Abstract
In vitro screening using the cell-free prion protein conversion system indicated that certain rodents may be susceptible to chronic wasting disease (CWD). Therefore, CWD isolates from mule deer, white-tailed deer, and elk were inoculated intracerebrally into various rodent species to assess the rodents' susceptibility and to develop new rodent models of CWD. The species inoculated were Syrian golden, Djungarian, Chinese, Siberian, and Armenian hamsters, transgenic mice expressing the Syrian golden hamster prion protein, and RML Swiss and C57BL10 wild-type mice. The transgenic mice and the Syrian golden, Chinese, Siberian, and Armenian hamsters had limited susceptibility to certain of the CWD inocula, as evidenced by incomplete attack rates and long incubation periods. For serial passages of CWD isolates in Syrian golden hamsters, incubation periods rapidly stabilized, with isolates having either short (85 to 89 days) or long (408 to 544 days) mean incubation periods and distinct neuropathological patterns. In contrast, wild-type mouse strains and Djungarian hamsters were not susceptible to CWD. These results show that CWD can be transmitted and adapted to some species of rodents and suggest that the cervid-derived CWD inocula may have contained or diverged into at least two distinct transmissible spongiform encephalopathy strains.
Chronic wasting disease (CWD) is a transmissible spongiform encephalopathy (TSE), or prion disease, that affects mule deer (Odocoileus hemionus), black-tailed deer (subspecies of Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), Rocky Mountain elk (Cervus elaphus nelsoni), and moose (Alces alces) (17). Other TSE diseases include scrapie in sheep and goats, transmissible mink encephalopathy (TME), bovine spongiform encephalopathy (BSE), and Creutzfeldt-Jakob disease in humans. Some TSE diseases, such as scrapie, BSE, and Creutzfeldt-Jakob disease, have been experimentally transmitted and adapted to rodents, yielding experimental animal models that have proved useful in the study of TSE diseases (4, 10, 13). Recently, CWD was transmitted to mice transgenic for cervid PrP (16a, 24). Although CWD has been transmitted to ferrets, and then from ferrets to Syrian golden (Sg) hamsters (2), there have been no reports of direct transmissions of CWD to any hamster species or wild-type mice (25).
An important and measurable occurrence in TSE diseases is the conversion of the normal host proteinase K (PK)-sensitive prion protein (PrP-sen) to an abnormal, disease-associated isoform that is characteristically PK resistant (PrP-res). One in vitro method that has been used to assess the potential interspecies transmissibility of TSE agents is a cell-free conversion (CFC) reaction in which PrP-res from one species is tested for its relative efficiency in converting PrP-sen of other species to PrP-res (20, 23). Such CFC reactions were found in the present study to predict the susceptibility of hamsters to CWD. Accordingly, CWD isolates were inoculated into various species of rodents, and some species proved to be modestly susceptible. The resulting rodent-adapted CWD models could be useful in comparative studies of TSE strains in vivo as well as for testing potential anti-TSE therapeutic agents.
MATERIALS AND METHODS
PrP-sen 35S labeling and purification.
The generation and detailed analyses of the cervid and human PrP-sen molecules have been described previously (6, 20). The PrP polymorphic types used in this study were as follows: elk e-GLSE and e-GMSE (PrP amino acid residues 96, 132, 138, and 226 are designated); md/wd-GMNQ and md/wd-GMSQ, which are identical in mule deer and white-tailed deer; wd-SMSQ, found only in white-tailed deer; and human, hu-M and hu-V (residue 129 is designated). The Sg hamster PrP-sen construct lacks a glycosylphosphatidylinositol (GPI) anchor (15). The 35S-PrP-sen molecules of the various species were immunopurified from various cultured cell lines labeled metabolically with [35S]methionine (EasyTag; Perkin-Elmer) (8), and each cell line used expressed one of the PrP-sen types. In order to simplify analysis of PrP conversion products, the 35S-PrP-sen molecules were radiolabeled in the presence of 10 μg/ml tunicamycin (Roche), an inhibitor of glycosylation. R521 antibody (Ab) (20) was used for immunoprecipitation of the various cervid 35S-PrP-sen molecules, and 3F4 monoclonal Ab (11) was used for the human and hamster PrP-sen molecules.
PrP-res purification.
Hamster PrP-res (ha263K) was purified from the brains of 263K-affected Sg hamsters (21). PrP-res isolates from brainstems of CWD-affected elk (eCWD), mule deer (mdCWD), and white-tailed deer (wdCWD) were the same as those used in a previous study (20) and were purified using the same method as that for ha263K.
CFC reactions.
CFC reaction methods have been described previously (20, 22) and are summarized here. For each CFC reaction, 250 ng of each of the purified, nonradiolabeled PrP-res molecules isolated from brain pools (20) was incubated with 20,000 to 30,000 cpm of each of the immunopurified 35S-PrP-sen molecules. PrP-res was pretreated with 2.0 to 2.5 M guanidine hydrochloride at 37°C for 1 h, added to the 35S-PrP-sen with final concentrations of 1 M guanidine-HCl, 50 mM sodium citrate, pH 6.0, 5 mM cetylpyridinium chloride, and 1.25% Sarkosyl, mixed, and incubated for an additional 72 h at 37°C. One-eleventh of each reaction mix was used for the mock (no-PK) digestion control, and the remainder was treated with 20 μg/ml PK for 1 h at 37°C. One microliter of 0.1 M Pefabloc (Roche) was added to each sample, and the samples were methanol precipitated, pelleted, boiled in polyacrylamide gel electrophoresis (PAGE) loading buffer, and run in precast 16% sodium dodecyl sulfate-PAGE (SDS-PAGE) gels (Invitrogen). A Storm phosphorimager (GE Healthcare) was used for detection, and ImageQuant software was used to quantitate radioactive PrP bands. The conversion efficiency of each reaction was the percentage of input 35S-PrP-sen (determined from the no-PK aliquot) that was converted to 16- to 18-kDa PK-resistant 35S-PrP bands (determined from the PK-treated aliquot).
Animals.
Sg hamsters (Mesocricetus auratus) were purchased from Harlan Sprague Dawley, Inc. Armenian (Cricetulus migratorious), Chinese (Cricetulus griseus), Djungarian (Phodopus campbelli), and Siberian (Phodopus sungorus) hamsters and RML Swiss mice and Tg (haPrP) mice (“Sg hamsterized;” also called Tg7-haPrP/moPrP−/− mice) (18) were bred at NIAID/Rocky Mountain Laboratories (RML). C57BL10 mice were purchased from Jackson Laboratory. Protocols for using animals in these studies were reviewed and approved by the NIAID/RML Animal Care and Use Committee and complied with relevant NIH guidelines. Animals were housed at NIAID/RML facilities accredited by AAALAC International.
