Abstract
A rat model of common bile duct ligation (BDL)-induced hepatic fibrosis was used to assess the expression and activities of collagen-degrading proteinases and their inhibitors during the progression of fibrosis. Expression of four members of the matrix metalloproteinase (MMP) family (MMP-2/gelatinase A, MMP-3, MMP-9/gelatinase B, and MMP-13) and three tissue inhibitors of metalloproteinases-1, -2, and -3 (TIMP-1, TIMP-2, and TIMP-3) were evaluated by Northern blot analysis of RNA from liver tissue isolated at 0, 2, 5, 10, 20, and 30 days after either a BDL or sham operation. In addition, we analyzed free gelatinase and TIMP activities by zymography and reverse zymography, respectively. We found that the proteolytic activities of MMP-2 and MMP-9 increased by 2 days after ligation, reached maximal levels at day 10, and remained high through the study period, whereas the gelatinolytic activities in plasma were unchanged. The increase in gelatinase activities was accompanied by an increase in the TIMP mRNA transcripts. TIMP-1 transcripts appeared at day 2, increased until day 10, and remained elevated throughout the study period. TIMP-2 and TIMP-3 transcripts become detectable on day 10 and remained stable afterwards. No corresponding increase in TIMP protein activity was detected by reverse zymography. This appears to result from the formation of TIMP/MMP complexes. These findings indicate a likely surplus in the BDL model of fibrosis of free gelatinases as compared with the TIMPs. Thus, excessive TIMP production is not a sufficient explanation for the observed extracellular matrix accumulation, but complex changes in the local MMP/TIMP balance may underlie the pathomechanisms of fibrosis.
Hepatic fibrosis is a condition that results in loss of normal liver cell function due to the disorganized over-accumulation of extracellular matrix (ECM) components in the liver. It is clear that the increased production of ECM components is responsible for the altered ECM metabolism in the fibrotic liver. 1 However, deregulation of the enzymatic machinery involved in ECM degradation may also be an important contributing factor in the pathogenesis of hepatic fibrosis and cirrhosis.
The main components of the extracellular matrix in normal liver are collagen types I, III, IV, V, and VI, although other types of collagen are present in smaller proportions. There are also many noncollagenous components, including fibronectin, laminin, tenascin, undulin, and entactin. 1 In a normal liver, these matrix components are constantly remodeled by matrix-degrading enzymes leading to a controlled deposition of matrix components. Of the several families of ECM-degradative enzymes, the matrix metalloproteinases (MMPs) are the most important, as collectively MMPs can degrade all of the protein components of the ECM. 2-4 MMPs are classified into four groups: 1) collagenases, which cleave collagen at a specific site in triple helical collagen fibrils resulting in fragments that are susceptible to thermal denaturation into gelatin, which can then be acted on by other groups of MMPs, predominantly the gelatinases, 2) gelatinases, which include gelatinase A (MMP-2) and gelatinase B (MMP-9), 3) stromelysins, and 4) The RXKR-motif-containing sub-family, which includes the membrane-type MMPs (MT-MMPs). 5 The activities of MMPs are regulated at three levels, namely, transcription and zymogen activation and through the action of a family of inhibitory proteins, ie, the tissue inhibitors of metalloproteinases (TIMPs). The TIMPs interact with a 1:1 stoichiometry with MMPs to inhibit their activity. 2,3
There have been four members of the TIMP family identified so far, designated TIMP-1, -2, -3, and -4. 6-9 The action of the TIMPs is considered to be quite broad, as TIMP-1, -2, and -3 are indistinguishable in their MMP-inhibitory abilities in solution-based assays. 7 Although there appears to be a similarity in activity of the TIMPs, there are differences in localization and regulation. TIMP-1 and TIMP-3 are inducible in response to phorbol myristate acetate (PMA) and many growth factors. 6,10-12 Alternatively, TIMP-2 is largely constitutively expressed in most cell types.
During the course of fibrosis and cirrhosis, all of the liver matrix proteins increase in abundance, but to varying extents. 13-15 Collagen I is the most up-regulated, with the percentage composition in the liver increasing 5- to 10-fold. 1 One recent study has focused on the expression levels of interstitial collagenase with respect to those of TIMP-1 in the bile duct ligation (BDL) and carbon tetrachloride models of experimental fibrosis. 16 This study found an increase in TIMP-1, whereas interstitial collagenase levels remained unchanged, suggesting that increased matrix deposition occurs due to the disruption of the balance between MMPs and TIMPs in favor of the inhibitors. Another group has analyzed the involvement of gelatinases in the dynamic alterations occurring during fibrosis, 17 finding an increase in both active and latent MMP-2 protein by gelatin zymography. These results collectively imply that normal maintenance of the ECM is disrupted, resulting in a disorganized and overabundant extracellular scaffold with a consequent impairment of liver cell functions.
To obtain a more detailed analysis of the dynamic interactions occurring between the MMPs and TIMPs during experimental hepatic fibrosis, we have systematically studied expression levels of MMP-2, -3, -9, and -13 and protein activity of MMP-2 and -9 as well as TIMP-1, -2, and -3 protein activity and expression at the mRNA level. Our data demonstrate that changes in the gelatinolytic activities, and TIMPs, occur rapidly after BDL and are sustained during the initial month of fibrogenesis. We discuss the implications of these findings and the effects of imbalance of gelatinase/TIMP levels in reference to hepatic fibrosis in a BDL model.
Materials and Methods
Animals
The BDL protocol was approved by the University of Calgary Faculty of Medicine Animal Care Committee, and all animals were treated humanely in accordance with guidelines established by the Canadian Council on Animal Care.
