Abstract
Pathogenic hantaviruses cause two human diseases: hantavirus pulmonary syndrome (HPS) and hemorrhagic fever with renal syndrome (HFRS). The hantavirus G1 protein contains a long, 142-amino-acid cytoplasmic tail, which in NY-1 virus (NY-1V) is ubiquitinated and proteasomally degraded (E. Geimonen, I. Fernandez, I. N. Gavrilovskaya, and E. R. Mackow, J. Virol. 77: 10760-10768, 2003). Here we report that the G1 cytoplasmic tails of pathogenic Andes (HPS) and Hantaan (HFRS) viruses are also degraded by the proteasome and that, in contrast, the G1 tail of nonpathogenic Prospect Hill virus (PHV) is stable and not proteasomally degraded. We determined that the signals which direct NY-1V G1 tail degradation are present in a hydrophobic region within the C-terminal 30 residues of the protein. In contrast to that of PHV, the NY-1V hydrophobic domain directs the proteasomal degradation of green fluorescent protein and constitutes an autonomous degradation signal, or “degron,” within the NY-1V G1 tail. Replacing 4 noncontiguous residues of the NY-1V G1 tail with residues present in the stable PHV G1 tail resulted in a NY-1V G1 tail that was not degraded by the proteasome. In contrast, changing a different but overlapping set of 4 PHV residues to corresponding NY-1V residues directed proteasomal degradation of the PHV G1 tail. The G1 tails of pathogenic, but not nonpathogenic, hantaviruses contain intervening hydrophilic residues within the C-terminal hydrophobic domain, and amino acid substitutions that alter the stability or degradation of NY-1V or PHV G1 tails result from removing or adding intervening hydrophilic residues. Our results identify residues that selectively direct the proteasomal degradation of pathogenic hantavirus G1 tails. Although a role for the proteasomal degradation of the G1 tail in HPS or HFRS is unclear, these findings link G1 tail degradation to viral pathogenesis and suggest that degrons within hantavirus G1 tails are potential virulence determinants.
Hantaviruses are members of the Bunyaviridae family and chronically infect their rodent or small mammal hosts in the absence of apparent disease (15, 27, 32, 35). Hantaviruses are zoonotically transmitted to humans and cause two discrete diseases, hemorrhagic fever with renal syndrome (HFRS) and hantavirus pulmonary syndrome (HPS), although vascular dysfunction and acute thrombocytopenia are common to both diseases (9, 11, 35, 43, 44). HFRS is caused by Hantaan virus (HTNV), Puumala virus, Seoul virus, and Dobrava/Belgrade virus and is commonly detected in Europe and Asia (24, 29, 32, 35). HPS occurs throughout the Americas and is caused by a number of hantaviruses, including Sin Nombre virus and NY-1 virus (NY-1V) in North America and Andes virus (ANDV) in South America (28, 36, 37, 39, 43, 44). In contrast to pathogenic hantaviruses, Prospect Hill virus (PHV) and Tula virus are not associated with any human disease (35, 41).
The hantavirus genome consists of three negative-sense RNA segments that are designated L, M, and S. The L segment encodes the viral RNA-dependent RNA polymerase; the M segment encodes two surface glycoproteins, G1 and G2; and the S segment encodes the nucleocapsid (N) protein (4). There is no evidence that hantaviruses express nonstructural proteins during infection, suggesting that structural proteins are likely to be multifunctional. The M segment of all hantaviruses is translated into a polyprotein that is cotranslationally cleaved into N-terminal G1 and C-terminal G2 glycoproteins (34). The G1G2 polyprotein is cleaved downstream of a conserved pentapeptide WAASA sequence, presumably by a signal peptidase complex, although the details of this process have not been defined (25). During infection, G1 and G2 form heterodimers that localize to the cis-Golgi compartment, and hantaviruses bud into the lumen of the Golgi complex (31, 38). G1G2 heterodimers are viral surface glycoproteins that define viral neutralization determinants (34), but apart from their obvious structural role, the function of G1 and G2 in hantavirus pathogenesis is unclear.
The G1 glycoprotein contains an N-terminal ectodomain followed by a transmembrane domain and a long, 142-residue cytoplasmic tail. The G1 cytoplasmic tails of all hantaviruses contain a membrane-proximal cysteine-rich region that is predicted to form a RING finger motif (16). In addition, HPS-causing hantaviruses contain an ITAM motif that is commonly found in immune cell receptors and is associated with phosphorylation-dependent immune cell signaling responses (17). The G1 cytoplasmic tail of pathogenic, but not nonpathogenic, hantaviruses is also reported to regulate cellular responses that mimic interferon-directed transcriptional responses following NY-1V and PHV infection of endothelial cells (1, 18). We previously reported that the NY-1V G1 cytoplasmic tail is ubiquitinated and degraded by the proteasome, suggesting that the G1 tail may regulate cellular or viral functions (16). Several viruses utilize the host proteasomal machinery to evade antiviral responses and encode RING finger-containing viral proteins that function as E3 ubiquitin ligases. N-terminal RING finger motifs of herpes simplex virus ICP0 and rotavirus NSP1 proteins, as well as the C-terminal RING finger domain of the poxvirus p28 protein, are virulence factors that mediate proteasomal degradation of self and target host cell proteins (3, 7, 21).
