Abstract
We have used Affymetrix high-density gene arrays to generate a temporal profile of gene expression during differentiation of UB/OC-1, a conditionally immortal cell line derived from the mouse cochlea. Gene expression was assessed daily for 14 days under differentiating conditions. The experiment was replicated in two separate populations of cells. Profiles for selected genes were correlated with those obtained by RT-PCR, TaqMan analysis, immunoblotting, and immunofluorescence. The results suggest that UB/OC-1 is derived from a population of nonsensory epithelial cells in the greater epithelial ridge that have the potential to differentiate into a hair-cell-like phenotype, without the intervention of Math1. Elements of the Notch signaling cascade were identified, including the receptor Notch3, with a transient up-regulation that suggests a role in hair cell differentiation. Several genes showed a profile similar to Notch3, including the transcriptional co-repressor Groucho1. UB/OC-1 also expressed Me1, a putative partner of Math1 that may confer competence to differentiate into hair cells. Cluster analysis revealed expression profiles for neural guidance genes associated with Gata3. The temporal dimension of this analysis provides a powerful tool to study genetic mechanisms that underlie the conversion of nonsensory epithelial cells into hair cells.
[The entire data set published in this paper has been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus Database (http://www.ncbi.nlm.nih.gov/geo/) under the series accession no. GSE36 and sample numbers contained therein. Supplementary material is available online at http://www.genome.org. The following individuals kindly provided reagents, samples or unpublished information as indicated in the paper: T. Hasson, C. Petit, and P. Matsudaira]
We sought to discover gene expression patterns associated with the early differentiation of mammalian auditory hair cells. We used a conditionally immortal cell line (UB/OC-1) selected from the cochlea of the Immortmouse at embryonic day 13 (E13), to represent the time at which hair cell progenitors cease proliferation and start to differentiate (Rivolta et al. 1998), and Affymetrix oligonucleotide arrays to assess gene expression in the cells during differentiation in vitro. The cell line UB/OC-1 was derived from Immortomouse embryos and can be induced to differentiate in a conditional manner. These mice have an immortalizing transgene, the temperature-sensitive variant tsA58, regulated by a γ-interferon-inducible promoter. When the cells are cultured at 33°C in the presence of γ-interferon, proliferation is maintained, but following removal of γ-interferon from the culture media and an increase in temperature to 39°C, proliferation ceases and the cells start to differentiate (Jat et al. 1991).
Synthetic oligonucleotide arrays and cDNA arrays have been used to analyze gene expression in a wide range of cells and tissues (Alon et al. 1999; Mochii et al. 1999; Perou et al. 2000; Ross et al. 2000). Although the technique is relatively simple (Lockhart et al. 1996; Kozian and Kirschbaum 1999; Lipshutz et al. 1999), the experimental design and methods of data analysis are critical. Clonal cell lines are valuable because the genetic programs within a cell are highly ordered and this is reflected in the coordinated expression of functionally related genes (Niehrs and Pollet 1999; Lockhart and Winzeler 2000). These ‘synexpression’ groups can most easily be explored by screening the genetic profile of a single cell type with time following a specific experimental manipulation (Hovland et al. 2001; Schulze et al. 2001). In a small, experimentally inaccessible organ like the mammalian inner ear, cell lines present a unique opportunity to investigate complex cellular processes and are well suited to drug discovery (Debouck and Goodfellow 1999).
There are only 6600 auditory hair cells in the mouse (Echteler et al. 1994). They are born between E12–E14 (Ruben 1967; Kelley et al. 1993) and differentiation proceeds from the base to the apex of the cochlea with a delay of ∼2–3 d (Lim and Anniko 1985; Zine and Romand 1996; Nishida et al. 1998). Cells mature around postnatal days P12–P14, when the ear becomes functional (Rubsamen and Lippe 1997; Kros et al 1998). Additional hair cells can be recruited during this period (Kelley et al. 1993; Zheng and Gao 2000), but there is no evidence for sensory cell replacement after P14. Progressive loss of hair cells accounts for the largest proportion of hearing loss, which affects more than 10% of the human population (Rubel 1997). One advantage of the experimental approach that we have adopted lies in the potential identification of key factors and signaling pathways that may allow therapeutic stimulation of hair cell replacement. As a developmental model, it also allows us to study transient events during differentiation of a single cell type.
We have investigated profiles of selected regulatory genes. These include the zinc finger transcription factor Gata3, which is expressed in sensory and nonsensory epithelial cells in the developing cochlea at E13 (Rivolta and Holley 1998; Karis et al. 2001; Lawoko-Kerali et al. 2002), the bHLH transcription factor Math1 (Bermingham et al. 1999; Lanford et al. 2000), and the associated genes Hes1 (Zheng et al. 2000; Zine et al. 2001) and Hes5 (Lanford et al. 2000; Zine et al. 2001). Math1 can induce hair cell differentiation in nonsensory embryonic epithelial cells from the greater epithelial ridge (GER; Zheng and Gao 2000) and its action appears to be opposed by Hes1 (Zheng et al. 2000). Hair cells and supporting cells share a common progenitor (Fekete et al. 1998; Lang and Fekete 2001) and cell specification depends upon a mechanism of lateral inhibition mediated by Notch1 and its ligands, Jagged1 and Jagged2 (Adam et al. 1998; Haddon et al. 1998; Lanford et al. 1999). We show that UB/OC-1 probably originates from the nonsensory epithelial cell population in the GER and that, when differentiated, closely resembles the hair cell phenotype. Thus it has considerable potential for studying the genetic processes underlying a potential mechanism for recruitment of replacement hair cells.
RESULTS
Transition from Proliferation at 33°C to Differentiation at 39°C
The transition from proliferating to differentiating conditions is a functional event that should be reflected in the gene expression profiles, thus presenting an opportunity to test the validity of the system. Genes associated with proliferation, including cyclins A, B1, B2, E, and F, were down-regulated at 39°C, whereas those associated with the quiescent state, including Gadd45, Chop-10, and Gas1, were up-regulated over 15-fold.
Expression levels for over 200 genes changed by more than fivefold during the experiment (Supplemental Table 1 available online at www.genome.org). At day 2, expression levels of 55 genes increased relative to day 0, whereas those for 36 genes decreased. At day 4, 119 genes increased and 36 decreased, whereas at day 9, 101 genes increased and 62 decreased. Twelve and 37 genes increased at day 2 and 4, respectively, and then dropped below the fivefold threshold, showing transient expression early in differentiation.
