Abstract
The mechanism of entry of hepatitis C virus (HCV) through interactions between the envelope glycoproteins and specific cell surface receptors remains unclear at this time. We have previously shown with the vesicular stomatitis virus (VSV)/HCV pseudotype model that the hypervariable region 1 of the HCV E2 envelope glycoprotein helps in binding with glycosaminoglycans present on the cell surface. In this study, we have examined the binding of HCV envelope glycoproteins with chemically modified derivatives of heparin. Furthermore, we have determined the functional relevance of the interaction of heparin derivatives with HCV envelope glycoproteins for infectivity by using a human immunodeficiency virus (HIV)/HCV pseudotype, a VSV/HCV pseudotype, and cell culture-grown HCV genotype 1a. Taken together, our results suggest that the HCV envelope glycoproteins rely upon O-sulfated esters of a heparin homologue to facilitate entry into mammalian cells.
We first reported the generation of a vesicular stomatitis virus (VSV)/hepatitis C virus (HCV) pseudotype to understand the role of the ectodomains of HCV envelope glycoproteins in relation to the initiation of virus infection (20). Since VSV particles assemble at the plasma membrane (1), we expressed chimeric HCV envelope glycoproteins (E1G and E2G) by appending the transmembrane domain and cytoplasmic tail of VSV glycoprotein at their C termini to provide membrane anchor signals. This modification allowed for the localization of the glycoproteins to the cell surface for efficient incorporation onto VSV pseudotype particles. Pseudotypes generated from either E1G or E2G which had been expressed individually displayed a low level of infectivity, while the presence of both envelope glycoproteins significantly increased the infectious titer of the pseudotype (26). Interestingly, each HCV envelope protein is suggested to have class I and/or class II fusion peptide motifs (10, 11, 12, 31), which may contribute to the infectivity of the parent virus. Murine leukemia virus (MuLV)- and human immunodeficiency virus (HIV)-derived pseudotypes were subsequently generated from human embryonic kidney epithelial cells (293T) by the expression of an unmodified HCV envelope genomic region (5, 15). Although there are apparent differences, the overall observations from MuLV- and HIV-derived pseudotypes were similar to observations from the VSV/HCV pseudotype (8, 26). We have previously observed that the E2 glycoprotein binds to cell surface glycosaminoglycans (GAGs) (7, 24). Further studies suggested that the hypervariable region 1 (HVR1) of E2 binds with heparin, and the infectivity of the VSV/HCV pseudotype is diminished in the presence of soluble GAGs or decreased after the enzymatic removal of heparin-like GAGs from the cell surface (7). A different group of investigators have suggested that HCV particles can be efficiently purified from patient sera by using heparin, and GAG binding sites are located within E2 (27, 32).
The binding of E2 with a heparin-like molecule appears to facilitate VSV-derived pseudotype attachment to susceptible mammalian cells, but E2 could not be identified as a single entry receptor (7). Other investigators have also reported that virus-like particles containing E2 and HCV from plasma of chronically infected patients interact with heparan sulfate (HS) proteoglycans for cellular binding (3, 13). CD81, a tetraspanin molecule present on some cell surfaces, has been implicated in facilitating HCV entry (21, 29, 33), although the mechanism of virus entry related to this protein remains unknown at this time. We have recently grown HCV genotype 1a in cell culture (17). In this study, we have analyzed the role of HCV envelope glycoproteins in the interaction with chemically modified heparin derivatives to further elucidate the mechanism of HCV entry by using HIV- and VSV-derived pseudotypes and cell culture-grown HCV genotype 1a.
MATERIALS AND METHODS
Reagents.
Heparin, oversulfated heparin, de-O-sulfated heparin, de-N-sulfated heparin, 2-O-desulfated heparin, 6-O-desulfated heparin, and desulfated heparin were procured from Neoparin Inc., San Leandro, CA. Mouse monoclonal antibody to HCV E1 (3D5/C3) and E2 (3E5-1) and affinity-purified E1/E2 or E2 glycoprotein of HCV genotype 1a produced in a CHO cell line were kindly provided by Michael Houghton (Chiron Corporation). A fluorescein-conjugated monoclonal antibody (C8A018F) to the HCV NS4 region (Biodesign) was used for the detection of HCV protein in focus-forming assays of virus-infected cells.
Enzyme-linked immunosorbent assay (ELISA).
Recombinant E2 glycoprotein was used in a polysaccharide binding assay as described previously (1). The monoclonal antibody to E2 (3E5-1) and a peroxidase-conjugated goat anti-mouse immunoglobulin G were used to detect the binding of E2 on polysaccharide-coated wells.
Generation of pseudotypes and cell culture-grown HCV.
The incorporation of HCV E1G and E2G chimeric glycoproteins onto a temperature-sensitive mutant of VSV (VSVts045) has been previously described (7, 20, 24, 25, 26). The titers of VSV-derived pseudotypes were determined by a plaque assay (20). HIV-derived pseudotypes were generated by the expression of the E1G and E2G gene constructs (26) in trans from HCV genotype 1a (GenBank accession number M62321) or from an unmodified E1-E2 genomic sequence (corresponding to amino acid residues 174 to 746) from H77C genotype 1a (GenBank accession number AF011751). A PCR-amplified genomic region was cloned into the pcDNA3 mammalian expression vector (Invitrogen, Carlsbad, CA) under the control of a cytomegalovirus promoter. HIV pseudotypes were generated by the cotransfection of a subclone of human embryonic kidney epithelial cells (293FT; Invitrogen) with equal quantities of plasmid DNA (4 μg total/35-mm2 dish) expressing the full-length HCV glycoprotein region, equal quantities of HCV glycoprotein chimeric constructs, or empty vector (negative control) and the envelope-defective pNL4.3.Luc.R−E− proviral genome by using Lipofectamine 2000 (15). Variations in p24 amounts (Beckman Coulter, Fullerton, CA) were observed in batches of HIV-derived pseudotypes, which could be due to differences in the efficiencies of pseudotype formation or the generation of bald particles. Therefore, to minimize artifacts, infectivity was expressed as relative luciferase units (RLU). Target cells were seeded into 24-well plates and incubated with viral supernatants in 3% fetal bovine serum-Dulbecco's modified Eagle's medium containing polybrene (5 μg/ml). Cells were washed and lysed with 50 μl of reporter lysis buffer after 72 h of incubation. Cell lysates (20 μl) were analyzed for luciferase activity by the addition of substrate (Promega, Madison, WI) in a luminometer (Lumat LB 9507; Berthold Technologies, Oak Ridge, TN).