Male Sprague-Dawley rats weighing 300 to 350 g were divided into three groups (three animals in each): 1) normal, 2) bile duct ligated, sacrificed at 2, 5, 10, 20, and 30 days after surgery, and 3) sham-operated rats, sacrificed at the same intervals. The animals were fed and watered ad libitum throughout the experiment. BDL is a well characterized and widely used model of experimental fibrosis and was performed in this study as previously described. 18 In brief, under halothane anesthesia, through a midline laparotomy, the extrahepatic common bile duct was double ligated with 30 silk and sectioned between the ligatures. The abdominal incision was sutured with silk, and the rats were allowed to recover in individual cages. Sham operated rats were treated in the same manner except that the bile duct was merely identified and gently manipulated but not ligated or sectioned. On the day of the studies, the rats were anesthetized with sodium pentobarbital (60 mg/kg intraperitoneally). The abdomen was opened through a midline incision, and before sacrifice, 1 ml of blood was obtained from the inferior vena cava using a 20-gauge needle. The blood was collected in heparinized tubes without EDTA and centrifuged, and the plasma was aliquotted and frozen at −70°C until testing.
The livers were flushed with 10 ml of cold phosphate-buffered saline through the portal vein to eliminate MMPs and TIMPs present in the blood. Liver fragments of 0.5 cm 3 were dissected out fresh and snap-frozen in plastic vials in liquid nitrogen.
Protein Isolation, Zymography, and Reverse Zymography
Frozen liver tissue in protein extraction buffer (1% Triton X-100, 500 mmol/L Tris/HCl, pH 7.6, 200 mmol/L NaCl, and 10 mmol/L CaCl2) was homogenized using an IKA-Ultra-Turrax T25 homogenizer. The homogenate was centrifuged at 12,000 rpm for 30 minutes at 4°C, and the supernatant was transferred to a clean tube and stored at −70°C. Homogenate protein content was determined using the Bradford procedure. 19 Proteins were analyzed for gelatinolytic activity by gelatin zymography using the previously described procedures. A total of 20 μg of protein extract or 5 μl of plasma (1/25 dilution) was separated by a 10% polyacrylamide gel, prepared as described. 20 Gelatin was included to a final concentration of 1 mg/ml. After electrophoresis, the gel was washed once for 15 minutes and then overnight in wash buffer (2.5% Triton X-100, 50 mmol/L Tris/HCl, pH 7.5, and 5 mmol/L CaCl2) to remove the sodium dodecyl sulfate (SDS). The gel was then rinsed three times in water, followed by a 24-hour incubation at 37°C in incubation buffer (50 mmol/L Tris/HCl, pH 7.5, and 5 mmol/L CaCl2). The gel was then stained for 4 hours in Coomassie Brilliant Blue. Reverse zymography was similarly performed with the exception that conditioned medium from baby hamster kidney (BHK) cells, which express gelatinase A, was included in the gel mix with the gelatin (1 ml of conditioned medium was added to 15 ml of the gel mix). After regeneration, the gelatinase A degrades the gelatin in all regions of the gel except in regions where there is TIMP activity. All washes and incubations were the same as zymography. As a positive control for zymography, conditioned medium from BHK cells expressing MMP-2 and transfected with MMP-9 was used. For reverse zymography, conditioned medium from BHK cells transfected with mouse TIMPs was used.
Complete gels were photographed, and the photos then digitized on a Hewlett Packard Scanjet 4c scanner. The images were then analyzed and quantified using the image analysis program NIH Image (National Institutes of Health, Bethesda, MD).
Western Blot
For Western blotting, 20 μg of protein from each sample were boiled for 3 minutes in the presence of SDS gel-loading buffer with 0.1 mol/L dithiothreitol and electrophoresed through a 12% SDS-polyacrylamide gel electrophoresis (PAGE) gel. The gel was run at 75 mA at room temperature. The proteins were transferred to nitrocellulose, blocked with 5% nonfat milk in Tris-buffered saline/Tween 20 (TBS-T), and incubated with 1:5000 diluted anti-mouse TIMP-1 antibodies (gift of Dr. G. Murphy, School of Biological Science, University of East Anglia, Norwich, UK) in TBS-T for 1 hour at room temperature. After washing, the blots were incubated with 1:5000 diluted rabbit anti-sheep antibody (catalog item 31480, Pierce, Rockford, IL) in TBS for 1 hour at room temperature. After repeated washes, the membranes were incubated in equal volumes of detection reagents 1 and 2 (ECL Western blotting detection reagents from Amersham Life Science, Arlington Heights, IL) for 1 minute at room temperature and immediately exposed to x-ray film. To confirm the specificity of binding, the anti-mouse TIMP-1 antibodies were preincubated for 2.5 hours with a Western blot containing 500 μl of 25X concentrated conditioned medium from BHK cells transfected with TIMP-1. Two Western blots were prepared under identical conditions. One was incubated with nonabsorbed antibodies and the other with the preabsorbed antibodies as described above.
RNA Isolation and Northern Hybridization
Total RNA was isolated by the acid-guanidinium-phenol-chloroform procedure of Chomczynski and Sacchi. 21 Ten micrograms of total RNA was electrophoresed on a 0.8% denaturing agarose gel and then transferred to nylon membranes (Duralon-UV, Stratagene, La Jolla, CA). Equal loading was determined by methylene blue staining of the membrane for 15 minutes and analysis of 28 S and 18 S rRNA. The membranes were then prehybridized in buffer containing 5X SSC, 50% (v/v) formamide, 10X Denhardt’s solution, and 0.1% SDS and then hybridized with nick-translated probes (specific activity, >10 8 cpm/μg) and washed in SDS/SSC solutions at 42°C (the highest stringency was 0.2X SSC/0.1% SDS). The probes used were generated from mouse TIMP-1, -2, and -3 plasmids, which have been described previously. 6,22 A partial mouse 72-kd gelatinase (MMP-2) clone was obtained by reverse transcription polymerase chain reaction from RNA isolated from mouse C3H 10T1/2 fibroblasts. The following oligonucleotides were used: 5′-GGCCCTGTCACTCCTGAGAT, corresponding to nucleotides 1337 to 1356 of the human MMP-2 sequence published by Collier et al 23 and 5′-oGCATCCAGGTTATCGGGGA, which is the reverse complement of the sequence from 1791 to 1810 of the same human sequence. The 475-bp polymerase chain reaction product was cloned into the EcoRI/HindIII sites of pGEM2 with the 5′end of the cDNA at the T7 promoter end of the multipurpose cloning site. The mouse gelatinase B (MMP-9) 3160-bp cDNA clone was a gift of Dr. Kenji Sugita, Division of Oncology, Department of Microbiology, Shionogi Research Laboratories, Osaka, Japan, and was described previously. 24 The rat stromelysin-1 (MMP-3) cDNA clone used was a 600-bp fragment generated by EcoRI and HindIII digestion of a full-length clone provided by Dr. Lynn Matrisian, Vanderbilt University, Nashville, TN. This fragment was cloned into pGEM-2 with the 5′ and 3′ ends of the insert in the EcoRI and HindIII sites of the vector, respectively. The mouse collagenase-3 (MMP-13) cDNA clone was a full-length construct designated pCLM11, which was a gift of Dr. Yves Eeckhout, University Catholique de Louvain, Brussels, Belgium.