Several loosely related groups of genes changed dramatically. Those encoding extracellular matrix (ECM) proteins such as decorin, Col6a1, Col3a1, matrix Gla protein, and OSF-2 (similar to fascilin, not to be confused with osf2/cbfa) increased from day 2, suggesting that the cells recondition their environment to undergo differentiation by secreting a different basement membrane. Col8a1, Col6a2, laminin β2 were detected at day 4 and laminin β3 at day 9. Genes encoding growth factors, including cellular retinol binding protein 1 (CRBP1), insulin-like growth factor binding protein 2 (IGFBP-2), and a member of the PDGF/VEGF family increased at day 2. At day 4, the cells expressed TGFβ3, whereas at day 9 they also expressed insulin-like growth factor binding protein 5 (IGFBP-5). The transcription factor C/EBPδ increased almost 10-fold at day 2 and remained high during late differentiation. The homeobox gene Prx2 was also up-regulated early in differentiation, but its level dropped below 5-fold by day 4, whereas goosecoid, another homeobox gene, peaked at day 4 with a 6-fold increase.
Characterization of UB/OC-1
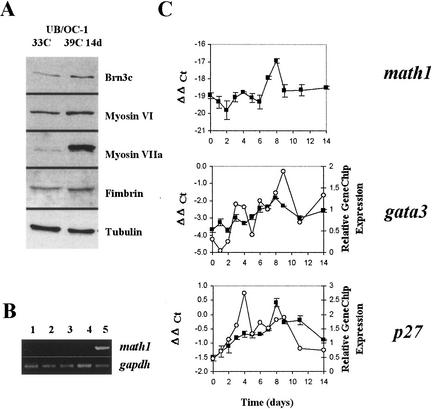
UB/OC-1 was originally selected because it expressed key markers for hair cell precursors (Rivolta et al. 1998). These included the transcription factor Brn3c (also known as Brn3.1), normally expressed from E14 (Ryan 1997) and essential for hair cell survival (Erkman et al. 1996; Xiang et al. 1997, 1998), the α9 subunit of the acetylcholine receptor (α9AChR), and the cytoskeletal proteins myosin VI, myosin VIIa, and fimbrin (Fig. 1A). The expression of these genes correlated with the developmental stage at which the cells were derived.
Figure 1.
(A) Western blot of protein samples extracted at 33°C and after 14 d at 39°C. (B) RT-PCR for math1. Lanes are 1, 33°C; 2, 2 d at 39°C; 3, 4 d at 39°C; 4, 14 d at 39°C; and 5, genomic DNA. (C) Plots comparing the expression profiles for math1, gata3, and p27Kip1obtained by TaqMan analysis (filled squares) and GeneChip microarray (open circles). Math1 plot includes only the TaqMan results because the array values were called absent and are below the normalized threshold. Scales for TaqMan are on the left axis (ΔΔ Ct), whereas those for relative gene chip expression are plotted on the right axis. Standard error bars are shown for the TaqMan assays. The absolute levels of math1 detected by TaqMan were extremely low. Compare the differences in scale for math1 and gata3. Each unit represents a difference of a power of 2, so at time point 0, gata3 expression is ∼214 greater than math1. Moreover, the values obtained for math1 were not different from those of the minus reverse transcriptase controls.
One unexpected finding was the absence of the proneural gene Math1, which is normally expressed in differentiating hair cells at E12.5–E13 (Bermingham et al. 1999; Lanford et al. 2000). It was recorded absent throughout the microarray analysis and in all samples assayed by RT-PCR (Fig. 1B). Controls with RNA minus reverse transcriptase (RT) and a mock cDNA reaction without RNA were also negative. Furthermore, the TaqMan analysis did not detect any expression above the background of the minus RT controls (Fig. 1C).
There was no obvious evidence for supporting cell markers in differentiated UB/OC-1. Immunoblots were negative for OCP-2, a protein highly expressed by supporting cells in vivo (Yoho et al. 1997). From the connexin family of molecules, only connexin 31 was detected at 33°C in the microarray, but it disappeared after 2 d at 39°C. The results for connexins 30, 26, and 43 were negative. α- and β-tectorins are specific to the ear and form part of the tectorial membrane (Legan et al. 1997; Rau et al. 1999). The microarrays were negative for α-tectorin at all time points. However, β-tectorin was detectable at 33°C and quickly down-regulated during differentiation. This ear-specific gene is normally expressed in the sensory epithelium at E12.5, and at E14.5 it is restricted to narrow bands that include the border cells along the inner edge of the inner hair cells, the pillar cells, and the third row of Deiters' cells (Rau et al. 1999). It is not expressed in hair cells.
The profiles obtained for Gata3 and p27Kip1 in the microarray were broadly similar to those obtained by TaqMan analysis (Fig 1C). These genes are important in inner ear development and their expression in UB/OC1 correlated with that described in vivo. Gata3 is normally expressed by all cells of the sensory epithelium and is down-regulated initially in differentiating hair cells (Rivolta and Holley 1998; Karis et al. 2001). In UB/OC-1, expression peaked at days 8–9 at 39°C and then dropped later to about half its peak level. For p27Kip1, there was an initial low level of expression at 33°C and the mRNA increased to peak transiently between days 4 and 8. In vivo, p27Kip1 normally appears around E14 in hair cells and supporting cells, but it is down-regulated in hair cells at ∼E16 (Chen and Segil 1999; Lowenheim et al. 1999). Thus the expression profile in the cell line resembled that in vivo, albeit on a slower timescale. This fact, already observed for other genes in this cell line, could prove useful in identifying and dissecting signaling cascade components that would normally have a very short and transient expression in vivo.
Global Cluster Analysis
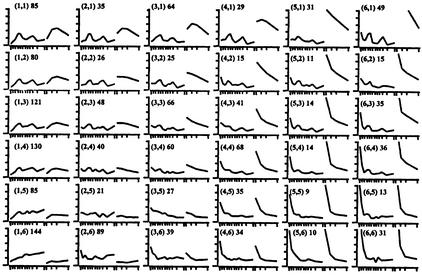
Global analysis of gene expression can reveal genes with clustered expression profiles that are likely to be functionally related. To establish such groups we employed self-organizing maps (SOMs). SOMs allow easy visualization of changing profiles through time and they place genes with similar profiles in adjacent groups. This produces a matrix with progressive, gradual transitions through the rows and columns. The 1675 genes that displayed a >2.5-fold change at any time point were clustered into a 6 × 6 lattice (Fig. 2; Supplemental Tables 2–37 available online).
Figure 2.
A 6 × 6 matrix displaying the clustered profiles of genes changing over 2.5-fold at any given point. Cluster number is enclosed by parentheses; total number of genes per cluster are outside parentheses. The Y-axis represents normalized relative expression, the X-axis shows both time courses (time course 1 following differentiation over a 14 d period containing 12 data points and time course 2 following differentiation over a 9 d period containing 4 data points). The average profile for each cluster is shown. Genes included in each cluster are listed in Supplemental Tables 2–37 available online.
The Cluster Containing Transcription Factor Gata3
In our SOM analysis, Gata3 located to cluster 1,4 in a group of 130 genes (Supplemental Table 5 available online). Potentially related patterns of gene expression from this set include those of Semaphorins A, E, and F; MEP (a member of the Eph family of receptors that lacks protein tyrosine kinase activity); the cellular retinol binding protein I (CRBPI); the putative regulator of transcription AES1; the homeobox gene Pbx1; and several ESTs similar to zinc finger genes.
