Abstract
Imaging of salivary gland tumours is a major challenge for radiologists due to the great variety of differential diagnoses. This article gives a short overview on the anatomy of the salivary glands, the epidemiology of salivary gland tumours as well as the clinical presentation and the different imaging modalities including new magnetic resonance techniques such as diffusion-weighted magnetic resonance imaging, dynamic contrast-enhanced magnetic resonance imaging and magnetic resonance spectroscopy applied in the work-up of salivary gland masses. The imaging features of different tumour types and their differential diagnoses are also discussed. Finally, staging classification and treatment options are presented.
Keywords: Imaging, salivary glands, magnetic resonance
Introduction
Salivary gland tumours account for only 3% of all tumours in the body and it is estimated that about 1% of all head and neck malignant neoplasms arise in the salivary glands.[1,2] However, the great variety of histological types makes them a major challenge for radiologists and clinicians. The large range of differential diagnoses influences not only prognosis but also treatment. Local excision or superficial parotidectomy is the surgical procedure of choice in patients with benign lesions; in the case of malignant tumours the patient has to undergo total parotidectomy, a more difficult procedure with the surgical risk of facial nerve palsy. Fine needle aspiration cytology (FNAC) is not always conclusive; there is a selection bias and if the tumour is located in the deep lobe, FNAC cannot be performed in all cases. Therefore, pre-operative imaging has a major role in surgical planning.
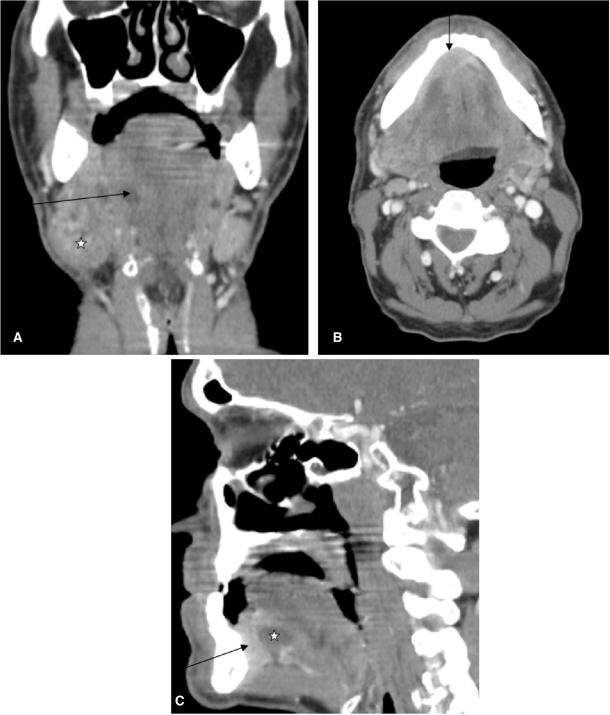
Only a few clinical symptoms, such as facial nerve palsy, in patients with parotid gland masses allow the diagnosis of malignancy. In most cases of palpable tumours the differentiation between benign and malignant is not possible by clinical examination only. In patients with swelling of the submandibular gland a tumour of the floor of the mouth has to be ruled out. Cancer arising in the anterior floor of the mouth can obstruct Wharton's duct and cause retro-obstructive submandibular sialadenitis mimicking inflammatory disease of the gland or metastatic adenopathy (Fig. 1).
Figure 1.
Coronal CT reconstruction (A) of a 59-year-old male patient presenting with a swelling at the angle of the mandible. Note the enlarged submandibular gland (asterisk) at the right side with dilated Wharton's duct (arrow). Axial CT scan (B) of the same patient shows a contrast enhancing mass (arrow) at the anterior floor of the mouth (squamous cell carcinoma) as aetiology of the dilated Wharton's duct and the retro-obstructive sialadenitis of the right submandibular gland. Sagittal reconstruction (C) again exemplifies the tumour at the anterior floor of the mouth (arrow) and the dilated Wharton's duct (asterisk).
The pre-operative information on whether a salivary gland tumour is benign or malignant may be helpful in assessing and establishing a policy towards lymph node dissection, in preventing treatment delay in case of malignancy, avoiding surgery for inflammatory disease and informing the patient more appropriately as to the treatment options and the possible risk of facial nerve injury.
Normal anatomy/epidemiology
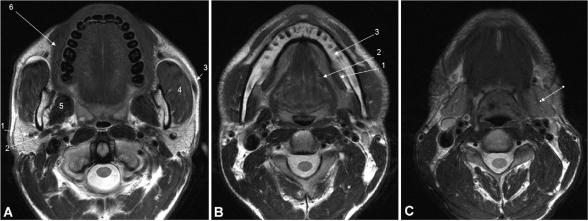
The major salivary glands consist of the paired bilateral parotid, submandibular and sublingual glands (Fig. 2); the minor salivary glands are dispersed beneath the mucosa of the oral cavity, palate, paranasal sinuses, pharynx, larynx, trachea and bronchi with a particular concentration in the buccal, labial, palatal, and lingual regions. In contrast to all other salivary glands the parotid gland is encapsulated after the development of the lymphatic system, therefore intraparotid lymph nodes and lymphatic channels are present.
Figure 2.
Axial T2-weighted MR image at (A) the midlevel of the parotid glands (arrows), with indications of the retromandibular vein (1), external carotid artery (2), Stensen's duct (3), masseter muscle (4), medial pterygoid muscle (5), and buccinator muscle (6). Axial T2-weighted MR image at (B) the level of the sublingual glands (arrows), with indications of mylohyoid muscle (1), hyoglossal muscle (2), and mandible (3). Axial T2-weighted MR image at (C) the level of the submandibular glands (arrows).