HCV genotype 1a (H77) was grown in immortalized human hepatocytes as recently described (17). Virus growth was measured in serial dilutions of cell culture supernatant filtered through a 0.45-μm-pore-size cellulose acetate membrane (Nalgene, Rochester, NY) by a fluorescent-focus-forming assay (17). The HCV titer was calculated as ∼105 focus-forming units (FFU)/ml.
Assay for the inhibition of virus infection.
Inhibitors were added to a predetermined titer of the HIV/HCV pseudotype (∼5 × 104 RLU/reaction), the VSV/HCV pseudotype (∼100 PFU/reaction), or cell culture-grown HCV (∼100 FFU/reaction), and the mixture was incubated for 1 h at 37°C. The inhibitor-virus mixture was added to the cell monolayer. Untreated viruses were used similarly for comparison. Cells were washed following incubation, and infectivity was determined as described previously (17, 18, 24).
Western blot analysis.
Culture fluid (10 ml) containing pseudotype virus or culture fluid from mock-transfected cells was clarified, pelleted by ultracentrifugation, and analyzed as previously described (26). Affinity-purified HCV E1 and E2 were used as envelope glycoprotein markers in sodium dodecyl sulfate-polyacrylamide gel electrophoresis. HCV E1 or E2 protein was detected using specific mouse monoclonal antibody and anti-mouse immunoglobulin conjugated to peroxidase, followed by chemiluminescence.
RESULTS
Characterization of the HIV/HCV pseudotype.
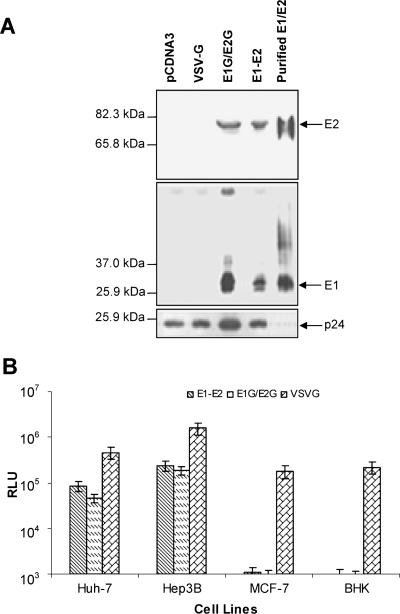
HIV-derived pseudotypes with a chimeric E1G-E2G sequence (26) or an unmodified E1-E2 sequence were generated to investigate the role of the ectodomains of E1 and E2 in virus entry. The plasmids expressing chimeric E1G and E2G from HCV genotype 1a (GenBank accession number M62321) under the control of a cytomegalovirus or MuLV promoter have been described previously (8, 26). These chimeric E1G and E2G expression plasmids differ from that used by Hsu et al. (15) in containing the signal sequences of E1 and E2 envelope proteins. The E1G and E2G constructs used by Hsu et al. (15) contained the signal sequence of VSV envelope glycoprotein. Western blot analysis demonstrated that both pseudotype preparations incorporated E1 and E2 glycoproteins onto HIV particles (Fig. 1A). Reprobing the blot with an antibody to p24 (HIV capsid protein) suggested a difference in the levels of HCV E1G/E2G and E1-E2, which may be due to variations in protein loads. HIV-derived pseudotypes generated from E1G-E2G and unmodified E1-E2 induced comparable levels of reporter luciferase activity (6 × 104 to 4 × 105 RLU) in Huh-7 and Hep3B cells (Fig. 1B), and these levels were much higher than those apparent in the nonhepatic MCF-7 and BHK cell lines (∼0.5× 103 to 1.2 × 103 RLU). The luciferase reporter activity of the HIV/VSVG pseudotype (as a positive control) in Hep3B cells was 5- to 10-fold higher than that observed in Huh-7, MCF-7, or BHK cells. This difference could be due to a variation in the transduction efficiencies of the lentivirus-mediated delivery system based on species and cell types (14, 16, 22), as it is not accurately reflected in the natural VSV infectious titers across these same cell lines (data not shown). Culture supernatant from cells transfected with empty-vector DNA as a negative control displayed an undetectable level (<102 RLU) of luciferase activity (data not shown). The results from this set of experiments indicated that HCV envelope glycoproteins from chimeric or unmodified sequences are incorporated onto HIV-derived pseudotype particles and these particles display a level of infectivity which is comparable to that of particles generated using the native envelope sequence.
FIG. 1.
