Abstract
In the present study, we evaluated the potential of bradykinin (BK) to induce the release of neutrophil and monocyte chemotactic activity (NCA and MCA) and cytokines from an alveolar type II epithelial cell line, A549 cells. BK stimulated A549 cells to release NCA and MCA in a dose- and time-dependent manner (P < 0.001). Checkerboard analysis revealed that both NCA and MCA involved chemotactic and chemokinetic activity. Molecular sieve column chromatography showed three molecular weight masses (near 19 kd, 8 kd, and 400 d) for NCA and several molecular weight peaks (near 66 kd, 25 kd, 19 kd, 16 kd, and 400 d) for MCA. The release of NCA and MCA was inhibited by cycloheximide and lipoxygenase inhibitors (P < 0.01). The NCA and MCA were inhibited by leukotriene B4 (LTB4) receptor antagonist (P < 0.01), and the concentration of LTB4 was high enough for NCA and MCA. Antibodies to interleukin (IL)-8 and granulocyte colony-stimulating factor (G-CSF) attenuated NCA (P < 0.01), and antibodies to monocyte chemotactic protein-1 (MCP-1), G-CSF, and transforming growth factor (TGF)-β attenuated MCA (P < 0.01). The levels of IL-8, G-CSF, MCP-1, and TGF-β increased time dependently (P < 0.01). BK also stimulated the release of ILeukin-6 from A549 cells (P < 0.001). The receptors responsible for the release of NCA, MCA, and individual chemokines involved both BKB1 and BKB2 receptors. These data suggest that BK may stimulate alveolar type II pneumocytes to release inflammatory cytokines, which then may modulate the lung inflammation.
Sequestration of peripheral blood neutrophils and monocytes within the lung is characteristic of a number of acute and chronic pulmonary diseases. 1-5 The presence of neutrophils is determined by the local generation of chemotactic agents, which direct neutrophil migration from the vascular compartment to the alveolar space along chemotactic gradients. The alveolar macrophage is also derived predominantly from differentiated peripheral blood monocytes and to a limited extent from local macrophage replication. 6-8 Although elicited neutrophils and macrophages serve a vital role in the host defense against a number of organisms, the presence of increased numbers of activated neutrophils and macrophages can lead to excessive tissue injury via the overzealous elaboration of inflammatory cytokines, proteolytic enzymes, and oxygen radicals. 2,9 Substantial investigation has focused on the alveolar macrophages as a primary source of chemotactic factors. 10-12 However, neutrophil and monocyte chemotactic activity (NCA and MCA) has been found to be produced by endothelial cells, 13 fibroblasts, 14 and pulmonary epithelial cells. 15-17
Alveolar type II epithelial cells (ATII cells) have been shown to play a key role in the maintenance of the alveolar space. ATII cells synthesize and secrete surfactant, control the volume and composition of the epithelial lining fluid, proliferate, and differentiate into type I alveolar epithelial cells after lung injury to maintain the integrity of the alveolar wall. 18 Moreover, ATII cells are located to have a role in modulating immunological activity in the alveolar space. In this setting, ATII cell line, A549 cells secreted monocyte chemotactic protein (MCP)-1, transforming growth factor (TGF)-β, and leukotriene (LT)B4 constitutively 19 and further secreted interleukin (IL)-8, 15,20 IL-6, 21 interferon, 22 and MCP-1 23 in response to IL-1β and tumor necrosis factor (TNF)-α, suggesting participation in the intra-alveolar cytokine network.
The activation of the kallikrein-kinin system in acute lung injury has long been recognized. Bradykinin (BK) is generated from kininogens by the actions of plasma and tissue kallikreins (kininogenases). 24,25 Its actions on pulmonary circulation and lung mechanics have been evaluated intensively. BK also stimulates alveolar macrophages and bronchial epithelial cells to release chemotactic factors for inflammatory cells. 26,27 Recently, BKB2 antagonist attenuates the acute lung injury induced by live Pseudomonas aeruginosa infusion, including the migration of neutrophils to the lung and lung sequestration of neutrophils. 28 In this context, BK may participate in the release of inflammatory mediators from lung cells.
Because the alveolar space is lined by epithelial cells, direct BK-epithelial cell contact, without intervening alveolar macrophages, is likely to occur. In the present study, we evaluated the potential of BK to stimulate ATII cells resulting in the release of inflammatory cytokines and chemokines. The results demonstrated that A549 cells released IL-6, IL-8, MCP-1, TGF-β, and granulocyte colony-stimulating factor (G-CSF) by BK. These data suggest that BK may play roles in stimulating ATII cells and mediating inflammatory responses in the lung.
Materials and Methods
Culture and Identification of Type II Alveolar Epithelial Cells
Because of difficulty in obtaining primary human type II epithelial cells of sufficient purity, A549 cells (American Type Culture Collection, Rockville, MD), from an alveolar type II cell line derived from an individual with alveolar carcinoma, 29 were used. These cells retained many of the characteristics of normal type II cells such as surfactant protein, cytoplasmic multilamellar inclusion bodies, and cuboidal appearance and had been extensively used to assess type II pneumocyte effecter cell functions. 15,21,28 A549 cells were grown as monolayers on 100-mm tissue culture dishes with supplemented F-12 medium as previously reported. 19 The cells from monolayers were harvested with trypsin (0.25%) and EDTA (0.1%) in PBS, centrifuged at low speed (250 × g for 5 minutes), and resuspended in fresh medium at the concentration of 1.0 × 10 6 cells/ml in 35-mm tissue culture dishes. The cells were grown to confluence on the dish during 5 to 7 days. After the cell reached confluence, the cells were used for the experiment.
Measurement of BK Concentration in A549 Cell Culture
We measured the concentration of BK at the selected time points (12, 14, 48, and 72 hours) to decide the exposure period of A549 cells to BK and determined the levels of BK in the supernatant fluids to estimate the half-life of BK. BK was measured by a highly sensitive radioimmunoassay as previously reported. 30
Exposure of A549 Cells to Bradykinin
Medium was removed from cells by washing twice with serum-free F-12, and cells were incubated with F-12 without fetal calf serum in the presence or absence of BK (0, 0.1, 1.0, 10, and 100 μmol/L; Sigma Chemical Co., St. Louis, MO) and cultured for 12, 24, 48, 72, and 96 hours. BK did not cause A549 cell injury (no deformity of cell shape and no detachment from tissue culture dish, and greater than 95% of cells were viable by trypan blue exclusion) after a 72-hour incubation at the maximal doses. The culture supernatant fluids were harvested and frozen at −80°C until assayed. At least seven separate A549 cell supernatant fluids were harvested from cultures for each experimental condition.
