Abstract
Delayed offline measurement of exhaled nitric oxide (eNO), although useful in environmental and clinical research, is limited by the instability of stored breath samples. The authors characterized sources of instability with the goal of minimizing them. Breath and other air samples were stored under various conditions, and NO levels were measured repeatedly over 1–7 d. Concentration change rates varied positively with temperature and negatively with initial NO level, thus “stable” levels reflected a balance of NO-adding and NO-removing processes. Storage under refrigeration for a standardized period of time can optimize offline eNO measurement, although samples at room temperature are effectively stable for several hours.
Keywords: air samples, breath samples, exhaled nitric oxide, NO
Measurement of exhaled nitric oxide (eNO) by chemiluminescence analysis is valuable for the rapid, noninvasive assessment of airway inflammation and other disease states.1–7 Increases or decreases in NO output from the bronchial passages, or from the alveolar region of the lungs, may have health significance. Routine eNO measurements applicable in environmental health screening cannot distinguish between bronchial and alveolar-region abnormalities; however, that issue can be addressed by measuring eNO over a range of expiratory flow rates.
Standardized eNO measurement methods need refinement because not all interferences are understood.4,5 In screening studies, collection of breath samples for offline measurement is often more practical than direct online measurement and provides similar sensitivity and specificity.8–11 Delayed offline measurements have been employed in small environmental epidemiologic studies12–14 and could be valuable in larger-scale epidemiologic and clinical studies; however, time-dependent physical and chemical processes may either increase or decrease measurable NO concentrations.15,16 Some investigators have reported that breath samples are stable for 24–48 h,4,17,18 but others found that NO concentration increases.8,19 Thus, interferences may differ under different circumstances and should be evaluated for any given collection/analysis protocol. For example, Bodini et al.20 evaluated the stability of breath samples collected in Mylar® balloons, with respect to time and temperature. In their system, NO appeared stable for about 9 h at temperatures of 4–37 °C, but increased between 9 and 48 h in samples with initially low concentrations. Use of a drying agent did not improve stability. We present herein a wider-ranging evaluation of a system intended for eNO sampling in field studies.
Materials and Method
Overall, we collected and stored 185 breath and other air samples under various conditions, and measured NO repeatedly over multiday periods (Table 1). A Bag Collection and Sampling Kit and 1.5–l aluminized Mylar® NO sampling bags (Sievers Instruments Division, Ionics, Inc., Boulder, CO) were used for most of the sample collection. In accordance with manufacturer’s instructions—based on American Thoracic Society recommendations4—subjects inspired maximally through an NO scrubber, then exhaled against resistance at 100 ml/s. Collection was delayed for more than 3 s to discard dead-space air. Bags were reused 10–20 times and flushed 3 times with NO-free air between uses. Differences between new (not flushed) and used bags were tested statistically. Samples were also collected in 46-cm and 23-cm-diameter aluminized Mylar® toy balloons, used only once. Samples were stored at 42, 22, 6, or −14 °C.
Table 1.
Classification of Nitric Oxide (NO) Samples (n = 185)
| Characteristic | Samples (n) |
|---|---|
| Source | |
| NO-free cylinder air (zero air) | 33 |
| Ambient air filtered through sampling kit | 7 |
| Ambient air | 15 |
| Adult volunteers (n = 10) | 72 |
| School-age volunteers (n = 28) | 58* |
| Container | |
| New Sievers breath-sampling bags | 21 |
| Used Sievers breath-sampling bags | 141 |
| Large (46-cm) balloons | 12† |
| Small (23-cm) balloons | 11 |
| Storage | |
| Protected from ambient NO | 12 |
| Temperature | |
| 42 °C | 18 |
| 22 °C | 50 |
| 6 °C | 107 |
| −14 °C | 10‡ |
All school-age volunteers’ samples were in used Sievers bags exposed to ambient NO and stored at 6 °C.
All large balloons were stored at 22 °C exposed to ambient NO.
All samples stored at −14 °C were in used Sievers bags exposed to ambient NO.
