
This European Molecular Biology Organization workshop on Hedgehog-Gli Signaling in Cancer and Stem Cells took place between 30 September and 4 October 2006 in Rome, Italy, and was organized by A. Gulino, H. Hahn, A. Ruiz í Altaba, R. Toftgard, F. Watt, P. Therond and I. Guerrero.
Introduction
During a wonderfully seasonal autumn, researchers from around the world gathered in Rome, the soul of Italy, for an EMBO workshop on Hedgehog (Hh) signalling in cancer and stem cells. The focus of this workshop was timely, as this area of research is responsible for the exponential growth—and increased research diversity—in the Hh field. Hh was first identified in a screen for genes required for Drosophila embryonic patterning (Nüsslein-Volhard & Wieschaus, 1980). Subsequent isolation of the affected hh gene and its encoded protein revealed a unique signalling molecule with related orthologues in vertebrates (Ingham & McMahon, 2001). Since its original description, the Hh pathway has received increasing attention, not only because of its involvement as a crucial regulator of embryonic organogenesis but also as an oncogenic pathway that has been implicated in several human tumours (Beachy et al, 2004; Pasca di Magliano & Hebrok, 2003; Robbins et al, 2005; Ruiz í Altaba et al, 2002). Despite extensive studies addressing the regulation and function of the pathway in different organs, the exact mechanisms by which Hh signals are transmitted and how they elicit diverse activities in a cell-specific manner remain obscure. Here, we review some of the recent advances in the Hh field that were discussed at this workshop and highlight some of the questions that remain.
Hedgehog signalling
Hh is translated into an approximately 47 kDa protein, which subsequently undergoes an intramolecular cleavage reaction that produces two discrete smaller proteins (Singh et al, 2006; Ingham & McMahon, 2001). The amino-terminal product is responsible for the biological activity of Hh, whereas the carboxy-terminal product functions as a cholesterol transferase, covalently modifying the N-terminal product on its terminal amino acid. Similar cleavage products have been described for the vertebrate homologues of Hh—Sonic Hedgehog (Shh), Indian Hedgehog (Ihh) and Desert Hedgehog. Besides being covalently modified by cholesterol, the N-terminal product of Hh proteins are also modified by a palmitoyl-group at its N-terminus. The cholesterol modification of Hh is thought to be important for its transport, whereas palmitoylation of Hh is thought to be required for high-level activity. These hydrophobic modifications of Hh are probably the driving evolutionary force behind many of the unanticipated mechanisms that have been revealed during the dissection of this novel signalling pathway.
Hh signalling is initiated when Hh binds to Patched (Ptc) and attenuates its function, which in the absence of Hh negatively regulates signalling (Ingham & McMahon, 2001). After Hh de-represses Ptc, the seven-transmembrane protein Smoothened (Smo), which is normally held in an inactive state by Ptc, is able to activate a signalling cascade that ultimately regulates the Gli family of transcription factors (Fig 1). The Drosophila Gli homologue cubitus interruptus (Ci) encodes a protein that acts as a transcriptional repressor in the absence of Hh and as a transcriptional activator in the presence of Hh. These two activities are conserved in the various vertebrate Gli family members, Gli 1–3, with the ratio of repressor and activator function varying for each Gli family member. Several proteins can modulate the ratio of Gli activator (GliA) to Gli repressor (GliR) activity and, in doing so, ultimately determine the level of Hh perceived by the cell. Therefore, the GliA:GliR ratio acts as a sort of biochemical rheostat, interpreting the response of a cell to different amounts of Hh.
Figure 1.
