Abstract
BACKGROUND
Heart failure (HF) patients display a number of breathing abnormalities including periodic breathing (PB) at rest. Although the mechanism(s) contributing to PB remain unclear we examined whether changes in pulmonary wedge pressure (PWP) and pulmonary vascular resistance (PVR) alter PB in patients with established HF.
METHODS
We studied 12 male HF patients (age=50±11 yrs; ejection fraction=18.3±3.8 %; NYHA class=3.2±0.4), with PB at rest, undergoing right heart catheterization with infusion of nitroprusside.
RESULTS
At baseline, HF patients displayed minute ventilation (VE) oscillations with amplitude of 5.5±2.7 L/min, (57±34 % of the average VE) and cycle length of 61±18 sec. Cardiac index (CI), PVR, and mean PWP averaged 2.0±0.4 L/min/m2, 281.9±214.9 dynes·sec·cm −5, and 28.3±5.4 mmHg, respectively. During nitroprusside infusion, CI increased to 3.1±0.6 L/min/m2, PVR decreased to 163.9±85.2 dynes·sec·cm −5 and PWP fell to 10.0±4.2 mmHg. Nitroprusside reduced the amplitude (2.6±2.4 L/min, 23±21 % of average VE, p<0.01) and cycle length (41.4±28.8 sec, p<0.01) of VE oscillations while abolishing oscillations in 3 patients. Although average VE and PaCO2 remained unchanged there was a significant increase in the ratio of tidal volume to inspiratory time (VT/TI; p<0.01) suggesting an increase in ventilatory drive. The change in the amplitude of VE oscillations was positively correlated with the change in PWP (r=0.75, p<0.01), negatively correlated with the change in PVR (r= −0.63, p<0.05), and not correlated with the change in CI.
CONCLUSIONS
These data suggest that PWP (left atrial pressure) may play a direct role in the PB observed in HF at rest.
Keywords: pulmonary circulation, hemodynamics, heart failure
INTRODUCTION
Patients with heart failure (HF) develop a number of breathing abnormalities. These include various forms of oscillatory breathing, such as central sleep apnea at night 1 and the periodic breathing (PB) observed during exercise 2,3. Less well described is the PB observed during wakefulness under resting conditions. This form of PB is associated with large oscillations in both tidal volume (VT) and ventilation (VE) (>25% of the average VE) with a cycle length often >60 sec 4. Importantly, periodic breathing during wakefulness has recently been shown to be associated with poor prognosis in this population 5.
Mechanisms stimulating the oscillatory changes in VT and VE remain unclear but are likely related to combined reductions in cardiac output (i.e. increased circulation time) 3,6–9 and increased ventilatory drive 4,8,10,11. Another mechanism that may influence ventilatory drive includes increased left atrial and pulmonary vascular pressures 12. In anesthetized dogs, it has been shown that an elevation of left atrial pressure independently contributes to an increase in breathing frequency 13. Others have proposed that elevated pulmonary pressures also results in hyperventilation and hypocapnia 14,15. This causal pathway suggests that elevated pulmonary pressures stimulate intrapulmonary J receptors located in close proximity to the pulmonary capillaries. Activation of the intrapulmonary J receptors elicits the transmission of neural impulses via afferent vagal C fibers to the ventilatory control center of the medulla.
Nitroprusside dilates the pulmonary and systemic vasculature and intravascular administration of the drug results in a drop in pulmonary vascular resistance, pulmonary wedge pressure and systemic vascular resistance 16. Whether an acute change in these pressures influences breathing pattern and the cycle length of PB at rest in HF is unclear. Thus, the purpose of this study was to examine the effects of acute nitroprusside infusion on breathing pattern in HF patients who demonstrate PB during wakefulness at rest. We hypothesized that acute reductions in left atrial pressure as assessed by pulmonary wedge pressure or improvements in cardiac output would result in reduction in the amplitude of, or elimination of the ventilatory oscillations.
METHODS
Population Characteristics
Twelve adult male patients from the Mayo Clinic Heart Failure Service undergoing clinically indicated right heart catheterization for potential cardiac transplantation and who demonstrated periodic breathing at rest (see below) volunteered for this study (Table 1). All participants gave written informed consent after being provided a description of study requirements. The study protocol was approved by the Mayo Clinic Institutional Review Board, all procedures followed institutional and HIPAA guidelines and the investigation conforms with the principles outlined in the Declaration of Helsinki.
TABLE 1.