At our laboratory, we collected breath repeatedly from nonsmoking adults—2 or more samples per person per session. We also collected breath from students 9–17 yr of age at school and at home, usually 2 samples in 1 testing session per person. As required by the local institutional review board for waiver of informed consent, samples were identified only by a code number which represented a given subject, a health classification as reported by the subject (i.e., healthy, asthma, other cardiorespiratory disease), and the time of collection.
Comparison samples included ambient indoor air, air filtered through the breath sampling kit, and NO-free zero air from a cylinder. Field-collected samples were cooled with Blue Ice packs (Rubbermaid, Wooster, OH) in transit, then stored at 6 °C.
Tests of ambient NO influence were conducted when daily peak ambient concentrations were several times higher than typical breath concentrations, using duplicate samples in similar containers. One sample was exposed to ambient air during storage; the other was protected in a resealable bag containing chemisorbent (Purafil, Inc., Norcross, GA) that captured 85%–95% of ambient NO.
NO was measured with a nitric oxide analyzer system (model 280i, Sievers Instruments, Inc., Boulder, CO), zero- and span-checked within 10 min of each reading. Samples were measured 2 to 3 times per day for 1–7 d, depending on the experiment. Laboratory-collected samples were first measured within 15 min; for field samples, the first measurement was delayed 2–14 h.
Statistical analysis
We evaluated the effects of sample source, initial concentration, container (i.e., breath sampling bag or toy balloon), storage temperature, and exposure to ambient NO by analyzing subsets of related data. Specifically, we summarized each sample result by linear regression of concentration vs. time, with the slope (ppb/d) representing the concentration change rate and the intercept (ppb) representing estimated eNO. We then tested influences of the independent variables (i.e., container, storage) by regression or ANOVA. Commercial statistical programs (BMDP Statistical Software, Cork, Ireland) were used for these analyses.
Results
NO in typical breath samples stored at 22 °C increased with time in approximately linear fashion (Figure 1). Among 38 human subjects, median eNO was 12 ppb (range = 3–85 ppb). The self-described asthmatic and healthy groups (n = 10 and 26, respectively) were not significantly different (p > 0.5 by Kruskal-Wallis ANOVA), perhaps because of the small sample size, asthmatic subjects with well-controlled disease, or self-described healthy subjects with undiagnosed conditions that increased eNO.
Fig. 1.
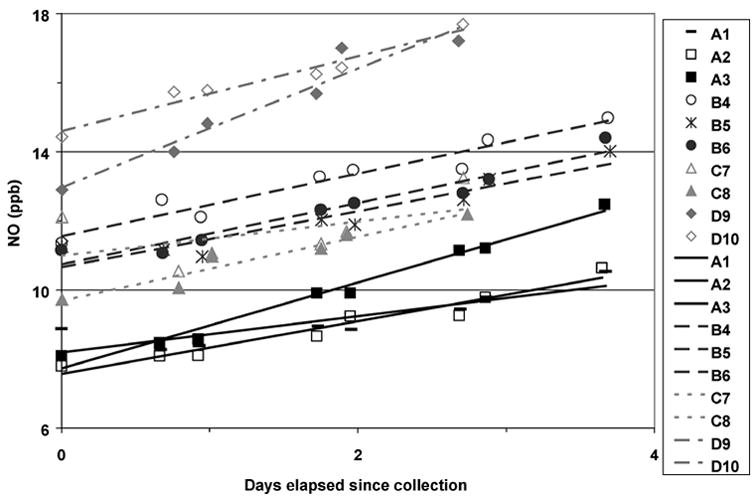
Nitric oxide (NO) concentration changes with time in samples from 3 healthy (A, B, C) and 1 mildly asthmatic (D) adult subjects in 10 different used Sievers bags (numbered 1–10) stored at 22 °C exposed to ambient NO.
