Abstract
Transcription factor SOX9 (sex-determining region Y-type high mobility group box 9) and its coactivators SOX5 and SOX6 (the SOX trio) induce early-stage chondrocyte differentiation and suppress its terminal stage. To identify possible targets of the SOX trio, we carried out a microarray analysis and identified S100A1 and S100B as possible target molecules. S100 protein expression was localized in late proliferative and pre-hypertrophic chondrocytes of the mouse growth plate. Overexpression of S100A1, S100B or their combination in cultured chondrogenic cells did not induce early differentiation, but suppressed hypertrophic differentiation and mineralization. Silencing of both S100A1 and S100B stimulated terminal differentiation and reversed the SOX-trio-mediated inhibition. Finally, luciferase reporter, electrophoretic mobility shift and chromatin immunoprecipitation analyses showed that transcription of both S100 proteins is induced by the SOX trio, and also identified their respective enhancer elements in the 5′-end flanking region. We conclude that S100A1 and S100B are transcriptional targets of the SOX trio and mediate its inhibition of terminal differentiation of chondrocytes.
Introduction
During endochondral ossification for skeletal development, cells in the mesenchymal condensations differentiate into chondrocytes that then undergo proliferation and differentiation into hypertrophic cells. The hypertrophic chondrocytes mineralize a surrounding matrix to provide a scaffold for osteoblastic cells. Many lines of evidence have shown that the sex-determining region Y-type high mobility group box (SOX) family transcription factors are required at sequential steps of the chondrocyte differentiation (Bi et al, 2001; Akiyama et al, 2002). Among them, SOX9 is known to be an essential transcription factor for mesenchymal condensation and subsequent chondrocyte differentiation at an early stage (Foster et al, 1994; Wagner et al, 1994; Ng et al, 1997). Two members of the SOX family, SOX5 and SOX6, are known to function as coactivators of SOX9 in chondrocyte differentiation (Lefebvre et al, 1998; Smits et al, 2001). Accumulated evidence on mutant mice with loss of function of the SOX factors has shown that they also function as potent inhibitors of terminal differentiation (Bi et al, 2001; Smits et al, 2001; Akiyama et al, 2002). We reported previously that the combination of SOX9, SOX5 and SOX6 (the SOX trio) highly stimulated the early stage of chondrocyte differentiation in several cultured cells, including nonchondrogenic cells (Ikeda et al, 2004). In addition, the SOX trio inhibited terminal differentiation of chondrocytes. This effect could potentially be explored to benefit regenerative medicine of permanent cartilage. The present study sought to identify transcriptional targets of the SOX trio by a microarray analysis, and further investigated their functional involvement in chondrocyte differentiation.
Results And Discussion
Induction of S100A1 and S100B by the SOX trio
A microarray analysis was performed using human mesenchymal stem cells (hMSCs) adenovirally transfected with the SOX trio or a LacZ control. We identified 17 and 373 genes that were upregulated more than five- and twofold, respectively, as well as 5 and 230 genes downregulated more than five- and twofold, respectively, by the SOX trio (supplementary Table online). Among them, S100A1 and S100B—S100 protein family members, which are the largest subgroup in the superfamily of proteins carrying the Ca2+-binding EF-hand motif—showed the strongest increases. Real-time reverse transcription–PCR (RT–PCR) analysis confirmed that S100A1 and S100B expression was induced by adenoviral transfection of SOX9 alone, and more strongly by that of the SOX trio—but not by SOX5 or SOX6 alone—in hMSCs, human dermal fibroblasts, chondrogenic cell line ATDC5 and nonchondrogenic cell lines HeLa and HuH-7 (Fig 1).
Figure 1.