Expression Profiles of Elements of the Notch Signaling System in UB/OC1
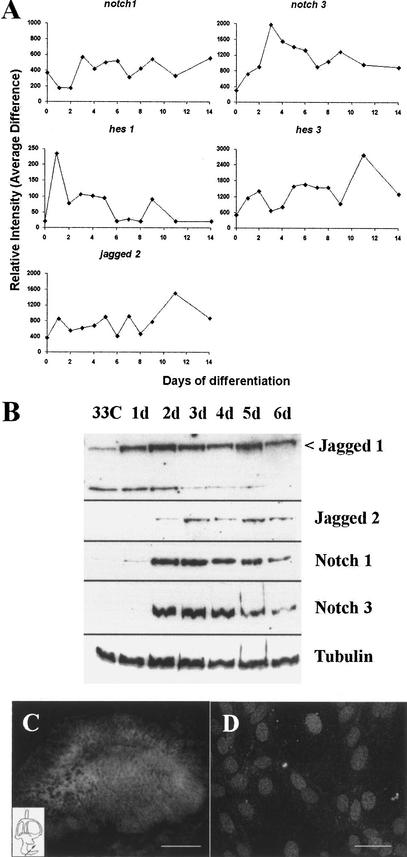
The notch signaling system plays an important role in the determination of cell fates in the inner ear (Adam et al. 1998; Haddon et al. 1998; Lanford et al. 1999) and four members of the notch receptor family, Notch1–4, have been described in the mouse (Weinmaster 1997). UB/OC-1 did not express Notch2 or Notch4 at any time point. Notch1 was present throughout the experiment, although at low levels under proliferative conditions, and it reached a peak after 3–4 d at 39°C (Fig. 3A). Notch3 was not expressed at 33°C but was detectable after 2 d at 39°C and reached a clear peak at days 3–4 (Fig. 3A). The expression of the notch genes on the arrays correlated well with results from immunoblotting. Notch3 expression was only detected from day 2 onward, whereas Notch1 was detectable at 33°C at low levels, increasing its expression at days 2–3 (Fig. 3B). The expression of Notch1 protein is reduced during late differentiation, being barely detectable after 14 d (not shown). This is in agreement with the expression profile described for differentiating hair cells in vivo (Zine et al. 2000).
Figure 3.
Notch signaling cascade in UB/OC-1. (A) Relative gene chip expression for Notch1, Notch3, Jagged2, hes1, and hes3. (B) Western blot for notch and jagged proteins on protein samples taken at daily intervals. (C) Cross-section of an E14.5 cochlear duct, showing immunolabeling for Notch3. (D) UB/OC1 at 39°C for 3 d, showing nuclear location of Notch3. Bars are 50 μm.
Hes genes are downstream effectors of the notch cascade. Hes1 was present at 33°C, increased sharply soon after the temperature switch, and then dropped at days 3–4 to lower levels, at which it remained constant until day 9 (Fig. 3A). Its expression was undetectable by day 14. In contrast, Hes3 steadily increased during differentiation and reached its peak at day 11 (Fig. 3A). Hes5 was not detected.
Of the notch ligands, Dll1 was not detected. Jagged2 expression peaked at day 11 (Fig. 3A) and on Western blots it was detected from day 2 (Fig. 3B). Jagged1 probe sets were not included in the MU11K microarray, so we explored its expression profile by Western blot. The anti-jag1 antibody identified a band of ∼190 Kilodalton (Kd) at 33°C that was up-regulated immediately after the temperature shift. A second band of ∼130 Kd was also detected. This smaller band was more obvious at 33°C but was progressively down-regulated and virtually undetectable after 6 d at 39°C (Fig. 3B). Proteolytic processing of the full-length ligand may have produced the 130-Kd band. The fact that the extracts were prepared in the presence of several protease inhibitors and that this band was systematically down-regulated with differentiation, suggests that the result reflected a real process within the cells rather than degradation during sample preparation.
Immunolocalization of Notch3
The pattern of distribution of Notch3 was investigated by immunolabeling on the cells and also on sections of the developing inner ear. In situ hybridization shows that it is expressed at E11.5 and E13.5 but is no longer detectable at E15.5 (Lardelli et al. 1994; Lindsell et al. 1996; Lewis et al. 1998). In our study, Notch3 was visualized in the epithelium of the inner ear at E14.5 (Fig. 3C). The receptor did not segregate to any particular region or cell type, being homogeneously expressed by the entire epithelial lining, but it was absent from the underlying mesenchyme. At E16.5, its expression was no longer detectable. In UB/OC-1 Notch3 was localized to the cell nuclei after 3 d at 39°C (Fig. 3D). This subcellular distribution suggests that at this time point it was activated, translocated to the nucleus, and engaged in signaling.
Genes Associated with Notch3
On the 6 × 6 SOM analysis, Notch3 clustered to the 1,2 group (Fig. 2). This cluster contained a total of 79 genes (Supplemental Table 3 available online.), including 32 ESTs with no significant homology in the database. The annotated genes included the transcription factors goosecoid, AREC3/six4, and Sox4, the cyclin-dependent kinase inhibitor p15ink4b and the vesicle trafficking gene Munc-18.
Other bHLH Genes
The list of genes expressed by UB/OC-1 was further explored for genes encoding a bHLH protein. Me1 (also known as tcf12-htf4-heb) was expressed at steady levels from day 0 and Stra13, a bHLH-gene inducible by retinoic acid, peaked late during differentiation at days 9–11.
DISCUSSION
One of the main applications of global analysis of gene expression using microarrays is to establish hypotheses that can then be tested experimentally. The data presented here have enabled us to characterize further the origin of the cell line UB/OC-1 and then to explore its properties in the context of normal development. Cloned cell lines of known origin allow us not only to identify genes not previously implicated in ear development, but also to propose potential synexpression groups with or without reference to known genes.
Identity and Origin of UB/OC-1
The results strongly suggest that UB/OC-1 was derived from epithelial cells within the GER that have the potential to differentiate into hair cells. Under differentiating conditions, they adopt a hair-cell-like phenotype. None of the supporting cell markers explored is expressed and the cells appear to commit uniformly to the same phenotype, as shown by the homogenous expression of brn3c (Rivolta et al. 1998; and this manuscript).
