Abstract
The hydrophobic patch of cyclins interacts with cyclin-dependent kinase (Cdk) substrates and p27-type Cdk inhibitors. Although this interaction is assumed to contribute to the specificity of different Cdk–Cyclin complexes, its role in specific steps of the cell cycle has not been demonstrated. Here, we show that in Drosophila the mitotic inhibitor Frühstart (Frs) binds specifically and with high affinity to the hydrophobic patch of cyclins. In contrast to p27-type Cdk inhibitors, Frs does not form a stable interaction with the catalytic centre of Cdk and allows phosphorylation of generic model substrates, such as histone H1. Consistent with a 2.5 times stronger binding to CycA than to CycE in vitro, ectopic expression of frs induces endocycles, in a manner similar to that reported previously for downregulation of CycA or Cdk1. We propose that binding of Frs to cyclins blocks the hydrophobic patch to interfere with Cdk1 substrate recognition.
Keywords: Drosophila, cell-cycle inhibitor, hydrophobic patch, cyclin, blastoderm
Introduction
The activity of cyclin-dependent kinase (Cdk) is controlled by several mechanisms. Inhibitors of the p14 family bind to Cdk6, leading to disruption of the Cdk6–cyclin (Cyc)D complex, whereas inhibitors of the p27 family form a stable association with the G1/S-phase-specific Cdk2–Cyclin complex by binding to the hydrophobic patch of cyclin and also to the catalytic centre of the kinase subunit (Pavletich, 1999). Common to G1/S- and G2/M-specific cyclins is the hydrophobic patch that acts as a binding site for some Cdk substrates and also for p27-type inhibitors (Zhu et al, 1995; Adams et al, 1996; Chen et al, 1996; Brown et al, 1999). The K/RxL motif (called KxL in the following) of substrates and p27-type inhibitors binds to a hydrophobic patch on the cyclin surface. Although this interaction has been studied in great detail (Adams et al, 1999; Lowe et al, 2002; Lacy et al, 2004), the role of the hydrophobic patch in substrate specificity of various Cdk–Cyclin complexes, and to which degree it is required for substrate phosphorylation (Wittenberg, 2005), remains to be established. It is also unknown whether the hydrophobic patch is essential for specific steps of the cell cycle.
The problem of specificity is best exemplified in budding yeast, which has only one Cdk, CDC28. By association with cyclins specific for G1 or G2, differential groups of substrates are assumed to be phosphorylated. By analysing the phosphorylation of a library of Cdk substrates in vitro, Loog & Morgan (2005) found that the G2/M-specific Cdk1–Clb2 (Clb2 for Cyclin B-like 2) phosphorylated all substrates equally well, whereas the S-phase-specific Cdk1–Clb5 showed preference for a small subset of substrates, but poorly phosphorylated most substrates. Efficient phosphorylation of these S-phase specific substrates depended on the hydrophobic patch of Clb5; therefore, it was proposed that the hydrophobic patch would contribute to the correct selection of S-phase-specific substrates.
The new protein Frühstart (Frs) and the pseudo-kinase Tribbles (Trbl) are specific cell-cycle regulators that are active in certain cell types and developmental stages (Lee & Orr-Weaver, 2003). Frs delays entry into mitosis in the mesoderm anlage during invagination and is involved in turning off the rapid nuclear division cycles after 13 rounds during the blastoderm stage (Seher & Leptin, 2000; Großhans & Wieschaus, 2000; Großhans et al, 2003). As no obvious motifs are present in the Frs primary structure, the molecular mechanism of cell-cycle inhibition by Frs is currently unknown.
Here, we show that frs inhibits mitotic entry but not G1/S phase. Consistent with the physiological function, preferential binding of Frs to CycA compared with CycE is shown. Complex formation requires the KxL motif of Frs and the hydrophobic patch of cyclins. But, in contrast to p27-type inhibitors, Frs does not associate with the kinase subunit.