Measurement of Neutrophil and Monocyte Chemotactic Activity
Polymorphonuclear leukocytes were purified from heparinized normal human blood by the method of Böyum. 31 Briefly, 15 ml of venous heparinized blood was suspended in the same volume of 3% dextran (Sigma) in isotonic saline for 30 minutes. The neutrophil-rich upper layer was aspirated and centrifuged at 400 × g for 5 minutes, and the cell pellet was resuspended in lysing solution consisting of 0.1% KHCO3 and 0.83% NH4Cl. The suspension was then centrifuged and washed three times in Hanks’ balanced salt solution (HBSS; GIBCO, Gaithersburg, MD). The viability of recovered neutrophils was >98% as assessed by trypan blue and erythrosin exclusion. The cells were suspended in Gey’s balanced salt solution (GIBCO) containing 2% bovine serum albumin (BSA; Sigma) at pH 7.2 to give a final concentration of 3.0 × 10 6 cells/ml.
Mononuclear cells were obtained for the chemotactic assay by Ficoll-Hypaque density centrifugation (Histopaque 1077, Sigma) to separate the red blood cells and neutrophils from mononuclear cells as previously reported. 19 The preparation routinely consisted of 30% monocytes and 70% lymphocytes determined by morphology and α-naphthyl acetate esterase staining (Sigma) with >98% viability as assessed by trypan blue and erythrosin exclusion. The cells were suspended in Gey’s balanced salt solution containing 2% BSA at pH 7.2 to give a final concentration of 5.0 × 10 6 cells/ml. These suspensions were used in the chemotaxis assay.
The chemotaxis assay was performed in 48-well microchemotaxis chambers (Neuroprobe, Cabin John, MD) as has been described. 32 The bottom wells of the chamber were filled with 25 μl of fluid containing the chemotactic stimulus. Each sample was tested in duplicate. A polycarbonate filter (Nucleopore Corp., Pleasanton, CA) with a pore size of 3 μm for neutrophil chemotaxis and 5 μm for monocyte chemotaxis was placed over the bottom wells. The silicon gasket and upper pieces of the chamber were applied, and the entire assembly was preincubated at 37°C in humidified air for 15 minutes before filling the upper wells with 50 μl of cell suspension. The chamber was incubated in humidified 5% CO2 at 37°C for 30 minutes for neutrophil assay and 90 minutes for monocyte chemotaxis. The chamber was disassembled after the incubation, and the filter was fixed, stained with Diff-Quik (American Scientific Products, McGaw Park, IL), and mounted on a glass slide. Cells that completely migrated through the filter were counted in five random high-power fields (HPF; ×1000) from each duplicate well. Chemotactic response was defined as the mean number of migrated cells per HPF. F-12 without fetal calf serum was incubated identically with A549 cells, and the supernatant fluids harvested were used to determine background neutrophil and monocyte migration. Formyl-methionyl-leucyl-phenylalanine (FMLP; 10−8 mol/L in F-12; Sigma) and normal human serum that was complement activated by incubation with Escherichia coli endotoxin and diluted 10-fold with F-12 were used as positive controls for both neutrophils and monocytes.
To determine whether the migration was due to movement along a concentration gradient (chemotaxis) or stimulation to randomly migrate (chemokinesis), a checkerboard analysis 33 was performed by A549 cell supernatant fluid harvested at 72 hours in response to 100 μmol/L BK. To do this, various concentrations of A549 cell supernatant fluids (1:27, 1:9, 1:3, and 1:1) were placed above the membrane with cells and below the membrane.
To ensure that monocytes, but not lymphocytes, were the primary cells that migrated, some of membranes were stained with α-naphthyl acetate esterase according to the manufacturer’s directions (Sigma).
Partial Characterization of NCA and MCA
Because NCA and MCA were detected in the A549 cell culture supernatant fluids, partial characterization of the released activity was performed using supernatant fluids harvested at 72 hours of incubation at the concentration of 100 μmol/L BK. Sensitivity to proteases was tested with trypsin treatment (final concentration, 100 μg/ml; Sigma) for 30 minutes at 37°C followed by the addition of 1.5 mol/L excess of soybean trypsin inhibitor (Sigma) to terminate the proteolytic activity before the chemotaxis assay. The lipid solubility of the activity was evaluated by mixing the A549 cell culture supernatant fluid twice with ethyl acetate, decanting the lipid phase after each extraction, evaporating the ethyl acetate to dryness, and resuspending the extracted material in F-12 used for the cell culture before the chemotaxis assay. Heat sensitivity was determined by heating the culture supernatant fluid at 98°C for 30 minutes.
Estimation of Molecular Size of the Chemotactic Activity by Column Chromatography
To determine the approximate molecular weight of the released chemotactic activity in the supernatant fluids harvested at 72 hours in response to 100 μmol/L BK, molecular sieve column chromatography was performed using Sephadex G-100 (25 × 1.25 cm; Pharmacia, Piscataway, NJ) at a flow rate of 6 ml/hour. The A549 cell culture supernatant fluid was eluted with PBS, and every fraction after the void volume was evaluated for NCA and MCA in duplicate.
Effects of Metabolic Inhibitors on the Release of NCA and MCA
Although A549 cells were capable of releasing lipoxygenase metabolites, which may account for the released NCA and MCA, the effects of nonspecific lipoxygenase inhibitors nordihydroguaiaretic acid (NDGA; 100 μmol/L; Sigma) and diethylcarbamazine (DEC; 1 mmol/L; Sigma) and 5-lipoxygenase inhibitor AA-861 (100 μmol/L; Takeda Pharmaceutical Co., Tokyo, Japan) were evaluated. The effect of the protein synthesis inhibitor cycloheximide (10 μg/ml; Sigma) was also evaluated. At these concentrations, NDGA, DEC, and AA-861 inhibited the release of LTB4 in other cell cultures in response to lipopolysaccharide and did not cause cytotoxicity to A549 cells after a 72-hour incubation. 26,27
Effects of BK Receptor Antagonists for the Release of NCA and MCA
To determine the receptor responsible for the release of chemotactic activity, BKB1 receptor antagonist Des-Arg9-[Leu8]-BK (Sigma) and BKB2 receptor antagonist, d-Arg-[Hyp, 3Thi5,8,d-Phe7]-BK (Sigma), at the concentration of 100 μmol/L, were evaluated, respectively. Thirty minutes before addition of 50 μmol/L BK, A549 cells were treated with BKB1 and BKB2 receptor antagonists and incubated for 72 hours.