Generation and characterization of HIV-derived pseudotype viruses. (A) Incorporation of unmodified and chimeric envelope glycoproteins of HCV onto HIV-derived pseudotype particles. Pelleted pseudotype virus was lysed, subjected to sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis, and transferred onto a nitrocellulose membrane for Western blot analysis using anti-E2 or anti-E1 specific monoclonal antibody. The positions of E2 and E1 are shown by arrows on the right. Viral proteins were authenticated from the positions of purified E1 and E2 glycoproteins and from molecular masses by comparison with prestained protein markers (Bio-Rad). The blot was reprobed with antibody to p24 for comparison of the levels of HCV envelope glycoproteins in the lanes. (B) Pseudotype viruses were examined for infectivity in Huh-7, Hep3B, MCF-7, and BHK cells by a luciferase reporter assay. Luciferase activities are presented as the means with standard deviations of results from five different experiments. The infectivity of the HIV-VSV glycoprotein pseudotype from diluted (1/100) culture medium was used as a positive control.
Sulfation is required for the binding of E2 to heparin.
Heparin is a complex polysaccharide composed of repeating disaccharides of uronic acid and glucosamine. The disaccharide units may be biosynthetically modified as N-acetyl or N-sulfate glucosamine, 2-O-sulfate uronic acid, and 6-O-sulfate and/or 3-O-sulfate glucosamine. In oversulfated heparin, all primary hydroxyls in glucosamine residues and a large proportion of secondary hydroxyl groups in disaccharide units are replaced. In de-N-sulfated heparin, the N-sulfated glucosamine residues of heparin are removed. In contrast, in de-O-sulfated heparin, all O-sulfate esters of heparin are removed without changing the backbone structure and most of the negative charges contributed by O-sulfates are eliminated.
Our previous findings (7) have suggested that an interaction of cell surface GAGs with E2 may facilitate virus entry. GAGs are long, unbranched polysaccharide chains consisting of repeating disaccharide units of an amino sugar (either N-acteylglucosamine or N-acteylgalactosamine) and a hexuronic acid (either glucuronic acid or its epimer, l-iduronic acid) that are covalently attached via an O-glycosidic linkage between xylose in the sugar chain and serine in the host cell membrane protein to form proteoglycans. During GAG synthesis, the chain of sugar residues may be further modified by N and/or O sulfation, de-N-acetylation, and variation in the chain length. While GAGs are found on the surfaces of almost all animal cells, the numbers and positions of sulfate groups and N-acetyl groups and chain lengths as well as the total numbers of saccharide chains per protein molecule contribute to the observed heterogeneity in GAG expression, which in turn determines the specificity of the individual GAG interactions with a wide variety of binding proteins (9). To identify the relevance of these modifications in relation to the E2-GAG interaction, we performed envelope glycoprotein binding experiments using chemically modified heparin derivatives to analyze their individual binding capacities as well as their abilities to act as competitive inhibitors.
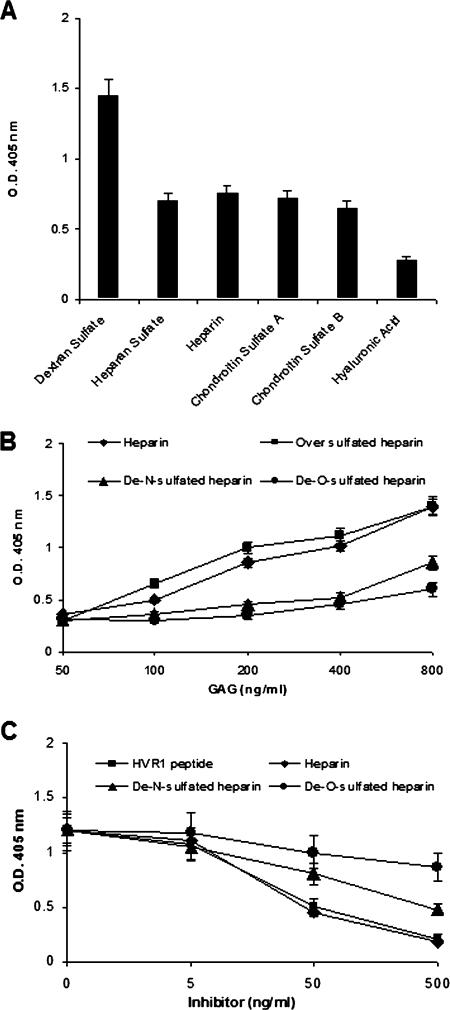
We first examined the interaction of complex carbohydrates with HCV E2 envelope glycoprotein by ELISA. The carbohydrates included dextran sulfate, heparan sulfate, heparin, chondroitin sulfate A (CS-A) and CS-B, and hyaluronic acid. Each of these complex carbohydrates has substantial sequence heterogeneity, with the exception of hyaluronic acid. Dextran sulfate displayed maximal binding with E2 (Fig. 2A), and a similar level of binding between E2 and GAGs (heparin, heparan sulfate, and chondroitin sulfate A and B) was observed. As these GAGs have similar levels of 2-O-sulfation, the results suggested that 2-O-sulfation is important for E2 binding. To further examine the importance of O and N sulfation of the GAG, we compared the binding activities of E2 with unmodified heparin and chemically modified heparin (oversulfated heparin, de-N-sulfated heparin, and de-O-sulfated heparin). Oversulfated heparin is a derivative of heparin which is further sulfated at the N position of glucuronic acid. De-N-sulfated heparin is a derivative in which N-sulfate groups of the N-sulfated glucosamine residues of heparin are removed. De-O-sulfated heparin is a derivative in which all O-sulfate esters of heparin have been removed without changing the backbone structure. Heparin or its derivatives were serially diluted and used to coat ELISA plates, and the plates were incubated with purified E2 protein. Absorbance was read against that of a negative control to calculate the concentration of E2 bound to heparin or its derivatives. Absorbance values were found to be linearly related to concentrations of heparin ranging between 50 and 800 ng/ml used for coating ELISA plates (Fig. 2B). Oversulfated heparin displayed a slightly higher level of E2 binding than unmodified heparin between 100 and 400 ng/ml. The de-O-sulfated heparin or de-N-sulfated heparin did not display a significant level of binding with E2 at concentrations of up to 400 ng/ml, while de-N-sulfated heparin retained a weak binding at a higher concentration (800 ng/ml).
