Abstract
Cellular content of cAMP generated by activation of adenylylcyclase (AC; EC 4.6.1.1) is a key determinant of functional responsiveness in the heart and other tissues. We have tested two hypotheses regarding the relationship between AC content and β-adrenergic receptor (βAR)-mediated signal transduction in cardiac myocytes. First, that AC content limits adrenergic signal transduction, and, second, that increased AC, independent of (βAR) number and G-protein content, yields a proportional increase in βAR-mediated transmembrane signaling. We used recombinant adenovirus to increase AC isoform VI (ACVI) expression in neonatal cardiac myocytes. Cells that overexpressed ACVI responded to agonist stimulation with marked increases in cAMP production in proportion to protein expressed. In parallel experiments performed on cells transfected with lacZ (control) or ACVI, [3H]forskolin binding, used to assess AC protein expression, was amplified 6-fold, while βAR-stimulated cAMP production from these cells was increased 7-fold. No changes in βAR number, or in the heterotrimeric GTP-binding proteins, Gαs or Gαi2, were observed. Previous studies indicate that increased cardiac expression of βAR or Gαs does not yield proportional increases in transmembrane adrenergic signaling. In contrast, the current data demonstrate that increased ACVI expression provides a proportional increase in β-adrenergic signal transduction. Our results show that the amount of AC sets a limit on transmembrane β-adrenergic signaling. We speculate that similar functional responses are possible in other cell types in which AC plays an important physiological role.
Cyclic adenosine 3′,5′-monophosphate (cAMP) is widely recognized as a second messenger that regulates intracellular metabolism, differentiated cell functions, and gene expression in mammalian cells. A large number of neurotransmitters and hormonal agonists act by means of membrane receptors and, in turn, heterotrimeric GTP-binding (G) proteins to regulate cAMP synthesis by adenylylcyclase (AC; EC 4.6.1.1). β-Adrenergic receptors (βARs) are among the most physiologically important type of receptors that promote cAMP formation. These receptors are of particular importance in the mammalian heart, where they regulate rate and force of cardiac contraction and relaxation.
The estimated molar proportions of the βAR⋅stimulatory GTP-binding protein (Gs)⋅AC complex suggest that βAR number or the amount of AC may set a limit on βAR-mediated transmembrane signaling. The molar proportions of these three elements in cardiac myocytes are 1:200:3 (βAR:Gs:AC) as estimated by radioligand binding (βAR), immunoblotting (Gs), and [3H]forskolin binding (AC) (1, 2). Previous efforts to increase transmembrane β-adrenergic signaling in the heart have examined the sequelae of βAR overexpression in cultured cardiac myocytes (3) and transgenic mice (4). Despite 20- to 200-fold overexpression of βAR protein, only 2-fold increases in cAMP production were achieved. Thus, increased βAR number does not provide a proportional increase in transmembrane signaling through AC. Similarly, cardiac directed overexpression of Gαs in transgenic mice only modestly increased cardiac adrenergic responsiveness (5). In these studies a fixed amount of AC may have limited the degree to which increased βAR or Gs expression could provoke a meaningful physiological response.
In contrast, manipulation of the amount of AC, independent of changes in receptor number or Gs, may provide a means for the same number of receptors to transduce signals more effectively. Data supporting this previously unexplored concept would provide a rationale for therapeutic strategies designed to amplify transmembrane signaling in the heart in disease states in which reduced adrenergic responsiveness occurs, such as congestive heart failure. In this regard, we and others recently have reported down-regulation of myocardial ACV and ACVI in animal models of heart failure (6, 7). To explore the influence of AC overexpression on transmembrane signaling, we chose to overexpress ACVI in cardiac myocytes because of potential inferences to cardiac pathophysiology. The relationship between AC content and signal transduction was the focus of the current study. We tested two hypotheses: first, that AC content limits adrenergic signal transduction; second, that increased AC, independent of βAR number and G-protein content, yields a proportional increase in βAR-mediated transmembrane signaling.
METHODS
Plasmid Construction and Recombinant Adenovirus Production.