Standard deviations of repeated eNO measurements in subjects tested on only 1 d were 0.1 to 4 ppb, with median near 1 ppb. For subjects tested on multiple days, standard deviations were 1–5 ppb, with median near 2 ppb. Thus, variability within subjects was similar to that found previously in online, or immediate offline, measurements.8,21
In 33 samples of zero air, estimated initial NO concentrations ranged from near zero to 3.5 ppb, indicating that some containers added NO or a positive-interfering substance to the sample soon after collection. These estimated initial concentrations differed significantly by container type: new sampling bag vs. used bag vs. balloon. Ambient air samples collected with the breath sampling kit showed essentially the same range (except for 1 high outlier, likely contaminated), confirming that the kit effectively removed ambient NO from inhaled air. In 15 samples of unfiltered ambient air, estimated initial NO concentrations ranged from 2 to 162 ppb. Duplicates agreed within 2% for all samples above 50 ppb, and within 8% for all but 1 sample at lower concentrations.
Analyses of pooled data (Table 2) employed stepwise regression with either initial NO concentration (estimated eNO) or concentration change rate as the dependent variable. The reference condition was zero air in a used Sievers bag at 6 °C unprotected from ambient NO. Candidate predictors included categorical variables to represent alternative temperatures, alternative sources (i.e., individual subjects, ambient air, filtered air), protection from ambient NO, and alternative containers. A continuous variable—duration of measurement—was included to verify that concentration change was consistently linear. If it was not, a significant effect of duration should have been found. Four ambient-air data points well above the highest breath NO concentrations were very influential; therefore, analyses were repeated without them.
Table 2.
Results of Stepwise Regression Analyses to Predict Nitric Oxide (NO) Concentration Change Rate (ppb/d) in Breath Samples: Estimate and Standard Error (SE) for Each Significant Predictor (p < 0.05)
| 160-ppb data excluded
|
160-ppb data included
|
|||
|---|---|---|---|---|
| Predictor | Estimate | SE | Estimate | SE |
| New bag | 0.45 | 0.16 | NS | |
| Large balloon | 0.71 | 0.22 | NS | |
| Storage temperature | ||||
| 42 °C | 2.75 | 0.17 | 2.74 | 0.23 |
| 22 °C | 0.44 | 0.13 | 0.61 | 0.16 |
| −14 °C | NS | −1.32 | 0.31 | |
| Subject’s eNO | ||||
| 29 ppb | −0.61 | 0.20 | NS | |
| 23 ppb | −1.23 | 0.46 | −1.30 | 0.64 |
| 63 ppb | −3.35 | 0.46 | −3.42 | 0.64 |
| 85 ppb | −4.05 | 0.46 | −4.11 | 0.64 |
| 61 ppb | −1.83 | 0.46 | −1.90 | 0.64 |
| 45 ppb | −1.16 | 0.46 | NS | |
| Ambient air | ||||
| 60 ppb NO | −2.70 | 0.38 | −2.77 | 0.46 |
| 160 ppb NO | NA | −20.93 | 0.48 | |
Notes: NS = not significant, NA = not applicable, and eNO = exhaled NO. Intercept (i.e., estimated rate of increase [ppb/d] under reference conditions [zero air, used bag, storage at 6 °C, unprotected]) was 0.24 or 0.31, respectively, with 160 ppb data excluded or included.
Table 2 shows significant predictors of concentration change rate. Intercepts (i.e., expected rates under the reference condition) were near zero. New Sievers bags or large balloons were associated with increases of less than 1 ppb/d, which were significant if data > 160 ppb were excluded. Storage at 22 °C also was associated with small increases, and storage at 42 °C with larger increases approaching 3 ppb/d. Storage at −14 °C had a negative effect. Other significant negative effects were related to sources (either subjects or ambient air) with high NO concentrations. Thus, samples initially low in NO showed increases over time (very slow at 6 °C), whereas samples initially high in NO showed decreases over time. Higher temperatures made the change rate more positive; lower temperatures made it more negative; and new sample containers—not previously flushed with zero air—made the change rate slightly more positive. New small balloons did not show the latter effect. Exposure to ambient NO during storage had no significant effect, confirming that neither bags nor balloons were prone to leakage.
