Abstract
It is unclear whether the intracardial immune reactivity after heart transplantation influences the peripheral immunological status (activation or nonresponsiveness) of the patient. Co-stimulation and activation-induced cell death (AICD) or apoptosis play an important role in determining the balance between lymphocyte reactivity and nonreactivity. Therefore, we studied the expression of co-stimulatory molecules and the process of apoptosis in biopsies of human heart allografts, using immunohistochemistry. Although a normal expression of co-stimulatory molecules on antigen-presenting cells was observed, the expression of their counter-structures on T cells was absent. This may be due to chronic T cell activation, which can lead to the induction of apoptosis via the Fas/Fas ligand pathway. In the infiltrates, a considerable percentage of the lymphocytes, but not the macrophages, were apoptotic. Apoptosis was confirmed by DNA fragmentation analysis. Increased numbers of Bax-expressing versus decreased numbers of Bcl2-expressing lymphocytes in comparison with normal lymphoid tissue confirmed a imbalance in favor of apoptosis. Apoptosis was biased towards CD4+ T cells (65.7% versus 26.6% in CD8+ T cells). Fas was expressed on most of the infiltrating cells. Fas ligand expression was also observed, not only on most of the T cells but also on all macrophages. Because macrophages were often detected in close contact with T cells, they may play a role in T cell regulation via the Fas/Fas ligand pathway. This study indicates that, during rejection, not only is tissue damage induced by infiltrating T cells, but also the infiltrating lymphocytes themselves are actively down-regulated (eg, AICD) by one another and by macrophages in the infiltrate. This regulatory process may affect the immunological status of the patient after heart transplantation.
Despite improved immunosuppressive regimens, human heart transplantation is still complicated by acute rejection episodes. Acute rejection, a T-cell-mediated process, occurs most frequently during the first months after transplantation. Most studies have concentrated on the induction of cytotoxicity against the graft and the production of cytokines within the graft. However, little is known about the immune-regulatory mechanisms occurring within the transplanted organ.
During rejection, T cells enter the graft. Activation of these T cells requires two signals. In addition to the interaction between the T cell receptor and the major histocompatibility complex on the antigen-presenting cell (APC), a second signal is required, which is provided through co-stimulatory molecules, present on both the T cell and on the APC. 1,2 The two most common pathways of co-stimulation are mediated by B7-1/B7-2 on the APC and CD28 or CTLA4 on the T cell and by CD40 on the APC and CD40 ligand (CD40L) on the T cell.
Absence of a co-stimulatory signal during primary activation will lead to a state of anergy, in which the T cells are unable to respond to a renewed antigen challenge. This anergic state can result in apoptosis of the T cell. 3,4 The role of this pathway in anergy induction toward allografts has been shown in rodents and primates; blocking the co-stimulatory pathway by treatment with CTLA4 Ig or anti-CD40L leads to prolonged or permanent acceptance of the allograft. 5,6 Human lymphocytes can be anergized in vitro, using the same treatment. 7 Anergy induction in vivo may eventually lead to donor-specific nonresponsiveness, resulting in a reduction of the number of rejection episodes later after transplantation.
In heart transplant recipients, this nonresponsiveness has been shown to be accompanied by a reduction in the frequency of donor-specific precursor cytotoxic T lymphocytes. 8 The cytotoxic T cells are effector cells in the rejection process, causing tissue damage inside the graft. The cytotoxicity can be mediated via the secretion of granzyme and perforin but also via the interaction between Fas on the target cell and Fas-ligand (FasL) on the T cell. Both mechanisms induce apoptosis in the target cell. 9,10 Fas is expressed constitutively on several cell types, including mouse heart tissue, but also on T cells. 10 After activation of the T cell, Fas expression is up-regulated. At the same time, FasL expression is induced. FasL can induce apoptosis in a Fas-expressing target cell, including the T cell itself. Therefore, the Fas/FasL pathway is not only involved in cytotoxicity, but has also been described as a pathway to down-regulate an ongoing immune response, so-called activation-induced cell death (AICD). 9,11
The process of apoptosis is strictly regulated. Two important regulating proteins are Bcl2 and Bax, both members of the Bcl2 gene family. Bcl2 protects, whereas Bax induces, apoptosis. Both molecules are localized in the inner mitochondrial membranes, the endoplasmic reticulum, and the perinuclear membrane. 12,13 The different family members can homo- and heterodimerize with one another. As long as heterodimers are present in excess, Bcl2 prevents the induction of apoptosis. However, when the expression of Bax increases, resulting in the formation of Bax homodimers, this can lead to the induction of the apoptotic pathway. 14,15 Therefore, the stochastic ratio of these inhibitors and activators inside a cell determines the sensitivity of a cell to undergo programmed cell death.
Many of the above mentioned processes are well studied in in vitro experiments. However, little is known about the occurrence of these immune-regulatory mechanisms within the graft in vivo after transplantation. Therefore, we used endomyocardial biopsies (EMBs) from heart transplantation patients to study the role of T cells and macrophages in the processes of co-stimulation and apoptosis inside the graft. We developed a double-immunofluorescence technique with a high sensitivity, which can be used for confocal laser scan microscopy (CLSM). This enabled us to link the expression of certain molecules on certain cells with the phenotype of those cells. To study the role of co-stimulatory molecules on macrophages and lymphocytes in the activation of T cells, the expression of CD28, CTLA4, and CD40L on T cells and B7-1, B7-2, and CD40 on APCs was analyzed in relation to different grades of rejection. To analyze whether AICD plays a role in T cell regulation, apoptosis was evaluated using the terminal deoxynucleotidyl transferase (TdT)-mediated biotin-dUTP nick end labeling (TUNEL) method and DNA fragmentation. For the same reason, the expression of Fas and FasL and Bcl2 and Bax and the production of granzyme B and perforin was studied to evaluate which mechanism may be responsible for the apoptosis and tissue destruction during different grades of rejection.
This study indicates that lymphocytes, infiltrating the cardiac graft during acute rejection, are actively down-regulated via AICD) by macrophages and by other lymphocytes in the infiltrate. This may affect the balance between T cell activation and inactivation, thereby influencing the immunological status of the patient after heart transplantation.
Materials and Methods
Endomyocardial Biopsies
EMBs were obtained from patients during the first months after allogeneic heart transplantation. Paraffin-embedded biopsies were histopathologically examined for rejection, according to the criteria of the International Society for Heart and Lung Transplantation. 16 Extra biopsies were snap-frozen in liquid nitrogen for research purposes. For this study, frozen and paraffin-embedded biopsies were used with grades of rejection varying from grade 0 (no rejection) to grade 3B (severe rejection).
As grading of the rejection is not feasible on frozen tissue, the number of CD3+ T cells per mm 2 was counted, as reported previously. 17 Biopsies were grouped according to these T cell numbers, as follows: −, no cells; ±, 1 to 100 cells/mm2; +, 101 to 200 cells/mm2; ++, >200 cells/mm2. These groups generally correlated with grading on the parallel paraffin-embedded EMBs. Absolute numbers of macrophages are difficult to determine, because they intermingle with surrounding cells due to their irregular shape. Therefore, the numbers of macrophages were semiquantitatively correlated to the T cell numbers and are indicated as follows: ±, few macrophages; +, intermediate number of macrophages; ++, high number of macrophages. Their number increased corresponding with the number of T cells.