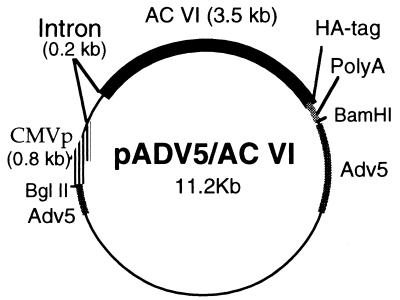
A murine ACVI cDNA (obtained from Dermott Cooper, University of Colorado, Denver) was modified by removing the 3′-untranslated region (Fig. 1). A subfragment containing the 3′ portion of ACVI with a XhoI site at its 5′ end was generated by using two PCR primers: ACVIPX, which anneals to ACVI cDNA at bases 2500–2521; and ACVIp3′HA, which contains sequences from the 3′ end of the ACVI coding region and sequences from human influenza hemagglutinin. The PCR product was digested with restriction enzymes XhoI and XbaI, whose sites were incorporated into the primers at the 3′ end. The 5′ portion of the ACVI fragment (base pairs −92 to +2500) was obtained by EcoRI and XhoI digestion of the full-length cDNA and was isolated on an agarose gel. Two ACVI fragments were then subcloned into an EcoRI–XbaI-digested adenovirus vector (pAd5CI) by three-molecule ligation. The pAd5/CI vector contains a cytomegalovirus immediate-early enhancer/promoter region (CMV promoter), a chimeric intron, and a multicloning site derived from pCI plasmid DNA (Promega). It also contains a bovine growth hormone poly(A) sequence and partial human adenovirus 5 sequences. The ACVI-containing vector was cotransfected (calcium phosphate) with pJM170 (which contains the adenovirus genome except the E1 region) into a human embryonic kidney cell line (H293). After recombination, plaques were selected and expanded in H293 cells, which have been transformed with adenovirus E1 and therefore provide this viral transcription factor in trans. Expression of ACVI was examined by reverse transcriptase (RT)-PCR and Western blot analysis. Virus was purified by cesium chloride ultracentrifugation and desalted by using Sephadex G-25 equilibrated with PBS. The viral concentration was determined by optical densitometry at 260 nm. Plaque-forming units (pfu) were assayed by plaque titration using H293 cells overlaid with agarose/Dulbecco’s modified Eagle’s medium (DMEM).
Figure 1.
Recombinant adenovirus expressing ACVI. CMVp, cytomegalovirus immediate-early enhancer/promoter region; HA, hemagglutinin protein of influenza virus; PolyA, poly(A) region of bovine growth hormone gene; Adv5, adenovirus 5.
Identification of ACVI-Expressing Clones.
To identify adenovirus clones that expressed ACVI, 16 clones were screened by RT-PCR using a pair of specific primers (ACVIPX and ACVIp3′HA) which hybridize to transgene ACVI but not endogenous ACVI in H293 cells. The 512-bp RT-PCR product was confirmed by digestion with the restriction enzyme ApaI to produce 312-bp and 200-bp fragments. Three of 16 clones expressed ACVI mRNA. When tested by transfection of these clones into cardiac myocytes, all three clones showed increased cAMP production in response to stimulation by isoproterenol (clone 1, 22-fold increase; clone 2, 15-fold increase; and clone 3, 12-fold increase) and forskolin (clone 1, 16-fold increase; clone 2, 11-fold increase; and clone 3, 13-fold increase). Clone 1 was selected for subsequent studies.
Cardiac Myocyte Preparation and Gene Transfer.
