Abstract
Cholesterol is known to play an important role in stabilizing particular cellular membrane structures, so-called lipid or membrane rafts. For several viruses, a dependence on cholesterol for virus entry and/or morphogenesis has been shown. Using flow cytometry and fluorescence microscopy, we demonstrate that infection of cells by canine distemper virus (CDV) was not impaired after cellular cholesterol had been depleted by the drug methyl-β-cyclodextrin. This effect was independent of the multiplicity of infection and the cellular receptor used for infection. However, cholesterol depletion of the viral envelope significantly reduced CDV infectivity. Replenishment by addition of exogenous cholesterol restored infectivity up to 80%. Thus, we conclude that CDV entry is dependent on cholesterol in the viral envelope. Furthermore, reduced syncytium formation was observed when the cells were cholesterol depleted during the course of the infection. This may be related to the observation that CDV envelope proteins H and F partitioned into cellular detergent-resistant membranes. Therefore, a role for lipid rafts during virus assembly and release as well is suggested.
Canine distemper virus (CDV) belongs to the family Paramyxoviridae and is classified within the genus Morbillivirus. This genus includes several closely related viruses, among them measles virus (MV). Two virus-encoded glycoproteins, the hemagglutinin (H) and the fusion (F) protein, are integrated into a lipid envelope that surrounds the ribonucleoprotein core. The H protein mediates adsorption to the cell by interaction with the cellular receptor, the signaling lymphocytic activating molecule (SLAM; also known as CD150) (43, 48). Upon receptor binding, a conformational change in the F protein initiates the fusion of the viral and plasma membranes (17).
The raft hypothesis claims that lipids and proteins are not distributed randomly in the plasma membrane but that sphingolipids and cholesterol form microdomains with an increased structural order, which are designated liquid ordered domains in model membranes. The tight packaging of the sphingolipids with their saturated fatty acid chains is maintained by the presence of cholesterol, and several proteins partition into these membrane domains (37). As a result of the tight packaging, the microdomains resist solubilization by anionic detergents at 4°C (6) and are therefore also designated detergent-resistant membranes (DRMs). Extraction of cholesterol by various chemicals destroys this order and renders the components of the microdomains soluble to detergents. DRMs may be the structural basis of membrane rafts. However, it should be noted that solubilization of proteins and lipids may differ depending on the type of detergent used. Therefore, different types of membrane rafts may exist.
The essential role of cholesterol in different aspects of the replication cycle has been clearly demonstrated for several viruses (reviewed in references 23, 27, and 40). Virus entry may be one critical step requiring cholesterol (32, 36). For enveloped viruses, two membranes are involved in the fusion process. Successful virus entry may require cholesterol in either membrane or in both or may be cholesterol independent. Murine leukemia virus is sensitive to cholesterol depletion from the cellular but not the viral membrane (21), whereas the reverse has been demonstrated for influenza virus (35, 39, 42). In contrast, human immunodeficiency virus (HIV) and herpes simplex virus require cholesterol in both membranes (4, 12, 18-20, 22), while vesicular stomatitis virus (VSV) replication appears to be completely cholesterol independent (7, 15, 31, 33).
There exist several reports concerning the importance of cholesterol/membrane rafts for the assembly of different paramyxoviruses. It has been shown that MV glycoproteins partially segregate into DRMs (24, 46). For Sendai virus, respiratory syncytial virus, and Newcastle disease virus (NDV), protein interactions with DRMs have been demonstrated (1, 9, 25). Recently, Laliberte et al. (16) investigated the role of lipid rafts for NDV assembly and also showed that NDV envelope cholesterol is not necessary for virus entry. However, there are no reports describing the role of cell membrane cholesterol/membrane rafts in paramyxovirus entry or the role of virion cholesterol in morbillivirus entry. Previous investigations report that cholesterol metabolism is changed in persistently MV infected cells (2, 3). We investigated the requirement for cholesterol within the viral envelope as well as in the cellular target membrane in the initiation of CDV infection. We show that CDV does not require cholesterol in the plasma membrane, but does require cholesterol in the viral envelope, for efficient infection of Vero cells. Furthermore, syncytium formation in CDV-infected cells is reduced when the cells are cholesterol depleted during the course of infection, suggesting a role of cholesterol in the CDV glycoprotein-containing membrane for an efficient attachment/fusion process. The functional importance of cholesterol for the CDV envelope proteins was supported by our finding that the CDV H and F proteins partially partitioned into DRMs. Therefore, membrane rafts might also be necessary during the assembly process.
MATERIALS AND METHODS
Cells and viruses.
Vero cells and B95a cells were cultured in Dulbecco's modified Eagle's medium (DMEM) containing 5% fetal calf serum (FCS) and were passaged twice a week. Vero cells constitutively expressing canine SLAM (Vero-SLAM cells) (49), were cultured like Vero cells with an additional 0.5 mg/ml Zeocin. MDCK II cells were cultured in Eagle's minimal essential medium containing 5% FCS and were passaged twice a week. Two recombinant strains of CDV containing insertions of enhanced green fluorescent protein (eGPF) were used. The insertion site for eGFP in strain Onderstepoort is in front of the nucleocapsid (N) gene, while it is in front of the polymerase (L) gene in the virulent strain 5804P (41, 47, 49). VSV strain Indiana, with eGFP inserted downstream of the G gene, was obtained from Gert Zimmer. The Beaudette strain of infectious bronchitis virus (IBV) was provided by Dave Cavanagh, Compton, United Kingdom. The rabbit antiserum against IBV was a kind gift from Christine Winter (51). All viruses were propagated and plaque-titrated with 0.8% (wt/vol) methylcellulose (Sigma) in DMEM on Vero cells.
Immunofluorescence assay.
For immunofluorescence analysis, cells grown on coverslips were fixed with 3% paraformaldehyde (Merck) in phosphate-buffered saline (PBS) for 20 min at room temperature. Cells infected with viruses expressing eGFP were mounted in Mowiol (Calbiochem). Cells infected with IBV were visualized by immunostaining. For this purpose, cells were permeabilized by treatment with 0.2% Triton X-100 (Serva) in PBS for 5 min at room temperature and washed three times with 1 ml PBS. Cells were incubated with 200 μl rabbit anti-IBV (α-IBV) serum, diluted 1:200 in PBS, for 1 h at room temperature and were subsequently washed three times with 1 ml PBS. Thereafter, cells were incubated with 200 μl of fluorescein isothiocyanate-conjugated goat α-rabbit immunoglobulin G (IgG) (Sigma), diluted 1:500 in PBS, for 1 h at room temperature, washed as before, and mounted in Mowiol (Calbiochem).
