Abstract
OBJECTIVE
A sonographically short cervix is a powerful predictor of spontaneous preterm delivery. However, the etiology and optimal management of a patient with a short cervix in the mid-trimester of pregnancy remain uncertain. Microbial invasion of the amniotic cavity (MIAC) and intra-amniotic inflammation are frequently present in patients with spontaneous preterm labor or acute cervical insufficiency. This study was conducted to determine the rate of MIAC and intra-amniotic inflammation in patients with a cervical length <25 mm in the mid-trimester.
STUDY DESIGN
A retrospective cohort study was conducted of patients referred to our high risk clinic because of a sonographic short cervix or a history of a previous preterm birth. Amniocenteses were performed for the evaluation of MIAC and for karyotype analysis in patients with a short cervix. Fluid was cultured for aerobic and anaerobic bacteria, as well as genital mycoplasmas. Patients with MIAC were treated with antibiotics selected by their physician.
RESULTS
Of 152 patients with a short cervix at 14–24 weeks, 57 had amniotic fluid analysis. The prevalence of MIAC was 9% (5/57). Among these patients, the rate of preterm delivery (<32 weeks) was 40% (2/5). Microorganisms isolated from amniotic fluid included Ureaplasma urealyticum (n=4) and Fusobacterium nucleatum (n=1). Patients with a positive culture for Ureaplasma urealyticum received intravenous Azithromycin. Three patients with Ureaplasma urealyticum had a sterile amniotic fluid culture after treatment, and subsequently delivered at term. The patient with Fusobacterium nucleatum developed clinical chorioamnionitis and was induced.
CONCLUSION
1) Sub-clinical MIAC was detected in 9% of patients with a sonographically short cervix (<25 mm); and 2) maternal parenteral treatment with antibiotics can eradicate MIAC caused by Ureaplasma urealyticum. This was associated with delivery at term in the three patients whose successful treatment was documented by microbiologic studies.
Keywords: Ultrasound, cervical length, preterm delivery, intra-amniotic infection, chorioamnionitis, cervical insufficiency, short cervix, microbial invasion of the amniotic cavity
Introduction
A sonographic short cervix is a powerful predictor of spontaneous preterm delivery [2, 3, 14, 15, 17, 43]. However, neither the etiology nor the optimal management of patients with a sonographic short cervix has been determined.
Intrauterine infection is a major cause of premature labor and delivery[8]. Previous studies have demonstrated that patients with a dilated cervix before 24 weeks of gestation have a very high incidence of sub-clinical microbial invasion of the amniotic cavity (MIAC) [23, 29]. Moreover, the lower the gestational age at which preterm delivery occurs, the higher the rate of MIAC [35, 48], intra-amniotic inflammation [55], and histologic chorioamnionitis [24, 37, 52].
This study was conducted to determine the rate of intra-amniotic infection in asymptomatic patients with a short cervix in the mid-trimester of pregnancy, and the effect of treatment with antibiotics in patients with a clinically silent intra-amniotic infection and a short cervix.
Population and Methods
A retrospective cohort study was conducted searching our perinatal database of patients referred to our high risk clinic at Hutzel Women’s Hospital (Detroit, MI) with the diagnosis of a sonographic short cervix who met the following criteria: (1) singleton gestation; (2) sonographic cervical length < 25 mm; (3) gestational age between 14 and 24 weeks; (4) asymptomatic; (5) cervical dilatation < 2 cm by digital examination; and (6) amniocentesis for microbiological studies of amniotic fluid. The study period was August 2002 to August 2004. Patients with preterm labor, preterm premature rupture of membranes (PROM), placenta previa, bleeding, or a cerclage in situ were excluded.
Amniocentesis was performed for clinical indications (e.g., to determine the microbial status of the amniotic cavity and karyotype analysis). Results of the amniocentesis were used in patient management (e.g., antibiotic administration). Amniocentesis in patients with a short cervix was undertaken as part of the standard obstetrical practice, in light of previous observations suggesting an association between a short cervix and histologic chorioamnionitis [11]. Patients were counseled by clinicians, and those who agreed to undergo an amniocentesis for clinical management were asked to donate amniotic fluid and allow collection of clinical information for research purposes, according to a protocol approved by the Wayne State University Institutional Review Board.
During the study period, our institution’s database recorded 152 patients with a sonographic cervical length < 25 mm and a gestational age between 14 and 24 weeks. Of these, 57 underwent an amniocentesis.
Retrieval of amniotic fluid
Amniotic fluid was retrieved by transabdominal amniocentesis under ultrasonographic guidance. The fluid was then transported to the laboratory in a capped plastic syringe and cultured for aerobic and anaerobic bacteria, as well as mycoplasmas. White blood cell count, glucose concentration and Gram stain for microorganisms were performed in amniotic fluid shortly after collection, using methods previously described [28, 31, 33].