Because EMBs are too small for elaborate studies, heart tissue with rejection grade 3A or 3B, obtained from four patients after autopsy, was used to develop the methodology and for the double-staining experiments.
Immunohistochemistry
Antibodies were titrated on tonsil and heart tissue to obtain optimal dilutions (Table 1) ▶ . For a basic evaluation, antibodies were applied using conventional immunoperoxidase staining. In brief, paraffin sections were deparaffinized and rehydrated. Endogenous peroxidase was blocked with 1.5% H2O2 in methanol for 30 minutes. For antigen retrieval, sections were boiled for 15 minutes in 10 mmol/L sodium citrate buffer (pH 6.0) or, only in the case of FasL, predigested with 2.5 × 10 6 U/L pepsin in 0.1 mmol/L glycine buffer (pH 2.0). Frozen sections were fixed in acetone for 10 minutes. After washing in PBS/Tween-20, sections were preabsorbed using 10% normal horse serum or normal goat serum for 15 minutes and incubated with the primary antibody, an diluted in PBS/1% bovine serum albumin (BSA), for 1 hour. The sections were washed and incubated with biotinylated horse anti-mouse antibody (1:500; lot F0425, Vector Laboratories, Burlingame, CA) or biotinylated goat anti-rabbit antibody (1:500; lot F505, Vector) in PBS/1% BSA for 30 minutes, washed again in PBS/Tween-20, and then incubated with horseradish peroxidase (HRP)-conjugated streptavidin (1:400; lot 14022721, Boehringer Mannheim, Mannheim, Germany) in PBS/1% BSA for 30 minutes. After development of the peroxidase with diaminobenzidine (DAB)/0.03% H2O2, the sections were counterstained with hematoxylin and embedded in dibutylphthalate polystyrene xylene (DPX). Omission of the primary antibody and an isotype-matched control antibody replacing the primary antibody served as negative controls. Only results that stand up to these controls are presented.
Table 1.
Antibodies Used for Immunohistochemistry
| Antibody | Dilution | Lot/clone | Cell markers used for double staining | |||||
|---|---|---|---|---|---|---|---|---|
| CD3a,b | CD4a | CD8a | CD68c | Ulexd | TUNEL | |||
| Co-stimulatory molecules | ||||||||
| CD28e | 1:25 | 05 | x | |||||
| CD28f | 1:5 | M014631 | ||||||
| CTLA4f | 1:10 | M014748 | ||||||
| CTLA4g | 1:1000 | 11D4 | ||||||
| CD40Lh | 1:25 | M91 | ||||||
| CD80i | 1:200 | B7-24 | x | x | ||||
| CD86f | 1:50 | 2331/Fun1 | x | x | ||||
| CD40j | 1:2000 | 5D12 | x | x | ||||
| Apoptosis and cytotoxicity | ||||||||
| Baxe | 1:1 | 01 | x | x | x | x | x | |
| Bax*†k | 1:5 | 1089501-4 | ||||||
| Bcl2†c | 1:100 | 105 | x | |||||
| Fasl | 1:100 | ws103 | ||||||
| FasLm | 1:40 | C047 | x | x | x | x | x | |
| Actin SMAd | 1:4000 | 29f4909 | x | |||||
| Desmin*n | 1:20 | 036-03 | x | |||||
| TUNEL | x | x | x | x | ||||
| Perforin†o | 1:10 | 303325 | ||||||
| Granzyme B†p | 1:500 | GrB7 | x | |||||
| CD57†e | 1:25 | 03.1 | x |
Indicated is which antibodies are used in different combinations in fluorescent double labeling (x). Dilutions are mentioned for single staining in conventional immunohistochemistry. For double immunofluorescence, dilutions were adapted to obtain proper signals. CD3, CD4, CD8, and CD68 were FITC conjugated, and Ulex (lot 534033) was TRITC conjugated. Cell markers and antibodies were obtained from the following sources: aCD3, lot 50527; CD4, lot 60442; CD8, lot 60992, Becton Dickinson, San Jose, CA; bCD3 in double-staining on paraffin sections: polyclonal rabbit antibody, 1:200, lot 102, Dako, Glostrup, Denmark; cCD68, lot 125(101), Dako; dSigma, St. Louis, MO; eImmunotech/Coulter, Marseille, France; fPharmingen, San Diego, CA; gBristol-Meyers, Seattle WA; hImmunex, Seattle WA; iInnogenetics, Gent, Belgium; jPanGenetics, Amsterdam, The Netherlands; kOncogene Research Products/Calbiochem, Cambridge, MA; l6th International Workshop HLDA; mSanta Cruz Biotechnology, Santa Cruz, CA; nEuro-Diagnostics, Apeldoorn, The Netherlands; oEndogen, Cambridge, MA; pgift from Dr. Kummer, Amsterdam, The Netherlands.
*Polyclonal rabbit antibody.
†Used on paraffin-embedded tissue.
Positive and Negative Control Tissues
As positive control tissues, tonsil was used as a normal lymphoid tissue, a muscle biopsy from a polymyositis patient was used as a control for inflammation in muscle tissue in comparison with heart muscle, and resected thyroid from a patient with Hashimoto’s thyroiditis was used, as in this disease thyrocytes have been described to constitutively express both Fas and FasL. 17 As negative control, healthy nontransplanted heart tissue obtained after autopsy was used.
Double Immunofluorescence Using Tyramide Amplification
Using the tyramide signal amplification (TSA) Direct- and Indirect kit (NEL 701/700, DuPont/NEN Life Science Products, Boston, MA), we developed a double-fluorescence technique that enabled us to combine any two monoclonal antibodies, provided that one of them is fluorescein isothiocyanate labeled. The kits can also be used for single-fluorescence techniques. The combinations of antibodies used in double-staining experiments are indicated in Table 1 ▶ . For double staining, frozen sections were fixed in acetone for 10 minutes and washed in PBS/Tween-20. The sections were incubated with the primary antibody, eg, a mouse antibody, diluted in PBS/1% human serum albumin (HSA) for 1 hour. The sections were washed three times for 5 minutes each in Tris-buffered saline (TBS)/Tween-20 buffer (0.1 mmol/L TBS, pH 7.5/0.01% Tween-20), and incubated with, in the case of a mouse antibody, HRP-conjugated rabbit anti-mouse antibody (RAMPO; 1:200, lot 020, Dako, Glostrup, Denmark) in TBS/10% human AB serum. After washing in TBS/Tween-20, the amplification of the signal was performed using the TSA Indirect kit, with biotinyl-conjugated tyramide diluted 1:50, according to the manufacturer’s instructions, for 8 minutes. The sections were washed in TBS, incubated with Texas-Red-conjugated streptavidin (1:250 in TBS; lot NEL 721, DuPont/NEN Life Science Products) for 30 minutes, and washed again in TBS. The residual RAMPO activity was blocked with PBS/1% H2O2 for 20 minutes. Sections were washed and preincubated with 10% normal mouse serum in TBS. A 1-hour incubation followed with a second FITC-labeled antibody of interest in PBS/1% HSA. The sections were washed in TBS/Tween-20 and incubated with HRP-conjugated rabbit-anti-FITC (1:200; lot 015(201), Dako) for 30 minutes. After washing, the FITC signal was amplified using the TSA Direct kit, with FITC-conjugated tyramide diluted 1:50, according to the manufacturer’s instructions, for 8 minutes. After a final washing step in TBS/Tween-20, sections were embedded in Vectashield.