Clinical Characteristics of the Patient Population
| Demographics | |
| Age (y) | 50 ± 11 |
| Height (m) | 176.7 ± 7.7 |
| Weight (kg) | 87.7 ± 17.0 |
| BMI (kg/m2) | 28.0 ± 4.9 |
| BSA (m2) | 2.1 ± 0.2 |
| LVEF (%) | 18.3 ± 3.8 |
| NYHA Class | Class III n=10
Class IV n=2 |
| CHF Etiology (Ischemic/Idiopathic) | 7 / 5 |
| Medications | |
| ACE Inhibitors | 8 (66.7) |
| Angiotensin II Receptor Blockers | 3 (25.0) |
| Aspirin | 8 (66.7) |
| β-Blockers | 7 (58.3) |
| Digitalis | 10 (83.3) |
| Diuretics | 12 (100) |
ACE, angiotensin converting enzyme; BMI, body mass index; BSA, body surface area; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association. Data are presented as Mean±SD or as number of participants (percentage of population), n=12
Hemodynamic Evaluation
Right sided heart catheterization was conducted in an environmentally controlled surgical procedure room in the AM with the patients in the resting supine position. After minimal sedation, so as to not influence ventilatory pattern, a 22 gauge indwelling arterial catheter was placed in the radial artery and a 7 French Swan-Ganz balloon-tipped catheter was introduced into the right internal jugular vein and advanced through the right side of the heart to the pulmonary artery. Systolic (sys), diastolic (dia) and mean right atrial (RAP), pulmonary artery (PAP), and pulmonary wedge pressures (PWP) were measured at baseline. Cardiac output (CO) was determined by the direct FICK method using measured oxygen consumption and mixed venous and arterial blood collected simultaneously from the pulmonary and radial arteries, respectively, for measurement of the partial pressure of oxygen (PvO2 and PaO2, respectively), carbon dioxide (PvCO2 and PaCO2, respectively), and oxygen saturation (SvO2 and SaO2, respectively). Systemic systolic (SBP), diastolic (DBP), and mean arterial (MAP) blood pressure were measured by a standard intra-arterial catheter pressure transducer. Stroke volume (SV) was calculated as CO divided by heart rate. Cardiac index (CI) was calculated as CO divided by body surface area (BSA). Systemic vascular resistance (SVR) was calculated as the difference of systemic mean arterial pressure and mean RAPmean divided by CO and multiplied by 80 (conversion from Woods units). Systemic vascular resistance index (SVRI) was calculated as SVR divided by BSA. Pulmonary vascular resistance (PVR) was calculated as the difference of PAPmean and PWPmean divided by CO, multiplied by 80. Arterial oxygen content (CaO2) was calculated using the following equation:
Mixed venous oxygen content (CvO2) was calculated as:
Systemic oxygen transport (SOT) was calculated as CaO2 multiplied by CI. After baseline measurements, intra-arterial nitroprusside infusion was initiated at 0.5 μg/kg/min. The dose was increased by 0.5 μg/kg/min every 2–3 minutes depending on patient response, with a maximum dose of 3.0 μg/kg/min. All baseline measurements were repeated at peak nitroprusside dosage.
Gas Exchange and Ventilatory Evaluation
Oxygen consumption (VO2), carbon dioxide production (VCO2), VE, VT, respiratory rate (RR), and partial pressure of end-tidal oxygen and carbon dioxide (PETO2 and PETCO2, respectively) were measured with a metabolic measurement system through a mouth piece and pneumotach while wearing a nose clip for the entire measurement period (MedGraphics CPX/D; Medical Graphics, St. Paul, MN). Manual volume calibration was performed with a 3 liter syringe while gas calibration was performed with gases of known concentration.
Definition of Periodic Breathing
Periodic Breathing was defined as consistently regular waxing and waning of VE and VT without phases of apnea at rest during wakefulness. We identified patients who demonstrated at least three consecutive cycles of clear ventilatory oscillations. The amplitude of the oscillations was quantified as the difference between the peak and nadir of VE. Patients demonstrating an amplitude of VE > 30% of the mean VE, as identified by Ponikowski and colleagues 3, were used in the analysis. Oscillatory cycle length was calculated as the difference in time between the onset of an increase in VE to the onset of an increase in VE of the next oscillation.
Statistical Analysis
Statistical analysis and graphic presentation were accomplished using Graphpad Prism® (v 4.0). The number needed for 90% power to detect statistical significance at an alpha level of 0.05 was calculated to be11 participants. Two-tailed paired t-tests were used to determine statistically significant differences between baseline and peak nitroprusside. Pearson’s correlation and linear regression analysis were used to determine the relationships between the measured variables. Statistical significance was set at an alpha level of 0.05. All data are presented as mean ± standard deviation (SD).
RESULTS
Population Characteristics
The clinical characteristics and medications in use by the patients at the time of the study are reported in Table 1. These patients were slightly overweight with significantly attenuated left ventricular ejection fraction (LVEF) of 18.3%. Of the 12 patients 10 were considered NYHA class III and 2 were class IV with 7 of the 12 patients presenting with ischemic (as opposed to idiopathic) etiology of heart failure.
Hemodynamic Response to Acute Nitroprusside Infusion
Hemodynamic characteristics of the population at baseline and peak nitroprusside infusion are reported in Table 2. Cardiac output increased by 64% (p<0.01) with nitroprusside infusion due to an increase in stroke volume (p<0.001) as heart rate decreased slightly (p=0.03). Systemic SBP and DBP decreased (p<0.001) resulting in a 30% fall in MAP (p<0.001). With this, both PAPsys and PAPdia (p<0.001) decreased from baseline to peak nitroprusside infusion resulting in a 39% reduction in PAPmean (p<0.001). Pulmonary vascular resistance was reduced by approximately 8% with nitroprusside infusion, demonstrating a trend towards reduction from baseline to peak nitroprusside infusion (p<0.06).
TABLE 2.