The parotid gland is the largest salivary gland and is located in the parotid space containing the facial nerve, the retromandibular vein, the external carotid artery and intraparotid lymph nodes (Fig. 2a). In about 20% of patients an accessory parotid gland can be observed superficial to the masseter muscle. The parotid gland is subdivided into deep and superficial lobes by the facial nerve and its branches for the purpose of surgical approach, however there is no anatomical division between lobes. The facial nerve cannot usually be visualised by imaging, however a virtual line drawn from the lateral border of the posterior belly of the digastric muscle and the retromandibular vein to the lateral edge of the mandible can be used as an anatomical landmark.[3] The use of the retromandibular vein as a marker for the facial nerve has been shown to be another sensitive method for identifying the location of parotid gland lesions.[4]
As a rule most parotid gland tumours are benign (80%) and only 20–25% are malignant; in smaller salivary glands the probability for malignancy increases to 40–50% in the submandibular gland and 50–81% in the sublingual and minor salivary glands.[2,5]
Salivary gland tumours are rare in children, however the frequency of malignancy is higher in children compared to adults. About 35% of all salivary gland tumours in children are malignant;[1] the most common malignant neoplasms are mucoepidermoid carcinomas.[3]
Both benign and malignant salivary gland masses show considerable overlap with regard to imaging appearance such as tumour margins, homogeneity and signal intensity. Malignancy is suggested if deep infiltration into the parapharyngeal space, muscles or bone and perineural spread is present; these findings are not observed in benign lesions.
Clinical presentation
The clinical presentation of parotid gland cancers is similar to that of benign parotid gland tumours. Most patients present with a painless palpable mass in the parotid or submandibular gland. Certain clinical symptoms however, such as pain, facial nerve palsy and enlarged lymph nodes may suggest malignancy of the parotid gland.[6–8] In the case of a submandibular mass, a tumour of the floor of the mouth or enlarged lymph nodes have to be ruled out as potential aetiology (Fig. 1).
Imaging modalities
In children and in pregnant women sonography is the first step, especially for lesions in the superficial lobe of the parotid gland. It can be used to distinguish focal from diffuse disease, assess adjacent vascular structures and vascularity, distinguish solid from cystic, guide fine-needle aspiration biopsy and perform nodal staging.[9,10] For further evaluation of the exact extent and nature of the tumours cross sectional imaging such as computed tompgraphy (CT) or magnetic resonance (MR) must be performed.
CT is the method of choice in patients suspicious for inflammatory disease (abscess, calculi, major salivary duct dilatation, and acute inflammation) or in patients with contraindication for MR imaging. For CT imaging both pre- and post-contrast studies must be performed in order to detect calcifications (pre-contrast) and enhancement pattern (post-contrast). Coronal and sagittal reconstructions can be helpful in the evaluation of perineural spread.
However, MR imaging (MRI) is the method of choice for patients with palpable masses and a strong suspicion that the lesion is neoplastic.[10] MRI gives information on the exact localisation and extent of the lesion, addresses neighbouring structures, and allows perineural spread,[11,12] bone invasion and meningeal infiltration to be assessed. Axial T1- and T2-weighted sequences allow the exact extent of the tumour, tumour margins and growth patterns to be determined, whereas T1-weighted sequences in the axial and coronal plane after gadolinium administration and with fat suppression allow determination of perineural spread along the cranial nerve VII (stylomastoid foramen), the cranial nerve V-3 (foramen ovale) or the cranial nerve V-2 (foramen rotundum) as well as tumour margins (sharp, fuzzy). Bone invasion can also be detected as a hypointense signal on T1-weighted sequences and contrast enhancement on post-contrast fat-suppressed images.
Recently, new MR technologies such as dynamic contrast-enhanced MRI (DCE-MRI), diffusion-weighted MRI (DW-MRI) and proton MR spectroscopy (MRS) have shown promising results in the differentiation between benign and malignant salivary gland tumours.[13–16] Malignant salivary gland tumours can be differentiated from pleomorphic adenomas but not from Whartin tumours using DCE-MRI at a time of peak enhancement of 120 s. A washout ratio of 30% enabled the additional differentiation between malignant and Whartin tumours.[13] Using time-signal intensity curves on the basis of time to peak enhancement of 120 s and a washout ratio of 30% had high sensitivity (91%) and specificity (91%) in the differentiation between benign and malignant tumours.
The apparent diffusion coefficient (ADC) as the quantitative parameter of DW-MRI was found to be significantly smaller in lymphomas than in carcinomas. The mean ADC of carcinomas has been shown to be significantly smaller than that of benign solid tumours; however the ADC value of Whartin tumours was even smaller than that of malignant tumours.[14,15] This finding might be attributed to the intense lymphoid accumulation in the stroma and proliferation of the epithelial component leading to a decrease in the extracellular extravascular space and therefore a decrease in ADC.[14] In another study of 33 pleomorphic adenomas and 13 malignant salivary gland tumours, an overlap in ADC values was observed in those pleomorphic adenomas that showed hypercellularity with less-myxoid stroma.[17] However, when comparing the ADC values of different DW-MRI studies, attention has to be paid to the underlying b-values. The lower the applied b-values the higher the influence of perfusion, whereas when applying high b-values only the ADC reflects mainly diffusion.[18] Therefore, the choice of various b-values might be helpful in the differentiation of benign and malignant tumours.
