Abstract
Natural killer (NK) cells play a pivotal role in the innate immune response to viral infections, particularly murine cytomegalovirus (MCMV) and human herpesviruses. In poxvirus infections, the role of NK cells is less clear. We examined disease progression in C57BL/6 mice after the removal of NK cells by both antibody depletion and genetic means. We found that NK cells were crucial for survival and the early control of virus replication in spleen and to a lesser extent in liver in C57BL/6 mice. Studies of various knockout mice suggested that γδ T cells and NKT cells are not important in the C57BL/6 mousepox model and CD4+ and CD8+ T cells do not exhibit antiviral activity at 6 days postinfection, when the absence of NK cells has a profound effect on virus titers in spleen and liver. NK cell cytotoxicity and/or gamma interferon (IFN-γ) secretion likely mediated the antiviral effect needed to control virus infectivity in target organs. Studies of the effects of ectromelia virus (ECTV) infection on NK cells demonstrated that NK cells proliferate within target tissues (spleen and liver) and become activated following a low-dose footpad infection, although the mechanism of activation appears distinct from the ligand-dependent activation observed with MCMV. NK cell IFN-γ secretion was detected by intracellular cytokine staining transiently at 32 to 72 h postinfection in the lymph node, suggesting a role in establishing a Th1 response. These results confirm a crucial role for NK cells in controlling an ECTV infection.
The possible use of variola virus as a bioterrorist weapon has renewed interest in understanding the pathogenesis of smallpox and in the development of antivirals, improved vaccines, and immunomodulatory therapies. Historically, mousepox has been used as the disease model of choice for smallpox and is the basis for the hypothesized events that occur from infection to the prodrome (12 to 14 days) (19, 20). Like smallpox, mousepox is a severe disease with high mortality that can be initiated with a small number of ectromelia virus (ECTV) particles (17). Mousepox affords the opportunity to examine the innate immune response to infection following a physiologically relevant dose of virus. The innate immune system provides the early defense against pathogens by controlling pathogen replication and directing the adaptive immune system to mount an antigen-specific attack. Natural killer (NK) cells represent an important component of the innate immune response, particularly against viral infections. The importance of these cells is immediately apparent in clinical cases where individuals selectively deficient in NK cells suffer severe recurrent viral infections (4).
NK cells have been implicated in recovery from mousepox. Studies of genetic resistance to mousepox in crosses of resistant and susceptible mice have led to the identification of four potential loci involved in the resistance to ECTV. The resistance to mousepox (rmp-1) locus on chromosome 6 controls replication of virus in the liver, and it may map to the NK gene complex, which encodes NK cell receptors. These data suggest a role for NK cells in resistance to severe ECTV-induced disease, possibly similar to that in murine cytomegalovirus (MCMV) infections of C57BL/6 mice where genetic resistance is mediated through the NKC locus, termed Ly49h (6, 13, 47).
Previous studies of mice lacking NK cells also suggest the importance of NK cells in the control of ECTV infection. Depletion of NK cells in C57BL/6 mice through the use of anti-asialo GM1 gammaglobulin increased the mortality of the normally resistant C57BL/6 mouse after a subcutaneous ECTV infection (25); however, treatment with anti-asialo GM1 also depletes CD8+ T cells, thus diminishing virus-specific cytotoxic lymphocyte responses and limiting the interpretation of this study (28). A similar study using both anti-asialo GM1 and anti-NK1.1 antibody treatment of C57BL/6 mice demonstrated viral loads in response to intravenous ECTV challenge in both liver and spleen that were higher than those of control antibody-treated mice (13). NK1.1 is the most specific serological marker for NK cells in C57BL/6 mice; however, anti-NK1.1 treatment may also affect NKT cells, a specialized class of T cells expressing both NK cell markers and T-cell receptors. NKT cells can play an important early role in the modulation of the immune response by producing both Th1 and Th2 cytokines and cytotoxic functions (3, 7, 30, 43). Studies of herpes simplex virus type 1 (HSV-1) (skin infection) and HSV-2 (vaginal infection) demonstrated increased virus titers in skin and spinal ganglia tissues and in vaginal washes of mice lacking CD1-restricted NKT cells compared to controls, as well as increased mortality following HSV-2 infections (2, 23). Recent studies in the hepatitis B virus transgenic mouse model showed that α-GalCer-activated NKT cells are capable of inhibiting virus replication (26). Thus, anti-asialo GM1 gammaglobulin or NK1.1 treatments are not specific for NK cells, and the role of other cell types cannot be ruled out.
Genetic approaches used to examine the importance of NK cells in mousepox also lack specificity. Jacoby et al. demonstrated that C57BL/6bg/bg mice were highly susceptible to lethal infection with ECTV (25); however, the bg/bg (beige) mutation affects granule exocytosis (24) and therefore affects both CD8+ T-cell and NK cell lytic activities. In addition, this mutation does not prevent NK cell secretion of gamma interferon (IFN-γ), which is a key mediator for recovery from ECTV infection. Perforin- and IFN-γ-deficient mice infected with ECTV succumb to a lethal infection with increased virus titers in target organs (36). Since both perforin and IFN-γ deficiencies affect the capabilities of both NK cells and cytotoxic T cells to exert antiviral activity, the specific contribution of NK cells to antiviral activity in these mice is difficult to determine.
In this study, we demonstrate that NK cells proliferate, accumulate, and are activated in spleen and liver (target organs) during ECTV infection in the C57BL/6 mouse. Using antibody therapy and genetic means, we demonstrate that the presence of NK cells, but not NKT cells, is crucial for the recovery of genetically resistant C57BL/6 mice from mousepox.
MATERIALS AND METHODS
Mice.
C57BL/6 female mice were purchased from Charles River Laboratories/NCI (Wilmington, MA). Mice that were NK deficient (NKD) due to an insertion of a transgenic construct were previously described (32, 33). CD1 knockout and Jα18 knockout mice were obtained from Randy Brutkiewicz at Indiana University. B6.CB17-PKRDSCID/SZJ (SCID) mice were obtained from Jackson Laboratory. All mice were used at 4 to 8 weeks of age. All animal experiments were carried out in accordance with Institutional Animal Care and Use Committee regulations (Saint Louis University protocol no. 1339).
Cell culture.
BS-C-1, a continuous African green monkey kidney cell line, and L929, a continuous fibroblast line from the C3H mouse, were maintained in Dulbecco's minimum essential medium (Cellgro) supplemented with 10% fetal calf serum, l-glutamine, and antibiotics. YAC-1, a line derived from Moloney murine leukemia virus-induced lymphoma in the A/Sn strain (H-2a) (31), and C1498, a line derived from an acute myeloid leukemia in the C57BL/6 strain (H-2b) (27), were maintained in RPMI 1640 (BioWhittaker) supplemented with 10% fetal calf serum, 50 μM 2-mercaptoethanol, l-glutamine, and antibiotics.