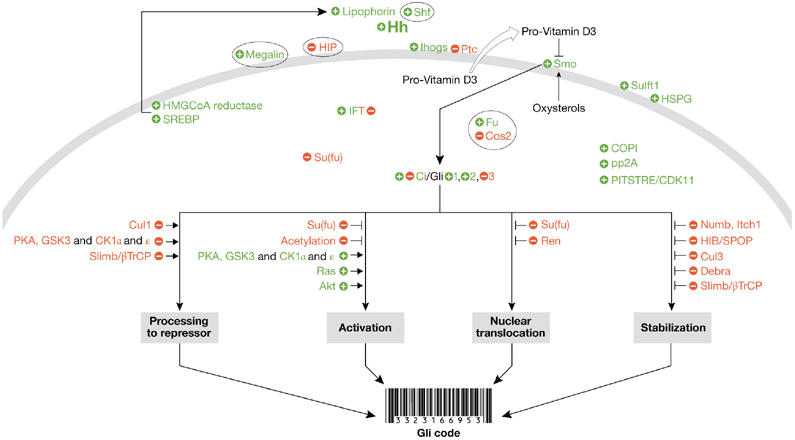
The Hedgehog signalling pathway. Positive regulators of the Hedgehog (Hh) pathway are shown in green, whereas negative regulators are shown in red. Although many of the components seem to have both positive and negative activities under different conditions, the component is colour-coded according to its predominant activity. Components of the pathway that do not seem to be conserved between Drosophila and mammals are shown in ovals. This figure is intended to summarize the key concepts about Hh signalling discussed at this workshop, and is not intended to represent all the known components of the pathway. Akt; cellular homologue of the transforming gene of the AKT8 provirus; β-TrCP; β-transducin repeat-containing protein; CDK11; cyclin-dependent kinase 11; Ci, Cubitus interruptus; CK1, casein kinase 1; COPI, coat protein 1; Cos2, Costal2; Cul, Cullin; Debra, determiner of breaking down of Ci activator; Fu, Fused; GSK3, glycogen synthase kinase 3; HIB, Hh-inducible BTB protein; HIP, Hh-interacting protein; HMGCoA, 3-hydroxy-3-methyl-glutaryl-CoA; HSPG, heparan sulphate proteoglycan; IFT, intraflagellar transport; Ihogs, Interference Hedgehogs; Itch, Itchy homologue E3 ubiquitin protein ligase; Numb, protein involved in asymmetric cell division; PITSTRE, a family of protein kinases containing this conserved sequence within its active site; PKA, protein kinase A; pp2A, protein phosphatase 2A; Ptc, Patched; Ras, monomeric G-protein; Ren, a gene that is upregulated by retinoic acid, epidermal growth factor and nerve growth factor; Shf, Shifted; Slimb, supernumerary limbs; Smo, Smoothened; SPOP, speckle-type POZ protein; SREBP, sterol regulatory element-binding protein; Su(fu), Suppressor of Fused; Sulft1, Sulfateless 1.
Extracellular Hedgehog signalling
At the workshop, fundamental questions were discussed about how secreted Hh proteins, which are post-translationally modified by both cholesterol and palmitate (Singh et al, 2006), are able to move many cell diameters from their site of synthesis (Torroja et al, 2005). Although it is now clearly established that Hh proteins are able to participate directly in long-range signalling, the mechanisms by which they do so remain controversial. Several viable, non-mutually exclusive, models have been proposed to account for the extracellular movement of these lipophilic proteins. One recent model (Panakova et al, 2005) suggests that Drosophila Hh moves through the extracellular environment as part of a large lipoprotein complex, nucleated by the abundant lipid-binding proteins apolipophorins (Drosophila apolipoproteins). If this is the case, I. Guerrero (Madrid, Spain) reasoned that modulating the expression levels of two Drosophila regulators of lipid homeostasis—3-hydroxy-3-methyl-glutaryl-CoA (HMGCoA) reductase and sterol regulatory element-binding protein (SREBP)—would affect Hh movement. Guerrero found that lowering the dosage of these genes rescued the phenotypes caused by increased Hh production in the wing, whereas increased expression of HMGCoA reductase increased the range of Hh movement in a sensitized mutant background, in which Hh stability is normally attenuated. Her group also showed that Ptc associates with and internalizes lipophorin in the presence or absence of Hh. Furthermore, a Ptc mutant with a defective sterol-sensing domain was also able to internalize and accumulate lipophorin even more efficiently than wild-type Ptc. These results are consistent with Ptc receiving an Hh–lipophorin complex, and with this form of Hh being capable of long-range signalling.