Results
frs inhibits entry into mitosis but not S phase
The precocious mitosis during ventral furrow formation in frs mutant embryos clearly shows the function of frs in the G2/M transition (Großhans & Wieschaus, 2000; Seher & Leptin, 2000). To test whether frs also inhibits G1-specific Cdk, we expressed frs in a striped pattern during mid-embryogenesis. This ectopic expression inhibited the last zygotic cell cycle, cycle 16, as shown by the halved cell density (Fig 1A). The reduced number of cells was not due to inhibition of S phase, as 5-bromodeoxyuridine (BrdU) was incorporated in S phase 16 in those embryos (Fig 1B,C). In contrast to frs expression, similar expression of dacapo, the p27 homologue as reported by Lane et al (1996), inhibits S phase, as measured by BrdU incorporation. These experiments show that frs affects cell-cycle progression, but not by inhibiting the G1/S transition.
Figure 1.
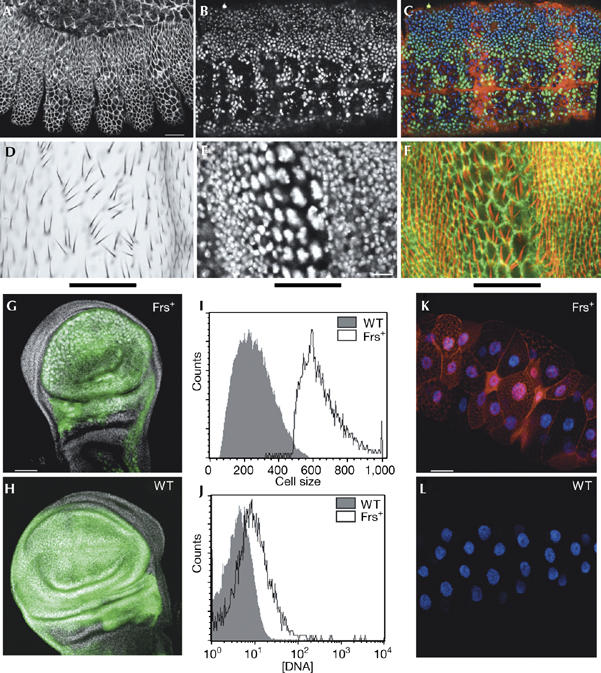
frs inhibits M phase but not S phase. (A–C) Genotype: pairedGal4/UASfrs[H]. (A) Cell number in the epidermal segments with frs expression was half that of neighbouring segments (38±3 compared with 20±3 cells per 34 μm2, n=10, stained for discs-large (Dlg). Scale bar, 20 μm. (B,C) Progression through S phase was marked by BrdU incorporation (25 min pulse). (B) BrdU, white. (C) BrdU, green; Frs, red; DNA, blue. (D–F) frs expressed in a stripe (indicated by the black bar) in pupal wing imaginal discs. Genotype: patchedGal4/UASfrs[J]. (D) Section of a differentiated wing. (E,F) Pupal discs (phalloidin, green; cell borders, red; higher optical section showing the trichomes and DAPI, white). Scale bar, 20 μm. (G–J) frs expressed in wing discs. Genotypes: (G) MS1096Gal4/+; UASfrs[C]/UASGFP, and (H) MS1096Gal4/+; UASGFP/+. The expression domain is indicated by GFP expression (green) and DNA (white). Scale bar, 50 μm. Cells were sorted by GFP expression and cell size, and analysed according to DNA content. (I) Cells from wild-type discs and size-selected population of large cells from the frs-expressing discs. (J) DNA content of the cell populations shown in (I). (K) frs expressed in third instar salivary glands in comparison with wild-type glands (L; DNA, white; Frs, red). Genotypes: (K) patchedGal4/UASfrs[C], (L) patchedGal4/UASGFP. Scale bar, 50 μm. BrdU, 5-bromodeoxyuridine; DAPI, 4′,6′-diamidino-2-phenylindol; Frs, Frühstart; GFP, green fluorescent protein; UAS, upstream activating sequence.
