Abstract
One central issue in schizophrenia research is to identify and characterize behavioral and biological markers that are intrinsic to the complex psychiatric disorder and that can serve as targets for detection, treatment, and prevention. A trait marker represents the properties of the behavioral and biological processes that play an antecedent, possibly causal, role in the pathophysiology of the psychiatric disorder, whereas a state marker reflects the status of clinical manifestations in patients. Certain visual functions, while deficient in schizophrenia, may be independent of psychosis. The question of what types of visual functions can serve as trait or state markers is beginning to be understood. Examining clinically unaffected relatives of schizophrenia patients and patients with bipolar disorder can provide information about the relationship between a schizophrenic disposition and visual response traits. In this effort, researchers found that motion integration is dysfunctional in schizophrenia patients but not in their relatives or bipolar patients, whereas motion discrimination is dysfunctional in schizophrenia patients and their relatives, but not in bipolar patients. By synthesizing these findings, this review suggests that distinguishing enduring trait markers from transient state markers for schizophrenia through visual processes is helpful for developing neurobiologically and psychologically based intervention strategies.
Keywords: Schizophrenic, visual functions, endophenotype, genetics, neurophysiology, behavior
INTRODUCTION
Despite extensive research since the modern characterization of schizophrenia [1, 2], the etiology of the disorder remains largely undetermined. Psychosis, the clinical hallmark of schizophrenia, has traditionally been a major target of investigation for understanding both the predisposition to, and the symptoms of the disorder. This symptom-based approach, while useful in understanding and treating less complex disorders, has not yet led us to a comprehensive picture of the pathophysiological processes underlying schizophrenia. One daunting obstacle for this approach is that psychosis may be merely one adverted effect of suffering from the disorder. Thus, focusing on psychosis appears to be too limited an approach for understanding the biological underpinnings of schizophrenia. The complexity associated with schizophrenia warrants a novel approach to unravel its altered biological processes.
In recent years, a new translational approach, which examines genetic, neurobiological, and behavioral responses concomitantly, has been applied, and has yielded significant progress. One important conceptual and empirical advance for schizophrenia research has been the distinction of trait vs. state markers. A trait is a behavioral characteristic brought about by the expression of a gene or many genes. Trait markers for schizophrenia refer to the properties of the behavioral and biological processes that play an antecedent, possibly causal, role in the predisposition to the psychiatric disorder, whereas state markers refer to the status of clinical manifestations in patients. Typically, though not necessarily, a trait characteristic is enduring and a state characteristic is transient. In the genetics of schizophrenia, another term, endophenotype, is often used to describe a trait or an enduring phenotype that is associated with the disorder, but is not immediately visible within the clinical domain [3]. The trait markers for schizophrenia we refer to here reflect a broader definition, which includes the manifestation of altered behavioral and biological processes that are linked not necessarily to psychosis, but to functional abnormalities that are at the core of the disorder. Trait markers are most useful when they are present in clinically unaffected relatives of schizophrenia patients (co-familial traits), and are not limited to those that co-segregate with psychosis.
Schizophrenia manifests itself through many altered responses that are mediated by genetic, neurobiological and psychological processes. One challenge of applying the translational approach in studying this complex mental disorder is to identify and characterize particular altered responses that can serve as trait markers. Studying trait markers, as opposed to state markers, will make the complex disorder more tractable at genetic, neurobiological and psychological levels [4–6].
In this article, we describe the progress in distinguishing trait markers from state markers for schizophrenia in the realm of visual processes. Not aiming for a general review of visual processing in schizophrenia, our selection of visual processes for this review was directed by the following guidelines:
Is the particular visual process altered in schizophrenia (integrity)?
Is the altered process associated with psychosis (generalized deficit or psychosis-independent deficit)?
Is the altered process present in patients and in clinically unaffected relatives (co-familiality)?
Does the altered process implicate a specific neural pathway in schizophrenia (pathophysiology)?
This paper discusses the associations between selected visual processes and the predisposition to, as opposed to the effect of, schizophrenia. We will not describe in detail the experimental paradigms that produced the results that elucidate this distinction, as these methods are well outlined in the original research articles. We will first discuss visual and visuomotor processes in schizophrenia, focusing on eye tracking. Then, the genetic neurophysiological and psychological basis of altered visual motion processing will be highlighted. We will then use visual motion processing as a model to demonstrate the case of trait vs. state markers at a perceptual level.
VISUAL AND VISUOMOTOR PROCESSES
Schizophrenia has long been recognized as a disorder of thought process. Whether and how basic sensory processes are involved in schizophrenia is now an active question in current research. Exploration in the past few decades has shown that several vision-related processes are altered in schizophrenia (Fig. 1). A prominent example is the dysfunction of eye tracking, an oculomotor response generated by visual motion signals. Extensive studies have shown that eye tracking dysfunction occurs not only in schizophrenia patients but also in their biological relatives [7–13]. It has also been demonstrated that eye tracking dysfunction is present in bipolar patients [14–16] but not, or to a much lesser extent, in relatives of bipolar patients [17]. The combination of these results provides unequivocal evidence that eye tracking dysfunction is a trait characteristic in schizophrenia [18–20]. Genetic linkage studies suggest that eye tracking dysfunction is a susceptibility marker for schizophrenia [21, 22].
Fig. (1).