CWD primary inocula.
New supplies and aseptic technique were used for the following preparations to minimize the potential for contamination from any other TSE source. In addition, inoculations for these experiments were done separately from any other TSE work. For each of the individual brain inocula used (see Fig. 3A), the brainstems of a CWD-affected elk, mule deer, and white-tailed deer were removed. The brainstems were confirmed to be CWD positive by histology and immunoblot analysis of PrPCWD, and homogenates were inoculated into Sg hamsters and RML and Tg (haPrP) mice. Each of the CWD brain pools used (see Fig. 3B) has been described previously and contains heterogeneous genotypes (20). Brain pool-derived homogenates were inoculated into Sg, Djungarian, Chinese, and Armenian hamsters and C57BL10 mice. For each of the inocula, 10% brain homogenates in Dulbecco's phosphate-buffered saline (DPBS; Invitrogen) were made using a separate, new Dounce (Wheaton) homogenizer. Suspensions were sonicated for 5 min at maximum power (Heat Systems-Ultrasonics), diluted to 1% in DPBS, and inoculated intracerebrally (i.c.; 0.05 ml per animal).
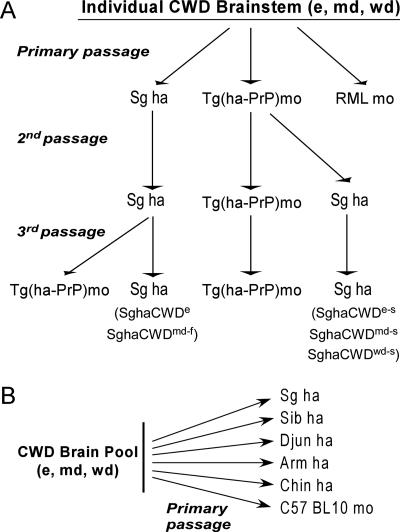
FIG. 3.
Passage history of CWD inocula in rodent species. Primary inocula used were brain homogenates of each species (e, elk; md, mule deer; and wd, white-tailed deer) from either individual CWD-positive animals (A) or brain pools from CWD-affected animals of each species (B). The individual brains were passaged serially three times in the rodent species indicated, whereas the pooled brain samples were passaged only one time in the various species shown. Since there were no positive RML mice from the primary passage of the individual positive cervid brains, no additional passes were done. ha, hamster; Sib, Siberian; Djun, Djungarian; Arm, Armenian; Chin, Chinese; mo, mouse.
Second- and third-passage inocula.
For the second passage, each brain from rodents inoculated with the individual animal primary inocula and suspected of being TSE positive by progressive signs of neurological disease was excised using new tools and divided sagittally. Half of each brain was used for immunohistochemical analysis (described below). A 20% homogenate was made from the other half in DPBS, using new disposable plastic pestles and microtubes (Kontes). A portion of this homogenate was used for PrP-res detection on immunoblots (see below), and another portion was diluted to 1%, with 0.05 ml inoculated i.c. into each animal as outlined in Fig. 3. The CWD brain pools from elk, mule deer, and white-tailed deer were passaged only once.
Immunoblot analysis of PrP-res from brain tissue.
Aliquots (0.1 ml) of 2% Triton X-100, 2% sodium deoxycholate, 0.2 M Tris-HCl, pH 8.3 (at 22°C), 0.3 M NaCl, and 0.01 M EDTA were added to 0.1-ml aliquots of the 20% brain homogenates described above. After incubation for 60 min at 22°C, the samples were placed on ice and sonicated in a cup horn at maximum power for 1 min. After centrifugation at 2,500 × g for 10 min at 4°C, the tubes were inverted three times to resuspend the softer portions of the pelleted material. The resulting supernatant suspensions, including all but the hardest parts of the pellets, were removed, and aliquots were saved at −20°C. A 15-μl aliquot of each suspension was digested by adding PK to 50 μg/ml and was incubated at 37°C for 60 min. One microliter of 0.1 M Pefabloc (Roche) was added, and the sample was held on ice for 5 min, followed by the addition of 25 μl of 2× PAGE loading buffer with 50 mM dithiothreitol and boiling for 5 min. Ten microliters of 0.25 M iodoacetamide (Sigma) was then added and incubated at 37°C for 10 min. A 10-μl aliquot of each sample was subjected to electrophoresis on 10% NuPAGE bis-Tris gels, using morpholineethanesulfonic acid (MES) running buffer (Invitrogen). Proteins were transferred to Immobilon-P (Millipore) membranes by semidry electroblotting. Membranes were immunostained using the following primary Abs (as described in references 20 and 22): monoclonal Ab 3F4 (11), rabbit antiserum 505 (against sheep peptide residues 100 to 111 [20]; generously provided by J. Langeveld, CIDC-Lelystad, The Netherlands), and rabbit antiserum R30 (against Sg hamster PrP residues 89 to 103) (9). The immunoblot was incubated with either alkaline phosphatase-conjugated anti-mouse or anti-rabbit immunoglobulin G (IgG; Zymed) secondary Ab, developed using AttoPhos (Promega) substrate, and scanned using a Storm fluorescence detection instrument (GE Healthcare).
Immunohistochemical analyses.
Brains were excised from PBS-perfused animals and divided sagittally. One-half was fixed in 3.7% phosphate-buffered formaldehyde for 3 to 5 days prior to routine dehydration and paraffin embedded. Sections were cut 4 to 6 μm thick and placed onto charged microscope slides. The remaining half was not fixed and was used for the biochemical analyses and serial passages described above. A Ventana NesES automated stainer was used for immunohistochemical staining of the sections. For PrP analysis, slides were deparaffinized, rehydrated with 0.1 mM citrate buffer, pH 6.0, and pretreated for 20 min at 120°C, using a decloaking chamber (Biocare). Using standard avidin-biotin technique (12), 3F4 was diluted 1:50, followed by biotinylated horse anti-mouse IgG at 1:250 (Vector Laboratories), Biogenex SS streptavidin (Biogenex), and amino carbazole as the substrate (Ventana). For glial fibrillary acidic protein (GFAP), there was no pretreatment, and the standard avidin-biotin technique was used with anti-GFAP at 1:1,000 (DAKO), biotinylated goat anti-rabbit IgG at 1:250 (Vector), and amino carbazole. Images were magnified at 40× and were captured on an Olympus BX51 light microscope, using MicroSuite software. For the images of whole brain sections, stained microscope slides were scanned using an Epson Expression 1640XL scanner at 1,400 dpi, and the images were processed using Adobe Photoshop software.
RESULTS
CFC reactions.