Similar analyses with eNO as the dependent variable (not tabulated) showed significant positive effects of new breath sampling bags (estimate = 1.9 ppb) and of storage at 22 °C (estimate = 1.5 ppb). The effect of storage at 42 °C was not significant.
In contrast to our results, Bodini et al.20 found no consistent effect of temperature on NO concentration. Their finding of increases in samples initially low in NO agrees with ours and with some earlier findings8,10,19; however, observed rates of increase differed. Although Bodini et al. did not comment on it, their high-concentration samples appeared to lose NO over time, as did ours.
We examined low-temperature effects further by comparing duplicate samples stored at 6 °C and −14 °C. As Table 3 indicates, change rates were more negative at −14 °C, and more negative for sources with higher initial concentrations, corroborating the pooled data analysis. Both temperature and source effects were significant by ANOVA.
Table 3.
Means and Standard Errors (SEs) for Nitric Oxide (NO) Concentration Change Rate (ppb/d) in Matched Samples Stored at −14 °C and 6 °C
| Storage at −14 °C
|
Storage at 6 °C
|
|||
|---|---|---|---|---|
| Source | Mean | SE | Mean | SE |
| Subject’s eNO | ||||
| 13 ppb | 0.12 | 0.12 | 0.05 | 0.01 |
| 16 ppb | −0.08 | 0.01 | −0.02 | 0.11 |
| 28 ppb | −0.62 | 0.09 | −0.11 | 0.18 |
| Zero air (initial estimate: 2 ppb NO) | 0.38 | 0.01 | 0.56 | 0.02 |
| Ambient air (initial estimate: 160 ppb NO) | −25.58 | 0.02 | −16.99 | 0.11 |
Note: eNO = exhaled NO.
Figure 2 illustrates the effects of storage temperature for the 146 samples with initial concentrations ≤ 20 ppb. Within that range, the effect of initial NO concentration as a covariate was not significant. Differences associated with temperature were significant (p < 0.001) by ANOVA, and were roughly consistent with temperature effects found in the pooled data analyses. Differences between breath and other air samples were small, but showed a significant (p < 0.001) dependence on temperature, inconsistent in direction.
Fig. 2.
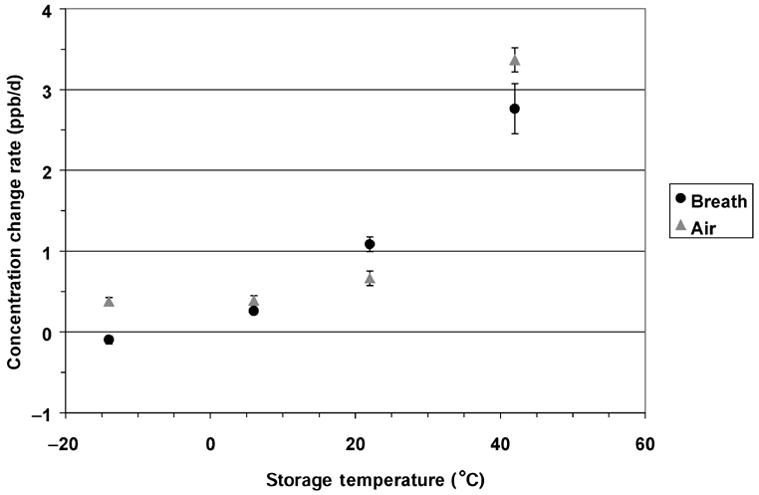
Rates of change in nitric oxide (NO) concentration at 4 storage temperatures, for all samples initially ≤ 20 ppb. Point = mean; flag = standard error.