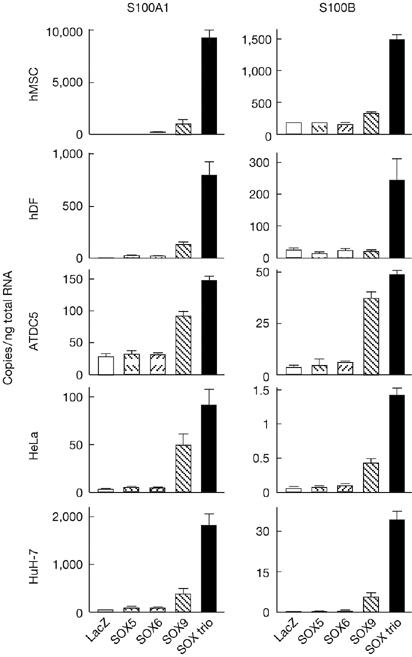
Expression of S100A1 and S100B is induced by SOX9 and the SOX trio in cultures of human mesenchymal stem cells, human dermal fibroblasts, ATDC5, HeLa and HuH-7 cells. The cells were adenovirally transfected with LacZ, SOX5, SOX6, SOX9 or the SOX trio, and the messenger RNA levels were determined by real-time reverse transcription–PCR analysis after 7 days of culture. Data are shown as means (bars)±s.d.s (error bars) for 4 wells/group. hDF, human dermal fibroblasts; hMSC, human mesenchymal stem cells; SOX, sex-determining region Y-type high mobility group box.
Expression patterns of S100A1 and S100B
To identify the expression patterns of S100A1 and S100B in the sequential differentiation stages of chondrocytes, we carried out immunohistochemistry (Fig 2A) and in situ hybridization (supplementary Fig S1 online) in the mouse growth plate. S100A1 and S100B were coexpressed with SOX9, SOX5 and SOX6 in differentiated cells—late proliferative and pre-hypertrophic chondrocytes—and moderately in the hypertrophic chondrocytes. When their subcellular localization was examined by immunocytochemistry in cultured retrovirally transduced ATDC5 cells, both S100 proteins were localized in the cytoplasm (Fig 2B).
Figure 2.
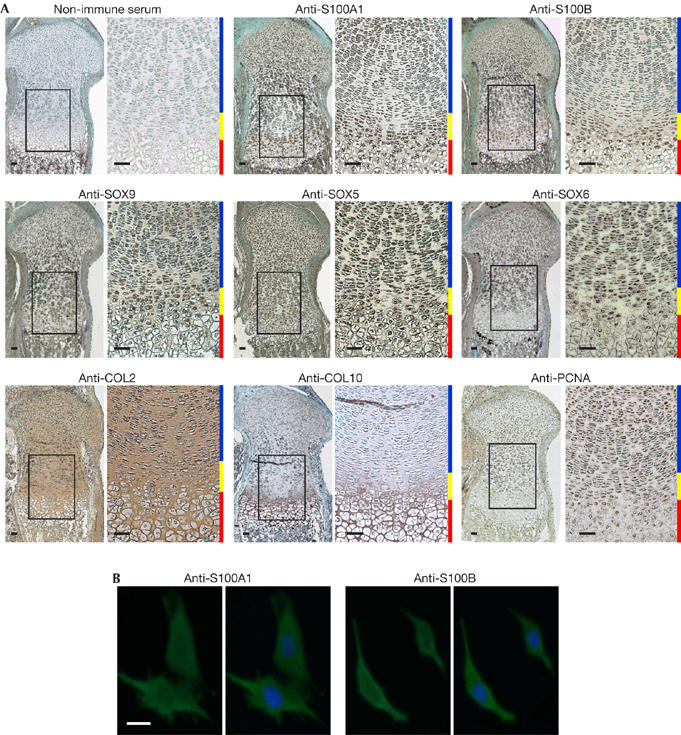
S100A1 and S100B proteins are detected in late proliferative and pre-hypertrophic chondrocytes of the growth plate and localize in the cytoplasm of ATDC5 cells. (A) Localization of the S100 and SOX proteins was examined by immunohistochemistry in the fetal mouse growth plate and compared with that of COL2, COL10 and PCNA as early differentiation, hypertrophic differentiation and proliferation markers, respectively. Inset boxes in the left-hand figures indicate the regions of the respective right-hand figures. Scale bars, 50 μm. Blue, yellow and red bars at the right-hand side of each pair of figures indicate layers of proliferative, pre-hypertrophic and hypertrophic zones, respectively. (B) Subcellular localization was examined by immunocytochemistry in ATDC5 cells transfected with S100A1 (left) or S100B (right) with or without nuclear counterstaining. Scale bar, 10 μm. COL2, type II collagen; COL10, type X collagen; PCNA, proliferating cell nuclear antigen; SOX, sex-determining region Y-type high mobility group box.