Hemodynamic Response to Acute Nitroprusside Infusion
| Variables | Baseline | Peak Nitroprusside | P-Value |
|---|---|---|---|
| Cardiac Output (L/min) | 4.1 ± 1.0 | 6.3 ± 1.4 | 0.01 |
| Heart Rate (bpm) | 85 ± 14 | 82 ± 14 | 0.03 |
| Stroke Volume (mL/bt) | 49.4 ± 16.3 | 86.6 ± 22.8 | 0.001 |
| Cardiac Index (L/min/m2) | 1.96 ± 0.41 | 3.06 ± 0.61 | 0.001 |
| O2 Pulse (mL O2/bt) | 3.22 ± 0.93 | 3.54 ± 1.17 | 0.25 |
| Systemic BP, systolic (mm Hg) | 116 ± 15 | 82 ± 17 | 0.001 |
| Systemic BP, diastolic (mm Hg) | 66 ± 8 | 44 ± 12 | 0.001 |
| Systemic BP, mean (mm Hg) | 82 ± 8 | 56 ± 13 | 0.001 |
| SVR (dynes·sec·cm −5) | 1491.8 ± 419.3 | 717.0 ± 351.5 | 0.001 |
| SVRI | 1080.6 ± 262.2 | 353.6 ± 179.5 | 0.001 |
| PAP, systolic (mm Hg) | 53.7 ± 15.1 | 36.7 ± 15.7 | 0.001 |
| PAP, diastolic (mm Hg) | 25.5 ± 5.7 | 13.8 ± 7.3 | 0.001 |
| PAP, mean (mm Hg) | 37.1 ± 8.5 | 23.0 ± 9.5 | 0.001 |
| PWP, mean (mm Hg) | 23.8 ± 5.4 | 10.0 ± 4.2 | 0.001 |
| PVR (dynes·sec·cm −5) | 281.9 ± 214.9 | 164.0 ± 85.2 | 0.06 |
BP, blood pressure; SVR, systemic vascular resistance; SVRI, systemic vascular resistance index; PAP, pulmonary artery pressure; PWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular pressure. Data are presented as Mean±SD, n=12.
Blood Gas Response to Acute Nitroprusside Infusion
The blood gas response to nitroprusside infusion is reported in Table 3. There was no significant change in the PaO2 or PaCO2, however the CaO2 decreased slightly (p<0.01) with nitroprusside infusion. Despite the slight reduction in CaO2, the much larger increase in cardiac index (driven by cardiac output) was sufficient to increase the SOT (p<0.001).
TABLE 3.
Blood Gas Response to Acute Nitroprusside Infusion
| Variables | Baseline | Peak Nitroprusside | P-Value |
|---|---|---|---|
| PaO2 (mm Hg) | 72.3 ± 16.6 | 70.9 ± 10.2 | 0.79 |
| PaCO2 (mm Hg) | 36.8 ± 4.7 | 35.1 ± 4.5 | 0.48 |
| CaO2 (mm Hg) | 17.5 ± 3.0 | 16.4 ± 3.1 | 0.01 |
| CvO2 (mmHg) | 10.8 ± 2.3 | 12.19 ± 2.8 | 0.001 |
| SaO2 (%) | 0.94 ± 0.03 | 0.95 ± 0.02 | 0.91 |
| SvO2 (%) | 0.59 ± 0.06 | 0.69 ± 0.05 | 0.001 |
| SOT (mL/min/m2) | 337.4 ± 69.2 | 524.1 ± 132.0 | 0.001 |
PaO2, arterial partial pressure of oxygen; PaCO2, arterial partial pressure of carbon dioxide; CaO2, arterial content of oxygen; CvO2, arterial content of carbon dioxide; SOT, systemic oxygen transport. Data are presented as Mean±SD, n=12.
Ventilatory Response to Acute Nitroprusside Infusion
Ventilatory and gas exchange characteristics at baseline and peak nitroprusside infusion are listed in Table 4. There was little change in VO2 with a minor increase in VCO2 resulting in an increase in RER (p<0.01). Tidal volume increased (p=0.04) while RR dropped slightly (p=0.26) resulting in a minimal change in VE (p=0.12). The PETO2 increased (p<0.05) while the PETCO2 decreased (p=0.02) from baseline to peak nitroprusside. With this, VD/VT demonstrated no change (p=0.31) while VT/TI (an index of ventilatory drive) increased with nitroprusside infusion (p<0.01).
TABLE 4.