Effects of LTB4 and Platelet-Activating Factor Receptor Antagonists on the Released NCA and MCA
Because nonspecific lipoxygenase and 5-lipoxygenase inhibitors inhibited the release of NCA and MCA, LTB4 receptor (ONO 4057, Ono Pharmaceutical Co., Tokyo, Japan) and platelet-activating factor (PAF) receptor antagonists (TCV 309, Takeda Pharmaceutical Co., Tokyo.) at the concentration of 10−5 mol/L were used to evaluate the responsible chemotactic activity in column chromatography-separated lowest molecular weight peak in BK-induced NCA and MCA.
Measurement of LTB4 and PAF in the Supernatant Fluid
The measurement of LTB4 was performed by radioimmunoassay (RIA) 19 as previously reported. Briefly, ethanol samples were centrifuged at 5500 × g at 0°C. The supernatants were evaporated under N2 gas at 37°C to cause ethanol evaporation. To each sample, 10 ml of distilled water was added. These samples were acidified to pH 4.0 with 0.1 mol/L hydrochloric acid and applied to Sep-pak C18 columns (Waters Associates, Milford, MA); the columns were washed with 10 ml of distilled water and 20 ml of petroleum ether and then eluted with 15 ml of methanol. These eluates were dried with N2 gas at 37°C and redissolved in 20 μl of methanol and 180 μl of RIA buffer (50 mmol/L Tris/HCl buffer containing 0.1% (w/v) gelatin, pH 8.6). Anti-LTB4 serum, [5,6,8,9,11,12,14,15,-3H(N)]LTB4, and synthetic LTB4 were purchased from Amersham Co. (Arlington Heights, IL). [3H]LTB4 was diluted in RIA buffer and aliquots (100 μl, containing approximately 4000 dpm) were mixed with 100 μl of standards or samples in disposable siliconized tubes. Anti-LTB4 serum diluted in RIA buffer (100 μl) and RIA buffer (100 μl) were added to give a total incubation volume of 400 μl, and the mixture was incubated at 4°C for 18 hours. Free LTB4 was adsorbed onto dextran-coated charcoal. The supernatant containing the antibody-bound LTB4 was decanted into a scintillation counter after centrifugation at 2000 × g for 15 minutes. Scintillation fluid (Aquazol 2, NEN Co., Boston, MA) was added, and radioactivity was counted by a scintillation counter (Tricarb-3255, Tackard Co., Downers Grove, IL) for 4 minutes.
PAF concentration in the supernatant fluids was measured by a scintillation proximity assay (SPA) system. This system combined the use of a high specific activity tritiated PAF tracer with an antibody specific for PAF and a PAF standard similar to the methods of measurement of LTB4.
Effects of Polyclonal Antibodies to IL-8, G-CSF, MCP-1, MIP-1α, GM-CSF, RANTES, and TGF-β on Released NCA and MCA
The neutralizing antibodies to IL-8, G-CSF, MCP-1, RANTES, GM-CSF, MIP-1α, and TGF-β were purchased from Genzyme (Cambridge, MA) and were added to the A549 cell supernatant fluids, which were harvested at 72 hours in response to 100 μmol/L BK, at the suggested concentration to inhibit these cytokines and incubated for 30 minutes in 37°C before chemotaxis. Then these samples were used for chemotactic assay. The antibodies to IL-8, G-CSF, TGF-β, and MCP-1 were tested by blocking the chemotactic response to neutrophils and monocytes. For all antibodies, referred to the manufacturer’s data for their validity. These antibodies did not influence the chemotactic response to endotoxin-activated serum (data not shown). For the nonspecific effects of IgG, nonimmune IgG (Genzyme) was used as a control antibody.
Measurement of IL-6, IL-8, G-CSF, MCP-1, MIP-1α, RANTES, G-CSF, and GM-CSF in the Supernatant Fluids
The concentrations of IL-6, IL-8, G-CSF, MCP-1, MIP-1α, RANTES, GM-CSF, and TGF-β in A549 cell supernatant fluids cultured for 72 hours at the concentration of 100 μmol/L BK were measured by ELISA according to manufacturers’ directions. MIP-1α, G-CSF, GM-CSF, and RANTES kits were purchased from Amersham (Little Chalfont, UK), and the minimal concentration detected by these methods was 46.9 pg/ml for MIP-1α, 31.9 pg/ml for G-CSF, 2.00 pg/ml for GM-CSF, and 15.6 pg/ml for RANTES. IL-6, IL-8, MCP-1, and TGF-β kits were purchased from R&D Systems (Minneapolis, MN), and the minimal concentrations for IL-6, IL-8, MCP-1, and TGF-β was 0.156 pg/ml, 10.0 pg/ml, 31.3 pg/ml, and 0.31 ng/ml, respectively.
Statistics
In experiments where multiple measurements were made, differences between groups were tested for significance using one-way analysis of variance with Duncan’s multiple range test applied to data at specific time and dose points. In experiments where a single measurement was made, the differences between groups were tested for significance using Student’s paired t-test. In all cases, a P value less than 0.05 was considered significant. Data in figures and tables were expressed as means ± SEM.
Results
Concentration of BK in A549 Cell Culture
BK at the initial concentration of 50 μg/ml declined to 29.8 ± 1.2 μg/ml at 12 hours, 18.4 ± 0.4 μg/ml at 24 hours, 4.11 ± 0.07 μg/ml at 48 hours, and 0.137 ± 0.008 μg/ml at 72 hours (n = 3 monolayers). Thus, the half-life was ∼15 hours, and the remaining level of BK after a 72-hour incubation was 0.27%. Because BK was retained in the supernatant fluids after 72 hours, the exposure of A549 cells to BK was observed after 96 hours.