FIG. 2.
Comparison of the binding patterns of HCV envelope glycoprotein(s) with GAGs and heparin derivatives. (A) Polysaccharides and GAGs (10 μg/well) were used to coat ELISA plates, and the binding of HCV E2 was measured. The binding of the HCV envelope glycoprotein was measured following incubation with a monoclonal antibody to E2 (3E5-1) and a second antibody-horseradish peroxidase conjugate. The absorbance was read at 405 nm, and optical densities (O.D.) of experimental samples are presented as the means, together with standard deviations, of results from three independent experiments after the subtraction of negative control values (<0.15). (B) Heparin and its derivatives at the indicated doses were used to coat ELISA plates, and the binding of HCV E2 was measured following incubation with a specific monoclonal antibody (3E5-1) as described above. (C) Competitive inhibition of E2 binding to heparin by chemically modified heparin derivatives and HVR1 peptide. An ELISA plate was coated with highly purified E2 recombinant protein of HCV. Serially diluted unlabeled HVR1, heparin, de-N-sulfated heparin, and de-O-sulfated heparin were used as competitors. Biotin-conjugated heparin at a fixed concentration was immediately added to the wells. The binding of biotinylated heparin was measured after incubation with streptavidin-horseradish peroxidase conjugate. Results are presented as the means together with the standard deviations of results from three independent assays after the subtraction of negative control values (<0.15).
The binding specificity of E2 in relation to the sulfated esters of heparin was elaborated by a competition ELISA using heparin, de-N-sulfated heparin, de-O-sulfated heparin, and HVR1 as competitors with a predetermined dose of biotinylated heparin (Fig. 2C). The biotinylated heparin was treated with serial dilutions of the competitors for 1 h at 37°C and added to E2-coated ELISA plates (1 μg/well). A dose-dependent inhibition of biotinylated-heparin binding to E2 was observed using an HVR1-specific peptide and heparin. However, de-N-sulfated heparin and de-O-sulfated heparin displayed lesser extents of inhibition than heparin. The results of the binding assay, and the competitive inhibition, suggested that the specific occupation of sulfate sites is required for the binding of E2 to heparin.
Sulfation of heparin is necessary for the inhibition of pseudotype infectivity.
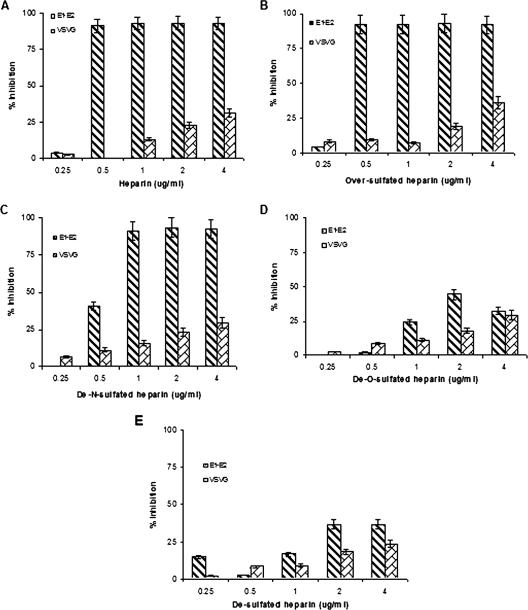
We have compared the inhibitory effects of heparin and its derivatives on the infectivities of pseudotypes. HIV/HCV E1-E2 and HIV/VSVG pseudotypes were incubated with various concentrations of heparin or its derivatives at 37°C and subsequently added to Huh-7 cells (Fig. 3). The 50% inhibitory concentration (IC50) for the HIV/HCV E1-E2 pseudotype was 0.5 μg/ml for heparin (Fig. 3A) and oversulfated heparin (Fig. 3B), while the HIV/VSVG pseudotype (control) did not display comparable inhibition at similar doses. De-N-sulfated heparin displayed similar inhibition of virus infectivity at concentrations between 1 and 4 μg/ml (Fig. 3C), while de-O-sulfated heparin (Fig. 3D) and desulfated heparin (Fig. 3E) did not display a significant inhibitory effect on pseudotype infection at concentrations between 0.25 and 4.0 μg/ml. We also utilized VSV pseudotypes to understand the interactions of the chimeric E1G and E2G glycoproteins with heparin or its derivatives (Fig. 4).
FIG. 3.
Effects of heparin derivatives on the inhibition of HIV/HCV E1-E2 pseudotype infection. Pseudotypes were incubated with heparin (A), oversulfated heparin (B), de-N-sulfated heparin (C), de-O-sulfated heparin (D), and desulfated heparin (E). A virus-antibody mixture was added to Huh-7 cells for infection, and the inhibitory effects of heparin and its derivatives on virus infectivity are shown. The effects of heparin derivatives on HIV/VSV glycoprotein pseudotype infectivity are shown in parallel as a control. The results are presented as means together with standard deviations of results from three independent assays.
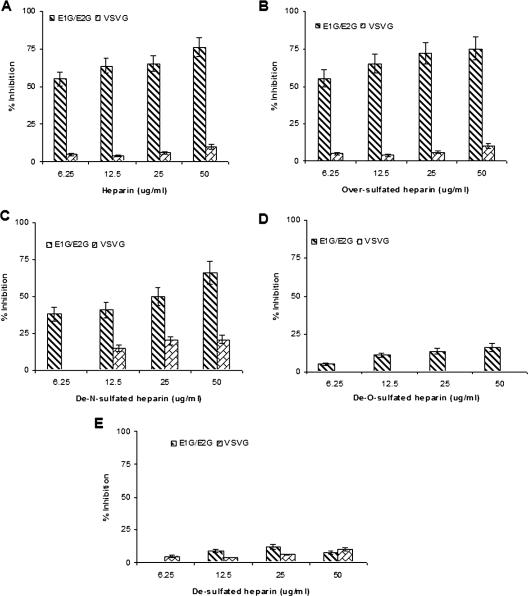
FIG. 4.