Proton MR spectroscopy performed on a small number of salivary gland tumours (9 malignant and 47 benign) suggested that by using choline/creatine (Cho/Cr) ratios greater than 2.4 at an echo time of 136 ms, a distinction between benign and malignant lesions is possible, while a ratio greater than 4.5 suggested the presence of a Whartin tumour.[16]
Differential diagnoses and imaging features of various tumour types
Benign masses
It is known that the greater the salivary glands the higher the probability that a lesion is benign, whereas in minor salivary glands the likelihood of malignancy increases.
First branchial cleft cysts typically manifest in middle-aged women with a history of recurrent parotid gland infection unresponsive to antibiotics. Occasionally a fistula can be observed at the angle of the mandible.
First branchial cleft cysts account for 8% of all branchial complex anomalies.[19] These cysts occur in or directly adjacent to the parotid gland. At ultrasound (US) these cysts may look solid due to internal haemorrhage or infection. Colour Doppler does not show any flow suggesting the cystic nature of the lesion. At CT or MRI, after administration of contrast medium, the cyst wall may be thickened and enhance due to infection. Increased signal intensity on T1-weighted sequences suggests prior bleeding or infection.
Haemangiomas manifest as unilateral parotid swelling shortly after birth. A bluish discoloration of the skin can often be seen. These lesions are the most common benign salivary gland masses in children with a female predilection.[3] They are classified as capillary or cavernous at pathology. About 90% represent congenital capillary haemangiomas which spontaneously regress until adolescence. Cavernous haemangiomas are rare, manifest in older children and do not undergo resolution. At CT or MRI, capillary haemangiomas are seen as well-defined masses with strong enhancement. Flow voids due to prominent vasculature are often present in or around the mass.[1,19]
Lymphangiomas are congenital malformations of the lymphatic system with an incidence of 90% at the age of 2 years.[1,3] Infection or haemorrhage may occur; however in contrast to haemangiomas spontaneous regression is rare. At imaging lymphangiomas consist of cystic areas and thin septations, but also solid enhancing portions. Haemorrhage can lead to fluid levels with variable signal intensities depending on the duration of the bleeding.
Pleomorphic adenoma (Table 1) usually appears in middle-aged people as a palpable slowly growing mass. It is the most common benign salivary gland tumour in adults (70–80% of all benign tumours of the major salivary glands) and the third most common in the paediatric population.[1,3] Most pleomorphic adenomas occur in the parotid gland (84%), 8% in the submanibular gland, 6.5% in the minor salivary glands and 0.5% in the sublingual glands.[1] The alternative term, mixed tumours, is attributed to the histological heterogeneity suggesting a variety of imaging findings. Pleomorphic adenomas may contain small calcifications. Imaging findings usually depend on tumour size. Small tumours are more homogeneous and well defined with strong enhancement after contrast medium administration in CT and MR, whereas larger tumours tend to have pedunculated outgrowth from the main lesion (lobulated contour) and are more heterogeneous including necrotic and haemorrhagic areas. The recurrence rate is reported to vary between 1% and 50% depending on the initial surgical procedure.[1] Recurrences are often multiple and clustered. There are three malignancies associated with pleomorphic adenoma: (1) the carcinoma ex-pleomorphic adenoma; (2) the rarer variant, the true malignant mixed tumour (carcinosarcoma); and (3) the metastasising mixed tumour.[1] The carcinoma ex-pleomorphic adenoma is a malignant change of a benign mixed tumour or a malignant tumour in a patient who previously underwent surgery for a pleomorphic adenoma. Carcinoma ex-pleomorphic adenomas (Fig. 3) have a high metastatic rate varying from 25% to 76% into regional lymph nodes, lungs, bones and brain.[1] The carcinosarcoma is rare and has a bad prognosis. The metastasising ‘benign’ mixed tumour is the rarest variant. Metastases may be multiple and occur in the lung, bone and soft tissue, often over decades.[1] Small benign mixed tumours are usually hypointense in T1- and hyperintense in T2-weighted sequences. Inhomogeneity is seen in larger tumours, whereas low signal intensity on T2-weighted images may be observed in carcinoma ex-pleomorphic adenomas.
Table 1.
Typical MRI features of the most frequent parotid gland tumours
| Parotid tumours | Defined border | Necrotic/cystic | T1-signal intensity | T2-signal intensity | Enhancement |
|---|---|---|---|---|---|
| Pleomorphic adenoma | + | −/+ | ↓ | ↑↑↑ | ↑↑ |
| Wharton's tumour | + | ++ | ↓ | ↓ | (↑) |
| Mucoepidemoid carcinoma | +/− | − | ↓(↑) | ↓(↑) | ↑ |
| Adenoid-cystic carcinoma | − | + | ↓ | ↑ | ↑↑ |
| Acinic cell carcinoma | −/+ | + | ↓ | ↑(↓) | (↑) |
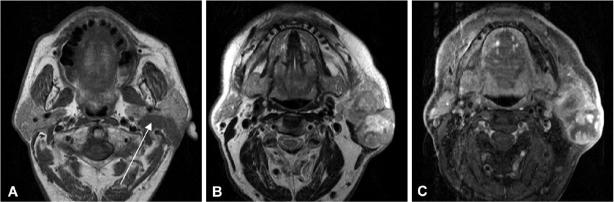
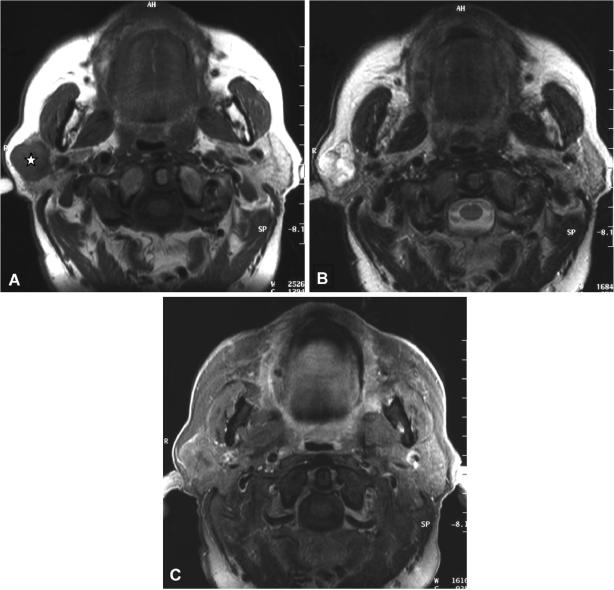
Figure 3.