In this study, we report that proteasomal degradation of the NY-1V G1 tail is independent of the RING finger motif and instead is mediated by residues within a C-terminal hydrophobic domain. We found that the G1 tails of pathogenic NY-1V, ANDV, and HTNV are degraded by the proteasome, while the G1 tail of nonpathogenic PHV is stable and not proteasomally degraded. Mutational analysis of NY-1V and PHV G1 tails identified residues that determine the stability and degradation of G1 tails and suggests that the hydrophobic moment of the G1 C-terminal domain regulates proteasomal degradation. Fusion of NY-1V, but not PHV, G1 C termini to green fluorescent protein (GFP) directed the proteasomal degradation of GFP. Thus, the C-terminal hydrophobic domain of pathogenic hantavirus G1 tails appears to define a “degron”-like domain that targets proteins to the proteasome. These findings link degradation of the G1 tail to HPS- and HFRS-causing hantaviruses and suggest that the G1 tail may be a virulence determinant that contributes to hantavirus pathogenesis.
MATERIALS AND METHODS
Cell culture and reagents.
COS7 cells were maintained in Dulbecco's modified Eagle's medium (Sigma) containing 10% fetal bovine serum (Sigma) and antibiotic antimycotic solution (100 U penicillin, 100 μg streptomycin, and 250 ng amphotericin B per ml [final concentration]; Sigma). The proteasomal inhibitor MG132 was purchased from Calbiochem, and cells were treated with 50 μM MG132 for 6 h as indicated. Anti-GAL4 (Santa Cruz), anti-β-actin (Sigma), and horseradish peroxidase-conjugated anti-mouse (Amersham) antibodies were used for Western blotting.
Plasmid construction and site-directed mutagenesis.
The G1 tails of NY-1V, PHV, ANDV, and HTNV were aligned using the ClustalW multiple sequence alignment tool (http://www.ebi.ac.uk). NY-1V, ANDV, HTNV, and PHV G1 tails were C-terminally fused in frame with the GAL4 protein in the pBIND mammalian expression vector (Promega) following PCR amplification with oligonucleotides containing BamHI and XbaI sites and verified by sequencing. Mutants derived from GAL4-tagged NY-1V and PHV G1 cytoplasmic tails were generated by site-directed mutagenesis using the QuikChange site-directed mutagenesis kit (Stratagene). pEGFP-C1 and pDsRed-Monomer-C1 were obtained from Clontech. Oligonucleotides containing EcoRI and BamHI restriction sites were used to amplify NY-1V and PHV G1 tails by PCR; the G1 tails were then directionally cloned into pEGFP-C1 and pDsRed-Monomer-C1 to fuse them in frame to the C termini of GFP and red fluorescent protein (RFP). Clones were sequenced to verify in-frame insertion.
Transfection.
Cells were plated 24 h prior to transfection at a concentration of 6 × 105 cells per well of a 6-well plate for optimum transfection efficiency. COS7 cells were transfected using 1 μg of plasmid DNA with FuGENE 6 (Roche), and protein expression was analyzed 48 h posttransfection by Western blot analysis or fluorescence microscopy.
Immunoblotting and fluorescence microscopy.
Six hours prior to lysis, COS7 cells were treated either with 50 μM MG132 or an equal amount of the diluent dimethyl sulfoxide (untreated) for 6 h. Cells were washed in cold phosphate-buffered saline, pH 7.0, and lysed at 4°C for 30 min in radioimmunoprecipitation assay buffer (150 mM sodium chloride, 50 mM Tris [pH 7.5], 0.2% sodium dodecyl sulfate [SDS], 0.5% deoxycholate, 1% NP-40) (16). Cells were collected by gentle scraping and clarified by centrifugation at 14,000 rpm and 4°C for 20 min. Lysates were assayed for total protein using a bicinchoninic acid protein assay kit (Pierce). Ten micrograms of total protein from each sample was separated on a 12% SDS-polyacrylamide gel electrophoresis (PAGE) gel and electroblotted onto a nitrocellulose membrane as previously described (16). Proteins were probed with an anti-GAL4 or anti-β-actin primary antibody and a horseradish peroxidase-conjugated anti-mouse secondary antibody and were detected by chemiluminescence using the ECL reagent (Amersham) as previously described (16).
For fluorescence detection of GFP- and RFP-tagged G1 tails, COS7 cells were transfected as described above, and 40 h posttransfection, MG132 or the diluent dimethyl sulfoxide was added. Six hours post-MG132 addition, fluorescence was analyzed using an inverted fluorescence microscope (Nikon). For quantification, fluorescent cells from five random fields were counted and the average plotted.
RESULTS
Contrasting proteasomal degradation of pathogenic and nonpathogenic hantavirus G1 tails.
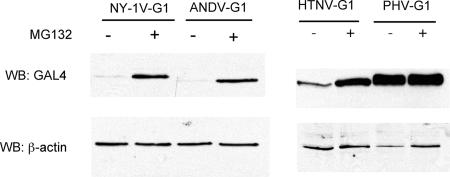
To determine whether proteasomal degradation of G1 cytoplasmic tails is common to all hantaviruses, we evaluated the stability of full-length G1 cytoplasmic tails from the hantaviruses ANDV, HTNV, and PHV. ANDV, HTNV, and PHV G1 tail proteins were C-terminally fused to GAL4 and transiently expressed in COS7 cells in the presence or absence of a proteasomal inhibitor. Like that of NY-1V, the ANDV G1 tail is detected only in the presence of a proteasomal inhibitor, suggesting that the ANDV G1 tail is proteasomally degraded (Fig. 1). In contrast, the G1 cytoplasmic tail of PHV is equivalently expressed in the presence or absence of a proteasomal inhibitor, indicating that the PHV G1 tail is stable and is not degraded by the proteasome (Fig. 1). The HTNV G1 tail is detected in the absence of the proteasome inhibitor, but the amount of the HTNV G1 tail is dramatically enhanced by the addition of the proteasome inhibitor (Fig. 1). These findings indicate that G1 cytoplasmic tails of pathogenic, but not nonpathogenic, hantaviruses are proteasomally degraded and suggest a fundamental difference between pathogenic and nonpathogenic hantaviruses.