Double staining of granzyme B or CD57 with CD3 was performed on paraffin sections. Sections were deparaffinized, rehydrated, and boiled for 15 minutes in 10 mmol/L sodium citrate buffer (pH 6.0) for antigen retrieval. Sections were preabsorbed with normal horse serum and incubated with primary antibodies diluted in PBS/1% BSA. After washing, an incubation with biotinylated horse anti-mouse was performed, followed by an incubation with tetramethylrhodamine isothiocyanate (TRITC)-conjugated streptavidin. Then the sections were washed and incubated with a polyclonal anti-CD3 antibody (1:40; Dako), followed by incubation with HRP-conjugated swine anti-rabbit antibody (1:50; Dako). This signal was visualized and amplified using the TSA Direct kit, resulting in a FITC labeling.
In Situ End Labeling of Fragmented DNA (TUNEL)
The TUNEL method was used, as described elsewhere, 18 to analyze apoptosis in EMB sections. For the longitudinal study of three patients, paraffin-embedded tissue sections were deparaffinized and rehydrated. Sections were then treated with proteinase K (20 μg/ml; Boehringer Mannheim) for 30 minutes. After washing in double-distilled water, endogenous peroxidase was blocked using PBS/2% H2O2 for 10 minutes. Sections were then presoaked in TdT buffer (0.5 mmol/L cacodylate, 1 mmol/L CoCl, 0.5 mmol/L dithiothreitol, 0.05% BSA, 0.15 mol/L NaCl) for 10 minutes. Frozen sections were fixed in acetone for 10 minutes at room temperature (RT) and then presoaked in TdT buffer. Sections were then incubated for 2 hours at 37°C in 25 μl of TdT solution, containing 1X terminal transferase buffer (Promega, Madison, WI), 0.5 nmol of biotin-dUTP (Boehringer Mannheim), and 5 to 10 U of TdT (Promega). After the TdT reaction, slides were soaked in TdT blocking buffer (300 mmol/L NaCl, 30 mmol/L tri-sodium citrate-2-hydrate), incubated with HRP-conjugated streptavidin for 30 minutes at RT, and developed for 10 minutes in phosphate-buffered citrate (pH 5.8) containing 0.6 mg/ml DAB. Nuclei were counterstained with hematoxylin.
For CLSM, sections were incubated with FITC-conjugated streptavidin (1:20; lot 14594222-09, Boehringer Mannheim) for 30 minutes at RT. Nuclei were counterstained with 4 μg/ml propidium iodide, washed in PBS, and mounted in Vectashield.
Positive controls were obtained by a DNAse I treatment for 60 minutes at 37°C (40 U/ml; Boehringer Mannheim). For negative controls, TdT was replaced by distilled water in the TdT solution.
Identification of the Phenotype of TUNEL-Positive Cells Using Double Labeling
Frozen sections were fixed in acetone for 10 minutes. After washing in PBS/Tween-20, sections were incubated for 1 hour with an unlabeled or FITC-conjugated primary antibody for phenotype analysis, diluted in PBS/1% HSA. After fixation in 4% formaldehyde for 10 minutes, the TUNEL reaction was performed as described above, using biotin-16-dUTP. The TUNEL reaction was developed using TRITC-conjugated streptavidin (1:10; lot A286-N596B, Southern Biotechnology Associates, Birmingham, AL). After washing, sections were incubated with secondary antibodies for 30 minutes. In the case of FITC-conjugated primary antibodies, the HRP-conjugated rabbit anti-FITC antibody was used. If primary antibodies were unlabeled, a RAMPO (1:100) or a HRP-conjugated swine anti-rabbit antibody (SWARPO, 1:100; lot 035(101), Dako) was used. For smooth muscle actin and desmin, a FITC-conjugated rabbit anti-mouse antibody (1:40; lot 082, Dako) and a FITC-conjugated horse anti-rabbit antibody (1:20; lot PK17-02-F10, CLB, Amsterdam, The Netherlands) was used, respectively. Except for these last two, all signals were amplified using the TSA Direct kit for 12 minutes, resulting in amplification of the FITC signal. All sections were washed and mounted in Vectashield.
DNA Fragmentation Analysis
From postmortem heart tissue without and with rejection (grade 3A/3B), 20 frozen sections of 10 μm were resuspended in 20 μl of solution A (10 mmol/L EDTA, 0.5 mmol/L Tris/HCl, 0.5% Sarcosyl, K 0.5 μg/ml proteinase) and incubated at 50°C for 60 minutes. Then 10 μl of DNAse-free RNAse was added (containing 5 μg of RNAse A, Boehringer Mannheim), followed by an incubation for 60 minutes at 50°C. Then 20 μl of solution B (40% sucrose containing Orange G, 10 mmol/L EDTA, and 1% low-melting-point agarose) was added at 56°C, and samples were loaded on a 1.5% agarose gel containing ethidium bromide. After polymerization of the samples, the gel was run and visualized under ultraviolet light.
In Situ Hybridization
In situ hybridization was performed for FasL using the same procedure as described previously. 19 The following primers for FasL were used in a polymerase chain reaction for the synthesis of the digoxigenin (Dig)-dUTP-labeled probe: 5′ primer, 5′-CAAGTCCAACTCAAGGTCCATGCC-3′; 3′ primer, 5′-CAGAGAGAGCTCAGATACGTTTGAC-3′. The probe was sequenced to confirm that the specific FasL product was amplified. After overnight hybridization of the probe on tissue sections, the signal was visualized using a mouse anti-Dig monoclonal antibody (1:50; lot 13026924-01, Boehringer Mannheim). The signal was amplified with RAMPO and SWARPO and developed with DAB. As negative controls, sections were pretreated overnight with RNAse T1 (100 U/ml; Boehringer Mannheim).
Statistical Analysis
The Mann-Whitney U test was used to compare the expression of molecules or apoptosis in different groups of cells or biopsies. 20 P values <0.05 were considered to be statistically significant.
Results
Expression of Co-Stimulatory Molecules
To study the in situ expression of co-stimulatory molecules during acute rejection of human heart allografts, we used 32 frozen EMBs from 17 patients, with and without signs of rejection. As described previously, the mononuclear cell infiltrate during acute rejection consists mainly of T cells and macrophages. The ratio CD4+ to CD8+ T cells is ∼2:1. 19
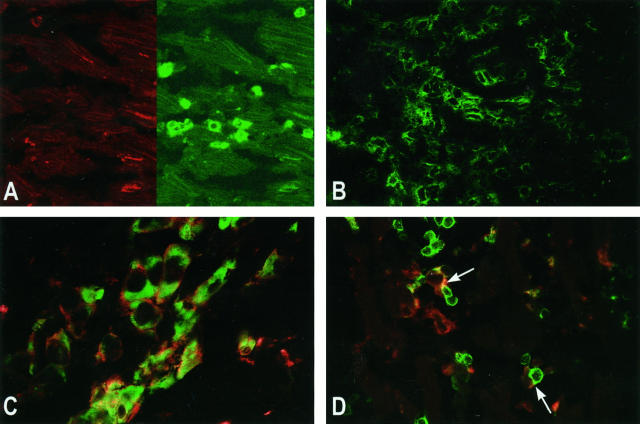
Expression of co-stimulatory molecules was studied by single- and double-immunofluorescence techniques, using tyramide amplification, and detection via CLSM. On T cells, no or hardly any expression of CD28 (Figure 1A) ▶ , and no expression of CTLA4 and CD40L was observed (not shown). These molecules were detected on T cells in control tonsil tissue (not shown). Only when a severe rejection infiltrate was present did a small proportion of T cells express CD28. In some Quilty lesions (mononuclear cell infiltrates in the endomyocardium not related to rejection), 21 expression of CD28 was observed (not shown). In positive control tissues containing activated T cells, taken from normal tonsil, polymyositis (Figure 1B) ▶ , and Hashimoto’s thyroiditis patients, an abundant expression of CD28 on T cells was observed. B7-1 (CD80) and B7-2 (CD86) were present on the majority of the macrophages, as confirmed by double-fluorescent staining with CD68 (Figure 1C) ▶ . CD40 was detectable both on macrophages and on endothelium (not shown). Despite the absence of co-stimulatory molecules on T cells, the T cells do make close contact with APCs, as shown by CLSM (Figure 1D) ▶ .