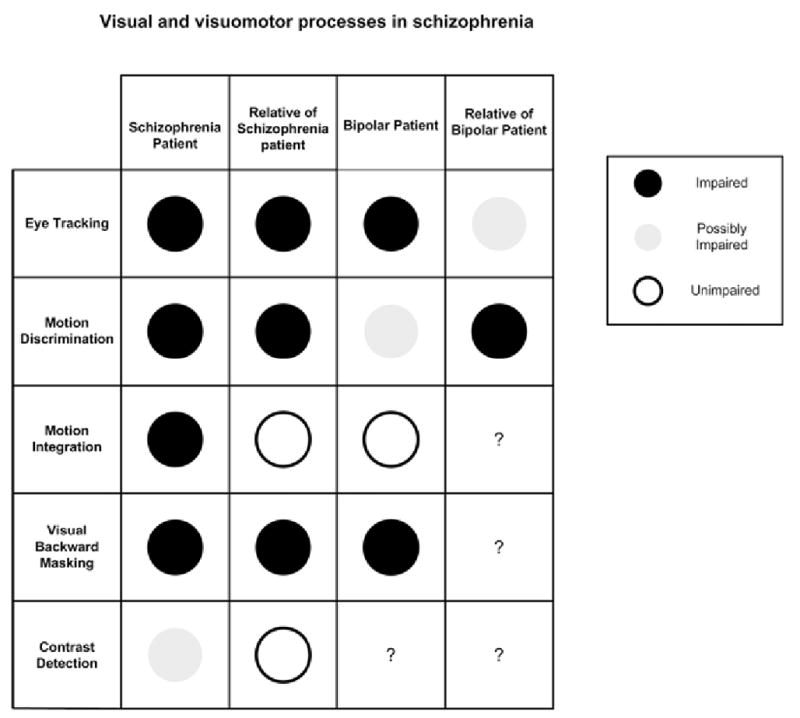
Functional integrity of selected visual and visuomotor processes in schizophrenia.
One challenge of further applying knowledge from eye tracking, a seemingly simple oculomotor behavior, to schizophrenia research is that the biological basis of the trait is actually complex. In order to follow a moving target with the eyes, the brain systems involved must first encode the information of visual motion, which occurs in the sensory cortex [23]. The encoded motion signals are then projected to the motor and the cognitive cortices to generate decision signals for initiating and maintaining eye movements [24]. As essential as visual motion signals are for initiating eye tracking, non-visual signals that temporally represent target movement are also necessary for maintaining eye tracking, insomuch as the external inputs of visual motion signals become unavailable when the eyes start to move and follow a moving target. Physiological and neurological studies have shown the involvement of multiple neural mechanisms in different aspects of eye tracking; disruption to any part of this cortical network causes eye tracking dysfunction [25–27].
In schizophrenia, multiple cortical areas, which process both visual and non-visual information [13, 28], have been implicated in eye tracking dysfunction [29–32]. The extensive exploration of eye tracking dysfunction as a trait marker for schizophrenia can serve as a useful exemplar for studying other visual processes.
Another visual process that has been extensively studied is visual backward masking. A visual masking paradigm measures how detection of one target is influenced by another target that is presented shortly after. Patients with schizophrenia and bipolar disorder showed altered behavioral responses in this measure [33]. This altered visual masking is independent of medication [34], and is accompanied by altered electrophysiological responses in schizophrenia patients [35]. Recent reports showed that the altered behavioral responses also occurred in clinically unaffected relatives of schizophrenia patients [36, 37]. These results suggest that altered visual backward masking is a trait characteristic for schizophrenia. However, visual backward masking may not be a schizophrenia-specific trait since bipolar patients were similarly affected.
In schizophrenia patients, perceptual deficiency is selective to some but not all visual signals [38]. One visual response that has been examined is contrast detection. Patients with schizophrenia showed reduced sensitivities in some studies [39], but not in others [40]. Several factors may contribute to these diverse results. For example, differences in the spatial and temporal pattern of the experimental stimuli have been shown to modulate visual contrast detection [41, 42]. In addition, various pharmacological treatments have been shown to affect contrast detection. For example, one study reported significantly different contrast detection abilities in groups of schizophrenia patients taking typical antipsychotics, atypical antipsychotics, and no antipsychotic medication [40]. Understanding the affects of neuromodulation on visual contrast detection will be important in determining whether or not contrast detection is a state or a trait marker for schizophrenia. One study addressed the state vs. trait question by comparing performance of schizophrenia patients and their relatives on contrast detection and reported that contrast detection in the relatives of schizophrenia patients was normal [40]. At this point, the question of a contrast detection deficit as a trait or state marker for schizophrenia remains open. With active examinations of these responses in relatives of schizophrenia patients and other psychiatric populations [43, 44], the value of these visual functions as trait or state markers for schizophrenia will become evident.
One key premise in studying visual and visuomotor functions is that certain behavioral responses could be more reflective of their underlying genetic and neurophysiological processes than other responses. In the case of schizophrenia, validating and specifying such behavioral responses are essential for understanding alterations at the genetic and neurophysiological levels. Patients’ behavioral responses may ultimately be the test for various pathophysiological models of schizophrenia, since psychosis, the clinical hallmark of the disorder, is extremely difficult to assess in animals.
One such behavioral response that we have been studying is motion perception. There are several vantage points to study visual motion processing in relation to schizophrenia. Broadly, motion perception has a well defined neural basis within the sensory cortex. Compared with other visual processes, motion perception seems more vulnerable to abnormalities of the brain [45]. Motion perception normally involves a straightforward behavioral task that does not depend substantially on cognitive processing. This relative independence makes performance in motion perception less contaminated by various cognitive deficits in schizophrenia. In addition, motion perception plays an important role in eye tracking, which is known to be impaired in schizophrenia. Studying motion perception may thus provide a sensitive measurement for assessing pathophysiological processes in schizophrenia. We will use this visual response as a means for exploring trait vs. state markers for schizophrenia, after outlining its underlying genetic and neurophysiological mechanisms (Fig. 2).
Fig. (2).