In Figure 3, rate of change is plotted vs. initial NO concentration for all samples stored at 6 °C. A quadratic regression model fit the data closely. (Although separate regression lines are shown for air and breath samples, their difference was not significant.) Assuming that NO was lost by the reaction 2 NO + O2 = 2 NO2, with O2 roughly 20% of the sample gas, these data yield a rate constant near 4 × 10−11 ppm−2 sec−1. This seems plausible at 6 °C, given that (a) rate constants between 1 and 2 × 10−11 ppm−2 sec−1 have been measured near 20 °C,22,23 and (b) the reaction rate increases as temperature falls.24 However, processes other than the O2 reaction likely influenced NO losses; otherwise, exhaled breath, because of its reduced O2 concentration, should have lost NO more slowly than other air samples. At −14 °C, the rate constant (estimated from relatively few samples) was about 50% larger than at 6 °C. At 22 °C and 42 °C, when reaction with O2 should be slow,22–24 rate of change showed no consistent relationship to initial concentration.
Fig. 3.
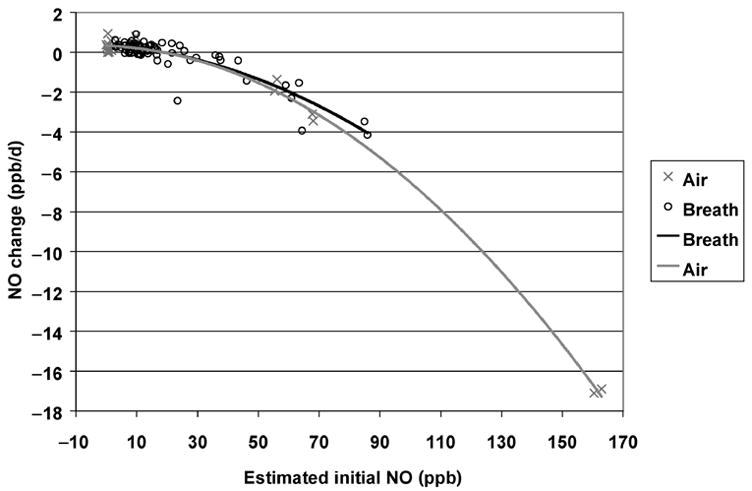
Rates of change in nitric oxide (NO) concentration as a function of initial NO concentration, for all samples stored at 6 °C. Lines indicate best-fit quadratic regressions for breath and other air samples. Difference between breath and other samples was not statistically significant.
Discussion
We demonstrated both positive and negative changes in NO concentration during eNO sample storage, which were partly predictable on the basis of temperature, initial concentration, and expected reaction with O2. Concentration increases were observed in some (not all) previous investigations, but the present study provides, to our knowledge, the first clear evidence of decreases in NO concentrations during storage. Apparently, multiple processes to produce and consume NO and/or interfering substances occur in aluminized Mylar® sample containers.
Our observations of relatively rapid NO increase in samples with low or zero initial concentrations extend the findings of Bodini et al.20 and support their interpretation that aluminized Mylar® containers desorb NO into collected samples at a rate dependent on the concentration gradient between the bulk sample and the interior surface. Prior observations of adsorption and desorption by sampling bags filled with NO at higher (ppm) concentrations19 also support this interpretation. In our sampling and storage methodology, desorption was temperature-dependent and differed by container type, whereas Bodini et al. found no consistent temperature dependence using containers different from ours. The contrasts among these findings—as well as previous inconsistent reports on concentration stability—indicate that surface effects differ in different collection systems. Because gas–gas or gas–particle reactions involving NO may well occur in breath or ambient air, concentrations might not be stable even in ideal sample containers.
Despite these problems, our results reaffirm that delayed offline eNO measurement can be useful for respiratory health evaluation. Variance of eNO estimates was not markedly larger than with online measurement. Concentration changes were slow enough to be neglected in samples kept for several hours at room temperature. If refrigerated at 6 °C, typical breath samples were stable (or concentration gains offset losses) for 48 h or longer. For refrigerated samples with atypically high NO concentrations, losses over time were appreciable but predictable from the aforementioned rate constant. Accordingly, refrigeration of samples after collection, and standardization of the delay time before analysis (e.g., at 24 ± 6 h), should avoid meaningful error in the ranking of individual or group mean eNO levels. Negative errors (relative to immediate measurements) would be appreciable in delayed analyses of samples with high initial concentrations, but could be corrected on the basis of the loss rate constant.