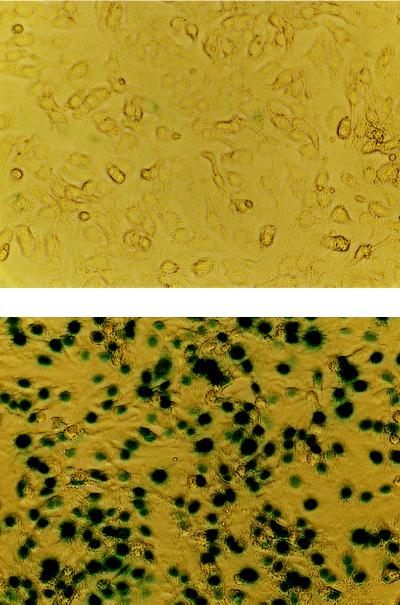
Hearts were removed from 1- to 2-day-old Sprague–Dawley rats, atria and great vessels were discarded, and ventricles were trisected. Myocardium was digested with collagenase II (Worthington) and pancreatin (GIBCO/BRL Life Technologies), and the myocardial cell suspension was centrifuged through Percoll (Pharmacia Biotechnology) step gradients to separate cardiac myocytes from other cells. Cells were plated (4 × 104 cells per cm2) in plates precoated with gelatin, incubated for 24 h, washed with serum-free medium, and maintained in 2% fetal bovine serum for 24 h (8). Gene transfer was performed 3 days after isolation by adding recombinant adenovirus expressing ACVI or lacZ (10 pfu per cell), and incubating for 20 h in DMEM containing 2% fetal bovine serum. Adenovirus was removed and cells were maintained for 24 h prior to study. The extent of gene transfer was evaluated by 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) staining of cells after gene transfer with lacZ. Preliminary studies established that at virus titers of 1, 10, and 100 pfu per cell, 95–100% of cardiac myocytes expressed β-galactosidase with no evidence of cytotoxicity (Fig. 2). The expression of β-galactosidase was undetectable in uninfected cells. We selected 10 pfu per cell for our studies.
Figure 2.
Neonatal cardiac myocytes before (Upper) and after (Lower) lacZ gene transfer. Gene transfer was performed by adding recombinant adenovirus expressing β-galactosidase (1 pfu per cell) and incubating for 20 h. Adenovirus then was removed and cells were maintained for 24 h. The extent of gene transfer was evaluated by 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) staining of cells after gene transfer with lacZ. Lower indicates >95% successful gene transfer. (×80.)
To determine whether lacZ gene transfer had a deleterious effect on cAMP production, we also studied untransfected cardiac myocytes. These studies showed that untransfected cells were indistinguishable from lacZ-transfected cells in cAMP production in response to 10 μM isoproterenol (untransfected, 1.5 ± 0.3 pmol/μg; lacZ, 2.0 ± 0.5 pmol/μg; triplicate samples) or forskolin (untransfected, 0.07 ± 0.01 pmol/μg; lacZ, 0.12 ± 0.04 pmol/μg; triplicate samples). Protein content per well was similar after gene transfer with lacZ and ACVI (lacZ, 32 ± 6 μg per well; ACVI, 35 ± 5 μg per well; n = 6 each group).
RT-PCR.
RT-PCR was used to identify transgene ACVI mRNA. Briefly, 1 μg of mRNA was mixed with 100 ng of the primer ACVI3′pHA in 11 μl, heated (70°C, 10 min), and quickly chilled on ice. Four microliters of buffer (250 mM Tris⋅HCl, pH 8.3/375 mM KCl/15 mM MgCl2), 2 μl of 0.1 M DTT, and 1 μl of 10 mM dNTP were added, and the reaction mixture was allowed to equilibrate (37°C, 2 min). Finally, 1 μl (200 units) of SuperScript II RNase H RT (GIBCO/BRL Life Technologies) was added; reaction duration was 1 h (37°C). Five microliters of RT product was used as template for PCR using the primers described above.
Northern Blot Analysis.
Total RNA was isolated from cardiac myocytes 48 h after gene transfer by using Trizon reagent (GIBCO/BRL Life Technologies). Twenty micrograms of denatured total RNA was electrophoresed in 1× Mops/EDTA buffer on a 1.0% agarose gel. RNA was transferred to a nylon membrane in 20× SCC and immobilized (80°C, 2 h), and the RNA on the membrane was hybridized with a randomly [32P]dCTP labeled cDNA probe for murine ACVI or glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Hybridization was carried out in Hood buffer (50% formamide/5× SSC/20 mM sodium phosphate, pH 6.7/7% SDS/1% polyethylene glycol 15,000–20,000/0.5% nonfat dry milk) at 42°C for 16 h. The membrane was washed once with 2× SSC/0.5% SDS for 30 min (25°C), and two or three times with 0.1× SSC/0.1% SDS for 30 min each (60–65°C) and exposed to x-ray film.
Western Blot Analysis.