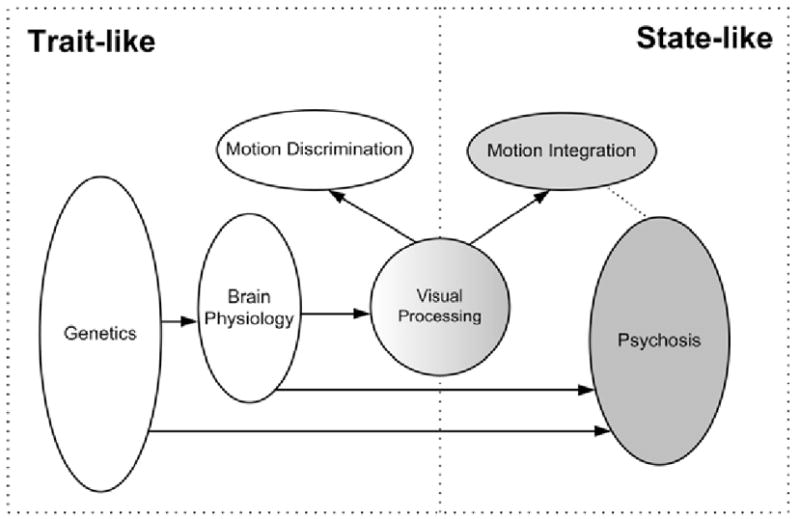
Visual trait versus state markers in relation to the biology of schizophrenia.
NEUROPHYSIOLOGY AND GENETICS OF VISUAL MOTION PROCESSING
Genetic factors play an essential role in determining the structure and function of the visual system. Before endeavoring to link visual responses and genetic factors in schizophrenia research, it is reasonable to ask, from a theoretical perspective, whether it is possible to study the genetics of motion processing by measuring behavioral responses. The genes essential for the development of neural pathways involved in visual processing have been screened and elicited in animal studies [46, 47]. Similar genetic studies have been conducted for motion processing [48]. Abnormal visual behaviors have been shown in patients with heritable vision-related diseases such as visual color deficiency [49]. These studies from various disciplines suggest that examining the relationships between certain visual behaviors and their genetic origins is becoming increasingly feasible.
At the neurochemical level, several types of neurotransmitters are involved in forming and shaping neural responses to visual signals. Dopamine, for example, acts to reduce the responsiveness of light-sensitive neurons in the retina and to uncouple electric junctions between neurons, to control neural amplification under different light conditions [50]. Glutamate, through NMDA and AMPA receptors, connects neighboring neurons at the retina and in the cortex to build direction selectivity, a special neural property that is fundamental for visual motion processing [51]. GABA, at a later stage of the visual system, selectively suppresses some neural responses but not others, allowing for more sophisticated non-linear neural computation in motion processing [52]. Increasing research efforts are being put towards the understanding of how diverse types of neurotransmissions regulate vision-related behaviors [53].
At the neurophysiological level, specific pathways for visual motion processing have been identified [54]. Specifically, neural processing of motion signals begins in subcortical areas such as the retina and lateral geniculate nucleus of the thalamus and in the striate cortex. The motion signals are then transmitted to Middle Temporal Area (MT), an extrastriate cortical area, for motion-specific processing. Other areas in the occipital, parietal and temporal cortices receive projections from the MT for motion-related cognitive and motor processing. These biological and behavioral results suggest that applying motion perception to studying the neural functions in schizophrenia is plausible.
The strong genetic and neurophysiological framework of motion processing provides a window to studying abnormal brain functioning [55] and genetics of the disorder [56, 57] in depth. Two challenges are immediate in applying motion perception to characterize trait vs. state markers for schizophrenia. The first one is to identify those visual motion functions that are altered and to examine the relationships between these functions and schizophrenic psychosis. The second one is to characterize those altered motion responses via a behavior-genetic analysis approach. Recent studies on motion perception in schizophrenia represent an effort in this direction.
VISUAL MOTION PERCEPTION
Studies on visual motion processing, motivated to understand the nature of eye tracking dysfunction, showed that motion discrimination (Fig. 3) was impaired in schizophrenia patients [28, 58] and in clinically unaffected relatives [28] whereas motion integration (Fig. 3) was impaired in schizophrenia patients [59–62], but not in relatives of schizophrenia patients or patients with bipolar disorder [62]. Since motion discrimination and motion integration are mediated by different cortical processes, this pattern of results has several implications in schizophrenia. First, the presence of altered motion discrimination in both schizophrenia patients and their relatives, but not in bipolar patients suggests that neither psychosis nor mood disturbances significantly affect the visual process. Second, motion discrimination may be a trait characteristic that is specific to schizophrenia. Third, motion integration may be specifically associated with schizophrenia psychosis, and thus may serve as a state marker for schizophrenia. In combination with neurophysiological knowledge of motion processing, these results suggest that the responses mediated by the cortical processes responsible for motion discrimination and those responsible for motion integration are indicators of different stages of the disorder, predisposition and psychosis, respectively.
Fig. (3).
