Abstract
The present study assessed whether advances in sleep times and circadian phase in older adults might be due to decreased responsiveness of the aging circadian clock to light. Sixteen young (29.3 ± 5.6 yrs) and 14 older adults (67.1 ± 7.4 yrs) were exposed to 4 hours of control dim (10 lux) or bright light (3,500 lux) during the night. Phase shifts of the melatonin rhythm were assessed from the nights before and after the light exposure. Bright light delayed the melatonin midpoint in both young and older adults (p < 0.001). Phase delays for the older subjects were not significantly different from those of the young subjects for either the bright or dim light conditions. The magnitude of phase delays was correlated with both sleep offset and phase angle in the older, but not the younger subjects. The present results indicate that at light intensities commonly used in research as well as clinical practice older adults are able to phase delay to the same extent as younger subjects.
Keywords: circadian, aging, light, advanced phase, melatonin rhythm, phase delay, phase shift, phase angle
Introduction
Aging is characterized by changes in both sleep quality and circadian rhythms.6, 43, 59 Complaints of sleep disturbance and awakenings during the night, daytime sleepiness, and the use of hypnotic medications increases with age.32, 38, 39, 50 Polysomnographic assessment supports the subjective decline in sleep quality. While elderly people may spend just as much time in bed, there is a marked reduction in slow wave sleep and more frequent periods of wakefulness.27, 46, 49 There is a strong association between sleep complaints and indicators of poor physical and mental health in this population, and sleep complaints often resolve with improvements in health.7, 31, 32, 42, 51, 60 However, poor health does not explain all of the sleep complaints of older adults. Both objective and subjective measures of sleep quality may still decline in older adults who remain healthy, and up to one quarter of very healthy older adults still have sleep complaints.7, 32, 42, 46
Elderly subjects, including those who remain very healthy into advanced age, frequently go to sleep and wake earlier, and show an advance in the timing of circadian rhythms in comparison with young adults.12, 22, 36, 44, 52, 57 It is well established that the timing of the circadian clock influences the ability to sleep, with sleep propensity at its maximum close to the nocturnal nadir of the core body temperature rhythm.24, 67 Based on the strong influence of circadian timing on sleep propensity, it has been hypothesized that age-related advances in circadian phase may contribute to the sleep maintenance insomnia and early morning awakenings frequently reported in this population.13, 14, 28, 43, 59
The cause of the age-related advance in circadian timing is not known. There are, however, several possible non-exclusive mechanisms that could contribute to advanced phase. These include a shorter period of the endogenous oscillator, reduced exposure to synchronizing stimuli (zeitgebers) such as light, or altered responsiveness to zeitgebers. Both circadian theory and empirical results indicate that a shorter (faster) circadian clock results in an advance in the timing of entrained circadian rhythms relative to the light-dark cycle (phase angle).20, 29, 47, 64 In addition, inter-individual variance in phase angle increases under low light conditions.64 It is notable that no difference in the intrinsic circadian period between young and older adults was found in an experimental protocol that desynchronizes the sleep/wake cycle from circadian rhythms.21 However, a shorter circadian period in older adults was found when subjects were able to self select their own sleep schedule.62
Advances in the timing of the sleep/wake cycle in elderly adults might also be due to age-related changes in either exposure or responsiveness of the circadian clock to light. It is well established that light is the primary zeitgeber for the circadian timing system, and that the effect of light on circadian timing depends on the time of the light exposure. Light exposure in the late afternoon to early night delays the circadian clock, while light in the morning shifts circadian rhythms to an earlier clock time.23, 25, 40 In humans, the transition point from delays to advances occurs near the temperature minimum, which occurs around 5:00 am in young adults and somewhat earlier in older adults.3, 22, 41 Stable entrainment to the light/dark cycle results from the combined action of phase delays and phase advances induced by daily light exposure.
Daily exposure to light can be substantially reduced in older adults and is a particular problem in nursing homes where patients may have little to no exposure to outdoor sunlight.2, 15 In patients with dementia the level of light exposure has been shown to be positively associated with stability of the rest-activity rhythm and with sleep consolidation.1, 58 Moreover, age-related lens pigmentation decreases the transmission of short wavelength light to the retina.9, 18, 48 Given that the circadian system is most sensitive to light in the short (blue) wavelength region, the reduced transmission of short wavelengths of light with age may be particularly detrimental for circadian timing in older adults.8, 18, 33, 34, 54 Together, these results suggest that reduced exposure to light at the level of the retina may also contribute to age-related changes in circadian phase.
There is also considerable evidence for age-related alterations in the responsiveness of the circadian clock to light from animal models. Aged rodents have smaller light-induced phase shifts of circadian activity rhythms and lower light-induced expression of immediate-early genes in the suprachiasmatic nucleus (SCN) when compared to young animals.5, 53, 66 In older humans, however, only phase advances were found to be decreased following three cycles of high-intensity long-duration light exposure.35 As concluded from that study, a reduction in phase advances without attenuation of phase delays cannot explain the advance in phase that is prevalent in older adults. Of several possible explanations that were put forward for the lack of age-related changes in phase delays was the possibility that the light stimulus (10,000 lux for 5 hours) was saturating, and therefore obscured age-related differences. In the present study we assessed whether phase delays would be reduced in older adults following exposure to a single 4-hour moderately bright pulse of light.
Methods
Subjects
Young and older volunteers were recruited from advertisements in newspapers, flyers, and talks at community centers. Young subjects were healthy with no current illness and no use of medications aside from birth control. Older subjects with unstable or serious illness, or who were taking beta-blockers, were excluded from participation. The older adults completed comprehensive neuropsychological testing at the Memory Disorders Research Core of the Alzheimer’s Disease Center at Northwestern University. In addition to testing, subjects were required to provide an informant who could answer questions about the subject’s functional capacity in daily living activities. The average score on the Mini-mental state exam was 28.4 ± 2.24, and all subjects but one scored above 26. This subject also had scores from the comprehensive neuropsychological test battery that were in the impaired range for age and education. The phase delay for this subject was close to the group average, and data from this subject was included in the analysis. Three other older adults who completed the study had scores from 1 - 4 components of the comprehensive test battery (visuoperceptual and/or learning/memory) that were in the impaired range for age and education. All other scores were within normal limits for the age group. These subjects were considered to have mild cognitive impairments without dementia, based on the absence of problems in daily living. None of the subjects were shift workers or had crossed more than two time zones in the two months prior to participation in the study. This research was approved by the Institutional Review Board and each subject provided written informed consent. 22 young and 24 older adults were enrolled in the study. Data from 10 older subjects were not included in the analysis due to melatonin levels that were too low to detect phase changes (n = 5); the inability to obtain blood samples throughout the night (n = 2); or other technical problems (n = 3). Three young subjects chose not to complete the study; data from 3 others were not included due to low melatonin levels (n = 2) or other technical problems (n = 1). Data presented are from the 16 young adults (8M, 8F, mean ± SD: 29.3 ± 5.6 yrs) and 14 older adults (7M, 7F, mean ± SD: 67.1 ± 7.4 yrs) who completed the study.