Cell lysates were prepared from virus-infected myocytes by using NP-40 lysis buffer [20 mM Hepes, pH 7.0/120 mM HCl/1 mM DTT/5 mM magnesium acetate/10% (vol/vol) glycerol/0.5% Nonidet P-40 containing proteinase inhibitors: 10 μg/ml each of leupeptin, aprotinin, and pepstatin, and 1 mg/ml Pefabloc (Boehringer Mannheim)] for 10 min on ice. The samples were centrifuged (13,800 × g) for 15 min (4°C). The pellet was resuspended in 1× SDS sample buffer (50 mM Tris⋅HCl, pH 6.8/100 mM DTT/2% SDS/0.1% bromophenol blue/10% glycerol). Samples were boiled (5 min) and cell lysates were loaded onto an SDS/7.5% polyacrylamide gel and subjected to electrophoresis. Protein was electrophoretically transferred (1 h, 100 V, 4°C) to nitrocellulose membranes in Tris/glycine buffer [25 mM Tris⋅HCl, pH 8.3/150 mM glycine/10% (vol/vol) methanol]. The membranes were treated with blocking buffer consisting of 5% nonfat dry milk in Tris/saline buffer (0.9% NaCl/10 mM Tris⋅HCl, pH 7.5). For detection of ACVI protein, membranes were incubated with anti-ACV/ACVI antibody (Santa Cruz Biotechnology) diluted 1:100 in blocking buffer for 1 h (25°C). For detection of Gαs and Gαi2, membranes were incubated with anti-Gαs or anti-Gαi2 antibodies as previously described (9). Primary antibodies were detected with goat anti-rabbit IgG conjugated to horseradish peroxidase (GIBCO/BRL Life Technologies) in blocking buffer. The antigen then was visualized with chemiluminescent substrates A and B (Kirkegaard & Perry Laboratories) and exposed to x-ray film.
[3H]Forskolin Binding.
[3H]Forskolin binding studies provided a means to evaluate the amount of AC available for activation during agonist-stimulated signaling. Previous data have documented that isoproterenol and GTP-dependent [3H]forskolin binding is a measure of Gαs coupling to AC (1, 2). As previously described (1, 2), myocytes were infected with adenovirus expressing either ACVI or β-galactosidase and cultured for 48 h in 12-well culture plates. Prior to assay, culture medium was aspirated and the cells were washed in reverse buffer (100 mM KCl/20 mM NaCl/1 mM NaH2PO4/20 mM Hepes/1 mM MgSO4, pH 7.4). Binding assays were initiated by the addition of saponin (20 μg/ml final), 20 nM [3H]forskolin, 1 μM 1,9-dideoxyforskolin (to reduce association of radiolabel with non-adenylylcyclase molecules) and, in some samples, 30 μM guanosine 5′-[γ-thio]triphosphate (GTP[γS]). Cells were incubated in a final volume of 0.5 ml for 15 min at 25°C. Reactions were terminated by aspiration of medium and cells were washed twice with ice-cold washing buffer (50 mM Tris⋅HCl/10 mM MgCl2, pH 7.4). The amount of [3H]forskolin associated with cells was determined by extraction of cells in 0.2% Triton X-100 and scintillation counting of the soluble cell extract.
cAMP Measurements.
Prior to treatment of cells, growth medium was removed and cells were equilibrated for 30 min (25°C) in serum-free and NaHCO3-free DMEM supplemented with 20 mM Hepes, pH 7.2. Subsequently, cells were incubated for 10 min (25°C) in fresh DMEM containing either 10 μM isoproterenol or 10 μM forskolin in the presence of 0.1 mM ascorbic acid (to prevent oxidization) and 250 μM isobutylmethylxanthine, a phosphodiesterase inhibitor. The reaction was terminated by aspiration of medium and addition of 7.5% ice-cold trichloroacetic acid (TCA). TCA extracts were frozen (−20°C) until assayed. Intracellular cAMP levels were determined by radioimmunoassay (Calbiochem) of TCA extracts after acetylation according to the protocol provided by the manufacturer. The sensitivity of this assay allowed for large dilution of TCA extracts such that extraction of the TCA with ether was unnecessary. Production of cAMP was normalized to the amount of acid-insoluble protein assayed by the Bio-Rad protein assay.
β-Adrenergic Receptor Binding Studies.
βARs were identified in radioligand binding experiments using [125I]iodocyanopindolol (ICYP; 30–240 pM); binding in the presence of 10−4 M isoproterenol defined nonspecific binding. Transfected cells (lacZ vs. ACVI) were lysed and membranes were prepared for radioligand binding (9); experiments were performed with triplicate samples. Data are reported as specifically bound ICYP (fmol/mg).