Histology
Fragments of livers were formalin fixed, processed for routine histology, and stained with hematoxylin and eosin, Sirius red, and Gomori’s trichrome.
Statistical Analysis
Differences between MMP-2 and -9 protein expressions in different groups were calculated by two-tailed between-groups t-tests using SPSS 4.0 for Macintosh.
Results
Gelatinase and TIMP Expression in BDL Liver Tissue
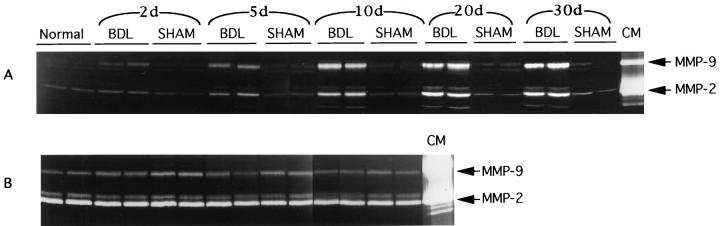
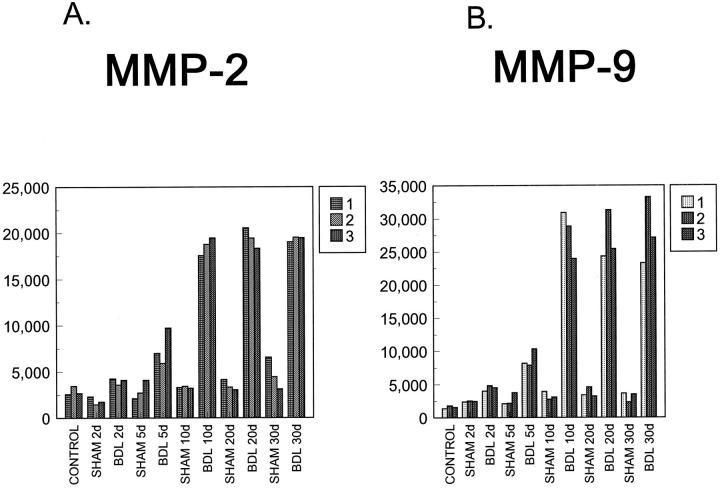
We have studied the levels of expression of MMP-2, -3, -9, and -13 and TIMP-1, -2, and -3 mRNAs in liver tissue from animals that have undergone BDL (three animals per group, at 2, 5, 10, 20, and 30 days after surgery) compared with sham-operated controls (same postoperative days as BDL). The study time course (2 to 30 days) was chosen to include both early and late fibrotic events. A very weak signal was seen for MMP-2 and -9 that was below publishable level. It showed no detectable differences between any of the groups. MMP-3 and -13 mRNA transcripts were not detected in any of the samples by Northern blot analysis (data not shown). This likely reflects low mRNA expression levels. Gelatin zymography analysis showed active MMP-2 and MMP-9 proteins (representative data shown in Figure 1A ▶ ). Activities of both gelatinases increased in BDL livers at day 2 after BDL, and a steady increase was noted until day 10, remaining stable afterwards (Figure 2, A and B) ▶ . Activities of MMP-2 and -9 were higher in BDL livers than in livers from control animals (not treated surgically) and from sham animals. For MMP-2, statistically significant differences were observed between control animals versus 10-day, 20-day, and 30-day BDL animals; differences were also seen between 2-day, 10-day, 20-day, and 30-day sham versus BDL animals. Additional differences were found and between 10-day, 20-day, and 30-day BDL versus 2-day and 5-day BDL animals (P ≤ 0.01). No significant differences were observed between days 10, 20, and 30 in BDL animals. For MMP-9, statistically significant differences were noted between control animals versus BDL 2-day, 5-day, 10-day, 20-day, and 30-day animals, between sham versus BDL 5-day, 10-day, 20-day, and 30-day animals, and between BDL 10-day, 20-day, and 30-day versus BDL 2-day and 5-day animals (P ≤ 0.01). A less significant difference (P = 0.012) was observed between 2-day BDL versus 2-day sham animals. No significant differences were noted between 10-day, 20-day, and 30-day BDL animals. Activated forms (lower molecular bands) of MMP-2 and -9 were observed in BDL animals (Figure 1A) ▶ . The appearance of increased levels of the active forms likely reflects a general increase in expression of gelatinase proforms and is therefore not the result of MMP activation in the post-BDL liver.
Figure 1.
Gelatin zymography analysis for gelatinolytic activities in liver tissue protein extracts (A) and plasma (B). Total protein extracts were isolated from normal (unmanipulated animals) 2, 5, 10, 20, and 30 day after sham and BDL rat livers, and plasma was obtained from the sham and BDL animals at days 2, 5, and 10 after surgery. Twenty micrograms of proteins and 5 μl of plasma were loaded onto SDS-PAGE gels and processed as described in Materials and Methods. Conditioned medium (CM) from BHK cells expressing MMP-2 and MMP-9 was included as a control for size comparison with sample gelatinolytic bands.