Axial T1-weighted (A), axial T2-weighted (B) and axial T1-weighted (C) contrast enhanced fat suppressed MR image of a 63-year-old male patient suffering from a carcinoma ex-pleomorphic adenoma of the left parotid gland. Please note extension into the deep parotid lobe (arrow) as well as infiltration of the subcutaneous tissue and skin. The tumour is heterogeneous on T2-weighted images (B) and shows large necrotic areas and irregular contours after contrast medium administration (C).
Whartin tumours (adenolymphoma or papillary cystadenoma lymphomatosum) (Table 1) often present as slow growing parotid gland masses in older men. It is the second most common benign parotid gland neoplasm in adults and children.[1,3] It is the most common neoplasm that manifests as multiple lesions and is bilateral in up to 10% of cases. Whartin tumours present as well-circumscribed partly cystic, partly solid lesions in CT or MRI, often located in the tail of the parotid gland. Enhancement after contrast medium administration is often relatively poor. In the differential diagnoses of multiple lesions, metastases, lymphoma or inflammatory disease must be considered.
Other benign tumours, such as oncocytomas, myoepitheliomas, monomorphic adenomas and basal cell adenomas, are relatively rare benign tumours of the parotid gland and lack typical imaging patterns.[20,21] Lipomas, however, can easily be diagnosed by CT or MRI thanks to their fat content with typical low attenuation values on CT and signal intensities isointense to fat on all pulse sequences on MR imaging.
Malignant masses
The clinical presentation of a patient with a rock-hard mass, often painful in the parotid or submandibular gland is suggestive of a malignant tumour. When facial nerve symptoms are also present, the lesion is highly suspicious for malignancy.
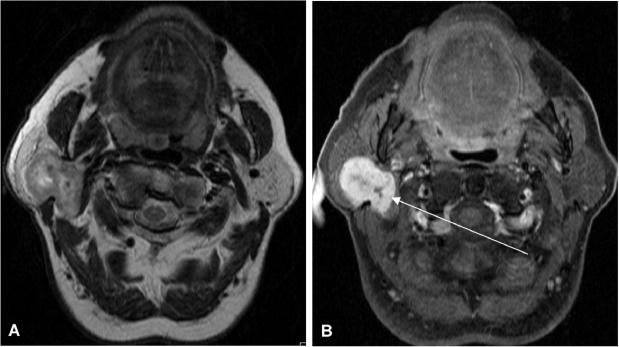
Mucoepidermoid carcinomas (Fig. 4, Table 1) represent about 30% of all salivary gland malignancies and are the most common malignant tumours in children and adults.[1,3,19] These tumours arise in the parotid gland (about 50%) and in the minor salivary glands (nearly 45%) mainly in the palate and buccal mucosa. The imaging features depend on the histological type. Low-grade lesions are well circumscribed, whereas high-grade lesions tend to have poor margins and infiltrate surrounding tissues. Low to intermediate signal intensity can be observed on T1- and T2-weighted images. These tumours metastasise primarily in the lymph nodes, bone and lung.[1] Perineural tumour spread along the course of the facial nerve into the mastoid segment of the temporal bone has to be ruled out.[19]
Figure 4.
Axial T1-weighted MR image (A) of a 26-year-old woman with a focal lesion in her left parotid gland. Note a homogonous hypointense solid lesion with slightly irregular margins (asterisk). Axial T2-weighted MR image (B) also depicts a slightly hypointense homogeneous mass. Contrast medium enhancement with fat suppression (C) at the same level shows strong and homogenous enhancement with slightly irregular margins suspicious for malignancy. This tumour turned out to be mucoepidermoid carcinoma on histology.
Adenoid cystic carcinoma (Fig. 5, Table 1) accounts for 2–6% of parotid gland tumours and is the most common malignancy in the submandibular gland (12% of all tumours) after mucoepidermoid carcinoma. These tumours occur mainly in the parotid gland, the submandibular gland, and the palate, with a worse prognosis when the tumour originates in the minor salivary glands. It usually presents as an infiltrating mass with a high propensity for perineural spread.[22] Perineural disease can also present with ‘skip’ lesions distally in a nerve that seems to be normal.
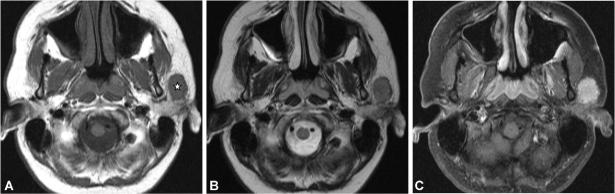
Figure 5.
Axial T2-weighted MR image (A) of a 61-year-old woman with an adenoid cystic carcinoma in the deep and superficial lobe of the right parotid gland. The tumour presents as a large heterogeneous mass. The T1-weighted contrast medium enhanced fat suppressed image (B) shows strong enhancement with hypointense areas in the centre and slightly irregular margins in the posterior part of the lesion (arrow).