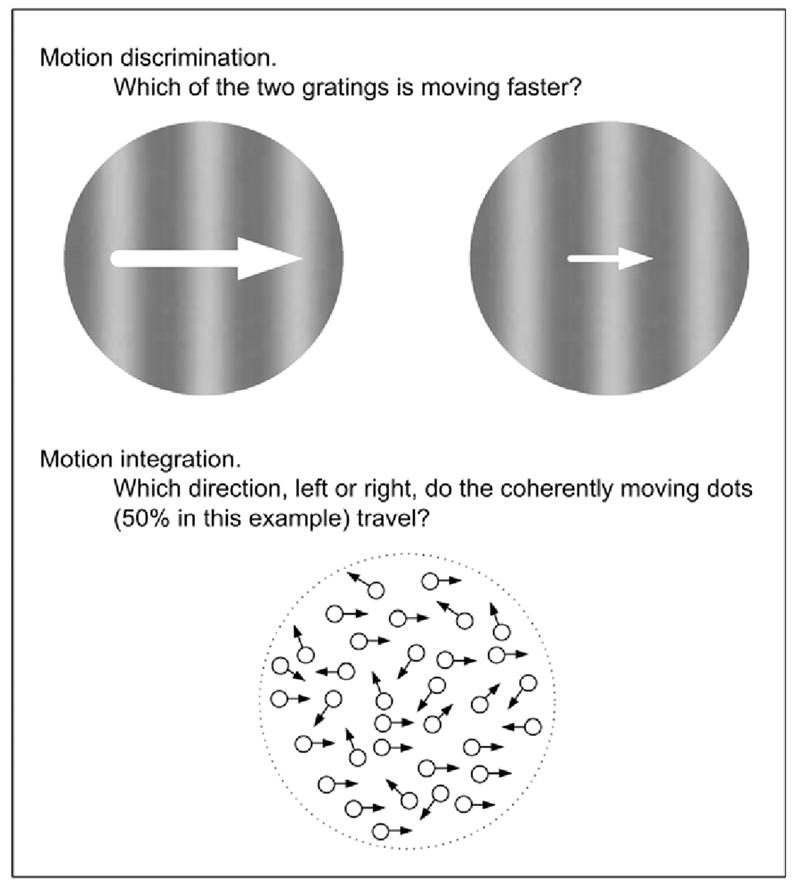
Schematic illustration of experimental paradigms used in studying visual motion processing in schizophrenia.
VISUAL RESPONSES AS TRAIT OR STATE MARKERS
In addition to comparing visual responses in different populations associated with schizophrenia (e.g., bipolar patients and relatives), another method for identifying altered visual responses as trait markers is to track their development and progression throughout the course of the illness.
With respect to development, children at high risk for schizophrenia show vision problems well before they develop any psychotic symptoms. As illustrated in an epidemiological study [63], the prevalence of visual difficulties is significantly higher in children who have a family history of schizophrenia. Further, visual difficulties are present more frequently than other sensory problems (such as auditory difficulties). These epidemiological results suggest that certain deficits in visual processing are more closely linked to schizophrenic predisposition than schizophrenic psychosis. Data on motion discrimination in children at high risk for schizophrenia are not available at present. However, data on motion discrimination in schizophrenia patients of different ages suggest that this visual process is altered regardless of age or stage of illness. A recent study showed that performance in motion discrimination in schizophrenia patients, while dysfunctional, is independent of age in the range of 18 to 55 years, whereas performance in normal controls gets worse starting at 45–55 years [64]. On the other hand, one report showed that the rate of age-related decline in other visual responses was more rapid in relatives of schizophrenia patients than in controls [65]. This pattern of result suggests that altered motion discrimination is a stable feature over the course of the illness, furthering the case that the visual response be used as a trait marker.
One relevant question concerns whether schizophrenia-related markers are modifiable by antipsychotic drugs. The distinction between trait and state markers implies that as the medications alleviate psychotic symptoms they may alter responses associated with state markers, because by definition the two sets of behavioral indexes are interdependent in patients. Pharmacological agents, such as antipsychotic drugs, can in principle modulate behavioral responses including those associated with trait markers for schizophrenia. Such modulation, however, should be independent of changes in psychotic symptoms or related state markers. Analogously, antipsychotic medications are known to have differential effects on abnormal behaviors of schizophrenia patients - these drugs are more effective in treating positive symptoms and less so in treating cognitive dysfunctions as well as negative symptoms [66]. How the antipsychotic medications affect visual responses, trait markers or not, remains a largely unexplored question [40]. One useful strategy in future studies will be to compare performance on a certain visual task before and after antipsychotic medications are administered in patients. This strategy may be complicated by the fact that a majority of patients are now treated with antipsychotics. However, many antipsychotic drugs, especially those of new generations such as clozapine, have a relatively short occupancy period. Such temporal dynamics suggest windows of opportunity in which effects of antipsychotic drugs may be minimal just before an individual dose is administered and maximal within a period of time after dose administration. Comparisons of the performance before and after the drugs are administered would provide a practical way to assess the drug effect in medicated patients. Another strategy will be to examine performance on a visual task in both patient and relative groups. If a trait marker manifests in both groups, comparing associated visual performance of the patients who are medicated with the relatives who are not would provide a good index on the effects of the drugs.
Another important and drug-relevant issue is whether and how the relationships between visual responses and neurochemical underpinnings inform neurochemical theories of schizophrenia. While current theories implicate several types of neurotransmissions such as dopamine, glutamate and GABA, empirical evidence is largely generated from neurochemical studies using either postmortem schizophrenic brains or animal models. As discussed earlier, these very same neurotransmissions also modulate visual responses. This heuristic link provides a basis for using visual perception as a means to test the theories directly in schizophrenia patients. Recent studies showed hypersensitivity in visual contrast detection in unmedicated schizophrenia patients and normal to hyposensitivity in patients who were treated with antipsychotic drugs (D2 receptor blockade) [40, 67]. This relation is consistent with the theory that abnormally high dopamine activity in the subcortical system may be associated with schizophrenia and antipsychotic drugs may act to reduce this activity [68]. When more research on this topic emerges, a different review may be warranted.
Much progress has been made towards understanding schizophrenia at a biological level via postmortem [69] and genetic studies [57]. It is important to synthesize such research with behavioral studies. Because of advancements in both basic and clinical research, the application of visual responses (e.g., motion discrimination and integration which have been extensively investigated psychophysically and physiologically) to schizophrenia research is becoming an increasingly powerful approach for probing into the underlying pathophysiological processes in schizophrenia. Using the methods described above, systematic exploration of visual responses will also be useful for understanding other sensory [70–72] and related cognitive processes [73–76] implicated in schizophrenia.