Release of NCA and MCA from A549 Cells in Response to BK
A549 cells released NCA in response to BK in a dose- and time-dependent fashion (P < 0.01, Figure 1, A and B ▶ ). The lowest concentration of BK to stimulate A549 cells was 0.1 μmol/L. Increasing concentrations of BK progressively increased the release of NCA up to 100 μmol/L. Although A549 cells released a small amount of NCA constitutively, the release of NCA began after a 12-hour exposure to BK, and the released activity was cumulative and continued to increase even after 72 hours (Figure 1B) ▶ .
Figure 1.
A: The dose-dependent release of neutrophil chemotactic activity in response to bradykinin from A549 cell monolayers after a 72-hour incubation (n = 8). Values are expressed as means ± SE. *P < 0.01 compared with medium alone. B: The time-related release of neutrophil chemotactic activity in response to 100 μmol/L bradykinin (•) from A549 cell monolayers (n = 8). ▪, baseline release of neutrophil chemotactic activity. Values are expressed as means ± SE. *P < 0.01 compared with supernatant fluids without incubation. ⋆P 0.01 compared with supernatant fluids without BK stimulation.
Although A549 cells also released MCA in the baseline condition as previously reported, 19 BK induced a dose-related release of MCA (P < 0.01; Figure 2A ▶ ). The lowest concentration of BK examined (0.01 μmol/L) induced a substantial release of MCA. Increasing concentrations progressively induced the release of MCA. The release of MCA began after a 12-hour exposure to BK (Figure 2B) ▶ . The released activity was cumulative and increased even after 72 hours.
Figure 2.
A: The dose-dependent release of monocyte chemotactic activity in response to bradykinin from A549 cell monolayers after a 72-hour incubation (n = 8). Values are expressed as means ± SE. *P < 0.01 compared with medium alone. B: The time-related release of monocyte chemotactic activity in response to 100 μmol/L bradykinin (•) from A549 cell monolayers (n = 8). ▪, baseline release of monocyte chemotactic activity. Values are expressed as means ± SE. *P < 0.01 compared with supernatant fluids without incubation. ⋆P < 0.01 compared with supernatant fluids without BK stimulation.
Confirmation that the migrated cells were monocytes was provided by the following lines of evidences: 1) >90% of the migrated cells appeared to be monocytes by light microscopy; 2) >90% of the migrated cells were esterase positive; and 3) lymphocytes purified by allowing the monocytes to attach to plastic and tested in the chemotaxis assay yielded 0% to 20% of the chemotactic activity of the monocyte preparation.
The chemotactic activities in response to FMLP and activated serum were 120.4 ± 8.7 neutrophils per HPF and 68.3 ± 3.2 monocytes per HPF and 170.6 ± 15.3 neutrophils per HPF and 75.3 ± 6.4 monocytes per HPF, respectively. BK itself in the culture medium without cells and incubated identically did not show any chemotactic activity for neutrophils and monocytes (data not shown).
Checkerboard analysis revealed that A549 cell supernatant fluids stimulated by BK induced neutrophil and monocyte migration in the presence and absence of concentration gradient across the membrane (data not shown). Thus, the migration in response to BK-stimulated A549 cell supernatant fluids was consistent with chemotactic and chemokinetic activity for both neutrophil and monocyte migration.
Partial Characterization of the Released NCA and MCA
The MCA and NCA were heterogeneous in their character. Both NCA and MCA were partially sensitive to heat, extractable to ethyl acetate, and digested by trypsin (Figure 3, A and B) ▶ .
Figure 3.
Partial characterization of the released neutrophil (A) and monocyte (B) chemotactic activity from A549 cell supernatant fluids harvested after a 72-hour incubation in response to 100 μmol/L bradykinin (n = 5). Values are expressed as means ± SE. *P < 0.01 compared with crude sample. EA, ethyl acetate.
Effects of Metabolic Inhibitors on the Release of NCA and MCA
Incubation of A549 cells with cycloheximide inhibited the release of both NCA and MCA (Figure 4, A and B) ▶ . Nonspecific lipoxygenase inhibitors NDGA and DEC and 5-lipoxygenase inhibitor AA-861 attenuated the release of NCA and MCA in response to 100 μmol/L BK (P < 0.01; Figure 4, A and B ▶ ). NDGA, DEC, and AA-861 did not have any effects on FMLP and activated-serum-induced neutrophil chemotaxis (data not shown).
Figure 4.
The effects of nordihydroguaiaretic acid (NDGA), diethylcarbamazine (DEC), AA-861, and cycloheximide on the release of neutrophil chemotactic activity (A) and monocyte chemotactic activity (B) in response to 100 μmol/L bradykinin for a 72-hour incubation from A549 cell monolayers (n = 5). Values are expressed as means ± SE. *P < 0.01 compared with stimulus alone.
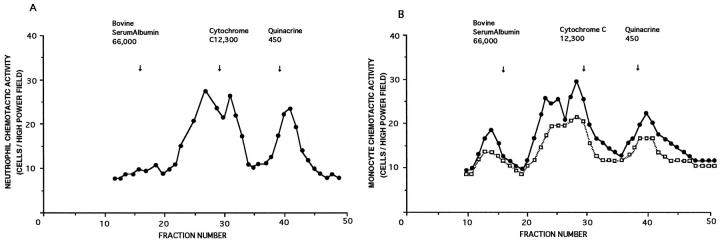
Molecular Size of Released Chemotactic Activity
The experiments using Sephadex-100 revealed that the released NCA was heterogeneous in size, with the estimated molecular weight at 19 kb, 81 kb, and 450 d after quinacrine (Figure 5A) ▶ . Although A549 cells released MCA in unstimulated supernatant fluids (Figure 5B) ▶ , the three molecular weight masses (25, 19, and 16 kd) before cytochrome C became prominent in response to BK (Figure 5B) ▶ . The addition of cycloheximide before BK treatment inhibited the release of these high molecular weight MCAs (data not shown).
Figure 5.