Flow cytometric analysis.
For flow cytometry, 2 × 105 cells were used. Adherent cells were washed three times with 1 ml PBS, and the supernatants were collected in a centrifuge tube. To detach the cells from the bottom of the well, 200 μl trypsin was added to each well and incubated at 37°C for 10 min. Thereafter, the cells were resuspended in 1 ml PBS, added to the supernatants of the preceding washing steps, and spun down at 400 × g for 7 min at 4°C. The pellet was resuspended in 1 ml PBS, transferred to a 1.5-ml reaction tube, and centrifuged at 420 × g for 7 min at 4°C. Cells infected with a virus expressing eGFP were resuspended in 400 μl 1% paraformaldehyde (Merck) in PBS, fixed for 2 h at 4°C, and analyzed by flow cytometry. IBV-infected cells were immunostained prior to flow cytometry. For this purpose, the cell pellets were incubated for 1 h at room temperature in 200 μl rabbit α-IBV serum, diluted 1:200 in PBS. Thereafter, cells were washed three times with 1 ml PBS and centrifuged at 420 × g for 7 min at 4°C. Cells were then incubated for 1 h at room temperature in 200 μl of fluorescein isothiocyanate-conjugated α-rabbit IgG as a secondary antibody (Sigma), diluted 1:500 in PBS. After three further washing steps, the cell pellets were resuspended in 400 μl PBS each and immediately analyzed by flow cytometry. As a negative control, mock-infected cells were immunostained.
Cholesterol depletion and virus entry assays. (i) Extraction of cellular cholesterol with MβCD.
In a total volume of 1 ml DMEM containing 5% FCS, 2 × 105 Vero cells were seeded per well of a 24-well plate and incubated in a CO2 incubator overnight. Cells were washed twice with 1 ml PBS, and concentrations of methyl-β-cyclodextrin (MβCD) (stock, 250 mM in double-distilled H2O; Sigma) ranging from 0 to 15 mM, diluted in DMEM, were added to each well in a total volume of 250 μl. After incubation for 30 min at 37°C, MβCD was removed by washing the cells three times with 1 ml PBS.
(ii) Determination of cell viability after cholesterol extraction.
Cell viability was determined by propidium iodide (PI) (Sigma) staining (28, 41). Cells were detached from the 24-well plate by incubation with 200 μl trypsin for 10 min at 37°C. After resuspension in 1 ml PBS, they were transferred to a 1.5-ml reaction tube and pelleted at 420 × g for 7 min at 4°C. For staining, cells were resuspended in 500 μl PBS, and 2 μg/ml PI was added. After incubation for 5 min at room temperature, the percentage of stained cells was determined by flow cytometry. Cells that were not stained with PI were used as a negative control to determine autofluorescence.
(iii) Measurement of cellular cholesterol.
To measure the effect of MβCD treatment, the cellular cholesterol content was determined using the “Cholesterin Farb Test” (Roche). A total of 2 × 107 Vero cells were seeded in a 162-cm2 cell culture flask in a total volume of 30 ml DMEM containing 5% FCS and incubated in a CO2 incubator overnight. The cell layer was washed twice with 20 ml PBS. Cells were treated either with 7.5 ml DMEM or with the same volume of DMEM containing 7.5 mM MβCD and were incubated on a rocking platform for 30 min at 37°C. Thereafter, the cells were washed three times and detached by treatment with 2 ml trypsin for 10 min at 37°C. The number of cells, resuspended in 10 ml PBS, was determined using a Thoma cell counting chamber. Equal cell numbers of both samples were spun down at 400 × g for 7 min at 4°C. The pellet was resuspended in 1 ml PBS and pelleted at 18,000 × g for 1 min at 4°C. Subsequently, the pellets were resuspended in 400 μl lysis buffer (20 mM Tris, 137 mM NaCl, 10% [vol/vol] glycerol, 1% [vol/vol] Triton X-100, 2 mM EDTA, 50 mM β-glycerolphosphate, 20 mM sodium pyrophosphate). Cell lysis was accomplished during a 20-min incubation at room temperature with vortexing of the cells every 5 min. The cholesterol content was determined, based on a colorimetric reaction, according to the manufacturer's instructions.
(iv) Infection efficiency after cholesterol depletion of cellular membranes.
Cells were either medium treated or cholesterol depleted as described above and subsequently either mock infected or infected with 250-μl virus dilutions in DMEM at multiplicities of infection (MOIs) of 0.001, 0.005, 0.01, 0.05, or 0.1, respectively, and incubated at 37°C for 1 h. After the adsorption period, cells were supplied with 1 ml methylcellulose (0.8% [wt/vol] in DMEM). Cells infected with VSV, IBV, and CDV-5804P were further processed 1 day postinfection, and cells infected with CDV-Onderstepoort were further processed 2 days postinfection, either for immunofluorescence analysis or for flow cytometry.
(v) Infection efficiency after cholesterol extraction from viral membranes.
A total of 2 × 105 Vero cells in a volume of 1 ml DMEM containing 5% FCS were seeded per well of a 24-well plate and incubated at 37°C overnight. The next day, the cell layer was washed three times with 1 ml PBS. Virus suspensions with a titer of 8 × 104 PFU/ml were treated for 30 min at 37°C with MβCD (Sigma) at concentrations ranging from 0 to 20 mM. Thereafter, 250 μl of the virus or an equal amount of medium was applied to the cell layer and incubated for 1 h at 37°C. Subsequently, cells were washed three times, overlaid with 1 ml methylcellulose (Sigma) (0.8% [wt/vol] in DMEM), and incubated as described above. Cells were processed 1 day postinfection either for immunofluorescence or for flow cytometry analysis as described before to determine the infection efficiency.
(vi) Replenishment of cholesterol.
After extraction of viral cholesterol as described above, virus was treated either with medium alone or with medium containing exogenous cholesterol (stock, 50 mM in ethanol; Sigma) at final concentrations ranging from 50 to 500 μM for 30 min at 37°C. Thereafter, cells were infected with these virus samples and prepared for immunofluorescence or flow cytometry analysis as described above.
(vii) Syncytium formation.