Identification and characterization of deficient visual responses and their neural correlates in schizophrenia may ultimately provide important clues for developing intervention strategies in helping patients. Understanding how neuromodulation affects deficient visual processing and its neural correlates in schizophrenia will be useful for identifying new and better targets for pharmacological treatment, as has recently begun to occur with sensory gating, another behavioral process that is compromised in schizophrenia. Extensive research into the neuromodulation of the sensory gating deficit has informed the search for pharmacological interventions that target the alpha-7 nicotinic receptor system [see 77 for a review]. In addition, characterization of visual responses and their neural correlations may be useful in another, more novel way. Basic research has shown that perceptual and cognitive capacities are plastic and adaptive not only in development period but also throughout adulthood [78, 79]. In healthy people, visual functions and cortical modulations can be enhanced through perceptual learning [80]. In light of such knowledge, the identification of vision-related trait markers would provide concrete targets for special neural training, analogous to cognitive rehabilitation, to improve patients’ performance starting at the sensory level.
CONCLUDING REMARKS
It is being increasingly recognized that schizophrenia is a pleiotropic disorder. The likely involvement of many genes and environmental factors makes it difficult to draw an outright picture of its underlying biological and behavioral mechanisms. With advances in basic research and the implementation of a new translational research strategy, we can move towards a genetically and neurobiologically based approach that focuses on those traits influenced by well-understood brain physiology and accessible genetic pathways. One important step in taking this novel approach is to differentiate and characterize trait and state markers for schizophrenia. Psychotic symptoms alone are not adequate for determining the genetic and neural basis of schizophrenia. Study of selected visual processes provides an opportunity to identify genetic variations within a range of visual phenotypes that are specific to schizophrenia. The results have pointed towards motion discrimination as a trait characteristic that may be useful for screening schizophrenia-related genes and for exploring neural mechanisms underlying the perceptual deficits in schizophrenia. Of foremost significance is the role that motion discrimination may play in linking genetics, neurophysiology and perceptual performance in schizophrenia. Future studies should underscore whether and to what extent the enduring trait markers for schizophrenia are modifiable and how they are related to state markers for schizophrenia. This information will help define schizophrenic phenotypes more precisely for use in genetic, physiological and psychological studies. Knowledge acquired from these studies will eventually be applied to unravel the pathophysiology at the core of schizophrenia, and to inform prevention and intervention strategies.
Acknowledgments
We thank two anonymous reviewers for helpful comments on the early version of the paper. The work was supported in part by UHS Grant MH 61824 and a faculty pilot research award (Harvard University).
References
- 1.Bleuler E. Dementia Praecox or the Group of Schizophrenias: I. New York: International Univ. Press; 1950. Originally published 1911. [Google Scholar]
- 2.Kraepelin E. Dementia Praecox and Paraphrenia. Chicago: Medical Books; 1919. [Google Scholar]
- 3.Gottesman II, Shields J. Genetic theorizing and schizophrenia. Br J Psychiatry. 1973;122:15–30. doi: 10.1192/bjp.122.1.15. [DOI] [PubMed] [Google Scholar]
- 4.Saccuzzo DP, Braff DL. Information-processing abnormalities: trait- and state-dependent components. Schizophr Bull. 1986;12:447–59. doi: 10.1093/schbul/12.3.447. [DOI] [PubMed] [Google Scholar]
- 5.Holzman PS. Behavioral markers of schizophrenia useful for genetic studies. J Psychiatr Res. 1992;26:427–45. doi: 10.1016/0022-3956(92)90044-o. [DOI] [PubMed] [Google Scholar]
- 6.Tamminga CA, Holcomb HH. Phenotype of schizophrenia: a review and formulation. Mol Psychiatry. 2005;10:27–39. doi: 10.1038/sj.mp.4001563. [DOI] [PubMed] [Google Scholar]
- 7.Holzman PS, Proctor LR, Hughes DW. Eye-tracking patterns in schizophrenia. Science. 1973;181:179–81. doi: 10.1126/science.181.4095.179. [DOI] [PubMed] [Google Scholar]
- 8.Holzman PS, Proctor LR, Levy DL, Yasillo NJ, Meltzer HY, Hurt SW. Eye-tracking dysfunctions in schizophrenic patients and their relatives. Arch Gen Psychiatry. 1974;31:143–51. doi: 10.1001/archpsyc.1974.01760140005001. [DOI] [PubMed] [Google Scholar]
- 9.Holzman PS, Kringlen E, Levy DL, Proctor LR, Haberman SJ, Yasillo NJ. Abnormal-pursuit eye movements in schizophrenia. Evidence for a genetic indicator. Arch Gen Psychiatry. 1977;34:802–5. doi: 10.1001/archpsyc.1977.01770190064005. [DOI] [PubMed] [Google Scholar]
- 10.Clementz BA, Grove WM, Iacono WG, Sweeney JA. Smooth-pursuit eye movement dysfunction and liability for schizophrenia: implications for genetic modeling. J Abnorm Psychol. 1992;101:117–29. doi: 10.1037//0021-843x.101.1.117. [DOI] [PubMed] [Google Scholar]