To assess the activity of frs in cells with a full cell cycle of G1 and G2 phases, we used imaginal discs of Drosophila larvae. Expression of frs in the wing imaginal discs induced morphological defects in pupal discs and adult wings (Fig 1D–F), which are consistent with inhibition of the mitotic Cdk1 (Sauer et al, 1995; Hayashi, 1996; Weigmann et al, 1997; Vidwans et al, 2002). The cells of wing imaginal discs were much larger and had bigger nuclei with strong DNA staining (Fig 1E–H), as confirmed by fluorescence-activated cell sorting analysis (Fig 1I,J). This phenotype produced groups of wing hairs with disturbed polarity in the differentiated wings of adults (Fig 1D,F). The higher DNA content suggests that frs induced endocycles. Conversely, expression of frs during endocycles in the larval salivary glands (Fig 1K,L) did not prevent the growth of the tissue, suggesting that ectopic expression of frs at the level applied did not stop normally occurring endocycles; however we, do not exclude a weak interference with Cdk2-controlled endocycles. Endocycles and similar morphological defects are also induced by downregulation of Cdk1 or CycA in Drosophila and mitotic Cdk in yeast (Moreno & Nurse, 1994; Sauer et al, 1995; Hayashi, 1996; Weigmann et al, 1997; Vidwans et al, 2002). The frs loss-of-function and gain-of-function phenotypes show preferential inhibition of the G2/M transition and thus specificity for the mitotic Cdk1 complexes.
Entry into mitosis during the zygotically controlled cell cycles is triggered by string (Drosophila melanogaster CDC25). To test whether frs interferes with the activation of Cdk1 by string, we coexpressed an allele of Cdk1 (Cdk1AF, ubiquitously induced by heat shock) that does not require dephosphorylation by string to be active with frs (in stripes with pairedGal4; Fig 2A–C). Almost no mitotic chromatin was detected in regions of the embryo with frs expression, whereas between the stripes of frs expression many mitotic chromosomes, which synchronously entered mitosis, were observed. Control embryos of the same stage with no Cdk1AF expression, showed fewer mitotic nuclei. Furthermore, we tested genetic interactions of frs and string in wings. As described before, ectopic expression of frs, as well as trbl, induces endocycles in wing imaginal discs. The resulting phenotype is not significantly altered by increasing or decreasing the expression of string, whereas overexpression of CycE suppresses the phenotype. By contrast, the phenotype caused by expression of trbl is modified by string but not by CycE. These experiments indicate that frs inhibits Cdk1 by a method other than interfering with activation by string.
Figure 2.
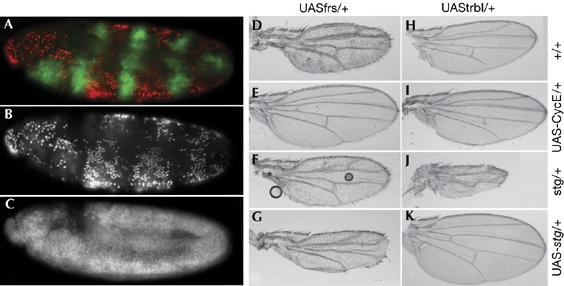
Frs inhibits the constitutively active Cdk1-AF allele and does not genetically interact with string. (A–C) Embryo expressing Cdk1-14A15F (uniformly by heat shock) and frs (UASfrs[H], in stripes with the pairedGal4) were stained for Frs (A, green), mitotic nuclei (pH3-S10; (A) red, (B) white and DNA (C, white). Expression of Cdk1-AF induces premature mitoses that are inhibited in the regions with Frs expression. (D–K) Wings of flies in which frs (D–G), trbl (H–K), CycE (E,I) or string (stg; G,K) was expressed with a Gal4 line specific for the wing blade (MS1096) or that was heterozygotes for string (F,J). Genetic interactions can be observed by the improved or worsened morphology. Cdk, cyclin-dependent kinase; CycE, cyclin E; Frs, Frühstart; trbl, tribbles; UAS, upstream activating sequence.
Frs associates with cyclins
To investigate the molecular mechanism of how Frs inhibits mitotic Cdk1 complexes, we identified interactors of Frs both biochemically and using a yeast two-hybrid screen. We isolated components of the nuclear transport machinery and the nuclear pore (supplementary Fig S1 online), which suggested that Frs shuttles between the nucleus and the cytoplasm. Furthermore, we isolated two independent clones of CycE with the yeast two-hybrid system (supplementary Fig S1 online). We confirmed the formation of Frs–Cyclin complexes in vitro. Beads loaded with GSTfrs (GST for glutathione-S-transferase) specifically associated with 35S-labelled CycA, CycB, CycB3 and CycE (Fig 3A). By contrast, we did not detect any binding of GSTfrs to Cdk1 (Fig 3A). Next, we concentrated on CycA as it is required—in contrast to CycB and CycB3—for mitotic entry in Drosophila (Lee & Orr-Weaver, 2003). The Frs–CycA–Cdk1 complex could be immunoprecipitated with CycA and Cdk1 antibodies from embryos (Fig 3B). By contrast, CycE immunoprecipitates did not contain detectable amounts of Frs, which suggests that Frs specifically associates with mitotic Cdk complexes in vivo.