To study the development of CDV-induced syncytium formation in the absence of cholesterol, 2 × 105 Vero cells in 1 ml DMEM containing 5% FCS were seeded per well of a 24-well plate and incubated at 37°C overnight. The next day, the cells were washed once with 1 ml PBS, and thereafter the cells were treated with 250 μl medium or infected with 250-μl virus dilutions at an MOI of 0.1 for 1 h at 37°C. Alternatively, cells were transfected with the pCG eukaryotic expression vectors carrying the CDV-Onderstepoort H and F genes (50) by using Lipofectamine (Invitrogen) according to the manufacturer's instructions. DNA uptake was allowed for 2 h. Subsequently, the virus inoculum or Lipofectamine/DNA, respectively, was removed by three washing steps as described before, and cellular cholesterol was extracted by treatment with 7.5 mM MβCD (Sigma) in a volume of 250 μl DMEM for 30 min at 37°C, while control cells were treated with an equal volume of medium. MβCD was removed by three washing steps as described before. To prevent replenishment of cellular cholesterol either by cholesterol uptake from the medium or by endogenous synthesis, cholesterol-depleted cells were provided with methylcellulose (Sigma) (0.8% [wt/vol] in DMEM) containing 5% delipidized FCS (PanBiotech), 10 μM mevastatin (Sigma), and 25 μM mevalonolactone (Sigma) (14). Control cells were overlaid with methylcellulose containing 5% FCS. After incubation at 37°C, cells were fixed 1 or 2 days postinfection with 3% paraformaldehyde (Merck) in PBS and mounted in Mowiol (Calbiochem).
(viii) Quantification of syncytia.
Syncytia were quantified by counting nuclei involved in syncytia using a fluorescence microscope, taking advantage of eGFP expression. Syncytia were defined as cells containing more than three nuclei. The size of syncytia was defined by the number of nuclei per syncytium, and the amount of syncytia was determined by the number of syncytia per counting grid.
Isolation of DRMs.
A total of 3 × 107 Vero cells were seeded per 162-cm2 flask in 20 ml DMEM containing 5% FCS and incubated overnight. The next day, the cells were infected with CDV-Onderstepoort at an MOI of 0.1 in a volume of 10 ml DMEM for 1 h at 37°C. Thereafter, the inoculum was replaced by 20 ml DMEM containing 5% FCS and incubated for 1 day. Subsequently, the cell layer was washed once with PBS and incubated with 5 ml of 10 mM EDTA in PBS for approximately 1 h to detach the cells. The cell number was determined, and cells were washed twice with cold PBS. A total of 3 × 107 cells were resuspended in 300 μl MB++ buffer (MB with 100 μg/ml DNase I [Sigma, Steinheim, Germany] and 5% [vol/vol] Complete protease inhibitor cocktail [Roche, Mannheim, Germany]) (MB buffer consists of 20 mM morpholineethanesulfonic acid, 150 mM NaCl, 1% [wt/vol] Brij 98, 0.02% [wt/vol] NaN3, pH 6.5) and kept on ice. After incubation for 16 h, the volume was adjusted to 500 μl with MB++ buffer and incubated for 5 min at 37°C. The sample was mixed with 500 μl 90% sucrose, overlaid with a linear (40 to 10%) sucrose gradient, and centrifuged in an SW41 rotor at 35,000 rpm for 20 h at 4°C. Fractions of 1 ml were collected from the top of the tube, and the pellet was resuspended in 1 ml MB+ buffer (MB++ without DNase I). Aliquots (10 μl) from each fraction were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane. The H and F proteins of CDV were detected by peptide-specific rabbit antisera (50); human Lamp-2 and human flotillin-2 were detected by commercial primary antibodies (Becton Dickinson, Heidelberg, Germany). Anti-mouse IgG conjugated with horseradish peroxidase or anti-rabbit IgG conjugated with horseradish peroxidase (DAKO Cytomation, Glostrup, Denmark) served as secondary antibodies. Proteins were visualized using BM chemiluminescence blotting substrate (POD; Roche) and a ChemiDoc imager (Bio-Rad).
RESULTS
Depletion of cholesterol from cell membranes does not prevent CDV entry.
Cholesterol is known to stabilize the tight packaging and thus the ordered structure of membrane rafts. To investigate the functional importance of these membrane domains, cholesterol can experimentally be depleted by MβCD (38). To demonstrate the efficiency of cholesterol reduction, we measured the cholesterol content of cellular membranes following MβCD treatment by using a cholesterol detection kit. Treatment of Vero cells with 7.5 mM MβCD efficiently reduced the cellular cholesterol content by about 45% (data not shown).
To rule out detrimental effects of MβCD on cell viability, which might result in impaired infection efficiency independent of cholesterol reduction, cell viability was measured by PI staining. Vero cells were treated with increasing concentrations of MβCD, prepared for flow cytometry, and stained with PI. Increasing concentrations of MβCD led to only a slight augmentation of the number of dead cells compared to treatment with medium alone (data not shown), indicating that MβCD treatment had no significant effect on cell viability.
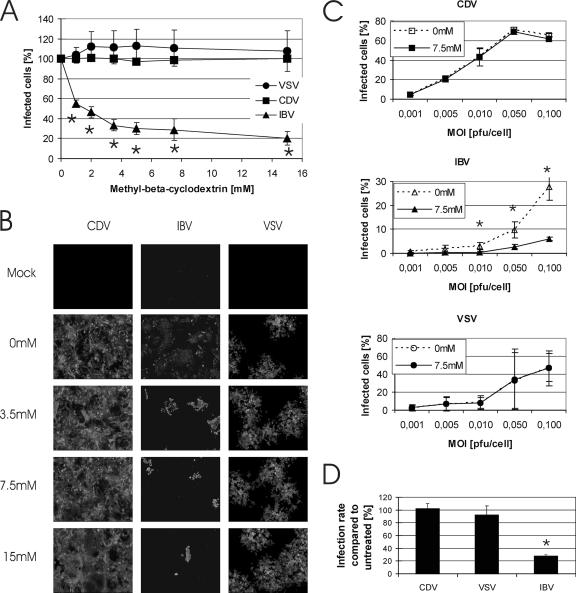
To investigate if cholesterol is essential for the entry of CDV-Onderstepoort into cells, we depleted cholesterol from Vero cells by applying up to 15 mM MβCD and subsequently infected the cells either with CDV-Onderstepoort or with the control viruses VSV and IBV, respectively, at an MOI of 0.1. As shown in Fig. 1A, the efficiency of CDV-Onderstepoort infection was not reduced when cells were cholesterol depleted at MβCD concentrations as high as 15 mM prior to infection. CDV resembled VSV, which is known to infect cells irrespective of cholesterol depletion (Fig. 1A) (45). By constrast, for murine hepatitis virus, a dependence on cellular cholesterol for efficient virus entry has been shown (45). Since this virus does not grow in Vero cells, we included another coronavirus, avian IBV, as a control virus. Infection by IBV was reduced by cholesterol depletion, even at MβCD concentrations as low as 1 mM (Fig. 1A). When Vero cells were pretreated with 7.5 mM MβCD, the infection efficiency of IBV decreased by about 70% as measured by flow cytometry (Fig. 1A). These results were confirmed by fluorescence microscopy for all three viruses tested (Fig. 1B).