FIG. 1.
G1 cytoplasmic tails of pathogenic HPS- and HFRS-causing hantaviruses are proteasomally degraded, while the G1 tail of nonpathogenic hantavirus is stable. One microgram of plasmid DNA expressing GAL4-tagged G1 tails of NY-1V, ANDV, HTNV, or PHV was transiently transfected into COS7 cells, and 42 h posttransfection, cells were either treated with 50 μM MG132 for 6 h (+) or left untreated (−). Lysate containing 10 μg of total protein was analyzed on 12% SDS-PAGE gels by immunoblotting with an anti-GAL4 antibody. β-Actin levels were evaluated to demonstrate comparable sample loading.
NY-1V but not PHV G1 tails direct the proteasomal degradation of GFP.
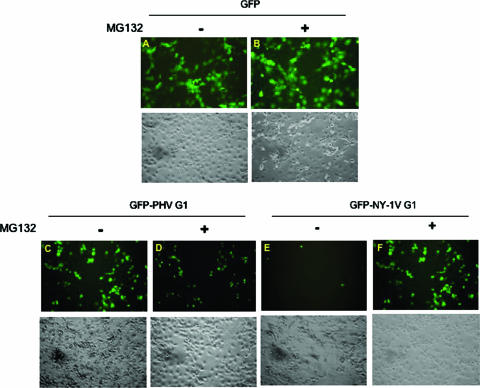
In order to rule out the involvement of the upstream GAL4 tag in G1 tail stability, we fused the G1 cytoplasmic regions from both NY-1V and PHV to the C terminus of enhanced green fluorescent protein (eGFP). Cells were transfected with equal amounts of plasmids expressing eGFP, eGFP-NY-1V-G1 tail, or eGFP-PHV-G1 tail. Fluorescence microscopy shows low expression levels of the eGFP-NY-1V-G1 tail, which increase dramatically following addition of a proteasomal inhibitor (Fig. 2E and F). In contrast, GFP or the GFP-PHV-G1 chimera was expressed similarly in the presence or absence of MG132 (Fig. 2A to D). These results are consistent with the degradation of NY-1V, but not PHV, GAL4-G1 chimeras and suggest a fundamental difference in the proteasomal degradation of pathogenic and nonpathogenic G1 tails.
FIG. 2.
The G1 tail of NY-1V, but not that of PHV, directs the chimeric GFP-G1 tail to the proteasome. One microgram of expression plasmid DNA encoding GFP, GFP-NY-1V-G1, or GFP-PHV-G1 was transfected into COS7 cells in the presence (+) or absence (−) of MG132. Fluorescence was detected 48 h posttransfection. (A and B) GFP; (C and D) GFP-PHV G1 tail; (E and F) GFP-NY-1V G1 tail. Each panel shows fluorescence (top) and phase-contrast (bottom) micrographs of the same field.
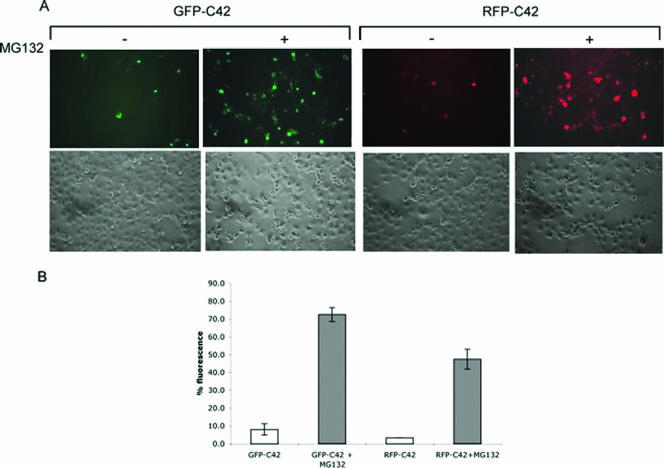
The C-terminal 42 residues of the NY-1V G1 tail direct GFP and RFP degradation.
To further analyze the NY-1V G1 tail domains that direct degradation, we fused the C-terminal 42 residues of the NY-1V G1 tail to GFP or RFP and analyzed protein stability in the presence or absence of MG132 by fluorescence microscopy (Fig. 3A). Expression of the GFP-C42 and RFP-C42 fluorescent chimeras is approximately 10-fold more when proteasomal degradation is inhibited (Fig. 3A and B). This indicates that the decreased fluorescence of NY-1V G1 tail constructs in the absence of the proteasomal inhibitor is not the result of poor expression but is due to rapid proteasomal turnover of chimeras containing the C-terminal 42 residues of the NY-1V G1 tail. Therefore, the C-terminal 42 residues of the NY-1V G1 tail are sufficient to target heterologous stable proteins for proteasomal degradation.
FIG. 3.
The C-terminal 42 residues of the NY-1V G1 cytoplasmic tail were fused downstream and in frame to GFP, or RFP. One microgram of each expression plasmid was transfected into COS7 cells, and fluorescence was detected in MG132-treated (+) or mock-treated (−) cells. (A) Fluorescence (top) and phase-contrast (bottom) images of the same field are presented. (B) Fluorescent cells with (shaded bars) or without (open bars) MG132 treatment were counted from five randomly chosen fields, and the averages were plotted.