In cultured ATDC5 cells, expression of endogenous S100A1 and S100B was increased with expression of SOX9, SOX5 and SOX6, as well as type II collagen (COL2), type X collagen (COL10) and RUNX2 (runt-related transcription factor 2) during chondrogenic and hypertrophic differentiation (supplementary Fig S2 online).
Functional contribution of S100A1 and S100B
To investigate the functional relevance of S100A1 and S100B to chondrocyte differentiation, we first established stable lines of ATDC5 cells overexpressing S100A1, S100B, a combination of both or a green fluorescent protein (GFP) control through retroviral transduction. Gene expression was confirmed by real-time RT–PCR, western blot and immunofluorescence analyses (supplementary Fig S3A online). Both COL2 messenger RNA level and Alcian blue staining in the four different cell lines were comparable with those of non-transfected parental cells (supplementary Fig S3B online), indicating that the S100 proteins did not induce early differentiation of chondrocytes. Considering that the SOX trio not only induces early differentiation, but also suppresses terminal differentiation (Ikeda et al, 2004), we then examined the effects of the S100 proteins on ATDC5 cells that were cultured in differentiation medium including insulin and inorganic phosphate. The mRNA levels of terminal differentiation markers COL10 and RUNX2, alkaline phosphatase (ALP) activity and Alizarin red staining were reduced in cells overexpressing S100A1, S100B or a combination of both (Fig 3A).
Figure 3.
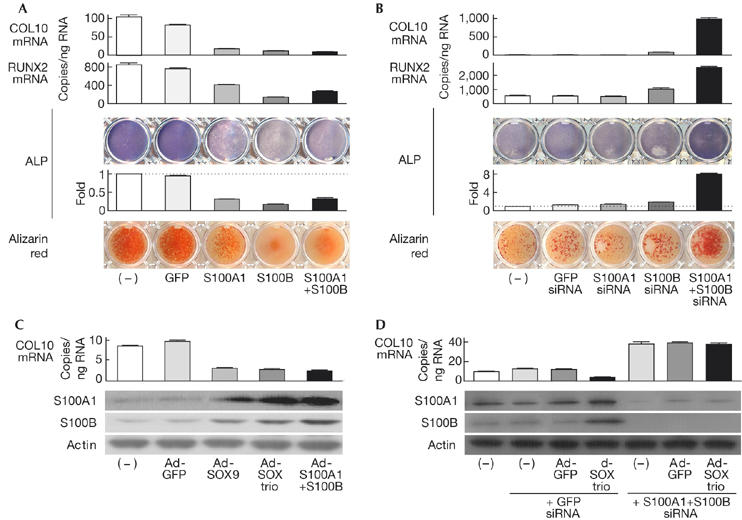
S100A1 and S100B mediate the suppression of terminal differentiation of chondrocytes by the SOX trio. (A) Messenger RNA levels of the terminal differentiation markers COL10 and RUNX2, ALP staining and activity, and Alizarin red staining in stable lines of ATDC5 cells with retroviral transfection of GFP, S100A1, S100B or a combination of both, and in non-transfected parental cells (–) after culture for 3 weeks with ITS (insulin, transferrin and sodium selenite) and 2 days with inorganic phosphate. (B) Analysis of the above markers in stable lines of ATDC5 cells retrovirally transfected with siRNA of GFP, S100A1, S100B or a combination of both, and in non-transfected parental cells (–) under the culture conditions given above. (C) COL10 mRNA level and S100A1 and S100B protein levels are shown as western blots in ATDC5 cells with adenoviral transfection of GFP, SOX9, the SOX trio or S100A1+S100B, and in non-transfected parental cells (–) after 10 days of a pellet culture. (D) Identical analysis in ATDC5 cells co-transfected with GFP or the SOX trio, and siRNA of GFP or S100A1+S100B, compared with those in non-transfected parental cells (–) after 10 days of a pellet culture. The graphs are expressed as means (bars)±s.d.s (error bars) for 3 wells/group. ALP, alkaline phosphatase; COL10, type X collagen; GFP, green fluorescent protein; PCNA, proliferating cell nuclear antigen; RUNX2, runt-related transcription factor 2; siRNA, small interfering RNA; SOX, sex-determining region Y-type high mobility group box.