Sonographic examination of the cervix
Sonographic evaluation of cervical length was conducted by transvaginal ultrasound. Sonographic examinations were performed with standard equipment (Advanced Technology Laboratories, Bothell, Washington; Seimens SI450, Issaquah, WA; Acuson XP128, Mountain View, CA). All examinations were performed by Registered Diagnostic Medical Sonographers and reviewed by a perinatologist. Transvaginal cervical length measurements were obtained using the technique described by Iams et al [17, 18].
Gestational age was determined by last menstrual period or by ultrasound, in the case where the discrepancy between the ultrasound and the menstrual dating was greater than two weeks. Patient clinical and demographic data, past obstetrical history, and pregnancy outcome were obtained by chart review.
Statistical Analysis
Comparisons were performed with Chi square, Fisher’s exact tests and Mann-Whitney U tests.
Results
One hundred fifty-two patients had a transvaginal cervical length < 25 mm at 14–24 weeks during the study period. Fifty-seven patients had amniotic fluid analyses. No patients had evidence of labor. Table 1 details the patient demographic data. The prevalence of positive amniotic fluid cultures was 9% (5/57). Among these patients with a positive amniotic fluid culture, the rate of preterm delivery at less than 32 weeks was 40% (2/5). Microorganisms isolated from amniotic fluid were Ureaplasma urealyticum (n=4) and Fusobacterium nucleatum (n=1). One patient had a positive culture for Staphylococcus aureus, but a negative Gram stain of amniotic fluid, no white blood cells, and a normal glucose determination. This patient was considered to have a contaminant and, therefore, was not included among individuals with a positive culture. No antibiotics were administered, and the patient delivered at 35 weeks with severe preeclampsia. The placenta showed no histologic evidence of chorioamnionitis.
Table 1.
Demographic data of the study population
| Parameter | n = 57 |
|---|---|
| Maternal age (years, mean ± SD) | 27 ± 9 |
| Parity | |
| 0 (%, n) | 53% (30) |
| 1 or more (%, n) | 47% (27) |
| History of preterm birth | |
| Yes | 58% (33) |
| No | 42% (24) |
| Ethnicity | |
| African American | 93% (53) |
| Caucasian | 3.5% (2) |
| Hispanic | 1.8% (1) |
| Asian | 1.8% (1) |
Four patients with a positive culture for Ureaplasma urealyticum received intravenous Azithromycin for seven days, and had a second amniocentesis after treatment. All patients had a second amniotic fluid culture which was negative for microorganisms. Three of the four women subsequently delivered at term, and one showed histologic evidence of chorioamnionitis. There was one case with a first positive culture for Ureaplasma urealyticum who had sterile amniotic fluid at the time of the second amniocentesis, but delivered preterm (7 weeks after the second amniocentesis). This patient had histologic evidence of chorioamnionitis.
One patient had a positive amniotic fluid culture for Fusobacterium nucleatum, developed clinical chorioamnionitis shortly after the amniocentesis, and labor was induced. The clinical and laboratory information for all cases with MIAC are displayed in Table 2. Table 3 displays the pregnancy outcome of the study population.
Table 2.
Clinical characteristics of patients with a short cervix (< 25 mm) and microbial invasion of the amniotic cavity
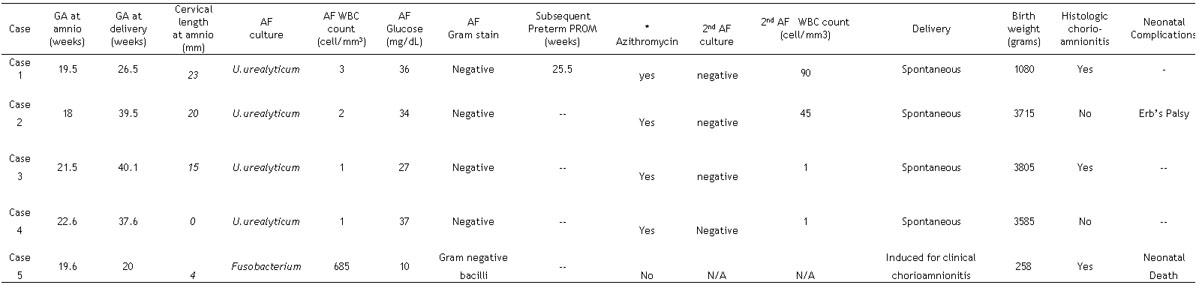
GA: gestational age; AF: amniotic fluid; WBC: white blood cell; amnio: amniocentesis; PROM: premature rupture of membranes.
Dosage = 1 gram intravenous every 24 hours X 7 days.
Table 3.
