST and STX. Glycobiology 8:415-424. [DOI] [PubMed] [Google Scholar]
- 59.Parry, C., S. Bell, T. Minson, and H. Browne. 2005. Herpes simplex virus type 1 glycoprotein H binds to αvβ3 integrins. J. Gen. Virol. 86:7-10. [DOI] [PubMed] [Google Scholar]
- 60.Patel, R. 2004. Antiviral agents for the prevention of the sexual transmission of herpes simplex in discordant couples. Curr. Opin. Infect. Dis. 17:45-48. [DOI] [PubMed] [Google Scholar]
- 61.Peng, T., M. Ponce de Leon, M. J. Novotny, H. Jiang, J. D. Lambris, G. Dubin, P. G. Spear, G. H. Cohen, and R. J. Eisenberg. 1998. Structural and antigenic analysis of a truncated form of the herpes simplex virus gH-gL complex. J. Virol. 72:6092-6103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perez-Romero, P., A. Perez, A. Capul, R. Montgomery, and A. O. Fuller. 2005. Herpes simplex virus entry mediator associates in infected cells in a complex with viral proteins gD and at least gH. J. Virol. 79:4540-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Puri, A., S. Grimaldi, and R. Blumenthal. 1992. Role of viral envelope sialic acid in membrane fusion mediated by the vesicular stomatitis virus envelope glycoprotein. Biochemistry 31:10108-10113. [DOI] [PubMed] [Google Scholar]
- 64.Reusser, P. 1998. Current concepts and challenges in the prevention and treatment of viral infections in immunocompromised cancer patients. Support Care Cancer 6:39-45. [DOI] [PubMed] [Google Scholar]
- 65.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2460. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott/The Williams & Wilkins Co., Philadelphia, PA.
- 66.Rux, A. H., W. T. Moore, J. D. Lambris, W. R. Abrams, C. Peng, H. M. Friedman, G. H. Cohen, and R. J. Eisenberg. 1996. Disulfide bond structure determination and biochemical analysis of glycoprotein C from herpes simplex virus. J. Virol. 70:5455-5465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scanlan, P. M., V. Tiwari, S. Bommireddy, and D. Shukla. 2003. Cellular expression of gH confers resistance to herpes simplex virus type-1 entry. Virology 312:14-24. [DOI] [PubMed] [Google Scholar]
- 68.Serafini-Cessi, F., F. Dall'Olio, N. Malagolini, L. Pereira, and G. Campadelli-Fiume. 1988. Comparative study on the O-linked oligosaccharides of glycoprotein D of herpes simplex virus types 1 and 2. J. Gen. Virol. 69:869-877. [DOI] [PubMed] [Google Scholar]
- 69.Shibuya, N., I. J. Goldstein, W. F. Broekaert, M. Nsimba-Ludaki, B. Peeters, and W. J. Peumans. 1987. The elderberry (Sambucus nigra L.) bark lectin recognizes the Neu5Ac(α2-6)Gal/GalNAc sequence. J. Biol. Chem. 262:1596-1601. [PubMed] [Google Scholar]
- 70.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 71.Sjoblom, I., M. Lundstrom, E. Sjogren-Jansson, J. C. Glorioso, S. Jeansson, and S. Olafsson. 1987. Demonstration and mapping of highly carbohydrate-dependent epitopes in the herpes simplex virus type-1 specified glycoprotein C. J. Gen. Virol. 68:545-554. [DOI] [PubMed] [Google Scholar]
- 72.Sodora, D. L., G. H. Cohen, M. I. Muggeridge, and R. J. Eisenberg. 1991. Absence of asparagine-linked oligosaccharides from glycoprotein D of herpes simplex virus type 1 results in a structurally altered but biologically active protein. J. Virol. 65:4424-4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spear, P. F., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 74.Spear, P. G., and R. Longnecker. 2003. Herpesvirus entry: an update. J. Virol. 77:10179-10185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spencer, B., S. Agarwala, L. Gentry, and C. R. Brandt. 2001. HSV-1 vector-delivered FGF2 to the retina is neuroprotective but does not preserve functional responses. Mol. Ther. 3:746-756. [DOI] [PubMed] [Google Scholar]
- 76.Stehle, T., Y. Yan, T. L. Benjamin, and S. C. Harrison. 1994. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature 369:160-163. [DOI] [PubMed] [Google Scholar]
- 77.Stehle, T., and S. C. Harrison. 1996. Crystal structures of murine polyomavirus in complex with straight-chain and branched-chain sialyloligosaccharide receptor fragments. Structure 4:183-194. [DOI] [PubMed] [Google Scholar]