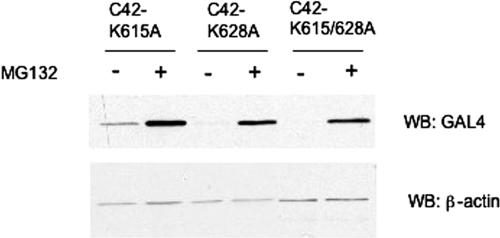
Lysines present within the NY-1V C-terminal 42 residues are not required for degradation.
Lysines are common sites of polyubiquitin chain attachment that mark proteins for proteasomal degradation. To determine whether lysines within the C-terminal 42 residues of the NY-1V G1 tail are sites for ubiquitin attachment, we mutated lysine 615 and 628 to alanine in GAL4-C42 and evaluated protein expression in the presence or absence of a proteasomal inhibitor (Fig. 4). Mutation of lysines 615 and 628 either singly or together had no detectable effect on the proteasomal degradation of GAL4-C42 (Fig. 4). These results suggest two possibilities: (i) that C-terminal residues of the NY-1V G1 tail function to direct the ubiquitination of upstream lysine residues in the G1 tail or reporter proteins or (ii) that sites other than the lysine residue(s) are targets of ubiquitin attachment within the C-terminal 42 residues of G1 (8).
FIG. 4.
The degradation signal in the C-terminal 42 residues of the NY-1V G1 tail is independent of lysines at positions 615 and 628. COS7 cells were transfected with 1 μg of plasmid DNA expressing C42 lysine mutants, and 6 h prior to lysis, 50 μM MG132 was added to selected wells (+). Ten micrograms of total protein was analyzed on a 12% SDS-PAGE gel by anti-GAL4 immunoblotting (WB). Blots were reprobed for β-actin to demonstrate comparable sample loading.
Degradation signals for the NY-1V G1 tail reside within the C-terminal hydrophobic domain.
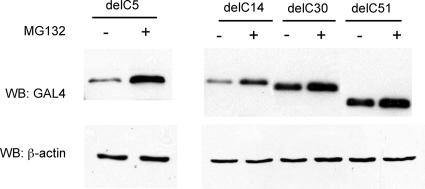
We have previously shown that deletion of 51 C-terminal residues of the NY-1V G1 tail (NY-1V-delC51) abrogates G1 tail degradation (16). In order to further delineate sequences that direct proteasomal degradation of the G1 tail, we evaluated the expression of C-terminally truncated mutants lacking 5, 14, or 30 residues at the G1 C terminus (NY-1V-delC5, -delC14, and -delC30). Figure 5 demonstrates that deleting 5 or 14 residues from the C terminus does not alter NY-1V G1 tail degradation. However, deletion of 30 residues from the C terminus (NY-1V-delC30) completely abolished proteasomal degradation of the NY-1V G1 tail (Fig. 5). Taken together, these results indicate that residues between 5 and 30 from the G1 C terminus are key determinants of G1 tail degradation.
FIG. 5.
The C-terminal 30 residues of NY-1V G1 tails direct proteasomal degradation. Deletion mutants lacking 5, 14, 30, or 51 residues from the NY-1V G1 tail C terminus were expressed in COS7 cells. Protein expression from MG132-treated (+) and untreated (−) cells was analyzed by Western blotting (WB) using an anti-GAL4 antibody. Blots were reprobed for β-actin to demonstrate comparable sample loading.
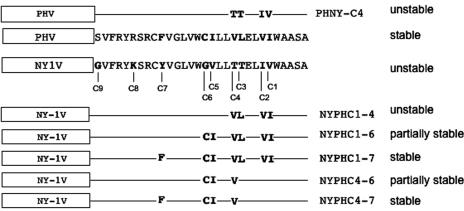
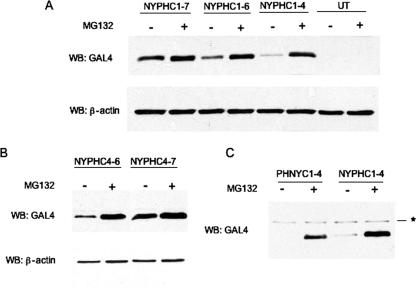
These results highlight an important role for the hydrophobic C-terminal 30 residues of the G1 tail in proteasome-directed degradation. Amino acid alignment of NY-1V and PHV G1 tails revealed nine differences within the C-terminal 30 residues (C1 to C9 from the C terminus) (Fig. 6). In order to identify critical residues that differentiate the stability properties of NY-1V and PHV G1 tails, we mutated C1 to C9 of the NY-1V G1 tail to homologous PHV G1 residues either sequentially or in groups (Fig. 6). As shown in Fig. 7A, substituting PHV residues within the NY-1V G1 tail at positions C1 to C4 (NYPHC1-4) did not alter G1 tail degradation, but substitution at positions C1 through C6 dramatically enhanced G1 tail stability (Fig. 7A). Compared to NYPHC1-4, more NYPHC1-6 protein is detected in the absence of MG132, although MG132 treatment enhances NYPHC1-6 accumulation, indicating partial proteasomal degradation of the protein. Substituting seven PHV G1 tail residues within the NY-1V G1 tail background (NYPHC1-7) completely blocks proteasome-dependent degradation of the NY-1V G1 tail (Fig. 7A). Together these results indicate that residues at positions C1 to C7 direct differences in the stability of the NY-1V and PHV G1 tails.
FIG. 6.
Schematic representation of NY-1V and PHV G1 tail mutants used in this study. The C-terminal 30 residues from NY-1V and PHV G1 tails were aligned using the ClustalW program, and differences at positions C1 to C9 are indicated (boldface). Mutations were incorporated into NY-1V (NYPH) or PHV (PHNY) as depicted, and a summary of mutant protein degradation by the proteasome is given on the right.