Effects of heparin derivatives on the inhibition of VSV/HCV E1G/E2G pseudotypes. Pseudotypes were incubated with heparin (A), oversulfated heparin (B), de-N-sulfated heparin (C), de-O-sulfated heparin (D), and desulfated heparin (E). A virus-antibody mixture was added to Huh-7 cells for infection, and the inhibitory effects of the heparin and its derivatives on virus infectivity are shown. The effects of heparin derivatives on VSV glycoprotein pseudotype infectivity are shown in parallel as a control. The results are presented as means together with standard deviations of results from three independent assays.
Heparin and oversulfated heparin inhibited VSV/HCV E1G/E2G pseudotype infectivity in a dose-dependent manner, and an IC50 of ∼6.25 μg/ml was noted (Fig. 4A and B). Similar analyses did not suggest a significant inhibitory effect on pseudotype virus harboring the VSV control glycoprotein. The use of de-N-sulfated heparin suggested a slight loss in HCV glycoprotein interaction and displayed an inhibitory effect of de-N-sulfated heparin at ≤25 μg/ml. However, de-O-sulfated heparin or desulfated heparin did not display a significant inhibitory effect upon the VSV/HCV E1G/E2G pseudotype (Fig. 4D and E). Results from these experiments indicated a strong specific inhibition of HIV-derived pseudotype infection at lower doses of heparin or its O-sulfated derivatives, while the use of the VSV-derived pseudotype required a higher quantity for the inhibition of infectivity.
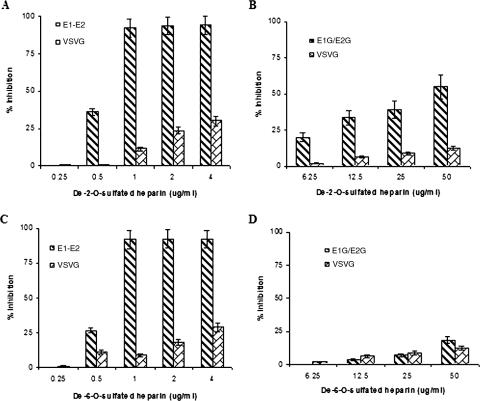
To further determine the specificities of O-sulfated heparin derivatives, pseudotypes were examined for the inhibition of infectivity by de-2-O-sulfated heparin or de-6-O-sulfated heparin (Fig. 5). The results from this inhibition study indicated that both heparin derivatives are effective in inhibiting HIV-derived pseudotypes with only a slight decrease in effectiveness compared to heparin (Fig. 5A and C). However, the removal of 6-O-sulfated esters from the heparin backbone reduced the ability to inhibit the infectivity of the VSV-derived pseudotype to a level similar to that apparent with de-O-sulfated heparin (Fig. 5B and D). The loss of 2-O-sulfation greatly reduced the inhibitory effect, but inhibition was still apparent at ≥50 μg/ml. On the other hand, both the heparin derivatives had relatively weak inhibitory effects on the glycoprotein used as a control. As in the observations described in the legend to Fig. 4, the sensitivities of the inhibitory effects of the heparin derivatives varied significantly based upon dose and the nature of the pseudotypes used in the studies. HIV-derived pseudotypes were highly sensitive to lower doses, while VSV-derived pseudotypes were inhibited with higher quantities of the heparin derivatives. The differences in susceptibilities of the pseudotypes could be due to the sensitivity of each assay or the numbers of glycoproteins incorporated onto virus particle surfaces by using each method.
FIG. 5.
Effect of O-sulfated heparin derivatives on the inhibition of HIV/HCV E1-E2 or VSV/HCV E1G/E2G pseudotypes. HIV-derived pseudotypes were incubated with de-2-O-sulfated and de-6-O-sulfated heparin (panels A and C, respectively), and the inhibitory effects upon pseudotype infectivity at different doses were determined. Similar analyses were performed with VSV-derived pseudotypes by using de-2-O-sulfated and de-6-O-sulfated heparin (panels B and D, respectively). The results are presented as means together with standard deviations of results from three independent assays.
O-sulfated heparin is effective in inhibiting infection with cell culture-derived HCV.
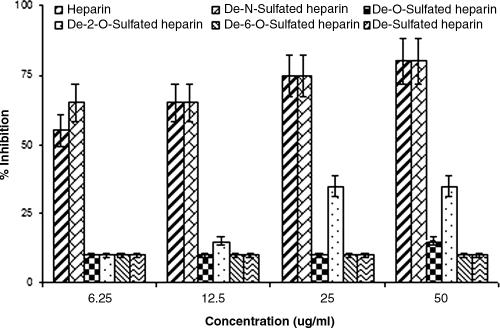
To examine if interactions with certain GAGs on the surfaces of hepatocytes may facilitate the attachment and entry of HCV genotype 1a, we performed an inhibition-of-infection study in the presence of heparin and its derivatives with immortalized human hepatocytes as previously described (17). The results (Fig. 6) suggested similar dose-dependent levels of inhibition of infection by heparin and de-N-sulfated heparin (IC50 of ≤6.25 μg/ml). However, the de-O-sulfation or desulfation of heparin significantly reduced the inhibitory effect on virus infection. Further, while de-2-O-sulfated heparin only partially inhibited virus infection, de-6-O-sulfated heparin tested at concentrations of up to 50 μg/ml failed to display any significant inhibitory effect upon HCV infection in this set of experiments. Typical results from the use of 50 μg/ml of GAGs in the inhibition of virus focus formation are shown (Fig. 7). Together, these data are in agreement with our results for the pseudotype virus and suggest the importance of 6-O-sulfated esters for the interaction of cell surface GAGs with HCV for attachment.