Statistics.
Data are reported as mean values ± 1 SD. Data were compared by using Student’s t test and, where appropriate, analyses of variance. The null hypothesis was rejected when P < 0.05.
RESULTS AND DISCUSSION
Adenovirus vectors provide a means to obtain highly efficient gene transfer (10). In the current study, we obtained >95% gene transfer in cultured neonatal rat cardiac myocytes (Fig. 2). We established that transgene DNA and mRNA were present by using PCR, RT-PCR, and Northern blotting (Fig. 3). Immunoblotting and [3H]forskolin binding were used to assess transgene protein expression, and both showed evidence for robust expression of transgene ACVI (Figs. 4 and 5).
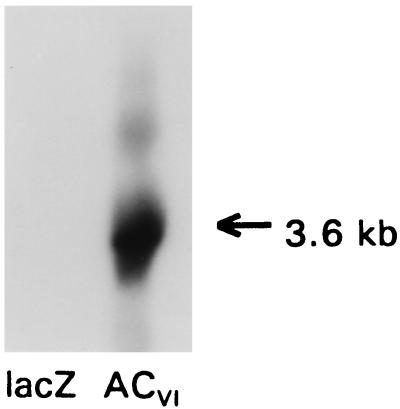
Figure 3.
Northern analysis. A murine ACVI cDNA probe was used in Northern blots prepared with RNA extracted from neonatal rat cardiac myocytes after gene transfer using recombinant adenovirus expressing lacZ or ACVI. Transgene mRNA was detectable in control cells only after prolonged exposure, reflecting the low abundance of endogenous ACVI. In contrast, after gene transfer with AdV.ACVI, abundant ACVI mRNA was evident. Equal RNA loading was documented by glyceraldehyde-3-phosphate dehydrogenase (GAPDH) controls (not shown). These data document robust transgene ACVI mRNA expression after gene transfer.
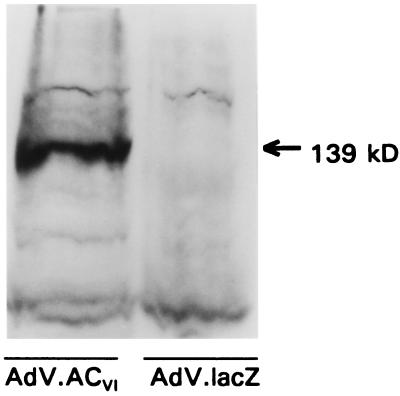
Figure 4.
Western analysis. A polyclonal antibody recognizing ACV and ACVI protein (Santa Cruz Biotechnology) was used in Western blots of lysates of neonatal rat cardiac myocytes after gene transfer using recombinant adenovirus expressing ACVI (AdV.ACVI) or lacZ (AdV.lacZ). As expected, transgene protein was undetectable in the control cells. In contrast, after gene transfer with AdV.ACVI, abundant ACVI protein was evident. The amount of total protein loaded was the same in both lanes. These data document robust transgene ACVI protein expression after gene transfer.
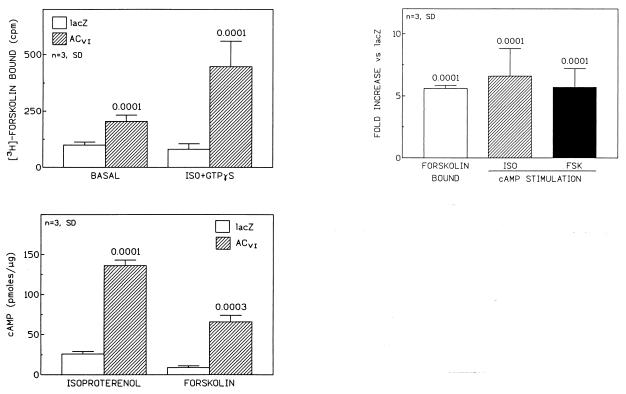
Figure 5.