Viruses.
Plaque-purified ECTV (Moscow strain) was propagated in murine L929 cells and purified as previously described (9). The virus titer was determined by using BS-C-1 monolayers.
Virus infection and NK1.1 antibody depletion of mice.
C57BL/6 mice were given an intraperitoneal injection of 100 μg of anti-NK1.1 (PK136) monoclonal antibody (MAb) or 100 μg of isotype control (clone MAR18.5, mouse anti-rat immunoglobulin G) MAb at t of −2. Depletion was confirmed at t of 2 days postinfection (p.i.) by flow cytometry using NK markers other than NK1.1. Two days following NK1.1 MAb treatment, the mice were challenged with various doses of ECTV by the footpad or intravenous routes of infection.
Measurement of viral titers in spleen and liver.
Spleens and livers were removed aseptically from mice and frozen at −70°C. The tissues were weighed and processed to a 10% (wt/vol) lysate. The lysate was frozen and thawed three times, sonicated, and titrated as described previously (29). Virus infectivity was expressed as log10 PFU/ml 10% tissue lysate.
Measurement of NK cell numbers in spleen, liver, and lymph node.
Female C57BL/6 mice were infected with ECTV at approximately 20 to 50 PFU per footpad at t of 0. Mice were sacrificed with ketamine and xylazine at t of 0, 2, 4, 6, and 9 days p.i., and the whole body was perfused with phosphate-buffered saline (PBS) and 1% fetal bovine serum. Spleens, livers, and popliteal lymph nodes were harvested and forced through a 100-μm filter (BD Falcon) to prepare single-cell suspensions. Spleen cell suspensions were then treated with ACK lysis buffer (BD Biosciences) to remove contaminating red blood cells. Contaminating hepatocytes in liver cell suspensions were excluded by a 30-min centrifugation at 1,500 rpm in a discontinuous RediGrad gradient (Amersham Biosciences). The hepatocyte fraction was discarded, and the remaining lymphocyte population was washed with flow buffer (PBS, 1% fetal bovine serum). Contaminating red blood cells were then lysed as described above. Live-cell concentrations were determined with a hematocytometer, and dead cells were excluded by trypan blue staining. The percentages of NK cells (NK1.1+ CD3−) in each organ were determined by flow cytometry. The total NK cell number in each organ was then determined by multiplying the percentage of NK cells by the total number of cells isolated from the organ.
Flow cytometry.
Leukocytes were blocked in anti-mouse CD16/CD32 (BD Biosciences) for 10 min at 4°C to prevent nonspecific antibody binding. Samples were then stained for 30 min, washed, and fixed in 1% formaldehyde (Polysciences, Inc., Warrenton, PA). Phycoerythrin (PE)-PK136 (anti-NK1.1), PerCP-Cy5.5-145-2C11 (anti-CD3), allophycocyanin (APC)-30-F11 (anti-CD45), and APC-XMG1.2 (anti-IFN-γ) were obtained from BD Biosciences (San Diego, CA). Intracellular cytokine staining of IFN-γ was performed as described by the manufacturer's manual (BD Biosciences). Cell proliferation was determined with the bromodeoxyuridine (BrdU) flow kit according to the manufacturer's instructions (BD Biosciences). Samples were run on a BD FACSCalibur system.
Preparation and staining of frozen sections.
Spleens and livers from naive and ECTV-infected mice were harvested 6 days p.i. and embedded in Tissue-Tek O.C.T. compound (Miles Laboratories, Elkhart, IN), rapidly frozen in liquid nitrogen-cooled isopentane, and stored at −80°C. Sections (5 μm) were cut, mounted, and fixed with acetone for 3 min at −20°C (38). Sections were rehydrated in PBS for 5 min, blocked with 10% normal mouse serum and 10% Tween in PBS for 20 min at room temperature, and incubated with anti-virus antibodies diluted in blocking reagent for 1 h at room temperature, and virus lesions were visualized with an anti-rabbit VectaStain alkaline phosphatase kit according to the manufacturer's instructions (Vector Laboratories, Burlingame, CA) and then washed and avidin and biotin blocked per the manufacturer's instructions (Vector Laboratories, Burlingame, CA). Sections were then incubated with antibodies against NK cells (clone 4D11; 1:50), CD4 T cells (BD Biosciences) (clone L3T4; 1:50), or CD8 T cells (BD Biosciences) (clone 53-5.8; 1:50) for 30 min at room temperature and incubated with biotinylated rabbit anti-rat immunoglobulin G (1:100) for 30 min at room temperature. Cell types were visualized with 3,3′-diaminobenzidine (DAB) substrate with a VectaStain hydrogen peroxidase kit according to the manufacturer's instructions. Sections were mounted in DakoCytomation aqueous mounting medium and visualized by bright-field microscopy. The numbers of NK cells, CD4+ T cells, and CD8+ T cells were counted in the 0.25 mm2 area that surrounded the viral lesions. As controls, we counted specific cell types in the 0.25-mm2 regions centered on NK, CD4+ T, or CD8+ T cells (distal from lesion) from uninfected and infected mice. A minimum of 20 regions were counted for each tissue.
NK cell cytotoxicity assays.
Tumor targets were labeled with sodium 51chromate for 60 min, washed, and plated (104 cells/well). Effector cells were added at various effector-to-target cell ratios in triplicate and incubated with the target cells for 4 h at 37°C. Plates were spun down, and supernatants were harvested and counted. Specific lysis was calculated as follows: % specific cytotoxicity = (experimental cpm − spontaneous cpm)/(maximum cpm − spontaneous cpm) × 100.
Statistics.
Mortality rates and virus titers were analyzed by two-tailed Student's t test (α = 0.05). A P value of 0.05 or less was considered significant.
RESULTS
NK cells are important for control of virus replication at early times following infection and for survival.
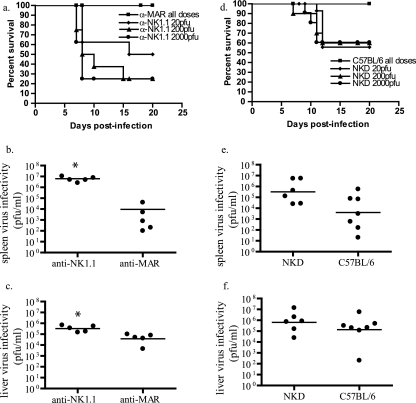
After depletion of NK cells by a nonspecific reagent, anti-asialo GM1, mortality rates and virus titers in tissues were increased following subcutaneous and intravenous ECTV challenge (13, 25, 28). To more-specifically determine the importance of NK cells in ECTV infection, we depleted NK cells utilizing MAb PK136 (α-NK1.1) prior to footpad infection of C57BL/6 mice with ECTV. NK1.1-depleted mice, compared to isotype controls, showed increased mortality at all tested ECTV doses (Fig. 1a). In concordance with the increased mortality rates, NK1.1 depletion resulted in increased splenic (Fig. 1b) and hepatic (Fig. 1c) virus titers compared to those of isotype controls at 6 days p.i. Splenic virus titers from NK-depleted animals were on average 3 logs higher than that of the isotype control mice, and hepatic titers were at least 1 log higher than controls.