In earlier experiments designed to identify and target proliferating precursors, whole organs of Corti from E13 Immortomice were cultured at 33°C with 100 U/mL γ-interferon to activate the immortalizing T-antigen. However, in the developing sensory epithelium the cell nuclei remained unlabeled by both BrdU and an antibody against the T-antigen. Furthermore, cuticular plates and hair bundles differentiated more or less normally (N. Grix, M. Rivolta and M. Holley, unpubl. results). In the adjacent nonsensory regions, including the GER and the lesser epithelial ridge (LER), the cell nuclei did express the T-antigen and labeled positively for BrdU. A possible interpretation is that the T-antigen is unable to override the controls imposed by intercellular interactions in at least some regions of the intact epithelia. Consequently, cell lines were derived from dissociated cultures and the exact origin of UB/OC-1 is thus unknown. However, the genetic profile suggests that UB/OC-1 originates from the GER. UB/OC-1 expresses Gata3 and cytokeratins, which are also expressed by sensory and nonsensory epithelial cells in the cochlear duct at the age of derivation (Rivolta and Holley 1998; Rivolta et al. 1998; Karis et al. 2001). It also expresses Hes1 and β-tectorin at 33°C. Hes1 is present mainly in the GER and the LER (Zheng et al. 2000; Zine et al. 2001), whereas β-tectorin appears only in the GER and the pillar cells during development (Rau et al. 1999). On the other hand, Hes5, which is normally expressed in the LER (Zheng et al. 2000; Zine et al. 2001), is not expressed by UB/OC-1 at any time point. Finally, Math1 is expressed in the prosensory cell population but not in the GER.
Certain Hair Cell Characteristics Are Independent of Math1 Expression
Our conclusion about the origin of UB/OC-1 is strengthened by the discovery that cells in the GER can become hair cells when transfected with Math1 (Zheng and Gao 2000). However, UB/OC-1 appears to achieve the expression of numerous hair cell markers without expressing Math1. Math1-null mice fail to generate hair cells (Bermingham et al 1999), implying that this gene is essential for hair cell production, but in chimeric mice where Math1−/− embryonic stem cells were introduced into wild type blastocysts, Math1−/− hair cells have been observed (Du et al. 2000). It thus is conceivable that the pro-hair-cell function assigned to Math1 is redundant and that other bHLH genes could compensate in its absence. Math1 may require an inductive signal that is missing in our in vitro system. In UB/OC-1 it is clear that Math-1 is not directly linked to the up-regulation of myo7a, brn3c, or the α9 AChR. However, Math1 could act by blocking the effect of negative regulators such as Hes5, which could directly repress the expression of hair cell genes in supporting cells.
The competence of cells in the GER to form hair cells must be intrinsic because the transfection of other cell types in the same preparations (such as non-neuronal ganglia cells and connective tissue) does not lead to the generation of new hair cells (Zheng and Gao 2000). It is known that Math1 requires a partner protein, namely members of the class A of HLH proteins (Akazawa et al 1995). The competence of the GER can then be interpreted as the ability of these cells to express such cofactors. With this aim we searched the expression profiles in the microarray for class A bHLH proteins that were present at any given point. We identified Me1, a putative partner of math1 that colocalizes with it during development of the granular cells of the cerebellum (Uittebogaard and Chiaramello 1999) and that is highly expressed in brain regions that display neuronal plasticity (Chiaramello et al. 1995). In UB/OC-1, Me1 is expressed at steady levels throughout differentiation.
Notch3 Could Mediate an Instructive Signal for Hair Cell Differentiation
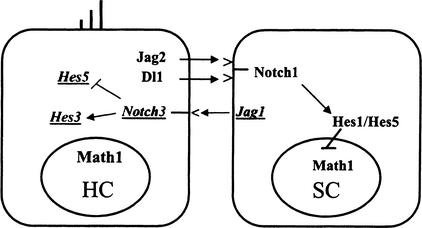
The expression of Notch3 could resolve an outstanding issue regarding the current model for notch signaling in the ear. In this model, Notch1 activation, mediated by Jagged2, inhibits the hair cell fate (Lanford et al. 1999; Zhang et al. 2000). The in vivo expression pattern of the notch ligand Jagged1 is not consistent with a simple model for lateral inhibition. Early in development, Jagged1 is expressed in the sensory patches (Morrison et al. 1999; Zine et al. 2000), whereas later its expression is segregated to the supporting cells, where it colocalizes with Notch1. The mouse mutants Htu and slalom, produced by missense mutations in Jagged1, show a reduction in the numbers of outer hair cells (Kiernan et al. 2001; Tsai et al. 2001), as opposed to the increases seen in loss-of-function studies for Jagged2, Notch1, and Hes1. Thus, Jagged1 appears to be signaling in the opposite direction to Jagged2. It has been proposed from gene reporter studies that Notch3 acts by interfering with Notch1 signaling, possibly by blocking Notch1-mediated Hes1 transcription (Beatus et al. 1999, 2001). In UB/OC-1, the level of Hes1 mRNA drops when Notch3 increases. It has been shown that activated Notch3 can induce astroglial differentiation (Tanigaki et al. 2001). Thus it is possible that Notch3 may act as an antagonist of Notch1, mediating an instructive rather than inhibitory signal for hair cell differentiation. In conclusion, we propose that Jagged1 could have an inductive effect on hair cell differentiation mediated by Notch3 (Fig. 4).
Figure 4.
A model for the proposed role of Notch3 in hair cell differentiation. The conventional lateral inhibition cascade includes Dll and Jag2, the ligands expressed by the hair cell that would activate Notch1 in the adjacent supporting cells. On the other hand, Jag1 expressed by the supporting cell could be activating Notch3 in the hair cells and oppose the effects of Notch1 in this cell type, either by repressing the transcription of Hes5 or by promoting differentiation through Hes3.
Several related genes cluster together with Notch3 or appear in nearby clusters in the SOM analysis, showing a peak of expression early during differentiation. Groucho1/tle1 is a transcriptional corepressor that interacts with HLH proteins (Fisher and Caudy 1998). It is up-regulated during early stages of neuronal differentiation in P19 cells exposed to retinoic acid (Husain et al. 1996; Yao et al. 1998), as well as in CFK2 cells, which are a model for chondrocytic determination (Yao et al. 1998). It is also transiently expressed during epithelial differentiation (Liu et al. 1996). Goosecoid (Gsc) is a homeobox-containing transcription factor that is expressed in the middle ear, as well as in the cochlear epithelia (Yamada et al. 1995; Rivera-Perez et al. 1999). Null-mutant mice have defects mainly in the malleus and tympanic membrane, as well as in the external canal (Yamada et al. 1995). In vitro, expression of Gsc can enhance neuronal differentiation (Sawada et al. 2000), whereas in Drosophila Gsc can interact with groucho (Jimenez et al. 1999). AREC3/Six4 is also a homebox-containing gene, homolog of the Drosophila gene sine oculis (Kawakami et al. 1996). It is up-regulated during muscle differentiation of C2C12 cells. In the chicken ear, cSix4 has been identified at the otic placodes and later to the ventral region of the otocyst that will develop into the cochlea (Esteve and Bovolenta 1999).
Another HLH gene that is up-regulated during differentiation in UB/OC-1 is Hes3. During development in vivo, Hes3 is expressed in the cochlear epithelium (Lobe 1997). Although there is no clear evidence that will support Hes3 as a positive instructor of differentiation, it has been proposed that the alternatively spliced variant Hes3a could inhibit the activity of negative repressors like Hes1 by competing for a cofactor, therefore promoting differentiation (Hirata et al. 2000).