- 78.Stehle, T., and S. C. Harrison. 1997. High-resolution structure of a polyomavirus VP-1oligosaccharide complex: implications for assembly and receptor binding. EMBO J. 16:5139-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stevens, J., A. L. Corper, C. F. Basler, J. K. Taubenberger, P. Palese, and I. A. Wilson. 2004. Structure of the uncleaved human H1 hemagglutinin from the extinct 1918 influenza virus. Science 303:1866-1870. [DOI] [PubMed] [Google Scholar]
- 80.Stewart, J. A., S. E. Reef, P. E. Pellett, L. Corey, and R. J. Whitley. 1995. Herpesvirus infections in persons infected with human immunodeficiency virus. Clin. Infect. Dis. 21:S114-S120. [DOI] [PubMed] [Google Scholar]
- 81.Sun, J., B. Barbeau, S. Sato, and M. J. Tremblay. 2001. Neuraminidase from a bacterial source enhances both HIV-1-mediated syncytium formation and the virus binding/entry process. Virology 284:26-36. [DOI] [PubMed] [Google Scholar]
- 82.Szele, F. G., J. J. Dowling, C. Gonzales, M. Theveniau, G. Rougon, and M. F. Chesselet. 1994. Pattern of expression of highly polysialylated neural cell adhesion molecule in the developing and adult rat striatum. Neuroscience 60:133-144. [DOI] [PubMed] [Google Scholar]
- 83.Thomas, J., and B. T. Rouse. 1997. Immunopathogenesis of herpetic ocular disease. Immunol. Res. 16:375-386. [DOI] [PubMed] [Google Scholar]
- 84.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uchida, Y., Y. Tsukada, and T. Sugimori. 1977. Distribution of neuraminidase in Arthrobacter and its purification by affinity chromatography. J. Biochem. 82:1425-1433. [DOI] [PubMed] [Google Scholar]
- 86.Uchida, Y., T. Sakada, and T. Sugimori. 1979. Enzymatic properties of neuraminidase from Arthrobacter ureafaciens. J. Biochem. 86:1573-1585. [DOI] [PubMed] [Google Scholar]
- 87.Visalli, R. J., and C. R. Brandt. 1993. The HSV-1 UL45 18KDa gene product is a true late protein and a component of the virion. Virus Res. 29:167-178. [DOI] [PubMed] [Google Scholar]
- 88.Visalli, R. J., and C. R. Brandt. 2002. Mutation of the HSV-1 KOS UL45 gene reveals multiplicity dependent effects on central nervous system growth. Arch. Virol. 147:519-532. [DOI] [PubMed] [Google Scholar]
- 89.Wang, W.-C., and R. D. Cummings. 1988. The immobilized leukoagglutinin from the seeds of Maackia amurensis binds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked α2,3 to penultimate galactose residues. J. Biol. Chem. 263:4576-4585. [PubMed] [Google Scholar]
- 90.Wang, W., A. M. Cole, T. Hong, A. J. Waring, and R. I. Lehrer. 2003. Retrocyclin, an antiretroviral θ-defensin, is a lectin. J. Immunol. 170:4708-4716. [DOI] [PubMed] [Google Scholar]
- 91.Wang, W., S. M. Owen, D. L. Rudolph, A. M. Cole, T. Hong, A. J. Waring, R. B. Lal, and R. I. Lehrer. 2004. Activity of α- and θ-defensins against primary isolates of HIV-1. J. Immunol. 173:515-520. [DOI] [PubMed] [Google Scholar]
- 92.Whitley, R. J. 1996. Herpes simplex viruses, p. 2297-2342. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, PA.
- 93.Winter, C., C. Schwegmann-Wessels, D. Cavanagh, U. Neuman, and G. Herrier. 2006. Sialic acid is a receptor determinant for infection of cell by avian infectious bronchitis virus. J. Gen. Virol. 87:1209-1216. [DOI] [PubMed] [Google Scholar]
- 94.Wood, M. J. 1996. Antivirals in the context of HIV disease. J. Antimicrob. Chemother. 37:S97-S112. [DOI] [PubMed] [Google Scholar]
- 95.Wu, Z., E. Miller, M. Agbandje-McKenna, and R. J. Samulski. 2006. α2,3 and α2,6 N-linked sialic acids facilitate efficient binding and transduction by adeno-associated virus types 1 and 6. J. Virol. 80:9093-9103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yasin, B., W. Wang, M. Pang, N. Chesenko, T. Hong, A. J. Waring, B. C. Herold, E. A. Wagar, and R. I. Lehrer. 2004. θ-Defensins protect cells from infection by herpes simplex virus by inhibiting viral adhesion and entry. J. Virol. 78:5147-5156. [DOI] [PMC free article] [PubMed] [Google Scholar]