- 11.Levin S, Luebke A, Zee DS, Hain TC, Robinson DA, Holzman PS. Smooth pursuit eye movements in schizophrenics: quantitative measurements with the search-coil technique. Vision Res. 1992;32:1009–14. doi: 10.1016/0022-3956(88)90005-2. [DOI] [PubMed] [Google Scholar]
- 12.Sweeney JA, Clementz BA, Escobar MD, Li S, Pauler DK, Haas GL. Mixture analysis of pursuit eye-tracking dysfunction in schizophrenia. Biol Psychiatry. 1993;34:331–40. doi: 10.1016/0006-3223(93)90090-z. [DOI] [PubMed] [Google Scholar]
- 13.Thaker GK, Ross DE, Cassady SL, et al. Smooth pursuit eye movements to extraretinal motion signals: deficits in relatives of patients with schizophrenia. Arch Gen Psychiatry. 1998;55:830–6. doi: 10.1001/archpsyc.55.9.830. [DOI] [PubMed] [Google Scholar]
- 14.Shagass C, Amadeo M, Overton D. Eye-tracking performance in psychiatric patients. Biol Psychiatry. 1974;9:245–60. [PubMed] [Google Scholar]
- 15.Levin S, Holzman PS, Rothenberg SJ, Lipton RB. Saccadic eye movements in psychotic patients. Psychiatry Res. 1981;5:47–58. doi: 10.1016/0165-1781(81)90060-3. [DOI] [PubMed] [Google Scholar]
- 16.Iacono WG, Pelequin LJ, Lumry AE, Valentine RH, Tuason VB. Eye tracking in patients with unipolar and bipolar affective disorders in remission. J Abnorm Psychol. 1982;91:35–44. doi: 10.1037//0021-843x.91.1.35. [DOI] [PubMed] [Google Scholar]
- 17.Levy DL, Yasillo NJ, Dorus E, et al. Relatives of unipolar and bipolar patients have normal pursuit. Psychiatry Res. 1983;10:285–93. doi: 10.1016/0165-1781(83)90075-6. [DOI] [PubMed] [Google Scholar]
- 18.Holzman PS, Kringlen E, Matthysse S, et al. A single dominant gene can account for eye tracking dysfunctions and schizophrenia in offspring of discordant twins. Arch Gen Psychiatry. 1988;45:641–7. doi: 10.1001/archpsyc.1988.01800310049006. [DOI] [PubMed] [Google Scholar]
- 19.Clementz BA, Grove WM, Iacono WG, Sweeney JA. Smooth-pursuit eye movement dysfunction and liability for schizophrenia: implications for genetic modeling. J Abnorm Psychol. 1992;101:117–29. doi: 10.1037//0021-843x.101.1.117. [DOI] [PubMed] [Google Scholar]
- 20.Thaker GK, Avila MT, Hong EL, Medoff DR, Ross DE, Adami HM. A model of smooth pursuit eye movement deficit associated with the schizophrenia phenotype. Psychophysiology. 2003;40:277–84. doi: 10.1111/1469-8986.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arolt V, Lencer R, Nolte A, et al. Eye tracking dysfunction is a putative phenotypic susceptibility marker of schizophrenia and maps to a locus on chromosome 6p in families with multiple occurrence of the disease. Am J Med Genet. 1996;67:564–79. doi: 10.1002/(SICI)1096-8628(19961122)67:6<564::AID-AJMG10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 22.Matthysse S, Holzman PS, Gusella JF, et al. Linkage of eye movement dysfunction to chromosome 6p in schizophrenia: additional evidence. Am J Med Genet B Neuropsychiatr Genet. 2004;128:30–6. doi: 10.1002/ajmg.b.30030. [DOI] [PubMed] [Google Scholar]
- 23.Newsome WT, Wurtz RH, Komatsu H. Relation of cortical areas MT and MST to pursuit eye movements. II. Differentiation of retinal from extraretinal inputs. J Neurophysiol. 1988;60:604–20. doi: 10.1152/jn.1988.60.2.604. [DOI] [PubMed] [Google Scholar]
- 24.Lisberger SG, Morris EJ, Tychsen L. Visual motion processing and sensory-motor integration for smooth pursuit eye movements. Annu Rev Neurosci. 1987;10:97–129. doi: 10.1146/annurev.ne.10.030187.000525. [DOI] [PubMed] [Google Scholar]
- 25.Dursteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988;60:940–65. doi: 10.1152/jn.1988.60.3.940. [DOI] [PubMed] [Google Scholar]
- 26.Morrow MJ, Sharpe JA. Retinotopic and directional deficits of smooth pursuit initiation after posterior cerebral hemispheric lesions. Neurology. 1993;43 (Pt 1):595–603. doi: 10.1212/wnl.43.3_part_1.595. [DOI] [PubMed] [Google Scholar]
- 27.Heide W, Kurzidim K, Kompf D. Deficits of smooth pursuit eye movements after frontal and parietal lesions. Brain. 1996;119 (Pt 6):1951–69. doi: 10.1093/brain/119.6.1951. [DOI] [PubMed] [Google Scholar]
- 28.Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Psychophysical isolation of a motion-processing deficit in schizophrenics and their relatives and its association with impaired smooth pursuit. Proc Natl Acad Sci USA. 1999;96:4724–9. doi: 10.1073/pnas.96.8.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Driscoll GA, Benkelfat C, Florencio PS, et al. Neural correlates of eye tracking deficits in first-degree relatives of schizophrenic patients: a positron emission tomography study. Arch Gen Psychiatry. 1999;56:1127–34. doi: 10.1001/archpsyc.56.12.1127. [DOI] [PubMed] [Google Scholar]
- 30.Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am J Psychiatry. 2004;161:315–21. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- 31.Hong LE, Tagamets M, Avila M, Wonodi I, Holcomb H, Thaker GK. Specific motion processing pathway deficit during eye tracking in schizophrenia: a performance-matched functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:726–32. doi: 10.1016/j.biopsych.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Lencer R, Nagel M, Sprenger A, Heide W, Binkofski F. Reduced neuronal activity in the V5 complex underlies smooth-pursuit deficit in schizophrenia: evidence from an fMRI study. Neuroimage. 2005;24:1256–9. doi: 10.1016/j.neuroimage.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 33.Green MF, Nuechterlein KH, Mintz J. Backward masking in schizophrenia and mania. I. Specifying a mechanism. Arch Gen Psychiatry. 1994;51:939–44. doi: 10.1001/archpsyc.1994.03950120011003. [DOI] [PubMed] [Google Scholar]