A: Molecular sieve column chromatographic finding of neutrophil chemotactic activity released from A549 cell monolayers in response to 100 μmol/L bradykinin for a 72-hour incubation. B: Molecular sieve column chromatographic finding of monocyte chemotactic activity released from A549 cell monolayer in response to 100 μmol/L bradykinin for a 72-hour incubation (•). □, molecular profiles of monocyte chemotactic activity under the unstimulated state. These data are the representative data of four experiments.
Effects of LTB4 and PAF Receptor Antagonists on the Lowest Molecular Weight NCA and MCA
The lowest molecular weight NCA and MCA separated by molecular sieve column chromatography was inhibited by the addition of LTB4 receptor antagonist ONO 4057 ∼70% for NCA and MCA (P < 0.01; Table 1 ▶ ). The effect of PAF receptor antagonist TCV 309 was not significant for NCA and MCA. Each receptor antagonist at the concentration of 10−5 mol/L completely inhibited the neutrophil migration in response to LTB4 and PAF at the concentration of 10−7 mol/L, respectively, but did not show any inhibitory effects on FMLP and activated-serum-induced neutrophil chemotaxis (data not shown).
Table 1.
Effects of Leukotriene B4 (ONO 4057) and Platelet-Activating Factor (TCV 309) Receptor Antagonists on the Column Chromatography-Separated Lowest Molecular Weight Neutrophil and Monocyte Chemotactic Activity
| NCA | MCA | |
|---|---|---|
| Crude fraction | 25.7 ± 3.5 | 22.4 ± 2.5 |
| Plus ONO 4057 | 14.5 ± 2.2* | 14.3 ± 2.2* |
| Plus TCV 309 | 23.9 ± 2.1 | 22.3 ± 1.3 |
| F-12 | 9.8 ± 2.3 | 8.6 ± 2.3 |
Values are expressed as means ± SE of the means (cells/high power field). F-12 expressed negative control chemotaxis. NCA and MCA, neutrophil and monocyte chemotactic activity, respectively.
*P < 0.01 compared with crude fraction; n = 4.
Concentrations of LTB4 and PAF in the Supernatant Fluids
The concentrations of LTB4 in the supernatant fluids in response to BK at the concentration of 100 μmol/L for 72 hours and control were 68.8 ± 12.4 pg/ml versus 55.4 ± 13.4 pg/ml (n = 6; P = 0.10). However, PAF in the supernatant fluids was not detected in response to BK stimulation (below 40 pg/ml).
Effects of Blocking Antibodies to IL-8, G-CSF, MCP-1, and TGF-β on NCA and MCA in the Supernatant Fluids
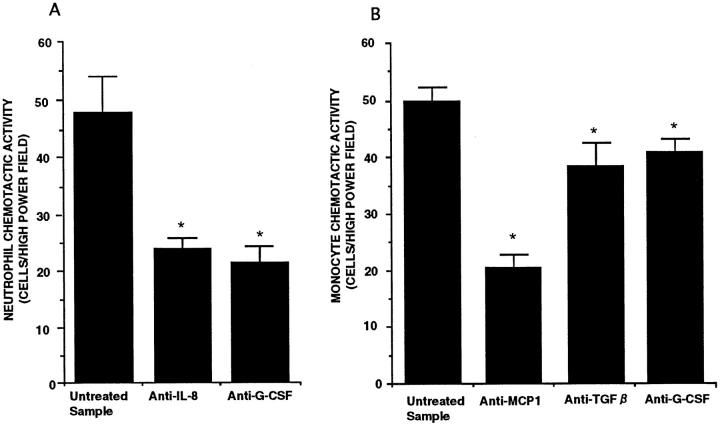
Anti-IL-8 antibody blocked NCA by 40%, and anti-G-CSF antibody attenuated NCA up to 50%. Anti-MCP-1 antibody inhibited 50% of total MCA, and anti-TGF-β and anti-G-CSF antibodies inhibited 10% of MCA (Figure 6, A and B) ▶ . These blocking antibodies blocked each corresponding molecular weight peak separated by molecular sieve column chromatography, respectively (data not shown). The nonimmune IgG was used to evaluate the effect of nonspecific antibody. Nonimmune IgG did not attenuate the NCA and MCA in the same BK-conditioned medium.
Figure 6.
The effects of blocking antibodies on neutrophil chemotactic activity (A) and monocyte chemotactic activity (B) released from A549 cell monolayers in response to 100 μmol/L bradykinin for a 72-hour incubation (n = 6). Values are expressed as means ± SE. *P < 0.01 compared with untreated sample.
Concentrations of IL-6, IL-8, G-CSF, MCP-1, MIP-1, GM-CSF, TGF-β, and RANTES in the Supernatant Fluids
The measurement of chemotactic cytokines by ELISA revealed that BK at the concentration of 100 μmol/L for 72 hours of incubation significantly stimulated the release of IL-8, G-CSF, MCP-1, and TGF-β from A549 cells (Table 2) ▶ . In contrast RANTES, MIP-1α, and GM-CSF were not detected in A549 cell supernatant fluids in response to BK and in the control condition. BK also stimulated the release of IL-6 (Table 2) ▶ .
Table 2.
Concentrations of Inflammatory Cytokines in A549 Cell Supernatant Fluids in Response to 100 μmol/L Bradykinin for 72 Hours of Incubation (n = 9)
| Control | Bradykinin | |
|---|---|---|
| IL-6 | 119 ± 24 | 371 ± 65† |
| IL-8 | 1169 ± 121 | 2294 ± 53† |
| MCP-1 | 1247 ± 36 | 3053 ± 445† |
| TGF-β | 550 ± 20 | 698 ± 30* |
| G-CSF | ND | 476 ± 70† |
| MIP-1α | ND | ND |
| GM-CSF | ND | ND |
| RANTES | ND | ND |
Values are mean ± SE of the means (pg/ml). ND, not detected.
*P < 0.01 compared with control.
†P < 0.001 compared with control.