Figure 2.
MMP-2 (A) and MMP-9 (B) gelatinolytic activities in liver tissue protein extracts. Gelatinolytic activities were measured by gelatin zymography (shown in Figure 1 ▶ ). The photographs of the gels were digitized on a Hewlett Packard Scanjet 4c scanner. The images were analyzed and quantified using the image analysis program NIH Image. Control indicates unmanipulated animals.
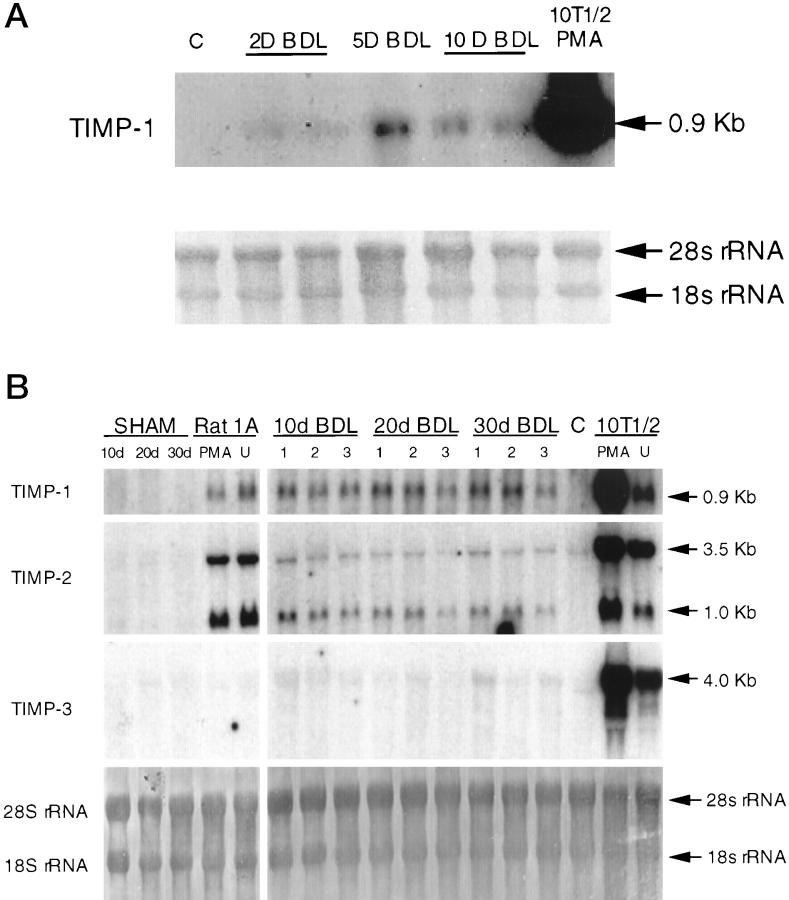
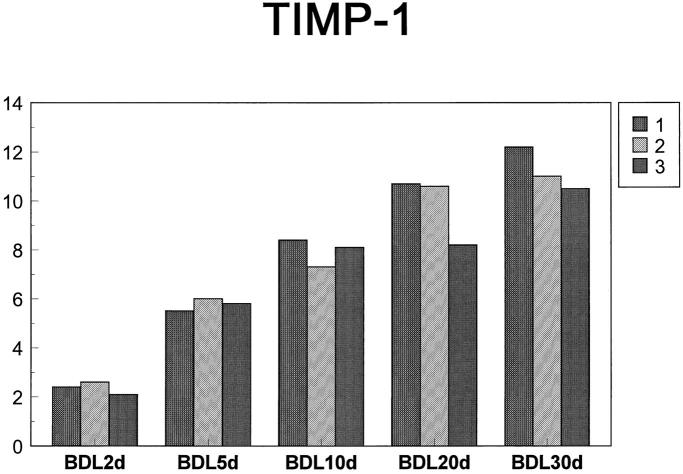
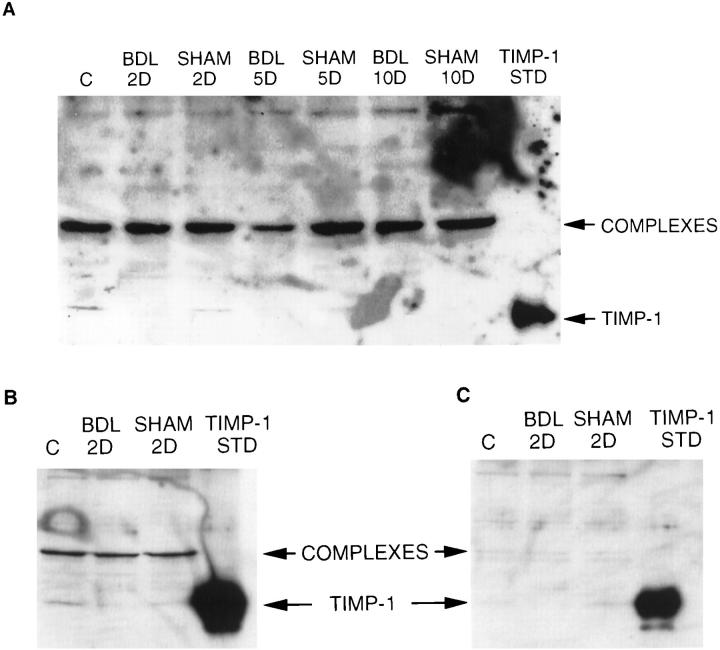
Northern blot analysis of TIMP mRNA transcripts showed an increase in TIMP-1 after BDL surgery (Figure 3, A and B) ▶ . TIMP-1 transcripts appeared on day 2 after BDL, increased until day 10 after BDL, and remained relatively stable afterwards (Figures 3 and 4) ▶ ▶ . The comparison of the densitometrically measured signals on the Northern blots showed statistically significant overexpression of TIMP-1 between BDL day 2 and BDL day 5 (P ≤ 0.0025) and between BDL day 5 and BDL day 10 (P ≤ 0.043). There were no significant differences between BDL day 10 and BDL day 20 levels or between BDL day 20 and BDL day 30 levels. A statistically significant difference was seen, however, when BDL day 10 levels were compared with BDL day 30 levels (p ≤ 0.018). No TIMP-1 transcripts were detected in control and sham-operated animals. Expression of TIMP-2 increased at 10 days after BDL and was not detected at either of the two earlier points, 2 and 5 days (data not shown). Of the two transcripts (3.5 and 1.0 kb), mainly the 1.0-kb transcript was increased in BDL livers (Figure 3B) ▶ . This lower molecular weight transcript was not detectable in the control animals, but it was present 10 days after BDL surgery, and the expression level remained constant until 30 days after BDL. Sham-operated animals had very low levels of both 3.5- and 1.0-kb transcripts. A slight up-regulation of TIMP-3 mRNA was also seen (Figure 3B) ▶ , although this was not as prominent as the changes seen for TIMP-1 and TIMP-2. Quantification of free TIMP activities in liver tissue by gelatin reverse zymography (Figure 5A) ▶ revealed that the changes in TIMP mRNA were not mirrored at the level of protein activity. The most prominent TIMP activity present in livers of control, sham-operated, and BDL animals co-migrated with unglycosylated TIMP-3 standard. Samples were also run on conventional SDS-PAGE gels (without reduction) that were Coomassie Blue stained to confirm that the bands were bona fide TIMP activities rather than abundant proteins (data not shown). The activity of TIMP-3 was unaffected by BDL surgery, as was also the case for TIMP-2 (Figure 5A) ▶ . No TIMP-1 activity was detected by reverse zymography (Figure 5A) ▶ , and Western blot analysis demonstrated that this TIMP was mainly present in the form of complexes (Figure 6) ▶ , which likely interferes with its detection by activity assays. The Western blots indicated the presence of TIMP-1 complexes, both in post-BDL and sham livers (Figure 6) ▶ . Free TIMP-1 (lower band) was seen only in normal controls and in sham-operated animals. Lack of a detectable free band in the BDL groups suggests that most of the produced TIMP-1 enters the complexes. Thus, little if any free TIMP-1 is available to exert its activities. These data agrees with the reverse zymography results, which failed to demonstrate any TIMP-1-related activity. The analysis of the composition of the TIMP-1 complexes seen on the Western blot is beyond the scope of this paper.