Next, to investigate the effects of loss of function of S100A1 and S100B, we established stable lines of ATDC5 cells expressing small interfering RNA of S100A1, S100B, a combination of both or a GFP control, and confirmed the silencing of the genes (supplementary Fig S4A online). Although none of the early differentiation markers above was much affected (supplementary Fig S4B online), the terminal differentiation markers were markedly enhanced when both S100A1 and S100B were silenced (Fig 3B).
Adenoviral transfection of S100A1+S100B suppressed COL10 expression similarly to expression of SOX9 or the SOX trio, and expression of SOX9 or the SOX trio induced S100A1 and S100B expression. These results indicate that the S100 proteins might mediate the inhibition of terminal differentiation by SOX signalling (Fig 3C). In fact, COL10 suppression by the SOX trio was not seen when S100A1 and S100B were silenced (Fig 3D). The recovery of COL10 by silencing the S100 proteins was reproducible in hMSCs (supplementary Fig S5 online).
Taking these data together, S100A1 and S100B are likely to mediate the suppression of terminal differentiation by the SOX trio, but seem not to be involved in induction of early chondrocyte differentiation.
Transcriptional induction of S100A1 and S100B
To examine the transcriptional regulation of S100A1, a luciferase-reporter gene construct containing a 1,000 bp fragment of the S100A1 5′-end flanking region was transfected into HeLa and HuH-7 cells. The transcriptional activity determined by the luciferase-reporter assay was enhanced by co-transfection with SOX9. Co-transfection of the SOX trio (but not of SOX5, SOX6 or the GFP control) further enhanced the transcriptional activity of the reporter (Fig 4A). Deletion analysis by a series of 5′-deletion constructs identified the core responsive element to SOX9 and the SOX trio to be between −135 and −95 region (A1 box). The tandem-repeat constructs of the A1 box responded to SOX9 and the SOX trio depending on its repeat number in both cell lines (Fig 4B). As this region contained paired SOX-binding motifs, surrounded by GC-rich sequences, site-directed mutagenesis of either or both of the motifs was carried out (m1, distal mutation; m2, proximal mutation; and m3, both mutations; Fig 4C, top panel). The transactivation abilities of SOX9 and the SOX trio were suppressed by m2 and m3, but not by m1, in the 1,000 bp fragment (Fig 4C, middle panel) and also in the four tandem-repeat constructs of A1 box (Fig 4C, bottom panel), indicating that the proximal motif is the core responsive element of SOX.
Figure 4.