(Upper Left) [3H]Forskolin binding. Net 10 μM isoproterenol- and 30 μM GTP[γS]-stimulated [3H]forskolin binding was increased after ACVI gene transfer. These data document that transgene ACVI is present and responsive to Gs–AC interaction. (Lower Left) cAMP production. Cardiac myocytes expressing transgene ACVI have increased adrenergic responsiveness to stimulation not only with 10 μM forskolin, reflecting increased amounts of AC, but also with 10 μM isoproterenol, indicating that newly synthesized AC is functionally coupled and recruitable through βAR stimulation. (Upper Right) Relationship between ACVI content and cAMP production. The graph displays three measures of altered adrenergic signaling ([3H]forskolin binding and isoproterenol- and forskolin-stimulated cAMP production). These data indicate that a proportional increase in AC content and enhanced adrenergic signaling has occurred. Net 30 μM GTP[γS]-stimulated [3H]forskolin binding was increased after ACVI gene transfer. Shown are mean values from three experiments, documenting that transgene ACVI is present and responsive to Gs–AC interaction. In all graphs bars represent mean values from three experiments and error bars denote 1 SD.
Cardiac myocytes that had received genes on the same day, from the same clone, underwent parallel studies designed to measure [3H]forskolin binding as well as agonist-stimulated cAMP production. The rationale for these studies was to obtain an assessment of the relationship between transgene protein expression and cAMP production. Net isoproterenol and GTP[γS]-stimulated [3H]forskolin binding was increased after ACVI gene transfer (lacZ: 81 ± 24 cpm; ACVI: 447 ± 113 cpm; n = 3; P < 0.0001). These data document that transgene ACVI is present and responsive to Gs–AC interaction (Fig. 5). ACVI gene transfer was associated with increased cAMP production when stimulated by isoproterenol (lacZ, 26 ± 3 pmol/μg; ACVI, 136 ± 7 pmol/μg; P < 0.0001) and by forskolin (lacZ, 9 ± 2 pmol/μg; ACVI, 66 ± 8 pmol/μg; P < 0.0001; n = 3; Fig. 5). These data document that cardiac myocytes expressing transgene ACVI have increased adrenergic responsiveness not only to forskolin stimulation, reflecting increased amounts of AC, but also to isoproterenol, indicating that newly synthesized AC is functionally coupled and recruitable through βAR stimulation.
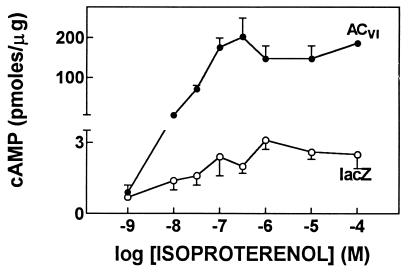
[3H]Forskolin binding and stimulation of cAMP formation were increased to a similar degree (6- to 7-fold), indicating that increased ACVI expression resulted in a proportional increase in transmembrane signaling (Fig. 5). Thus, we have shown that gene transfer of ACVI results in robust transgene expression and proportional increases in protein and enzyme activation. Moreover, in spite of the prominent increase in maximal cAMP synthesis, the apparent EC50 for isoproterenol-stimulated cAMP production was unchanged (lacZ, 16 ± 13 nM; ACVI, 32 ± 19 nM; Fig. 6). In a set of experiments where ACVI protein expression was increased 2.1 ± 0.4-fold, there was a 1.9 ± 0.2-fold increase in isoproterenol-stimulated cAMP production (n = 3). These data provide additional confirmation that increased AC expression is associated with a proportional increase in isoproterenol-stimulated cAMP production.
Figure 6.
Isoproterenol stimulation. Neonatal rat cardiac myocytes underwent gene transfer using recombinant adenovirus expressing lacZ or ACVI. After gene transfer with ACVI (vs. lacZ), there is an obvious increase in cAMP produced through a wide range of isoproterenol concentrations. The apparent EC50 for isoproterenol-stimulated cAMP production was unchanged.