Pregnancy outcome of patients with a short cervix (< 25 mm)
| Parameter | All patients (n=57) | MIAC (n=5) | No MIAC (n=52) |
|---|---|---|---|
| Gestational age at amniocentesis [weeks, mean ± sd, median (range)] | 20.3 + 1.9 | 20.3 ± 1.7 | 20.3 ± 2.0 |
| 20.4 (15.2–23.5) | 20.4 (18–22.6) | 20.4 (15.2–23.5) | |
| Spontaneous preterm delivery | |||
| ≤ 35 weeks | 45.6% (26/57) | 40% (2/5) | 46% (24/52) |
| ≤ 32 weeks | 31.6% (18/57) | 40% (2/5) | 31% (16/52) |
| ≤ 28 weeks | 22.8% (13/57) | 40% (2/5) | 21% (11/52) |
| Clinical chorioamnionitis | 1.8% (1/57) | 20% (1/5) | 0% (0/52) |
| Histological chorioamnionitis | 54% (26/48) | 60% (3/5) | 53% (23/43) |
| Gestational age at delivery [weeks, mean ± sd, median(range)] | 33.5 ± 6.2 | 33.2 ± 8.1 | 33.5 ± 6.5 |
| 36 (18–42) | 36.5 (20–40.1) | 36 (18–42) | |
MIAC: microbial invasion of the amniotic cavity; all comparisons were non-significant (p>0.05).
Comment
Principal findings of this study
1) Nine percent of women with a sonographic short cervix (defined as a cervical length < of 25 mm) had MIAC; 2) MIAC was sub-clinical in nature, as none of the patients showed evidence of clinical chorioamnionitis at the time of presentation; 3) the most frequent organism isolated from the amniotic fluid was Ureaplasma urealyticum; and 4) treatment with antibiotics administered to the mother was associated with eradication of the Ureaplasma urealyticum in three of four cases, and these patients delivered at term. One patient with a positive Ureaplasma urealyticum culture who received treatment and subsequently had a negative amniotic fluid culture delivered preterm, indicating that treatment was not successful in all cases. Our findings suggest that a short cervix, detected with ultrasound, can be the only clinical manifestation of MIAC, and that intra-amniotic infection in the mid-trimester of pregnancy can be treated with antibiotics.
The clinical significance of a short cervix detected with ultrasound
The shorter the sonographic cervical length in the mid-trimester of pregnancy, the higher the likelihood of spontaneous preterm delivery [1–3, 14, 15, 17, 19, 43, 45, 46]. However, there is no agreement as to what is a sonographic short cervix. For example, Iams et al. [17] reported the results of a prospective study of low-risk women for preterm delivery who underwent sonographic examination at 24 and 26–28 weeks of gestation. The authors concluded that a cervix of 26 mm or shorter at 24 weeks increased the risk for spontaneous preterm delivery (Relative risk:6.19; 95% CI: 3.84–9.97) [17]. However, the prevalence of spontaneous preterm delivery (defined as < 35 weeks) in this study was 4.3%, while the positive predictive value was 17.8% for a cervical length ≤ 25 mm at 24 weeks gestation [17]. Other investigators, including members of our group, have proposed a cut-off of 15 mm, because a cervical length of 15 mm or less is associated with nearly a 50% risk of spontaneous preterm delivery at 32 weeks of gestation or less when neonatal morbidity is substantial [14, 15].
It is clear, however, that sonographic cervical length is not a screening test for spontaneous preterm delivery, as only a fraction of all patients who have a spontaneous preterm birth have a short cervix in the mid-trimester. Previous studies conducted at our institution indicated that only 8% of all patients with a preterm delivery at less than 32 weeks of gestation have a cervical length of 15 mm or less [14]. For this reason, we advocate that a sonographic cervical length is only a method for risk assessment for spontaneous preterm delivery and not a screening test. Cervical length can modify the a priori risk for preterm delivery. For example, a woman with a history of preterm delivery or one with a twin or triplet gestation will have a higher risk for preterm delivery than a patient without such history and the same cervical length [7, 12, 13, 22, 27, 40, 41, 44, 47, 49].
Why do patients have a short cervix?
The causes of a sonographic short cervix are largely unknown. A congenital short cervix has been reported in patients exposed to diethylstilbestrol (DES)[38], though this is an extremely rare occurrence nowadays. Previous studies suggest that maternal age, body mass index, ethnicity, and obstetrical history are independent explanatory variables of sonographically determined cervical length [16]. One interpretation of these observations is that the combination of genetic and environmental factors may play a role in determining cervical length. For instance, pregnant adolescents, undernourished women (low body mass index), individuals of African-Caribbean or African-American origin, and those with a previous preterm delivery have a higher risk of spontaneous preterm birth [4, 16]. It is possible that these factors confer risk by altering, among other biological determinants of preterm birth, cervical length.