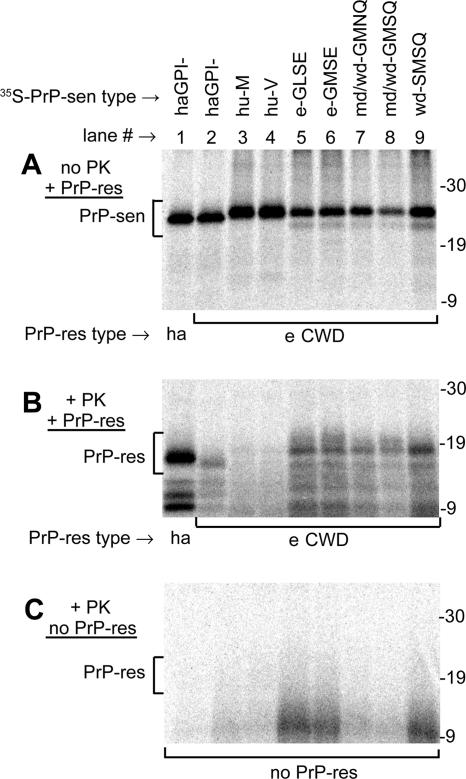
To test initially for the likelihood that hamsters might be susceptible to CWD, interspecies CFC reactions were done. Purified PrP-res from CWD-affected cervid brain tissue (PrPCWD) was incubated with unglycosylated and immunopurified 35S-labeled PrP-sen from hamsters, humans, and cervids and then digested with PK to detect newly formed 35S-PrP-res (Fig. 1). Although the hamster PrP-sen (Fig. 1A, lanes 1 and 2) used in these reactions lacked the GPI anchor, the other PrP-sen molecules (Fig. 1A, lanes 3 to 9) used did not. Previous studies have shown that this difference does not significantly affect conversion efficiency, at least for hamster PrP (15, 16). Furthermore, glycosylation does not significantly affect conversion efficiencies under these conditions (22).
FIG. 1.
CFC reactions of various species' PrP-sen molecules induced by PrP-res. PrP-res isolated from either Sg hamsters (ha; lane 1 of each panel) or CWD-affected elk (e CWD; lanes 2 to 9) was incubated with various immunopurified 35S-PrP-sen molecules, identified at the tops of the lanes. (A) Aliquots (1/11) of each reaction mix that were not PK treated. The PrP-sen bands (bracketed; 22 to 26 kDa) were not glycosylated, as explained in Results. (B) Remaining portion of each reaction mix, treated with PK, resulting in the radiolabeled PrP-sen molecules being converted to PK-resistant PrP bands (PrP-res bracket; 16 to 18 kDa). (C) Parallel set of PK-digested reaction mixes incubated without PrP-res. The following PrP-sen molecules (descriptions are given in reference 20) were used in the conversion reactions: cervid PrP polymorphic types present in elk (e-GLSE and e-GMSE; lanes 5 and 6), PrP types present in mule deer and white-tailed deer (md/wd-GMNQ and md/wd-GMSQ; lanes 7 and 8) or white-tailed deer only (wd-SMSQ; lane 9), human Met/Met (hu-M; lane 3) and human Val/Val (hu-V; lane 4) PrP (amino acid residue 129 is given), and Sg hamster PrP lacking the GPI anchor (lanes 1 and 2; haGPI−). The md/wd GMNQ (lane 7) has recently been shown to be only the predicted translation product of an unexpressed pseudogene in deer (6). All data except those using hamster PrP molecules were previously published (20) and are shown here only to compare interspecies conversion efficiencies. The hamster conversion reactions were done at the same time as the other conversion reactions, using identical PrP-res isolates. The migration of molecular mass standards, in kilodaltons, is shown to the right of each panel.
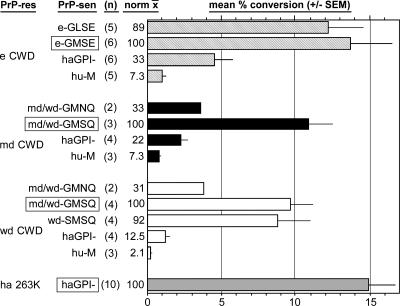
PK-resistant 35S-PrP bands (Fig. 1B, PrP-res bracket) that were 6 to 8 kDa smaller than the 35S-PrP-sen precursors (Fig. 1A, PrP-sen bracket) were of primary interest because they reflect the 6- to 8-kDa amino-terminal truncation that is observed with the PK digestion of PrP-res isolated from the brains of the TSE-affected species used for these studies (20). These CFC reactions show that elk PrPCWD (eCWD) induced the conversion of cervid, human, and hamster 35S-PrP-sen molecules to 35S-PrP-res (16- to 18-kDa PrP-res [brackets in Fig. 1B]). Cervid and human 35S-PrP-sen molecules were the most and least efficiently converted, respectively (20). Similar results were observed for CFC reactions done using mule deer (mdCWD) and white-tailed deer (wdCWD) PrPCWD (Fig. 2). The hamster 35S-PrP-sen was converted with intermediate efficiency by all PrPCWD isolates (e-, md-, and wdCWD) and with a much higher efficiency by hamster PrP-res (ha 263K) (Fig. 2). For unknown reasons, the conversion products from PrPCWD reaction mixes incubated with hamster 35S-PrP-sen migrated slightly faster by SDS-PAGE than those induced by hamster PrP-res (Fig. 1B, lanes 1 and 2). In the absence of PrP-res, 16- to 18-kDa PK-resistant 35S-PrP bands were not observed (Fig. 1C). The observation that the PrPCWD preparations converted hamster 35S-PrP-sen suggested that hamsters might be susceptible to CWD infection.
FIG. 2.
Relative efficiencies of conversion reactions, examples of which are shown in Fig. 1. The efficiency for each conversion reaction was determined by quantitation of the amount of input radiolabeled 35S-PrP-sen converted to 35S-PrP-res bands, as described in Materials and Methods. Efficiencies are expressed as the mean percent conversion of 35S-PrP-sen to 35S-PrP-res for 2 to 10 replicate experiments (n), with standard errors of the means (SEM) shown by the error bars. For each PrP-res species, the mean efficiency was normalized (norm x) to the mean conversion efficiency of the homologous (boxed) PrP-sen. All data except those using hamster PrP molecules were previously published (20) and are shown here for comparison.
Passages of CWD isolates into Sg hamsters.
Based on the CFC results, we attempted to transmit different CWD isolates to various species of hamsters as diagrammed in Fig. 3. Immunoblotting showed that PK-treated aliquots of the primary inocula from individual cervid animals (Fig. 4A, lanes e, md, and wd) and brain pools (20) contained PrPCWD, as evidenced by immunoreactivity with antiserum 505, which detects PrP molecules from all of these species (20) (Fig. 4A, left panel). The same samples were not immunoreactive with monoclonal Ab 3F4, which detects PK-treated Sg hamster 263K PrP-res (Fig. 4A, right panel). Thus, the 3F4 Ab was used to detect newly formed PrP-res in the host and to discriminate it from the primary inocula. Since 3F4 does not detect mouse or Chinese hamster PrP, PrP-res from these species was detected on immunoblots using R30 (data not shown), which, like 3F4, does not react with cervid PrP.