Gata3 and Semaphorins
Gata3 has a broad pattern of expression in the ear (Rivolta and Holley 1998; Karis et al. 2001; Lawoko-Kerali et al. 2002), but there is strong evidence that it is involved in neural pathfinding (Karis et al. 2001). In this context it may be important to note that three semaphorin genes (semaA, semaE, and semaF) clustered to the same group. Semaphorins are signaling molecules involved in axon pathfinding, mainly acting as a repeling cue for the guidance of axons during development (Tamagnone and Comoglio 2000). During innervation of the organ of Corti, Gata3 is down-regulated in hair cells but remains expressed by the surrounding supporting cells. If these semaphorin genes are under Gata3 regulation, it could be hypothesized that they are preventing the axons from establishing synaptic contacts with the supporting cells.
An understanding of the genetic programs that underlie the conversion of nonsensory to sensory cell phenotypes could be essential for developing therapeutic approaches to sensory regeneration in the inner ear. We have shown that the combination of a characterized cell line and gene array technology can yield robust insights into such programs. The data allows us to form sound hypotheses for the functions of developmentally important genes, illustrated here by Math1, Gata3, and Notch3, that can be tested experimentally either in vivo or in vitro.
METHODS
Cell Culture
UB/OC-1 and culture conditions have been described previously (Rivolta et al. 1998). Proliferation was maintained by culturing cells in Minimal Essential Medium with Earle's Salts and Glutamax I (MEM, Life Technologies), 10% fetal calf serum (FCS, Life Technologies), and 50 U/mL of γ-Interferon (γ-IFN, Life Technologies) at 33°C. Differentiation was induced by replacing the media without γ-IFN and then transferring the dishes to 39°C. RNA samples were collected at day 0 (before transfer to differentiating conditions) and then at 24-h intervals up to 14 d after the temperature shift. Two independent time courses were performed and two separate sets of RNA samples were collected.
RNA Isolation and Array Hybridization
Total RNA was prepared with a Qiagen RNeasy mini-kit according to the manufacturer's instructions. cRNA was prepared as described in the Affymetrix GeneChip Expression Analysis Technical Manual. GeneChip oligonucleotide arrays (Affymetrix), composed of 13,179 probe sets representing over 11,000 genes and ESTs (MU11K; chips A and B) were used for hybridization. A comprehensive list of genes present in the chips and search tools to scan through their contents are available at http://www.netaffx.com. Hybridizations were performed according to the Affymetrix GeneChip Expression Analysis Technical Manual. Arrays were scanned using an Affymetrix confocal scanner. Array hybridization was performed at the time points 0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, and 14 d for the first experiment (Time Course 1) and at 0, 2, 4, and 9 d for the second experiment (Time Course 2).
Analysis of GeneChip Data
Scanned output files were visually inspected for hybridization artifacts and then analyzed with GeneChip v. 3 (Affymetrix). Arrays were scaled to an average intensity of 300 and analyzed independently. The expression data for each array was saved as an Excel file containing expression values (Average Difference) and detection of RNA species (Absence Call). These parameters were calculated by GeneChip according to standard Affymetrix procedures. Comparison files were generated with GeneChip for each differentiating time course, using day 0 as a baseline. These comparison files contained a parameter that measured whether gene expression had changed between two time points (Difference Call). For further data mining, clustering, and presentation, tab delimited Affymetrix Excel files were imported into GeneSpring (v. 3.2.11–4.0.0, Silicon Genetics) after values for Average Difference of <20 were given an arbitrary value of 20. In GeneSpring, expression profiles were normalized per time point, making the median of all measurements 1, and normalized per gene to give a median value of 1 for each gene expression profile. These normalizations reduced the influence of outliers and allowed the visualization of expression profiles for different genes on the same axis.
Criteria for Gene Selection
The following criteria were set for determining which genes were responsive to differentiation in UB/OC-1. Gene lists were generated for genes with Difference Calls that represented expression changes in at least one time point with respect to day 0 in Time Course 1. This gene list was further filtered in GeneSpring by applying a 2.5-fold change restriction in at least one time point for Time Course 1. However, lists were also scanned for potentially interesting genes based on their Presence/Absence Call, despite their fold change.
Gene Clustering
Clustering was performed using a SOM algorithm in GeneSpring. SOMs have the benefit of being a well-studied method of clustering and have been shown to be able to extract physiologically relevant patterns of gene expression in an experimental setting (Tamayo et al. 1999). All genes modulated by at least 2.5-fold in Time Course 1 were clustered according to the relative behavior of each gene in both time courses.
Fold Change Lists
Restrictions were applied to Time Courses 1 and 2 and days 0, 2, 4, and 9 using the Affymetrix generated metrics Fold Change, Absolute Call, and Difference Call. Lists were generated of genes with expression that changed measurably (Difference Call) by at least 2.5-fold (Fold Change) compared to day 0, were detected above background in at least one of the time points (called Present in the Absolute Call), and were maintained in both experiments for each day. These lists were further restricted by including only those genes that had an Average Fold Change ≥5-fold.
TaqMan Assay
TaqMan 5′ nuclease fluorogenic quantitative PCR assays were performed using the TaqMan Gold RT-PCR kit (PE Applied Biosystems) according to manufacturer's instructions. Total mRNA was prepared from UB/OC-1 grown and harvested as described above. Reverse transcription was performed with 2 μg of total RNA using random hexamer primers and identical reactions were performed in the absence of reverse transcriptase. Assays were performed in triplicate using an ABI Prism 7700 Sequence Detector (PE Applied Biosystems), VIC-labeled ribosomal RNA control reagents (PE Applied Biosystems), and the following oligonucleotides (5′ to 3′; MWG Biotech UK):
forward primer CTGAAAACTGAGACAACCAAATGCreverse primer AAGGGTGCAGGGATATTTGTCA
FAM-labeled probe CTAGCGCGCGGGAAGCCCC.
GATA-3:
forward primer GAGTCTCCAAGTGTGCGAAGAGT
reverse primer TCGGGCTTCATGATACTGCTC
FAM-labeled probe TCCGACCCCTTCTACTTGCGTTTTTCG.
G1 cyclin-Cdk protein kinase inhibitor p27:
forward primer CTTCCGCCTGCAGAAATCTC
reverse primer CAGTGCTTCTCCAAGTCCCG
FAM-labeled probe TCGGCCCGGTCAATCATGAAGAACT
RT-PCR
End-point RT-PCR was performed on samples taken at 33°C and after 2, 4, and 14 d at 39°C. Controls included genomic DNA and samples without reverse transcriptase. Math1 primers and cycling conditions were taken from Ben-Arie et al. (1996). Gapdh primers used as a normalizing control were as previously described (Rivolta et al. 1998).