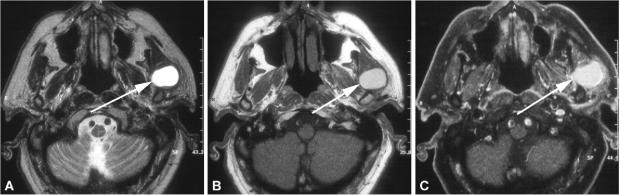
Most acinic cell carcinomas (Fig. 6, Table 1) present in the 4th to 6th decades of life with a relatively equal sex distribution, with a slight female preponderance.[23] These tumours are usually painless and slowly growing masses. Acinic cell carcinomas are the second most common parotid gland malignancy in children.[1] Acinic cell carcinoma represents only 2–4% of all major salivary gland malignancies.[1] These tumours occur mainly in the parotid gland (83%) with the rest arising mostly intraorally or in the submandibular gland[24,25] and they represent about 15–17% of all malignant parotid gland tumours.[1] It is the second most common multiple or bilateral tumour after Whartin's tumour. The typical features of these tumours are circumscribed solid or partially cystic lesions which may have a thin or incomplete capsule. The imaging features are non-specific and differential diagnosis of pleomorphic adenoma or Whartin's tumour may be impossible by CT or conventional MRI.[26] In a series of 12 patients with acinic cell carcinomas, solid lesions with low-attenuating portions and no calcifications could be observed.[26] Metastases to regional lymph nodes are reported in 10–19% of patients, and distant metastases mainly to lung and bone occur in 15%.[27]
Figure 6.
Axial T1- (A) and T2-weighted (B) MR images of a 52-year-old man with an acinic cell carcinoma of the superficial lobe of the right parotid gland (asterisk). The polylobular lesion is circumscribed and shows a hypointense signal on the T1-weighted image and a hyperintense signal on the T2-weighted image. Contrast medium enhancement (C) is only fair and the lesion shows an incomplete capsule in the posterior part.
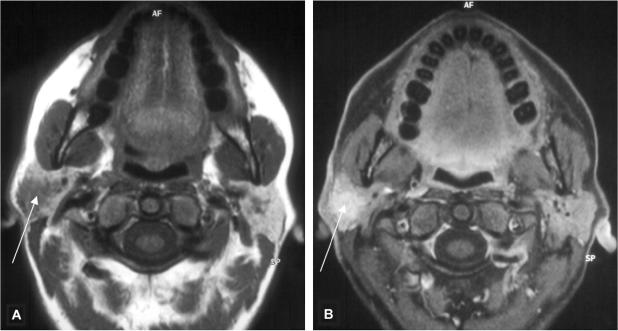
Salivary duct carcinoma (Fig. 7) is an uncommon but highly aggressive malignant tumour mainly of the parotid gland with a male predilection. MR features have been analysed in a study performed on nine salivary gland tumours.[28] Ill-defined margins and low to moderately high signal intensity on T2-weighted images could be observed in all patients on MR imaging. Furthermore the low ADC values (1.22 × 10−3 mm2/s) as well as early enhancement with a low wash-out ratio on DCE-MRI were other factors suggestive of malignancy in this investigation.[28] Differential diagnosis to a benign salivary gland cyst can be difficult on non-contrast images only (Fig. 8). Perineural spread as well as lymph node involvement (70%) are frequent findings observed in this particular tumour type.[1]
Figure 7.
Axial T2-weighted MR image (A) of a 73-year-old male patient with a histologically proven salivary duct carcinoma (arrows) of the deep lobe of the parotid gland displacing the superficial lobe of the parotid gland laterally. The lesion is also well delineated and hyperintense on the T1-weighted images (B) suggesting previous bleeding. Only theT1-weighted contrast-enhanced fat saturated MR image (C) reveals slightly irregular margins of the tumour, which could not be appreciated on the native images suggestive of malignancy.
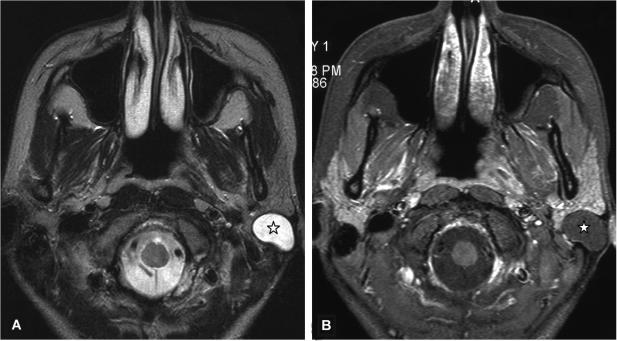
Figure 8.
Axial T2-weighted MR image (A) of a 25-year-old woman with an epidermoid cyst in the superficial lobe (asterisk) presented as a well-delineated and homogeneous hyperintense lesion. The T1-weighted contrast-enhanced fat suppressed MR image (B) at the same level shows a homogeneous hypointense lesion without contrast medium enhancement and well-delineated margins.
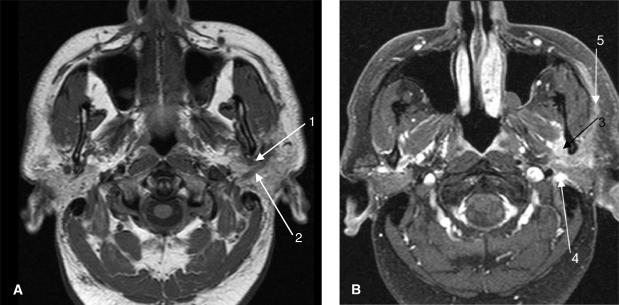
Polymorphous low-grade adenocarcinomas are characterised by an infiltrative growth pattern and perineural spread is common (Fig. 9).[1] This tumour entity usually presents as an indolent slow-growing lesion. The majority of these tumours arise in the palate, but may also occur in the cheek, lip, and parotid gland.