Experimental Protocol
Prior to admission, each subject underwent a 3 week baseline period in which they were instructed to maintain a regular, self-selected sleep schedule (varying no more than ± 30 minutes from their habitual sleep time). Subjects maintained sleep logs during this 3-week period. On the week prior to their stay subjects wore an activity monitor (Cambridge Neurotechnology L.T.D., Cambridge, England) on their wrist to objectively verify sleep and wake times. The sleep period during the hospital stay was determined from the self-selected habitual sleep time prior to admission.
At the end of the 3 week baseline subjects were admitted to the General Clinical Research Center (GCRC) at Northwestern Memorial Hospital for a 4 night/ 3 day hospital stay under constant conditions 56, 63. The experimental protocol is illustrated in Figure 1. Subjects were admitted to the GCRC during the evening hours. Light levels were maintained at 10 lux during waking hours, and turned off by nursing staff during the 8 hour sleep period. Core body temperature was collected at one-minute intervals throughout the study with a rectal thermistor and data collection unit (Mini-Logger, Mini-Mitter, Inc., Bend, OR). Subjects were provided with small snacks (150-250 Kcal, based on normal food intake, 50% carbohydrate, 20% protein, and 30% fat) every two hours throughout the study. Water was available ad lib. Subjects remained in a semi-recumbent or seated position throughout the study with the exception of one daily shower and voiding as necessary. They were not allowed to engage in strenuous activity.
Figure 1.
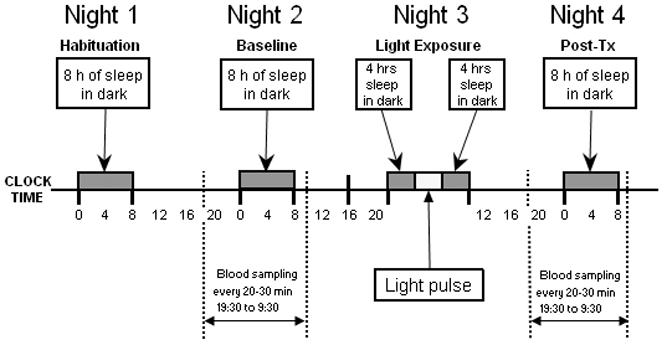
Schematic of the experimental protocol. Subjects were admitted for 4 nights and 3 days under constant conditions during daytime hours with 8 hours sleep in dark at their habitual time (dark bars). Half of the subjects also participated in a 4 night/3 day dim control condition (see methods). Blood samples were taken throughout the baseline and post-treatment nights to assess light-induced changes in the circadian melatonin rhythm. On the third night, subjects were exposed to 4 hours of dim control or bright light (3,500 lux for 3 h, plus 1 hour of intermediate illumination), beginning 5 hours before the temperature minimum.
The first night of admission was a habituation night to adjust to the experimental conditions. On the second day of the study, blood was sampled throughout the night, beginning at 19:30 until 9:30 the following morning for baseline measurement of the circadian melatonin profile. Subjects received a sterile heparin-lock catheter for intravenous blood sampling in the forearm vein 2 hours before the beginning of blood sampling. During periods of wake, blood was sampled from a stopcock attached directly to the intravenous catheter. During sleep periods blood was sampled through tubing extending to an adjacent room so as not to disturb the subject. The intravenous line was kept patent with a slow drip of heparinized saline (750 IU heparin in 9.0 g NACl/l).
On the third night, subjects went to bed earlier than usual for a 4 hour sleep period, then were awoken for exposure to bright fluorescent light via commercially available light-boxes (Sunbox, Co, Gaithersburg, MD: 3500 lux for 3 hours, with a ramp up and ramp down period of 15 minutes at approximately 1000 lux and 15 minutes at approximately 2000 lux). Thus, the total duration of light exposure was 4 h. The light exposure began 5 hours prior to the individual’s core body temperature minimum (Tmin) on the baseline night (described below), a time known to induce maximal phase delays (Van Cauter, 1999). Upon completion of light exposure, subjects were allowed to sleep for an additional 4 hours. Blood sampling was resumed from 19:30 until 9:30 on the post-treatment night to assess the effect of bright light exposure on the timing of the melatonin rhythm.
Half of the subjects (8 young and 7 elderly) also participated in a control condition where they were awakened and exposed to background dim light (10 lux) for 4 hours at the same time of the night (beginning 5 hours before Tmin). For 77% of the subjects the light condition was their first or only admission. There was a minimum of 3 weeks between each admission, with an average interval of 9.75 ± 10.6 weeks. The older group (n=14) was initially asked to participate in a single admission with bright light. Seven of the 14 older subjects were brought back at a later time in order to verify that there was no difference between young and old groups in the response to the dim light. Despite the variability in the interval between the bright and dim light exposure, this limitation did not affect interpretation of the results because the bright light admissions were conducted concurrently and there was no significant phase change in response to the dim light for either group.
Circadian Phase Assessments
Blood samples (2 ml) were collected on the nights before and after light exposure at 20 - 60 min intervals (q 20 min in the evening, q 60 min in the middle of the night, and q 30 min in the morning). The sampling interval was variable in order to reduce the total volume of blood sampled throughout the night while maximizing the detection of phase changes on the rising and then declining portions of the melatonin profile. The plasma collected was frozen at -80 ° C for later radioimmunoassay of melatonin. Plasma melatonin levels were measured with a double-antibody RIA using commercially available reagents (Stockgrand, Guilford, Surrey, UK) 56. The lower limit of sensitivity of the assay was 2.5 pg/ml. The intra-assay coefficient of variation averaged 17.5% for values <10 pg/ml, 8.6% in the range of 10-30 pg/ml, and 5.2% for values >30 pg/ml. The inter-assay coefficient of variation averaged 20% for values <10 pg/ml and 13.5% for values ≥ 10 pg/ml. All samples from the same subject were measured in the same assay.
Phase shifts were calculated from the midpoint of the melatonin rhythm, as this phase marker provides a more reliable measure of circadian phase than the core body temperature rhythm 3. Briefly, melatonin levels in plasma were adjusted to a percent of maximum (3 highest values), smoothed with a Lowess (Cleveland) curve fitting procedure, and interpolated at 1-minute intervals. The time that the rising phase of the melatonin profile (dim light melatonin onset) reached and remained above 50% of maximum levels (DLMO 50%) and the time that the declining phase of the melatonin profile reached and remained below 50% of maximum levels (DLMOff 50%) was determined for each subject. The melatonin midpoint was the average of DLMO 50% and DLMOff 50%. In two of the young subjects (bright light) and 4 of the older subjects (dim light) the offset of the melatonin profile was not obtained due to technical difficulties. For these subjects, the value of DLMO 50% was used.