FIG. 4.
Analysis of PrP-res from sequential passages of CWD inocula in Sg hamsters and Tg (haPrP) mice. (A) Fluorescent immunoblots of SDS-PAGE gels show PK-resistant PrP in CWD primary inocula from individual elk (e), mule deer (md), and white-tailed deer (wd). Equal aliquots of the primary inocula were immunoblotted and analyzed with either 505 antiserum for the left blot or 3F4 Ab for the right blot. The 3F4 Ab detected 263K PrP-res from Sg hamster brain (ha), but it did not detect the PrPCWD in any of the primary inocula. (B and C) Immunoblots of representative examples of first and second serial passages, respectively, into either Sg hamsters or Tg (haPrP) mice, analyzed using 3F4. The sources indicated in panels B and C identify the primary CWD passage inocula. The migration of molecular mass standards, in kilodaltons, is shown to the right of each panel.
Upon primary passage of the various individual cervid inocula into Sg hamsters, only two hamsters inoculated with mule deer CWD showed clinical signs of TSE disease during an observation period of up to 2 years (Table 1, primary passage column, 172 and 326 days to death postinoculation [dpi]). Although they did not show evident clinical signs of TSE disease, two additional Sg hamsters for each of the md- and eCWD inoculations showed disease-associated PrP-res-positive immunoblots, indicating subclinical infections. None of the Sg hamsters inoculated with wdCWD showed any signs of neurological disease or tested positive for PrP-res on immunoblots.
TABLE 1.
Serial passage details of individual CWD-positive cervid animal brain homogenates inoculated into Sg hamsters
| CWD inoculum | Primary passage
|
Second passage
|
Third Passage
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species inoculateda | No. of animals with TSE signs/no. inoculatedb | No. of blot-positive animals/no. testedc | Incubation time (dpi) of blot-positive samplesf | Donor animal inoculum (dpi)e | Species inoculateda | No. of animals with TSE signs/no. inoculatedb | No. of blot-positive animals/no. tested | Incubation time of animals with TSE signs (mean dpi ± SD) | Donor animal inoculume | Species inoculateda | No. of animals with TSE signs/no. inoculatedb | Incubation time of animals with TSE signs (mean dpi ± SD) | |
| e | Sg ha | 0/7 | 2/7 | 381, 668 | e 1 (381) | Sg ha | 8/8 | 6/6 | 427 ± 53 | e 1a | Sg ha | 6/6 | 463 ± 21 |
| e 1b | Sg ha | 4/4 | 423 ± 28 | ||||||||||
| e 1b | Tg (haPrP) mo | 3/3 | 376 ± 8 | ||||||||||
| md | Sg ha | 2/7 | 4/7 | 172,* 274, 326,* 393 (291 ± 93) | md 1 (172) | Sg ha | 8/8 | 2/2 | 85 ± 0 | md 1a | Sg ha | 6/6 | 89 ± 0 |
| md 2 (326) | Sg ha | 8/8 | 2/2 | 85 ± 0 | md 2a | Sg ha | 6/6 | 89 ± 0 | |||||
| wd | Sg ha | 0/8 | 0/8 | Noned | Not done | Not done | |||||||
Sg ha, Sg hamster; Tg (haPrP) mo, Tg mouse with mouse PrP gene knocked out and Sg hamster PrP gene inserted (18).
For each group, 12 animals were inoculated for the first passage, 8 animals were inoculated for the second passage, and six Sg hamsters or four Tg (haPrP) mice were inoculated for the third passage. Animals lost due to intercurrent deaths were not included in these data. Intercurrent deaths are defined as animals that died without neurological signs prior to the date that the first animal in the group was determined to be TSE positive by either neurological signs or brain PrP-res by immunoblot analysis.
During the first passage, all animals with neurological signs were PrP-res positive, but not all brain PrP-res immunoblot-positive animals had neurological signs.
The animals in this group did not have neurologic signs and were not positive for brain PrP-res during their life span (range, 351 to 553 dpi; mean, 451 ± 62 dpi).
The code designates the individual Tg (haPrP) mouse or Sg hamster that was used as a source of inoculum in the passage specified. For example, “e 1” designates an individual TSE-positive Sg hamster that had been inoculated with eCWD and was used as the source of inoculum for a second passage; “e 1a” designates an individual Sg hamster from the second passage that had been inoculated from the “e1” Sg hamster.
The days to death for the individual animals displaying neurological signs consistent with TSE disease are indicated by asterisks. Brain homogenates from each of these were inoculated into groups of eight Sg hamsters for the second passage.
When the TSE-positive Sg hamsters from the primary passage of eCWD were passaged a second and then a third time, all recipients showed clinical signs after prolonged mean incubation periods of ≥423 dpi (Table 1, second- and third-passage columns), and all of the brains tested were PrP-res positive on immunoblots. Passage of an inoculum from one of the second-passage eCWD-inoculated positive hamsters into Tg (haPrP) mice gave a slightly shorter mean incubation period (Table 1, third-passage column). Second and third passages of mdCWD into Sg hamsters gave much shorter incubation periods, averaging 85 to 89 dpi, suggesting that the mule deer-derived CWD isolate was a different and much faster (f) isolate (SghaCWDmd-f) than that obtained from the eCWD inoculum (SghaCWDe). The TSE neurological signs of infection with this fast isolate differed from those for the SghaCWDe isolate, with affected animals presenting initially with a waddling gait, head bobbing, and unkempt appearance that developed into severe ataxia to the point of repeated falling and loss of the righting reflex. Approximately 2 to 3 weeks after the first neurological signs were observed, these animals became recumbent and were euthanized. In contrast, SghaCWDe-infected animals presented with a more subtle neurologic disorder that progressed at a much lower rate. After a period of 1.5 to 3 months, the disease typically progressed to hind leg paralysis, increased ataxia, tremors, and eventually, wasting.
Passages into “Sg-hamsterized” Tg (haPrP) mice.