FIG. 7.
Stability-determining amino acids in pathogenic and nonpathogenic G1 tails. (A) Equivalent amounts of the NY-1V and PHV G1 tail mutants indicated were transiently expressed in COS7 cells in the presence (+) or absence (−) of MG132. Protein expression levels were determined by immunoblotting (WB) using anti-GAL4 antibodies. UT, untransfected COS7 lysate. Blots were reprobed for β-actin as an internal loading control. (B) Replacement of residues C1 to C7 and C4 to C7 in the NY-1V G1 tail with PHV residues enhances NY-1V G1 stability. (C) Mutations at residues C1 to C4 alter the stable PHV G1 tail into a proteasomally degraded chimera. Asterisk indicates a nonspecific cellular band.
Since NYPHC1-4 is unstable, we made additional mutants to further identify critical residues that determine NY-1V G1 tail instability. Expression of NYPHC4-6 and NYPHC4-7 (Fig. 7B) indicated that NYPHC4-6 is partially stable, while NYPHC4-7 is completely stable, in the absence of proteasome inhibitors (Fig. 7B). This indicates that substituting 4 PHV G1 tail residues for residues in the NY-1V G1 tail (positions C4 to C7) stabilizes the NY-1V G1 tail. Collectively, our results indicate an important role for amino acids Y632, G638, V639, and T642 in the degradation of the NY-1V G1 tail.
Four NY-1V residue changes direct PHV G1 tail degradation by the proteasome.
In order to define residues critical for PHV G1 tail stability, we reciprocally mutated the C-terminal residues of the PHV G1 tail to homologous residues from NY-1V. We found that substituting NY-1V residues C1 to C4 for PHV residues transforms the stable PHV-G1 tail into a proteasomally degraded protein (Fig. 7C). Therefore, while stabilization of the NY-1V G1 tail requires mutations at residues C4 to C7, destabilization of the PHV G1 tail is achieved by mutations at C1 to C4. These results demonstrate the role of residues at the C termini of the PHV and NY-1V G1 tails that regulate proteasomal degradation, and they further suggest that the C termini of pathogenic hantavirus G1 tails contain degron-like components (14, 42) that direct the G1 tail to the proteasome.
DISCUSSION
During hantavirus infection, oligomeric complexes of viral surface glycoproteins G1 and G2 accumulate within the cis-Golgi compartment and mediate virus maturation (31, 38). G1 contains a 142-residue C-terminal cytoplasmic tail with the potential to mediate viral assembly, regulate cellular responses, and limit protein expression levels. We previously demonstrated that the G1 cytoplasmic tail from pathogenic NY-1V is ubiquitinated and degraded by the proteasome (16). In this report, we have further analyzed G1 tail degradation from pathogenic and nonpathogenic hantaviruses, and our findings demonstrate the contrasting stabilities of pathogenic and nonpathogenic hantavirus G1 tails.
Like that of NY-1V, the G1 tails of pathogenic ANDV and HTNV are degraded proteasomally, whereas the G1 tail of the nonpathogenic hantavirus, PHV, is stable. Thus, it appears that proteasomal degradation of the G1 cytoplasmic tail is unique and conserved among pathogenic hantaviruses. Despite degradation differences, G1 tails from both NY-1V and PHV appeared to display identical punctate perinuclear localization, similar to G1 localization reported following expression and hantavirus infection (31). The identical localization pattern of NY-1V and PHV G1 tails suggests that the subcellular targeting of the GFP-G1 tail chimeras is conserved and specific. This finding also indicates that proteasomal degradation of the NY-1V G1 tail is not the result of aberrant intracellular localization of the protein.
The NY-1V G1 tail contains a RING finger motif in its N-terminal domain, and RING finger motifs are known to recruit ubiquitinating enzymes and to direct the proteasomal degradation of proteins (26). However, our deletional analysis of the NY-1V G1 tail (Fig. 5) demonstrates that the degradation signal within the G1 tail resides within the last 30 residues and does not require the presence of the RING finger domain. These results indicate that the RING finger domain in the NY-1V G1 tail does not function as an E3 ubiquitin ligase in directing proteasomal degradation.
The NY-1V and PHV G1 tails differ at 9 positions in their C-terminal 30 residues, and a systematic analysis of PHV and NY-1V G1 tail mutants resulted in the proteasomal degradation of the PHV G1 tail when NY-1V residues were substituted at positions V642T, I643T, V646I, and I647V. Reciprocal substitution of a partially overlapping set of PHV G1 tail residues (T642V, V639I, G638C, and Y632F) for NY-1V residues rendered the NY-1V G1 tail stable. Substitutions involving Val and Ile are biochemically conservative, suggesting that changes in the threonines (T642, T643), glycine (G638), or tyrosine (Y632) residues are likely to be critical to G1 protein degradation. Changing cysteine 638 alone had no effect on proteasomal degradation, suggesting that a potential disulfide bond in this region does not contribute to protein stability. Taken together, these results suggest a conformation-dependent signal at the C terminus of G1 that directs proteasomal degradation.
Interestingly, mutation of lysines within the 30 C-terminal residues of the NY-1V G1 tail did not alter protein stability, indicating that lysines within this domain do not appear to play a role in G1 tail degradation. Although cysteines have recently been implicated as sites for ubiquitin addition, cysteine residues in NY-1V and PHV G1 tails are conserved and unlikely to determine hantavirus-specific ubiquitination and degradation differences (8). It is possible that ubiquitination occurs at residues outside the C30 region, and a recent study of serum- and glucocorticoid-induced protein kinase-1 (SGK-1) degradation has shown that the site of protein ubiquitination is discrete from the hydrophobic 6-amino-acid motif (GMVAIL) that directs its proteasomal degradation (5).