Figure 9.
Axial non-enhanced T1-weighted MR image (A) from a 48-year-old man with facial nerve paralysis with undifferentiated carcinoma of the left parotid gland. The image demonstrates a soft tissue mass adjacent to the left mandible posteriorly that extends along the course of the auriculotemporal nerve[1] as well as the facial nerve.[2] T1-weighted contrast enhanced MR image (B) after fat suppression shows an enhancing inhomogeneous soft tissue mass with perineural spread along the auriculotemporal nerve (V3)[3] as well as the facial nerve posteriorly in the stylomastoid foramen[4] and anteriorly along the peripheral lobe of the parotid gland.[5]
Adenocarcinomas, not otherwise specified, are usually painful and rapidly growing tumours; however, occasionally they may also present as painless slow-growing masses. Adenocarcinomas can be classified according to their histological findings: grade 1 tumours are circumscribed and minimally invasive, grade 2 tumours are in between grade 1 and grade 3, whereas grade 3 tumours are more solid with a greater mitotic rate. Survival rates are reported to be poorer for high-grade tumours.[1]
Primary squamous cell carcinomas occur typically in men in their sixth and seventh decades and present as rapidly growing tumours often fixed to the soft tissues and skin.[1] Primary squamous cell carcinoma can arise in the salivary glands due to squamous metaplasia occurring in patients with chronic inflammation, however metastatic squamous cell carcinoma is more common by far. The typical imaging features are necrotic areas in solid tumours best recognised in fat suppressed T1-weighted MR images after administration of contrast medium or in enhanced CT images.
Metastases to salivary glands are mainly observed in the parotid gland due to the presence of intraglandular lymph nodes, which drain the face, external ear, and scalp.[1] Skin malignancies (melanoma, squamous cell carcinomas) are the most common primary tumours metastasising to the salivary glands, therefore careful clinical examination has to be performed. However, other malignancies, such as renal cell carcinomas, lung, breast and gastrointestinal carcinomas can also metastasise to the parotid gland or periparotid lymph nodes.
Primary lymphoma of the salivary glands is rare and can only be diagnosed if there is no evidence of intra-or extraglandular nodal involvement. These lymphomas are considered as extranodal marginal zone B-cell lymphomas. The incidence of salivary gland involvement varies between 1 and 8% of the patients with lymphomas; all forms of Hodgkin's and non-Hodgkin's lymphomas have been reported.[1] In the case of secondary lymphomatous involvement of the salivary glands, the parotid gland is involved in about 80%. In patients with Sjögren's syndrome the prevalence of non-Hodgkin's lymphoma is about 44 times greater compared to control subjects,[1] therefore lymphoma has to be ruled out by imaging in this particular patient population.
The benign lymphoepithelial lesion (BLEL) or Godwin's tumour represents a manifestation of autoimmune disease in the salivary glands.[1] This benign entity may be difficult to distinguish from malignant tumours due to its focal character, contrast medium enhancement and its irregular margins (Fig. 10).
Figure 10.
Axial T1-weighted MR image (A) of a 46-year-old women presenting with a painful lesion in the right parotid gland. A focal lesion with irregular margins can be seen (arrow). The lesion shows strong contrast medium enhancement on the fat suppressed images (B). On histology this lesion turned out to be a benign lymphoepithelial lesion (BLEL).
Staging classification
T-staging is based on tumour size and whether there is extraparenchymal extension or involvement of adjacent structures (Table 2). Lymphatic spread (Table 3) occurs in a predictable way usually to periparotid lymph nodes and level 2 and 3 lymph nodes.
Table 2.
TNM classification (2002): primary tumour (T)
| T1 | Tumour 2 cm or less in greatest dimension without extraparenchymal extension |
| T2 | Tumour more than 2 cm but not more than 4 cm in greatest dimension without extraparenchymal extension |
| T3 | Tumour more than 4 cm and/or tumour with extraparenchymal extension |
| T4a | Tumour invades skin, mandible, ear canal, or facial nerve |
| T4b | Tumour invades base of skull, pterygoid plates, or encases carotid artery |
Table 3.
TNM classification (2002): nodal (N) involvement
| N1 | Single ipsilateral node smaller than 3 cm |
| N2A | Single ipsilateral node larger than 3 cm but smaller than 6 cm |
| N2B | Multiple ipsilateral nodes smaller than 6 cm |
| N2C | Bilateral/contralateral nodes smaller than 6 cm |
| N3 | Any node larger than 6 cm |
Treatment options
Superficial parotidectomy is the most common surgical technique in patients with benign parotid gland lesions located in the superficial lobe of the parotid gland. In a few centres, T1/T2 low grade malignancies are also treated by superficial parotidectomy;[29,30] in most centres all parotid cancers are treated by conservative or radical parotidectomy.[31,32]
In contrast to an established elective lymph node dissection for N0 in patients with squamous cell carcinoma of head and neck tumours, the indication for parotid carcinomas is still under debate. Most authors advocate performing an elective neck dissection in case of T3/T4 tumours, high grade carcinoma and perilymphatic invasion.[33–35]
In T3/T4 and high grade primary parotid gland tumours with lymph node metastases, adjuvant radiation therapy of the primary tumour as well as of the neck must be performed. As the local recurrence rate of T1/T2 primary parotid carcinomas is reported to be between 7 and 15%,[6,36–38] post-operative radiation therapy should probably be performed not only in primary malignant parotid tumours with adverse prognostic factors but also in T2N0 carcinomas and in several T1 tumours.