FIG. 6.
Inhibitory effect of heparin and its derivatives upon the infectivity of cell culture-grown HCV genotype 1a. Cell culture-grown virus was incubated with GAGs at the indicated doses prior to addition to immortalized human hepatocytes. Cells were incubated for 3 days, and the fluorescent-focus formation was determined by immunofluorescence using NS4-fluorescein isothiocyanate-conjugated monoclonal antibody (Biodesign). Nuclear staining was performed with TO-PRO-3 (Molecular Probes). The percent inhibition was calculated based on the number of fluorescent foci compared to that in the untreated positive control. The results are presented as means together with standard deviations of results from triplicate experiments.
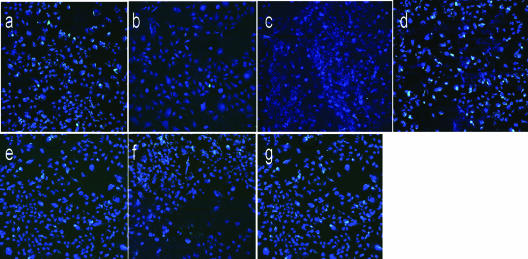
FIG. 7.
Results from a typical focus-forming assay of HCV H77 genotype 1a with and without GAG, as described in the legend to Fig. 6, are shown. Fluorescent-focus formation in the untreated positive control (a) and inhibition by 50 μg/ml of heparin (b), de-N-sulfated heparin (c), de-O-sulfated heparin (d), de-2-O-sulfated heparin (e), de-6-O-sulfated heparin (f), and de-sulfated heparin (g) are shown.
DISCUSSION
We have utilized two different pseudotype systems and cell culture-grown HCV genotype 1a to understand the nature of the interactions of HCV envelope glycoproteins with cell surface molecules in facilitating the entry of pseudotype or cell culture-grown HCV into susceptible host cells. Our results from HIV-derived pseudotypes harboring chimeric E1G-E2G clearly displayed the ability to infect Huh-7 and Hep3B cells. Thus, the replacement of the C-terminal regions of HCV E1 and E2 glycoproteins with that of VSV glycoprotein does not appear to enhance or abolish infectivity in the HIV-derived pseudotype model. The reason for this discordance with results in an earlier report (15) remains unknown at this time. The chimeric glycoproteins used by Hsu et al. (15) contained a signal peptide sequence of VSV glycoprotein unlike our chimeric E1G-E2G constructs (26). A recent study from Pfeiffer et al. (28) demonstrated that the replacement of an HIV envelope signal peptide sequence with heterologous signal peptides, derived from erythropoietin and tissue plasminogen activator, decreases the infectivity of HIV. The decrease is due to the incomplete removal of the signal peptide from the mature protein and/or the incomplete folding of the HIV envelope protein for premature removal of the signal peptide. Our earlier observations (20, 26) and a report from a different group of investigators (23) also indicated that the ectodomains of the HCV envelope glycoproteins are sufficient for the infectivity of the VSV/HCV pseudotype.
In this study, we have determined the structural requirements of HS by using chemically modified heparin as an analogue of cell surface-expressed heparan sulfate to analyze HCV glycoprotein binding. The HIV pseudotype displayed high sensitivity to the inhibition of infection by GAGs compared to the VSV pseudotype, and the saturation threshold appeared to be narrower with the HIV pseudotype than with the other infectious systems. The inhibitory effect of heparin or its derivatives at lower concentrations could be due to a lower level of incorporation of the natural endoplasmic reticulum-resident HCV envelope glycoproteins onto the HIV particle surface or due to a change in the glycosylation pattern of the E2 protein, as well as a higher sensitivity for readout of HIV-derived pseudotypes. Recently, Koutsoudakis et al. (19) have reported that the interactions of HCV with cell surface-resident GAGs aid in the efficient infection of Huh-7 cells and that CD81 acts during a postattachment step. They have demonstrated that heparin, but not other soluble GAGs which differ in their degree of sulfation and the composition of disaccharide units, competes with HCV particles for productive entry into Huh-7-Lunet cells, suggesting that interactions with a specific, highly sulfated type of GAG may contribute to efficient HCV infection. This observation agrees with our previous and present findings that the HCV envelope glycoproteins play a significant role in binding with heparin and may facilitate virus infection (7, 26). Therefore, synergism between observations from cell culture-grown virus and those from pseudotype models is emerging.