FIG. 1.
Lack of NK cells increases susceptibility of resistant C57BL/6 mice to lethal disease. (a) C57BL/6 mice were treated with either anti-NK1.1 or an isotype control anti-MAR. Anti-NK1.1 MAb-treated and control-treated C57BL/6 mice were infected with various doses of ECTV in the footpad two days following MAb treatment, and mortality after infection was recorded. Six days following infection with 50 PFU of ECTV, spleens (b) and livers (c) from anti-NK1.1 MAb-treated and control-treated mice were harvested and virus titers were determined by a virus plaque assay. Circles represent individual animals and lines represent means. (n = 4 to 5 mice/group; representative of two experiments; *, P = 0.03). (d) Transgenic NKD mice and C57BL/6 controls were infected with various doses of ECTV in the footpad, and mortality was recorded. Six days following infection with 50 PFU of ECTV, spleens (e) and livers (f) from infected NKD and control mice were harvested and virus titers were determined by plaque assay (n = 6 to 7; representative of two experiments).
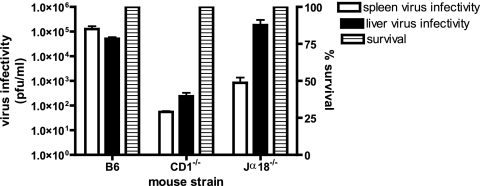
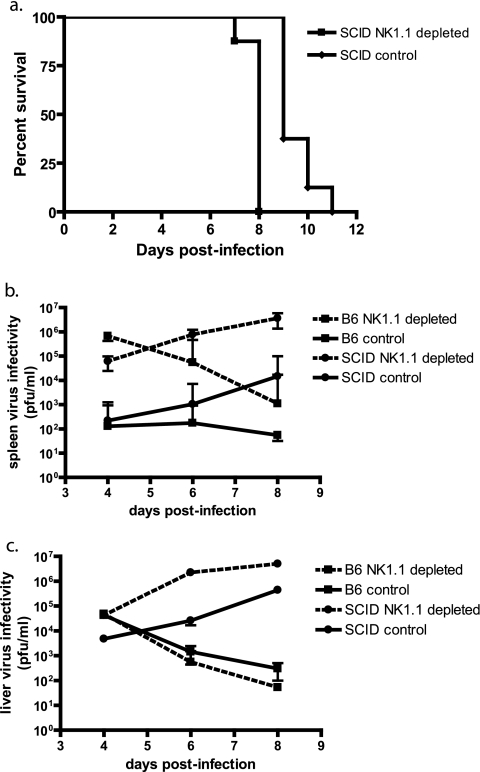
NK1.1 depletion is more specific than asialo GM1 depletion but may also deplete NKT cells. To investigate whether the observed results were skewed by effects on NKT cells, we examined CD1 knockout (35) and Jα18 knockout (11) mice that specifically lack CD1-restricted NKT cells and NKT cells utilizing the Jα18 chain in their T-cell receptors, respectively, but have normal levels of NK, B, and T cells. Footpad challenges of CD1 and Jα18 knockout mice with ECTV resulted in no significant morbidity, weight loss, or mortality over the course of the experiment, and liver and splenic virus titers were less than or equal to those of intact C57BL/6 mice (Fig. 2). The reason for the dramatic reduction of spleen titers in CD1 and Jα18 knockout mice is not known. In addition, we examined transgenic NKD mice, which have deficient NK cells but retain normal numbers of T cells, B cells, and NKT cells (32). Compared to C57BL/6 mice, NKD mice showed greater mortality over the tested range of virus challenge doses (Fig. 1d) and increased virus titers in spleen (Fig. 1e) and liver (Fig. 1f) at 6 days p.i. These data further support a role of NK and not NKT cells in controlling ECTV replication and aiding in recovery from infection. Furthermore, NK1.1-depleted SCID mice that lack mature B, T, and NKT cells demonstrated results similar to those seen with NKD- and NK1.1-depleted C57BL/6 mice, with increased spleen and liver virus titers compared to controls, as well as a significantly shorter mean time to death (P < 0.001; Fig. 3). Taken together these data support an important role of NK cells in the control of ECTV replication at 6 days p.i. but do not indicate if this effect was through direct antiviral activity of NK cells or by an indirect effect on other cell populations.
FIG. 2.
Lack of NKT cells does not increase susceptibility to ECTV infection. CD1 knockout, Jα18 knockout, and intact mice were infected with 50 PFU of ECTV in the footpad. Mortality was recorded, and virus titers in spleens and livers were determined by plaque assays.
FIG. 3.
Lack of NK cells in SCID mice increases severity of ECTV infection. (a) SCID and C57BL/6 mice were treated with either anti-NK1.1 MAb or anti-MAR isotype control 2 days prior to intravenous infection with 300 PFU of ECTV, and mortality was recorded (n = 8; representative of two experiments; P < 0.001). Infected mice were sacrificed at 6 days p.i., and virus titers in spleens (b) and livers (c) were determined by plaque assays (n = 8; representative of two experiments).
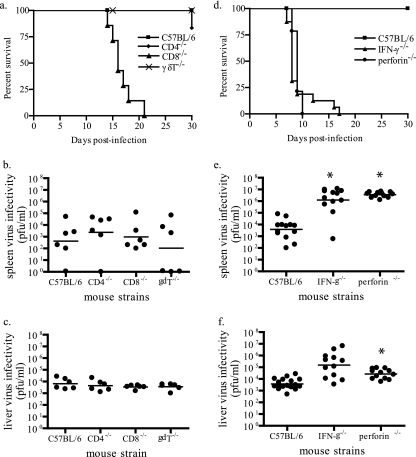
To determine if this effect was through direct antiviral activity of NK cells or by an indirect effect(s) on other cell populations, we examined mortality and splenic and liver titers at 6 days p.i. in ECTV infections of C57BL/6 strains that lack γδ T cells, CD4+ T cells, and CD8+ T cells. Only the CD8+ T-cell knockout strain demonstrated a significant increase in mortality compared to controls over the observation period (Fig. 4a). However, none of the knockout strains demonstrated increased splenic (Fig. 4b) or hepatic (Fig. 4c) virus titers at 6 days p.i. These results suggest that γδ T cells, CD4+ T cells, and CD8+ T cells are not directly or indirectly required for controlling virus replication in the spleen or liver at 6 days p.i., even though the CD4+ and CD8+ cell types are required for recovery from infection (18, 28). Because CD8+ and CD4+ T cells did not significantly contribute to the control of virus replication at 6 days pi, antiviral activities shared by NK and T cells could be attributed to NK cells at this time point. We tested the importance of NK cytolytic activity and IFN-γ secretion using IFN-γ−/− and perforin−/− mouse strains on a C57BL/6 background. Following infection, both strains demonstrated a significant increase in mortality after ECTV infection (Fig. 4d) and increased splenic (Fig. 4e) and liver (Fig. 4f) virus titers at 6 days p.i. compared to controls. Taken together, these results suggest a role for NK cells, perforin, and IFN-γ in controlling early virus replication and recovery from ECTV infection. At this level of the analysis, the data are consistent with the lack of an important role of NKT and γδ T cells in the early control of virus replication and recovery from ECTV infection in the C57BL/6 mouse, as well as the lack of an important role for T cells in the early control of virus replication.