FIG. 1.
Effect of cholesterol depletion of Vero cells on CDV-Onderstepoort infection. (A and B) Effect of cholesterol depletion of Vero cells by increasing MβCD concentrations on CDV infection efficiency. Vero cells were depleted of cholesterol by increasing MβCD concentrations for 30 min at 37°C and were subsequently infected with CDV-Onderstepoort (squares), VSV (circles), or IBV (triangles). VSV and IBV samples were prepared for quantification by flow cytometry (A) or fluorescence microscopy (B) 1 day postinfection, and CDV-Onderstepoort samples were prepared 2 days postinfection. Infection rates without MβCD obtained by flow cytometry were set to 100%. Results are means from three independent experiments with standard deviations. Asterisks indicate significance (P < 0.05) by the t test. (C) Effect of cholesterol depletion of Vero cells by 7.5 mM MβCD on CDV-Onderstepoort, IBV, or VSV infection at different MOIs. Vero cells were either treated with medium or cholesterol depleted by treatment with 7.5 mM MβCD and were subsequently infected with increasing MOIs of CDV-Onderstepoort, IBV, or VSV. Quantification was done by flow cytometry. Results are means from three independent experiments with standard deviations. Asterisks indicate significance (P < 0.05) by the t test. (D) Vero cells were treated with medium or 7.5 mM MβCD prior to infection with CDV-Onderstepoort, IBV, or VSV at an MOI of 0.001. Plaque numbers were counted and set to 100% for untreated samples. Results are means from three independent experiments with standard deviations. Asterisks indicate significant values (P < 0.05) by the t test.
To rule out the possibility that infection of Vero cells after cholesterol depletion is MOI dependent, cells were pretreated with medium or 7.5 mM MβCD and subsequently infected at MOIs ranging from 0.1 to 0.001. The efficiency of IBV infection of MβCD-treated cells was reduced for every MOI tested, while the infection efficiencies of VSV and CDV-Onderstepoort remained unchanged as determined by flow cytometry (Fig. 1C, compare the dashed line with the continuous line in every graph). These results were confirmed by fluorescence microscopy and were additionally quantified by plaque assays at an MOI of 0.001, which also revealed a 75% reduction for IBV but no significant reduction for CDV or VSV, respectively (Fig. 1D). We therefore conclude that CDV-Onderstepoort infection is independent of plasma membrane cholesterol.
Receptor usage does not affect the cholesterol independence of CDV entry.
The only known CDV receptor is SLAM (43). SLAM is expressed in subsets of lymphocytes and on some macrophages/monocytes (8, 41, 44). The CDV-Onderstepoort strain has a long passage history in Vero cells, which are of epithelial origin and have been shown not to express SLAM (10). Thus, entry of CDV-Onderstepoort into Vero cells is mediated by a different receptor.
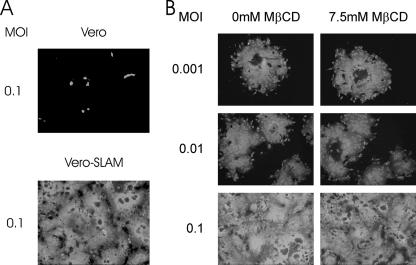
To investigate whether SLAM-mediated CDV entry is also independent of cellular cholesterol, we used the recombinant CDV wild-type strain (CDV-5804P) which had been modified by reverse genetics to express eGFP (47). As expected from results obtained with the virulent strain CDV-5804P(wt), which does not express eGFP (49), only a few eGFP-expressing cells were observed when Vero cells were inoculated with CDV-5804P, whereas Vero-SLAM cells were infected with high efficiency (Fig. 2A). Thus, CDV-5804P obviously infects Vero-SLAM cells almost exclusively by using SLAM as receptor. Vero-SLAM cells were then pretreated with increasing MβCD concentrations; infected with either CDV-Onderstepoort, CDV-5804P, VSV, or IBV; and analyzed for infection efficiency as before. Since infection of Vero-SLAM cells with either CDV strain resulted in a strong cytopathic effect, including cell lysis, which made quantification by flow cytometry difficult, the effect of cholesterol depletion was determined using fluorescence microscopy. As shown in Fig. 2B, infection with CDV-5804P was not affected by pretreatment of the cells with 7.5 mM MβCD, independently of the MOI used. When infections by CDV-Onderstepoort, IBV, or VSV were analyzed by fluorescence microscopy, the same results as for Vero cells were obtained (Fig. 1A; also data not shown). These results demonstrate that CDV entry is independent of cellular cholesterol regardless of whether SLAM or a still unidentified receptor on epithelial cells is used.
FIG. 2.
Effect of cholesterol depletion of Vero-SLAM cells on infection with CDV-5804P. (A) CDV-5804P infection of Vero cells and Vero-SLAM cells. Vero cells and Vero-SLAM cells were infected at an MOI of 0.1 and fixed 1 day postinfection. Infection was monitored by fluorescence microscopy. Results are representative of three independent experiments. (B) Infection efficiency of CDV-5804P for cholesterol-depleted Vero-SLAM cells. Vero-SLAM cells were treated with medium or 7.5 mM MβCD prior to infection with CDV-5804P at an MOI of 0.1, 0.01, or 0.001. Infection efficiencies were monitored by fluorescence microscopy. Results are representative of three independent experiments.
Depletion of cholesterol from the viral envelope impairs CDV infection.
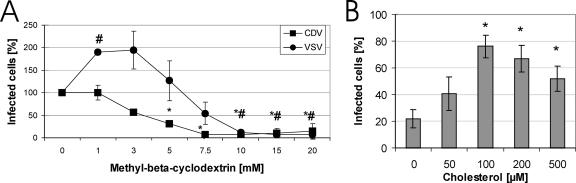
To analyze whether cholesterol in the CDV envelope is necessary for virus entry, we depleted virions by MβCD treatment prior to infection. VSV was included as a control, since it is known to bud from nonraft membrane regions (7, 33). As shown in Fig. 3A, CDV-Onderstepoort infection was inhibited by cholesterol depletion as determined by flow cytometry. Interestingly, VSV infection was somewhat enhanced by MβCD concentrations between 1 and 3 mM. However, MβCD concentrations higher than 5 mM resulted in decreased infection efficiency even for VSV. These results were confirmed by fluorescence microscopy (data not shown).