At the time of the preparation of this paper, Barth et al. (4) had reported that four viral epitopes overlapping the E2 HVR1 and E2-CD81 binding domains mediate HS binding. The HCV-HS binding requires a specific HS structure that includes N-sulfated groups and a minimum of 10 to 14 saccharide subunits. They had also shown the binding of E1 to heparin, but to a lesser extent, as we previously observed (24). A direct comparison of our experimental data with those from Barth et al. (4) is somewhat difficult, although there are elements to clarify these different conclusions. As the only apparent difference between the working materials of our two groups is the subtype of recombinant HCV envelope glycoproteins and HCV pseudotype particles used, the reason behind these differences remains to be determined. Further, in the inhibition of retrovirus-derived HCV pseudotypes, Barth et al. utilized heparins with different degrees of sulfation (but not the desulfated heparin derivatives included in our work) at a single concentration of 10 μg/ml. Furthermore, CS-A has the same degree of binding to E2 as other forms of GAGs except for hyaluronic acid (Fig. 2A), suggesting that CS-A should inhibit HCV attachment and entry. Yet in the study by Barth et al., CS-A did not inhibit HCV pseudotype particle entry. A more directly comparable difference is the binding to E2 of heparin and its de-O-sulfated form (Fig. 2B). Our results presented here using a lower concentration of heparin derivatives were unable to indicate a significant difference in the binding of de-N- and de-O-sulfated GAGs. In fact, all evidence indicated a slightly higher level of reactivity for the binding of de-N-sulfated heparin that was linear. While the de-O-sulfated heparin did not bind to E2, in the Barth et al. study, both de-2-O-sulfated and de-6-O-sulfated forms blocked E2 binding to target cells. Barth et al. have observed the binding properties of insect cell-derived HCV-like particles and individual recombinant E1 and E2 glycoproteins from HCV genotype 1b (isolate BE11) to determine specific reactivities to heparin derivatives. In these experiments, the presence of N-sulfated groups appeared to be necessary for GAG-HCV binding, while the inhibition of infection with HCV pseudotype particles by these derivatives was not investigated. To further clarify the discrepancies between our observations and those of Barth et al., each of the heparin derivatives used in our study was further analyzed for the ability to inhibit infection with cell culture-derived HCV and two separate pseudotype systems. Our results further indicated the importance of O-sulfated groups for the interaction of cell surface GAGs with HCV for attachment.
A number of different cell surface molecules have been proposed for the attachment of HCV, suggesting that multiple interactions with HCV envelope glycoproteins may occur (2, 3, 5, 6, 7, 15, 24, 26). However, none of the studies so far have demonstrated a complete receptor function for HCV entry into mammalian cells. We have previously shown with the VSV-derived pseudotypes that the inhibition of low-density lipoprotein receptor activity may partially interfere with E1 pseudotype infectivity while the inhibition of a CD81 interaction disrupts E2 pseudotype infectivity, as well as the infectivity of pseudotypes expressing both envelope glycoprotein ectodomains (24, 26). However, a high-affinity binding of E2 to CD81 is not a common feature of all HCV genotypes and the level of HCV entry has also been suggested to correlate with low-density lipoprotein receptor expression (30). The results from our present study suggest that E2 displays a loss of binding with desulfated heparin and that the infectivity of HIV- or VSV-derived pseudotypes and cell culture grown-HCV genotype 1a is inhibited by heparin but not by desulfated heparin. Our results from heparin derivatives clearly indicate that O-sulfated heparin is more effective in inhibiting infection with both pseudotypes and cell culture-derived HCV. Further studies should help in understanding the role of HCV envelope glycoproteins in the orchestration of virus entry through a specific receptor(s) into mammalian cells.
Acknowledgments
We are grateful to Michael Houghton for providing affinity-purified HCV envelope glycoproteins and monoclonal antibodies and Charles M. Rice for providing the full-length clone of HCV 1a H77. We thank Ratna B. Ray for helpful suggestions and Lin Cowick for preparation of the manuscript.
This research was supported by grants 1R43AI068194-01 (A.B.) and AI068769 (R.R.) from the National Institutes of Health.
Footnotes
Published ahead of print on 7 February 2007.
REFERENCES
- 1.Alban, S., and R. Gastpar. 2001. Development of SPC-ELISA: a new assay principle for the study of sulfated polysaccharide-protein interactions. J. Biomol. Screen. 6:393-400. [DOI] [PubMed] [Google Scholar]
- 2.Andre, P., G. Perlemuter, A. Budkowska, C. Brechot, and V. Lotteau. 2005. Hepatitis C virus particles and lipoprotein metabolism. Semin. Liver Dis. 25:93-104. [DOI] [PubMed] [Google Scholar]
- 3.Barth, H., C. Schafer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 278:41003-41012. [DOI] [PubMed] [Google Scholar]
- 4.Barth, H., E. K. Schnober, F. Zhang, R. J. Linhardt, E. Depla, B. Boson, F. L. Cosset, A. H. Patel, H. E. Blum, and T. F. Baumert. 2006. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J. Virol. 80:10579-10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197:633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartosch, B., G. Verney, M. Dreux, P. Donot, Y. Morice, F. Penin, J. M. Pawlotsky, D. Lavillette, and F. L. Cosset. 2005. An interplay between hypervariable region 1 of the hepatitis C virus E2 glycoprotein, the scavenger receptor BI, and high-density lipoprotein promotes both enhancement of infection and protection against neutralizing antibodies. J. Virol. 79:8217-8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu, A., A. Beyene, K. Meyer, and R. Ray. 2004. The hypervariable region 1 of the E2 glycoprotein of hepatitis C virus binds to glycosaminoglycans, but this binding does not lead to infection in a pseudotype system. J. Virol. 78:4478-4486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beyene, A., A. Basu, K. Meyer, and R. Ray. 2004. Influence of N-linked glycans on intracellular transport of hepatitis C virus E1 chimeric glycoprotein and its role in pseudotype virus infectivity. Virology 324:273-285. [DOI] [PubMed] [Google Scholar]