At the plasma membrane
Accumulating evidence from several groups has shown that the primary cilia found on most vertebrate cells have an important, but undefined, role in many signalling pathways (Satir & Christensen, 2007; Singla & Reiter, 2006). At this workshop, additional evidence supporting the important role of primary cilia in Hh signalling was presented. Primary cilia are regulated by large protein complexes involved in intraflagellar transport (IFT), which function in the retrograde and anterograde movement of cargo within the primary cilia. Several mutations encoding proteins involved in anterograde IFT have been described, which result in mice with Hh loss-of-function phenotypes. J. Eggenschwiler (Princeton, NJ, USA) presented a new mouse mutant, called Sister of Open Brain, that exhibited a Hh-like gain-of-function phenotype. The gene mutated in these mice mapped to the IFT122 locus and represents a component of the retrograde IFT complex. Eggenschwiler suggested that mice lacking IFT122 function have impaired retrograde transport within their primary cilia, which allows an accumulation of positive activators of the Hh pathway. Interestingly, although dynein heavy chain (Dnchc2) is also required for retrograde transport, Dnchc2 mouse mutants have the opposite role in Hh signalling from that of IFT122, in that Dnchc2 is required for activation rather than repression of the pathway. Two additional groups (J. Reiter, San Francisco, CA, USA; L. Milenkovic, Palo Alto, CA, USA) showed the dynamic nature of Smo localization in the primary cilia of cells treated with Hh or various Smo antagonists, using real-time imaging of Smo and photo-bleaching experiments. Ptc was also shown to be enriched in primary cilia, in which it is proposed to inhibit Hh signalling by preventing the enrichment of Smo. These presentations prompted much discussion about the mechanistic role of primary cilia in Hh signalling, and whether such mutations result directly in defective Hh signalling or whether they disrupt some more global cytoskeletal process that in turn disrupts the pathway.
Exciting recent work has identified a new class of transmembrane proteins—named Interference Hedgehogs (Ihogs) in Drosophila, and cell adhesion molecule-related/downregulated by oncogenes (CDO) and brother of CDO (BOC) in vertebrates—that are involved in Hh signalling (Tenzen et al, 2006; Yao et al, 2006; Zhang et al, 2006). These proteins modulate Hh signalling by acting directly as co-receptors, as well as acting indirectly at an additional step downstream of Ptc. R. Krauss (New York, NY, USA) described how BOC and CDO seem to modulate Hh signalling in a spatial and temporal manner. For example, although many Hh-dependent processes, such as limb development, seemed normal in mice lacking CDO, these mice developed facial characteristics similar to those found in holoprosencephaly patients who have inherited or spontaneous sonic hedgehog (SHH) mutations.
Another area that stimulated much discussion was the role of naturally occurring 3β-hydroxysteroids in regulating Smo function, in a manner mimicked by the teratogenic 3β-hydroxysteroid cyclopamine. M. Peppelenbosch (Amsterdam, The Netherlands) showed that these naturally occurring 3β-hydroxysteroid derivatives exist in many forms. Depending on their particular structure, they are able to activate, inhibit or have no effect on Smo. Peppelenbosch proposed that Ptc pumps a sub-class of 3β-hydroxysteroids, such as pro-vitamin D3, into the extracellular space, where they seem to inhibit Smo function. Consistent with this suggestion, pro-vitamin D3 is able to inhibit Shh function in vitro and in vivo, and does so in a manner as potent as cyclopamine.