Tmin on the baseline (second) night was estimated using both Cleveland and Cosine procedures. The 24 hour profiles of temperature were edited to remove obvious artifacts and fit using the Cleveland regression procedure and by fit of a simple Cosine curve, demasked for sleep, with software provided by C. Eastman.19, 37 In most cases, the average of the two measures was used as the estimate of Tmin. However, in some cases there was a large discrepancy (e.g. > 1 h) in estimates of Tmin derived from the Cleveland and Cosine procedures. Discrepancies were due to a section of missing data, a low amplitude rhythm, or a modest rise in temperature in the middle of the night, which affected the two curve fitting procedures differently. In order to ensure that the light exposure was within the phase delay zone in these cases (assuming a crossover point from delays to advances around Tmin), we used the earlier of the two phase estimates. We report the time of Tmin in this report as the demasked Cosine value, as recent analyses indicate that the baseline circadian temperature rhythm analyzed with a simple cosine curve is a more reliable estimate of circadian phase than when estimated by the Cleveland curve fitting procedure.3
Statistical Analysis
The magnitude of phase shifts following exposure to bright light or control light were determined by the difference in the timing of the baseline and post-treatment melatonin midpoint. Results, presented as average ± sd, were analyzed by multifactor repeated measures ANOVA (young vs. old, light vs. control) with NCSS 2000 (NCSS Statistical Software, Kaysville, UT). Baseline vs. post-treatment change in phase was compared by pairwise comparisons. The timing of the individual baseline phase markers, and timing of light exposure in the two groups was compared by t-test. A modification of the O’Brien method was used to provide a global statistic of phase differences between the two groups.11, 45 The multiple endpoints of circadian phase (sleep onset, sleep offset, DLMO 50%, melatonin midpoint, DLMoff 50%, Tmin) were converted to ranks. The ranks were then summed over all of the endpoints for each subject, and a one-tailed Wilcoxin signed-rank test was conducted on the summed ranks.
Results
Average sleep times and circadian phase are depicted in Fig. 2. The sleep onset of the older subjects, as measured by actigraphy on the week prior to admission, was 1 hour earlier than the young adults (p = 0.011). There was a trend towards earlier timing of sleep offset and circadian phase markers in the older subjects, however, none of these differences reached statistical significance when analyzed individually (Table 1). When all 6 phase markers were assessed together as a global assessment of sleep and circadian phase, the phase of the older subjects was earlier than that of young subjects (p = 0.052).
Figure 2.
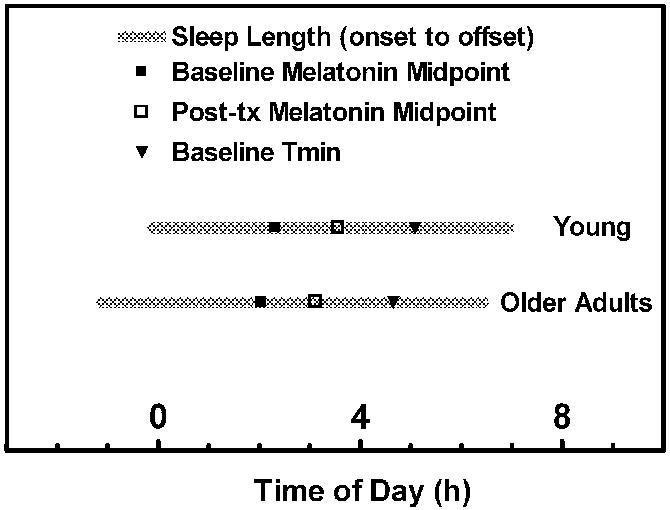
Average sleep times and circadian phase markers for the young and older subjects. The interval between sleep onset and sleep offset, measured by actigraphy on the week prior to admission, is depicted by the gray bar. The timing of Tmin (▼) and melatonin midpoint on the baseline night (■) and on the night following the light exposure (□) are indicated. The global timing of sleep (onset and offset) and circadian phase markers (DLMO 50%, melatonin midpoint, DLMOff 50%, Tmin) at baseline was earlier in the older adults (p = 0.052). The phase angles between the circadian phase markers and the timing of sleep were not different for the two groups.
Table 1.
Sleep Times and Circadian Phase
| Young | Older | |
|---|---|---|
| Sleep Onset | 23.89 ± 0.87 | 22.88 ± 1.02* |
| Sleep Offset | 6.95 ± 0.63 | 6.44 ± 1.27 |
| Baseline Tmin | 5.09 ± 1.80 | 4.65 ± 1.62 |
| Baseline DLMO 50% | 22.74 ± 1.42 | 22.19 ± 1.33 |
| Baseline Melatonin Midpoint | 2.32 ± 1.36 | 2.04 ± 1.18 |
| Post-tx Melatonin Midpoint | 3.57 ± 1.41 | 3.12 ± 1.29 |
Times are in decimal clock time ± SD
p = 0.011
The phase angles between the circadian phase markers and the timing of sleep were comparable in the two groups (Fig. 2). In the young subjects, DLMO 50% was 1.34 ± 1.80 h prior to sleep onset and 8.19 ± 1.54 h prior to sleep offset. In the older adults, DLMO 50% was 0.86 ± 1.76 h prior to sleep onset and 8.42 ± 1.65 h prior to sleep offset. Melatonin midpoint was about 4 1/2 h prior to sleep offset in both groups (Young: 4.46 ± 1.29 h; Older: 4.55 ± 1.37 h). Tmin was 1.42 ± 1.34 h prior to sleep offset in young subjects, and 1.99 ± 1.83 h prior to sleep offset in older adults.
The time of light exposure was 0.83 h earlier in the older subjects (1.0 ± 1.20 h vs. 1.83 ± 1.06 h, p = 0.054), with light exposure occurring at a comparable time in the two groups relative to Tmin (Young: -3.20 ± 1.17 h, Older: -3.56 ± 0.65 h before Tmin), DLMO 50% (Young: 2.81 ± 1.27 h, Older: 3.08 ± 1.22 h after DLMO 50%), or melatonin midpoint (Young: -0.54 ± 1.05 h, Older: -1.04 ± 1.16 h prior to melatonin midpoint).
Baseline and post-treatment melatonin profiles for the 4 conditions are shown in Fig. 3. Although there was a slight delay drift of the average melatonin profile from baseline to posttreatment in the control dim light condition, this delay was not statistically significant (Fig. 4, n = 7 - 8). Bright light exposure delayed the melatonin rhythm in both the young [F(1,25) = 139.86, p < 0.001] and older [F(1,27) = 43.05, p < 0.001] groups. For both groups, the magnitude of phase delays following bright light exposure were significantly greater than following the dim control condition [F(1,44) = 26.31, p < 0.001]. Phase delays for the older subjects were not statistically different from those of young subjects for either treatment condition (Fig. 4). Power analysis indicated that with an alpha value of 0.05 (two-sided) and a beta value of 0.80, the study was powered to detect a difference of 0.52 h.