Figure 3.
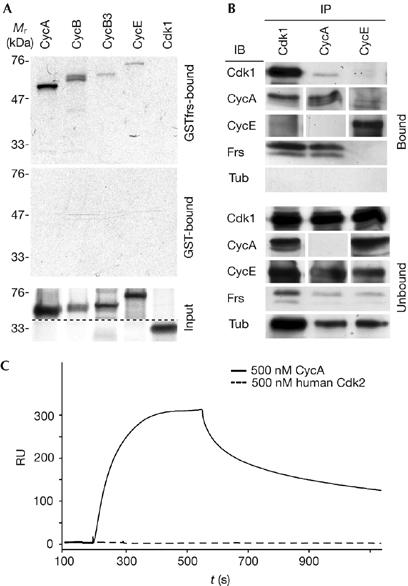
Frs binds to cyclins with high affinity. (A) Frs binds cyclins but not Cdk1 in vitro. 35S-labelled cyclins or Cdk1 were incubated with beads loaded with GSTfrs or GST. Bound (50%) and unbound (100%) fractions were analysed by SDS–PAGE and autoradiography. (B) Frs associates with the CycA–Cdk1 complex in vivo. Cdk1, CycA or CycE immunoprecipitates obtained from lysed embryos (stage 5) were analysed by western blotting with the indicated antibodies. To achieve higher loading for CycA and CycE, a second immunoprecipitation was performed with the unbound fraction with CycA and CycE antibodies. Loading per slot in embryo equivalents (bound/unbound): Cdk1 (50/50), CycA (2,000/2,000), CycE (2,000/2,000), Frs (2,000/50), tubulin (10/10). (C) Quantitative analysis of complex formation with immobilized Frs (H10ZZ-frs) by surface plasmon resonance with human Cdk2 (500 nM) and CycAΔN170 (500 nM). Cdk, cyclin-dependent kinase; Cyc, cyclin; Frs, Frühstart; GST, glutathione-S-transferase; IB, immunoblottting; IP, immunoprecipitation; RU, response units; SDS–PAGE, SDS–polyacrylamide gel electrophoresis.
To quantify complex formation and to assess the extent to which Frs binds preferentially to mitotic cyclins, we measured the kinetic parameters of complex formation with amino-terminally truncated cyclins by surface plasmon resonance (SPR; Fig 3C; Table 1; supplementary Fig S2 online). We detected binding of CycAΔN170 to immobilized Frs with a nanomolar binding constant (KD=38 nM; Fig 3C; Table 1), whereas the interaction of human (h) Cdk2 and Frs could not be measured (KD>1 μM; Fig 3C). In comparison, as determined by isothermic calorimetry, the cyclin-dependent kinase inhibitor p27 bound to both hCycA and hCdk2 with nanomolar binding constants (KD(hCycA)=25 nM and KD(hCdk2)=70 nM; Lacy et al, 2004). These and our data show that Frs binds as tightly as p27 to CycA, indicating that Frs is able to block the binding site of cyclins. In contrast to p27, Frs does not stably bind to the kinase subunit. Furthermore, binding of Frs to the Cdk–CycAΔN170 complex was no stronger than its binding to isolated CycAΔN170 (Table 1; supplementary Fig S2 online). To address the cell-cycle specificity of Frs, we determined the binding constant of Frs to the G1/S cyclin CycEΔN300. We measured a binding constant of KD=96 nM (Fig 3C; Table 1), showing that Frs preferentially binds to CycA compared with CycE, and thus showing a molecular specificity of Frs; however, this is not absolute. Both binding constants are in the range of the estimated concentration of Frs (c≈100 nM; supplementary Fig S2 online) during mid-cellularization, when frs expression reaches its highest values (Großhans et al, 2003).
Table 1.