Since the uterine cervix is a connective tissue structure[20], lack of appropriate nutrition during development (in utero or during critical periods of reproductive development, such as adolescence) conceivably may lead to inadequate extracellular matrix deposition in the cervix, and this may predispose to a short cervix. For example, a low body mass index (below 19.8) is an independent risk factor for preterm delivery (OR: 3.6, 95% CI: 1.6–8.0) [39]. However, this association may operate through a short cervix. If the low body mass index is due to environmental factors, the short cervix could be an acquired phenomenon. The possibility that maternal under-nutrition or fetal growth restriction may predispose to a short cervix needs to be considered.
Several authors have documented a relationship between a previous obstetrical history of preterm birth and cervical length after pregnancy [10, 18]. Iams et al. reported a study of cervical length in patients with: 1) a previous history on cervical insufficiency; 2) a previous preterm delivery ≤ 26 weeks; 3) a previous preterm delivery at 27–32 weeks; 4) a previous preterm delivery at 33–35 weeks; and 5) a control group of women with previous term delivery. A strong relationship was established between cervical length in the index pregnancy and previous obstetric history. This relationship appears linear and patients considered to have a typical history of an incompetent cervix did not constitute a unique group[18]. Similar results have been reported by Guzman et al. who described a strong relationship between previous obstetric history and cervical length in the subsequent pregnancy [10]. Specifically, they observed that the frequency of a short cervix (cervical length < 2cm) or progressive shortening of the cervix to a length of < 2cm was associated with the gestational age at delivery in the previous pregnancy [10]. Collectively, these studies suggest a relationship between a history of preterm delivery and the cervical length in a subsequent pregnancy. A short cervical length in the subsequent pregnancy may reflect the result of genetic and environmental factors predisposing to preterm delivery, which can express themselves in changes in cervical structure.
Another cause for a short cervix is premature cervical ripening in response to a pathologic stimulus such as infection. Previous studies have indicated that up to 50% of patients presenting with painless cervical dilatation before 24 weeks of gestation have a positive amniotic fluid culture for microorganisms [23, 29]. In these cases, infection can be a cause of cervical insufficiency, or may be secondary to prolonged exposure of chorioamniotic membranes to the microbial flora present in the lower genital tract [29].
The prevalence of MIAC in patients with a short cervix compared to other complications of pregnancy
The frequency of MIAC in patients with an asymptomatic short cervix is quite similar to that of patients presenting with preterm labor and intact membranes [35], but lower than those with preterm PROM (30%) [36] and acute cervical insufficiency (50%) [29].
Ureaplasma urealyticum: the most common microorganism found in the amniotic cavity in women with a sonographic short cervix
The observations reported herein that Ureaplasma urealyticum is the most common microorganism found in the amniotic cavity is consistent with those made in cases of patients in preterm labor with intact membranes, preterm PROM, acute cervical insufficiency, term PROM, and vaginal bleeding [8, 32, 51, 53, 54, 56]. The reasons why Ureaplasma urealyticum is a frequent isolate from the amniotic cavity are unknown. This organism, found in the lower genital tract of up to 80% of normal pregnant women, has been detected in endometrial cultures [5, 21, 42]. It is possible that Ureaplasma urealyticum gains access to the endometrial cavity with sperm in the non-pregnant state, to cause endometritis and, subsequently, an intra-amniotic infection during pregnancy. The other alternative is that Ureaplasma urealyticum is prone to gain access from the lower genital tract to the amniotic cavity in some women, while not in others. If this is the case, the factors responsible for this susceptibility need to be understood.
The clinical significance of microbial invasion in patients with a short cervix
Gray et al. reported that all women with a positive amniotic fluid culture for Ureaplasma urealyticum at the time of genetic amniocentesis delivered a preterm neonate with histologic evidence of chorioamnionitis [9]. This study, however, did not contain information about cervical length.
We were not able to examine the natural history of MIAC in the context of this study, as the results were used for patient management. Patients with MIAC were treated with antibiotics. The current ethical framework governing research in human subjects indicates that the fetus exposed to an invasive procedure must have the possibility of benefiting from the information acquired from the procedure. Therefore, we find it difficult to envision the circumstances in which MIAC can be identified and then an intervention withheld to determine the natural history of this condition. The second limitation of this study is that we have used microbial culture rather than molecular microbiological techniques. We have previously demonstrated that standard culture techniques underestimate the rate of microbial footprints in the amniotic cavity, and that patients with bacterial footprints but negative amniotic fluid cultures have a similar outcome to those with a positive amniotic fluid culture for bacteria [53, 54]. Thus, the current study provides a minimum estimate of the frequency of MIAC in women with a short cervix. It is, however, possible that the rate of microbial invasion may increase with the use of molecular microbiologic techniques for the detection of bacteria and viruses [6, 25, 26, 50, 53].