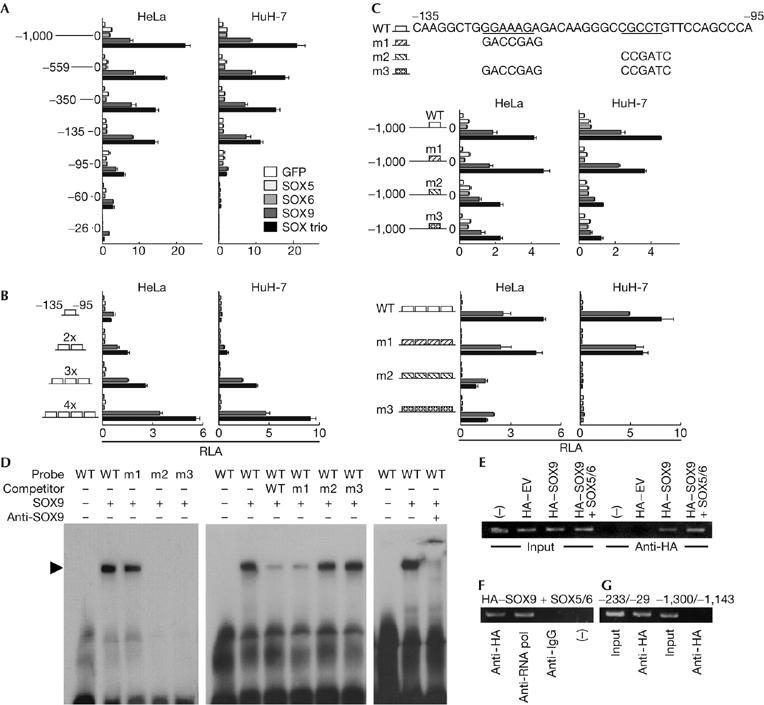
The SOX trio transcriptionally induces S100A1 through activation of a SOX9 enhancer element in the 5′-end flanking region. (A) Deletion analysis using luciferase-reporter constructs containing a 1,000 bp S100A1 5′-end flanking region and the series of deletion fragments in HeLa and HuH-7 cells transfected with green fluorescent protein (GFP), SOX5, SOX6, SOX9 or the SOX trio. (B) Dose–response analysis of the tandem repeats of the identified responsive element (−135/−95; A1 box) in the transfected cells. (C) Site-directed mutagenesis analysis of either (m1 and m2) or both (m3) of the SOX-binding motifs in the A1 box in the transfected cells. Mutagenesis analysis was performed in the 1,000 bp construct (top) and in the tandem-repeat construct (bottom). (D) Electrophoretic mobility shift assay for specific binding of the SOX9 protein with the wild-type (WT) and the mutated (m1, m2 and m3) oligonucleotide probes of the A1 box (left). Cold competition with 50-fold excess of unlabelled WT and mutated probes (middle) is shown. Supershift by a SOX9 antibody of the complex of SOX9 protein and the WT probe is presented. (E) Chromatin immunoprecipitation assays for specific binding of SOX9 to the A1 box. Cell lysates of ATDC5 cells transfected with haemagglutinin (HA)-tagged empty vector (HA–EV) or HA-tagged SOX9 in the presence or absence of SOX5 and SOX6 (HA–SOX9+SOX5/6 or HA–SOX9, respectively) were amplified by a primer set spanning the A1 box (−233/−29) before (input) and after (anti-HA) immunoprecipitation with an HA antibody. (F) Immunoprecipitation of lysates of ATDC5 cells transfected with HA–SOX9 and SOX5/6 was carried out using an HA antibody, a positive control RNA polymerase II antibody (anti-RNA pol), a negative control non-immune IgG antibody (anti-IG), or without antibody (–), and amplified with the primer set mentioned above. (G) Amplification of lysates of ATDC5 cells transfected with HA–SOX9 and SOX5/6 was carried out using a primer set spanning (−233/−29) or not spanning (−1,300/−1,143) the A1 box, before (input) and after (anti-HA) immunoprecipitation with an HA antibody. The graphs are expressed as means (bars)±s.d.s (error bars) for 3 wells/group. SOX, sex-determining region Y-type high mobility group box.
Electrophoretic mobility shift assay (EMSA) showed specific binding of in vitro-translated SOX9 with the wild-type and the m1 A1 box oligonucleotide probes, but not with the m2 or m3 probe (Fig 4D, left panel). Neither SOX5 nor SOX6 protein bound any of the oligonucleotide probes (data not shown). Cold competition with 50-fold excess of an unlabelled wild-type and m1 probe (but not with the unlabelled m2 or m3 probe) suppressed formation of the complex, confirming the specific binding of the proximal motif (Fig 4D, middle panel). Specificity of SOX9 binding was further verified by antibody supershift (Fig 4D, right panel).