FIG. 4.
γδ T cells (gdT), CD4+ T cells, and CD8+ T cells do not appear important in early control of ECTV infection despite the critical need for IFN-γ and perforin. (a) C57BL/6 controls and strains lacking CD8+, CD4+, and γδ T cells were infected with 50 PFU of ECTV by the footpad route, and mortality was recorded (n = 8 to 12 mice). Infected mice were sacrificed at 6 days p.i., and virus titers in spleens (b) and livers (c) were determined by plaque assays (n = 5 to 6 mice). (d) C57BL/6 controls and strains lacking IFN-γ and perforin were infected via the footpad route with 50 PFU of ECTV, and mortality was recorded (n = 8 to 12 mice). Infected mice were sacrificed at 6 days p.i., and virus titers in spleens (e) and livers (f) were determined by plaque assays (n = 10 to 12 mice; *, indicates a P value of 0.03 for spleen IFN-γ, 0.003 for spleen perforin, and 0.01 for liver perforin).
NK cell populations expand in response to ECTV infection and accumulate near virus foci.
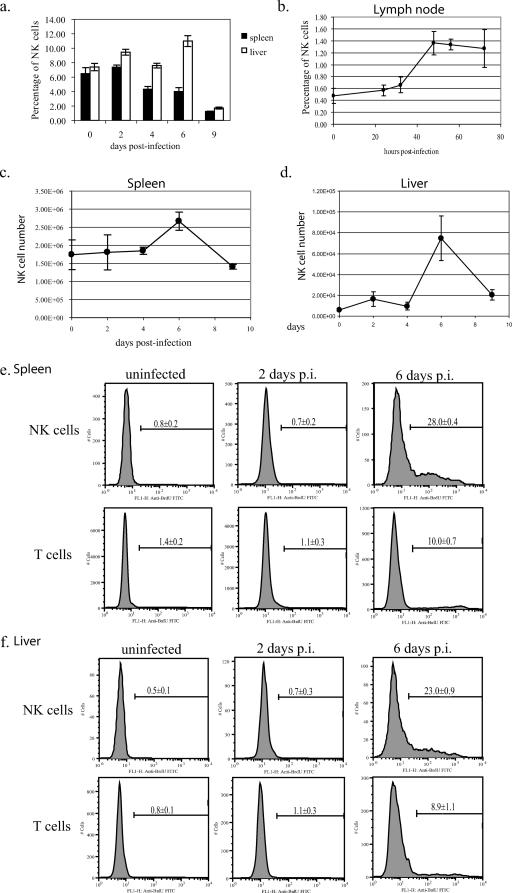
If NK cells are important in the recovery from ECTV infection, NK cells should increase in number and be in an activated state in tissues supporting active virus replication. To test this hypothesis, we infected C57BL/6 mice and followed the NK cell number in the draining lymph nodes, spleens, and livers by flow cytometry (NK1.1+ CD3−). NK cell number increased in the draining popliteal lymph nodes 48 h p.i., from 3.91 × 103 ± 1.61 × 103 in uninfected mice to 2.11 × 104 ± 6.84 × 103 cells in infected mice, a 5.4-fold increase. The percentage of NK cells in lymph nodes increased from 0.48% ± 0.13% in controls to 1.36% ± 0.2% in infected mice, a 2.8-fold increase (Fig. 5b). The splenic NK cell number at 6 days p.i. peaked at 2.5 × 106 ± 0.3 × 106 cells compared to 1.3 × 106 ± 0.4 × 106 cells in uninfected animals, a 1.9-fold increase (Fig. 5c). This increase in splenic NK cell number was accompanied by a decrease in the percentage of NK cells from 6.5% ± 0.5% to 4.0% ± 0.5% (P = 0.003) at day 6 p.i. (Fig. 5a), the result of a dramatic increase in splenic T cells. Similar to the spleen, the NK cell number in the liver also increased at 6 days p.i. from 6.2 × 103 ± 9.0 × 102 to 7.5 × 104 ± 2.1 × 104, a 12-fold increase (Fig. 5d; P = 0.002); however, in contrast to the spleen, the percentage of NK cells also increased from 7.3% ± 0.5% to 11.0% ± 0.8% (P = 0.0002) (Fig. 5a).
FIG. 5.
NK cell populations expand in response to ECTV infection. C57BL/6 mice were infected with 50 PFU of ECTV in the footpad and sacrificed at various times p.i. Spleens, livers, and popliteal lymph nodes were harvested, and NK cell populations were analyzed by flow cytometry (a to d) (n = 5 to 14 mice/time point; representative of two experiments). Flow cytometric analysis of BrdU incorporation into splenic (e) and liver (f) NK and T cells was performed on tissues isolated at 2 and 6 days p.i. (n = 5 mice/time point; representative of two experiments).
The increase in NK cell number seen in liver and spleen may result from recruitment or proliferation. To distinguish between these possibilities, we measured the proliferation of NK cells during ECTV infection using a 3-h BrdU incorporation period. Uninfected C57BL/6 mice demonstrated 0.8% ± 0.2% and 0.5% ± 0.1% BrdU-positive NK cells in spleen and liver, respectively, while day 6 ECTV-infected mice exhibited 28.0% ± 0.4% (Fig. 5e) and 23.0% ± 0.9% (Fig. 5f) BrdU-positive NK cells in spleen and liver, respectively. We further investigated the role of NK cell recruitment to target organs by performing adoptive transfer studies. NK1.1+ CD3− splenocytes isolated from ECTV-infected mice were adoptively transferred into naive or ECTV-infected C57BL/6 mice. During the 3-h posttransfer (equivalent to BrdU labeling time), adoptively transferred NK cells did not preferentially recruit to the spleen or liver of infected or naive C57BL/6 mice, suggesting that a large part of the increase in NK cell number in spleen and liver was a result of proliferation of NK cells rather than recruitment (data not shown).