Frühstart binds with high affinity to cyclins
| Protein | koff (× 10−3 s−1) | s.d. | kon (× 104 M−1 s−1) | s.d. | Kd (nM) | s.d. |
|---|---|---|---|---|---|---|
| CycAΔN170 | 2.80 | 0.01 | 7.30 | 0.03 | 38 | 0.2 |
| CycEΔN300 | 6.78 | 0.05 | 7.04 | 0.09 | 96 | 1.4 |
| human Cdk2-CycAΔN170 | 4.39 | 0.04 | 4.00 | 0.03 | 100 | 1.0 |
s.d. standard deviation.
The KxL motif of Frs is required for mitotic inhibiton
To improve analysis of the interaction, we mapped the important residues of Frs and cyclin that are required for the interaction. In an unbiased approach, we screened a library of random frs mutants for clones that had lost the ability to bind to CycE but not to Nup50 (Nuclear pore protein 50), in the yeast two-hybrid system (Fig 4A). The isolated clones either encoded carboxy-terminal truncations or contained mutations in one of the last six codons or the stop codon (Fig 4A), indicating that the C-terminal part of Frs is required for binding to CycE. Close examination of the C-terminal sequence showed a KxL motif including a phenylalanyl residue at position+2 and a basic residue at position −3 (Fig 4B; supplementary Fig S2 online). This motif has been found in the cyclin-dependent kinase inhibitor and Cdk substrates and has been thoroughly analysed (Fig 4B; Moreno & Nurse, 1994; Zhu et al, 1995; Adams et al, 1996; Chen et al, 1996; Sanchez-Diaz et al, 1998; Brown et al, 1999; Foley et al, 1999; Lowe et al, 2002). Substituting the basic and hydrophobic residues of the KxL motif with alanine (Frs86ASA) severely affected the Frs–Cyclin complex formation in the two-hybrid assay (Fig 4C), as well as in the in vitro binding assay (Fig 4D). Furthermore, a peptide comprising the C-terminal residues of Frs with the KxL motif interfered with Frs–CycA association, although competition was effective only at high concentrations (Fig 4C). To confirm that Frs binds to the hydrophobic patch, three hydrophobic amino-acid residues of CycA were mutated to alanine (Schulman et al, 1998). This mutation did not interfere with complex formation of CycA with hCdk2 but compromised binding to Frs (Fig 4D). In conclusion, we were able to identify a KxL motif at the C terminus of Frs that is required for binding to the hydrophobic patch of CycA.
Figure 4.

The carboxy-terminal KxL motif of Frs binds to the hydrophobic patch of Cyclin A. (A) Interaction mutants of Frs. Schematic structure of Frs showing the position of the predicted nuclear export signal (NES), the Cdk1 phosphorylation sites (TP), the predicted nuclear localization signal (NLS) and the cyclin binding site (KxL). The positions of multiple mutations in clones defective in Nup50 or CycE interaction are shown by black tick marks. New C-terminal sequences caused by frameshift mutations are shown in red. Regions with clustered mutations are marked in red and blue text. (B) Alanyl substitutions in the KxL motif and putative NES do not interact with CycE or Nup50, as assayed by the two-hybrid system in yeast. Blue staining of the lacZ reporter indicates interaction. (C) A peptide with the 15 C-terminal residues of Frs interferes with binding of 35S-labelled CycA to GSTfrs. Quantification of the autoradiograph is indicated. Note that the peptide concentrations are much higher than the binding constant of full-length Frs. Residues of Frs outside the C-terminal portion might contribute to complex formation. (D) The hydrophobic patch of CycA is required for Frs binding. 35S-labelled CycA, Cdk1 or CycA-AAA (three residues of the hydrophobic patch mutated to alanine) were incubated with beads loaded with GSTfrs, GSTfrs86ASA or H10ZZhuCdk2. Bound (50%) and unbound (100%) fractions were analysed by SDS–PAGE and autoradiography. (E) Frs inhibits CDK1 phosphorylation. Cdk immunoprecipitates from embryonic lysates were incubated with 3 μM of the indicated substrate (lamin, histone H1 and Rb) and the indicated concentrations of GST, GSTfrs or GSTfrs86ASA in kinase buffer containing [32P]ATP. The reaction products were analysed by SDS–PAGE and autoradiography. Relative quantification of [32P] incorporation is indicated below the respective bands. Km values are approximately 1 μM (GSTfrs), 10 μM (GSTfrsASA), 2 μM (histone H1) and 49 μM (lamin; Stuurman, 1997). Cdk, cyclin-dependent kinase; Cyc, cyclin; Frs, Frühstart; GST, glutathione-S-transferase; Nup50, Nuclear pore protein 50; SDS–PAGE, SDS–polyacrylamide gel electrophoresis.