In summary, numerous sources of variability may influence the delayed offline measurement of eNO. Resulting random or systematic errors may differ in magnitude and direction, depending on the characteristics of the specific breath collection/NO analysis system. In the system we describe, gain of NO (probably by desorption) predominated at or above room temperature, but loss (probably by gas-phase reaction) predominated at lower temperatures. Loss was more predictable, so that refrigeration of samples between collection and analysis optimized the precision of NO measurement. In any event, valid comparisons within a group of samples require that they all be stored at similar temperatures for similar time intervals prior to analysis. Our results give confidence that, with appropriate sample handling, useful eNO estimates are obtainable even with 2–3 d delay in analysis. However, given the inconsistencies among prior studies, and the diversity of sampling and NO measurement techniques, any offline measurement system should undergo a careful evaluation of its potential sources of error, over the entire range of conditions under which it may be used.
Acknowledgments
The authors thank Karen R. Anderson, Kenneth W. Clark, Kimberly J. Hudson, and Sheryl L. Terrell for invaluable advice and technical help. They thank staff and volunteer subjects of the USC Children’s Health Study, and staff and family members of the Environmental Health Service, for their participation.
Contributor Information
WILLIAM S. LINN, Environmental Health Service, Los Amigos Research and Education Institute, Downey, California and Department of Preventive Medicine, Keck School of Medicine, University of Southern California, Los Angeles, California
MARISELA AVILA, Environmental Health Service, Los Amigos Research and Education Institute, Downey, California.
HENRY GONG, JR., Environmental Health Service, Los Amigos Research and Education Institute, Downey, California and Departments of Medicine and Preventive, Medicine Keck School of Medicine, University of Southern California, Los Angeles, California
References
- 1.Van Amsterdam JG, Nierkens S, Vos SG, et al. Exhaled nitric oxide: a novel biomarker of adverse respiratory health effects in epidemiological studies. Arch Environ Health. 2000;55:418–23. doi: 10.1080/00039890009604040. [DOI] [PubMed] [Google Scholar]
- 2.Kharitonov SA, Barnes PJ. Clinical aspects of exhaled nitric oxide. Eur Respir J. 2000;16:781–92. doi: 10.1183/09031936.00.16478100. [DOI] [PubMed] [Google Scholar]
- 3.Kharitonov SA, Barnes PJ. State of the art: exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–722. doi: 10.1164/ajrccm.163.7.2009041. [DOI] [PubMed] [Google Scholar]
- 4.American Thoracic Society. Recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide in adults and children—1999. Am J Respir Crit Care Med. 1999;160:2104–17. doi: 10.1164/ajrccm.160.6.ats8-99. [DOI] [PubMed] [Google Scholar]
- 5.Baraldi E, de Jongste JC. Measurement of exhaled nitric oxide in children, 2001. Eur Respir J. 2002;20:223–37. doi: 10.1183/09031936.02.00293102. [DOI] [PubMed] [Google Scholar]
- 6.Payne DN. Nitric oxide in allergic airway inflammation. Curr Opin Allergy Clin Immunol. 2003;3:133–7. doi: 10.1097/00130832-200304000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Ricciardolo FLM. Multiple roles of nitric oxide in the airways. Thorax. 2003;58:175–82. doi: 10.1136/thorax.58.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Silkoff PE, Stevens A, Pak J, et al. A method for the standardized offline collection of exhaled nitric oxide. Chest. 1999;116:754–9. doi: 10.1378/chest.116.3.754. [DOI] [PubMed] [Google Scholar]