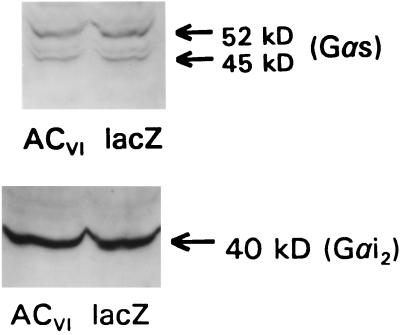
The manipulation of AC expression, with attendant alterations in cAMP production, might be expected to influence the expression of other elements in transmembrane signaling through interaction with cAMP response elements that govern gene transcription (11). Radioligand binding assays identified similar amounts of specifically bound ICYP per mg of membrane protein in plates of cardiac myocytes infected with adenovirus expressing lacZ (Bmax, 26 fmol/mg; Kd, 43 pM) or ACVI (Bmax, 29 fmol/mg; Kd, 78 pM). These data indicate that βAR number was unchanged by gene transfer of ACVI. To determine whether increased AC content affected G-protein content, we performed immunoblotting studies with antibodies directed against Gαs and Gαi2. These assays identified similar amounts of Gαs and Gαi2 (Fig. 7). Thus, the contents of Gαs and Gαi2 were not changed by gene transfer of ACVI. Quantitatively, βAR number and G-protein content were unchanged, and there was a marked increase in G-protein/AC ratio after gene transfer.
Figure 7.
G-protein Western analysis. Polyclonal antibodies recognizing Gαs (Upper) and Gαi2 (Lower) were used in Western blots of lysates of neonatal rat cardiac myocytes after gene transfer using recombinant adenovirus expressing ACVI (AdV.ACVI) or lacZ (AdV.lacZ). Equal amounts of protein were loaded in each lane. Gene transfer had no effect on immunodetectable Gαs or Gαi2 content. These data indicate that substantial expression of ACVI transgene protein does not alter Gαs or Gαi2 content.
Despite unchanged numbers of cell surface receptors responding to agonist stimulation, and unchanged G-protein content, we were able to amplify cAMP production through βAR stimulation manyfold (Figs. 5 and 6). A cell’s responsiveness to βAR-stimulation, it appears, can be markedly influenced by increasing the expression of the effector (AC) without changing the amount of the receptor. Thus, enhanced adrenergic signaling resulted from ACVI gene transfer and functional coupling of endogenous βARs to ACVI and not from alterations in proximal elements in the pathway.
The molar proportions of the β-adrenergic signaling pathway, estimated to be 1:200:3 (βAR:Gs:AC) in studies conducted on isolated rat cardiac myocytes and other cells (1, 2) suggest that βAR number or the amount of AC may limit βAR-mediated transmembrane signaling. However, previous studies in the heart have shown that βAR overexpression does not yield a proportional increase in agonist-promoted stimulation of cAMP production (3, 4). Similarly, overexpression of cardiac Gαs has limited effects on adrenergic responsiveness (5). In contrast, the present study indicates that overexpression of ACVI in cardiac myocytes is associated with a robust amplification of βAR-mediated signaling.
The range of relative increases (ACVI vs. lacZ) in adrenergic signaling in the different experiments we conducted deserves comment. Depending on the experiment, our data show 6- to 50-fold amplification of transmembrane signaling. Variations in cell preparation, viral titer, and cAMP assays may contribute to the range in cAMP production that we found. Previous studies examining augmentation of transmembrane adrenergic signaling by altering βAR or Gs expression (3–5) or inhibiting G-protein coupled receptor kinase function (12) typically achieve no more than a 2-fold increase in cAMP production.
Previous studies expressing AC isoforms in cell lines generally have been limited to phenotypic characterization of the transfected isoform (13–15). Krupinski et al. expressed ACVI in H293 cells and showed a response to isoproterenol that was inhibitable by carbachol (14). No previous study has used AC overexpression in primary cultures of differentiated cells to amplify βAR-mediated transmembrane signaling, and none have examined the relationship between the content of AC, βAR, G proteins, and cAMP formation. The other major AC isoform in mammalian heart is type V, an isoform that exhibits functional redundancy with type VI (both AC isoforms are unaffected by Gβγ, inhibitable by Gαi, and stimulated by Gαs). We speculate that type V would have similar effects if overexpressed in cardiac myocytes, but these data, to our knowledge, have not been reported in the literature.
In conclusion, we have shown that gene transfer of ACVI into neonatal cardiac myocytes is associated with robust expression of transgene ACVI. Cells that overexpress ACVI respond to agonist stimulation with marked increases in cAMP production proportional to the degree of increased protein expressed. These data indicate that the amount of AC sets a limit on transmembrane β-adrenergic signaling, and that using ACVI for gene transfer is a rational approach to obtain proportional increases in β-adrenergic signal transduction. Our findings are significant because they establish the importance of ACVI content in modulating transmembrane β-adrenergic signaling, and because they suggest a therapeutic target for increasing transmembrane signaling in cardiac conditions associated with desensitization or loss of cAMP formation. We speculate that similar responses are possible in other tissues and organs in which AC plays a physiological role.