Intracellular signal transduction
Several new Hh signalling components were discussed that were isolated through various high-throughput strategies, including RNA interference screens in Drosophila cells (K. Nybakken, Boston, MA, USA; G. Hausmann, Zurich, Switzerland) and cDNA expression screens in mammalian cells (J. Taipale, Helsinki, Finland). These screens identified several protein kinases, including casein kinase II α subunit (CK2), PITSLRE protein kinase β 1 (CDK11) and cyclin-dependent kinase 9 (CDK9)/cyclin T, and at least one protein phosphatase, pp2A, that act as positive regulators of Hh signalling. Additional membrane vesicular transport components of the Hh pathway were also identified, including the coat protein 1 (COP1) complex, which regulates transport from the Golgi apparatus to the endoplasmic reticulum. Although the role of the COP1 complex in Hh signalling is unknown, this positive effect on Hh signalling seems to be evolutionarily conserved between Drosophila and mammals. Evidence was also provided supporting a role for RNA-processing enzymes in Hh signalling, as overexpression of the RNA splicing enzyme SF2 expanded markers of Hh signal activation in Drosophila wing imaginal discs (Nybakken).
A recurrent theme at this meeting centered on how other signalling pathways communicate with the Hh pathway. The epidermal growth factor (EGF) and insulin-like growth factor (IGF) signalling pathways also seem to function as important modulators of Hh signalling by regulating Gli activity through Ras and the mitogen-activated protein (MAP) kinase or serine-threonine kinase AKT pathways (A. Ruiz í Altaba, Geneva, Switzerland; C. Emerson Jr, Watertown, MA, USA; A. Kenney, New York, NY, USA). This modulation of Hh signalling by other signalling pathways was context-dependent, occurring in some tissues or cell lines but not in others. Furthermore, F. Aberger (Salzburg, Austria) suggested that the combination of Hh and EGF could activate a unique set of target genes, which EGF or Hh were unable to activate alone. Other factors suggested to have a role in Hh pathway communication were protein kinase C δ (Emerson), Numb (Gulino), insulin receptor substrate 1 (IRS1; Kenney) and secreted frizzled-related protein 1 (sFRP1; J. Xie, Galveston, TX, USA).
Modulating Gli function
Another recurrent theme of this meeting was the identification and characterization of factors that directly modify the function of the Ci/Gli family of transcription factors. In general, these modulating factors affect the stability, activity, nuclear localization or partial proteolytic processing of Ci/Gli family members. J. Jiang (Dallas, TX, USA) described a new Hh-inducible Cullin 3 (Cul3) adaptor protein for Ci, called Hedgehog-inducible BTB protein (HIB)/Roadkill, that degrades full-length Ci. HIB/Roadkill directly links Ci to other components of the Cul3-based E3 ligase complex. The mammalian orthologue of HIB—speckle-type POZ protein (SPOP)—also degrades Ci/Gli family members when they are expressed in Drosophila. Interestingly, the HIB/Cul3 pathway is independent of the Gli phosphorylation status, a clear distinction from the previously described Slimb/β-transducin repeat-containing protein (β-TrCP) degradation pathway that is activated on sequential phosphorylation of Gli by protein kinase A (PKA), glycogen synthase kinase 3β (GSK3β), and casein kinase I (CK1).
T. Oro (Palo Alto, CA, USA) showed that the stability of Gli1 is an important determinant of tumour latency in mouse basal cell carcinoma (BCC). Oro described two intrinsic regulators of Gli1 stability—a β-TrCP-dependent, C-terminal domain present in Gli1 and Gli2, and an SPOP/E3 ligase-dependent N-terminal domain present in all three Gli proteins. Transgenic mice expressing Gli1 mutants that lack these domains exhibited decreased tumour latency.