Tg (haPrP) mice overexpressing Sg hamster PrP on a mouse PrP null background have shorter incubation periods than do Sg hamsters when inoculated with 263K hamster-adapted scrapie (18). Based on this observation, we suspected that Tg (haPrP) mice may have a more rapid disease response to CWD infection than that of Sg hamsters. Therefore, the same cervid inocula used for the Sg hamsters were used for the Tg (haPrP) mice (Fig. 3A, primary passage). After inoculation with brain homogenates from each of the CWD-affected cervid species, approximately one-third of the Tg (haPrP) mice showed clinical signs of TSE disease after extended mean incubation periods ranging from 585 to 668 dpi (Table 2, primary passage column), and a majority (62 to 88%) of these mice were positive for brain PrP-res by immunoblot analysis (examples are shown in Fig. 4B). Second and third serial passages into Tg (haPrP) mice caused clinical disease in all of the recipients and reductions in average incubation periods of the various groups to 185 to 282 dpi. Sg hamsters receiving second and third passages from clinically affected Tg (haPrP) mice were much slower (s) to develop disease (408 to 544 dpi) with elk (SghaCWDe-s), mule deer (SghaCWDmd-s), or white-tailed deer (SghaCWDwd-s)-derived CWD isolates than when Tg (haPrP) mice were inoculated with the same material. The longer incubation periods observed for the Sg hamsters compared to those for the Tg (haPrP) mice remained stable upon additional passages (Table 2, second- and third-passage columns). These hamsters displayed neurological signs and disease courses that were similar, if not identical, to those seen with the SghaCWDe isolate described above. The brains of all mice and hamsters analyzed for PrP-res on immunoblots were positive (examples are given in Fig. 4B and C). The clinical presentation of all affected Tg (haPrP) mice was similar to that for the SghaCWDe isolate, with subtle neurological disturbances and a prolonged disease course lasting at least 1 to 2 months. The Tg (haPrP) mice also exhibited kyphosis, a tiptoed gait, hind leg clasp when suspended by the tail, and eventually hind leg paralysis in most of the animals. This disease course contrasted with that seen with 263K scrapie-inoculated Tg (haPrP) mice, in which subtle neurological symptoms are followed rapidly by death, within 2 to 4 days (18).
TABLE 2.
Serial passage details of individual CWD-positive cervid animal brain homogenates inoculated into Tg (haPrP) mice
| CWD inoculum | Primary passage
|
Second passage
|
Third passage
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species inoculateda | No. of animals with TSE signs/no. inoculatedb | No. of blot-positive animals/no. tested | Incubation time (dpi) of TSE-positive sample (mean ± SD) | Time (dpi) of blot-positive samples (mean ± SD) | Donor animal inoculumc | Species inoculateda | No. of animals with TSE signs/no. inoculatedb | No. of blot-positive animals/no. tested | Incubation time of animals with TSE signs (mean dpi ± SD) | Donor animal inoculumc | Species inoculateda | No. of animals with TSE signs/no. inoculatedb | Incubation time of animals with TSE signs (mean dpi ± SD) | |
| e | Tg (haPrP) mo | 3/9 | 8/9 | 632 ± 89 | 585 ± 142 | e 1 | Tg (haPrP) mo | 7/7 | 3/3 | 282 ± 39 | e 1a | Tg (haPrP) mo | 4/4 | 203 ± 9 |
| e 1b | Tg (haPrP) mo | 5/5 | 202 ± 7 | |||||||||||
| e 1 | Sg ha | 5/6 | 4/5d | 509 ± 70 | e 1c | Sg ha | 5/5 | 543 ± 39 | ||||||
| e 1d | Sg ha | 5/5 | 526 ± 90 | |||||||||||
| e 2 | Tg (haPrP) mo | 8/8 | 3/3 | 215 ± 9 | e 2a | Tg (haPrP) mo | 4/4 | 210 ± 10 | ||||||
| e 2b | Tg (haPrP) mo | 4/4 | 185 ± 16 | |||||||||||
| e 3 | Tg (haPrP) mo | 7/7 | 3/3 | 212 ± 10 | e 3a | Tg (haPrP) mo | 6/6 | 247 ± 36 | ||||||
| e 3b | Tg (haPrP) mo | 3/3 | 204 ± 9 | |||||||||||
| md | Tg (haPrP) mo | 3/8 | 5/8 | 726 ± 47 | 668 ± 105 | md 1 | Tg (haPrP) mo | 8/8 | 3/3 | 202 ± 8 | md 1a | Tg (haPrP) mo | 4/4 | 213 ± 5 |
| md 1b | Tg (haPrP) mo | 4/4 | 259 ± 33 | |||||||||||
| md 1 | Sg ha | 7/7 | 7/7 | 485 ± 44 | md 1c | Sg ha | 4/4 | 544 ± 110 | ||||||
| md 1d | Sg ha | 2/2 | 540 ± 614 | |||||||||||
| md 2 | Tg (haPrP) mo | 8/8 | 4/4 | 209 ± 28 | md 2a | Tg (haPrP) mo | 3/3 | 228 ± 15 | ||||||
| md 2b | Tg (haPrP) mo | 4/4 | 212 ± 14 | |||||||||||
| wd | Tg (haPrP) mo | 4/9 | 8/9 | 632 ± 73 | 621 ± 80 | wd 1 | Tg (haPrP) mo | 7/7 | 4/4 | 272 ± 62 | wd 1a | Tg (haPrP) mo | 4/4 | 212 ± 32 |
| wd 1b | Tg (haPrP) mo | 4/4 | 237 ± 13 | |||||||||||
| wd 1 | Sg ha | 5/5 | 5/5 | 462 ± 3 | wd 1c | Sg ha | 3/3 | 408 ± 42 | ||||||
| wd 1d | Sg ha | 4/4 | 436 ± 15 | |||||||||||
Abbreviations used are the same as those in Table 1.
For each group, 12 animals were inoculated for the first passage, 8 animals were inoculated for the second passage, and 4 to 6 animals were inoculated for the third passage. Intercurrent deaths, as defined in Table 1, are not included in these data.
The code designates the individual Tg (haPrP) mouse or Sg hamster that was used as a source of inoculum in the passage specified. For example, “e 1” designates an individual TSE-positive Tg (haPrP) mouse that was inoculated with eCWD and was used as the source of inoculum for a second passage; “e 1a” designates an individual Tg (haPrP) mouse from the second passage that had been inoculated from the e 1 Tg (haPrP) mouse.
The blot-negative animal (second passage, e 1 inoculated into Sg hamster) was also negative for neurological signs and likely died due to another undetermined cause.
Immunohistological analyses of different isolates.