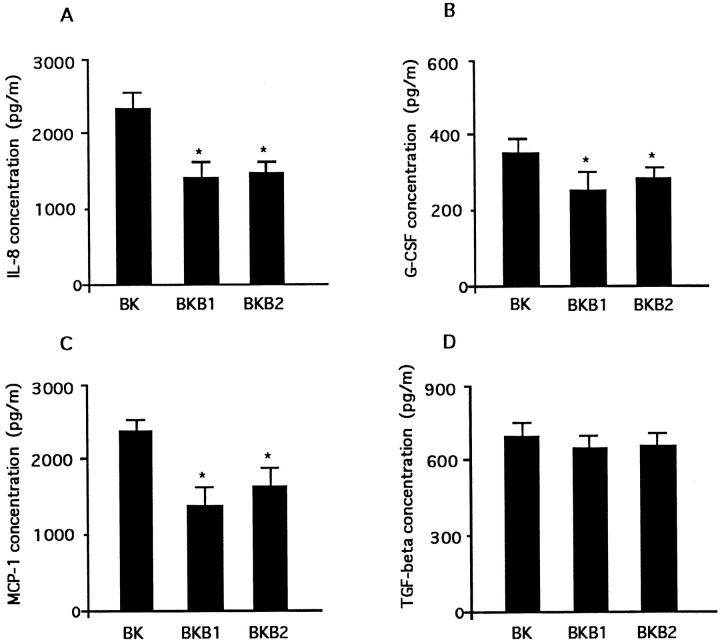
Because NCA consisted of IL-8 and G-CSF, and because MCA consisted of MCP-1, G-CSF, and TGF-β, we evaluated the individual time courses of mediators that constituted chemotactic activity (Figure 7, A–D) ▶ . All chemokines showed time-dependent increases.
Figure 7.
The individual time courses of chemokines that comprised chemotactic activity. The release of IL-8 (A), G-CSF (B), MCP-1 (C), and TGF-β (D) from A549 cell monolayer in response to 100 μmol/L bradykinin (n = 6).
Inhibition of the Release of NCA, MCA, and Individual Chemokines by Both BKB1 and BKB2 Receptor Antagonists
Both BKB1 and BKB2 receptor antagonists significantly inhibited the release of NCA and MCA equally harvested after 72 hours of incubation (Figure 8, A and B) ▶ . The individual chemokines, ie, IL-8, MCP-1, and G-CSF, but not TGF-β, were inhibited significantly by BKB1 and BKB2 receptor antagonists (Figure 9, A–D) ▶ .
Figure 8.
The effects of bradykinin B1 (BKB1) and B2 (BKB2) receptor antagonists on the release of NCA (A) and MCA (B) from A549 cell monolayer after a 72-hour incubation (n = 5). *P < 0.01 compared with bradykinin alone.
Figure 9.
The effects of bradykinin B1 (BKB1) and B2 (BKB2) receptor antagonists on the release of IL-8 (A), G-CSF (B), MCP-1 (C), and TGF-β (D) from A549 cell monolayer after a 72-hour incubation (n = 5). *P < 0.01 compared with bradykinin alone.
Discussion
BK is thought to be a potent inflammatory mediator in lung diseases. 24,25 Turino and co-workers suggested that immunoreactive kinins and kininogenase activity were present in BALF and plasma obtained from patients with lung inflammation. 25 BK is generated from kininogens by the actions of plasma and tissue kallikreins (kininogenases). 24,25 Although its action includes vasodilation, vascular leakage, and contraction of smooth muscles, 24,25,34 BK stimulated alveolar macrophages and bronchial epithelial cells to release NCA and MCA. 26,27 In the present study, BK induced the release of chemokines from A549 cells, suggesting that BK has the potential to stimulate ATII cells resulting in the release of NCA and MCA and contribute to lung inflammation besides its direct effects on lung cells.
The identification of NCA and MCA is not complete. It is reported that ATII cells have the potential to release IL-8, 15,20 MCP-1, 23 and IL-6 21 in response to TNF-α, IL-1β, and asbestos stimulation. In the present study, the blocking antibody to IL-8, G-CSF, MCP-1, and TGF-β attenuated the chemotactic activity, and BK significantly stimulated the release of IL-8, G-CSF, MCP-1, and TGF-β from A549 cells. Although the highest molecular weight peaks of MCA were undetermined, these data suggest that these cytokines may be predominantly responsible for the NCA and MCA.
Although the releasing potential of IL-6, G-CSF, IL-8, MCP-1, and TGF-β from A549 cells by BK was less than that observed by TNF-α and IL-1 stimulation (unpublished data), the release of NCA and MCA was increased three- to fourfold compared with the constitutive release of chemotactic activity. The chemotactic potential of released activity from A549 cells was more than the released activity from 10 6 alveolar macrophages per culture dish in response to endotoxin (unpublished data). Thus, BK may contribute to the recruitment of inflammatory cells into the alveolar space by stimulating ATII cells.
BK has been reported to have several potent effects on airway functions, many of which are thought to be mediated via the BKB2 receptor. The existence of the BKB2 receptor has been mapped out in humans and guinea pig lungs by autoradiography with [3H]BK. 35 BKB1 receptor ligands do not displace [3H]BK binding in trachea and lung, indicating the absence of BKB1 receptors and the presence of BKB2 receptors in the healthy lung. 36 However, the BKB1 receptor antagonist Des-Arg9-[Leu8]-BK significantly inhibited airway hyperresponsiveness and neutrophilia induced by antigen challenge. 37 Although the BK receptor on A549 cells is not identified, the present study suggests that both BKB1 and BKB2 receptors are concerned with the release of NCA and MCA. Thus, the receptor responsible for the release of IL-8, G-CSF, and MCP-1 may involve both BKB1 and BKB2 receptors.
IL-6 is a cytokine involved in the regulation of the immune response and inflammation. IL-6 modulates proliferation of lymphoid and nonlymphoid cells. 38 Although IL-6 is usually thought of as a pro-inflammatory cytokine, it has been suggested that IL-6 may act as an inhibitor of local acute inflammation, particularly in the lung. 39 However, the concentration of IL-6 is elevated in patients with various lung diseases. 40,41 Although alveolar macrophages are certainly involved in the generation of alveolar IL-6, as they are known as potent producers of IL-6, 42 the possible contribution to IL-6 production by ATII cells in response to BK may support the regulation of the alveolar IL-6 production.
TGF-β induces monocyte chemotaxis at concentrations from 0.1 to 10 pg/ml. 43 At higher concentrations, the chemotactic response of monocytes declined. Wakefield and co-workers reported that the biologically inactive form of TGF-β, which constitutes more than 98% of autocrine TGF-β secreted by all of 12 different cell types assayed, was shown to be unable to bind to the receptor without previous activation. 44 Although the concentrations of TGF-β were far above the monocyte chemotactic range, ie, 500 to 700 pg/ml in the present study, the active form of TGF-β was less than 2%. This may account for the concentration discrepancy of TGF-β-induced monocyte chemotaxis.