Figure 3.
TIMP expression in BDL livers. Northern blot analysis of total RNA from rat liver tissue at 2, 5, and 10 days after BDL (A) and 10, 20, and 30 days after BDL (B). Ten micrograms RNA from normal liver (C) was included as a control in addition to mouse (10T1/2) and rat (Rat 1A) fibroblasts either unstimulated (U) or phorbol ester (phorbol myristate acetate) treated. Filters were hybridized to nick-translated mouse TIMP-1, -2, and -3 probes as described in Materials and Methods. As a loading control, the filters were stained with methylene blue, which allows visualization of 28 S and 18 S rRNAs.
Figure 4.
TIMP-1 expression in BDL livers. TIMP-1 mRNA levels were measured by Northern blot (shown in Figure 3 ▶ ). The gels were photographed, and the photos were then digitized on a Hewlett Packard Scanjet 4c scanner. The images were then analyzed and quantified using the image analysis program NIH Image. TIMP-1 values were normalized to 28 S rRNA values.
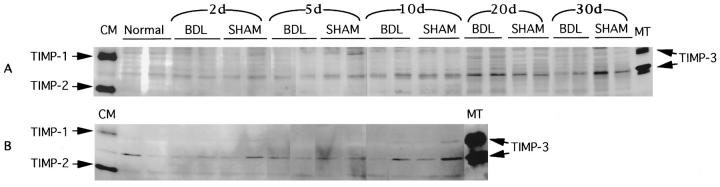
Figure 5.
Reverse gelatin zymography analysis for inhibition of gelatinolytic activities in liver tissue protein extracts (A) and plasma (B). Proteins from liver tissue extracts and plasma were loaded onto SDS-PAGE gels and processed for reverse gelatin zymography as described in Materials and Methods. Tissue from unmanipulated rats (normal) was included as a control. Conditioned media from TIMP-1/TIMP-2-expressing BHK cells (CM) or TIMP-1/TIMP-3-overexpressing BHK cells (MT) were included as size markers for identification of TIMP proteins. The divergent migration patterns of TIMP-1 and -2 result from their different glycosylation (TIMP-1 is double glycosylated; TIMP-3 exists in glycosylated and nonglycosylated forms, hence two separate bands).
Figure 6.
Western blot analysis of TIMP-1 expression in BDL and sham livers. A: Western blot was performed as described in Materials and Methods. Polyclonal anti-mouse TIMP-1 antibodies (gift from Dr. G. Murphy, School of Biological Science, University of East Anglia, Norwich, UK) were used. The position of TIMP-1 was established by comparison with a standard (TIMP-1, STD-conditioned medium from BHK cells transfected with mouse TIMP-1; C, control normal liver). B and C: Two Western blots were prepared at the same time under the same conditions, containing the same samples. The blot on the left (B) was incubated with anti-mouse TIMP-1 antibodies as in A, and the blot on the right (C) with antibodies preabsorbed with TIMP-1-rich conditioned medium (as described in the Materials and Methods). The greater than 10-fold reduction in the intensity of TIMP-1 signal, after TIMP-1-rich conditioned medium preabsorption, indicates the specificity of antibody binding.