- 34.Butler PD, Harkavy-Friedman JM, Amador XF, Gorman JM. Backward masking in schizophrenia: relationship to medication status, neuropsychological functioning, and dopamine metabolism. Biol Psychiatry. 1996;40:295–8. doi: 10.1016/0006-3223(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 35.Schechter I, Butler PD, Silipo G, Zemon V, Javitt DC. Magnocellular and parvocellular contributions to backward masking dysfunction in schizophrenia. Schizophr Res. 2003;64:91–101. doi: 10.1016/s0920-9964(03)00008-2. [DOI] [PubMed] [Google Scholar]
- 36.Green MF, Nuechterlein KH, Breitmeyer B. Backward masking performance in unaffected siblings of schizophrenic patients. Evidence for a vulnerability indicator. Arch Gen Psychiatry. 1997;54:465–72. doi: 10.1001/archpsyc.1997.01830170091012. [DOI] [PubMed] [Google Scholar]
- 37.Green MF, Nuechterlein KH, Breitmeyer B, Mintz J. Forward and Backward Visual Masking in Unaffected Siblings of Schizophrenic Patients. Biol Psychiatry. 2006;59:446–51. doi: 10.1016/j.biopsych.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 38.O'Donnell BF, Swearer JM, Smith LT, Nestor PG, Shenton ME, McCarley RW. Selective deficits in visual perception and recognition in schizophrenia. Am J Psychiatry. 1996;153:687–92. doi: 10.1176/ajp.153.5.687. [DOI] [PubMed] [Google Scholar]
- 39.Slaghuis WL. Contrast sensitivity for stationary and drifting spatial frequency gratings in positive- and negative-symptom schizophrenia. J Abnorm Psychol. 1998;107:49–62. doi: 10.1037//0021-843x.107.1.49. [DOI] [PubMed] [Google Scholar]
- 40.Chen Y, Levy DL, Sheremata S, Nakayama K, Matthysse S, Holzman PS. Effects of typical, atypical, and no antipsychotic drugs on visual contrast detection in schizophrenia. Am J Psychiatry. 2003;160:1795–801. doi: 10.1176/appi.ajp.160.10.1795. [DOI] [PubMed] [Google Scholar]
- 41.Kelly DH. Motion and vision. II. Stabilized spatio-temporal threshold surface. J Opt Soc Am. 1979;69:1340–9. doi: 10.1364/josa.69.001340. [DOI] [PubMed] [Google Scholar]
- 42.Watson AB, Barlow HB, Robson JG. What does the eye see best? Nature. 1983;302:419–22. doi: 10.1038/302419a0. [DOI] [PubMed] [Google Scholar]
- 43.Keri S, Kelemen O, Benedek G, Janka Z. Different trait markers for schizophrenia and bipolar disorder: a neurocognitive approach. Psychol Med. 2001;31:915–22. doi: 10.1017/s0033291701004068. [DOI] [PubMed] [Google Scholar]
- 44.Keri S, Kelemen O, Benedek G, Janka Z. Vernier threshold in patients with schizophrenia and in their unaffected siblings. Neuropsychology. 2004;18:537–42. doi: 10.1037/0894-4105.18.3.537. [DOI] [PubMed] [Google Scholar]
- 45.Braddick O, Atkinson J, Wattam-Bell J. Normal and anomalous development of visual motion processing: motion coherence and 'dorsal-stream vulnerability'. Neuropsychologia. 2003;41:1769–84. doi: 10.1016/s0028-3932(03)00178-7. [DOI] [PubMed] [Google Scholar]
- 46.Li L, Dowling JE. A dominant form of inherited retinal degeneration caused by a non-photoreceptor cell-specific mutation. Proc Natl Acad Sci USA. 1997;94:11645–50. doi: 10.1073/pnas.94.21.11645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neuhauss SC. Behavioral genetic approaches to visual system development and function in zebrafish. J Neurobiol. 2003;54:148–60. doi: 10.1002/neu.10165. [DOI] [PubMed] [Google Scholar]
- 48.Baier H. Zebrafish on the move: towards a behavior-genetic analysis of vertebrate vision. Curr Opin Neurobiol. 2000;10:451–5. doi: 10.1016/s0959-4388(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 49.Tovee MJ. The molecular genetics and evolution of primate colour vision. Trends Neurosci. 1994;17:30–7. doi: 10.1016/0166-2236(94)90032-9. [DOI] [PubMed] [Google Scholar]
- 50.Dowling JE. Functional and pharmacological organization of the retina: dopamine, interplexiform cells, and neuromodulation. Res Publ Assoc Res Nerv Ment Dis. 1990;67:1–18. [PubMed] [Google Scholar]
- 51.Rivadulla C, Sharma J, Sur M. Specific roles of NMDA and AMPA receptors in direction-selective and spatial phase-selective responses in visual cortex. J Neurosci. 2001;21:1710–9. doi: 10.1523/JNEUROSCI.21-05-01710.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Egelhaaf M, Borst A, Pilz B. The role of GABA in detecting visual motion. Brain Res. 1990;509:156–60. doi: 10.1016/0006-8993(90)90325-6. [DOI] [PubMed] [Google Scholar]
- 53.Carter OL, Pettigrew JD, Hasler F, Wallis GM, Liu GB, Hell D, Vollenweider FX. Modulating the rate and rhythmicity of perceptual rivalry alternations with the mixed 5-HT2A and 5-HT1A agonist psilocybin. Neuropsychopharmacology. 2005;30:1154–62. doi: 10.1038/sj.npp.1300621. [DOI] [PubMed] [Google Scholar]
- 54.Maunsell JH, Newsome WT. Related Articles, Visual processing in monkey extrastriate cortex. Annu Rev Neurosci. 1987;10:363–401. doi: 10.1146/annurev.ne.10.030187.002051. [DOI] [PubMed] [Google Scholar]
- 55.Andreasen NC. Linking mind and brain in the study of mental illnesses: a project for a scientific psychopathology. Science. 1997;275:1586–93. doi: 10.1126/science.275.5306.1586. [DOI] [PubMed] [Google Scholar]