Wang and co-workers reported the chemotactic activity of recombinant human G-CSF for neutrophils and monocytes. 45 G-CSF was active in inducing neutrophil migration at concentrations more than 10 to 100 U/ml (7 to 70 ng/ml). The concentration of G-CSF in the supernatant fluids released from A549 cells was relatively low. However, the blocking antibody of G-CSF inhibited chemotactic responses of neutrophils by 40% and monocytes up to 10%. The discrepancy of G-CSF concentration for neutrophil migration compared with the present data might be due to the differences of neutrophil preparation, because human recombinant G-CSF induced neutrophil migration at 10 to 1000 pg/ml in our laboratory. The concentration of G-CSF in the A549 cell supernatant fluids induced by BK reached this chemotactic concentration.
In conclusion, BK induced the release of NCA and MCA from A549 cells. The released NCA and MCA in response to BK were both chemotactic and chemokinetic. Although the released activity was heterogeneous in its character, LTB4 receptor antagonist and blocking antibodies to IL-8, G-CSF, MCP-1, and TGF-β significantly inhibited the chemotactic response, and the releases of IL-6, IL-8, G-CSF, MCP-1, and TGF-β were significantly augmented in response to BK stimulation. These data suggest that BK may stimulate ATII cells to release NCA, MCA, and inflammatory cytokines and may modulate lung inflammation in the lung diseases.
Footnotes
Address reprint requests to Dr. Sekiya Koyama, The First Department of Internal Medicine, Shinshu University School of Medicine, 3-1-1 Asahi, Matsumoto 390, Japan.
References
- 1.Weiland JE, Davis WB, Holter JF, Mohammed JR, Dorinsky PM, Gadek JE: Lung neutrophils in the adult respiratory distress syndrome: clinical and pathophysiological significance. Am Rev Respir Dis 1986, 133:218-225 [DOI] [PubMed] [Google Scholar]
- 2.Hunninghake GW, Garrett KC, Richerson HB, Fantone JC, Ward PA, Rennard SI, Bitterman PB, Crystal RG: Pathogenesis of the granulomatous lung disease. Am Rev Respir Dis 1984, 130:476-496 [DOI] [PubMed] [Google Scholar]
- 3.Hunninghake GW, Gadek JE, Lawley TJ, Crystal RG: Mechanisms of neutrophil accumulation in the lungs of patients with idiopathic pulmonary fibrosis. J Clin Invest 1981, 68:S259-S269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blusse van Oud Alblas A, van der Linden-Schrever B, van Furth R: Origin and kinetics of pulmonary macrophages during an inflammatory reaction induced by intra-alveolar administration of aerosolized heat-killed BCG. Am Rev Respir Dis 1983, 128:276-281 [DOI] [PubMed] [Google Scholar]
- 5.Adanson IYR, Bowden JH: Role of monocytes and interstitial cell cells in the generation of alveolar macrophages. II. Kinetic studies after carbon loading. Lab Invest 1980, 42:518-531 [PubMed] [Google Scholar]
- 6.Shellito J, Esparza C, Armstrong C: Maintenance of the normal alveolar macrophage cell population. Am Rev Respir Dis 1987, 135:78-82 [DOI] [PubMed] [Google Scholar]
- 7.Blusse van Oud Alblas A, Mattie H, van Furth R: A quantitative evaluation of pulmonary macrophage kinetics. Cell Tissue Kinet 1983, 16:211-219 [PubMed] [Google Scholar]
- 8.Bitterman PB, Saltzman LE, Adelberg S, Ferrans VJ, Crystal RG: Alveolar macrophage replication: one mechanism for the expansion of the mononuclear phagocyte population in the chronically inflamed lung. J Clin Invest 1984, 74:460-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sibille Y, Reynolds HY: Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am Rev Respir Dis 1990, 141:471-501 [DOI] [PubMed] [Google Scholar]
- 10.Nathan CF: Secretory products of macrophages. J Clin Invest 1987, 79:319-326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merill WW, Naegel GP, Matthay RA, Reynolds HY: Alveolar macrophage-derived chemotactic factor for neutrophils: kinetics of in vitro production and partial characterization. J Clin Invest 1980, 65:268-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hunninghake GW, Gadek JE, Fales HM, Crystal GR: Human alveolar macrophage-derived chemotactic factor for neutrophils: stimuli and partial characterizatioKunkel SL, Showell HJ, Remick DG, Phan SH, Wardn. J Clin Invest 1980, 66:473-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strieter RM, Kunkel SL, Showell HJ, Remick DG, Phan SH, Ward PA, Marks RM: Endothelial cell gene expression of a neutrophil chemotactic factor by TNF-alpha, LPS, and IL-1 beta. Science 1989, 243:1467-1469 [DOI] [PubMed] [Google Scholar]
- 14.Strieter RM, Showel HJ, Remnick DG, Lynch JP, III, Genord M, Raiford C, Eskandari M, Marjs RM, Kunkel SL: Monokine-induced neutrophil chemotactic factor gene expression in human fibroblasts. J Biol Chem 1989, 264:10621-10626 [PubMed] [Google Scholar]
- 15.Standiford TJ, Kunkel SL, Basha MB, Chensue SW, Lynch JP, III, Toews GB, Westwick J, Strieter RM: Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest 1990, 86:1945-1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakamura H, Yoshimura K, Jahhe HA, Crystal RG: Interleukin-8 expression in human bronchial epithelial cells. J Biol Chem 1991, 266:19611-19617 [PubMed] [Google Scholar]
- 17.Koyama S, Rennard SI, Leikauf GD, Shoji S, Von Essen S, Claasen L, Robbins RA: Endotoxin stimulates bronchial epithelial cells to release chemotactic factor for neutrophils. J Immunol 1991, 147:4293-4301 [PubMed] [Google Scholar]
- 18.Mason J, Williams MC: Type II alveolar cell: defender of the alveolus. Am Rev Respir Dis 1977, 116:81-91 [DOI] [PubMed] [Google Scholar]