Conclusion
The exact pre-operative evaluation of salivary gland tumours remains a major challenge. MR imaging is the method of choice in patients with palpable salivary gland masses to assess the exact extent of tumours, the invasion of neighbouring structures, perineural spread and lymph node staging. The differentiation of benign and malignant masses is often impossible, however new MR techniques such as DCE-MRI, DW-MRI and MR spectroscopy have already shown promising results and further research with larger scale studies on these particular MR methods have to be performed.
Acknowledgments
The author would like to warmly thank Peter Zbaeren, MD, and Andreas Christe, MD, for their helpful comments and Frederik De Keyzer for his editorial assistance in the preparation of the manuscript
References
- 1.Som PM, Curtin HD. Head and neck imaging. 3rd. Vol. 2. St. Louis, MO: Mosby; 1996. pp. 877–912. [Google Scholar]
- 2.Batsakis JG. Tumors of the head and neck: clinical and pathological considerations. 2nd. Baltimore, MD: Williams & Willkins; 1979. pp. 1–120. [Google Scholar]
- 3.Lowe LH, Stokes LS, Johnson JE, et al. Swelling at the angle of the mandible: imaging of the pediatric parotid gland and periparotid region. Radiographics. 2001;21:1211–27. doi: 10.1148/radiographics.21.5.g01se171211. [DOI] [PubMed] [Google Scholar]
- 4.Divi V, Fatt MA, Teknos TN, Mukherji SK. Use of cross-sectional imaging in predicting surgical location of parotid neoplasms. J Comput Assist Tomogr. 2005;29:315–19. doi: 10.1097/01.rct.0000161758.25130.34. [DOI] [PubMed] [Google Scholar]
- 5.Freling NJ, Molenaar WM, Vermey A, et al. Malignant parotid tumors: clinical use of MR imaging and histologic correlation. Radiology. 1992;185:691–6. doi: 10.1148/radiology.185.3.1438746. [DOI] [PubMed] [Google Scholar]
- 6.Witten J, Hybert F, Hansen HS. Treatment of malignant tumors in the parotid glands. Cancer. 1990;65:2515–20. doi: 10.1002/1097-0142(19900601)65:11<2515::aid-cncr2820651121>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 7.Pederson D, Overgaard J, Sogaard H, Elbond O, Overgaard M. Malignant parotid tumors in 110 consecutive patients: treatment results and prognosis. Laryngoscope. 1992;102:1064–9. doi: 10.1288/00005537-199209000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Zbaren P, Schupbach J, Nuyens M, Stauffer E, Greiner R, Hausler R. Carcinoma of the parotid gland. Am J Surg. 2003;186:57–62. doi: 10.1016/s0002-9610(03)00105-3. [DOI] [PubMed] [Google Scholar]
- 9.Gritzmann N, Rettenbacher T, Hollerweger A, Macheiner P, Hubner E. Sonography of the salivary glands. Eur Radiol. 2003;13:964–75. doi: 10.1007/s00330-002-1586-9. [DOI] [PubMed] [Google Scholar]
- 10.Yousem DM, Kraut MA, Chalian AA. Major salivary gland imaging. Radiology. 2000;216:19–29. doi: 10.1148/radiology.216.1.r00jl4519. [DOI] [PubMed] [Google Scholar]
- 11.Parker GD, Harnsberger HR. Clinical-radiologic issues in perineural tumor spread of malignant diseases of the extracranial head and neck. Radiographics. 1991;11:383–99. doi: 10.1148/radiographics.11.3.1852933. [DOI] [PubMed] [Google Scholar]
- 12.Barakos JA, Dillon WP, Chem WM. Orbit, skull base, and pharynx: contrast-enhanced fat suppression MR imaging. Radiology. 1991;179:191–8. doi: 10.1148/radiology.179.1.2006277. [DOI] [PubMed] [Google Scholar]
- 13.Yabuuchi H, Fukuya T, Tajima T, Hachitanda Y, Tomita K, Koga M. Salivary gland tumors: diagnostic value of gadolinium-enhanced dynamic MR imaging with histopathologic correlation. Radiology. 2003;226:345–54. doi: 10.1148/radiol.2262011486. [DOI] [PubMed] [Google Scholar]
- 14.Wang J, Takashima S, Takayama F, et al. Head and neck lesions: characterization with diffusion-weighted echo-planar MR imaging. Radiology. 2001;220:621–30. doi: 10.1148/radiol.2202010063. [DOI] [PubMed] [Google Scholar]
- 15.Habermann CR, Gossrau P, Graessner J, et al. Diffusion-weighted echo-planar MRI: a valuable tool for differentiating primary parotid gland tumors? RoFo. 2005;177:940–5. doi: 10.1055/s-2005-858297. [DOI] [PubMed] [Google Scholar]
- 16.King AD, Yeung DK, Ahuja AT, et al. Salivary gland tumors at in vivo proton MR spectroscopy. Radiology. 2005;237:563–9. doi: 10.1148/radiol.2372041309. [DOI] [PubMed] [Google Scholar]
- 17.Motoori K, Yamamoto S, Ueda T, et al. Inter- and intratumoral variability in magnetic resonance imaging of pleomorphic adenoma: an attempt to interpret the variable magnetic resonance findings. J Comput Assist Tomogr. 2004;28:233–46. doi: 10.1097/00004728-200403000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Thoeny HC, De Keyzer F, Boesch C, Hermans R. Diffusion-weighted imaging of the parotid gland: influence of the choice of b-values on the apparent diffusion coefficient value. J Magn Reson Imaging. 2004;20:786–90. doi: 10.1002/jmri.20196. [DOI] [PubMed] [Google Scholar]