Figure 3.
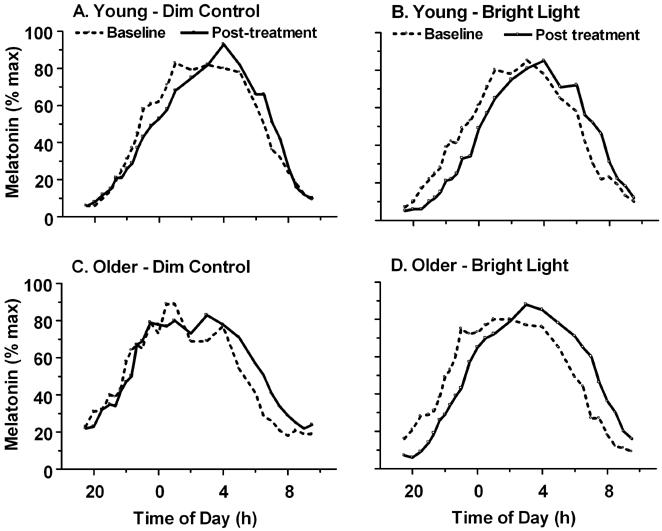
Treatment-induced changes of the nocturnal melatonin rhythm in young (A, B) and older (C, D) adults. Subjects were exposed to 4 hours of dim control (A, C) or bright (B, D) light on the treatment night. Melatonin levels, measured throughout the night prior to (dashed) and following (solid line) the treatment night, are plotted as a percent of maximum.
Figure 4.
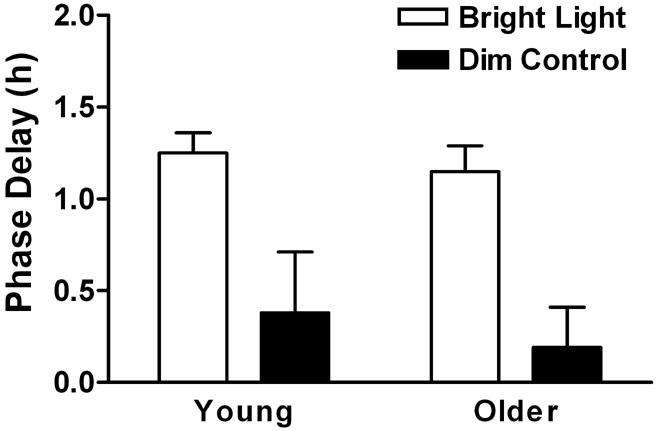
Treatment-induced phase delays of the melatonin midpoint. 4 hours exposure to bright light (open bars) delayed the melatonin rhythm in both the young (p < 0.001, n = 16) and older (p < 0.001, n = 14) groups. The melatonin midpoint did not significantly change after awakening for 4 hours under dim light (n = 7 - 8). Phase delays following bright light exposure were significantly greater than following the dim control condition (p < 0.001). The magnitude of phase delays were not significantly different between the young and older adults.
Correlation analyses were conducted in order to assess whether the variability in the magnitude of light-induced phase delays was associated with the timing of the light exposure or baseline circadian phase parameters. There was no association between the magnitude of phase delays and the timing of the light exposure relative to circadian phase at baseline (e.g. the time of light exposure relative to baseline Tmin, melatonin midpoint, or sleep offset). There was also no association between the magnitude of light-induced phase delays and baseline circadian phase as measured by Tmin or melatonin midpoint. However, the magnitude of light-induced phase delays was significantly correlated with average sleep offset on the week prior to admission in the older (r = 0.70, 0 = 0.008), but not in the young subjects (Fig. 5).
Figure 5.
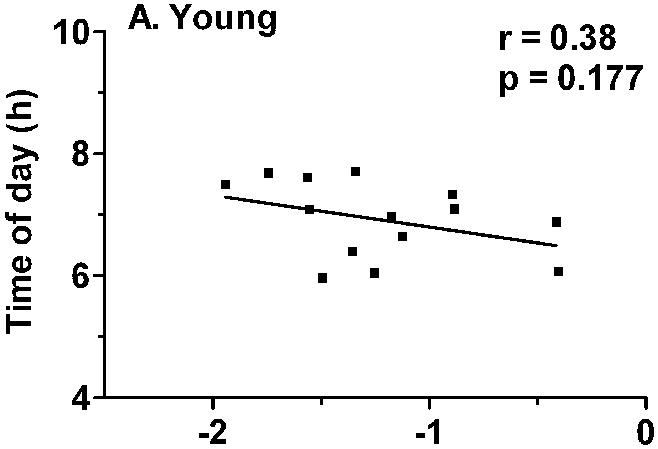
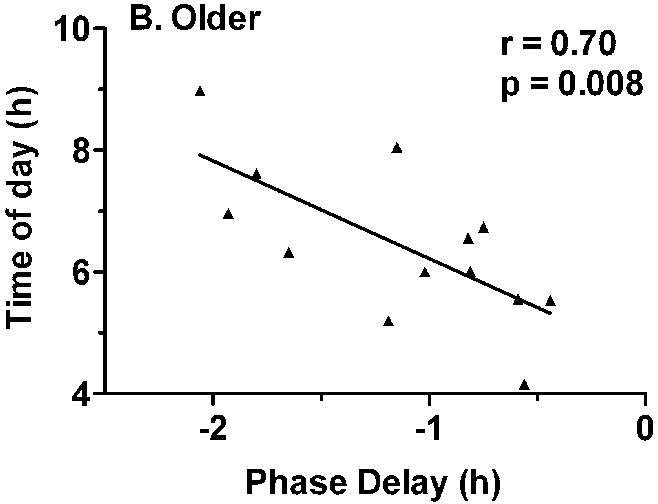
Correlation between habitual sleep offset and the magnitude of light-induced phase delays. A) There was no association between sleep offset and phase delays in the younger subjects. B) In the older subjects, the magnitude of phase delays was significantly correlated with habitual time of awakening (r = 0.70, p = 0.008), with earlier wake time associated with smaller phase delays.
The magnitude of phase delays were not correlated with sleep duration in either group, but significant correlations were observed between the magnitude of phase delays and phase angle in the older adults (Tmin to sleep offset: r = 0.56, p = 0.048; Melatonin midpoint to sleep offset, r = 0.68, p = 0.010, Fig. 6). The older subjects who woke early and/or had early sleep offset relative to circadian phase tended to have small magnitude phase delays, while those subjects who woke up later tended to have larger magnitude phase delays (Fig. 7). Similar relationships were not observed for the younger subjects.
Figure 6.