Chromatin immunoprecipitation (ChIP) also showed binding of SOX9 to the S100A1 regulatory region including the A1 box in the presence or absence of SOX5 and SOX6 (Fig 4E). Specificity was confirmed as SOX9 because it was not immunoprecipitated by the non-immune IgG antibody (Fig 4F) and no amplification was seen with a primer set that does not span the A1 box (Fig 4G).
Similar assays were carried out to examine the transcriptional regulation of S100B by the SOX trio in HeLa and HuH-7 cells (supplementary Fig S6 online). Deletion, tandem-repeat and site-directed mutagenesis analyses of the luciferase-reporter assay indicated the transcriptional induction of S100B by the SOX trio and identified the core enhancer element of SOX9 between the −181 and −129 region in the 5′-end flanking region. EMSA and ChIP analyses also confirmed the specific binding of SOX9 to the enhancer motif.
Speculation
We conclude that S100A1 and S100B are transcriptional targets of the SOX trio and mediate its inhibition of terminal differentiation. This study identified, for the first time to our knowledge, SOX9 or SOX trio targets other than matrix proteins such as COL2 and aggrecan (Lefebvre et al, 1998; Sekiya et al, 2000). Interestingly, gain of function of S100A1 or S100B inhibited chondrocyte differentiation; however, loss of function of either did not appear to significantly affect chondrocyte differentiation, whereas combined loss of function of both proteins enhanced it (Fig 3A,B). This might be due to the functional redundancy between S100A1 and S100B in chondrocyte differentiation, which is supported by previous reports that a single deletion of S100A1 or S100B in mice caused no abnormality in skeletal development or growth (Xiong et al, 2000; Du et al, 2002).
As the SOX trio provides signals that are sufficient for the induction of permanent cartilage in vitro (Ikeda et al, 2004), understanding of the molecular network related to the SOX–S100 signal will greatly benefit the advancement of cartilage regenerative medicine. Considering that induction of S100A1 and S100B by the SOX trio is not specific to chondrogenic cells (Fig 1), and that both S100 and SOX proteins are expressed in various organs, the SOX–S100 signal might be important in cellular functions other than chondrocyte differentiation in other tissues.
Methods
Cell cultures. hMSCs, human dermal fibroblasts, HuH-7 and HeLa cells were cultured in high-glucose DMEM with 10% FBS. ATDC5 cells were grown and maintained in DMEM/F12 (1:1) with 5% FBS. To determine early differentiation, the ATDC5 cells cultured in the presence of ITS (insulin, transferrin and sodium selenite) supplement (Sigma, St Louis, MO, USA) for 1 week were stained with 0.1% Alcian blue 8GS (Sigma) in 0.1 M HCl. To induce hypertrophic differentiation and mineralization, ATDC5 cells were cultured in the presence of ITS supplement for 3 weeks and then the medium was replaced by α-MEM/5% FBS with 4 mM inorganic phosphate and cells were grown for 2 days, as described previously (Magne et al, 2003). ALP staining was carried out using a solution containing 0.01% naphtol AS-MX phosphate disodium salt, 1% N,N-dimethyl-formamide and 0.06% fast blue BB (Sigma). ALP activity was measured with a Lab Assay ALP kit (Wako, Osaka, Japan), according to the manufacturer's instructions. In addition, the cells were stained with 2% Alizarin red S solution.
Immunostaining. Tissues from C57BL6 mouse embryos (embryonic day 17.5) were fixed in 4% paraformaldehyde/PBS overnight at 4°C, embedded in paraffin and cut into 5 μm sections. Sections were incubated overnight at 4°C with primary antibodies against S100A1 (1:200; Upstate, Charlottesville, VA, USA), S100B (1:200; Sigma), SOX5, SOX6, SOX9 (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), COL2, COL10 (1:500; LSL), proliferating cell nuclear antigen (1:200; Cell Signaling Technology, Danvers, MA, USA) or non-immune rabbit serum (1:200). The localizations were detected with horseradish peroxidase-conjugated secondary antibodies (Promega, Madison, WI, USA). For immunocytochemistry, ATDC5 cells, which were retrovirally transduced with S100A1 or S100B, were fixed in 4% paraformaldehyde/PBS for 10 min and incubated with the primary antibodies at 25°C for 1 h. A secondary antibody conjugated with Alexa 488 (Molecular Probes, Eugene, OR, USA) was used for fluorescent visualization and the nucleus was counterstained with Hoechst 33258 (Sigma).