Figure 1.
Expression of co-stimulatory molecules on infiltrating cells in heart allografts (rejection grade 3A), detected by confocal laser scan microscopy. A: CD28 (red, left) is not expressed on the T cells (CD3, green, right). Original magnification, ×170. B: As control; Expression of CD28 (green) in polymyositis is abundantly present on most of the T cells. Original magnification, ×170. C: B7-2 (CD86, red) is expressed on most of the macrophages (CD68, green) in the rejection infiltrate. Original magnification, ×310. D: Despite the absence of CD28 on the T cells, antigen-presenting cells (CD86, red) are observed in close contact with T cells (CD3, green) (arrows). Original magnification, ×170. Immunofluorescent single or double labeling was done using indirect (CD86) or direct (CD28, CD68, and CD3) tyramide signal amplification. For indirect amplification, tissues were incubated with HRP-conjugated secondary antibodies. For direct amplification, a FITC-conjugated primary antibody was used.
These data were (semiquantified in relation to the grade of rejection, as indicated in Table 2 ▶ . The absence of co-stimulatory molecules on T cells was independent of the rejection grade. The expression of co-stimulatory molecules on the APCs correlated with the number of macrophages present in the mononuclear cell infiltrate. The data were confirmed by immunofluorescent double labeling.
Table 2.
Expression of Co-Stimulatory Molecules in Heart Biopsies
| Number of biopsies | CD3 | CD68 | T cells | Antigen-presenting cells | ||||
|---|---|---|---|---|---|---|---|---|
| CD28 | CTLA4 | CD40L | B7-1 | B7-2 | CD40 | |||
| 7 | − | ± | − | − | − | ± | − | ± |
| 7 | ± | + | − | − | − | ± | ± | + |
| 10 | + | + | − | − | − | ± | ± | + |
| 8 | ++ | ++ | ± | − | − | + | + | ++ |
The number of CD3+ T cells per mm2 tissue was counted. Biopsies are grouped according to these T cell numbers (−, no cells; ±, 1 to 100 cells/mm2; +, 101 to 200 cells/mm2; ++, >200 cells/mm2), as grading of the rejection is not feasible on frozen tissue. The numbers of macrophages were semiquantitatively correlated to the T cell numbers and are indicated with a range: ±, few macrophages; +, intermediate number of macrophages, ++, high number of macrophages. Their number increased corresponding with the number of T cells. The number of cells expressing a given co-stimulatory molecule is indicated in the same range as the number of T cells or macrophages.
Although the expression of CD28 inside the graft was absent or low, this was not reflected in the periphery. FACS analysis of peripheral blood mononuclear cells from 20 patients, followed from before transplantation until 1 year after transplantation, showed a normal and constant distribution of the expression of CD28 within the CD4+ and CD8+ T cell subsets (data not shown).
Detection of Apoptosis in the Graft Myocardial Tissue
To evaluate whether apoptosis occurred, which cells were apoptotic, and where they were localized, postmortem heart tissue with rejection grade 3A or 3B from several patients after transplantation and normal nontransplanted heart tissue was studied, using an in situ end-labeling method (TUNEL). In normal nontransplanted heart tissue, no TUNEL positivity was seen in myocytes. In heart tissue with moderate to severe rejection, which according to the histopathological diagnosis exhibited muscle damage, TUNEL-positive myocytes were indeed detected, localized in the areas surrounding the cellular infiltrates. However, apart from myocytes, the infiltrating cells themselves demonstrated a high level of apoptosis (Figure 2A) ▶ . Propidium iodide counterstaining revealed that ∼50% of the mononuclear cells in the infiltrate were apoptotic (Figure 2B) ▶ . This was not observed in healthy heart tissue or in tonsil, polymyositis, or Hashimoto’s thyroiditis biopsies (not shown).
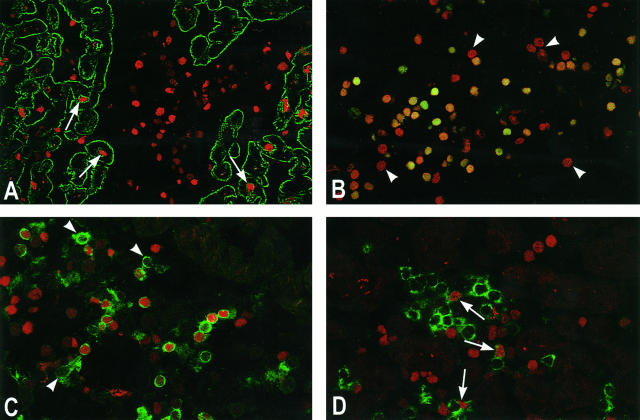
Figure 2.
Apoptosis during heart allograft rejection. A: Apoptosis (TUNEL, red) is observed in myocytes (desmin, green) during severe rejection (arrows). However, the majority of apoptotic cells is observed in the infiltrate (center). Original magnification, ×100. B: Propidium iodide counterstaining (red) of the TUNEL-positive cells (green) reveals that ∼50% of all infiltrating cells are apoptotic. Double-positive cells stain orange to yellow. Deep-red nuclei illustrate non-apoptotic cells, some of which are indicated with arrowheads. Original magnification, ×150. C: Apoptosis in the CD4+ T cells. Approximately 65% of the CD4+ T cells (green) are apoptotic (TUNEL, red). Some non-apoptotic cells are indicated (arrowheads). Original magnification, ×170. D: Apoptosis in the CD8+ T cells. Only ∼25% of the CD8+ T cells (green) are apoptotic (TUNEL, red). Some apoptotic CD8+ cells are indicated (arrows). Original magnification, ×200. For biotin-dUTP-labeled DNA (TUNEL), detection was by FITC- or TRITC-conjugated streptavidin. For desmin, detection of primary antibody was by FITC-conjugated horse anti-rabbit antibody. For CD4 and CD8, direct staining with FITC-conjugated primary antibodies was done, followed by direct tyramide signal amplification.
To identify the phenotype of the apoptotic cells, double staining with TUNEL reaction and CD4, CD8, or CD68 was performed and analyzed by CLSM. The double staining revealed that the apoptosis was restricted to lymphocytes. Within the lymphocytes, a significant skewing of the apoptosis was observed toward the CD4+ T cells compared with the CD8+ T cells (65.7 ± 9.6% versus 26.6 ± 15.8%; P < 0.001), shown in Figure 2, C and D ▶ .