The C-terminal 30 residues of the NY-1V G1 tail contains a stretch of highly hydrophobic residues that are also present in PHV, ANDV, and HTNV proteins. In pathogenic, but not nonpathogenic, hantavirus G1 tails, the C-terminal hydrophobic domain contains intervening hydrophilic or polar residues that could potentially interfere with the hydrophobic moment of this region. Introducing a hydrophilic residue into the hydrophobic domain of the PHV G1 tail (V642T) renders it unstable, whereas mutations that stabilize the NY-1V G1 tail result in a continuous hydrophobic linear sequence. Although we have not addressed specific residue requirements of ANDV and HTNV G1 tails, a similar insertion of hydrophilic polar residues into the C-terminal hydrophobic domain is present (T635 and S645 in HTNV) and may direct degradation.
Proteasomal degradation of proteins is a multistep process involving the ubiquitination of target proteins in some cases through the recognition of degradation signals, or “degrons,” within proteins (42). Degrons are often composed of multiple, dispersed interacting elements within a region of the protein that act in concert to direct proteasomal degradation. The N-terminal 128 amino acids of Myc and the N-terminal 14 to 32 residues of Deg1 contain signals that promote their degradation (13, 19, 23, 33). The hydrophobic face of an amphipathic helix in Deg1 is important for its degradation, and the hydrophobicity of a 28-residue degron within the HMG coenzyme A reductase isozyme, rather than a specific motif, directs its degradation (14, 23). Similarly, it appears that the G1 tails of hantaviruses contain a degron whose function depends on a hydrophobic domain at the C terminus of G1. Like other degrons, the G1 tail C-terminal region of pathogenic hantaviruses can autonomously target stable GAL4, GFP, and RFP for degradation without the participation of upstream G1 tail sequences (20). Future studies to identify cellular proteins that interact with C-terminal domains of pathogenic hantavirus G1 tails will be crucial to elucidating the mechanism by which the degron functions to target the G1 tail to the proteasome.
Several viruses encode proteins that mediate host cell protein degradation, thereby modulating host cell responses and contributing to viral persistence and disease. The E6 protein of human papillomavirus (HPV) targets cellular proteins including p53 for degradation and is a potential determinant of HPV-mediated disease (10). The kK3 and kK5 proteins of the Kaposi-sarcoma herpesvirus, and the US2 and U11 proteins of human cytomegalovirus, specifically direct the degradation of major histocompatibility complex class I molecules, thereby evading host immune surveillance and establishing viral persistence (2, 22, 30, 40). We previously reported that the NY-1V G1 tail specifically interacts with cellular Src and Syk family kinases that regulate immune cell function (17). Hantaviruses also persistently infect their animal hosts, suggesting that these small RNA viruses have the ability to evade host immune responses, like human herpesviruses and papillomaviruses (6, 12). Another intriguing possibility is that proteasomal degradation is part of a viral strategy to limit the levels of G1 protein available for viral assembly or antigen presentation, in order to establish viral persistence within hosts. It is also possible that degradation products may enhance antigen presentation. Further studies directed toward major histocompatibility complex class I proteins may shed light on the two possibilities, since these may be important in hantavirus persistance and clearance.
Viral regulation of cellular interferon responses is nearly universal and is often a key determinant of viral disease. The rotavirus NSP1 protein has been shown to regulate interferon (IFN) signaling responses by targeting interferon regulatory factor 3 (IRF3) and IRF7 for proteasomal degradation (3). We recently determined that the NY-1V G1 tail, but not the PHV G1 tail, blocks RIG-I- and TBK-1-directed IFN induction (1). These findings suggest that the altered stability of the PHV and NY-1V G1 tails may differentially regulate cell-signaling responses that contribute to viral pathogenesis. Although it is not clear what G1 tail domain is required for regulating IFN responses or whether the degron in the G1 tail plays a role in IFN regulation, the G1 tail appears to be a multifunctional hantavirus virulence determinant, and future studies are likely to define the mechanisms by which the G1 tail regulates cellular responses.
Acknowledgments
We thank Irina Gavrilovskaya and Peter Alff for helpful discussions and critical reviews of the manuscript.
This work was supported by National Institutes of Health grants R01AI47873, PO1AI055621, and U54AI57158 (Northeast Biodefense Center [director, W. I. Lipkin]) and by a Veterans Affairs Merit Award.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Alff, P. J., I. N. Gavrilovskaya, E. Gorbunova, K. Endriss, Y. Chong, E. Geimonen, N. Sen, N. C. Reich, and E. R. Mackow. 2006. The pathogenic NY-1 hantavirus G1 cytoplasmic tail inhibits RIG-I- and TBK-1-directed interferon responses. J. Virol. 80:9676-9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barel, M. T., G. C. Hassink, S. van Voorden, and E. J. Wiertz. 2006. Human cytomegalovirus-encoded US2 and US11 target unassembled MHC class I heavy chains for degradation. Mol. Immunol. 43:1258-1266. [DOI] [PubMed] [Google Scholar]
- 3.Barro, M., and J. T. Patton. 2005. Rotavirus nonstructural protein 1 subverts innate immune response by inducing degradation of IFN regulatory factor 3. Proc. Natl. Acad. Sci. USA 102:4114-4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bishop, D. 1996. Biology and molecular biology of bunyaviruses, p. 19-62. In R. M. Elliot (ed.), The Bunyaviridae. Plenum Press, New York, NY.