Ventilatory and Gas Exchange Response to Acute Nitroprusside Infusion
| Variables | Baseline | Peak Nitroprusside | P-Value |
|---|---|---|---|
| VO2 (mL/min) | 266.1 ± 57.1 | 277.8 ± 50.9 | 0.59 |
| VCO2 (mL/min) | 226.2 ± 59.4 | 259.8 ± 56.4 | 0.14 |
| RER | 0.85 ± 0.07 | 0.93 ± 0.08 | 0.01 |
| VE (L/min) | 10.0 ± 2.3 | 11.6 ± 2.3 | 0.12 |
| VE/VCO2 | 44.8 ± 6.0 | 44.9 ± 6.0 | 0.94 |
| VT (mL/min) | 523.1 ± 172.5 | 716.1 ± 421.7 | 0.04 |
| RR (breaths/min) | 20.3 ± 5.1 | 19.2 ± 6.9 | 0.26 |
| PETO2 (mm Hg) | 97.9 ± 17.3 | 106.3 ± 6.5 | 0.05 |
| PETCO2 (mm Hg) | 32.6 ± 4.0 | 31.1 ± 3.6 | 0.02 |
| VD/VT | 0.47 ± 0.07 | 0.46 ± 0.06 | 0.31 |
| VT/TI | 515.2 ± 103.7 | 635.3 ± 125.8 | 0.01 |
| Amplitude of Ventilatory Oscillations (peak-nadir, L/min) | 5.5 ± 2.7 | 2.6 ± 2.4 | 0.001 |
| Amplitude of Ventilatory Oscillations (% of mean VE) | 57.5 ± 33.6 | 22.5 ± 20.8 | 0.001 |
| Cycle Length of Ventilatory Oscillations (sec) | 60.8 ± 17.6 | 41.4 ± 28.8 | 0.01 |
VO2, volume of oxygen consumed; VCO2, volume of carbon dioxide produced; RER, respiratory exchange ratio; VE, minute ventilation; VT, tidal volume; RR, respiratory rate; PETO2, partial pressure of end tidal oxygen; PETCO2, partial pressure of end tidal carbon dioxide. Data are presented as Mean±SD, n=12.
Influence of Nitroprusside on Periodic Breathing
The amplitude of the oscillations of VE (both absolute and as a % of mean VE) decreased significantly with nitroprusside infusion (Table 4). Cycle length also decreased in conjunction with the drop in amplitude (p<0.01). Interestingly, the decline in amplitude and cycle length occurred in all patients with nitroprusside infusion and in 3 of the 12 patients the periodic breathing was transiently abolished. On average, nitroprusside infusion resulted in a 3 L/min and 19 sec reduction in amplitude and cycle length, respectively. The amplitude as a % of the mean VE was reduced by approximately 35% resulting in a 53% change from baseline.
Relationship between Hemodynamic Changes and Ventilatory Measures of Periodic Breathing with Nitroprusside
In attempt to determine the relationship between hemodynamic changes to alterations in PB, we determined correlations between various pulmonary and systemic hemodynamic measurements such as PVR, mean PAP, PWP, CO, CI, arterial blood gases, and SOT and the changes in amplitude and cycle length of PB using linear regression. The strongest relationship was seen between the change in PWP and the change in amplitude of VE oscillations. Figure 1A suggests a close relationship between the decrease in PWP and decrease amplitude of VE oscillations. Figure 1B demonstrates a close negative relationship between changes in PVR as it relates to change in amplitude of VE oscillations. Interestingly, this figure illustrates that with a greater reduction in PVR there is less reduction in amplitude of VEoscillations. We did not see a significant relationship between the change in amplitude of VE oscillations and mean PAP (r=0.13, p=0.69), CO (r=0.18, p=0.58), cardiac index (r=0.15, p=0.63), metabolic demand (VO2, r=0.003, p=0.99), or SOT (r=0.31, p=0.42).
FIGURE 1.
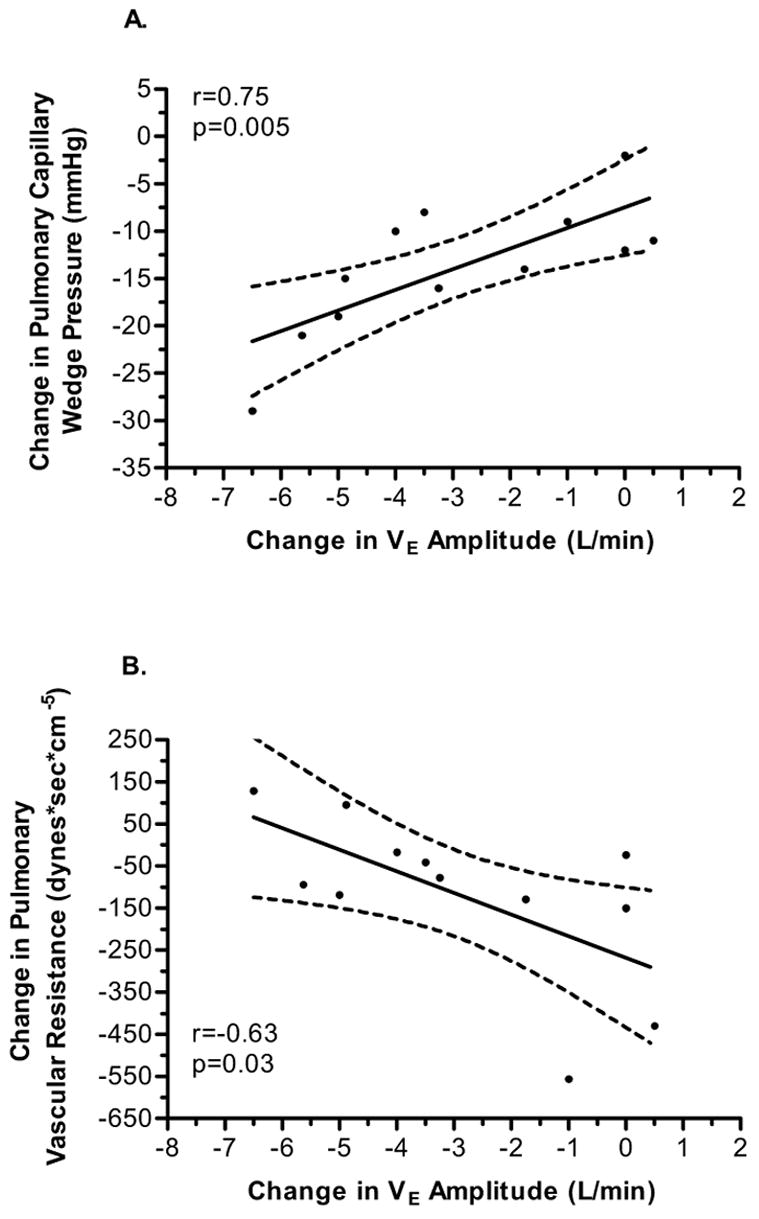
A. Correlation between the change in mean pulmonary wedge pressure and amplitude of minute ventilation oscillations. The solid line represents the least square regression line and the dashed lines represent the 95% confidence interval of the regression line. B. Correlation between the change in pulmonary vascular resistance and amplitude of minute ventilation oscillations. The solid line represents the least square regression line and the dashed lines represent the 95% confidence interval of the regression line.