Plasma TIMP and MMP Activities in BDL Rat Livers
Because the observed increases in MMP-2 and MMP-9 activity might be attributable to inflammation and changes in MMP levels in the plasma, we tested plasma gelatinase and TIMP levels in BDL rats. We observed no detectable change in plasma gelatinase levels after BDL or sham surgery (Figure 1B) ▶ . Plasma TIMP levels, however, did seem to be variable (Figure 5B) ▶ , but this variability showed no pattern with respect to the BDL surgery and likely represents animal-to-animal variation. TIMP-1 is undetectable except in a few samples, including both BDL and sham-operated animals. TIMP-3 shows similar variability, again with no correlation to surgery, whereas TIMP-2 levels are constant irrespective of which treatment was given. These results demonstrate that the changes in TIMP and MMP levels detected in this study are attributable to actual changes within the liver and are not due to plasma enzymatic activity changes in MMP and TIMP levels within the circulating blood.
Histology
After BDL (days 2 and 5), there was widening of portal triads due mainly to bile ductular proliferation and the presence of inflammatory infiltrate. Periportal fibrosis occurred around days 10 to 14 after BDL and progressed to established fibrosis.
Discussion
The net deposition of the ECM, which occurs during hepatic fibrosis, has generated a great deal of interest in ECM-regulatory enzymes and proteins. We have elected to study the effect of experimental hepatic fibrosis on gelatinase expression and activity, as compared with their inhibitors. Although several papers reported the expression and activity of MMPs and TIMPs in both BDL and carbon tetrachloride injury, we have approached the BDL model from a point of view of MMP/TIMP interactions. This approach differs from previous studies that generally examined MMPs and their inhibitors in isolation. The importance of studying the MMPs and TIMPs as an interactive group becomes apparent after analyzing the complex interactions that occur in the process of MMP regulation and activation. MMPs are regulated at the transcriptional level, resulting in changes in the net amounts of protein present. There are also two other mechanisms of regulation: first, the MMPs are secreted as inactive proenzymes requiring activation to obtain enzymatic activity, and second, TIMP/MMP complexes form that block the degradative function of MMPs. Our previous data have shown that there may be no correlation between MMP mRNA expression and enzymatic activity, likely due to either a block in the MMP activation pathway or to the formation of complexes with the TIMPs. 25 This indicates that an increase in gene expression does not always translate into increased activity.
Our study is the first to examine the expression of TIMPs at the level of mRNA expression and protein activity in conjunction with the gelatinolytic activity of MMPs. Expression of both TIMP-1 and TIMP-2 mRNA levels were increased in liver samples after an induction of experimental hepatic fibrosis relative to sham or control tissue; however, there was no detectable change in TIMP-3. These results are in agreement with previously reported data. 26,27 These data lend support to the hypothesis that increases in TIMP expression may contribute to the net deposition of ECM in the fibrotic liver. At the level of protein activity, however, we were surprised by the lack of change in TIMP activities after BDL in light of the increased expression of TIMP-1 and TIMP-2 mRNA. These seemingly contradictory results can be explained by looking at the dynamic interaction between the MMP family of enzymes and their inhibitors. TIMP-1 and -2 are known to form specific complexes with the proenzyme forms of MMP-9 and MMP-2, respectively. 28,29 Therefore, the overexpression of both MMP-2 and MMP-9, as shown by zymographic detection of elevated levels of free latent and active forms of the gelatinases in livers of BDL animals, might be sequestering the available TIMP into MMP/TIMP complexes. This would not allow the TIMP to be identified using reverse gelatin zymography, thus accounting for the lack of correlation between TIMP activity and mRNA levels. This is a likely explanation in view of the fact that MMP-2 and TIMP-2 were shown by Herbst et al 26 to co-localize in liver sections. The demonstration of free TIMP-1 in the sham groups but only TIMP-1 in complexes in the BDL animals in our Western blot studies (Figure 6) ▶ further support such a mechanism. It is also theoretically possible that the lack of change in TIMP activity may reflect a translational control mechanism, but no precedent for this has been seen in any studies of TIMP gene regulation.
To expand the study of possible interactions between MMPs and TIMPs, we also looked at the expression of MMP-3 and -13, but no signal was detected for either of the two enzymes in any of the groups. Because of the inflammation and tissue trauma associated with BDL surgery, we were concerned that the observed changes in MMP and TIMP activity might be due to increased gelatinolytic or inhibitory activity within the blood. Such a phenomenon has been detected previously in association with hepatic disease, where an increase in serum TIMP-1 has been identified in human patients with chronic hepatitis, alcoholic cirrhosis, and primary biliary cirrhosis. 30,31 Increased levels of gelatinases in the circulation could be due to MMPs released from inflammatory cells, polymorphonuclear leukocytes in particular, or from liver-specific cells, either parenchymal or nonparenchymal.
To rule out the possibility that the increases in gelatinolytic activity were not from contamination of gelatinase- or TIMP-rich plasma or associated with an inflammatory response, several measures were taken to control plasma levels in the liver samples. First, the liver samples were perfused with saline before harvesting to eliminate most of the blood. Histological examination of BDL liver demonstrates that the polymorphonuclear portal infiltrate was seen by day 2 and did not increase appreciably (data not shown). In view of increasing gelatinolytic activity throughout the period of the study, this observation further argues against the role of leukocytes as the main source of active MMPs. Additionally, plasma from both BDL and sham-operated animals was analyzed for any alterations in gelatinolytic activity after induction of experimental hepatic fibrosis. Gelatin zymography and reverse gelatin zymography demonstrated no change in gelatinolytic activity or any TIMP activity in the plasma after BDL surgery. Therefore, we postulate that the observed changes in the gelatinolytic activity in the liver tissue are independent of the gelatinase levels in circulating blood. Although TIMP-1 is detectable in serum of patients with hepatic disease, we found no recognizable changes in the activity of the plasma in the BDL model of hepatic fibrosis. Such discrepancy between the reported results from human sera and our analysis may simply result from the duration of the process. The patients with hepatic disease have a chronic process of several years duration or more, whereas the animal samples represent fibrosis of up to 1 month duration only.