The processing of Gli2 and Gli3 into their proteolysed repressor forms is also β-TrCP-dependent. B. Wang (New York, NY, USA) presented data that reveal a process by which Gli2 and Gli3 remain partly intact after moving through the proteasome. A discrete 197 amino-acid domain found in Gli2 and Gli3 is required for this processing. This domain is also able to protect other exogenous proteins from complete proteolysis by the proteasome. Gli3 is efficiently processed in vivo into its repressor form, whereas the majority of Gli2 remains full-length. Surprisingly, a two amino-acid difference in this 197 amino-acid domain between Gli2 and Gli3 seems to regulate the relative efficiency with which the two are processed into their respective repressor forms. Gulino also provided evidence for additional mechanisms that regulate Gli1 stability. Gli1 activity, but not Gli2 or Gli3, is also regulated by the Itchy homologue ITCH, which is a HECT-type E3 ubiquitin ligase. ITCH-mediated ubiquitination of Gli1 results in protein degradation. Interestingly, this process seems to be enhanced by Numb, a protein previously shown to be involved in asymmetric cell division. Numb acts as an adaptor protein that binds to both Gli1 and ITCH; therefore, forming a complex that targets Gli1 for degradation. The importance of this interaction is supported by the observation that forced expression of Numb in medulloblastoma cells promotes growth arrest and neuronal differentiation by antagonizing Gli1 activity. Gulino also showed that Gli1 activity can be attenuated by acetylation. He showed that Gli1 is acetylated, probably through the activity of p300 and p300/CBP-associated factor (PCAF), and that the histone deacetylase inhibitor trichostatin A reduces both Gli1 transcriptional activity in a luciferase reporter assay and the expression of Hh target genes.
Ruiz í Altaba proposed that the expression and post-translational stabilization of the various Gli family members results in a unique Gli code, which ultimately creates a distinct combination of transcriptional activators and repressors that results in a specific biological readout. J. Briscoe (London, UK) presented additional evidence showing that a series of Gli3 mutants, which induced various degrees of Gli activity, activated various Hh concentration-dependent readouts in the chick neural tube. Therefore, this correlation between Gli activation in vitro and the induction of Hh-dependent readouts in the ventral neural tube provides in vivo evidence that substantiates the Gli-code model.
Hedgehog signalling as an oncogenic pathway
Initially, the activity of the Hh signalling pathway was considered to be confined to embryonic development; however, more recently, numerous studies have indicated that the pathway remains active in a subset of adult cells and that its deregulation can cause cancer. Several important findings about the various types of Hh-responsive tumours were presented at this meeting. Ruiz í Altaba showed data about the role of Gli signalling in glioma. Results from his group indicate that human glioma cells require the Smoothened Drosophila homologue (SMOH) and GLI1 expression for survival. Ruiz í Altaba also presented data that indicate the presence of cells with stem-cell properties within glioma tumours. These cells express the surface antigen CD133 and depend on Hh signalling for self-renewal, survival and the sustained expression of stemness genes, including Nanog and Nestin. Hh inhibition in these cells inhibits glioma growth and invasion after xenotransplantation. To understand the consequences of elevated Hh signalling in the adult mammary gland, R. Toftgard (Stockholm, Sweden) ectopically expressed Gli1 under the control of mouse mammary tumour virus promoter elements. Transgenic mice were marked by lactation defects, and histological analysis showed that cells in mature mammary glands had lost their differentiation status and instead expressed markers of undifferentiated progenitor cells. Furthermore, a mixed set of tumours with myoepithelial and ductal differentiation properties were found, suggesting that they might have derived from these undifferentiated progenitor cells. These data, together with the finding that normal luminal and myoepithelial cells express Gli3, indicate that Hh signalling needs to be tightly controlled in the adult mammary gland to prevent expansion of progenitor cells that carry a tumorigenic potential. A possible role for the Hh pathway in the proliferation of adult myogenic progenitors—satellite cells—was discussed by H. Hahn (Göttingen, Germany); however, the exact contribution of satellite cells to the formation of rhabdomyosarcomas and the potential role of Hh signalling in this process remains to be established.
J. Olson (Seattle, WA, USA) described a mouse model of medulloblastoma that is based on ectopic Hh activation through the expression of a constitutively active version of Smo under the control of the NeuroD2 promoter in neural cells. His data indicate that the transcription factor N-myc is an essential downstream effector during tumorigenesis. Conditional inactivation of N-myc in NeuroD-SmoA1 mice completely prevents tumour formation, a finding that could be explored for therapeutic interventions. E. Epstein's group (San Francisco, CA, USA) has been studying the role of Hh signalling in BCC. Their results indicate that treatment of Ptc+/− transgenic mice with the topical retinoid tazarotene results in a marked reduction of BCC lesions that have formed after ultraviolet or ionizing radiation. In addition, treatment with this retinoic acid receptor β/γ ligand prevented the reappearance of tumours, suggesting a complete loss of tumour cells. Given these encouraging results, a clinical trial testing the efficacy of tazarotene on human BCC has been initiated.