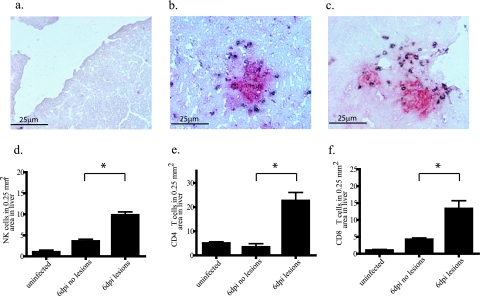
Immunohistochemistry was utilized to determine if the increased numbers of NK cells in the spleens and livers of infected mice associate with viral lesions. In agreement with the data in Fig. 5d, liver sections from ECTV-infected C57BL/6 mice (6 days p.i.) demonstrated an increase in NK cells over uninfected controls (Fig. 6d). NK cells were not uniformly distributed through the tissue. Instead, 9.8 ± 0.8 NK cells per 0.25 mm2 were observed in areas with lesions compared to 3.6 ± 1.5 cells in similar areas lacking virus lesions (Fig. 6d; P = 0.001). Similar results were found for T cells with 22.6 ± 3.4 CD4+ and 13.3 ± 2.4 CD8+ T cells in areas with lesions and 6.4 ± 3.4 CD4+ (Fig. 6e; P = 0.0004) and 4.2 ± 3.4 CD8+ (Fig. 6f; P = 0.002) T cells in areas without lesions. NK cells, CD4+ T cells, and CD8+ T cells were also assessed in ECTV-infected spleens (6 days p.i.) (data not shown). Due to the large numbers of these cells in ECTV-infected spleens, as well as uninfected spleens, it was difficult to correlate these cell types and virus lesions at 6 days p.i. Taken together, these results suggest that NK cells are important in controlling early virus replication and do so by targeting areas of the liver containing virus lesions.
FIG. 6.
Association of hepatic NK cells with ECTV lesions. C57BL/6 mice were infected or mock infected with 50 PFU of ECTV in the footpad and sacrificed at 6 days p.i. Livers were embedded in embedding media, sectioned at 5 μm, and stained for orthopoxvirus antigens (red) and NK, CD4, or CD8 antigens (brown). (a) Uninfected; (b and d) anti-NK1.1, infected (*, P = 0.0001); (c and e) anti-CD4, infected; (f) anti-CD8, infected (*, P = 0.002).
NK cells are activated in response to ectromelia virus infection.
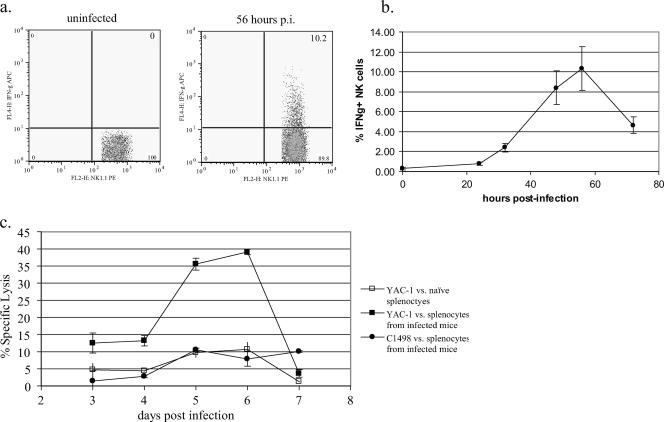
To determine if NK cells induced during infection were activated, we analyzed the two main NK cell effector functions: (i) IFN-γ secretion and (ii) cytolytic activity (48). Detectable NK cell-associated IFN-γ synthesis in the popliteal lymph nodes was extremely brief; first detected at 32 h p.i. (2.4% ± 0.4%), peaking at 56 h p.i. (10.3% ± 2.2%), and decreasing by 72 h p.i. (4.7% ± 0.8%) (Fig. 7a and b). At these time points, the popliteal lymph node contained very few infected cells and NK cells demonstrated minimal proliferation and activation in the spleen, liver, and peripheral blood (data not shown). NK cell-associated IFN-γ was not detectable by intracellular cytokine staining at any time point after ECTV infection in the spleen (data not shown).
FIG. 7.
NK cells demonstrate increased IFN-γ production and cytolytic activity in response to ECTV infection. C57BL/6 mice were infected with 50 PFU of ECTV in the footpad and sacrificed at various times p.i. Spleens and popliteal lymph nodes were harvested, and NK cell IFN-γ secretion and lytic activity were measured. (a) NK cell IFN-γ production at 56 h p.i. (n = 4 to 5 mice/time point; representative of two experiments). (b) Kinetics of NK cell-associated IFN-γ production in draining lymph nodes (n = 4 to 5 mice/time point; representative of two experiments). (c) Splenocyte lytic activity at 6 days p.i. (n = 3 to 4 mice/time point).
To assess the cytolytic activity of NK cells during ECTV infection, we performed NK cell cytolytic 51Cr release assays. Previous studies have determined peak NK lytic activity against the YAC-1 cells (ECTV 103 PFU footpad infection) occurred 5 days p.i. (8). We utilized YAC-1 (H-2a T lymphoma line) and C1498 (H-2b T lymphoma line) cells to test splenocytes from both naive and ECTV-infected C57BL/6 mice (20 PFU footpad infection). Peak NK cell lytic activity occurred 6 days p.i. with 39.1% ± 0.8% specific lysis at an effector-to-target cell ratio of 30 (Fig. 7c). Specific lysis of YAC-1 cells by splenocytes from naive mice and specific lysis of C1498 cells by infected splenocytes did not increase above 10% at any of the time points. These results suggest that NK cells present within the spleen at 6 days p.i. are activated and capable of cytolysis, compared to NK cells within the spleen in naive mice, which represent a larger percentage of the splenocyte population compared to the numbers of NK cells in day-6-infected mice.
DISCUSSION
Natural killer cells, although best known for their ability to recognize and eliminate tumor cells, also play an important role in the innate immune response to viral infections. These lymphocytes can be activated by a number of distinct mechanisms and once activated produce cytokines and perform cytolytic functions (49). The importance of these cells is immediately apparent in clinical cases of humans selectively deficient in NK cells. These patients suffer severe recurrent viral infections, including herpesvirus and cytomegalovirus infections (4).
The role of NK cells in recovery from viral infections has been most thoroughly studied with herpesviruses. HSV-1 ocular infection of NK1.1-depleted C57BL/6 mice or HSV-2 vaginal infection of interleukin-15 (IL-15)−/− C57BL/6 mice lacking NK and NKT cells are more severe, with increased mortality and corneal scarring or increased virus titers in vaginal washings (2, 22). MCMV intraperitoneal infections nonspecifically activate NK cells during the first 48 h of infection. Later in the infection (day 4), a subset of NK cells expressing the activation receptor Ly49H are specifically expanded (14). The gene for Ly49H maps to the Cmv1 autosomal dominant locus and is responsible for the specific survival of C57BL/6 mice after MCMV challenge (12, 41). Further studies have identified MCMV protein m157 as the ligand responsible for triggering the Ly49H receptor and the subsequent specific proliferation and activation of the Ly49H+ subset of NK cells (44).