To test whether binding to cyclin is essential for frs function in vivo, we introduced the mutated KxL motif into a genomic rescue construct and tested its ability to complement the ventral furrow phenotype of frs (Großhans et al, 2003). We found that mutations in the putative nuclear export signal (frs11AxxA) did not affect frs function, whereas Frs with a mutated KxL motif was not active (Table 2; supplementary Fig S3 online). These data indicate that frs requires binding to the hydrophobic patch of cyclins in vivo.
Table 2.
The KxL motif is required for frs function
| Genotype of male | Normal ventral furrow | Open ventral furrow | Penetrance*(%) |
|---|---|---|---|
| Df frs/TM3 | 100 | 78 | 88 |
| frs+; Df frs/TM3 | 120 | 20 | 28 |
| frs86ASA; Df frs/TM3 | 59 | 53 | 94 |
| frs11AxxA; Df frs/TM3 | 121 | 19 | 27 |
| frs22A48A; Df frs/TM3 | 55 | 25 | 62 |
*As 50% of the embryos were marked with the reporter gene in a cross with compound females, only 50% of the unmarked embryos that were scored have the expected genotype; the penetrance number takes this into account; Df, deficiency chromosome; Frs, Frühstart; TM, third multiple 3 balancer chromosome.
Frs inhibits Cdk kinase activity in vitro
Frs does not stably bind to the kinase subunit; therefore, we investigated whether Frs would interfere with Cdk kinase activity. In an in vitro kinase assay, we compared the effect of Frs on the phosphorylation of model substrates (histone H1 (calf), Rb (human) and LaminDmO (Drosophila)) by Cdk1 obtained by immunoprecipitation from embryonic lysates (Fig 4E; supplementary Fig S4 online). We found that the Cdk1 and Cdk2 kinase activities were inhibited by increasing concentrations of GSTfrs. However, significant inhibition was observed only at concentrations of Frs that were more than 10 times higher than the binding constant of Frs to CycA. As Frs is itself a Cdk1 substrate, inhibition might be caused by substrate competition (supplementary Figs S4,S5 online). We tested whether phosphorylation of Frs is important for its function. In the cyclin binding assay, we did not observe a differential behaviour of pre-phosphorylated Frs (supplementary Fig S5 online). Furthermore, a transgene of frs, with mutated phosphorylation sites, at least in part complemented the frs mutant phenotype (Table 2; supplementary Fig S3 online), indicating that phosphorylation of Frs by Cdk1 does not have an important role.
Discussion
In summary, Frs binds specifically and tightly to the hydrophobic patch of cyclins. Although Frs does not inhibit model substrate phosphorylation by Cdk complexes at nanomolar concentrations in vitro, it specifically inhibits mitotic Cdk1–Cyclin function in vivo. The question is how Frs functions. Cdk complexes can shuttle between the cytoplasm and the nucleus during interphase and remain in the nucleus early in prophase. In principle, blocking of the hydrophobic patch might affect the nuclear accumulation of Cdk during prophase or might affect the interaction of Cdk1 with other regulators, such as String/Twine or Wee1/Myt1 (Myt for membrane-associated tyrosine and threonine specific cdc2 inhibitory protein kinase). Frs might interfere with Cdk1 activation by the phosphatase String; however, this model is less likely as frs inhibits the Cdk1-AF allele that cannot be inhibited by phosphorylation.