Treatment with antibiotics can eradicate MIAC in women with a short cervix
An important observation of our study is that three patients with positive amniotic fluid cultures for Ureaplasma urealyticum underwent treatment with intravenous Azithromycin, and a repeat amniocentesis demonstrated that the amniotic cavity had become sterile and the patient delivered at term. This information is consistent with two previous reports that parenteral antibiotic therapy can eradicate MIAC [30, 34]. However, the significance of this report is that, in the past, maternal treatment has accomplished eradication in patients who had preterm PROM, and this time it was accomplished in women with intact membranes. This result suggests that maternal antibiotic administration can successfully eradicate MIAC in patients in the mid-trimester of pregnancy. The implications of this observation are substantial, since Gray et al reported a 0.37% (9/2461) prevalence of positive cultures for Ureaplasma urealyticum in amniotic fluid samples obtained during second trimester genetic amniocenteses. After exclusion of a therapeutic abortion case, all women (8/8) with positive amniotic fluid cultures had either a fetal loss within four weeks of amniocentesis (n=6) or preterm delivery (n=2). All had histologic evidence of chorioamnionitis [9]. Our findings suggest that treatment with antibiotics may be successful. A limitation of our study is that the natural history of intra-amniotic infection in women with intact membranes is not known. The traditional assumption has been that once MIAC has occurred, it has a progressive course. Still, it is possible that in certain patients the host defense mechanisms suffice to control some cases of MIAC, and in such cases intra-amniotic inflammation/infection could be transient and not lead inevitably to preterm delivery. It is important to note that one patient with a positive amniotic fluid culture with Ureaplasma urealyticum was treated with antibiotics and had a second amniocentesis, suggesting eradication of the infection within the amniotic cavity, and still had a preterm delivery. Noteworthy is the fact that while the number of white blood cells in the first amniocentesis was 3 per mm3, the second amniocentesis performed after treatment had 90 white blood cells per mm3, but a negative culture. These results suggest that treatment will not be successful in all cases. It is conceivable that the microorganisms were eradicated or that the inoculum size was decreased to such a degree that a positive culture could not be obtained. Another possibility is that the antibiotic used for the treatment remained in the amniotic fluid and interfered with the growth of the microorganism in culture. The net result may have been a negative culture. However, progressive intra-amniotic inflammation occurred and resulted in a preterm delivery. Indeed, the white blood cell count in the second amniocentesis increased dramatically, and the placenta of the patient in question showed histologic evidence of chorioamnionitis. The clinical implications of these observations are that not all patients will be successfully treated, and that care needs to be exercised in monitoring the inflammatory response and optimizing the methods for the identification of microorganisms in the amniotic cavity. This information is important for both patient care and the improvement of the treatment of intra-amniotic infection/inflammation.
In conclusion, this study demonstratesthat a short cervix may be the only manifestation of sub-clinical MIAC, and that eradication of these infections is possible and may be associated with a normal term delivery.
Acknowledgments
This research was supported [in part] by the Intramural Research Program of the National Institute of Child Health and Human Development, NIH, DHHS. Dr. Sonia Hassan is a Women’s Reproductive Health Research Scholar whose research is sponsored by a grant from the National Institutes of Health/NICHD Grant #2K12HD01254-06.
References
- 1.Andersen HF. Transvaginal and transabdominal ultrasonography of the uterine cervix during pregnancy. J Clin Ultrasound. 1991;19:77. doi: 10.1002/jcu.1870190204. [DOI] [PubMed] [Google Scholar]
- 2.Andersen HF, Nugent CE, Wanty SD, Hayashi RH. Prediction of risk for preterm delivery by ultrasonographic measurement of cervical length. Am J Obstet Gynecol. 1990;163:859. doi: 10.1016/0002-9378(90)91084-p. [DOI] [PubMed] [Google Scholar]