DISCUSSION
Primary Findings
The present study evaluated the ventilatory and oscillatory breathing pattern response to acute nitroprusside infusion in a group of NYHA class III and IV HF patients who demonstrated PB at rest. Our results suggest that the acute infusion of nitroprusside in this population results in a clear reduction of both pulmonary and systemic vascular resistance while significantly increasing CI and SOT. These changes were coupled with a reduction in the amplitude and length of the oscillatory cycles in VE.
Potential Mechanisms
Oscillatory ventilation (PB) has been reported in up to 64% of patients with HF patients 17, and is linked to severity of disease 18. Oscillatory ventilation observed during wakefulness has more recently been shown to be particularly predictive of a poor prognosis 5. However, the specific causes of this oscillatory breathing pattern remain unclear. Previous studies have suggested the augmented breathing pattern may be related to: instability of the ventilatory control system 8,9,19,20, oscillations in stroke volume and cardiac output that may influence ventilation independently 3, alterations in pulmonary blood flow 21, or activity of a central ventilatory pacemaker that is set into oscillation (or whose normally weak oscillatory activity is greatly enhanced, i.e. Mayer waves) 22.
Alterations in Ventilatory Control
There are several potential mechanisms that can lead to increased ventilatory drive in HF patients including: 1) increased left atrial pressure influencing stretch receptors involved in ventilation 13,23, 2) pulmonary vascular congestion and potentially subclinical edema which may stimulate pulmonary J receptors or irritant receptors involved in causing a more tachypneic ventilatory response 14, 3) enhanced ergoreceptor activation in the peripheral muscles 4 or other neurohumoral interactions 24, and 4) low perfusion to the carotid bodies (a hypoxic condition) either by direct hypoxia or reduced perfusion due to reduced cardiac output 10,25.
Animal studies have suggested that elevated left atrial and pulmonary pressures may directly stimulate hyperventilation and hypocapnia leading to PaCO2 levels that fall below the apneic threshold, thus triggering cyclic breathing patterns 13–15,23,26–28. Solin and colleagues 12 confirmed this in HF patients with central sleep apnea demonstrating that patients with central sleep apnea had significantly higher pulmonary artery pressures and PWP (a surrogate measure of left atrial pressure) compared to patients with obstructive sleep apnea or no evidence of disordered breathing. From this it was suggested that pulmonary congestion, a result of elevated left atrial pressure in heart failure, may be a direct stimulus of pulmonary vagal afferent nerve fibers resulting in hyperventilation and hypocapnia and subsequent cyclic apneas. However, whether left atrial pressure independently contributed to the response remains unclear. Recently Chennuel et al. 28 have shown in the dog model of HF that elevations in left atrial pressure resulted in increased gain of the ventilatory response to CO2 below eupnea thereby narrowing the CO2 reserve. These authors suggest that the narrowing of CO2 reserve resulted in a predisposition toward an apnea and consequently breathing instability. Our data partially support this hypothesis in that acute infusion of nitroprusside significantly reduced PWP which was positively related to the reduction in ventilatory oscillation amplitude.
Interestingly however, we found that patients who had the largest reduction in PWP but least reduction in PVR had the greatest reduction in amplitude of ventilatory oscillations. The drop in PWP without a marked drop in PVR could be explained by a direct vasodilatory effect of nitroprusside on the systemic vasculature in patients with relatively fixed pulmonary vascular resistance. We did note a marked drop in systemic vascular resistance and arterial pressure with a proportionately greater increase (64%) in CO, relative to the fall in mean PAP, after the nitroprusside infusion. Our results are consistent with a reduction in stimulation of pulmonary J receptors resulting from a reduced PWP with secondary changes in PVR and mean PAP.