- 9.Choppin, P. W., and R. W. Compans. 1970. Phenotypic mixing of envelope proteins of the parainfluenza virus SV5 and vesicular stomatitis virus. J. Virol. 5:609-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drummer, H. E., and P. Poumbourios. 2004. Hepatitis C virus glycoprotein E2 contains a membrane-proximal heptad repeat sequence that is essential for E1E2 glycoprotein heterodimerization and viral entry. J. Biol. Chem. 279:30066-30072. [DOI] [PubMed] [Google Scholar]
- 11.Flint, M., C. Maidens, L. D. Loomis-Price, C. Shotton, J. Dubuisson, P. Monk, A. Higginbottom, S. Levy, and J. A. McKeating. 1999. Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J. Virol. 73:6235-6244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garry, R. F., and S. Dash. 2003. Proteomics computational analyses suggest that hepatitis C virus E1 and pestivirus E2 envelope glycoproteins are truncated class II fusion proteins. Virology 307:255-265. [DOI] [PubMed] [Google Scholar]
- 13.Germi, R., J.-M. Crance, D. Garin, J. Guimet, J. H. Lortat-Jacob, R. W.-H. Ruigrok, J.-P. Zarski, and E. Drouet. 2002. Cellular glycosaminoglycans and low density lipoprotein receptor are involved in hepatitis C virus adsorption. J. Med. Virol. 68:206-215. [DOI] [PubMed] [Google Scholar]
- 14.Ginn, S. L., J. Fleming, P. B. Rowe, and I. E. Alexander. 2003. Promoter interference mediated by the U3 region in early-generation HIV-1-derived lentivirus vectors can influence detection of transgene expression in a cell-type and species-specific manner. Hum. Gene Ther. 14:1127-1137. [DOI] [PubMed] [Google Scholar]
- 15.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. M. Rice, and J. A. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 100:7271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ikeda, Y., M. K. Collins, P. A. Radcliffe, K. A. Mitrophanous, and Y. Takeuchi. 2002. Gene transduction efficiency in cells of different species by HIV and EIAV vectors. Gene Ther. 9:932-938. [DOI] [PubMed] [Google Scholar]
- 17.Kanda, T., A. Basu, R. Steele, T. Wakita, J. S. Ryerse, R. Ray, and R. B. Ray. 2006. Generation of infectious hepatitis C virus in immortalized human hepatocytes. J. Virol. 80:4633-4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanda, T., R. Steele, R. Ray, and R. B. Ray. 2007. Small interfering RNA targeted to hepatitis C virus 5′-nontranslated region exerts potent antiviral effect. J. Virol. 81:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koutsoudakis, G., A. Kaul, E. Steinmann, S. Kallis, V. Lohmann, T. Pietschmann, and R. Bartenschlager. 2006. Characterization of the early steps of hepatitis C virus infection by using luciferase reporter viruses. J. Virol. 80:5308-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lagging, L. M., K. Meyer, R. J. Owens, and R. Ray. 1998. Functional role of hepatitis C virus chimeric glycoproteins in the infectivity of pseudotyped virus. J. Virol. 72:3539-3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lindenbach, B. D., M. J. Evans, A. J. Syder, B. Wolk, T. L. Tellinghuisen, C. C. Liu, T. Maruyama, R. O. Hynes, D. R. Burton, J. A. McKeating, and C. M. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309:623-626. [DOI] [PubMed] [Google Scholar]
- 22.Lois, C., E. J. Hong, S. Pease, E. J. Brown, and D. Baltimore. 2002. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295:868-872. [DOI] [PubMed] [Google Scholar]
- 23.Matsuura, Y., H. Tani, K. Suzuki, T. Kimura-Someya, R. Suzuki, H. Aizaki, K. Ishii, K. Moriishi, C. S. Robison, M. A. Whitt, and T. Miyamura. 2001. Characterization of pseudotype VSV possessing HCV envelope proteins. Virology 286:263-275. [DOI] [PubMed] [Google Scholar]
- 24.Meyer, K., A. Basu, and R. Ray. 2000. Functional features of hepatitis C virus glycoproteins for pseudotype virus entry into mammalian cells. Virology 276:214-226. [DOI] [PubMed] [Google Scholar]
- 25.Meyer, K., A. Basu, C. T. Przysiecki, L. M. Lagging, A. D. M. Di Bisceglie, A. J. Conley, and R. Ray. 2002. Complement-mediated enhancement of antibody function for neutralization of pseudotype virus containing hepatitis C virus E2 chimeric glycoprotein. J. Virol. 76:2150-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer, K., A. Beyene, T. L. Bowlin, A. Basu, and R. Ray. 2004. Coexpression of hepatitis C virus E1 and E2 chimeric envelope glycoproteins displays separable ligand sensitivity and increases pseudotype infectious titer. J. Virol. 78:12838-12847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olenina, L. V., T. I. Kuzmina, B. N. Sobolev, T. E. Kuraeva, E. F. Kolesanova, and A. I. Archakov. 2005. Identification of glycosaminoglycan-binding sites within hepatitis C virus envelope glycoprotein E2. J. Viral Hepat. 12:584-593. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer, T., T. Pisch, G. Devitt, D. Holtkotte, and V. Bosch. 2006. Effects of signal peptide exchange on HIV-1 glycoprotein expression and viral infectivity in mammalian cells. FEBS Lett. 580:3775-3778. [DOI] [PubMed] [Google Scholar]
- 29.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11:791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wunschmann, S., J. D. Medh, D. Klinzmann, W. N. Schmidt, and J. T. Stapleton. 2000. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J. Virol. 74:10055-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yagnik, A. T., A. Lahm, A. Meola, R. M. Roccasecca, B. B. Ercole, A. Nicosia, and A. Tramontano. 2000. A model for the hepatitis C virus envelope glycoprotein E2. Proteins 40:355-366. [DOI] [PubMed] [Google Scholar]
- 32.Zahn, A., and J. P. Allain. 2005. Hepatitis C virus and hepatitis B virus bind to heparin: purification of largely IgG-free virions from infected plasma by heparin chromatography. J. Gen. Virol. 86:677-685. [DOI] [PubMed] [Google Scholar]
- 33.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 102:9294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]