Luciferase assay. The luciferase assay was carried out with a dual-luciferase-reporter assay system (Promega) and a Lumat LB 9507 (Berthold, Bad Wildbad, Germany).
Electrophoretic mobility shift assay. SOX9 protein was prepared by in vitro translation using TNT T7 Quick System (Promega) and pCITE4 vector (Novagen, Milwaukee, WI, USA) into which SOX9 complementary DNA was cloned. The translation product was verified by western blotting. EMSA was carried out using a DIG gel shift kit (Roche, Mannheim, Germany) according to the manufacturer's instructions.
Chromatin immunoprecipitation assay. The ChIP assay was carried out with an EZ ChIP kit (Upstate) according to the manufacturer's instructions.
Other experiments. The detailed methods for construction of expression vectors, cell cultures, microarray analysis, real-time RT–PCR, Western blotting, in situ hybridization, luciferase assay, EMSA and ChIP assay are described in the supplementary information online.
Supplementary information is available at EMBO reports online (http://www.emboreports.org).
Supplementary Material
supplementary Information
Acknowledgments
This study was supported by a grant-in-aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology (nos. 16390430, 17591549 and 18209047).
References
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B (2002) The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev 16: 2813–2828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B (2001) Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci USA 98: 6698–6703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du XJ, Cole TJ, Tenis N, Gao XM, Kontgen F, Kemp BE, Heierhorst J (2002) Impaired cardiac contractility response to hemodynamic stress in S100A1-deficient mice. Mol Cell Biol 22: 2821–2829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JW et al. (1994) Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372: 525–530 [DOI] [PubMed] [Google Scholar]
- Ikeda T, Kamekura S, Mabuchi A, Kou I, Seki S, Takato T, Nakamura K, Kawaguchi H, Ikegawa S, Chung UI (2004) The combination of SOX5, SOX6, and SOX9 (the SOX trio) provides signals sufficient for induction of permanent cartilage. Arthritis Rheum 50: 3561–3573 [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Li P, de Crombrugghe B (1998) A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J 17: 5718–5733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magne D et al. (2003) Phosphate is a specific signal for ATDC5 chondrocyte maturation and apoptosis-associated mineralization: possible implication of apoptosis in the regulation of endochondral ossification. J Bone Miner Res 18: 1430–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng LJ, Wheatley S, Muscat GE, Conway-Campbell J, Bowles J, Wright E, Bell DM, Tam PP, Cheah KS, Koopman P (1997) SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev Biol 183: 108–121 [DOI] [PubMed] [Google Scholar]
- Sekiya I, Tsuji K, Koopman P, Watanabe H, Yamada Y, Shinomiya K, Nifuji A, Noda M (2000) SOX9 enhances aggrecan gene promoter/enhancer activity and is up-regulated by retinoic acid in a cartilage-derived cell line, TC6. J Biol Chem 275: 10738–10744 [DOI] [PubMed] [Google Scholar]
- Smits P, Li P, Mandel J, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B, Lefebvre V (2001) The transcription factors L-Sox5 and Sox6 are essential for cartilage formation. Dev Cell 1: 277–290 [DOI] [PubMed] [Google Scholar]
- Wagner T et al. (1994) Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79: 1111–1120 [DOI] [PubMed] [Google Scholar]
- Xiong Z, O'Hanlon D, Becker LE, Roder J, MacDonald JF, Marks A (2000) Enhanced calcium transients in glial cells in neonatal cerebellar cultures derived from S100B null mice. Exp Cell Res 257: 281–289 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
supplementary Information