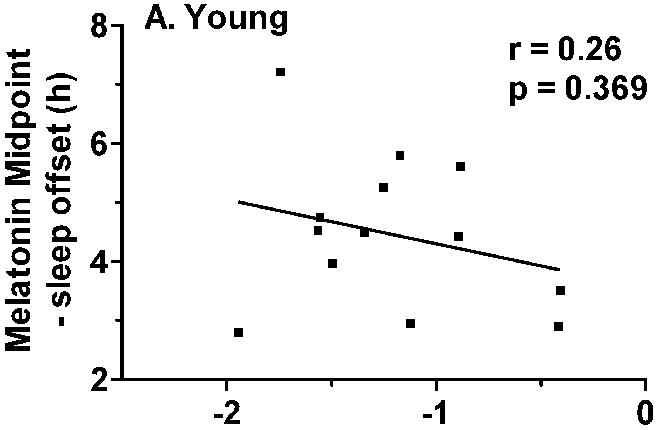
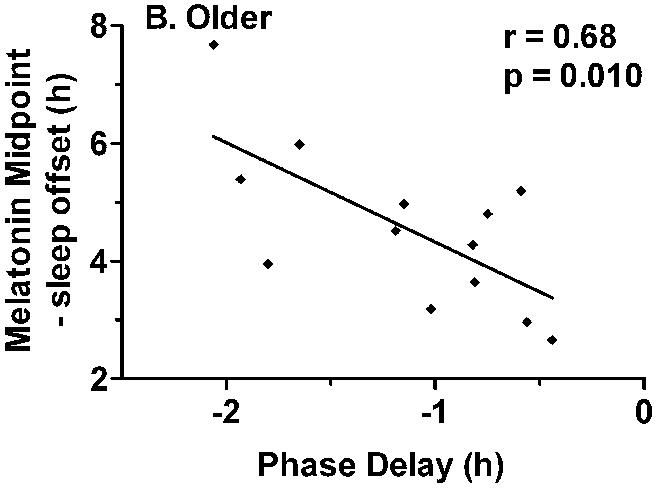
Correlation between phase angle and the magnitude of light-induced phase delays. Phase angle was calculated as the interval between melatonin midpoint and habitual sleep offset. A) There was no association between phase angle and phase delays in the younger subjects. B) In the older group of subjects, the magnitude of phase delays was significantly correlated with phase angle r = 0.68, p = 0.010). A shorter interval between melatonin midpoint and sleep offset was associated with smaller phase delays.
Figure 7.
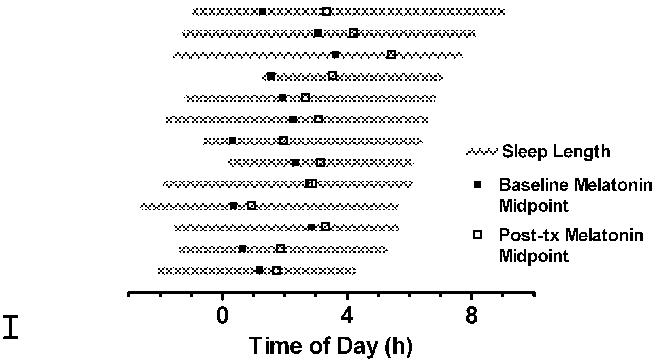
Illustration of the association between phase delays of the melatonin midpoint and sleep parameters in individual older subjects. Habitual sleep onset and sleep offset is depicted by the beginning and end of the gray bar. Phase delays are the difference between baseline (■) and post-treatment (□) melatonin midpoints. Both early wake times and/or a smaller interval between sleep offset and baseline circadian phase (e.g. melatonin midpoint) were associated with smaller phase shifts.
Discussion
This study compared the effects of moderately-bright broad spectrum light and control dim light exposure on phase delays of the circadian melatonin rhythm in young and older adults studied under regular sleep/wake schedules. The timing of both sleep and circadian phase markers of the older adults was earlier than that of the younger subjects. Light-induced phase delays were greater than following dim light exposure for both age groups. Age-related differences in the magnitude of phase delays following either the bright or dim light conditions were not observed. There was, however, an association between the magnitude of phase delays and both phase angle and habitual wake time in the older subjects.
Numerous studies have reported advances in the timing of sleep and circadian phase with age.22, 36, 43, 44, 57 An advance in phase could theoretically be related to a shortening of circadian period, an alteration in the phase angle between circadian phase and sleep timing, or an alteration in the response to light. Advanced circadian phase can be associated with a shorter circadian period in humans, although the relationship is weaker in older adults.20, 29 When measured under a forced desynchrony protocol, average circadian period was found not to differ between young and older adults.21 However, a shortened period of the temperature, but not the sleep/wake rhythm, was shown in older adults under free running conditions.62 It is notable that a shortening of circadian period without a change in sleep period also increases the phase angle between circadian phase and sleep.62 In the present study we found that the timing of both sleep and circadian phase advanced, without changing the relation between the two measures.
Based on the established relationship between circadian phase and sleep propensity,24, 67 the advanced circadian phase of older adults has been postulated to contribute to impaired sleep maintenance in this population.14, 22, 28 This hypothesis was supported by findings that sleep of older people is particularly disrupted when scheduled on the rising limb of the temperature rhythm.28 In addition, the timing of sleep and the timing of the body temperature rhythm are correlated, and exposure to light in the evening both delays Tmin and improves sleep efficiency.13, 14 However, light-induced delays in Tmin and improvements in sleep efficiency are not correlated, and other studies have shown either no age-related phase-angle differences between circadian phase markers and wake time or even a reduced interval between the two measures.13, 17, 30, 55
Several studies specifically assessed whether impaired sleep consolidation in older adults was related to alterations in the phase angle between circadian phase and sleep timing. A report of 60 healthy adults, age 40 - 84 with some subjects selected for advanced sleep phase, found that although there was no significant difference in Tmin between sleep disturbed and non-sleep disturbed older adults, the earlier the Tmin, the more disturbed, and shorter, was a subject’s sleep.16 In contrast, in a group of 48 healthy elderly subjects there was no association between the temperature-wake phase angle and sleep consolidation,17 and a study of 42 healthy elderly subjects with reductions in sleep maintenance showed that the shorter the interval between melatonin midpoint and wake, the greater the reduction in sleep maintenance.55 Together, these studies suggest that although advances in circadian phase may reinforce difficulty in maintaining sleep, phase changes alone do not explain sleep maintenance insomnia in older adults.
The present study tested the hypothesis that the advanced phase of older adults is a result of a reduction in the phase delaying response to evening light. The finding in this report that young and older subjects do not differ in their phase delaying response to a single 4 hour moderately-bright light pulse is similar to results from Klerman and colleagues.35 In the present study we did not assess the phase advancing response to light in the morning. Klerman et al, however, administered 5 hours of light exposure (∼10,000 lux) over three consecutive days in either the phase advance or phase delay zone. They observed a significant age-related reduction in the magnitude of phase advances, but not phase delays. Thus, results of both of these studies do not support the hypothesis that the advanced phase of older adults is due to a major reduction in the phase delaying response to light or an increase in the phase advancing effects of light. The present results further demonstrate that the circadian clock of older adults is able to phase delay in response to light of an intensity and duration similar to that used in clinical practice.