Oscillatory ventilation also may be related to direct modulation of peripheral chemosensitivity. Fanfulla et al. 10 demonstrated that patients with daytime breathing disorders have pronounced chronic hypocapnia. These authors suggest that the reduction in CO, typical of heart failure, results in reduced transport of oxygen to the periphery. This reduction in peripheral oxygen transport and resultant chronic mild hypoxia of chemoreceptors may be a stimulus respiratory drive leading to chronic hyperventilation and subsequent hypocapnia. In the present study, most of our patients were hypocapnic and had only mild reductions in arterial oxygen content and thus there was not a significant relationship between the change in SOT and change in ventilatory oscillation amplitude or cycle length with nitroprusside infusion. Although these results do not definitively rule out the hypothesis that chronic hypoxia results in overstimulation of peripheral chemoreceptors, it does suggest that this mechanism may not play a major role in the attenuation of periodic breathing during acute nitroprusside infusion.
Mortara et al. 6 have suggested that the Cheyne-Stokes respiration during daytime wakefulness in heart failure patients may largely be related to hemodynamic dysfunction. Specifically, these authors suggest a combination of elevated of PWP and reduction of cardiac index that results in prolonged circulation time may be significant contributors to abnormal breathing patterns. Others have also concluded prolonged circulation time is a major contributor to PB 8,9. Our data support the theory that elevated PWP (a surrogate of LA pressure) may play a role as a trigger for oscillatory ventilation. Interestingly however, the results of the present study demonstrate that although the change in PWP was related to the change in amplitude of the ventilatory oscillations, the significant increase in cardiac index that occurred during peak nitroprusside infusion was not significantly correlated to the changes in amplitude or cycle length of the ventilatory oscillations. Importantly, the small sample size of this study and redundancy of physiologic systems may have masked the importance of augmented cardiac index and subsequently circulation time.
Oscillations in Cardiac Output and a Central Ventilatory Pacemaker
Ben-Dov and colleagues 3 have suggested that oscillations in cardiac output may directly influence the oscillations seen during periodic breathing through changes in pulmonary blood flow. These authors report that heart failure patients who demonstrate periodic breathing also exhibit oscillations in VO2 of similar magnitude and duration with slightly offset phasic pattern suggesting that the cardiac output oscillations may promote an oscillatory pattern of blood flow to the pulmonary circulation. To the contrary however, Francis et al. 11 have suggested that the oscillations in VO2 may be a result of, rather than a contributor to, ventilatory oscillations. Unfortunately, the ability to precisely measure stroke volume and cardiac output on a beat to beat basis, the inability to separate the influence of breathing pattern on cardiac function due to respiratory variations in intrathoracic and abdominal pressure 29 as well as competition for intrathoracic space between the heart and lungs 30 limits the ability to specifically delineate the magnitude of contribution of cardiac output oscillations to periodic breathing.
With regards to a central ventilatory pacemaker, Preiss and colleagues have demonstrated that arterial pressure oscillations occur in a very similar manner to that of ventilatory oscillations although both have been shown to persist independently. These authors suggest that both the vasomotor and respiratory control centers may be influenced by a central pacemaker or central oscillator capable of modulating neural activity 22,31.
Conceptual Model for Control of Periodic Breathing
Although the factors regulating or contributing to oscillatory breathing in CHF are complex, the ventilatory system can effectively be modeled as a simple, closed-loop feedback controller with the CNS providing ventilatory drive via neural output to respiratory muscles 8,9,19. A model such as this includes a central “controller” represented by the CNS and a “plant” represented by the ventilatory muscles, lungs and chest wall. The controller takes feedback from the periphery, (e.g. chemo-, metabo-, ergo-, baro-, or other receptors capable of conveying information to the control center), integrates it with the regulatory ventilatory drive, and produces phasic neural output directly to the ventilatory muscles, in effect driving ventilation. The plant element of the model receives this neural output from the controller and attempts to effect a change in blood gas concentration through ventilation. Ordinarily, this system provides highly efficient control of blood PO2 and PCO2 levels through its negative feedback mechanism; however, for the system to remain stable, the feedback information sent back to the CNS from the periphery (i.e. changes in blood PO2 and PCO2) must be such that it matches temporally with the regulatory activity of the CNS controller.
In chronic disease states such as HF, a potential contributor to the alterations in matching between the peripheral feedback and regulatory activity of the CNS includes circulatory delay 8,9,20,32,33. When blood transport slows (i.e. increased circulatory delay), it is possible for both the information from the periphery to the carotid receptors as well as information from the lungs to the CNS to arrive out of phase with the regulatory activity of the CNS. It has been suggested that increased transit time combined with increased ventilatory drive can lead to oscillatory breathing, however the magnitude of the increase in transit time often reported in mathematical modeling studies may be out of physiologic range. 8,9,19,20
Limitations
A potential limitation to this study is the relatively small sample size and homogeneity of the study population as all participants in this study were Caucasian men. As such, these findings should be confirmed in a larger non-selective cohort of HF patients. Another potential limitation remains the difficulty of deciphering the specific mechanisms involved in PB in human subjects. However, studies in the intact human are inherently complex due to the multiple redundancies in physiologic systems and thus small but key advances in mechanistic understanding remain essential to unveiling true physiologic interactions. We feel that, the results of this study demonstrate important progress in the involvement of left atrial pressure as one potential contributory mechanism of PB with therapeutic potential.