FIG. 3.
Role of CDV-Onderstepoort envelope cholesterol in infection. (A) Infection efficiency of cholesterol-depleted CDV on Vero cells. Envelope cholesterol of CDV or VSV was depleted by treatment with increasing MβCD concentrations, and these viruses were subsequently used to infect Vero cells. The number of infected cells was measured by flow cytometry 1 day postinfection. Infection rates without MβCD were set to 100%. Results are means from three independent experiments with standard deviations. Significant values (P < 0.05) by the t test are indicated by asterisks for CDV and by number signs (#) for VSV. (B) Infection efficiency of cholesterol-depleted CDV after replenishment with exogenous cholesterol. CDV was cholesterol depleted by treatment with 5 mM MβCD. Prior to infection of Vero cells, cholesterol was replenished by increasing concentrations of exogenous cholesterol. Infection efficiencies were determined by flow cytometry 1 day postinfection. Infection rates for control virions, treated neither with MβCD nor with cholesterol, were set to 100%. Results are means from three independent experiments with standard deviations. Asterisks indicate significant values (P < 0.05) by the t test.
To demonstrate that the decrease in CDV-Onderstepoort infection efficiency was caused by the reduced cholesterol content in the virus envelope, we attempted to reverse the effect by supplementing with exogenous cholesterol. Addition of 100 μM cholesterol after depletion with 5 mM MβCD restored CDV infection efficiency to almost 80% as demonstrated by flow cytometry (Fig. 3B) and fluorescence microscopy (data not shown). These observations indicate that the interaction of CDV with target cells is dependent on cholesterol in the virus envelope.
Syncytium formation by CDV is reduced upon depletion of cellular cholesterol.
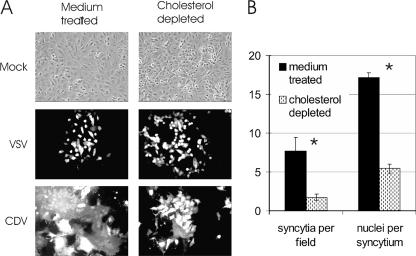
The importance of cholesterol for the fusion activity of CDV was determined by measuring the effect of depletion of cellular cholesterol on the ability of the viral glycoproteins H and F to form syncytia. Vero cells were infected with CDV or VSV at an MOI of 0.1, and in order to prevent cholesterol replenishment during the time necessary for viral replication, cells were maintained in delipidized serum and 10 μM mevastatin, an inhibitor of cholesterol synthesis, as well as 25 μM of the metabolic intermediate mevalonolactone, to prevent shutdown of all metabolic pathways downstream of the mevastatin block. Cells were prepared for fluorescence microscopy 1 and 2 days postinfection and were analyzed for syncytium or plaque formation, respectively. As shown in Fig. 4A (top panels), Vero cells grown under cholesterol starvation conditions showed the same microscopic appearance as Vero cells grown under normal conditions, and cell viability remained within the normal range as determined by flow cytometry after PI staining (data not shown). VSV replication was not inhibited (Fig. 4A), and plaque formation 1 (Fig. 4A) and 2 (data not shown) days postinfection was similar in cholesterol-depleted and normal cells. In contrast, the level of syncytium formation by CDV-infected cells was strongly reduced in cholesterol-depleted Vero cells, as shown in Fig. 4A. By quantification, we measured a 78% reduction in the number of syncytia and a 68% reduction in the number of nuclei per syncytium in cholesterol-depleted cells 1 day postinfection (Fig. 4B). Syncytium formation in Vero cells transfected with pCG plasmids carrying the CDV H and F genes was also significantly reduced (data not shown). If mevastatin was administered without mevalonolactone, cell morphology 2 days postinfection was slightly altered and syncytium formation in CDV-infected cells was decreased compared to that in untreated cells (data not shown). These findings indicate that cholesterol is also needed for downstream events during viral replication.
FIG. 4.
Effect of cholesterol depletion of cells during the course of CDV infection on syncytium formation. (A) Syncytium formation of CDV grown on Vero cells under cholesterol starvation conditions. Vero cells either were infected with CDV or VSV or were mock infected as a control. One hour postinfection, cells were cholesterol depleted by incubation with MβCD following treatment with mevastatin and mevalonolactone and addition of delipidized FCS to prevent replenishment of cholesterol. Alternatively, cells were medium treated and subsequently provided with a medium containing normal FCS. VSV-infected cells were fixed and prepared for immunofluorescence 1 day postinfection; CDV-infected cells were prepared 2 days postinfection. Images are representative of three independent experiments. (B) Quantification of syncytium formation following CDV-Onderstepoort infection. The size and number of syncytia were determined following CDV-Onderstepoort infection of Vero cells grown normally or under cholesterol starvation conditions during the course of infection. Syncytia were defined as cells with more than three nuclei. The size of syncytia was defined as the number of nuclei per cell and the amount as the number of syncytia per counting square. Results are means from five counting grids from three independent experiments, with standard deviations. Asterisks indicate significant (P < 0.05) values by the t test.
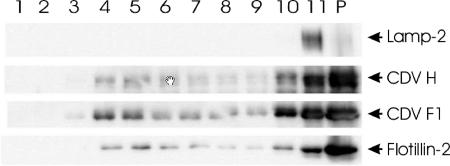
CDV envelope proteins partition into cellular DRMs.
The functional role of cholesterol for CDV attachment/fusion suggests an association of the envelope proteins with cellular membrane rafts. To obtain experimental evidence, DRMs of CDV-infected Vero cells were isolated by lysis with 1% Brij 98 and subjected to linear sucrose gradient centrifugation. As shown in Fig. 5, the CDV H and F proteins partitioned into low-density fractions (fractions 4 and 5) in a pattern similar to that of flotillin-2, a protein known to be associated with DRMs (5). In contrast, Lamp, a protein known not to be associated with DRMs (14), was detected exclusively in high-density fractions (fraction 11).
FIG. 5.