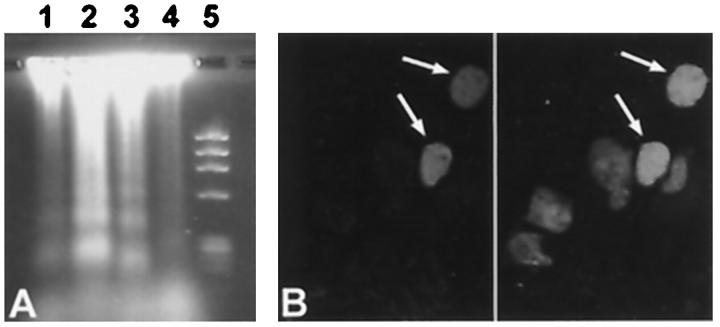
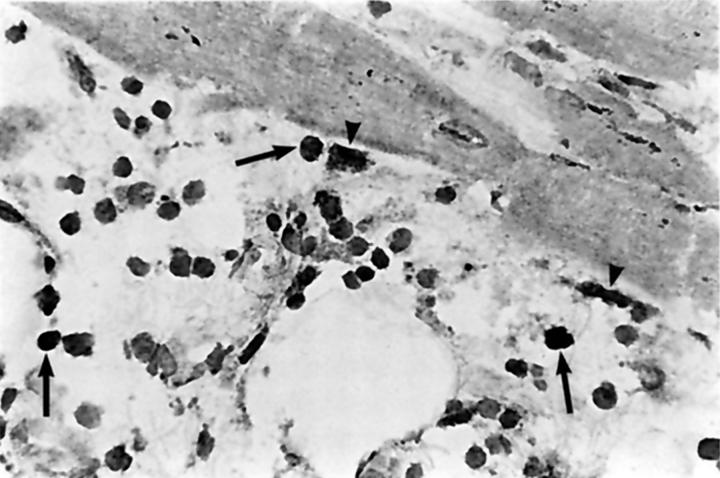
To confirm that the observed TUNEL positivity indeed demonstrated apoptosis, DNA fragmentation analysis was performed. Electrophoresis of DNA, isolated from three hearts with rejection grade 3A or 3B, obtained after autopsy, showed the ladder pattern characteristic for apoptosis. This was not seen in normal heart tissue (Figure 3A) ▶ . In addition, one of the morphological characteristics of apoptosis, chromatin condensation in the nucleus, was confirmed by CLSM in part of the TUNEL-positive cells (Figure 3B) ▶ .
Figure 3.
Apoptosis, demonstrated by DNA-ladder analysis and nuclear condensation. A: DNA fragmentation analysis of heart tissue during allograft rejection. DNA was isolated from heart tissue during moderate to severe rejection and from healthy heart tissue. After electrophoresis on an agarose gel, the DNA ladder, characteristic for apoptosis, was observed in hearts with a rejection, but not in normal, non-transplanted heart tissue. Lanes 1 and 2, rejection grade 3A; lane 3, rejection grade 3B; lane 4, healthy heart; lane 5, DNA marker (φX 174). B: Nuclear condensation, one of the morphological characteristics of apoptosis, was observed in apoptotic cells. In situ end labeling of the DNA (TUNEL) demonstrates apoptotic cells (left panel, arrows) . Propidium iodide counterstaining of the nuclei (right panel) shows chromatin condensation in the TUNEL-positive cells (arrows) and a normal chromatin distribution in the non-apoptotic cells. Original magnification, ×550. For biotin-dUTP-labeled DNA (TUNEL), detection was by TRITC-conjugated streptavidin.
Observations in Sequential Endomyocardial Biopsies
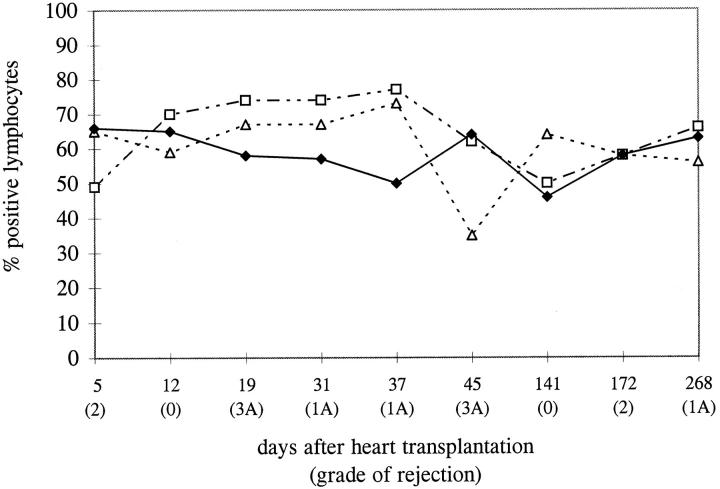
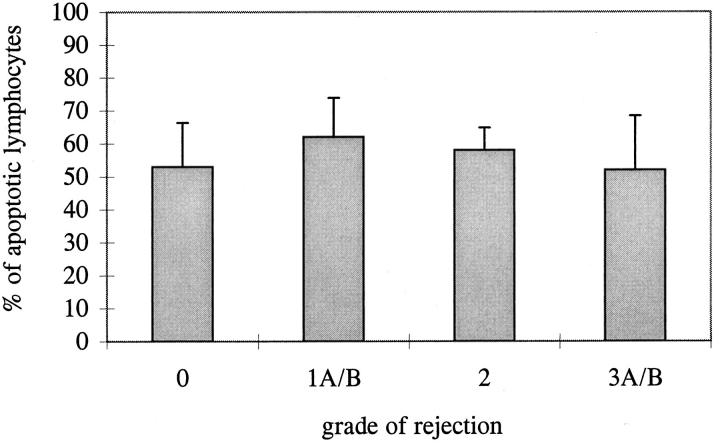
The process of apoptosis induction and regulation in the allograft in time was studied in 24 paraffin-embedded EMBs from three patients, taken at different times after transplantation. The mononuclear cell infiltrates in biopsies with different grades of rejection were analyzed for the presence or absence of molecules involved in the induction and regulation of apoptosis (Bcl2 and Bax). For this part of the study, TUNEL was performed on paraffin-embedded tissue sections to enable us to compare the TUNEL positivity to the expression of the other molecules, as the Bcl2 antibody could be applied only on paraffin-embedded tissue. For Bax, two antibodies were available, one of which could be used on paraffin-embedded tissue. The applied pretreatment of the tissue sections for the TUNEL reaction was sufficient to give comparable results for paraffin-embedded and frozen tissue. Paraffin sections were stained, and the percentage of cells positive for Bcl2, Bax, and TUNEL was counted in triplicate in at least 100 cells per biopsy. The three patients showed similar patterns. Our observations in one of the three patients are shown in Figure 4 ▶ . The percentage of cells expressing Bcl2 and Bax tended to be stable in time, varying between 40% and 70% of the infiltrating cells. At some time points a change in the expression of the molecules seemed to occur in parallel with a change in the percentage of apoptotic cells. However, overall, no clear-cut correlation between the expression of these molecules, the presence of apoptosis, and the grade of rejection was observed. Although the percentage of apoptotic cells did not alter, the absolute numbers of apoptotic cells did correlate with the grade of rejection, as with an increasing grade of rejection the number of infiltrating cells increases. If all biopsies from these patients were combined and grouped according to the grade of rejection, it was confirmed that no significant correlation existed between the percentage of apoptotic cells and the grade of rejection (Figure 5 ▶ ; P > 0.05 for all groups). The same conclusion could be drawn for the expression of Bcl2 and Bax (data not shown).