Alternatively, the hydrophobic patch might be important for substrate specificity of mitotic Cdks. The hydrophobic patch would not be essential for the recognition of our model substrates, but only for the—yet unknown—subset of mitotic Cdk1 substrates that triggers mitosis. Such a model is consistent with the situation in yeast, in which the phosphorylation of many substrates might not depend on the hydrophobic patch of the cyclins Clb2 and Clb5. Only a subset of the Clb5 substrates requires the hydrophobic patch for efficient phosphorylation (Loog & Morgan, 2005). In contrast to Drosophila, in which blocking of the hydrophobic patch by Frs inhibits M phase, the hydrophobic patch in yeast seems to be essential for efficient selection of S-phase-specific substrates. Although the mitotic inhibitors substrate inhibitor of Cdk (Sic1), replication uncoupled from mitosis 1 (Rum1) and roughex (Rux) are not structural homologues of Frs (Moreno & Nurse, 1994; Sanchez-Diaz et al, 1998; Foley et al, 1999), they might share the mechanism of Cdk inhibition, in that they might also block the hydrophobic patch of mitotic cyclins to prevent efficient phosphorylation of some Cdk substrates.
Independent of the actual mechanism of Cdk1 inhibition, our study suggests that in contrast to yeast, in which the hydrophobic patch seems to be essential for efficient selection of S-phase-specific but not M-phase-specific substrates, in Drosophila, the hydrophobic patch might be important for controlling entry into mitosis. Identification of the Cdk1 substrates by proteomic studies will allow a more systematic investigation of the role of the hydrophobic patch in cyclin-specific substrate selection.
Methods
The experimental procedures and materials are described in detail in the supplementary information online.
The experiments with flies were performed by using standard protocols. The frs complementation test is described by Großhans et al (2003). Frs-interacting clones were isolated from a ovarian complementary DNA library with lex-frs, LEU2 and lacZ reporter genes (Großhans et al, 1999). Frs interaction mutants were isolated from a library produced by error-prone PCR by screening with Nup50 and CycE preys.
Purified GST proteins were cleaved using thrombin and further purified by gel filtration. The H10ZZhuCdk2 protein was expressed from a bicistronic plasmid with Cdk activating protein kinase (CAK) kinase. Binding tests were performed with 35S-labelled proteins and GST fusion proteins on glutathione–Sepharose, and analysed by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and phospho-imaging. The peptide corresponding to the C-terminal residues of Frs was YEADKNFIKARKSLNF. Immunoprecipitates with protein A–Sepharose were subjected to western blotting or kinase assays. Kinase assays were performed with equivalents of 50 embryos for 10 min at 25°C, if not otherwise noted. The sample was analysed by SDS–PAGE, and signal was detected and quantified by a phospho-imager.
For SPR, H10ZZfrs was immobilized on the flow cells of a CM5 sensor chip (Biacore, Freiburg, Germany). The rate constants were calculated by fitting to 1:1 Langmuir binding model.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
Supplemental Data
Acknowledgments
We thank D. Görlich, K. Kapp, M. Seedorf and the members of the laboratory for discussions, advice and suggestions on the manuscript. We received materials from D. Görlich, N. Johnson, C. Lehner, F. Sprenger, the Drosophila stock centre in Bloomington, USA and the Hybridoma Centre in Iowa, USA. This study was supported by the Emmy Noether-Programm of the Deutsche Forschungsgemeinschaft (J.G.).
References
- Adams PD, Sellers WR, Sharma SK, Wu AD, Nalin CM, Kaelin WGJ (1996) Identification of a cyclin–cdk2 recognition motif present in substrates and p21-like cyclin-dependent kinase inhibitors. Mol Cell Biol 1: 6623–6633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams PD, Li X, Sellers WR, Baker KB, Leng X, Harper JW, Taya Y, Kaelin WGJ (1999) Retinoblastoma protein contains a C-terminal motif that targets it for phosphorylation by cyclin–cdk complexes. Mol Cell Biol 19: 1068–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NR, Noble ME, Endicott JA, Johnson LN (1999) The structural basis for specificity of substrate and recruitment peptides for cyclin-dependent kinases. Nat Cell Biol 1: 438–443 [DOI] [PubMed] [Google Scholar]