In this study we have shown NK, and not NKT, cells to be important in the recovery from ECTV infection. The absence of NK cells due either to antibody depletion or to genetic means leads to an increase in splenic and hepatic virus burden, resulting in increased morbidity and a ∼50% mortality rate over a 100-fold range of viral challenge doses. Early during the course of ECTV infection (32 to 56 h), the NK cell population expands and becomes activated transiently in the popliteal lymph node draining the site of infection; however, peak proliferation and activation are seen 6 days p.i. in the spleen and liver. At 6 days p.i. NK cells, and not CD4+ or CD8+ T cells, appear to control virus replication, as hepatic and splenic titers increase upon depletion of NK cells but not CD4+ or CD8+ T cells. Interestingly, γδ T cells do not appear to contribute at all to recovery from infection. These studies correlated quite well with studies of NK cell migration early after vaccinia virus infection. C57BL/6 mice demonstrated increased NK cell number and NK cell proliferation in response to vaccinia virus infection, although the time course for vaccinia virus was earlier than that seen with ectromelia. These differences may be attributed to the high dose of vaccinia used as well as the intraperitoneal route used to infect with vaccinia (40).
The mode of activation of NK cells during ECTV infection remains unclear. Studies of other viruses have demonstrated specific and nonspecific activation mechanisms for NK cells. During MCMV infection, the viral protein m157 ligates the NK surface receptor Ly49H, activating NK cell cytotoxicity and the production of IFN-γ (14). The NK cell receptor NCR1 has been shown to be crucial during influenza A infection, and the presence of this receptor rescues C57BL/6 mice from influenza-induced death (21). Activation of NK cells in HSV infections may be nonspecific, resulting from decreased major histocompatibility complex (MHC) expression (loss of inhibitory signaling) and increased NKG2D expression (increased activation signaling) (16). Similar to HSV, vaccinia virus-induced activation of human NK cells may result from decreased MHC expression, leading to the loss of inhibition and increased cytolytic activity of NK cells expressing high levels of the inhibitory receptor NKG2A (5). However, studies of vaccinia virus have also demonstrated that the activating receptors NKp30, NKp44, and NKp46 are important for recognition of vaccinia virus-infected cells by human NK cells (10), suggesting that the activation of NK cells during vaccinia virus infection may be a result of multiple modes of activation.
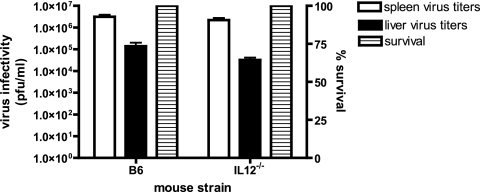
The activation of NK cells during ECTV infections appears to be distinct from that of MCMV. During MCMV infection, NK cells produce a short burst of IFN-γ, peaking at 40 h postinfection in the spleen (14). IFN-γ production can also be seen with the incubation of naive NK cells with MCMV-infected bone marrow-derived macrophages (15). Like MCMV, NK cell-associated IFN-γ was also detected early and transiently but in this case in the lymph node draining the site of ECTV infection; however, no NK cells secreting IFN-γ were detected in the spleen. The difference in tissue distribution of detectable IFN-γ-secreting NK cells in MCMV and ECTV infections may be due to the route of infection, as MCMV and ECTV are administered intraperitoneally and by footpad, respectively. Coculture of naive NK cells with ECTV-infected bone marrow-derived macrophages, however, did not result in detectable IFN-γ production (A. K. Parker and R. M. L. Buller, unpublished data), suggesting that the early production of IFN-γ in the lymph node was not simply due to a viral component interacting with NK cell activation receptors, as is the case for MCMV. Supporting this notion was the lack of virus-infected cells in the popliteal lymph node draining the site of infection at 56 h p.i., when we detected peak numbers of NK1.1 IFN-γ-positive cells. In addition, the NK cell-activating cytokine IL-12 is vitally important in MCMV infection but appears to have little importance during ECTV infection. Serum IL-12 levels peak approximately 2 days p.i. during MCMV infection, whereas ECTV did not elicit detectable serum IL-12 (J. Schriewer and R. M. L Buller, unpublished data). MCMV infection of IL-12−/− C57BL/6 mice resulted in increased mortality (39), and the presence of IL-12 is necessary for NK cell IFN-γ production but not NK cell cytotoxicity (37). In contrast, ECTV infection of IL-12−/− C57BL/6 mice resulted in no mortality, and day 6 splenic and hepatic virus titers were similar to those seen in C57BL/6 mice (Fig. 8). This argues that, unlike MCMV, IL-12 is not essential for the generation of NK cell responses during ECTV infection. Together these results strongly suggest a method of NK cell activation in ECTV infection that is distinct from MCMV.
FIG. 8.
IL-12 does not appear to play a role in controlling ECTV infection. IL-12 knockout and C57BL/6 mice were infected with 50 PFU of ECTV by the footpad route. Mortality was recorded, and virus titers in spleen and liver were determined by plaque assays.
Activated NK cells likely control ECTV replication by direct antiviral and indirect immune modulator activities. ECTV-infected C57BL/6 IFN-γ−/− mice demonstrated increased virus titers in spleen and liver at 6 days p.i., whereas similar specimens from infected CD4−/− and CD8−/− T-cell C57BL/6 mouse strains did not. NK cells are a likely source of this IFN-γ that is responsible for controlling virus replication before 6 days p.i. Although NK cell-associated IFN-γ is undetectable in the spleen and liver by intracellular cytokine staining after ECTV infection, low-level IFN-γ production by NK cells is inferred by the reduction of IFN-γ in spleen and liver lysates in mice treated with NK1.1 antibody compared to the levels in controls (cytokine bead array assay; Parker and Buller, unpublished). IFN-γ could activate macrophages in lymphoid tissues or Kupffer cells in liver to produce antiviral molecules such as nitric oxide and activate RNase L, PKR, and cytosine deaminase pathways which limit virus replication and dissemination. Similar to IFN-γ−/− mice, perforin−/− mice have increased mortality and splenic and hepatic virus titers at 6 days following ECTV infection. At 6 days p.i., CD8+ T-cell cytolytic activity is undetectable in spleens under the employed experimental conditions, suggesting a role of NK cytolytic activity in controlling ECTV replication (S. J. Haskett, A. K. Parker, and R. M. L. Buller, unpublished data). Studies of several systems, including Leishmania major, have shown NK cell IFN-γ to be important for inducing Th1 responses and early resistance (1, 42, 45, 46). In a model studying Th1 polarization, injection of mature dendritic cells induced rapid recruitment of NK cells to lymph nodes and stimulated IFN-γ production (34). By analogy, NK cell secretory IFN-γ in the draining lymph nodes early in an ECTV infection may be important for the priming of a Th1 response.