Figure 4.
A representative longitudinal analysis of apoptosis and apoptosis-regulating molecules in EMBs after heart transplantation. Paraffin-embedded biopsies, taken at different time points after transplantation, were analyzed for the expression of apoptosis-regulating molecules, Bcl2 (▵) and Bax (□), in relation to the presence of apoptosis (♦) in the infiltrating cells (conventional immunoperoxidase using DAB development). The percentage of positive cells, counted in triplicate in each biopsy, is indicated. Although the percentage of positive lymphocytes is relatively constant, the absolute numbers of positive cells do differ, as the number of infiltrating cells increases with increasing severity of the rejection process.
Figure 5.
Mean percentage of apoptotic lymphocytes in biopsies with different grades of rejection. The percentage of apoptotic cells was counted in a total of 24 paraffin-embedded biopsies, after TUNEL staining with DAB development. The mean ± SE is indicated, obtained from the mean percentage of apoptotic cells of all biopsies with a certain grade of rejection. Each biopsy is counted in triplicate. No significant difference between the different grades of rejection was observed.
Induction and Regulation of Apoptosis
To further characterize the cells expressing FasL, Bcl2, and Bax, the phenotype of mononuclear infiltrating cells was determined in heart tissue with rejection grade 3A/B, using double immunofluorescence. Double staining for TUNEL and FasL demonstrated that all apoptotic cells expressed FasL (Figure 6A) ▶ . Not only apoptotic cells expressed FasL; the majority of CD4+ T cells and CD8+ and, surprisingly, all CD68+ macrophages expressed FasL (Figure 6, B and C) ▶ . ISH confirmed that FasL was expressed in lymphocytes and macrophages (Figure 7) ▶ . In Hashimoto’s thyroiditis, FasL expression was observed on follicular epithelial cells, as described previously, 17 but not on the infiltrating T cells (not shown). FasL is able to induce apoptosis in Fas-bearing cells. Staining for Fas showed that, indeed, the majority of the infiltrating cells, both T cells and macrophages, expressed Fas (Figure 6D) ▶ .
Figure 6.
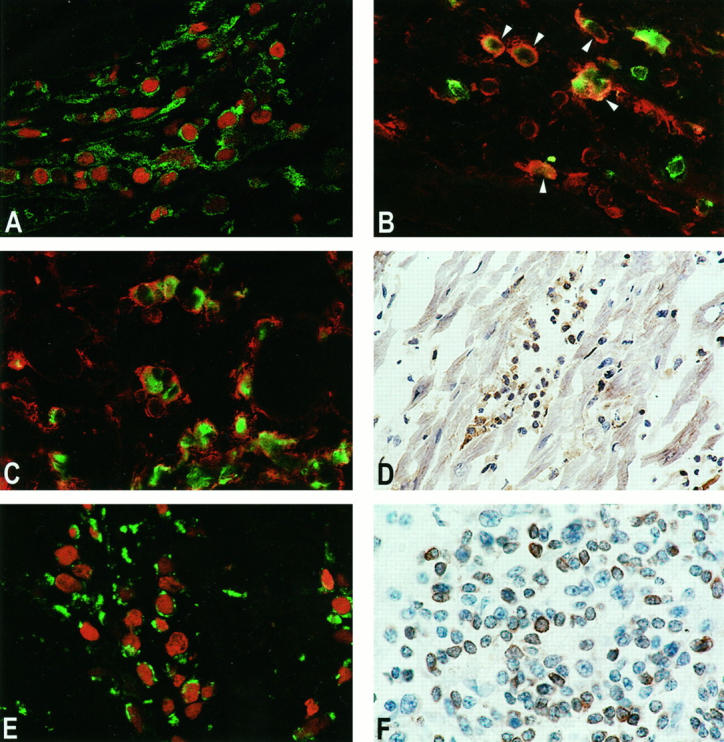
Regulation of apoptosis in EMBs during heart allograft rejection. A: FasL expression on apoptotic cells. Double staining for FasL (green) and TUNEL (red) demonstrated that virtually all apoptotic cells expressed FasL. Original magnification, ×280. B: FasL expression on CD4+ T cells. The majority of CD4+ T cells (green) co-expressed FasL (red, arrowheads). Original magnification, ×210. C: FasL expression on macrophages. All CD68+ macrophages (green) co-expressed FasL (red). Original magnification, ×190. D: Fas expression, shown by conventional immunoperoxidase staining (brown). Most of the infiltrating cells expressed Fas. Original magnification, ×100. E: Bax expression in apoptotic cells. Bax (green) was expressed on 50% to 70% of all apoptotic cells (TUNEL, red). Original magnification, ×330. F: Bcl2 expression in the infiltrate. Approximately 50% of all cells expressed Bcl2 (brown staining). Original magnification, ×250. For FasL, detection was by SWARPO and direct (green) or indirect (red) signal amplification. For TUNEL, detection was by TRITC-conjugated streptavidin. For CD4 and CD68, staining was with FITC-conjugated antibodies and direct signal amplification. For Bax, detection was by RAMPO and direct signal amplification. For Fas and Bcl2, detection was by biotinylated horse anti-mouse antibody and HRP-conjugated streptavidin, developed with DAB.
Figure 7.
In situ hybridization (ISH) for FasL on the mRNA level. The ISH confirmed that FasL was expressed in lymphocytes and macrophages in the mononuclear cell infiltrate during rejection. Some positive lymphocytes (arrows) and macrophages (arrowheads) are indicated. Original magnification, ×150. Detection of Dig-labeled probes was by mouse anti-Dig antibody, RAMPO and SWARPO, developed with DAB.
Regulation of apoptosis may occur via Bax and Bcl2. Bax was expressed in the majority of apoptotic cells (Figure 6E) ▶ , in contrast to, eg, tonsil, where hardly any expression of Bax could be observed. Bcl2 on the other hand was observed in most T lymphocytes in tonsil, but only in ∼50% of the lymphocytes in the heart biopsies (Figure 6F) ▶ . The expression of Bcl2 in T cells in heart biopsies was not correlated with the presence or absence of apoptosis in these cells, as seen by double staining with TUNEL.
Analysis of Cytotoxicity and Activation in the Allograft
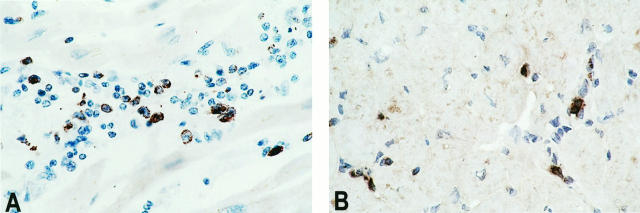
A common mechanism of cytotoxicity in the process of allograft rejection and induction of myocyte damage is via the secretion of perforin and granzyme B. To evaluate whether this mechanism plays a role in the induction of the observed myocyte damage, expression of granzyme B and perforin was analyzed in 29 and 19 EMBs, respectively, with different grades of rejection. Both proteins were detected in part of the infiltrating cells (Figure 8, A and B) ▶ . In general, the number of positive cells correlated with the severity of the rejection reaction. Granzyme B was produced by T cells, as confirmed by double staining with CD3 (not shown). The number of CD3+ CD57+ activated cytotoxic T cells was comparable to the number of granzyme-B- and perforin-producing cells and confirmed the presence of activated cytotoxic T cells (not shown).