- 5.Bogusz, A. M., D. R. Brickley, T. Pew, and S. D. Conzen. 2006. A novel N-terminal hydrophobic motif mediates constitutive degradation of serum- and glucocorticoid-induced kinase-1 by the ubiquitin-proteasome pathway. FEBS J. 273:2913-2928. [DOI] [PubMed] [Google Scholar]
- 6.Botten, J., K. Mirowsky, D. Kusewitt, C. Ye, K. Gottlieb, J. Prescott, and B. Hjelle. 2003. Persistent Sin Nombre virus infection in the deer mouse (Peromyscus maniculatus) model: sites of replication and strand-specific expression. J. Virol. 77:1540-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadwell, K., and L. Coscoy. 2005. Ubiquitination on nonlysine residues by a viral E3 ubiquitin ligase. Science 309:127-130. [DOI] [PubMed] [Google Scholar]
- 9.Cosgriff, T. M. 1991. Mechanisms of disease in Hantavirus infection: pathophysiology of hemorrhagic fever with renal syndrome. Rev. Infect. Dis. 13:97-107. [DOI] [PubMed] [Google Scholar]
- 10.Crook, T., J. A. Tidy, and K. H. Vousden. 1991. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell 67:547-556. [DOI] [PubMed] [Google Scholar]
- 11.Duchin, J. S., F. T. Koster, C. J. Peters, G. L. Simpson, B. Tempest, S. R. Zaki, T. G. Ksiazek, P. E. Rollin, S. Nichol, E. T. Umland, et al. 1994. Hantavirus pulmonary syndrome: a clinical description of 17 patients with a newly recognized disease. N. Engl. J. Med. 330:949-955. [DOI] [PubMed] [Google Scholar]
- 12.Ehrlich, R. 1997. Modulation of antigen processing and presentation by persistent virus infections and in tumors. Hum. Immunol. 54:104-116. [DOI] [PubMed] [Google Scholar]
- 13.Flinn, E. M., C. M. Busch, and A. P. Wright. 1998. myc boxes, which are conserved in myc family proteins, are signals for protein degradation via the proteasome. Mol. Cell. Biol. 18:5961-5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gardner, R. G., and R. Y. Hampton. 1999. A ‘distributed degron’ allows regulated entry into the ER degradation pathway. EMBO J. 18:5994-6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gavrilovskaya, I. N., N. S. Apekina, A. Myasnikov Yu, A. D. Bernshtein, E. V. Ryltseva, E. A. Gorbachkova, and M. P. Chumakov. 1983. Features of circulation of hemorrhagic fever with renal syndrome (HFRS) virus among small mammals in the European U.S.S.R. Arch. Virol. 75:313-316. [DOI] [PubMed] [Google Scholar]
- 16.Geimonen, E., I. Fernandez, I. N. Gavrilovskaya, and E. R. Mackow. 2003. Tyrosine residues direct the ubiquitination and degradation of the NY-1 hantavirus G1 cytoplasmic tail. J. Virol. 77:10760-10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geimonen, E., R. LaMonica, K. Springer, Y. Farooqui, I. N. Gavrilovskaya, and E. R. Mackow. 2003. Hantavirus pulmonary syndrome-associated hantaviruses contain conserved and functional ITAM signaling elements. J. Virol. 77:1638-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geimonen, E., S. Neff, T. Raymond, S. S. Kocer, I. N. Gavrilovskaya, and E. R. Mackow. 2002. Pathogenic and nonpathogenic hantaviruses differentially regulate endothelial cell responses. Proc. Natl. Acad. Sci. USA 99:13837-13842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst, A., S. E. Salghetti, S. Y. Kim, and W. P. Tansey. 2004. Multiple cell-type-specific elements regulate Myc protein stability. Oncogene 23:3863-3871. [DOI] [PubMed] [Google Scholar]
- 20.Hochstrasser, M., and A. Varshavsky. 1990. In vivo degradation of a transcriptional regulator: the yeast alpha 2 repressor. Cell 61:697-708. [DOI] [PubMed] [Google Scholar]
- 21.Huang, J., Q. Huang, X. Zhou, M. M. Shen, A. Yen, S. X. Yu, G. Dong, K. Qu, P. Huang, E. M. Anderson, S. Daniel-Issakani, R. M. Buller, D. G. Payan, and H. H. Lu. 2004. The poxvirus p28 virulence factor is an E3 ubiquitin ligase. J. Biol. Chem. 279:54110-54116. [DOI] [PubMed] [Google Scholar]
- 22.Ishido, S., C. Wang, B. S. Lee, G. B. Cohen, and J. U. Jung. 2000. Downregulation of major histocompatibility complex class I molecules by Kaposi's sarcoma-associated herpesvirus K3 and K5 proteins. J. Virol. 74:5300-5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Johnson, P. R., R. Swanson, L. Rakhilina, and M. Hochstrasser. 1998. Degradation signal masking by heterodimerization of MATα2 and MATa1 blocks their mutual destruction by the ubiquitin-proteasome pathway. Cell 94:217-227. [DOI] [PubMed] [Google Scholar]
- 24.Lee, H. W. 1982. Hemorrhagic fever with renal syndrome (HFRS). Scand. J. Infect. Dis. Suppl. 36:82-85. [PubMed] [Google Scholar]
- 25.Lober, C., B. Anheier, S. Lindow, H. D. Klenk, and H. Feldmann. 2001. The Hantaan virus glycoprotein precursor is cleaved at the conserved pentapeptide WAASA. Virology 289:224-229. [DOI] [PubMed] [Google Scholar]