ECTV infection of mice provides valuable insights into the pathogenesis of smallpox. Our data suggest a crucial role for NK cells in recovery from mousepox and by analogy may also be important in the recovery from smallpox. Immunomodulators capable of activating NK cells could prove beneficial for decreasing virus burden early during orthopoxvirus infections.
Acknowledgments
This work was supported by NIAID NOI-AI-15436 (R.M.L.B.), the American Heart Association Heartland Predoctoral Fellowship (A.K.P.), and National Institutes of Health grant U54 AI057160 to the Midwest Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research (MRCE) (R.M.L.B. and W.M.Y.).
We thank Monica Allen for administrative assistance, Ed Hembrador for assaying virus infectivity, Erika Holroyd for antibody purification, Michael Orihuela for genotyping NKD mice and NK-depleted mice, and Sherri Koehm, Joy Eslick, and Scott Haskett for assistance with flow cytometry.
Footnotes
Published ahead of print on 7 February 2007.
REFERENCES
- 1.Afkarian, M., J. R. Sedy, J. Yang, N. G. Jacobson, N. Cereb, S. Y. Yang, T. L. Murphy, and K. M. Murphy. 2002. T-bet is a STAT1-induced regulator of IL-12R expression in naive CD4+ T cells. Nat. Immunol. 3:549-557. [DOI] [PubMed] [Google Scholar]
- 2.Ashkar, A. A., and K. L. Rosenthal. 2003. Interleukin-15 and natural killer and NKT cells play a critical role in innate protection against genital herpes simplex virus type 2 infection. J. Virol. 77:10168-10171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bendelac, A., M. N. Rivera, S. H. Park, and J. H. Roark. 1997. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu. Rev. Immunol. 15:535-562. [DOI] [PubMed] [Google Scholar]
- 4.Biron, C. A., K. S. Byron, and J. L. Sullivan. 1989. Severe herepesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320:1731-1735. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, C. R., T. Elliott, P. Parham, and S. I. Khakoo. 2006. The inhibitory receptor NKG2A determines lysis of vaccinia virus-infected autologous targets by NK cells. J. Immunol. 176:1141-1147. [DOI] [PubMed] [Google Scholar]
- 6.Brownstein, D. G., and L. Gras. 1997. Differential pathogenesis of lethal mousepox in congenic DBA/2 mice implicates natural killer cell receptor NKR-P1 in necrotizing hepatitis and the fifth component of complement in recruitment of circulating leukocytes to spleen. Am. J. Pathol. 150:1407-1420. [PMC free article] [PubMed] [Google Scholar]
- 7.Brutkiewicz, R. R., Y. Lin, S. Cho, Y. K. Hwang, V. Sriram, and T. J. Roberts. 2003. CD1d-mediated antigen presentation to natural killer T (NKT) cells. Crit. Rev. Immunol. 23:403-419. [DOI] [PubMed] [Google Scholar]
- 8.Chaudhri, G., V. Panchanathan, R. M. Buller, A. J. van den Eertwegh, E. Claassen, J. Zhou, R. de Chazal, J. D. Laman, and G. Karupiah. 2004. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc. Natl. Acad. Sci. USA 101:9057-9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, W., R. Drillien, D. Spehner, and R. M. Buller. 1992. Restricted replication of ectromelia virus in cell culture correlates with mutations in virus-encoded host range gene. Virology 187:433-442. [DOI] [PubMed] [Google Scholar]
- 10.Chisholm, S. E., and H. T. Reyburn. 2006. Recognition of vaccinia virus-infected cells by human natural killer cells depends on natural cytotoxicity receptors. J. Virol. 80:2225-2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cui, J., T. Shin, T. Kawano, H. Sato, E. Kondo, I. Toura, Y. Kaneko, H. Koseki, M. Kanno, and M. Taniguchi. 1997. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science 278:1623-1626. [DOI] [PubMed] [Google Scholar]
- 12.Daniels, K. A., G. Devora, W. C. Lai, C. L. O'Donnell, M. Bennett, and R. M. Welsh. 2001. Murine cytomegalovirus is regulated by a discrete subset of natural killer cells reactive with monoclonal antibody to Ly49H. J. Exp. Med. 194:29-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delano, M. L., and D. G. Brownstein. 1995. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus replication by cells with an NK phenotype. J. Virol. 69:5875-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dokun, A. O., S. Kim, H. R. Smith, H. S. Kang, D. T. Chu, and W. M. Yokoyama. 2001. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol. 2:951-956. [DOI] [PubMed] [Google Scholar]
- 15.Dorner, B. G., H. R. Smith, A. R. French, S. Kim, J. Poursine-Laurent, D. L. Beckman, J. T. Pingel, R. A. Kroczek, and W. M. Yokoyama. 2004. Coordinate expression of cytokines and chemokines by NK cells during murine cytomegalovirus infection. J. Immunol. 172:3119-3131. [DOI] [PubMed] [Google Scholar]
- 16.Duerst, R. J., and L. A. Morrison. 2003. Innate immunity to herpes simplex virus type 2. Viral Immunol. 16:475-490. [DOI] [PubMed] [Google Scholar]
- 17.Esteban, D. J., and R. M. Buller. 2005. Ectromelia virus: the causative agent of mousepox. J. Gen. Virol. 86:2645-2659. [DOI] [PubMed] [Google Scholar]
- 18.Fang, M., and L. J. Sigal. 2005. Antibodies and CD8+ T cells are complementary and essential for natural resistance to a highly lethal cytopathic virus. J. Immunol. 175:6829-6836. [DOI] [PubMed] [Google Scholar]
- 19.Fenner, F. 1948. Pathogenesis of the acute exanthems. Lancet 2:915-920. [DOI] [PubMed] [Google Scholar]
- 20.Fenner, F., D. A. Henderson, A. Arita, Z. Jezek, and I. D. Ladnyi. 1988. Smallpox and its eradication. World Heath Organization, Geneva, Switzerland.