Immunoblotting and Immunolabeling
These procedures were performed as previously described (Rivolta et al. 1998; Lawlor et al. 1999). Primary antibodies used were as follows: brn3c (rabbit polyclonal, Babco); myosin VI (rabbit polyclonal, a kind gift from T. Hasson, UCSD), myosin VIIa (rabbit polyclonal, a kind gift from C. Petit, Institut Pasteur), fimbrin (rabbit polyclonal, a kind gift from P. Matsudaira, Whitehead Institute for Biomedical Research). Polyclonal antibodies to Jagged1, Jagged2, Notch1, and Notch3 were from Santa Cruz Biotech and the tubulin Ab-4 antibody (Clone DM1A + DM1B) was from Neomarkers.
WEB SITE REFERENCES
http://www.ncbi.nlm.nih.gov/geo/; National Center for Biothechnology Information (NCBI) Gene Expression Omnibus Database.
http://www.netaffx.com; Affymetrix home page.
Acknowledgments
This work was supported by grants from The Wellcome Trust (grant no. 042065) and from Pfizer Global Research. We thank Dr. Frank Burslem at Pfizer for technical guidance.
The publication costs of this article were defrayed in part by payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 USC section 1734 solely to indicate this fact.
Footnotes
E-MAIL m.n.rivolta@sheffield.ac.uk; FAX (44) 0-114-222-2360.
Article and publication are at http://www.genome.org/cgi/doi/10.1101/gr.225602.
REFERENCES
- Adam J, Myat A, Le Roux I, Eddison M, Henrique D, Ish-Horowicz D, Lewis J. Cell fate choices and the expression of Notch, Delta, and Serrate homologues in the chick inner ear: Parallels with Drosophila sense-organ development. Development. 1998;125:4645–4654. doi: 10.1242/dev.125.23.4645. [DOI] [PubMed] [Google Scholar]
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem. 1995;270:8730–8738. doi: 10.1074/jbc.270.15.8730. [DOI] [PubMed] [Google Scholar]
- Alon U, Barkai N, Notterman DA, Gish K, Ybarra S, Mack D, Levine AJ. Broad patterns of gene expression revealed by clustering analysis of tumor and normal colon tissues probed by oligonucleotide arrays. Proc Natl Acad Sci. 1999;96:6745–6750. doi: 10.1073/pnas.96.12.6745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatus P, Lundkvist J, Oberg C, Lendahl U. The notch 3 intracellular domain represses notch 1–mediated activation through Hairy/Enhancer of split (HES) promoters. Development. 1999;126:3925–3935. doi: 10.1242/dev.126.17.3925. [DOI] [PubMed] [Google Scholar]
- Beatus P, Lundkvist J, Oberg C, Pedersen K, Lendahl U. The origin of the ankyrin repeat region in Notch intracellular domains is critical for regulation of HES promoter activity. Mech Dev. 2001;104:3–20. doi: 10.1016/s0925-4773(01)00373-2. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, McCall AE, Berkman S, Eichele G, Bellen HJ, Zoghbi HY. Evolutionary conservation of sequence and expression of the bHLH protein Atonal suggests a conserved role in neurogenesis. Hum Mol Genet. 1996;5:1207–1216. doi: 10.1093/hmg/5.9.1207. [DOI] [PubMed] [Google Scholar]
- Bermingham NA, Hassan BA, Price SD, Vollrath MA, Ben-Arie N, Eatock RA, Bellen HJ, Lysakowski A, Zoghbi HY. Math1: An essential gene for the generation of inner ear hair cells. Science. 1999;284:1837–1841. doi: 10.1126/science.284.5421.1837. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. (p27 Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chiaramello A, Soosaar A, Neuman T, Zuber MX. Differential expression and distinct DNA-binding specificity of ME1a and ME2 suggest a unique role during differentiation and neuronal plasticity. Mol Brain Res. 1995;29:107–118. doi: 10.1016/0169-328x(94)00236-8. [DOI] [PubMed] [Google Scholar]
- Debouck C, Goodfellow PN. DNA microarrays in drug discovery and development. Nat Genet. 1999;21:48–50. doi: 10.1038/4475. [DOI] [PubMed] [Google Scholar]
- Du XP, Jensen P, Goldowitz D, Hamre KM. Inner ear hair cell development in math1−/− chimeric mice. 30th Annual Meeting of the Society for Neuroscience, New Orleans, LA. 2000. [Google Scholar]
- Echteler SM, Fay RR, Popper AN. Structure of the mammalian cochlea. In: Fay RR, et al., editors. Comparative hearing: Mammals, Springer handbook of auditory research. Vol. 4. New York: Springer-Verlag; 1994. pp. 134–171. [Google Scholar]
- Erkman L, McEvilly RJ, Luo L, Ryan AK, Hooshmand F, O'Connell SM, Keithley EM, Rapaport DH, Ryan AF, Rosenfeld MG. Role of transcription factors Brn-3.1 and Brn-3.2 in auditory and visual system development. Nature. 1996;381:603–606. doi: 10.1038/381603a0. [DOI] [PubMed] [Google Scholar]
- Esteve P, Bovolenta P. cSix4, a member of the six gene family of transcription factors, is expressed during placode and somite development. Mech Dev. 1999;85:161–165. doi: 10.1016/s0925-4773(99)00079-9. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Muthukumar S, Karagogeos D. Hair cells and supporting cells share a common progenitor in the avian inner ear. J Neurosci. 1998;18:7811–7821. doi: 10.1523/JNEUROSCI.18-19-07811.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher AL, Caudy M. Groucho proteins: Transcriptional corepressors for specific subsets of DNA-binding transcription factors in vertebrates and invertebrates. Genes & Dev. 1998;12:1931–1940. doi: 10.1101/gad.12.13.1931. [DOI] [PubMed] [Google Scholar]
- Haddon C, Jiang YJ, Smithers L, Lewis J. δ-Notch signaling and the patterning of sensory cell differentiation in the zebrafish ear: Evidence from the mind bomb mutant. Development. 1998;125:4637–4644. doi: 10.1242/dev.125.23.4637. [DOI] [PubMed] [Google Scholar]
- Hirata H, Ohtsuka T, Bessho Y, Kageyama R. Generation of structurally and functionally distinct factors from the basic helix-loop-helix gene Hes3 by alternative first exons. J Biol Chem. 2000;275:19083–19089. doi: 10.1074/jbc.M001075200. [DOI] [PubMed] [Google Scholar]
- Hovland AR, Nahreini P, Andreatta CP, Edwards-Prasad J, Prasad KN. Identifying genes involved in regulating differentiation of neuroblastoma cells. J Neurosci Res. 2001;64:302–310. doi: 10.1002/jnr.1079. [DOI] [PubMed] [Google Scholar]