- 3.Cook CM, Ellwood DA. The cervix as a predictor of preterm delivery in ‘at-risk’ women. Ultrasound Obstet Gynecol. 2000;15:109. doi: 10.1046/j.1469-0705.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 4.Dijkstra K, Janssen HC, Kuczynski E, Lockwood CJ. Cervical length in uncomplicated pregnancy: A study of sociodemographic predictors of cervical changes across gestation. Am J Obstet Gynecol. 1999;180:639. doi: 10.1016/s0002-9378(99)70267-x. [DOI] [PubMed] [Google Scholar]
- 5.Eschenbach DA, Rosene K, Tompkins LS, Watkins H, Gravett MG. Endometrial cultures obtained by a triple-lumen method from afebrile and febrile postpartum women. J Infect Dis. 1986;153:1038. doi: 10.1093/infdis/153.6.1038. [DOI] [PubMed] [Google Scholar]
- 6.Gerber S, Vial Y, Hohlfeld P, Witkin SS. Detection of Ureaplasma urealyticum in second-trimester amniotic fluid by polymerase chain reaction correlates with subsequent preterm labor and delivery. J Infect Dis. 2003;187:518. doi: 10.1086/368205. [DOI] [PubMed] [Google Scholar]
- 7.Goldenberg RL, Iams JD, Miodovnik M, Van Dorsten JP, Thurnau G, Bottoms S, Mercer BM, Meis PJ, Moawad AH, Das A, Caritis SN, McNellis D. The preterm prediction study: risk factors in twin gestations. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1996;175:1047. doi: 10.1016/s0002-9378(96)80051-2. [DOI] [PubMed] [Google Scholar]
- 8.Gomez R, R Romero, M Mazor, F Ghezzi, C David, BH Yoon: The role of infection in preterm labor and delivery. In: Elder, M. G., Romero, R., and Lamont, R. F.: Preterm labor. Churchill Livingstone, New York, NY 1997
- 9.Gray DJ, Robinson HB, Malone J, Thomson RB., Jr Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn. 1992;12:111. doi: 10.1002/pd.1970120206. [DOI] [PubMed] [Google Scholar]
- 10.Guzman ER, Mellon R, Vintzileos AM, Ananth CV, Walters C, Gipson K. Relationship between endocervical canal length between 15–24 weeks gestation and obstetric history. J Matern Fetal Med. 1998;7:269. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<269::AID-MFM3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Guzman ER, Shen-Schwarz S, Benito C, Vintzileos AM, Lake M, Lai YL. The relationship between placental histology and cervical ultrasonography in women at risk for pregnancy loss and spontaneous preterm birth. Am J Obstet Gynecol. 1999;181:793. doi: 10.1016/s0002-9378(99)70303-0. [DOI] [PubMed] [Google Scholar]
- 12.Guzman ER, Walters C, O’Reilly-Green C, Kinzler WL, Waldron R, Nigam J, Vintzileos AM. Use of cervical ultrasonography in prediction of spontaneous preterm birth in twin gestations. Am J Obstet Gynecol. 2000;183:1103. doi: 10.1067/mob.2000.108896. [DOI] [PubMed] [Google Scholar]
- 13.Guzman ER, Walters C, O’Reilly-Green C, Meirowitz NB, Gipson K, Nigam J, Vintzileos AM. Use of cervical ultrasonography in prediction of spontaneous preterm birth in triplet gestations. Am J Obstet Gynecol. 2000;183:1108. doi: 10.1067/mob.2000.108875. [DOI] [PubMed] [Google Scholar]
- 14.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, Wolfe HM. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 15.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 16.Heath VC, Southall TR, Souka AP, Novakov A, Nicolaides KH. Cervical length at 23 weeks of gestation: relation to demographic characteristics and previous obstetric history. Ultrasound Obstet Gynecol. 1998;12:304. doi: 10.1046/j.1469-0705.1998.12050304.x. [DOI] [PubMed] [Google Scholar]
- 17.Iams JD, Goldenberg RL, Meis PJ, Mercer BM, Moawad A, Das A, Thom E, McNellis D, Copper RL, Johnson F, Roberts JM. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 18.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 19.Kushnir O, Vigil DA, Izquierdo L, Schiff M, Curet LB. Vaginal ultrasonographic assessment of cervical length changes during normal pregnancy. Am J Obstet Gynecol. 1990;162:991. doi: 10.1016/0002-9378(90)91302-s. [DOI] [PubMed] [Google Scholar]
- 20.Leppert PC. Anatomy and physiology of cervical ripening. Clin Obstet Gynecol. 1995;38:267. doi: 10.1097/00003081-199506000-00009. [DOI] [PubMed] [Google Scholar]
- 21.Luton D, Ville Y, Luton-Sigy A, Cousin C, Narraido B, Fassasi-Jarretou A, Escarguel C. Prevalence and influence of Mycoplasma hominis and Ureaplasma urealyticum in 218 African pregnant women and their infants. Eur J Obstet Gynecol Reprod Biol. 1994;56:95. doi: 10.1016/0028-2243(94)90263-1. [DOI] [PubMed] [Google Scholar]