A. Dlugosz (Ann Arbor, MI, USA) described efforts to modulate Hh signalling and BCC formation in the skin of transgenic mice. In his model, ectopic expression of an artificially truncated version of Gli2, which lacks an N-terminal repressor domain, in the epidermis depends on the presence of doxycycline, therefore allowing temporal and spatial control of gene expression. Forced expression of Gli2 results in BCC that disappear on doxycycline removal. However, reactivation of Gli2 expression results in the formation of some tumours at the same location as the previous lesions. These data complement those described by Epstein, which indicate that prolonged Hh activation might change the properties of some progenitor cells in the skin. Although these cells are non-proliferative in the absence of excessive Hh signalling, reactivation of the pathway might result in rapid BCC formation. It should also be noted that Ruiz í Altaba showed a role for Hh signalling in melanomas, a lethal form of skin cancer. Melanoma cells are characterized by active Hh signalling, and cyclopamine treatment blocks melanoma growth after xenotransplantation into mice. Further evidence for a wider role of the pathway in other tumours came from Xie, who analysed the expression of Hh components in various gastrointestinal and hepatocellular tumours. His data support the idea that a subset of hepatocellular carcinomas express SHH, GLI1 and Patched homologue 1(PTCH1), and that pathway inhibition of tumour cell lines with cyclopamine reduces cell proliferation. Future studies, including the generation of mouse models with ectopic Hh expression in liver, will show whether deregulation of the pathway is sufficient to cause hepatocellular carcinoma.
W. Bushman (Madison, WI, USA) discussed the role of Hh in prostate cancer. His data suggest that in this particular cancer, epithelial expression of Hh regulates the expression of target genes in the surrounding stroma in a paracrine manner. However, this issue remains controversial as other groups have previously provided evidence for autocrine Hh signalling within the prostate epithelium. Nonetheless, similar paracrine effects of Hh signalling on tumour stroma are also suspected for other cancers, such as pancreatic adenocarcinoma (M. Hebrok, San Francisco, CA, USA; F. de Sauvage, San Francisco, CA, USA), and increasing evidence suggests that Hh signalling has different roles in distinct tumours. Detailed analysis of the Hh response in the distinct cell types within a given tumour will be crucial, an idea that was supported by G. van den Brink (Leiden, The Netherlands) who presented data that indicate an inhibitory effect of increased Hh signalling on the growth of intestinal tumours driven by deregulated Wnt signalling.
Modulation of Hedgehog signalling for therapeutics
An important aspect of Hh signalling that was reiterated throughout the meeting is its diversity of functions, which depend on the state and cellular context of the receiving cells. For example, Hh signalling seems to have a positive role in regulating cell proliferation and therefore maintenance of several adult organ progenitor cells. By contrast, overt activation results in tumour formation in other tissues. An important aspect of future research will be to modulate Hh signalling in a tissue- and cell-specific manner either to harness the potential of the pathway for regenerative purposes or to block unwanted tumorigenesis. Several speakers, including D. Robbins (Hanover, NH, USA), M. Lauth (Stockholm, Sweden), and de Sauvage, described the use of new Hh inhibitors that could be used to combat the unwanted effects of the pathway in human tumorigenesis. Although the exact mechanisms by which Hh activity is blocked still need to be explored, it seems that some of these compounds block downstream of Smo, which was the target of the first generation of Hh antagonists. Further work is required to address the specificity of these new compounds, but the fact that Hh signalling can be blocked at different levels is encouraging and might result in improved treatment options for tumours that depend on sustained Hh activity. Although the idea of Hh inhibition for therapeutic intervention has received broad support, controversies remain about the target cells affected by Hh inhibition. Future studies will need to address the roles of cell-autonomous Hh signalling in epithelial-derived tumours and non-cell autonomous signalling in surrounding stromal cells. Defining the exact role of Hh signalling in the diverse tumours in which the pathway has been implicated is one of the main challenges in designing more effective treatments based on Hh inhibition.