Figure 8.
Production of granzyme B and perforin during rejection. A: Granzyme B is detected in part of the lymphocytes during rejection (brown staining). Original magnification, ×150. B: Perforin is produced to the same extent as granzyme B, in part of the infiltrating cells (brown staining). Original magnification, ×150. Detection in paraffin-embedded tissue was by biotinylated horse anti-mouse antibody and HRP-conjugated streptavidin, developed with DAB.
Discussion
For the treatment of allograft rejection, the most important complication of organ transplantation, currently only nonspecific immunosuppressive drugs are available. The major disadvantages of these drugs are the severe side effects, such as infections and tumor induction. The ultimate goal of the research in this field is to develop more specific immunosuppressive treatments that will lead to alloantigen-specific tolerance. What is known so far about the pathways leading to alloantigen-specific tolerance stems from in vitro studies and experimental animal models. To gain a better understanding of the relevance of these pathways for human organ transplantation, it is necessary to evaluate the process of immunoregulation in the in vivo situation. Therefore, in the present study, human EMBs, obtained after human heart transplantation, were investigated for the expression of co-stimulatory molecules, the presence of apoptosis, and the regulation of apoptosis during allograft rejection. We developed a sensitive double-immunofluorescent staining technique, which enabled us to characterize the cells expressing relevant molecules.
In the mononuclear cell infiltrate in the transplanted heart, we observed a low expression of co-stimulatory molecules, especially on the T cells, compared with other inflammatory infiltrates in, eg, polymyositis and Hashimoto’s thyroiditis. FACS analysis of the expression of CD28 on peripheral blood lymphocytes (PBLs) demonstrated that the absence of CD28 expression in the allograft was not reflected in the periphery. If co-stimulation is absent during primary activation of the T cells by the alloantigens in the heart, this will lead to anergy in the infiltrating T cells. However, considering the fact that these biopsies were taken during active acute rejection and contained both large infiltrates and areas of myocyte damage, this does not seem likely. Perhaps only part of the T cells is anergized, which could account for the decrease of the frequency of rejection episodes later after transplantation. A similar phenomenon of strongly reduced expression of CD28 has also been observed in HIV infection and Chagas’ disease, and resided mainly in the CD8+ T cells. This, too, has been associated with anergy induction but, according to others, also to chronic activation of the T cells. 22,23 Down-regulation of CD28 as a result of the interaction between B7 and CD28 during T cell activation has also been shown in vitro. 24 It can be assumed that in cardiac graft rejection, the T cells in the graft infiltrate have been continuously triggered by alloantigens, not only, or even mainly, in the allograft itself but also in draining lymph nodes, resulting in the observed low expression of CD28 and probably also in the observed absence of CTLA4 and CD40L.
To down-regulate an immune response, apoptosis is induced in activated T cells via the Fas/FasL pathway, so-called activation-induced cell death (AICD). 9,11 Surprisingly, in the EMBs, ∼50% of all infiltrating cells were apoptotic, irrespective of the severity of the rejection reaction. Because the absolute number of infiltrating cells increases with increasing rejection grade, the absolute number of apoptotic cells did correlate with the grade of rejection. Apoptosis was restricted to T cells. Chromatin condensation and DNA fragmentation analysis confirmed the presence of apoptosis. The percentage of apoptotic cells over time and in different grades of rejection was quite constant. In other inflammatory infiltrates, used as controls for this study (polymyositis and Hashimoto’s thyroiditis), hardly any apoptotic cell was observed. In these tissues, there was a strong CD28 expression. Whether the mutual correlation between absence of CD28 and presence of apoptosis is based upon a causal relationship needs further investigation.
The percentage of apoptotic cells in our study is unexpectedly high, and of the same order of magnitude as in in vitro induced apoptosis, after, eg, anti-Fas or antigen-induced T cell apoptosis. 25,26 In murine cardiac allografts, only a low number of apoptotic cells was observed in the cellular infiltrate during rejection. 27 In human heart allografts, apoptosis has been observed in myocytes and some other cell types. 28 Recently, a very high level of apoptosis in heart-infiltrating cells during rejection was described. 29 Because in both studies, these cells were not further characterized, it is hard to compare these results in more detail with our data. Still, their results confirm that apoptosis occurs in the heart-infiltrating cells. In coronary artery disease after heart transplantation, apoptosis was observed in endothelial cells, but also in 18% to 78% of the perivascular T cells. 30 In kidney allografts, however, apoptotic epithelial cells were observed, but apoptotic lymphocytes were rare. 31 The inflammatory T cell populations present in our control tissues also showed much lower levels of apoptosis, compared with the rejection infiltrates. Clearly, a large variation in the level of apoptosis is observed in different studies and under different circumstances. Besides the effect of different experimental approaches on the level of apoptosis, other factors have been suggested to influence apoptosis. One important factor is the tissue microenvironment. For example, the presence of stromal cells, and production of different cytokines in this microenvironment, may play a role in the induction or inhibition of apoptosis. 32 To elucidate the role of the microenvironment in the regulation of apoptosis observed in our study, additional investigations are necessary.
Macrophages are able to induce apoptosis in activated T cells. In vitro, PBLs exposed to alloantigen presented by macrophages were shown to be selectively depleted of the alloantigen-specific T cells. 33 In vivo, activated macrophages were observed in lymph nodes of HIV-infected individuals, where apoptosis has been described to occur. 34 In vitro, using PBLs of these patients, macrophages induced apoptosis. 35 In that situation, apoptosis was mediated by FasL and TNF. The mononuclear cell infiltrates in our EMBs contain a high number of macrophages. We showed previously that a considerable number of lymphocytes in the infiltrate express TNF receptors. 36 We demonstrate here that not only Fas but also FasL is expressed on most of the infiltrating cells, including macrophages. Via FasL, these macrophages can induce apoptosis in Fas-bearing T cells. That apoptosis is present only in T cells, and not in macrophages, may be due to a combination of the expression of Fas and FasL and the lack of co-stimulatory molecules on the T cells, which may render them more sensitive to the induction of apoptosis. CD4+ T cells have been described to be more susceptible to AICD via Fas/FasL than CD8+ T cells. 37 In PBLs from HIV patients, macrophages induced apoptosis mainly in CD4+ T cells. 35 In our EMBs, T cells were often seen in close contact with macrophages, and apoptosis was biased towards the CD4+ T cells. These observations indicate that macrophages may play an important role in the regulation of especially the CD4+ T cells by the induction of apoptosis.
Apoptosis is a tightly regulated process, which involves an intricate network of regulatory proteins. Two important proteins are Bcl2, which inhibits, and Bax, which promotes apoptosis. Conflicting data are available about the relevance of Bcl2 and Bax for the regulation of apoptosis mediated via the Fas/FasL pathway. It was demonstrated that induction of apoptosis through Fas did not alter the expression of Bcl2 and Bax. 38 Others, however, showed that Bcl2 did partially inhibit Fas-induced apoptosis. 39 These differences are probably the result of different in vitro approaches. In the present study, Bcl2 was expressed on nearly all T cells in normal lymphoid tissue. In the EMBs, however, only ∼50% of the infiltrating cells expressed Bcl2. On the contrary, Bax was hardly detectable in T cells in lymphoid tissue but was expressed in about 50% to 80% of the infiltrating cells in the EMB. A double staining for Bcl2 with TUNEL showed no clear correlation between apoptosis and the presence or absence of these proteins at the individual cell level. Most apoptotic cells expressed Bax, whereas Bcl2 was expressed in both apoptotic and non-apoptotic cells. Although in general a tendency toward a reduced percentage of T cells expressing Bcl2 and an increased percentage of T cells expressing Bax was seen, as compared with the expression in normal lymphoid tissue, a more quantitative measurement would be needed to confirm a relationship with apoptosis.