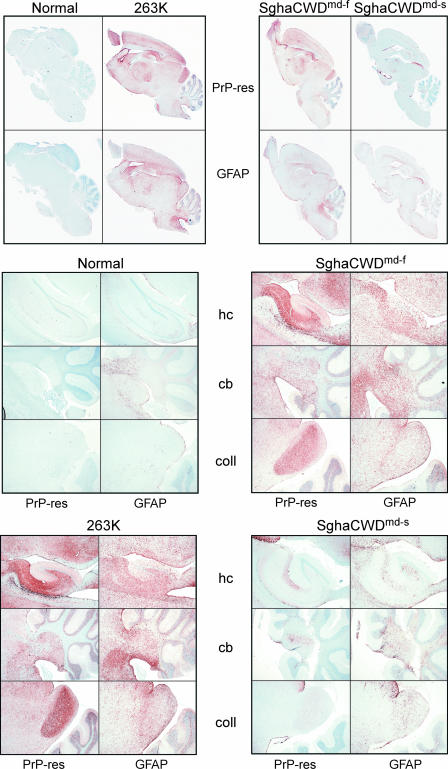
Immunohistological analyses of brain sections from Sg hamsters clinically affected with the faster isolate, SghaCWDmd-f, and with the slower isolates first passaged through the Tg (haPrP) mice, i.e., SghaCWDe-s, SghaCWDmd-s, and SghaCWDwd-s, revealed different patterns of PrP-res accumulation and gliosis (Fig. 5, PrP-res and GFAP panels, respectively). PrP-res was detected using 3F4 Ab, and gliosis was detected with anti-GFAP Ab as described in Materials and Methods. Immunohistological data are shown for sagittal sections of brains of an uninfected (normal) Sg hamster and a terminally affected 263K Sg hamster, SghaCWDmd-f, which was generated by serial passage of mdCWD through two Sg hamsters, and SghaCWDmd-s, which was generated by sequential passages, first into Tg (haPrP) mice and then into Sg hamster. Both PrP-res deposition and gliosis were widespread in the brains of 263K- and SghaCWDmd-f-affected hamsters, whereas in the SghaCWDmd-s-affected hamster they were not. The hippocampus, cerebellum, and caudal colliculus regions are also shown at higher magnification for more detailed analysis. SghaCWDe-s and SghaCWDwd-s showed patterns of PrP-res deposition and gliosis indistinguishable from those of SghaCWDmd-s shown in Fig. 5 (data not shown).
FIG. 5.
Immunohistochemical analysis of brain sections from slow (SghaCWDmd-s) and fast (SghaCWDmd-f) isolates compared to uninfected (normal) and 263K-infected Sg hamster brains. PrP-res deposition was visualized in sagittal sections of the various brains, using 3F4 Ab (“PrP-res” panels). Using adjacent brain sections, the extent of gliosis was visualized using anti-GFAP Ab (“GFAP” panels). Scanned whole-brain images are shown in the upper panels. For the middle and lower panels, hippocampus (hc), cerebellum (cb), and caudal colliculus (coll) regions were magnified at ×40 for more detailed analysis. The images shown are representative of the following numbers of brains analyzed: two normal brains, two 263K-infected brains, three each of second- and third-passage SghaCWDmd-f-infected brains, and two each of third-passage SghaCWDmd-s-, SghaCWDe-s-, and SghaCWDwd-s-infected brains.
Passages of CWD into various hamster species.
In order to determine whether other hamster species may be susceptible to CWD and result in a useful rodent-adapted CWD animal model, brain homogenates isolated from elk, mule deer, or white-tailed deer brain pools were inoculated into several other hamster species (Table 3). In contrast to the transmission experiments using CWD isolates from individual elk, mule, and white-tailed deer (Table 1), none of the Sg hamsters inoculated with the CWD brain pools developed clinical disease within their life span, and no PrP-res was detected by immunoblot analysis of their brains. There was also no evidence of transmission to Djungarian hamsters. In contrast, there were obvious clinical signs in a majority of Chinese hamsters inoculated with the e- and mdCWD brain pools, though not with the wdCWD pool. Most of the clinically positive Chinese hamster brains tested were PrP-res positive by immunoblot analysis. With Siberian hamsters, there was one animal that received the mdCWD pool inoculum that was positive for both neurological signs of TSE disease and PrP-res. Another Siberian hamster was confirmed to be positive from the white-tailed deer pool inoculum. A single Armenian hamster was clinically suspect and immunoblot positive for the eCWD pool inoculum. All of the clinically infected animals initially presented with rapid tremors and ataxia that progressively worsened for 2 to 4 weeks until the animals became recumbent and were euthanized.
TABLE 3.
Passage details of CWD-positive cervid brain homogenates inoculated into various hamster and wild-type mouse species
| CWD inoculum | Species inoculateda | No. of animals with TSE signs/no. inoculatedb | No. of blot-positive animals/no. tested | Incubation time of animals with TSE signs (mean dpi ± SD)c | Life span (dpi, mean ± SD) | Life span range (dpi) |
|---|---|---|---|---|---|---|
| e | Sg ha | 0/12 | 0/2 | None positive | 628 ± 98 | 480-751 |
| md | Sg ha | 0/12 | 0/2 | None positive | 591 ± 98 | 469-693 |
| wd | Sg ha | 0/12 | 0/3 | None positive | 510 ± 95 | 430-644 |
| e | Djun ha | 0/4 | Not done | None positive | 500 ± 95 | 384-615 |
| md | Djun ha | 0/10 | Not done | None positive | 507 ± 97 | 399-659 |
| wd | Djun ha | 0/1 | Not done | None positive | 476 | 476 |
| e | Chin ha | 6/8 | 4/5e | 626 ± 80 | 730 ± 220 | 555-1,133 |
| md | Chin ha | 6/8 | 6/7e | 648 ± 58 | 690 ± 166 | 563-1,133 |
| wd | Chin ha | 0/6 | Not done | None positive | 722 ± 239 | 448-1,133 |
| e | Sib ha | 0/2 | Not done | None positive | 532 ± 69 | 483-581 |
| md | Sib ha | 1/3 | 1/3e | 464 | 482 ± 23 | 464-509 |
| wd | Sib ha | 1/4 | 1/4e | 735 | 638 ± 118 | 499-739 |
| e | Arm ha | 1/4 | 1/2e | 745 | 669 ± 121 | 490-745 |
| md | Arm ha | 0/15 | 0/1 | None positive | 625 ± 96 | 348-704 |
| wd | Arm ha | 0/8 | 0/8 | None positive | 587 ± 40 | 532-649 |
| ed | RML mo | 0/9 | 0/9 | None positive | 592 ± 80 | 488-736 |
| mdd | RML mo | 0/6 | 0/6 | None positive | 679 ± 93 | 488-805 |
| wdd | RML mo | 0/6 | 0/6 | None positive | 730 ± 101 | 562-893 |
| e | C57BL10 mo | 0/15 | 0/2 | None positive | 724 ± 107 | 588-837 |
| md | C57BL10 mo | 0/16 | 0/2 | None positive | 657 ± 169 | 368-837 |
| wd | C57BL10 mo | 0/14 | 0/2 | None positive | 772 ± 125 | 368-837 |
Abbreviations are the same as those in Table 1. Djun, Djungarian; Chin, Chinese; Sib, Siberian; Arm, Armenian; RML mo, Rocky Mountain Laboratory mouse.