- 22.Maymon R, Herman A, Jauniaux E, Frenkel J, Ariely S, Sherman D. Transvaginal sonographic assessment of cervical length changes during triplet gestation. Hum Reprod. 2001;16:956. doi: 10.1093/humrep/16.5.956. [DOI] [PubMed] [Google Scholar]
- 23.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol. 2000;95:652. doi: 10.1016/s0029-7844(99)00633-x. [DOI] [PubMed] [Google Scholar]
- 24.Mooney EE, J Padfield, SJ Robboy: Nidation and placenta. In: Robboy, S. J., Anderson, M. C., and Russell, P.: Pathology of the female reporductive tract. Churchill Livingstone, New York, NY 2002
- 25.Oyarzun E, Yamamoto M, Kato S, Gomez R, Lizama L, Moenne A. Specific detection of 16 microorganisms in amniotic fluid by polymerase chain reaction and its correlation with preterm delivery occurrence. Am J Obstet Gynecol. 1998;179:1115. doi: 10.1016/s0002-9378(98)70115-2. [DOI] [PubMed] [Google Scholar]
- 26.Perni SC, Vardhana S, Korneeva I, Tuttle SL, Paraskevas LR, Chasen ST, Kalish RB, Witkin SS. Mycoplasma hominis and Ureaplasma urealyticum in midtrimester amniotic fluid: association with amniotic fluid cytokine levels and pregnancy outcome. Am J Obstet Gynecol. 2004;191:1382. doi: 10.1016/j.ajog.2004.05.070. [DOI] [PubMed] [Google Scholar]
- 27.Ramin KD, Ogburn PL, Jr, Mulholland TA, Breckle RJ, Ramsey PS. Ultrasonographic assessment of cervical length in triplet pregnancies. Am J Obstet Gynecol. 1999;180:1442. doi: 10.1016/s0002-9378(99)70033-5. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Emamian M, Quintero R, Wan M, Hobbins JC, Mazor M, Edberg S. The value and limitations of the Gram stain examination in the diagnosis of intraamniotic infection. Am J Obstet Gynecol. 1988;159:114. doi: 10.1016/0002-9378(88)90503-0. [DOI] [PubMed] [Google Scholar]
- 29.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y, Mazor M, Treadwell MC, Cotton DB. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol. 1992;167:1086. doi: 10.1016/s0002-9378(12)80043-3. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Hagay Z, Nores J, Sepulveda W, Mazor M. Eradication of Ureaplasma urealyticum from the amniotic fluid with transplacental antibiotic treatment. Am J Obstet Gynecol. 1992;166:618. doi: 10.1016/0002-9378(92)91686-5. [DOI] [PubMed] [Google Scholar]
- 31.Romero R, Jimenez C, Lohda AK, Nores J, Hanaoka S, Avila C, Callahan R, Mazor M, Hobbins JC, Diamond MP. Amniotic fluid glucose concentration: a rapid and simple method for the detection of intraamniotic infection in preterm labor. Am J Obstet Gynecol. 1990;163:968. doi: 10.1016/0002-9378(90)91106-m. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A, Parra M, Behnke E, Montiel F, Cassell GH. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol. 1992;166:129. doi: 10.1016/0002-9378(92)91845-2. [DOI] [PubMed] [Google Scholar]
- 33.Romero R, Quintero R, Nores J, Avila C, Mazor M, Hanaoka S, Hagay Z, Merchant L, Hobbins JC. Amniotic fluid white blood cell count: a rapid and simple test to diagnose microbial invasion of the amniotic cavity and predict preterm delivery. Am J Obstet Gynecol. 1991;165:821. doi: 10.1016/0002-9378(91)90423-o. [DOI] [PubMed] [Google Scholar]
- 34.Romero R, Scioscia AL, Edberg SC, Hobbins JC. Use of parenteral antibiotic therapy to eradicate bacterial colonization of amniotic fluid in premature rupture of membranes. Obstet Gynecol. 1986;67:15S. doi: 10.1097/00006250-198603001-00005. [DOI] [PubMed] [Google Scholar]
- 35.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R, Sabo V, Athanassiadis AP, Hobbins JC. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Yoon BH, Mazor M, Gomez R, Gonzalez R, Diamond MP, Baumann P, Araneda H, Kenney JS, Cotton DB. A comparative study of the diagnostic performance of amniotic fluid glucose, white blood cell count, interleukin-6, and gram stain in the detection of microbial invasion in patients with preterm premature rupture of membranes. Am J Obstet Gynecol. 1993;169:839. doi: 10.1016/0002-9378(93)90014-a. [DOI] [PubMed] [Google Scholar]
- 37.Russell P. Inflammatory lesions of the human placenta. III. The histopathology of villitis of unknown aetiology. Placenta. 1980;1:227. doi: 10.1016/s0143-4004(80)80005-1. [DOI] [PubMed] [Google Scholar]
- 38.Salle B, Sergeant P, Awada A, Bied-Damon V, Gaucherand P, Boisson C, Guibaud S, Benchaib M, Rudigoz RC. Transvaginal ultrasound studies of vascular and morphological changes in uteri exposed to diethylstilbestrol in utero. Hum Reprod. 1996;11:2531. doi: 10.1093/oxfordjournals.humrep.a019153. [DOI] [PubMed] [Google Scholar]