Recent studies have clearly demonstrated the cellular sources responsible for the increased MMP and TIMP expression 26,32-34 and suggested the co-localization of the intracytoplasmic MMP-2/TIMP signals, indicating the formation of MMP-2/TIMP-2 complexes. These complexes may act synergistically with TIMP-1 in its inhibition of MMP-1. 26
The results of our study are in agreement with the previous reports indicating marked alterations in the expression of the MMPs and their inhibitors after BDL, which takes place within the first week after BDL. We have demonstrated a net increase in gelatinolytic activity without any corresponding increases in detectable TIMP activity. These findings suggest that increased TIMP expression does not result in increased inhibitory activity, and free TIMPs are therefore not sufficient to effect an increased matrix deposition during hepatic fibrosis. Detection of the formation of MMP/TIMP complexes, seen in previous studies, suggests that part of the inhibitory activities of MMPs are likely related to the presence of MMP-2/TIMP-2 complexes. In view of previously generated data, which shows up-regulation of MMP-2 and MMP-9 up to 100 days of experimental fibrosis, 17 it is likely that fibrosis is the result of excessive collagen deposition, which is not readily degraded by increased levels of gelatinases. As previous groups 15,27 have observed a lack of up-regulation of MMP-1 (interstitial collagenase) after experimental hepatic fibrosis (in agreement with our present study) or autopsy material from human cirrhotic and fibrotic livers, it is possible that increased collagen (mainly type I) is deposited, but it makes a poor substrate for the gelatinases without previous processing by MMP-1.
We have shown that important information can be gained by a comparison between mRNA expression and functional protein activity. In the systems characterized by complex interactions, the overexpression at the mRNA level does not always indicate increased biological activity. Through a detailed examination of the two major components of the ECM remodeling system, the MMPs and the TIMPs, we have demonstrated the importance of studying all of the components of such a system rather than its individual elements.
Footnotes
Address reprint requests to Dr. Stefan J. Urbanski, Foothills Provincial General Hospital, 1403 29 Street N.W., Calgary, AB, Canada T2N 2T9. E-mail: stefan.urbanski@crha-health.ab.ca.
Supported by funding from operating grants from the Medical Research Council of Canada to S.S. Lee, A.E. Kossakowska, and D.R. Edwards and from the Human Frontier Science Program to D.R. Edwards. D.R. Edwards is a senior scholar of the Alberta Heritage Foundation for Medical Research. B.W. Phillips is the recipient of a studentship from the Medical Research Council of Canada.
References
- 1.Schuppan D: Structure of the extracellular matrix in normal and fibrotic liver: collagens and glycoproteins. Semin Liver Dis 1990, 10:1-10 [DOI] [PubMed] [Google Scholar]
- 2.Matrisian L: Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet 1990, 6:121-125 [DOI] [PubMed] [Google Scholar]
- 3.Birkedal-Hansen H, Moore WGI, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA: Matrix metalloproteinases: a review. Clin Rev Oral Biol Med 1993, 4:197-250 [DOI] [PubMed] [Google Scholar]
- 4.Woessner JF: Matrix metalloproteinases and their inhibitors in connective tissue remodelling. FASEB J 1991, 5:2145-2154 [PubMed] [Google Scholar]
- 5.Sato H, Takino T, Okada Y, Cao J, Shinagawa A, Yamamoto E, Seiki M: A matrix metalloproteinase expressed on the surface of invasive tumour cells. Nature 1994, 370:61-65 [DOI] [PubMed] [Google Scholar]
- 6.Leco KJ, Khoka R, Pavloff N, Hawkes SP, Edwards DR: Tissue inhibitor of metalloproteinases (TIMP-3) is an extracellular matrix-associated protein with a distinctive pattern of expression in mouse cells and tissues. J Biol Chem 1994, 269:9352-9360 [PubMed] [Google Scholar]
- 7.Apte SS, Olsen BR, Murphy G: The gene structure of tissue inhibitor of metalloproteinases (TIMP-3) and its inhibitory activities define the distinct TIMP gene family. J Biol Chem 1995, 270:14313-14318 [DOI] [PubMed] [Google Scholar]
- 8.Stetler-Stevenson WG, Krutzsch HC, Liotta LA: TIMP-2: identification and characterization of a new member of the metalloproteinase inhibitor family. Matrix Suppl 1992, 1:299-306 [PubMed] [Google Scholar]
- 9.Leco KJ, Apte SA, Taniguchi GT, Hawkes SP, Khokha R, Schultz G, Edwards DR: Murine tissue inhibitor of metalloproteinases-4 (TIMP-4): cDNA isolation and expression in adult mouse tissues. FEBS Lett 1997, 401:213-217 [DOI] [PubMed] [Google Scholar]
- 10.Edwards DR, Waterhouse P, Holman ML, Denhardt DT: A growth responsive gene (16C8) in normal mouse fibroblasts homologous to a human collagenase inhibitor with erythroid-potentiating activity: evidence for inducible and constitutive transcripts. Nucleic Acids Res 1986, 14:8863-8878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards DR, Rocheleau H, Sharma R, Wills AJ, Cowie A, Hassel JA, Heath JK: Involvement of AP-1 and PEA3 binding sites in the regulation of murine tissue inhibitor of metalloproteinases-1 (TIMP-a) transcription. Biochem Biophys Acta 1992, 1171:41-55 [DOI] [PubMed] [Google Scholar]