For each group, 12 to 16 animals were inoculated. Animals lost due to intercurrent deaths (as defined in Table 1) are not included in these data.
None positive, none of the animals in the group were TSE positive by either neurological signs or immunoblot analysis for brain PrP-res during their life span.
RML mice were inoculated with the individual CWD-positive cervid brain homogenates used for both the Sg hamsters in Table 1 and the Tg (haPrP) mice in Table 2.
The blot-positive animals also showed neurological signs consistent with TSE disease, while the blot-negative animals did not.
Passages into wild-type mice.
The inocula used for the RML outbred mice and C57BL10 inbred mice were from the CWD-affected individual animal brains and the CWD brain pools, respectively (Table 3). None of the animals of either mouse species developed any neurological signs within their life span (ranging from 488 to 893 dpi), and none were immunoblot positive for brain PrP-res.
DISCUSSION
We have shown that CWD from one or more cervid species can be transmitted to Sg, Chinese, Siberian, and Armenian hamsters and to Tg mice that express Sg hamster prion protein. Transmission of CWD to Sg hamsters was attempted previously without generating disease, most likely because the animals in that study were incubated for only 1 year (2). In the present study, inoculated animals were observed for periods exceeding 2 years for some animals. We found CWD transmission, with the highest attack rates in Chinese hamsters and Tg (haPrP) mice. The other rodent species had much lower attack rates or were not susceptible. The incomplete attack rates for the hamster species and Tg (haPrP) mice indicated that the cervid CWD inocula contained an average of only roughly 1 ID50 (the dose that would infect 50% of the animals) for these species. However, these inocula produced disease in Tg mice expressing deer PrP at a 100% attack rate, indicating that the titer for mice with homologous PrP is greater (R. E. Race, K. Meade-White, and B. Chesebro, unpublished data). The lack of transmission of some of the cervid CWD inocula to the other rodent species could be due to small differences in inoculum titers or to heterogeneity in the PrP sequences in the pooled inocula rather than to fundamental differences in host susceptibility. The amounts of normal host PrP expressed in the different hamster species are similar (K. Meade-White and R. E. Race, unpublished data) and are not likely an explanation for the different susceptibilities. Due to the low attack rates and long incubation periods seen with primary passages from cervids, none of these rodent species would be practical for use in direct bioassays for cervid CWD.
Nonetheless, the rodent-adapted CWD models we have developed may be useful to experimentally analyze TSE species and strain differences. Despite the low initial attack rates for the first passage of CWD into Sg hamsters, CWD isolates derived initially from elk and mule deer readily adapted to hamsters, as evidenced by the 100% infection rate on second and third passages. The average incubation periods were similar for the second and third passages but considerably shorter than that for the first passage for the Sg hamsters, suggesting that any species barrier to infection (formally, the shortening of the incubation period between the first and subsequent passages in a new species) was overcome quickly.
When mdCWD was serially passaged in Sg hamsters, an isolate, SghaCWDmd-f, was obtained that had a relatively short incubation period. When the same inoculum was passaged first into Tg (haPrP) mice followed by serial passage in Sg hamsters, an isolate with a fivefold longer incubation period developed, namely, SghaCWDmd-s. The CWD inocula from elk and white-tailed deer led only to the slow isolates SghaCWDe-s and SghaCWDwd-s, which were indistinguishable from the slow mule deer isolate SghaCWDmd-s. The markedly different incubation periods of these two isolates from the mdCWD inoculum, as well as the distinct clinical signs and patterns of brain pathology and PrP-res deposition, raise the possibility that different strains of CWD isolates may exist, at least in mule deer, which in turn can lead to distinct CWD strains in Sg hamsters. Another possibility is that the strains diverged upon introduction into Sg hamsters, as suggested for the HY and DY Sg hamster strains from TME inoculum-infected mink brain homogenates (1).
Differences in PrP-res glycoform patterns analyzed from several CWD-affected deer and elk also suggested that CWD strains in mule deer may be more heterogeneous than those in elk (19). Others have also found evidence of CWD strains (16a). Curiously, however, this apparent strain difference was not manifested when the identical mdCWD inoculum was serially passaged through only one recipient species. Serial passage in Sg hamsters yielded only the fast-growing isolate (Table 1 and Fig. 3), while passage first through Tg (haPrP) mice and then into Sg hamsters yielded only the slow-growing isolate (Table 2 and Fig. 3). With this in mind, it is important to consider other possible explanations for these results. One possibility is that the CWD isolate might be able to undergo a stochastic change into a more rapid and aggressive strain in Sg hamsters and that this happened to occur after the mdCWD inoculations. This would be similar to the emergence of fast (HY) and slow (DY) strains upon inoculation of TME isolates into Sg hamsters (5). These strains developed even when a clonal isolate of the TME inoculum was used, suggesting that they arose in the recipient Sg hamsters rather than in the mink source (1).
Finally, although extensive precautions were taken, we cannot formally prove that inadvertent contamination of the mdCWD inoculum with the hamster-derived 263K strain did not occur, which potentially could yield short incubation period passages in Sg hamsters (Table 1). However, the incubation periods observed with the CWD passages (85 to 89 days) were significantly longer than the 263K incubation periods observed in our lab (70 to 75 days), and no mock-infected controls became sick during their life span. Also, we saw no 263K-like infectivity develop in the highly susceptible Tg (haPrP) mice, even though we used the identical primary inoculum for both recipient species. Interestingly, the similarity of the Sg hamster-adapted CWD fast-growing isolate and 263K might be due to a common origin, since there is circumstantial evidence that CWD arose from cervid exposure to sheep scrapie, which was also the origin of the 263K strain in hamsters (14). Furthermore, the Hyper strain derived from TME inoculations has 263K-like strain characteristics in Sg hamsters (5). Thus, it would appear that both CWD and TME transmissions into Sg hamsters can result in divergent fast and slow strains.
Acknowledgments
This research project was funded in part by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases (NIAID), NIH, by the Colorado Division of Wildlife, by the University of Wyoming, and by the Wyoming Game and Fish Department.
We particularly thank Sue Priola, Valerie Sim, and Brent Race for helpful discussions and critical readings of the manuscript. We also thank John Coe for his expertise in establishing colonies of the various hamster species at RML that were used in these studies and Anita Mora and Gary Hettrick for critical assistance with photographic images and graphics. We greatly appreciate the animal care provided by the Rocky Mountain Veterinary Branch/NIAID/NIH, in particular Ed Schreckendgust and Rod Parker.
Footnotes
Published ahead of print on 7 February 2007.