Summary and Conclusions
The results of this study suggest that the amplitude of ventilatory oscillations in heart failure patients that demonstrate periodic breathing at rest is positively related to PWP and inversely related to PVR. Although numerous mechanisms are postulated to be associated with the etiology of ventilatory oscillations in CHF including delayed circulation time and factors influencing ventilatory drive (e.g. chemoreceptor sensitivity), these data suggest that LA pressure receptors have a contributory impact in modulating ventilatory control independent of the overall influence on ventilatory drive and that the ventilatory oscillations may also have been strongly influenced by a change in transit time.
Acknowledgments
This work was supported in part by: NIH grants HL71478 and HL07111. General Clinical Research Center Program, NCRR/NIH #: M01-RR00585.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
The authors of this manuscript do not have any conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 2.Corra U, Giordano A, Bosimini E, Mezzani A, Piepoli M, Coats AJ, Giannuzzi P. Oscillatory ventilation during exercise in patients with chronic heart failure: clinical correlates and prognostic implications. Chest. 2002;121:1572–80. doi: 10.1378/chest.121.5.1572. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Dov I, Sietsema KE, Casaburi R, Wasserman K. Evidence that circulatory oscillations accompany ventilatory oscillations during exercise in patients with heart failure. Am Rev Respir Dis. 1992;145:776–81. doi: 10.1164/ajrccm/145.4_Pt_1.776. [DOI] [PubMed] [Google Scholar]
- 4.Ponikowski P, Anker SD, Chua TP, Francis D, Banasiak W, Poole-Wilson PA, Coats AJ, Piepoli M. Oscillatory breathing patterns during wakefulness in patients with chronic heart failure: clinical implications and role of augmented peripheral chemosensitivity. Circulation. 1999;100:2418–24. doi: 10.1161/01.cir.100.24.2418. [DOI] [PubMed] [Google Scholar]
- 5.Koike A, Shimizu N, Tajima A, Aizawa T, Fu LT, Watanabe H, Itoh H. Relation between oscillatory ventilation at rest before cardiopulmonary exercise testing and prognosis in patients with left ventricular dysfunction. Chest. 2003;123:372–9. doi: 10.1378/chest.123.2.372. [DOI] [PubMed] [Google Scholar]
- 6.Mortara A, Sleight P, Pinna GD, Maestri R, Capomolla S, Febo O, La Rovere MT, Cobelli F. Association between hemodynamic impairment and Cheyne-Stokes respiration and periodic breathing in chronic stable congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;84:900–4. doi: 10.1016/s0002-9149(99)00462-2. [DOI] [PubMed] [Google Scholar]
- 7.Jones PW, Huszczuk A, Wasserman K. Cardiac output as a controller of ventilation through changes in right ventricular load. J Appl Physiol. 1982;53:218–224. doi: 10.1152/jappl.1982.53.1.218. [DOI] [PubMed] [Google Scholar]
- 8.Francis DP, Willson K, Davies LC, Coats AJS, Piepoli M. Quantitative General Theory for Periodic Breathing in Chronic Heart Failure and its Clinical Implications. Circulation. 2000;102:2214–2221. doi: 10.1161/01.cir.102.18.2214. [DOI] [PubMed] [Google Scholar]
- 9.Carley DW, Shannon DC. A minimal mathematical model of human periodic breathing. J Appl Physiol. 1988;65:1400–1409. doi: 10.1152/jappl.1988.65.3.1400. [DOI] [PubMed] [Google Scholar]
- 10.Fanfulla F, Mortara A, Maestri R, Pinna GD, Bruschi C, Cobelli F, Rampulla C. The development of hyperventilation in patients with chronic heart failure and Cheyne-Strokes respiration: a possible role of chronic hypoxia. Chest. 1998;114:1083–90. doi: 10.1378/chest.114.4.1083. [DOI] [PubMed] [Google Scholar]
- 11.Francis DP, Davies LC, Piepoli M, Rauchhaus M, Ponikowski P, Coats AJS. Origin of Oscillatory Kinetics of Respiratory Gas Exchange in Chronic Heart Failure. Circulation. 1999;100:1065–1070. doi: 10.1161/01.cir.100.10.1065. [DOI] [PubMed] [Google Scholar]
- 12.Solin P, Bergin P, Richardson M, Kaye DM, Walters EH, Naughton MT. Influence of pulmonary capillary wedge pressure on central apnea in heart failure. Circulation. 1999;99:1574–9. doi: 10.1161/01.cir.99.12.1574. [DOI] [PubMed] [Google Scholar]
- 13.Lloyd TC., Jr Effect of increased left atrial pressure on breathing frequency in anesthetized dog. J Appl Physiol. 1990;69:1973–1980. doi: 10.1152/jappl.1990.69.6.1973. [DOI] [PubMed] [Google Scholar]
- 14.Roberts AM, Bhattacharya J, Schultz HD, Coleridge HM, Coleridge JC. Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ Res. 1986;58:512–22. doi: 10.1161/01.res.58.4.512. [DOI] [PubMed] [Google Scholar]