Distribution of the CDV envelope proteins in DRMs. CDV-infected Vero cells were lysed in 1% Brij 98 and subjected to linear sucrose gradient centrifugation. Aliquots of the gradient and pellet (P) fractions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The CDV proteins H and F as well as the cellular proteins Lamp-2 and flotillin-2 were detected by Western blot analysis.
DISCUSSION
The importance of cholesterol in either the viral envelope or the target cell membrane has been demonstrated for several viruses. For HIV and herpes simplex virus, the infection is sensitive to variations in both the viral and the cellular membranes (4, 12, 18-20, 22), while for some viruses, the critical importance of cholesterol is restricted to one of the two membranes. Influenza virus (strain WSN) is inhibited only by a reduction in the cholesterol content of the viral membrane (39), while the infectivity of ecotropic murine leukemia virus is exclusively affected by depleting cholesterol from the target cell (21).
Role of cellular cholesterol in CDV entry.
Here, we have analyzed the importance of cholesterol in viral and cellular membranes for the entry of a paramyxovirus. We found that cholesterol reduction in the viral membrane decreased CDV infectivity, whereas changes in the plasma membrane of the target cell did not affect infection with CDV. To rule out artifacts, we included two control viruses: VSV, which is known to retain infectivity following depletion of cellular cholesterol prior to infection (34, 45), as a negative control and IBV as a positive control. IBV, the type species of the genus Coronavirus, which belongs to antigenic group III, was chosen because of its relationship with murine hepatitis virus, an antigenic group II coronavirus, whose sensitivity to cholesterol depletion of the target cell membrane has been demonstrated (45). In contrast to the murine virus, IBV strain Beaudette replicates efficiently in Vero cells, thus permitting a direct comparison of all the viruses used in our study. Our initial characterization of this virus confirmed its sensitivity to cholesterol depletion of the plasma membrane, indicating that this may be a general feature of coronaviruses.
The sensitivity of a virus to cholesterol depletion from the target cell membrane may be explained by an association of virus receptors with cholesterol-enriched microdomains or DRMs. In the case of HIV, the attachment receptor, CD4, is located in DRMs, and the secondary receptors enabling the fusion reaction, CXCR4 and CCR5, have been shown to be recruited into such microdomains after the binding of HIV (22, 28). Whether or not SLAM resides in DRMs has not yet been analyzed. The cholesterol-independent infection of the wild-type strain CDV 5804P, mediated via SLAM as a receptor, as well as that of the vaccine strain CDV-Onderstepoort, mediated via a still unidentified receptor, suggests that both cellular receptors used are not located in cholesterol-enriched microdomains.
Role of envelope cholesterol in CDV entry.
In contrast to our finding regarding the target cell membrane, cholesterol depletion from the viral envelope had a significant effect on CDV infectivity. Again, VSV served as a negative control, because reducing the cholesterol content in the viral membrane was not expected to decrease infectivity, for the following reasons: (i) VSV contains only a minor amount of lipids in DRMs (31, 33); (ii) VSV buds from nonraft domains (7); and (iii) the surface protein G is not located in DRMs (9, 26, 33, 46). In fact, treatment with low concentrations of MβCD even enhanced infectivity twofold. This finding suggests that the microenvironment of the G protein is changed after cholesterol depletion and that this, in turn, may be responsible for the increase in infectivity. However, at high MβCD concentrations, infection efficiency was decreased compared to that of untreated virus. A similar effect has also been reported for HIV and simian immunodeficiency virus; it was explained by perforation of the viral membrane and subsequent loss of the genome (12). The effect of MβCD on CDV cannot be explained by perforation of the viral envelope, because the reduction in infectivity was already observed at lower drug concentrations. Furthermore, the inhibitory effect of MβCD was reversible, since exogenous addition of cholesterol resulted in restoration of infectivity. This observation is not compatible with a perforation of the viral envelope. Laliberte et al. (16) reported recently that NDV obtained from cholesterol-depleted cells had reduced infectivity. However, when NDV virions were cholesterol depleted, infectivity was unaffected compared to that of untreated virions. This is in contrast to our observation with CDV and may reflect differences between the two viruses. However, the distribution of NDV HN and F proteins into DRMs found by Laliberte et al. (16) resembles the distribution of the CDV H and F proteins. The necessity of virus envelope cholesterol for efficient cell entry suggests the existence of membrane rafts in the virus envelope. Since the viral envelope originates from the cellular membrane during virus assembly, an involvement of membrane rafts during the CDV assembly and budding step is probable. The incorporation of the CDV envelope proteins into DRMs, shown here, as well as the strong evidence for membrane rafts being the assembly site for NDV, MV, respiratory syncytial virus, and Sendai virus (1, 11, 13, 16, 24, 46), also supports this idea.
Role of cholesterol in late steps in CDV replication.
The importance of viral envelope cholesterol for CDV infectivity and a role for cholesterol during CDV assembly are also supported by the results of our functional assay, which showed that cholesterol depletion from cellular membranes harboring the CDV envelope proteins resulted in decreased syncytium formation. This effect was observed for infected cells as well as for cells transfected with plasmids harboring the genes coding for CDV envelope proteins H and F. The results can be explained by localization of one or both of the viral envelope proteins in cholesterol-enriched microdomains. In agreement with this, we showed that both proteins partially partitioned into DRMs. A partial association of the envelope proteins with DRMs has also been shown for other paramyxoviruses, namely, MV, respiratory syncytial virus, Sendai virus, and NDV (1, 9, 13, 16, 24, 25, 46), and for other viruses such as influenza virus (42).
Incorporation of the envelope proteins into DRMs or interaction with cholesterol may be necessary for virus entry for several reasons. For influenza virus it has been shown that depletion of cholesterol from the viral envelope reduces infectivity (39) and that this effect was caused by inhibition of the fusion activity, whereas the attachment process and endocytotic uptake were not affected (39). Alternatively, cholesterol may be necessary for the correct conformation of proteins, as has been shown for the chemokine receptors CXCR4 and CCR5 (29, 30). CDV-induced fusion, as well as the correct conformation of the CDV envelope proteins, may depend on cholesterol. Membrane rafts are also known to exert a concentrating effect on proteins by including some while excluding others. Influenza virus relies on membrane rafts to concentrate the HA protein to efficiently mediate fusion (42). For measles virus, it has been shown that the F protein drags the H protein in membrane rafts (46). In the case of morbilliviruses, membrane rafts may accumulate the virus envelope proteins, thereby facilitating the close contact between the proteins necessary for a fusion process to occur. In addition, cholesterol may favor a special conformation of the CDV envelope proteins. Efficient attachment/fusion, either during virus entry into a cell or during syncytium formation, might be dependent on a membrane raft environment that brings the proteins into close contact and that may support molecular interactions. This concentrating effect of the CDV proteins might also be beneficial during the assembly process.