In summary, the Rome meeting on Hh-Gli signalling provided an excellent overview of the present state of this field. The fact that the annual publications on Hh signalling have increased from fewer than 20 in the early 1990s to more than 500 in 2006 is a strong indicator of the increasing interest in this signalling pathway. Although exciting advances have been made, the meeting also provided us with a clear understanding of the remaining questions that need to be addressed before this information can be used to develop curative or preventative strategies for the various human pathologies in which Hh signalling is deregulated.

David J. Robbins

Matthias Hebrok
Acknowledgments
We are grateful to the organizers for putting together an outstanding ensemble of speakers in a remarkable setting. We apologize that, because of strict size limitations, we were not able to discuss all the excellent lectures presented at this meeting. Work in D.J.R.'s laboratory is supported by grants from the National Institutes of Health (NIH; GM064011 & CA82628). M.H.'s laboratory is also supported by grants from the NIH (DK60533 & CA112537). We also thank members of the Hebrok and Robbins laboratories for their insightful comments during the writing of this report.
References
- Beachy PA, Karhadkar SS, Berman DM (2004) Tissue repair and stem cell renewal in carcinogenesis. Nature 432: 324–331 [DOI] [PubMed] [Google Scholar]
- Ingham PW, McMahon AP (2001) Hedgehog signalling in animal development: paradigms and principles. Genes Dev 15: 3059–3087 [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E (1980) Mutations affecting segment number and polarity in Drosophila . Nature 287: 795–801 [DOI] [PubMed] [Google Scholar]
- Panakova D, Sprong H, Marois E, Thiele C, Eaton S (2005) Lipoprotein particles are required for Hedgehog and Wingless signalling. Nature 435: 58–65 [DOI] [PubMed] [Google Scholar]
- Pasca di Magliano M, Hebrok M (2003) Hedgehog signalling in cancer formation and maintenance. Nat Rev Cancer 3: 903–911 [DOI] [PubMed] [Google Scholar]
- Robbins DJ, Goetz JA, Yuan Z, Stegman MA (2005) Inhibitors of the Hedgehog signal transduction pathway. Current Cancer Therapy Review 1: 227–288 [Google Scholar]
- Ruiz í Altaba A, Sanchez P, Dahmane N (2002) Gli and Hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer 2: 361–372 [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST (2007) Overview of structure and function of mammalian cilia. Annu Rev Physiol 69: 377–400 [DOI] [PubMed] [Google Scholar]
- Singh S, Goetz JA, Robbins DJ (2006) Sonic hedgehog. AfCS-Nature Molecule Pages, doi:10.1038/mp.a002208.01
- Singla V, Reiter JF (2006) The primary cilium as the cell's antenna: signalling at a sensory organelle. Science 313: 629–633 [DOI] [PubMed] [Google Scholar]
- Tenzen T, Allen BL, Cole F, Kang JS, Krauss RS, McMahon AP (2006) The cell surface membrane proteins Cdo and Boc are components and targets of the Hedgehog signalling pathway and feedback network in mice. Dev Cell 10: 647–656 [DOI] [PubMed] [Google Scholar]
- Torroja C, Gorfinkiel N, Guerrero I (2005) Mechanisms of Hedgehog gradient formation and interpretation. J Neurobiol 64: 334–356 [DOI] [PubMed] [Google Scholar]
- Yao S, Lum L, Beachy P (2006) The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell 125: 343–357 [DOI] [PubMed] [Google Scholar]
- Zhang W, Kang JS, Cole F, Yi MJ, Krauss RS (2006) Cdo functions at multiple points in the Sonic Hedgehog pathway, and Cdo-deficient mice accurately model human holoprosencephaly. Dev Cell 10: 657–665 [DOI] [PubMed] [Google Scholar]