- 21.Gazit, R., R. Gruda, M. Elboim, T. I. Arnon, G. Katz, H. Achdout, J. Hanna, U. Qimron, G. Landau, E. Greenbaum, Z. Zakay-Rones, A. Porgador, and O. Mandelboim. 2006. Lethal influenza infection in the absence of the natural killer cell receptor gene Ncr1. Nat. Immunol. 7:517-523. [DOI] [PubMed] [Google Scholar]
- 22.Ghiasi, H., S. Cai, G. C. Perng, A. B. Nesburn, and S. L. Wechsler. 2000. The role of natural killer cells in protection of mice against death and corneal scarring following ocular HSV-1 infection. Antiviral Res. 45:33-45. [DOI] [PubMed] [Google Scholar]
- 23.Grubor-Bauk, B., A. Simmons, G. Mayrhofer, and P. G. Speck. 2003. Impaired clearance of herpes simplex virus type 1 from mice lacking CD1d or NKT cells expressing the semivariant V alpha 14-J alpha 281 TCR. J. Immunol. 170:1430-1434. [DOI] [PubMed] [Google Scholar]
- 24.Huynh, C., D. Roth, D. M. Ward, J. Kaplan, and N. W. Andrews. 2004. Defective lysosomal exocytosis and plasma membrane repair in Chediak-Higashi/beige cells. Proc. Natl. Acad. Sci. USA 101:16795-16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby, R. O., P. N. Bhatt, and D. G. Brownstein. 1989. Evidence that NK cells and interferon are required for genetic resistance to lethal infection with ectromelia virus. Arch. Virol. 108:49-58. [DOI] [PubMed] [Google Scholar]
- 26.Kakimi, K., L. G. Guidotti, Y. Koezuka, and F. V. Chisari. 2000. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 192:921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karlhofer, F. M., R. K. Ribaudo, and W. M. Yokoyama. 1992. MHC class I alloantigen specificity of Ly-49+ IL-2-activated natural killer cells. Nature 358:66-70. [DOI] [PubMed] [Google Scholar]
- 28.Karupiah, G., R. M. Buller, N. Van Rooijen, C. J. Duarte, and J. Chen. 1996. Different roles for CD4+ and CD8+ T lymphocytes and macrophage subsets in the control of a generalized virus infection. J. Virol. 70:8301-8309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Karupiah, G., T. N. Fredrickson, K. L. Holmes, L. H. Khairallah, and R. M. Buller. 1993. Importance of interferons in recovery from mousepox. J. Virol. 67:4214-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawano, T., J. Cui, Y. Koezuka, I. Toura, Y. Kaneko, H. Sato, E. Kondo, M. Harada, H. Koseki, T. Nakayama, Y. Tanaka, and M. Taniguchi. 1998. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Valpha14 NKT cells. Proc. Natl. Acad. Sci. USA 95:5690-5693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiessling, R., E. Klein, and H. Wigzell. 1975. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 5:112-117. [DOI] [PubMed] [Google Scholar]
- 32.Kim, S., K. Iizuka, H. L. Aguila, I. L. Weissman, and W. M. Yokoyama. 2000. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc. Natl. Acad. Sci. USA 97:2731-2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim, S., Y. J. Song, D. A. Higuchi, H. P. Kang, J. R. Pratt, L. Yang, C. M. Hong, J. Poursine-Laurent, K. Iizuka, A. R. French, J. B. Sunwoo, S. Ishii, A. M. Reimold, and W. M. Yokoyama. 2006. Arrested natural killer cell development associated with transgene insertion into the Atf2 locus. Blood 107:1024-1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin-Fontecha, A., L. L. Thomsen, S. Brett, C. Gerard, M. Lipp, A. Lanzavecchia, and F. Sallusto. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 5:1260-1265. [DOI] [PubMed] [Google Scholar]
- 35.Mendiratta, S. K., W. D. Martin, S. Hong, A. Boesteanu, S. Joyce, and L. Van Kaer. 1997. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity 6:469-477. [DOI] [PubMed] [Google Scholar]
- 36.Mullbacher, A., R. T. Hla, C. Museteanu, and M. M. Simon. 1999. Perforin is essential for control of ectromelia virus but not related poxviruses in mice. J. Virol. 73:1665-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen, K. B., T. P. Salazar-Mather, M. Y. Dalod, J. B. Van Deusen, X. Q. Wei, F. Y. Liew, M. A. Caligiuri, J. E. Durbin, and C. A. Biron. 2002. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 169:4279-4287. [DOI] [PubMed] [Google Scholar]
- 38.Norbury, C. C., D. Malide, J. S. Gibbs, J. R. Bennink, and J. W. Yewdell. 2002. Visualizing priming of virus-specific CD8+ T cells by infected dendritic cells in vivo. Nat. Immunol. 3:265-271. [DOI] [PubMed] [Google Scholar]
- 39.Pien, G. C., A. R. Satoskar, K. Takeda, S. Akira, and C. A. Biron. 2000. Cutting edge: selective IL-18 requirements for induction of compartmental IFN-gamma responses during viral infection. J. Immunol. 165:4787-4791. [DOI] [PubMed] [Google Scholar]
- 40.Prlic, M., J. Gibbs, and S. C. Jameson. 2005. Characteristics of NK cell migration early after vaccinia infection. J. Immunol. 175:2152-2157. [DOI] [PubMed] [Google Scholar]
- 41.Scalzo, A. A., N. A. Fitzgerald, A. Simmons, A. B. La Vista, and G. R. Shellam. 1990. Cmv-1, a genetic locus that controls murine cytomegalovirus replication in the spleen. J. Exp. Med. 171:1469-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharton, T. M., and P. Scott. 1993. Natural killer cells are a source of interferon gamma that drives differentiation of CD4+ T cell subsets and induces early resistance to Leishmania major in mice. J. Exp. Med. 178:567-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seaman, W. E. 2000. Natural killer cells and natural killer T cells. Arthritis Rheum. 43:1204-1217. [DOI] [PubMed] [Google Scholar]
- 44.Smith, H. R., J. W. Heusel, I. K. Mehta, S. Kim, B. G. Dorner, O. V. Naidenko, K. Iizuka, H. Furukawa, D. L. Beckman, J. T. Pingel, A. A. Scalzo, D. H. Fremont, and W. M. Yokoyama. 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. USA 99:8826-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Snijders, A., P. Kalinski, C. M. Hilkens, and M. L. Kapsenberg. 1998. High-level IL-12 production by human dendritic cells requires two signals. Int. Immunol. 10:1593-1598. [DOI] [PubMed] [Google Scholar]
- 46.Szabo, S. J., B. M. Sullivan, C. Stemmann, A. R. Satoskar, B. P. Sleckman, and L. H. Glimcher. 2002. Distinct effects of T-bet in TH1 lineage commitment and IFN-gamma production in CD4 and CD8 T cells. Science 295:338-342. [DOI] [PubMed] [Google Scholar]
- 47.Wallace, G. D., R. M. Buller, and H. C. Morse III. 1985. Genetic determinants of resistance to ectromelia (mousepox) virus-induced mortality. J. Virol. 55:890-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yokoyama, W. M., S. Kim, and A. R. French. 2004. The dynamic life of natural killer cells. Annu. Rev. Immunol. 22:405-429. [DOI] [PubMed] [Google Scholar]
- 49.Yokoyama, W. M., and A. A. Scalzo. 2002. Natural killer cell activation receptors in innate immunity to infection. Microbes Infect. 4:1513-1521. [DOI] [PubMed] [Google Scholar]