- 19.Koyama S, Sato E, Nomura H, Kubo K, Nagai S, Izumi T: Type II pneumocytes release chemoattractant activity for monocytes constitutively. Am J Physiol 1997, 272:L830-L837 [DOI] [PubMed] [Google Scholar]
- 20.Rosental GJ, Germolec DR, Blazka ME, Corsini E, Simeonova P, Pollock P, Kong LY, Kwon J, Luster MI: Asbestos stimulates IL-8 production from human lung epithelial cells. J Immunol 1994, 153:3237-3244 [PubMed] [Google Scholar]
- 21.Crestani B, Cornilett P, Dehoux M, Rolland C, Guenounou M, Aubier M: Alveolar type II epithelial cells produce interleukin-6 in vitro and in vivo: regulation by alveolar macrophage secretory products. J Clin Invest 1994, 94:731-740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hahon N, Castranova V: Interferon production in rat type II pneumocytes and alveolar macrophages. Exp Lung Res 1989, 15:429-445 [DOI] [PubMed] [Google Scholar]
- 23.Standiford TJ, Kunkel SL, Phan SH, Rollins BJ, Strieter RM: Alveolar macrophage-derived cytokines induces monocyte chemoattractant protein-I expression from human pulmonary type II-like epithelial cells. J Biol Chem 1991, 266:9912-9918 [PubMed] [Google Scholar]
- 24.Proud D, Kaplan AP: Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol 1988, 6:49-83 [DOI] [PubMed] [Google Scholar]
- 25.Turino GM, Rodrigues JR, Greenbaum LM, Mandel I: Mechanisms of pulmonary injury. Am J Med 1974, 57:493-505 [DOI] [PubMed] [Google Scholar]
- 26.Sato E, Koyama S, Nomura H, Kubo K, Sekiguchi M: Bradykinin stimulates alveolar macrophages to release neutrophil, monocyte, and eosinophil chemotactic activity. J Immunol 1996, 157:3122-3129 [PubMed] [Google Scholar]
- 27.Koyama S, Rennard SI, Robbins RA: Bradykinin stimulates bronchial epithelial cells to release neutrophil and monocyte chemotactic activity. Am J Physiol 1995, L38–L44 [DOI] [PubMed]
- 28.Riding PC, Blocher CR, Fisher BJ, Fowler AA, III, Sugerman HJ: Beneficial effects of a bradykinin antagonist in a model of gram-negative sepsis. J Trauma 1995, 39:81-89 [DOI] [PubMed] [Google Scholar]
- 29.Leiber M, Smith B, Szakal A, Nelson-Rees W, Todaro G: A continuous tumor cell line from human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer 1976, 17:62-70 [DOI] [PubMed] [Google Scholar]
- 30.Ando T, Shimamoto K, Nakahashi Y, Nishitani T, Hosoda S, Ishida H, Tanaka S, Iimura O: Kinins. Fritz H Dietze G Fiedler F Haberland GL eds. Recent Progress on Kinins. 1982, :pp 222-232 Birkhauser Verlag, Boston [Google Scholar]
- 31.Böyum A: Isolation of mononuclear cells from human blood. Scand J Invest 1968, 21:77-98 [PubMed] [Google Scholar]
- 32.Harrath L, Falk W, Leonard EJ: Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multi well assembly. J Immunol Methods 1980, 37:39-45 [DOI] [PubMed] [Google Scholar]
- 33.Zigmond SH, Hirsch GJ: Leukocyte locomotion and chemotaxis: new methods for evaluation and demonstration of cell-derived chemotactic factor. J Exp Med 1973, 137:387-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fuller RW, Dixon CMS, Cuss FMC, Barnes PJ: Bradykinin-induced bronchoconstriction in humans: mode of action. Am Rev Respir Dis 1987, 135:176-180 [DOI] [PubMed] [Google Scholar]
- 35.Mak JCW, Barnes PJ: Autoradiographic visualization of bradykinin receptors in human and guinea pig lung. Eur J Pharmacol 1991, 194:37-44 [DOI] [PubMed] [Google Scholar]
- 36.Farmer SG, Burch RM, Meeker SN, Willikins DE: Evidence for a pulmonary bradykinin B3 receptor. Mol Pharmacol 1989, 36:1-7 [PubMed] [Google Scholar]
- 37.Farmer SG, Willikins DE, Meeker SA, Seeds EA, Page CP: Effects of bradykinin receptor antagonists on antigen-induced respiratory distress, airway hyperresponsiveness and eosinophilia guinea-pigs. Br J Pharmacol 1992, 107:65-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krueger J, Ray A, Sehgal PB: Expression and function of interleukin-6 in epithelial cells. J Cell Biochem 1991, 45:327-334 [DOI] [PubMed] [Google Scholar]
- 39.Ulich TR, Yin S, Guo K, Yi ES, Remick D, Del Castillo J: Intratracheal injection of endotoxin and cytokines. II. Interleukin-6 and transforming growth factor-β inhibit acute inflammation. Am J Pathol 1991, 138:1097-1101 [PMC free article] [PubMed] [Google Scholar]
- 40.Jones KP, Reynolds SP, Capper SJ, Kalinka S, Edwards JH, Davis BH: Measurement of interleukin-6 in bronchoalveolar lavage fluid by radioimmunoassay: differences between patients with interstitial lung disease and control subjects. Clin Exp Immunol 1991, 83:30-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grigg JM, Barber A: Increased levels of bronchoalveolar lavage fluid interleukin-6 in preterm ventilated infants after prolonged rupture on membrane. Am Rev Respir Dis 1992, 145:782-786 [DOI] [PubMed] [Google Scholar]
- 42.Soliman DM, Twigg HII: Cigarette smoking decreases bioactive interleukin-6 secretion by alveolar macrophages. Am J Physiol 1992, 236:L471-L478 [DOI] [PubMed] [Google Scholar]
- 43.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB: Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA 1987, 84:5788-5792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wakefield LM, Smith DM, Masui T, Harris CC, Sporn MB: Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol 1987, 105:965-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang JM, Chen ZG, Colella S, Bonilla MA, Welte K, Bordignon C, Mantovani A: Chemotactic activity of recombinant human granulocyte colony-stimulating factor. Blood 1988, 72:1456-1480 [PubMed] [Google Scholar]