We have shown that cholesterol depletion of the viral envelope reduces the infectivity of CDV and that CDV-induced syncytium formation in cholesterol-depleted cells is also reduced. This may be due to the involvement of cholesterol in the maintenance of membrane rafts, which also harbor the CDV envelope proteins H and F.
Acknowledgments
We thank Gert Zimmer, Dave Cavanagh, and Christine Winter for kindly providing the VSV-eGFP, IBV, and the rabbit α-IBV antiserum, respectively. We thank Robert Lindner for the introduction to the technique of DRM isolation.
This work was supported by a grant from the Center for Infection Biology, Hannover Medical School, Hannover, Germany.
Footnotes
Published ahead of print on 31 January 2007.
REFERENCES
- 1.Ali, A., and D. P. Nayak. 2000. Assembly of Sendai virus: M protein interacts with F and HN proteins and with the cytoplasmic tail and transmembrane domain of F protein. Virology 276:289-303. [DOI] [PubMed] [Google Scholar]
- 2.Anderton, P., T. F. Wild, and G. Zwingelstein. 1983. Accumulation of radiolabelled fatty acids in the neutral fraction of measles virus persistently infected BGM cells. Biochem. Biophys. Res. Commun. 112:29-34. [DOI] [PubMed] [Google Scholar]
- 3.Anderton, P., T. F. Wild, and G. Zwingelstein. 1983. Measles-virus-persistent infection in BGM cells; modification of the incorporation of [3H]arachidonic acid and [14C]stearic acid into lipids. Biochem. J. 214:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bender, F. C., J. C. Whitbeck, D. L. Ponce, H. Lou, R. J. Eisenberg, and G. H. Cohen. 2003. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 77:9542-9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bickel, P. E., P. E. Scherer, J. E. Schnitzer, P. Oh, M. P. Lisanti, and H. F. Lodish. 1997. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J. Biol. Chem. 272:13793-13802. [DOI] [PubMed] [Google Scholar]
- 6.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 7.Brown, E. L., and D. S. Lyles. 2003. A novel method for analysis of membrane microdomains: vesicular stomatitis virus glycoprotein microdomains change in size during infection, and those outside of budding sites resemble sites of virus budding. Virology 310:343-358. [DOI] [PubMed] [Google Scholar]
- 8.Cocks, B. G., C. C. Chang, J. M. Carballido, H. Yssel, J. E. de Vries, and G. Aversa. 1995. A novel receptor involved in T-cell activation. Nature 376:260-263. [DOI] [PubMed] [Google Scholar]
- 9.Dolganiuc, V., L. McGinnes, E. J. Luna, and T. G. Morrison. 2003. Role of the cytoplasmic domain of the Newcastle disease virus fusion protein in association with lipid rafts. J. Virol. 77:12968-12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erlenhoefer, C., W. J. Wurzer, S. Loffler, S. Schneider-Schaulies, V. ter Meulen, and J. Schneider-Schaulies. 2001. CD150 (SLAM) is a receptor for measles virus but is not involved in viral contact-mediated proliferation inhibition. J. Virol. 75:4499-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleming, E. H., A. A. Kolokoltsov, R. A. Davey, J. E. Nichols, and N. J. Roberts, Jr. 2006. Respiratory syncytial virus F envelope protein associates with lipid rafts without requirement for other virus proteins. J. Virol. 80:12160-12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham, D. R., E. Chertova, J. M. Hilburn, L. O. Arthur, and J. E. Hildreth. 2003. Cholesterol depletion of human immunodeficiency virus type 1 and simian immunodeficiency virus with beta-cyclodextrin inactivates and permeabilizes the virions: evidence for virion-associated lipid rafts. J. Virol. 77:8237-8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henderson, G., J. Murray, and R. P. Yeo. 2002. Sorting of the respiratory syncytial virus matrix protein into detergent-resistant structures is dependent on cell-surface expression of the glycoproteins. Virology 300:244-254. [DOI] [PubMed] [Google Scholar]
- 14.Karacsonyi, C., R. Knorr, A. Fulbier, and R. Lindner. 2004. Association of major histocompatibility complex II with cholesterol- and sphingolipid-rich membranes precedes peptide loading. J. Biol. Chem. 279:34818-34826. [DOI] [PubMed] [Google Scholar]
- 15.Katzman, R. B., and R. Longnecker. 2003. Cholesterol-dependent infection of Burkitt's lymphoma cell lines by Epstein-Barr virus. J. Gen. Virol. 84:2987-2992. [DOI] [PubMed] [Google Scholar]
- 16.Laliberte, J. P., L. W. McGinnes, M. E. Peeples, and T. G. Morrison. 2006. Integrity of membrane lipid rafts is necessary for the ordered assembly and release of infectious Newcastle disease virus particles. J. Virol. 80:10652-10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb, R. A., R. G. Paterson, and T. S. Jardetzky. 2006. Paramyxovirus membrane fusion: lessons from the F and HN atomic structures. Virology 344:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee, G. E., G. A. Church, and D. W. Wilson. 2003. A subpopulation of tegument protein vhs localizes to detergent-insoluble lipid rafts in herpes simplex virus-infected cells. J. Virol. 77:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao, Z., L. M. Cimakasky, R. Hampton, D. H. Nguyen, and J. E. Hildreth. 2001. Lipid rafts and HIV pathogenesis: host membrane cholesterol is required for infection by HIV type 1. AIDS Res. Hum. Retrovir. 17:1009-1019. [DOI] [PubMed] [Google Scholar]
- 20.Liao, Z., D. R. Graham, and J. E. Hildreth. 2003. Lipid rafts and HIV pathogenesis: virion-associated cholesterol is required for fusion and infection of susceptible cells. AIDS Res. Hum. Retrovir. 19:675-687. [DOI] [PubMed] [Google Scholar]
- 21.Lu, X., Y. Xiong, and J. Silver. 2002. Asymmetric requirement for cholesterol in receptor-bearing but not envelope-bearing membranes for fusion mediated by ecotropic murine leukemia virus. J. Virol. 76:6701-6709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manes, S., G. Del Real, R. A. Lacalle, P. Lucas, C. Gomez-Mouton, S. Sanchez-Palomino, R. Delgado, J. Alcami, E. Mira, and A. Martinez. 2000. Membrane raft microdomains mediate lateral assemblies required for HIV-1 infection. EMBO Rep. 1:190-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manes, S., G. del Real, and C. Martinez-A. 2003. Pathogens: raft hijackers. Nat. Rev. Immunol. 3:557-568. [DOI] [PubMed] [Google Scholar]