- 12.Ponton A, Coulombe B, Steyaert A, Williams BR, Skup D: Basal expression of the gene (TIMP) encoding the murine tissue inhibitor of metalloproteinases is mediated through AP1- and CCAAT-binding factors. Gene 1992, 116:187-194 [DOI] [PubMed] [Google Scholar]
- 13.Arthur MJP: Matrix degradation in the liver. Semin Liver Dis 1990, 10:47-55 [DOI] [PubMed] [Google Scholar]
- 14.Arthur MJP, Iredale JP: Hepatic lipocytes, TIMP-1 and liver fibrosis. J R Coll Physicians Lond 1994, 28:200-208 [PMC free article] [PubMed] [Google Scholar]
- 15.Milani S, Herbst H, Schuppan D, Hahn E, Stein H: In situ hybridization for procollagen types I, III, and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology 1989, 10:84-92 [DOI] [PubMed] [Google Scholar]
- 16.Iredale JP, Benyon C, Arthur MJP, Ferris WF, Alcolado R, Winwood PJ, Clark N, Murphy G: Tissue inhibitor of metalloproteinases-1 messenger RNA in experimental liver injury and fibrosis. Hepatology 1996, 24:176-184 [DOI] [PubMed] [Google Scholar]
- 17.Takahara T, Furui K, Funaki J: Nakayama y, Itoh H, Miyabayashi C, Sato H, Seiki M, Ooshima A, Watanabe A: Increased expression of matrix metalloproteinases-II in experimental liver fibrosis in rats. Hepatology 1995, 21:787-795 [PubMed] [Google Scholar]
- 18.Lee SS, Huang M, Ma Z, Rorstad O: Vasoactive intestinal peptide in cirrhotic rats: hemodynamic effects and mesenteric arterial receptor characteristics. Hepatology 1996, 23:1174-1180 [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM: A rapid and sensitive method for the quantification of microgram quantities of protein utilizes the principle of protein dye binding. Anal Biochem 1980, 102:196-202 [DOI] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual. ed 2 1989, Cold Spring Harbor Laboratory Press, Cold Spring Harbor NY
- 21.Chomczynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 22.Leco KJ, Hayden LJ, Sharma RR, Rocheleau H, Greenberg AH, Edwards DR: Differential regulation of TIMP-1 and TIMP-2 mRNA expression in normal and Ha-ras-transformed murine fibroblasts. Gene 1992, 117:209-217 [DOI] [PubMed] [Google Scholar]
- 23.Collier IE, Wilhelm SM, Eisen AZ, Marmer BL, Grant GA, Seltzer JL, Kronberger A, He C, Bauer EA, Goldberg GI: H-ras oncogene-transformed human bronchial epithelial cells (TBE-1) secrete a single metalloprotease capable of degrading basement membrane collagen. J Biol Chem 1988, 14:6579-6587 [PubMed] [Google Scholar]
- 24.Tanaka H, Hojo K, Yoshida H, Yoshioka T, Sugita K: Molecular cloning and expression of the mouse 105-kd gelatinase cDNA+. Biol Biophys Res Commun 1993, 190:732-740 [DOI] [PubMed] [Google Scholar]
- 25.Urbanski SJ, Hinek A, Lim MS, Kossakowska AE: Expression of gelatinase A and B mRNA does not correlate with their enzymatic activity in human sporadic colorectal neoplasia. Lab Invest 1995, 72:70A7837793 [Google Scholar]
- 26.Herbst H, Wege T, Milani S, Pellegrini G, Orzechowski HD, Bechstein WO, Neuhaus P, Gressner AM, Schuppan D: Tissue inhibitor of metalloproteinase-1 and -2 RNA expression in rat and human liver fibrosis. Am J Pathol 1997, 150:1647-1659 [PMC free article] [PubMed] [Google Scholar]
- 27.Roeb E, Purucker E, Breuer B, Nguyen H, Heinrich PC, Rose-John S, Matern S: TIMP expression in toxic and cholestatic liver injury in rat. J Hepatol 1997, 27:535-544 [DOI] [PubMed] [Google Scholar]
- 28.Kolkenbrook H, Hecker-Kia A, Orgel D, Ruppitsch W, Ulrich N: Activity of ternary gelatinase A-TIMP-2 matrix metalloproteinase complex. Biol Chem Hoppe-Seyler 1994, 375:589-5995 [DOI] [PubMed] [Google Scholar]
- 29.Boker KHW, Albrecht KA, Karbakhsh-Raveri B, Buttner J, Manns MP, Lichtinghagen R: Expression of metalloproteinase-inhibitors TIMP-1, -2, -3 in normal and fibrotic human liver. Hepatology 1995, 22:370A [Google Scholar]
- 30.Murawaki Y, Yamamoto H, Kawasaki H, Shima H: Serum tissue inhibitor of metalloproteinases in patients with chronic liver disease and with hepatocellular carcinoma. Clin Chim Acta 1993, 218:47-58 [DOI] [PubMed] [Google Scholar]
- 31.Li J, Rosman AS, Lio MA, Nagai Y, Lieber CS: Tissue inhibitor of metalloproteinase is increased in the serum of precirrhotic and cirrhotic alcoholic patients and can serve as a marker of fibrosis. Hepatology 1994, 19:1418-1423 [PubMed] [Google Scholar]
- 32.Winwood PJ, Schuppan D, Iredale JP, Kawser CA, Docherty AJP, Arthur MJP: Kupffer cell-derived 95-kd type IV collagenase/gelatinase B: characterization and expression in cultured cells. Hepatology 1995, 22:304-315 [PubMed] [Google Scholar]
- 33.Theret N, Musso O, L’Helgoualc’h A, Clement B: Activation of matrix metalloproteinase-2 from hepatic stellate cells requires interactions with hepatocytes. Am J Pathol 1997, 150:51-58 [PMC free article] [PubMed] [Google Scholar]
- 34.Arthur MJP, Stanley A, Iredale JP, Rafferty JA, Hembry RM, Friedman SL: Secretion of 72 kd type IV collagenase/gelatinase by cultured human lipocytes. Biochem J 1992, 287:701-707 [DOI] [PMC free article] [PubMed] [Google Scholar]