REFERENCES
- 1.Bartz, J. C., R. A. Bessen, D. McKenzie, R. F. Marsh, and J. M. Aiken. 2000. Adaptation and selection of prion protein strain conformations following interspecies transmission of transmissible mink encephalopathy. J. Virol. 74:5542-5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartz, J. C., R. F. Marsh, D. I. McKenzie, and J. M. Aiken. 1998. The host range of chronic wasting disease is altered on passage in ferrets. Virology 251:297-301. [DOI] [PubMed] [Google Scholar]
- 3.Reference deleted.
- 4.Bessen, R. A., and R. F. Marsh. 1994. Distinct PrP properties suggest the molecular basis of strain variation in transmissible mink encephalopathy. J. Virol. 68:7859-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bessen, R. A., and R. F. Marsh. 1992. Identification of two biologically distinct strains of transmissible mink encephalopathy in hamsters. J. Gen. Virol. 73:329-334. [DOI] [PubMed] [Google Scholar]
- 6.Brayton, K. A., K. I. O'Rourke, A. K. Lyda, M. W. Miller, and D. P. Knowles. 2004. A processed pseudogene contributes to apparent mule deer prion gene heterogeneity. Gene 326:167-173. [DOI] [PubMed] [Google Scholar]
- 7.Browning, S. R., G. L. Mason, T. Seward, M. Green, G. A. Eliason, C. Mathiason, M. W. Miller, E. S. Williams, E. Hoover, and G. C. Telling. 2004. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J. Virol. 78:13345-13350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caughey, B., M. Horiuchi, R. Demaimay, and G. J. Raymond. 1999. Assays of protease-resistant prion protein and its formation. Methods Enzymol. 309:122-133. [DOI] [PubMed] [Google Scholar]
- 9.Caughey, B., G. J. Raymond, D. Ernst, and R. E. Race. 1991. N-terminal truncation of the scrapie-associated form of PrP by lysosomal protease(s): implications regarding the site of conversion of PrP to the protease-resistant state. J. Virol. 65:6597-6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandler, R. L. 1963. Experimental scrapie in the mouse. Res. Vet. Sci. 4:276-285. [Google Scholar]
- 11.Kascsak, R. J., R. Rubenstein, P. A. Merz, M. Tonna-DeMasi, R. Fersko, R. I. Carp, H. M. Wisniewski, and H. Diringer. 1987. Mouse polyclonal and monoclonal antibody to scrapie-associated fibril proteins. J. Virol. 61:3688-3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kercher, L., C. Favara, C. C. Chan, R. Race, and B. Chesebro. 2004. Differences in scrapie-induced pathology of the retina and brain in transgenic mice that express hamster prion protein in neurons, astrocytes, or multiple cell types. Am. J. Pathol. 165:2055-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kimberlin, R. H., and C. A. Walker. 1977. Characteristics of a short incubation model of scrapie in the golden hamster. J. Gen. Virol. 34:295-304. [DOI] [PubMed] [Google Scholar]
- 14.Kimberlin, R. H., and C. A. Walker. 1978. Evidence that the transmission of one source of scrapie agent to hamsters involves separation of agent strains from a mixture. J. Gen. Virol. 39:487-496. [DOI] [PubMed] [Google Scholar]
- 15.Kocisko, D. A., J. H. Come, S. A. Priola, B. Chesebro, G. J. Raymond, P. T. Lansbury, and B. Caughey. 1994. Cell-free formation of protease-resistant prion protein. Nature 370:471-474. [DOI] [PubMed] [Google Scholar]
- 16.Kocisko, D. A., S. A. Priola, G. J. Raymond, B. Chesebro, P. T. Lansbury, Jr., and B. Caughey. 1995. Species specificity in the cell-free conversion of prion protein to protease-resistant forms: a model for the scrapie species barrier. Proc. Natl. Acad. Sci. USA 92:3923-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16a.LaFauci, G., R. I. Carp, J. C. Meeker, X Ye, J. I. Kim, M. Natelli, M. Cedeno, R. B. Petersen, R. Kascsak, and R. Rubenstein. 2006. Passage of chronic wasting disease prion into transgenic mice expressing Rocky Mountain elk (Cervus elephus nelsoni) PrPc. J. Gen. Virol. 87:3772-3780. [DOI] [PubMed] [Google Scholar]
- 17.Miller, M. W., and E. S. Williams. 2004. Chronic wasting disease of cervids. Curr. Top. Microbiol. Immunol. 284:193-214. [DOI] [PubMed] [Google Scholar]
- 18.Race, R., M. Oldstone, and B. Chesebro. 2000. Entry versus blockade of brain infection following oral or intraperitoneal scrapie administration: role of prion protein expression in peripheral nerves and spleen. J. Virol. 74:828-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Race, R. E., A. Raines, T. G. Baron, M. W. Miller, A. Jenny, and E. S. Williams. 2002. Comparison of abnormal prion protein glycoform patterns from transmissible spongiform encephalopathy agent-infected deer, elk, sheep, and cattle. J. Virol. 76:12365-12368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raymond, G. J., A. Bossers, L. D. Raymond, K. I. O'Rourke, L. E. McHolland, P. K. Bryant III, M. W. Miller, E. S. Williams, M. Smits, and B. Caughey. 2000. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO J. 19:4425-4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raymond, G. J., and J. Chabry. 2004. Purification of the pathological isoform of prion protein (PrPSc or PrPres) from transmissible spongiform encephalopathy-affected brain tissue, p. 16-26. In S. Lehmann and J. Grassi (ed.), Techniques in prion research. Birkhauser Verlag, Basel, Switzerland.
- 22.Raymond, G. J., J. Hope, D. A. Kocisko, S. A. Priola, L. D. Raymond, A. Bossers, J. Ironside, R. G. Will, S. G. Chen, R. B. Petersen, P. Gambetti, R. Rubenstein, M. A. Smits, P. T. Lansbury, Jr., and B. Caughey. 1997. Molecular assessment of the transmissibilities of BSE and scrapie to humans. Nature 388:285-288. [DOI] [PubMed] [Google Scholar]
- 23.Silveira, J. R., B. Caughey, and G. S. Baron. 2004. Prion protein and the molecular features of transmissible spongiform encephalopathy agents. Curr. Top. Microbiol. Immunol. 284:1-50. [DOI] [PubMed] [Google Scholar]
- 24.Tamguney, G., K. Giles, E. Bouzamondo-Bernstein, P. J. Bosque, M. W. Miller, J. Safar, S. J. DeArmond, and S. B. Prusiner. 2006. Transmission of elk and deer prions to transgenic mice. J. Virol. 80:9104-9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams, E. S., and S. Young. 1992. Spongiform encephalopathies in Cervidae. Rev. Sci. Tech. 11:551-567. [DOI] [PubMed] [Google Scholar]