- 24.Manie, S. N., S. Debreyne, S. Vincent, and D. Gerlier. 2000. Measles virus structural components are enriched into lipid raft microdomains: a potential cellular location for virus assembly. J. Virol. 74:305-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marty, A., J. Meanger, J. Mills, B. Shields, and R. Ghildyal. 2004. Association of matrix protein of respiratory syncytial virus with the host cell membrane of infected cells. Arch. Virol. 149:199-210. [DOI] [PubMed] [Google Scholar]
- 26.Meder, D., M. J. Moreno, P. Verkade, W. L. Vaz, and K. Simons. 2006. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc. Natl. Acad. Sci. USA 103:329-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nayak, D. P., and S. Barman. 2002. Role of lipid rafts in virus assembly and budding. Adv. Virus Res. 58:1-28. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen, D. H., B. Giri, G. Collins, and D. D. Taub. 2005. Dynamic reorganization of chemokine receptors, cholesterol, lipid rafts, and adhesion molecules to sites of CD4 engagement. Exp. Cell Res. 304:559-569. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen, D. H., and D. Taub. 2002. Cholesterol is essential for macrophage inflammatory protein 1 beta binding and conformational integrity of CC chemokine receptor 5. Blood 99:4298-4306. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen, D. H., and D. Taub. 2002. CXCR4 function requires membrane cholesterol: implications for HIV infection. J. Immunol. 168:4121-4126. [DOI] [PubMed] [Google Scholar]
- 31.Pessin, J. E., and M. Glaser. 1980. Budding of Rous sarcoma virus and vesicular stomatitis virus from localized lipid regions in the plasma membrane of chicken embryo fibroblasts. J. Biol. Chem. 255:9044-9050. [PubMed] [Google Scholar]
- 32.Rawat, S. S., M. Viard, S. A. Gallo, A. Rein, R. Blumenthal, and A. Puri. 2003. Modulation of entry of enveloped viruses by cholesterol and sphingolipids. Mol. Membr. Biol. 20:243-254. [DOI] [PubMed] [Google Scholar]
- 33.Scheiffele, P., A. Rietveld, T. Wilk, and K. Simons. 1999. Influenza viruses select ordered lipid domains during budding from the plasma membrane. J. Biol. Chem. 274:2038-2044. [DOI] [PubMed] [Google Scholar]
- 34.Shah, W. A., H. Peng, and S. Carbonetto. 2006. Role of non-raft cholesterol in lymphocytic choriomeningitis virus infection via alpha-dystroglycan. J. Gen. Virol. 87:673-678. [DOI] [PubMed] [Google Scholar]
- 35.Sieczkarski, S. B., and G. R. Whittaker. 2002. Influenza virus can enter and infect cells in the absence of clathrin-mediated endocytosis. J. Virol. 76:10455-10464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simons, K., and E. Ikonen. 2000. How cells handle cholesterol. Science 290:1721-1726. [DOI] [PubMed] [Google Scholar]
- 37.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 38.Simons, K., and W. L. Vaz. 2004. Model systems, lipid rafts, and cell membranes. Annu. Rev. Biophys. Biomol. Struct. 33:269-295. [DOI] [PubMed] [Google Scholar]
- 39.Sun, X. J., and G. R. Whittaker. 2003. Role for influenza virus envelope cholesterol in virus entry and infection. J. Virol. 77:12543-12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suomalainen, M. 2002. Lipid rafts and assembly of enveloped viruses. Traffic 3:705-709. [DOI] [PubMed] [Google Scholar]
- 41.Suter, S. E., M. B. Chein, V. von Messling, B. Yip, R. Cattaneo, W. Vernau, B. R. Madewell, and C. A. London. 2005. In vitro canine distemper virus infection of canine lymphoid cells: a prelude to oncolytic therapy for lymphoma. Clin. Cancer Res. 11:1579-1587. [DOI] [PubMed] [Google Scholar]
- 42.Takeda, M., G. P. Leser, C. J. Russell, and R. A. Lamb. 2003. Influenza virus hemagglutinin concentrates in lipid raft microdomains for efficient viral fusion. Proc. Natl. Acad. Sci. USA 100:14610-14617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tatsuo, H., N. Ono, and Y. Yanagi. 2001. Morbilliviruses use signaling lymphocyte activation molecules (CD150) as cellular receptors. J. Virol. 75:5842-5850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theil, D., C. Farina, and E. Meinl. 2005. Differential expression of CD150 (SLAM) on monocytes and macrophages in chronic inflammatory contexts: abundant in Crohn's disease, but not in multiple sclerosis. J. Clin. Pathol. 58:110-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thorp, E. B., and T. M. Gallagher. 2004. Requirements for CEACAMs and cholesterol during murine coronavirus cell entry. J. Virol. 78:2682-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincent, S., D. Gerlier, and S. N. Manie. 2000. Measles virus assembly within membrane rafts. J. Virol. 74:9911-9915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.von Messling, V., D. Milosevic, and R. Cattaneo. 2004. Tropism illuminated: lymphocyte-based pathways blazed by lethal morbillivirus through the host immune system. Proc. Natl. Acad. Sci. USA 101:14216-14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.von Messling, V., N. Oezguen, Q. Zheng, S. Vongpunsawad, W. Braun, and R. Cattaneo. 2005. Nearby clusters of hemagglutinin residues sustain SLAM-dependent canine distemper virus entry in peripheral blood mononuclear cells. J. Virol. 79:5857-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.von Messling, V., C. Springfeld, P. Devaux, and R. Cattaneo. 2003. A ferret model of canine distemper virus virulence and immunosuppression. J. Virol. 77:12579-12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.von Messling, V., G. Zimmer, G. Herrler, L. Haas, and R. Cattaneo. 2001. The hemagglutinin of canine distemper virus determines tropism and cytopathogenicity. J. Virol. 75:6418-6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winter, C., C. Schwegmann-Wessels, D. Cavanagh, U. Neumann, and G. Herrler. 2006. Sialic acid is a receptor determinant for infection of cells by avian infectious bronchitis virus. J. Gen. Virol. 87:1209-1216. [DOI] [PubMed] [Google Scholar]