Results from these human studies contrast with those obtained in rodents, in which age decreases phased shifts of circadian activity rhythms and immediate-early gene expression in the SCN.5, 53, 66 Old hamsters were shown to be approximately 20 times less sensitive to the phase advancing effects of low intensity monochromatic light.65, 66 At higher light intensities, however, this age-related difference was not observed. We recently reported that in humans the duration of light exposure has a greater impact on the magnitude of phase shifts than the intensity of the light exposure.4 It is possible, therefore, that the 4 - 5 hours of light exposure used in both the present study and that of Klerman and colleagues elicited phase delays that were close to maximal, thus obscuring age-related differences. The possibility of age-related differences with shorter durations, lower levels, or different times of light exposure within the circadian cycle has yet to be tested.
The average daily exposure to light can be dramatically reduced in older populations, and pigmentation of the lens with age causes a further reduction in transmittance of short wavelength light to the retina.2, 9, 15, 18 Given that short wavelength (blue) light is the most potent wavelength region for the circadian clock, reduced transmission of this wavelength may have particular consequences for circadian timing of older adults.8, 18, 54 In support of this hypothesis, two recent studies found that short-wavelength light-induced melatonin suppression is reduced in older adults.33, 34 Thus, while it remains possible that reduced responsiveness to low daily light exposure might contribute to the advance in phase observed in older adults, the present results clearly indicate that given light of adequate intensity and duration, older adults are able to phase delay to the same extent as younger subjects.
The magnitude of phase delays in the older subjects was, however, related to both the timing of sleep offset and the phase angle between circadian phase markers and sleep offset. It is possible that similar associations were not observed in the younger subjects due to restriction of sleep offset times by work and school schedules. Those older adults who habitually woke early and/or had a shorter interval between circadian rhythms and sleep offset also showed the smallest phase delays in response to light exposure (Fig. 7). When young subjects are given a short 6-hour sleep period over 14 nights, light-induced phase advances are only half the magnitude as when the same subjects are allowed a 9-hour sleep period.10 It may also be relevant that these young subjects were required to go to bed at the same time for both conditions, but woke up to 3 hours later during the long nights. In the present study, we found a significant association between the magnitude of phase delays and wake time in older adults, but not between the magnitude of phase delays and sleep duration. Together, these results indicate that sleep duration and/or habitual wake time may alter the responsiveness of the clock to light. Individual differences in period may also contribute to variability in the phase shifting effects of light,25 while morning light exposure, increased sleepiness in the evening, and/or an effect of sleep state itself on the circadian clock could also contribute an advance in sleep and circadian phase.26, 28
It is also important to note that while older adults tend to be, on average, early in phase, not all older adults are phase advanced relative to young controls.30, 41, 61 The substantial variability in sleep length and circadian phase in the older adults in the present report is consistent with data reported by Duffy and colleagues, who found that sleep length and Tmin were more variable in older than in young subjects.30 The present results suggest that older adults may show predictable variability in the magnitude of phase delays in response to light. Thus, in addition to assessing whether the response to light of shorter wavelengths, lower intensity, and/or shorter duration is altered, future studies should examine whether those older adults with advanced wake times respond to light in a manner different from those older adults whose sleep offset remains similar to that of the younger population.
Acknowledgments
We thank the research subject volunteers for their participation in these studies, the administrators and staff of the General Clinical Research Center, and Dr. Nancy Johnson from the Memory Disorders Research Core for assistance with the Neuropsychological testing. Dr. Borko Jovanovich provided statistical consulting. This work was supported in part by a grant from the national Center on Clinical Research Resources (NCRR-0048), Public Health Service Grants R01 HL67604, P01 AG11412, and K01 AG00810 and an Alzheimer’s Disease Core Center grant (AG13854) from the National Institute on Aging to Northwestern University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, Levi L. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1:22–36. doi: 10.1207/S15402010BSM0101_4. [DOI] [PubMed] [Google Scholar]
- 2.Ancoli-Israel S, Kripke D, Jones D, Parker L, Hanger M. Light exposure and sleep in nursing home patients. Soc Light Treatment Biol Rhythms Abstr. 1991;3:18. [Google Scholar]
- 3.Benloucif S, Guico MJ, Reid KJ, Wolfe LF. L’Hermite-Balériaux M, and Zee PC. Stability of melatonin and temperature as circadian phase markers and their relation to sleep times in humans. J Biol Rhythms. 2005;20:178–188. doi: 10.1177/0748730404273983. [DOI] [PubMed] [Google Scholar]
- 4.Benloucif S, Guico MJ, Wolfe LF, L’Hermite-Baleriaux M, Zee PC. Effect of increasing light intensity vs. increasing light duration on phase shifts of the circadian clock of humans. Sleep. 2003;26:A103. [Google Scholar]
- 5.Benloucif S, Masana MI, Dubocovich ML. Light-induced phase shifts of circadian activity rhythms and immediate early gene expression in the suprachiasmatic nucleus are attenuated in old C3H/HeN mice. Brain Res. 1997;747:34–42. doi: 10.1016/s0006-8993(96)01182-1. [DOI] [PubMed] [Google Scholar]
- 6.Bliwise DL. Sleep in normal aging and dementia. Sleep. 1993;16:40–81. doi: 10.1093/sleep/16.1.40. [DOI] [PubMed] [Google Scholar]