- Husain J, Lo R, Grbavec D, Stifani S. Affinity for the nuclear compartment and expression during cell differentiation implicate phosphorylated Groucho/TLE1 forms of higher molecular mass in nuclear functions. Biochem J. 1996;317:523–531. doi: 10.1042/bj3170523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat PS, Noble MD, Ataliotis P, Tanaka Y, Yannoutsos N, Larsen L, Kioussis D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc Natl Acad Sci. 1991;88:5096–5100. doi: 10.1073/pnas.88.12.5096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez G, Verrijzer CP, Ish-Horowicz D. A conserved motif in goosecoid mediates groucho-dependent repression in Drosophila embryos. Mol Cell Biol. 1999;19:2080–2087. doi: 10.1128/mcb.19.3.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karis A, Pata I, van Doorninck JH, Grosveld F, de Zeeuw CI, de Caprona D, Fritzsch B. Transcription factor GATA-3 alters pathway selection of olivocochlear neurons and affects morphogenesis of the ear. J Comp Neurol. 2001;429:615–630. doi: 10.1002/1096-9861(20010122)429:4<615::aid-cne8>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kawakami K, Ohto H, Ikeda K, Roeder RG. Structure, function and expression of a murine homeobox protein AREC3, a homologue of Drosophila sine oculis gene product, and implication in development. Nucleic Acids Res. 1996;24:303–310. doi: 10.1093/nar/24.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MW, Xu X-M, Wagner MA, Warchol ME, Corwin JT. The developing organ of Corti contains retinoic acid and forms supernumerary hair cells in response to exogenous retinoic acid in culture. Development. 1993;119:1041–1053. doi: 10.1242/dev.119.4.1041. [DOI] [PubMed] [Google Scholar]
- Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, Steel KP, Hrabe de Angelis M. The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci. 2001;98:3873–3878. doi: 10.1073/pnas.071496998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozian DH, Kirschbaum BJ. Comparative gene-expression analysis. Trends Biotechnol. 1999;17:73–78. doi: 10.1016/s0167-7799(98)01292-x. [DOI] [PubMed] [Google Scholar]
- Kros CJ, Ruppersberg JP, Rüsch A. Expression of a potassium current in inner hair cells during development of hearing in mice. Nature. 1998;394:281–284. doi: 10.1038/28401. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, Gridley T, Kelley MW. Notch signaling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21:289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- Lanford PJ, Shailam R, Norton C R, Gridley T, Kelley MW. Expression of Math1 and HES5 in the cochleae of wild-type and Jag2 mutant mice. JARO. 2000;1:161–171. doi: 10.1007/s101620010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H, Fekete DM. Lineage analysis in the chicken inner ear shows differences in clonal dispersion for epithelial, neuronal, and mesenchymal cells. Dev Biol. 2001;234:120–137. doi: 10.1006/dbio.2001.0248. [DOI] [PubMed] [Google Scholar]
- Lardelli M, Dahlstrand J, Lendahl U. The novel Notch homologue mouse Notch 3 lacks specific epidermal growth factor-repeats and is expressed in proliferating neuroepithelium. Mech Dev. 1994;46:123–136. doi: 10.1016/0925-4773(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Lawlor P, Marcotti W, Rivolta MN, Kros C, Holley MC. Differentiation of mammalian vestibular hair cells from conditionally immortal, postnatal supporting cells. J Neurosci. 1999;19:9445–9458. doi: 10.1523/JNEUROSCI.19-21-09445.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawoko-Kerali G, Rivolta MN, Holley MC. Expression of the transcription factors GATA3 and Pax2 during development of the mammalian inner ear. J Comp Neurol. 2002;442:378–391. doi: 10.1002/cne.10088. [DOI] [PubMed] [Google Scholar]
- Legan PK, Rau A, Keen JN, Richardson GP. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J Biol Chem. 1997;272:8791–8801. doi: 10.1074/jbc.272.13.8791. [DOI] [PubMed] [Google Scholar]
- Lewis AK, Frantz GD, Carpenter DA, de Sauvage FJ, Gao WQ. Distinct expression patterns of notch family receptors and ligands during development of the mammalian inner ear. Mech Dev. 1998;78:159–163. doi: 10.1016/s0925-4773(98)00165-8. [DOI] [PubMed] [Google Scholar]
- Lim DJ, Anniko M. Developmental morphology of the mouse inner ear. A scanning electron microscopic observation. Acta Otolaryngol (Suppl.) 1985;422:1–69. [PubMed] [Google Scholar]
- Lindsell CE, Boulter J, diSibio G, Gossler A, Weinmaster G. Expression patterns of Jagged, Delta1, Notch1, Notch2, and Notch3 genes identify ligand-receptor pairs that may function in neural development. Mol Cell Neurosci. 1996;8:14–27. doi: 10.1006/mcne.1996.0040. [DOI] [PubMed] [Google Scholar]
- Lipshutz RJ, Fodor SPA, Gingeras TR, Lockhart DJ. High density synthetic oligonucleotide arrays. Nat Genet. 1999;21:20–24. doi: 10.1038/4447. [DOI] [PubMed] [Google Scholar]
- Liu Y, Dehni G, Purcell KJ, Sokolow J, Carcangiu ML, Artavanis-Tsakonas S, Stifani S. Epithelial expression and chromosomal location of human TLE genes: Implications for notch signaling and neoplasia. Genomics. 1996;31:58–64. doi: 10.1006/geno.1996.0009. [DOI] [PubMed] [Google Scholar]
- Lobe CG. Expression of the helix-loop-helix factor, Hes3, during embryo development suggests a role in early midbrain-hindbrain patterning. Mech Dev. 1997;62:227–237. doi: 10.1016/s0925-4773(97)00665-5. [DOI] [PubMed] [Google Scholar]
- Lockhart DJ, Winzeler EA. Genomics, gene expression and DNA arrays. Nature. 2000;405:827–836. doi: 10.1038/35015701. [DOI] [PubMed] [Google Scholar]
- Lockhart DJ, Dong H, Byrne MC, Follettie MT, Gallo MV, Chee MS, Mittmann M, Wang C, Kobayashi M, Horton H, et al. Expression monitoring by hybridization to high-density oligonucleotide arrays. Nature Biotechnol. 1996;14:1675–1680. doi: 10.1038/nbt1296-1675. [DOI] [PubMed] [Google Scholar]
- Lowenheim H, Furness DN, Kil J, Zinn C, Gultig K, Fero ML, Frost D, Gummer AW, Roberts JM, Rubel EW, et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc Natl Acad Sci. 1999;96:4084–4088. doi: 10.1073/pnas.96.7.4084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochii M, Yoshida S, Morita K, Kohara Y, Ueno N. Identification of transforming growth factor-β-regulated genes in caenorhabditis elegans by differential hybridization of arrayed cDNAs. Proc Natl Acad Sci. 1999;96:15020–15025. doi: 10.1073/pnas.96.26.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison A, Hodgetts C, Gossler A, Hrabe de Angelis M, Lewis J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech Dev. 1999;84:169–172. doi: 10.1016/s0925-4773(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Niehrs C, Pollet N. Synexpression groups in eukaryotes. Nature. 1999;402:483–487. doi: 10.1038/990025. [DOI] [PubMed] [Google Scholar]