- Chen J, Saha P, Kornbluth S, Dynlacht BD, Dutta A (1996) Cyclin-binding motifs are essential for the function of p21CIP1. Mol Cell Biol 16: 4673–4682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley E, O'Farrell PH, Sprenger F (1999) Rux is a cyclin-dependent kinase inhibitor (CKI) specific for mitotic cyclin–Cdk complexes. Curr Biol 9: 1392–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Großhans J, Wieschaus E (2000) A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell 101: 523–531 [DOI] [PubMed] [Google Scholar]
- Großhans J, Schnorrer F, Nüsslein-Volhard C (1999) Oligomerisation of Tube and Pelle leads to nuclear localisation of Dorsal. Mech Dev 81: 127–138 [DOI] [PubMed] [Google Scholar]
- Großhans J, Müller A, Wieschaus E (2003) Control of cleavage cycles in Drosophila embryos by frühstart. Dev Cell 5: 285–294 [DOI] [PubMed] [Google Scholar]
- Hayashi S (1996) A Cdc2 dependent checkpoint maintains diploidy in Drosophila. Development 122: 1051–1058 [DOI] [PubMed] [Google Scholar]
- Lacy ER, Filippov I, Lewis WS, Otieno S, Xiao L, Weiss S, Hengst L, Kriwacki RW (2004) p27 binds cyclin–CDK complexes through a sequential mechanism involving binding-induced protein folding. Nat Struct Mol Biol 11: 358–364 [DOI] [PubMed] [Google Scholar]
- Lane ME, Sauer K, Wallace K, Jan YN, Lehner CF, Vaessin H (1996) Dacapo, a cyclin-dependent kinase inhibitor, stops cell proliferation during Drosophila development. Cell 87: 1225–1235 [DOI] [PubMed] [Google Scholar]
- Lee LA, Orr-Weaver TL (2003) Regulation of cell cycles in Drosophila development: intrinsic and extrinsic cues. Annu Rev Genet 37: 545–578 [DOI] [PubMed] [Google Scholar]
- Loog M, Morgan DO (2005) Cyclin specificity in the phosphorylation of cyclin-dependent kinase substrates. Nature 434: 104–108 [DOI] [PubMed] [Google Scholar]
- Lowe ED, Tews I, Cheng KY, Brown NR, Gul S, Noble ME, Gamblin SJ, Johnson LN (2002) Specificity determinants of recruitment peptides bound to phospo-CDK2/cyclin A. Biochemistry 41: 15625–15634 [DOI] [PubMed] [Google Scholar]
- Moreno S, Nurse P (1994) Regulation of progression through the G1 phase of the cell cycle by the rum1+ gene. Nature 367: 219–220 [DOI] [PubMed] [Google Scholar]
- Pavletich NP (1999) Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators, and Cip and INK4 inhibitors. J Mol Biol 287: 821–828 [DOI] [PubMed] [Google Scholar]
- Sanchez-Diaz A, Gonzalez I, Arellano M, Moreno S (1998) The Cdk inhibitors p25rum1 and p40SIC1 are functional homologues that play similar roles in the regulation of the cell cycle in fission and budding yeast. J Cell Sci 111: 843–851 [DOI] [PubMed] [Google Scholar]
- Sauer K, Knoblich JA, Richardson H, Lehner CF (1995) Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev 9: 1327–1339 [DOI] [PubMed] [Google Scholar]
- Schulman BA, Lindstrom DL, Harlow E (1998) Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc Natl Acad Sci USA 95: 10453–10458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seher TC, Leptin M (2000) Tribbles, a cell-cycle brake that coordinates proliferation and morphogenesis during Drosophila gastrulation. Curr Biol 10: 623–629 [DOI] [PubMed] [Google Scholar]
- Stuurman N (1997) Identification of a conserved phosphorylation site modulating nuclear lamin polymerization. FEBS Lett 401: 171–174 [DOI] [PubMed] [Google Scholar]
- Vidwans SJ, DiGregorio PJ, Shermoen AW, Foat B, Iwasa J, Yakubovich N, O'Farrell PH (2002) Sister chromatids fail to separate during an induced endoreplication cycle in Drosophila embryos. Curr Biol 12: 829–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigmann K, Cohen SM, Lehner CF (1997) Cell cycle progression, growth and patterning in imaginal discs despite inhibition of cell division after inactivation of Drosophila Cdc2 kinase. Development 124: 3555–3563 [DOI] [PubMed] [Google Scholar]
- Wittenberg C (2005) Cyclin guides the way. Nature 434: 34–35 [DOI] [PubMed] [Google Scholar]
- Zhu L, Harlow E, Dynlacht BD (1995) p107 uses a p21CIP1-related domain to bind cyclin/cdk2 and regulate interactions with E2F. Genes Dev 9: 1740–1752 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Data