- 7.Bliwise DL, King AC, Harris RB, Haskell WL. Prevalence of self-reported poor sleep in a healthy population aged 50-65. Soc Sci Med. 1992;34:49–55. doi: 10.1016/0277-9536(92)90066-y. [DOI] [PubMed] [Google Scholar]
- 8.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brainard GC, Rollag MD, Hanifin JP. Photic regulation of melatonin in humans: ocular and neural signal transduction. J Biol Rhythms. 1997;12:537–546. doi: 10.1177/074873049701200608. [DOI] [PubMed] [Google Scholar]
- 10.Burgess HJ, Eastman CI. Short nights attenuate light-induced circadian phase advances in humans. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2005-0536. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buxton OM, Copinschi G, Van Onderbergen A, Karrison TG, Van Cauter E. A benzodiazepine hypnotic facilitates adaptation of circadian rhythms and sleep-wake homeostatis to an eight hour delay shift simulating westward jet lag. Sleep. 2000;23:915–927. [PubMed] [Google Scholar]
- 12.Buysse DJ, Browman KE, Monk TH, Reynolds CF, 3rd, Fasiczka AL, Kupfer DJ. Napping and 24-hour sleep/wake patterns in healthy elderly and young adults. J Am Geriatr Soc. 1992;40:779–786. doi: 10.1111/j.1532-5415.1992.tb01849.x. [DOI] [PubMed] [Google Scholar]
- 13.Campbell SS, Dawson D, Anderson MW. Alleviation of sleep maintenance insomnia with timed exposure to bright light. J Am Geriatr Soc. 1993;41:829–836. doi: 10.1111/j.1532-5415.1993.tb06179.x. [DOI] [PubMed] [Google Scholar]
- 14.Campbell SS, Gillin JC, Kripke DF, Erikson P, Clopton P. Gender differences in the circadian temperature rhythms of healthy elderly subjects: relationships to sleep quality. Sleep. 1989;12:529–536. [PubMed] [Google Scholar]
- 15.Campbell SS, Kripke DF, Gillin JC, Hrubovcak JC. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiol Behav. 1988;42:141–144. doi: 10.1016/0031-9384(88)90289-2. [DOI] [PubMed] [Google Scholar]
- 16.Campbell SS, Murphy PJ. Relationships between sleep and body temperature in middle-aged and older subjects. J Am Geriatr Soc. 1998;46:458–462. doi: 10.1111/j.1532-5415.1998.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 17.Carrier J, Monk TH, Reynolds CF, 3rd, Buysse DJ, Kupfer DJ. Are age differences in sleep due to phase differences in the output of the circadian timing system? Chronobiol Int. 1999;16:79–91. doi: 10.3109/07420529908998714. [DOI] [PubMed] [Google Scholar]
- 18.Charman WN. Age, lens transmittance, and the possible effects of light on melatonin suppression. Ophthalmic Physiol Opt. 2003;23:181–187. doi: 10.1046/j.1475-1313.2003.00105.x. [DOI] [PubMed] [Google Scholar]
- 19.Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979;74:829–836. [Google Scholar]
- 20.Czeisler CA, Allan JS, Strogatz SH, Ronda JM, Sanchez R, Rios CD, Freitag WO, Richardson GS, Kronauer RE. Bright light resets the human circadian pacemaker independent of the timing of the sleep-wake cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 21.Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- 22.Czeisler CA, Dumont M, Duffy JF, Steinberg JD, Richardson GS, Brown EN, Sanchez R, Rios CD, Ronda JM. Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340:933–936. doi: 10.1016/0140-6736(92)92817-y. [DOI] [PubMed] [Google Scholar]
- 23.Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- 24.Czeisler CA, Weitzman E, Moore-Ede MC, Zimmerman JC, Knauer RS. Human sleep: its duration and organization depend on its circadian phase. Science. 1980;210:1264–1267. doi: 10.1126/science.7434029. [DOI] [PubMed] [Google Scholar]
- 25.Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents. II. The variability of phase response curves. J Comp Physiol. 1976;106:253–266. [Google Scholar]
- 26.Deboer T, Vansteensel MJ, Detari L, Meijer JH. Sleep states alter activity of suprachiasmatic nucleus neurons. Nat Neurosci. 2003;6:1086–1090. doi: 10.1038/nn1122. [DOI] [PubMed] [Google Scholar]
- 27.Dement WC, Miles LE, Carskadon MA. “White paper” on sleep and aging. J Am Geriatr Soc. 1982;30:25–50. doi: 10.1111/j.1532-5415.1982.tb03700.x. [DOI] [PubMed] [Google Scholar]
- 28.Dijk DJ, Duffy JF, Czeisler CA. Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol Int. 2000;17:285–311. doi: 10.1081/cbi-100101049. [DOI] [PubMed] [Google Scholar]
- 29.Duffy JF, Czeisler CA. Age-related change in the relationship between circadian period, circadian phase, and diurnal preference in humans. Neurosci Lett. 2002;318:117–120. doi: 10.1016/s0304-3940(01)02427-2. [DOI] [PubMed] [Google Scholar]
- 30.Duffy JF, Dijk DJ, Klerman EB, Czeisler CA. Later endogenous circadian temperature nadir relative to an earlier wake time in older people. Am J Physiol. 1998;275:R1478–1487. doi: 10.1152/ajpregu.1998.275.5.r1478. [DOI] [PubMed] [Google Scholar]
- 31.Foley DJ, Monjan A, Simonsick EM, Wallace RB, Blazer DG. Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep. 1999;22(Suppl 2):S366–372. [PubMed] [Google Scholar]
- 32.Foley DJ, Monjan AA, Brown SL, Simonsick EM, Wallace RB, Blazer DG. Sleep complaints among elderly persons: an epidemiologic study of three communities. Sleep. 1995;18:425–432. doi: 10.1093/sleep/18.6.425. [DOI] [PubMed] [Google Scholar]
- 33.Green KA, Wolfe LF, Gaskill MB, Goldman N, Zee PC, Benloucif S. Comparison of melatonin suppression by blue wavelength light in young and older adults. Sleep. 2004;27:A76. [Google Scholar]
- 34.Herljevic M, Middleton B, Thapan K, Skene DJ. Light-induced melatonin suppression: age-related reduction in response to short wavelength light. Exp Gerontol. 2005;40:237–242. doi: 10.1016/j.exger.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Klerman EB, Duffy JF, Dijk DJ, Czeisler CA. Circadian phase resetting in older people by ocular bright light exposure. J Investig Med. 2001;49:30–40. doi: 10.2310/6650.2001.34088. [DOI] [PubMed] [Google Scholar]
- 36.Lieberman HR, Wurtman JJ, Teicher MH. Circadian rhythms of activity in healthy young and elderly humans. Neurobiol Aging. 1989;10:259–265. doi: 10.1016/0197-4580(89)90060-2. [DOI] [PubMed] [Google Scholar]
- 37.Martin SK, Eastman CI. Medium-intensity light produces circadian rhythm adaptation to simulated night-shift work. Sleep. 1998;21:154–165. [PubMed] [Google Scholar]
- 38.Middlekoop HAM, Smilde-van den Doel DA, Neven AK, Kamphuisen HAC, Springer CP. Subjective sleep characteristics of 1,485 males and females aged 50-93: Effects of sex and age, and factors related to self-evaluated quality of sleep. J Gerontol: Med Sci. 1996;51A:M108–M115. doi: 10.1093/gerona/51a.3.m108. [DOI] [PubMed] [Google Scholar]