- 56.Gottesman II, Shields J. Schizophrenia: The epigenetic puzzle. Cambridge England: Cambridge University Press; 1982. [Google Scholar]
- 57.Owen MJ, Williams NM, O'Donovan MC. The molecular genetics of schizophrenia: new findings promise new insights. Mol Psychiatry. 2004;9:14–27. doi: 10.1038/sj.mp.4001444. [DOI] [PubMed] [Google Scholar]
- 58.Kim D, Wylie G, Pasternak R, Butler PD, Javitt DC. Magnocellular contributions to impaired motion processing in schizophrenia. Schizophr Res. 2006;82:1–8. doi: 10.1016/j.schres.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stuve TA, Friedman L, Jesberger JA, Gilmore GC, Strauss ME, Meltzer HY. The relationship between smooth pursuit performance, motion perception and sustained visual attention in patients with schizophrenia and normal controls. Psychol Med. 1997;27:143–52. doi: 10.1017/s0033291796004230. [DOI] [PubMed] [Google Scholar]
- 60.Li CS. Impaired detection of visual motion in schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26:929–34. doi: 10.1016/s0278-5846(02)00207-5. [DOI] [PubMed] [Google Scholar]
- 61.Chen Y, Nakayama K, Levy DL, Matthysse S, Holzman PS. Processing of global, but not local, motion direction is deficient in schizophrenia. Schizophr Res. 2003;61:215–27. doi: 10.1016/s0920-9964(02)00222-0. [DOI] [PubMed] [Google Scholar]
- 62.Chen Y, Bidwell LC, Holzman PS. Visual motion integration in schizophrenia patients, their first-degree relatives, and patients with bipolar disorder. Schizophr Res. 2005;74:271–81. doi: 10.1016/j.schres.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Henriksson KM, McNeil TF. Health and development in the first 4 years of life in offspring of women with schizophrenia and affective psychoses: Well-Baby Clinic information. Schizophr Res. 2004;70:39–48. doi: 10.1016/j.schres.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Bidwell LC, Holzman PS, Chen Y. Aging and motion discrimination in normal adults and schizophrenia patients. Psychiatry Res. doi: 10.1016/j.psychres.2005.05.019. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bedwell JS, Esposito S, Miller LS. Accelerated age-related decline of visual information processing in first-degree relatives of persons with schizophrenia. Psychiatry Res. 2004;125:225–35. doi: 10.1016/j.psychres.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 66.Kopala LC, Fredrikson D, Good KP, Honer WG. Symptoms in neuroleptic-naive, first-episode schizophrenia: response to risperidone. Biol Psychiatry. 1996;39:296–8. doi: 10.1016/0006-3223(95)00500-5. [DOI] [PubMed] [Google Scholar]
- 67.Keri S, Antal A, Szekeres G, Benedek G, Janka Z. Transient visual channel functions in schizophrenia. Int J Psychophysiol. 1998;30:170. [Google Scholar]
- 68.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148:1474–86. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 69.Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–69. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 70.Saccuzzo DP, Braff DL. Early information processing deficit in schizophrenia. New findings using schizophrenic subgroups and manic control subjects. Arch Gen Psychiatry. 1981;38:175–9. doi: 10.1001/archpsyc.1981.01780270061008. [DOI] [PubMed] [Google Scholar]
- 71.Freedman R, Adler LE, Gerhardt GA, et al. Neurobiological studies of sensory gating in schizophrenia. Schizophr Bull. 1987;13:669–78. doi: 10.1093/schbul/13.4.669. [DOI] [PubMed] [Google Scholar]
- 72.Javitt DC, Shelley AM, Silipo G, Lieberman JA. Deficits in auditory and visual context-dependent processing in schizophrenia: defining the pattern. Arch Gen Psychiatry. 2000;57:1131–7. doi: 10.1001/archpsyc.57.12.1131. [DOI] [PubMed] [Google Scholar]
- 73.Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Arch Gen Psychiatry. 1995;52:821–8. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- 74.Silverstein SM, Kovacs I, Corry R, Valone C. Perceptual organization, the disorganization syndrome, and context processing in chronic schizophrenia. Schizophr Res. 2000;43:11–20. doi: 10.1016/s0920-9964(99)00180-2. [DOI] [PubMed] [Google Scholar]
- 75.Kelemen O, Erdelyi R, Pataki I, Benedek G, Janka Z, Keri S. Theory of mind and motion perception in schizophrenia. Neuropsychology. 2005;19:494–500. doi: 10.1037/0894-4105.19.4.494. [DOI] [PubMed] [Google Scholar]
- 76.Kim J, Doop ML, Blake R, Park S. Impaired visual recognition of biological motion in schizophrenia. Schizophr Res. 2005;77:299–307. doi: 10.1016/j.schres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 77.Martin LF, Kem WR, Freedman R. Alpha-7 nicotinic receptor agonists: potential new candidates for the treatment of schizophrenia. Psychopharmacol. 2004;174:54–64. doi: 10.1007/s00213-003-1750-1. Berl. [DOI] [PubMed] [Google Scholar]
- 78.Levine SM. Adult visual system plasticity. J Am Optom Assoc. 1988;59:135–9. [PubMed] [Google Scholar]
- 79.Gilbert CD. Plasticity in visual perception and physiology. Curr Opin Neurobiol. 1996;6:269–74. doi: 10.1016/s0959-4388(96)80083-3. [DOI] [PubMed] [Google Scholar]
- 80.Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc Natl Acad Sci USA. 2002;99:17137–42. doi: 10.1073/pnas.242414599. [DOI] [PMC free article] [PubMed] [Google Scholar]