- 15.Paintal AS. Mechanism of stimulation of type J pulmonary receptors. J Physiol. 1969;203:511–32. doi: 10.1113/jphysiol.1969.sp008877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa H, Kumada T, Matsuda Y, Katayama K, Fujii T, Yoshino F, Kohno M, Moritani K, Miura T, Kusukawa R. Hemodynamic effects of nitroprusside on cardiovascular system. Jpn Circ J. 1984;48:405–13. doi: 10.1253/jcj.48.405. [DOI] [PubMed] [Google Scholar]
- 17.Mortara A, Sleight P, Pinna GD, Maestri R, Prpa A, La Rovere MT, Cobelli F, Tavazzi L. Abnormal awake respiratory patterns are common in chronic heart failure and may prevent evaluation of autonomic tone by measures of heart rate variability. Circulation. 1997;96:246–252. doi: 10.1161/01.cir.96.1.246. [DOI] [PubMed] [Google Scholar]
- 18.Andreas S, Hagenah G, Moller C, Werner GS, Kreuzer H. Cheyne-stokes respiration and prognosis in congestive heart failure. The American Journal of Cardiology. 1996;78:1260–1264. doi: 10.1016/s0002-9149(96)00608-x. [DOI] [PubMed] [Google Scholar]
- 19.Khoo MC, Kronauer RE, Strohl KP, Slutsky AS. Factors inducing periodic breathing in humans: a general model. J Appl Physiol. 1982;53:644–659. doi: 10.1152/jappl.1982.53.3.644. [DOI] [PubMed] [Google Scholar]
- 20.Khoo MCK. Determinants of ventilatory instability and variability. Respiration Physiology. 2000;122:167–182. doi: 10.1016/s0034-5687(00)00157-2. [DOI] [PubMed] [Google Scholar]
- 21.Yajima T, Koike A, Sugimoto K, Miyahara Y, Marumo F, Hiroe M. Mechanism of periodic breathing in patients with cardiovascular disease. Chest. 1994;106:142–6. doi: 10.1378/chest.106.1.142. [DOI] [PubMed] [Google Scholar]
- 22.Preiss G, Iscoe S, Polosa C. Analysis of a periodic breathing pattern associated with Mayer waves. Am J Physiol. 1975;228:768–74. doi: 10.1152/ajplegacy.1975.228.3.768. [DOI] [PubMed] [Google Scholar]
- 23.Kostreva DR, Hopp FA, Zuperku EJ, Kampine JP. Apnea, tachypnea, and hypotension elicited by cardiac vagal afferents. J Appl Physiol. 1979;47:312–318. doi: 10.1152/jappl.1979.47.2.312. [DOI] [PubMed] [Google Scholar]
- 24.Abraham MR, Olson LJ, Joyner MJ, Turner ST, Beck KC, Johnson BD. Angiotensin-converting enzyme genotype modulates pulmonary function and exercise capacity in treated patients with congestive stable heart failure. Circulation. 2002;106:1794–1799. doi: 10.1161/01.cir.0000031735.86021.79. [DOI] [PubMed] [Google Scholar]
- 25.Mortara A, Bernardi L, Pinna GD, Spadacini G, Maestri R, Dambacher M, Muller C, Sleight P, Tavazzi L, Roskamm H, Frey AW. Alterations of breathing in chronic heart failure: clinical relevance of arterial oxygen saturation instability. Clin Sci (Lond) 1996;91:72–4. doi: 10.1042/cs0910072supp. Suppl. [DOI] [PubMed] [Google Scholar]
- 26.Paintal AS. A study of right and left atrial receptors. J Physiol. 1953;120:596–610. doi: 10.1113/jphysiol.1953.sp004920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dempsey JA. Crossing the apnoeic threshold: causes and consequences. Exp Physiol. 2005;90:13–24. doi: 10.1113/expphysiol.2004.028985. [DOI] [PubMed] [Google Scholar]
- 28.Chennuel B, Smith C, Skatrud J, Henderson K, Dempsey J. Increased propensity for apnea in response to acute elevations in left atrial pressure during sleep in the dog. J Appl Physiol. 2006;101:76–83. doi: 10.1152/japplphysiol.01617.2005. [DOI] [PubMed] [Google Scholar]
- 29.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol. 2004;96:1920–1927. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- 30.Olson TP, Beck KC, JB J, BD J. Competition for Intrathoracic Space Reduces Lung Capacity in Patients with Chronic Heart Failure: A Radiographic Study. Chest. 2006 doi: 10.1378/chest.130.1.164. In Press. [DOI] [PubMed] [Google Scholar]
- 31.Preiss G, Kirchner F, Polosa C. Patterning of sympathetic preganglionic neuron firing by the central respiratory drive. Brain Res. 1975;87:363–74. doi: 10.1016/0006-8993(75)90434-5. [DOI] [PubMed] [Google Scholar]
- 32.Pryor WW. Cheyne-Stokes respiration in patients with cardiac enlargement and prolonged circulation time. Circulation. 1951;4:233–238. doi: 10.1161/01.cir.4.2.233. [DOI] [PubMed] [Google Scholar]
- 33.Guyton AC, Crowell JW, Moore JW. Basic oscillating mechanism of Cheyne-Stokes breathing. Am J Physiol. 1956;187:395–398. doi: 10.1152/ajplegacy.1956.187.2.395. [DOI] [PubMed] [Google Scholar]