- 39.Miles LE, Dement WC. Sleep and aging. Sleep. 1980;3:1–220. [PubMed] [Google Scholar]
- 40.Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- 41.Monk TH, Buysse DJ, Reynolds CF, 3rd, Kupfer DJ, Houck PR. Circadian temperature rhythms of older people. Exp Gerontol. 1995;30:455–474. doi: 10.1016/0531-5565(95)00007-4. [DOI] [PubMed] [Google Scholar]
- 42.Morin CM, Gramling SE. Sleep patterns and aging: comparison of older adults with and without insomnia complaints. Psychol Aging. 1989;4:290–294. doi: 10.1037//0882-7974.4.3.290. [DOI] [PubMed] [Google Scholar]
- 43.Myers BL, Badia P. Changes in circadian rhythms and sleep quality with aging: mechanisms and interventions [published erratum appears in Neurosci Biobehav Rev 1996 Summer;20(2):I-IV] Neurosci Biobehav Rev. 1995;19:553–571. doi: 10.1016/0149-7634(95)00018-6. [DOI] [PubMed] [Google Scholar]
- 44.Nakazawa Y, Nonaka K, Nishida N, Hayashida N, Miyahara Y, Kotorii T, Matsuoka K. Comparison of body temperature rhythms between healthy elderly and healthy young adults. Jpn J Psychiatry Neurol. 1991;45:37–43. doi: 10.1111/j.1440-1819.1991.tb00503.x. [DOI] [PubMed] [Google Scholar]
- 45.O’Brien PC. Procedures for comparing samples with multiple en-points. Biometrics. 1984;40:1079–1087. [PubMed] [Google Scholar]
- 46.Ohayon MM, Carskadon MA, Guilleminault C, Vitiello MV. Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep. 2004;27:1255–1273. doi: 10.1093/sleep/27.7.1255. [DOI] [PubMed] [Google Scholar]
- 47.Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J Comp Physiol. 1976;106:291–331. [Google Scholar]
- 48.Pokorny J, Smith VC, Lutze M. Aging of the human lens. Applied Optics. 1987;26:1437–1440. doi: 10.1364/AO.26.001437. [DOI] [PubMed] [Google Scholar]
- 49.Prinz PN. Sleep patterns in the healthy aged: Relationship with intellectual function. J Gerontol. 1977;32:179–186. [Google Scholar]
- 50.Prinz PN. Sleep and sleep disorders in older adults. J Clin Neurophysiol. 1995;12:139–146. doi: 10.1097/00004691-199503000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Quan SF, Katz R, Olson J, Bonekat W, Enright PL, Young T, Newman A. Factors associated with incidence and persistence of symptoms of disturbed sleep in an elderly cohort: the Cardiovascular. Health Study. 2005;329:163–172. doi: 10.1097/00000441-200504000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Reynolds CFd, Jennings JR, Hoch CC, Monk TH, Berman SR, Hall FT, Matzzie JV, Buysse DJ, Kupfer DJ. Daytime sleepiness in the healthy “old old”: a comparison with young adults. J Am Geriatr Soc. 1991;39:957–962. doi: 10.1111/j.1532-5415.1991.tb04041.x. [DOI] [PubMed] [Google Scholar]
- 53.Sutin EL, Dement WC, Heller HC, Kilduff TS. Light-induced gene expression in the suprachiasmatic nucleus of young and aging rats. Neurobiol Aging. 1993;14:441–446. doi: 10.1016/0197-4580(93)90102-h. [DOI] [PubMed] [Google Scholar]
- 54.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tozawa T, Mishima K, Satoh K, Echizenya M, Shimizu T, Hishikawa Y. Stability of sleep timing against the melatonin secretion rhythm with advancing age: clinical implications. J Clin Endocrinol Metab. 2003;88:4689–4695. doi: 10.1210/jc.2003-030147. [DOI] [PubMed] [Google Scholar]
- 56.Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L’Hermite-Baleriaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol. 1994;266:E953–963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- 57.van Coevorden A, Mockel J, Laurent E, Kerkhofs M, L’Hermite-Baleriaux M, Decoster C, Neve P, Van Cauter E. Neuroendocrine rhythms and sleep in aging men. Am J Physiol. 1991;260:E651–661. doi: 10.1152/ajpendo.1991.260.4.E651. [DOI] [PubMed] [Google Scholar]
- 58.Van Someren EJ, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41:955–963. doi: 10.1016/S0006-3223(97)89928-3. [DOI] [PubMed] [Google Scholar]
- 59.van Someren EJ, Mirmiran M, Swaab DF. Non-pharmacological treatment of sleep and wake disturbances in aging and Alzheimer’s disease: chronobiological perspectives. Behav Brain Res. 1993;57:235–253. doi: 10.1016/0166-4328(93)90140-l. [DOI] [PubMed] [Google Scholar]
- 60.Vitiello MV, Moe KE, Prinz PN. Sleep complaints cosegregate with illness in older adults: clinical research informed by and informing epidemiological studies of sleep. J Psychosom Res. 2002;53:555–559. doi: 10.1016/s0022-3999(02)00435-x. [DOI] [PubMed] [Google Scholar]
- 61.Vitiello MV, Smallwood RG, Avery DH, Pascualy RA, Martin DC, Prinz PN. Circadian temperature rhythms in young adult and aged men. Neurobiol Aging. 1986;7:97–100. doi: 10.1016/0197-4580(86)90146-6. [DOI] [PubMed] [Google Scholar]
- 62.Weitzman ED, Moline ML, Czeisler CA, Zimmerman JC. Chronobiology of aging: temperature, sleep-wake rhythms and entrainment. Neurobiol Aging. 1982;3:299–309. doi: 10.1016/0197-4580(82)90018-5. [DOI] [PubMed] [Google Scholar]
- 63.Wirz-Justice A, Werth E, Renz C, Muller S, Krauchi K. No evidence for a phase delay in human circadian rhythms after a single morning melatonin administration. J Pineal Res. 2002;32:1–5. doi: 10.1034/j.1600-079x.2002.10808.x. [DOI] [PubMed] [Google Scholar]
- 64.Wright KP, Jr., Gronfier C, Duffy JF, Czeisler CA. Intrinsic period and light intensity determine the phase relationship between melatonin and sleep in humans. J Biol Rhythms. 2005;20:168–177. doi: 10.1177/0748730404274265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Brainard GC, Zee PC, Pinto LH, Takahashi JS, Turek FW. Effects of aging on lens transmittance and retinal input to the suprachiasmatic nucleus in golden hamsters. Neurosci Lett. 1998;258:167–170. doi: 10.1016/s0304-3940(98)00887-8. [DOI] [PubMed] [Google Scholar]
- 66.Zhang Y, Kornhauser JM, Zee PC, Mayo KE, Takahashi JS, Turek FW. Effects of aging on light-induced phase-shifting of circadian behavioral rhythms, fos expression and CREB phosphorylation in the hamster suprachiasmatic nucleus. Neuroscience. 1996;70:951–961. doi: 10.1016/0306-4522(95)00408-4. [DOI] [PubMed] [Google Scholar]
- 67.Zulley J, Wever R, Aschoff J. The dependence of onset and duration of sleep on the circadian rhythm of rectal temperature. Pflugers Arch. 1981;391:314–318. doi: 10.1007/BF00581514. [DOI] [PubMed] [Google Scholar]