- 26.Lorick, K. L., J. P. Jensen, S. Fang, A. M. Ong, S. Hatakeyama, and A. M. Weissman. 1999. RING fingers mediate ubiquitin-conjugating enzyme (E2)-dependent ubiquitination. Proc. Natl. Acad. Sci. USA 96:11364-11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills, J. N., J. M. Johnson, T. G. Ksiazek, B. A. Ellis, P. E. Rollin, T. L. Yates, M. O. Mann, M. R. Johnson, M. L. Campbell, J. Miyashiro, M. Patrick, M. Zyzak, D. Lavender, M. G. Novak, K. Schmidt, C. J. Peters, and J. E. Childs. 1998. A survey of hantavirus antibody in small-mammal populations in selected United States National Parks. Am. J. Trop. Med. Hyg. 58:525-532. [DOI] [PubMed] [Google Scholar]
- 28.Nichol, S. T., C. F. Spiropoulou, S. Morzunov, P. E. Rollin, T. G. Ksiazek, H. Feldmann, A. Sanchez, J. Childs, S. Zaki, and C. J. Peters. 1993. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science 262:914-917. [DOI] [PubMed] [Google Scholar]
- 29.Nuti, M., M. Agostini, E. Albini, T. Avsic-Zupanc, and A. Kraigher. 1992. Antibodies against the Hantaan virus in Italian ex-soldiers stationed in the Balkans during World War II. Ann. Ig. 4:23-28. (In Italian.) [PubMed] [Google Scholar]
- 30.Oresic, K., V. Noriega, L. Andrews, and D. Tortorella. 2006. A structural determinant of human cytomegalovirus US2 dictates the down-regulation of class I major histocompatibility molecules. J. Biol. Chem. 281:19395-19406. [DOI] [PubMed] [Google Scholar]
- 31.Pensiero, M. N., and J. Hay. 1992. The Hantaan virus M-segment glycoproteins G1 and G2 can be expressed independently. J. Virol. 66:1907-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plyusnin, A., O. Vapalahti, and A. Vaheri. 1996. Hantaviruses: genome structure, expression and evolution. J. Gen. Virol. 77:2677-2687. [DOI] [PubMed] [Google Scholar]
- 33.Salghetti, S. E., S. Y. Kim, and W. P. Tansey. 1999. Destruction of Myc by ubiquitin-mediated proteolysis: cancer-associated and transforming mutations stabilize Myc. EMBO J. 18:717-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmaljohn, C. 1996. Bunyaviridae and their replication, p. 1447-1471. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, PA. [Google Scholar]
- 35.Schmaljohn, C., and B. Hjelle. 1997. Hantaviruses: a global disease problem. Emerg. Infect. Dis. 3:95-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song, J. W., L. J. Baek, I. N. Gavrilovskaya, E. R. Mackow, B. Hjelle, and R. Yanagihara. 1996. Sequence analysis of the complete S genomic segment of a newly identified hantavirus isolated from the white-footed mouse (Peromyscus leucopus): phylogenetic relationship with other sigmodontine rodent-borne hantaviruses. Virus Genes 12:249-256. [DOI] [PubMed] [Google Scholar]
- 37.Sosa-Estani, S., V. P. Martinez, M. Gonzalez Della Valle, A. Edelstein, S. Miguel, P. J. Padula, M. L. Cacase, and E. L. Segura. 2002. Hantavirus in human and rodent population in an endemic area for hantavirus pulmonary syndrome in Argentina. Medicina (Buenos Aires). 62:1-8. (In Spanish.) [PubMed] [Google Scholar]
- 38.Spiropoulou, C. F., C. S. Goldsmith, T. R. Shoemaker, C. J. Peters, and R. W. Compans. 2003. Sin Nombre virus glycoprotein trafficking. Virology 308:48-63. [DOI] [PubMed] [Google Scholar]
- 39.Tager Frey, M., P. C. Vial, C. H. Castillo, P. M. Godoy, B. Hjelle, and M. G. Ferres. 2003. Hantavirus prevalence in the IX Region of Chile. Emerg. Infect. Dis. 9:827-832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Wal, F. J., M. Kikkert, and E. Wiertz. 2002. The HCMV gene products US2 and US11 target MHC class I molecules for degradation in the cytosol. Curr. Top. Microbiol. Immunol. 269:37-55. [DOI] [PubMed] [Google Scholar]
- 41.Vapalahti, O., A. Lundkvist, S. K. Kukkonen, Y. Cheng, M. Gilljam, M. Kanerva, T. Manni, M. Pejcoch, J. Niemimaa, A. Kaikusalo, H. Henttonen, A. Vaheri, and A. Plyusnin. 1996. Isolation and characterization of Tula virus, a distinct serotype in the genus Hantavirus, family Bunyaviridae. J. Gen. Virol. 77:3063-3067. [DOI] [PubMed] [Google Scholar]
- 42.Varshavsky, A. 1991. Naming a targeting signal. Cell 64:13-15. [DOI] [PubMed] [Google Scholar]
- 43.Wells, R. M., J. Young, R. J. Williams, L. R. Armstrong, K. Busico, A. S. Khan, T. G. Ksiazek, P. E. Rollin, S. R. Zaki, S. T. Nichol, and C. J. Peters. 1997. Hantavirus transmission in the United States. Emerg. Infect. Dis. 3:361-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaki, S. R., P. W. Greer, L. M. Coffield, C. S. Goldsmith, K. B. Nolte, K. Foucar, R. M. Feddersen, R. E. Zumwalt, G. L. Miller, A. S. Khan, et al. 1995. Hantavirus pulmonary syndrome. Pathogenesis of an emerging infectious disease. Am. J. Pathol. 146:552-579. [PMC free article] [PubMed] [Google Scholar]