Acknowledgments
We thank Jinyao Zhou and Zhen Li for expert technical support and Dr. Tamsin Lisa Kelly for reviewing the manuscript. This research was supported by a Merit Award from the Department of Veterans Affairs (H.K.H.), Specialized Center of Research on Congestive Heart Failure 1 P50 HL-53773-01 (P.A.I. and H.K.H.), and National Institutes of Health Research Career Development Award HL02812-01 (H.K.H.). Additional support was provided by Individual National Research Service Award HL09097-01 (P.P.) and Institutional National Research Service Award HL07444 (M.G.). Portions of this research were supported by an unrestricted grant from Collateral Therapeutics, Inc., La Jolla, CA; H.K.H. has a proprietary interest in Collateral Therapeutics.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: AC, adenylylcyclase; ACVI, adenylylcyclase type VI; βAR, β-adrenergic receptor; RT, reverse transcriptase; pfu, plaque-forming unit(s); GTP[γS], guanosine 5′-[γ-thio]triphosphate; ICYP, iodocyanopindolol.
References
- 1.Alousi A, Jasper J R, Insel P A, Motulsky H J. FASEB J. 1992;5:2300–2303. doi: 10.1096/fasebj.5.9.1650314. [DOI] [PubMed] [Google Scholar]
- 2.Post S R, Hilal-Dandan R, Urasawa K, Brunton L L, Insel P A. Biochem J. 1995;311:75–80. doi: 10.1042/bj3110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drazner M H, Peppel K C, Dyer S, Grant A O, Koch W J, Lefkowitz R J. J Clin Invest. 1997;99:288–296. doi: 10.1172/JCI119157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milano C A, Allen L F, Rockman H A, Dolber P C, McMinn T R, Chien K R, Johnson T D, Bond R A, Lefkowitz R J. Science. 1994;264:582–586. doi: 10.1126/science.8160017. [DOI] [PubMed] [Google Scholar]
- 5.Gaudin C, Ishikawa Y, Wight D C, Mahdavi V, Nadal-Ginard B, Wagner T E, Vatner D E, Homcy C J. J Clin Invest. 1995;95:1676–1683. doi: 10.1172/JCI117843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ping P, Anzai T, Gao M, Hammond H K. Am J Physiol. 1997;273:H707–H717. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
- 7.Ishikawa Y, Sorota S, Kiuchi K, Shannon R P, Komamura K, Katsushika S, Vatner D E, Vatner S F, Homcy C J. J Clin Invest. 1994;93:2224–2229. doi: 10.1172/JCI117219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knowlton K U, Barachini E, Ross R S, Harris A N, Henderson S A, Evans S M, Glembotski C C, Chien K R. J Biol Chem. 1991;266:7759–7768. [PubMed] [Google Scholar]
- 9.Ping P, Gelzer-Bell R, Roth D A, Kiel D, Insel P A, Hammond H K. J Clin Invest. 1995;95:1271–1280. doi: 10.1172/JCI117777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McGrory W J, Bautista D S, Graham F L. Virology. 1988;163:614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- 11.Vallejo M. J Neuroendocrinol. 1994;6:587–596. doi: 10.1111/j.1365-2826.1994.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 12.Koch W J, Rockman H A, Samama P, Hamilton R A, Bond R A, Milano C A, Lefkowitz R J. Science. 1995;268:1350–1353. doi: 10.1126/science.7761854. [DOI] [PubMed] [Google Scholar]
- 13.Yoshimura M, Cooper D M. Proc Natl Acad Sci USA. 1992;89:6716–6720. doi: 10.1073/pnas.89.15.6716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krupinski J, Lehman T C, Frankenfield C D, Zwaagstra J C, Watson P A. J Biol Chem. 1992;267:24858–24862. [PubMed] [Google Scholar]
- 15.MacEwan D J, Kim G D, Milligan G. Biochem J. 1996;318:1033–1039. doi: 10.1042/bj3181033. [DOI] [PMC free article] [PubMed] [Google Scholar]