- 19.Harnsberger HR. Handbook of head and neck imaging. 2nd. St. Louis, MO: Mosby; 1990. pp. 60–73. [Google Scholar]
- 20.Yerli H, Teksam M, Aydin E, Coskun M, Ozdemir H, Agildere AM. Basal cell adenoma of the parotid gland: dynamic CT and MRI findings. Br J Radiol. 2005;78:642–5. doi: 10.1259/bjr/32453517. [DOI] [PubMed] [Google Scholar]
- 21.Lee MW, Nam SY, Choi HJ, Choi JH, Moon KC, Koh JK. Myoepithelioma of parotid gland presenting as infra-auricular subcutaneous mass. J Cutan Pathol. 2005;32:240–4. doi: 10.1111/j.0303-6987.2005.00293.x. [DOI] [PubMed] [Google Scholar]
- 22.Sigal R, Monnet O, De Baere T, et al. Adenoid cystic carcinoma of the head and neck: evaluation with MR imaging and clinical-pathologic correlation in 27 patients. Radiology. 1992;184:95–101. doi: 10.1148/radiology.184.1.1319079. [DOI] [PubMed] [Google Scholar]
- 23.Batsakis JG, Luna MA, el-Naggar AK. Histopathologic grading of salivary gland neoplasms: II. Acinic cell carcinomas. Ann Otol Rhinol Laryngol. 1990;99:929–33. doi: 10.1177/000348949009901115. [DOI] [PubMed] [Google Scholar]
- 24.Spiro RH, Huvos AG, Strong EW. Acinic cell carcinoma of salivary origin. A clinicopathologic of 67 cases. Cancer. 1978;41:924–35. doi: 10.1002/1097-0142(197803)41:3<924::aid-cncr2820410321>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Laskawi R, Rodel R, Zirk A, Arglebe C. Retrospectively analysis of 35 patients with acinic cell carcinoma of the parotid gland. J Oral Maxillofac Surg. 1998;56:440–3. doi: 10.1016/s0278-2391(98)90708-x. [DOI] [PubMed] [Google Scholar]
- 26.Suh S, Seol HY, Kim TK, et al. Acinic cell carcinoma of the head and neck: radiology-pathologic correlation. J Comput Assist Tomogr. 2005;29:121–6. doi: 10.1097/01.rct.0000150141.14113.ab. [DOI] [PubMed] [Google Scholar]
- 27.Batsakis JG, Chinn EK, Weimert TA, Work WP, Krause CJ. Acinic cell carcinoma: a clinicopathologic study of thirty-five cases. J Laryngol Otol. 1979;93:325–40. doi: 10.1017/s0022215100087107. [DOI] [PubMed] [Google Scholar]
- 28.Motoori K, Iida Y, Nagai Y, et al. MR imaging of salivary duct carcinoma. AJNR Am J Neuroradiol. 2005;26:1201–6. [PMC free article] [PubMed] [Google Scholar]
- 29.Harish K. Management of primary malignant epithelial parotid tumors. Surg Oncol. 2004;13:7–16. doi: 10.1016/j.suronc.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 30.Witt RL. Major salivary gland cancer. Surg Oncol Clin N Am. 2004;13:113–27. doi: 10.1016/S1055-3207(03)00126-1. [DOI] [PubMed] [Google Scholar]
- 31.Vander Poorten VL, Balm AJM, Hilgers FJM. Management of cancer of the parotid gland. Curr Opin Otolaryngol Head Neck Surg. 2002;10:134–44. [Google Scholar]
- 32.Guntinas-Lichius O, Klussman JP, Schroeder U, Quante G, Jungehuelsing M, Stennert E. Primary parotid malignoma surgery in patients with normal preoperative facial nerve function: outcome and long-term postoperative facial nerve function. Laryngoscope. 2004;114:949–56. doi: 10.1097/00005537-200405000-00032. [DOI] [PubMed] [Google Scholar]
- 33.Spiro RH, Armstrong J, Harrison L, Geller HL, Lin SY, String EW. Carcinoma of major salivary glands. Recent trends. Arch Otolaryngol Head Neck Surg. 1989;115:316–21. doi: 10.1001/archotol.1989.01860270058015. [DOI] [PubMed] [Google Scholar]
- 34.Armstrong JG, Harrison LB, Thaler HT, et al. The indications for elective treatment of the neck in cancer of the major salivary glands. Cancer. 1992;69:615–19. doi: 10.1002/1097-0142(19920201)69:3<615::aid-cncr2820690303>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 35.Medina JE. Neck dissection in the treatment of cancer of major salivary glands. Otolaryngol Clin North Am. 1998;31:815–22. doi: 10.1016/s0030-6665(05)70089-x. [DOI] [PubMed] [Google Scholar]
- 36.Tran L, Sadeghi A, Hanson D, et al. Major salivary gland tumors: treatment results and prognostic factors. Laryngoscope. 1986;96:1139–44. doi: 10.1288/00005537-198610000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Tullio A, Marchetti C, Sesenna E, Brusati R, Cocchi R, Eusebi V. Treatment of carcinoma of the parotid gland: the results of a multicenter study. J Oral Maxillofac Surg. 2001;59:263–70. doi: 10.1053/joms.2001.20986. [DOI] [PubMed] [Google Scholar]
- 38.Zbaren P, Schupbach J, Nuyens M, Stauffer E. Elective neck dissection versus observation in primary parotid carcinoma. Otolaryngol Head Neck Surg. 2005;132:387–91. doi: 10.1016/j.otohns.2004.09.029. [DOI] [PubMed] [Google Scholar]