- Nishida Y, Rivolta MN, Holley MC. Timed markers for the differentiation of the cuticular plate and stereocilia in hair cells from the mouse inner ear. J Comp Neurol. 1998;395:18–28. [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- Rau A, Legan PK, Richardson GP. Tectorin mRNA expression is spatially and temporally restricted during mouse inner ear development. J Comp Neurol. 1999;405:271–280. [PubMed] [Google Scholar]
- Rivera-Perez JA, Wakamiya M, Behringer RR. Goosecoid acts cell autonomously in mesenchyme-derived tissues during craniofacial development. Development. 1999;126:3811–3821. doi: 10.1242/dev.126.17.3811. [DOI] [PubMed] [Google Scholar]
- Rivolta MN, Holley MC. GATA3 is downregulated during hair cell differentiation in the mouse cochlea. J Neurocytol. 1998;27:637–647. doi: 10.1023/a:1006951813063. [DOI] [PubMed] [Google Scholar]
- Rivolta MN, Grix N, Lawlor P, Ashmore JF, Jagger D, Holley MC. Auditory hair cell precursors immortalized from the mammalian inner ear. Proc R Soc Lond B. 1998;265:1595–1603. doi: 10.1098/rspb.1998.0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, et al. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24:227–235. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- Rubel EW. Overview: Personal views on the study of auditory system development. In: Rubel EW, et al., editors. Development of the auditory system, Springer handbook of auditory research. Vol. 9. New York: Springer-Verlag; 1997. pp. 1–11. [Google Scholar]
- Ruben RJ. Development of the inner ear of the mouse: A radioautographic study of terminal mitoses. Acta Otolaryngol (Suppl.) 1967;220:1–44. [PubMed] [Google Scholar]
- Rubsamen R, Lippe WR. The development of cochlear function. In: Rubel EW, et al., editors. Development of the auditory system, Springer handbook of auditory research. Vol. 9. New York: Springer-Verlag; 1997. pp. 193–270. [Google Scholar]
- Ryan AF. Transcription factors and the control of inner ear development. Sem Cell Dev Biol. 1997;8:249–256. doi: 10.1006/scdb.1997.0146. [DOI] [PubMed] [Google Scholar]
- Sawada K, Konishi Y, Tominaga M, Watanabe Y, Hirano J, Inoue S, Kageyama R, Blum M, Tominaga A. Goosecoid suppresses cell growth and enhances neuronal differentiation of PC12 cells. J Cell Sci. 2000;113:2705–2713. doi: 10.1242/jcs.113.15.2705. [DOI] [PubMed] [Google Scholar]
- Schulze A, Lehmann K, Jefferies HB, McMahon M, Downward J. Analysis of the transcriptional program induced by Raf in epithelial cells. Genes & Dev. 2001;15:981–994. doi: 10.1101/gad.191101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Comoglio PM. Signaling by semaphorin receptors: Cell guidance and beyond. Trends Cell Biol. 2000;10:377–383. doi: 10.1016/s0962-8924(00)01816-x. [DOI] [PubMed] [Google Scholar]
- Tamayo P, Slonim D, Mesirov J, Zhu Q, Kitareewan S, Dmitrovsky E, Lander ES, Golub TR. Interpreting patterns of gene expression with self-organizing maps: Methods and application to hematopoietic differentiation. Proc Natl Acad Sci. 1999;96:2907–2912. doi: 10.1073/pnas.96.6.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanigaki K, Nogaki F, Takahashi J, Tashiro K, Kurooka H, Honjo T. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron. 2001;29:45–55. doi: 10.1016/s0896-6273(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Tsai H, Hardisty R, Rhodes C, Kiernan A, Roby P, Tymowska-Lalanne Z, Mburu P, Rastan S, Hunter A, Brown SDM, et al. The mouse slalom mutant demonstrates a role for Jagged1 in neuroepithelial patterning in the organ of Corti. Hum Mol Genet. 2001;10:507–512. doi: 10.1093/hmg/10.5.507. [DOI] [PubMed] [Google Scholar]
- Uittenbogaard M, Chiaramello A. Expression of the basic helix-loop-helix ME1 E-protein during development and aging of the murine cerebellum. Neurosci Lett. 1999;274:191–194. doi: 10.1016/s0304-3940(99)00710-7. [DOI] [PubMed] [Google Scholar]
- Weinmaster G. The ins and outs of notch signaling. Mol Cell Neurosci. 1997;9:91–102. doi: 10.1006/mcne.1997.0612. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Li D, Chen ZY, Zhou L, O'Malley BW, Klein W, Nathans J. Essential role of POU-domain factor Brn-3c in auditory and vestibular hair cell development. Proc Natl Acad Sci. 1997;94:9445–9450. doi: 10.1073/pnas.94.17.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Gao WQ, Hasson T, Shin JJ. Requirement for Brn-3c in maturation and survival, but not in fate determination of inner ear hair cells. Development. 1998;125:3935–3946. doi: 10.1242/dev.125.20.3935. [DOI] [PubMed] [Google Scholar]
- Yamada G, Mansouri A, Torres M, Stuart ET, Blum M, Schultz M, De Robertis EM, Gruss P. Targeted mutation of the murine goosecoid gene results in craniofacial defects and neonatal death. Development. 1995;121:2917–2922. doi: 10.1242/dev.121.9.2917. [DOI] [PubMed] [Google Scholar]
- Yao J, Liu Y, Husain J, Lo R, Palaparti A, Henderson J, Stifani S. Combinatorial expression patterns of individual TLE proteins during cell determination and differentiation suggest non-redundant functions for mammalian homologs of Drosophila Groucho. Dev Growth Differ. 1998;40:133–146. doi: 10.1046/j.1440-169x.1998.00003.x. [DOI] [PubMed] [Google Scholar]
- Yoho ER, Thomopoulos GN, Thalmann I, Thalmann R, Schulte BA. Localization of organ of Corti protein II in the adult and developing gerbil cochlea. Hear Res. 1997;104:47–56. doi: 10.1016/s0378-5955(96)00183-9. [DOI] [PubMed] [Google Scholar]
- Zhang N, Martin GV, Kelley MW, Gridley T. A mutation in the Lunatic fringe gene suppresses the effects of a Jagged2 mutation on inner hair cell development in the cochlea. Curr Biol. 2000;10:659–662. doi: 10.1016/s0960-9822(00)00522-4. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3:580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- Zheng JL, Shou J, Guillemot F, Kageyama R, Gao WQ. Hes1 is a negative regulator of inner ear hair cell differentiation. Development. 2000;127:4551–4560. doi: 10.1242/dev.127.21.4551. [DOI] [PubMed] [Google Scholar]
- Zine A, Romand R. Development of the auditory receptors of the rat: A SEM study. Brain Res. 1996;721:49–58. doi: 10.1016/0006-8993(96)00147-3. [DOI] [PubMed] [Google Scholar]
- Zine A, Van De Water T, Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127:3373–3383. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]
- Zine A, Aubert A, Qiu J, Therianos S, Guillemot F, Kageyama R, de Ribaupierre F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J Neurosci. 2001;21:4712–4720. doi: 10.1523/JNEUROSCI.21-13-04712.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]