- 39.Schieve LA, Cogswell ME, Scanlon KS, Perry G, Ferre C, Blackmore-Prince C, Yu SM, Rosenberg D. Prepregnancy body mass index and pregnancy weight gain: associations with preterm delivery. The NMIHS Collaborative Study Group. Obstet Gynecol. 2000;96:194. doi: 10.1016/s0029-7844(00)00883-8. [DOI] [PubMed] [Google Scholar]
- 40.Skentou C, Souka AP, To MS, Liao AW, Nicolaides KH. Prediction of preterm delivery in twins by cervical assessment at 23 weeks. Ultrasound Obstet Gynecol. 2001;17:7. doi: 10.1046/j.1469-0705.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 41.Souka AP, Heath V, Flint S, Sevastopoulou I, Nicolaides KH. Cervical length at 23 weeks in twins in predicting spontaneous preterm delivery. Obstet Gynecol. 1999;94:450. doi: 10.1016/s0029-7844(99)00277-x. [DOI] [PubMed] [Google Scholar]
- 42.Stray-Pedersen B, Bruu AL, Molne K. Infertility and uterine colonization with Ureaplasma urealyticum. Acta Obstet Gynecol Scand. 1982;61:21. doi: 10.3109/00016348209156945. [DOI] [PubMed] [Google Scholar]
- 43.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks’ gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 44.To MS, Skentou C, Cicero S, Liao AW, Nicolaides KH. Cervical length at 23 weeks in triplets: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 2000;16:515. doi: 10.1046/j.1469-0705.2000.00293.x. [DOI] [PubMed] [Google Scholar]
- 45.Tongsong T, Kamprapanth P, Pitaksakorn J. Cervical length in normal pregnancy as measured by transvaginal sonography. Int J Gynaecol Obstet. 1997;58:313. doi: 10.1016/s0020-7292(97)00108-2. [DOI] [PubMed] [Google Scholar]
- 46.Tongsong T, Kamprapanth P, Srisomboon J, Wanapirak C, Piyamongkol W, Sirichotiyakul S. Single transvaginal sonographic measurement of cervical length early in the third trimester as a predictor of preterm delivery. Obstet Gynecol. 1995;86:184. doi: 10.1016/0029-7844(95)00152-h. [DOI] [PubMed] [Google Scholar]
- 47.Vayssiere C, Favre R, Audibert F, Chauvet MP, Gaucherand P, Tardif D, Grange G, Novoa A, Descamps P, Perdu M, Andrini E, Janse-Marec J, Maillard F, Nisand I. Cervical length and funneling at 22 and 27 weeks to predict spontaneous birth before 32 weeks in twin pregnancies: a French prospective multicenter study. Am J Obstet Gynecol. 2002;187:1596. doi: 10.1067/mob.2002.127380. [DOI] [PubMed] [Google Scholar]
- 48.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol. 1992;79:351. doi: 10.1097/00006250-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 49.Yang JH, Kuhlman K, Daly S, Berghella V. Prediction of preterm birth by second trimester cervical sonography in twin pregnancies. Ultrasound Obstet Gynecol. 2000;15:288. doi: 10.1046/j.1469-0705.2000.00087.x. [DOI] [PubMed] [Google Scholar]
- 50.Yankowitz J, Weinter CP, Henderson J, Grant S, Towbin JA. Outcome of low-risk pregnancies with evidence of intraamniotic viral infection detected by PCR on amniotic obtained at second trimester genetic amniocentesis. J Soc Gynecol Investig. 1996;3:132. [Google Scholar]
- 51.Yoon BH, Chang JW, Romero R. Isolation of Ureaplasma urealyticum from the amniotic cavity and adverse outcome in preterm labor. Obstet Gynecol. 1998;92:77. doi: 10.1016/s0029-7844(98)00122-7. [DOI] [PubMed] [Google Scholar]
- 52.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH, Syn HC. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol. 1995;172:960. doi: 10.1016/0002-9378(95)90028-4. [DOI] [PubMed] [Google Scholar]
- 53.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS, Jun JK. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol. 2000;183:1130. doi: 10.1067/mob.2000.109036. [DOI] [PubMed] [Google Scholar]
- 54.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY, Jun JK. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol. 2003;189:919. doi: 10.1067/s0002-9378(03)00839-1. [DOI] [PubMed] [Google Scholar]
- 55.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, Jun JK. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 56.Yoon BH, Romero R, Park JS, Chang JW, Kim YA, Kim JC, Kim KS. Microbial invasion of the amniotic cavity with Ureaplasma urealyticum is associated with a robust host response in fetal, amniotic, and maternal compartments. Am J Obstet Gynecol. 1998;179:1254. doi: 10.1016/s0002-9378(98)70142-5. [DOI] [PubMed] [Google Scholar]