- 9.Canady RG, Platts-Mills T, Murphy A, et al. Vital capacity reservoir and online measurement of childhood nitrosopnea are linearly related: clinical implications. Am J Respir Crit Care Med. 1999;159:311–4. doi: 10.1164/ajrccm.159.1.9803034. [DOI] [PubMed] [Google Scholar]
- 10.Barreto M, Villa MP, Martella S, et al. Offline exhaled nitric oxide measurements in children. Pediatr Pulmonol. 2001;32:159–67. doi: 10.1002/ppul.1102. [DOI] [PubMed] [Google Scholar]
- 11.Deykin A, Massaro AF, Drazen JM, et al. Exhaled nitric oxide as a diagnostic test for asthma. Am J Respir Crit Care Med. 2002;165:1597–1601. doi: 10.1164/rccm.2201081. [DOI] [PubMed] [Google Scholar]
- 12.Van Amsterdam JG, Verlaan BP, Van Loveren H, et al. Air pollution is associated with increased level of exhaled nitric oxide in nonsmoking healthy subjects. Arch Environ Health. 1999;54:331–5. doi: 10.1080/00039899909602496. [DOI] [PubMed] [Google Scholar]
- 13.Steerenberg PA, Nierkens S, Fischer PH, et al. Traffic-related air pollution affects peak expiratory flow, exhaled nitric oxide, and inflammatory nasal markers. Arch Environ Health. 2001;56:167–74. doi: 10.1080/00039890109604069. [DOI] [PubMed] [Google Scholar]
- 14.Koenig JQ, Jansen K, Mar TF, et al. Exhaled nitric oxide is associated with outdoor, indoor, and personal PM2.5 in children with asthma in a panel study. Am J Respir Crit Care Med. 2003;167:A34. [Google Scholar]
- 15.Van der Mark TW, Kort E, Meijer RJ, et al. Water vapour and carbon dioxide decrease nitric oxide readings. Eur Respir J. 1997;10:2120–3. doi: 10.1183/09031936.97.10092120. [DOI] [PubMed] [Google Scholar]
- 16.Binding N, Muller W, Czeschinski PA, et al. NO chemiluminescence in exhaled air: interference of compounds from endogenous or exogenous sources. Eur Respir J. 2000;16:499–503. doi: 10.1034/j.1399-3003.2000.016003499.x. [DOI] [PubMed] [Google Scholar]
- 17.Paredi P, Loukides S, Ward S, et al. Exhalation flow and pressure controlled reservoir collection of exhaled nitric oxide for remote and delayed analysis. Thorax. 1998;53:775–9. doi: 10.1136/thx.53.9.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Djupesland PG, Qian W, Haight JS. A new method for the remote collection of nasal and exhaled nitric oxide. Chest. 2001;120:1645–50. doi: 10.1378/chest.120.5.1645. [DOI] [PubMed] [Google Scholar]
- 19.Sievers Instruments, Inc. Nitric Oxide Analyzer NOA 280i Operation and Maintenance Manual. Boulder, CO: Sievers Instruments Division, Ionics, Inc; 2000. pp. 8.1–8.16. [Google Scholar]
- 20.Bodini A, Pijnenburg MW, Boner AL, et al. Exhaled nitric oxide in Mylar balloons: influence of storage time, humidity and temperature. Mediators Inflamm. 2003;12:47–9. doi: 10.1080/0962935031000096971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kharitonov SA, Gonio F, Kelly C, et al. Reproducibility of exhaled nitric oxide measurements in healthy and asthmatic adults and children. Eur Respir J. 2003;21:433–8. doi: 10.1183/09031936.03.00066903a. [DOI] [PubMed] [Google Scholar]
- 22.Nishimura M, Hess D, Kacmarek RM, et al. Nitrogen dioxide production during mechanical ventilation with nitric oxide in adults. Anaesthesiology. 1995;82:1246–54. doi: 10.1097/00000542-199505000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Sokol GM, van Meurs KP, Wright LL, et al. Nitrogen dioxide formation during nitric oxide therapy. Clin Chem. 1999;45:382–7. [PubMed] [Google Scholar]
- 24.Atkins PW. Physical Chemistry. 4. Oxford, UK: Oxford University Press; 1990. p. 802. [Google Scholar]