One special factor may play a role in the process of co-stimulation and apoptosis in our heart transplant recipients. These patients need life-long immunosuppressive treatment, which includes cyclosporin A (CsA). Several effects of CsA have been described. CsA inhibits the expression of cytokines, particularly IL-2. 40 CsA may therefore be responsible for the low level of the expression of cytokines in the EMB, which we described previously. 19 Cytokine deprivation is a possible mechanism leading to apoptosis. 41,42 Recently, CsA was also described to inhibit the expression of CTLA4. 43 A similar inhibitory effect may be involved in the low expression of co-stimulatory molecules on T cells observed in our study, which may lead to apoptosis. Apart from the indirect effect of CsA on the induction of apoptosis, several in vitro studies describe a direct modulation of apoptosis by CsA. 44,45 To what extent CsA is involved, either directly or indirectly, in the apoptosis observed by us is difficult to determine from our presented data.
In end-stage heart failure, such as idiopathic dilated cardiomyopathy, damage of myocytes was found to result from apoptosis. 46,47 In heart transplantation, myocyte damage is one of the criteria for the diagnosis of the severity of the rejection process. In our study, apoptosis of myocytes was seen during moderate to severe rejection. It must be assumed that this damage, at least in large part, results from the action of perforin and granzyme B, produced by cytotoxic T cells, detected in the infiltrates. The number of perforin- and granzyme-B-producing cells was comparable to the number of CD57+ T cells, which confirmed the presence of activated cytotoxic T cells. Apparently, rejection still continues, despite the large amount of apoptosis. This could be due to the fact that only a minority of the CD8+ T cells is affected by apoptosis.
In the present study, we demonstrate that the expression of co-stimulatory molecules, especially on T cells, is absent or low. This may be one of the factors involved in the high frequency of apoptosis we observe, which shows a preference for CD4+ T cells. Considering the abundant expression of FasL, the apoptosis seems to be mediated via the Fas/FasL pathway and is probably induced by macrophages, which all express FasL. In vitro experiments will be needed to further characterize the mechanisms and the cells involved in the induction of apoptosis in the heart allografts. The fact that the level of apoptosis is constant in different grades of rejection indicates that the diagnostic value of our observations is at least limited. The diagnosis of heart allograft rejection is based on morphological criteria: the presence of a mononuclear cell infiltrate and signs of myocyte damage. It is, however, not possible to judge, by these criteria, the quantitative and qualitative aspects of the anti-graft activity of the infiltrate and the regulation of the rejection process. Our study indicates that during allograft rejection the infiltrating mononuclear cells not only play a role in the rejection process, but that they are also involved in the immunoregulation of one another during the ongoing immune response.
Acknowledgments
We thank Immunex and Innogenetics for the generous supply of the CD40L and CD80 antibodies. We thank Dr. Kummer for the granzyme B antibody. Dr. J. Hoogendijk and Ms. M. van der Meulen are greatly acknowledged for providing us with muscle tissue from a polymyositis patient. We thank Ms. F. Veninga for her technical assistance. We are grateful to Dr. R. Goldschmeding for critical reading of the manuscript.
Footnotes
Address reprint requests to Dr. Roel De Weger, Department of Pathology (H04.312), University Hospital, P.O. Box 85.500, 3508 GA Utrecht, The Netherlands. E-mail: R.deWeger@lab.azu.nl.
Supported by the University of Utrecht and the University Hospital.
References
- 1.Hathcock KS, Laszlo G, Pucillo C, Linsley P, Hodes RJ: Comparative analysis of B7–1 and B7–2 costimulatory ligands: expression and function. J Exp Med 1994, 180:631-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ding L, Shevach EM: Activation of CD4+ T cells by delivery of the B7 costimulatory signal on bystander antigen-presenting cells (trans-costimulation). Eur J Immunol 1994, 24:859-866 [DOI] [PubMed] [Google Scholar]
- 3.Boussiotis VA, Freeman GJ, Gray G, Gribben J, Nadler LM: B7 but not intercellular adhesion molecule-1 costimulation prevents the induction of human alloantigen-specific tolerance. J Exp Med 1993, 178:1753-1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.June CH, Bluestone JA, Nadler LM, Thompson CB: The B7 and CD28 receptor families. Immunol Today 1994, 15:321-331 [DOI] [PubMed] [Google Scholar]
- 5.Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Jr, Knechtle SJ: CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA 1997, 94:8789-8794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lakkis FD, Konieczny BT, Saleem S, Baddoura FK, Linsley PS, Alexander DZ, Lowry RB, Pearson TC, Larsen CP: Blocking the CD28–B7 T cell costimulation pathway induces long term cardiac allograft acceptance in the absence of IL-4. J Immunol 1997, 158:2443-2448 [PubMed] [Google Scholar]
- 7.Van Gool SW, de Boer M, Ceuppens JL: The combination of anti-B7 monoclonal antibody and cyclosporin A induces alloantigen-specific anergy during a primary mixed lymphocyte reaction. J Exp Med 1994, 179:715-720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu H, Robertus M, De Jonge N, Gmelig-Meyling FHJ, Van der Meulen A, Schuurman HJ, Doornewaard H, Van Prooijen HC, De Weger RA: Reduction of donor-specific cytotoxic T lymphocyte precursors in peripheral blood of allografted heart recipients. Transplantation 1994, 58:1263-1268 [PubMed] [Google Scholar]
- 9.Nagata S, Golstein P: The Fas death factor. Science 1995, 267:1449-1456 [DOI] [PubMed] [Google Scholar]
- 10.Kummer JA, Wever PC, Kamp AM, ten Berge IJM, Hack CE, Weening JJ: Expression of granzyme A and B proteins by cytotoxic lymphocytes involved in acute renal allograft rejection. Kidney Int 1995, 47:70-77 [DOI] [PubMed] [Google Scholar]
- 11.Alderson MR, Tough TW, Davis-Smith T, Braddy S, Falk B, Schooley KA, Goodwin RG, Smith CA, Ramsdell F, Lynch DH: Fas Ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med 1995, 181:71-77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobson MD, Burne JF, King MP, Miyashita T, Reed JC, Raff MC: Bcl-2 blocks apoptosis in cells lacking mitochondrial DNA. Nature 1993, 361:365-369 [DOI] [PubMed] [Google Scholar]
- 13.Oltvai ZN, Milliman CL, Korsmeyer SJ: Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell 1993, 74:609-619 [DOI] [PubMed] [Google Scholar]
- 14.Nag B, Kendrick T, Arimilli S, Yu SCT, Sriram S: Soluble MHC II-peptide complexes induce antigen-specific apoptosis in T cells. Cell Immunol 1996, 170:25-33 [DOI] [PubMed] [Google Scholar]
- 15.Osborne BA: Apoptosis and the maintenance of homeostasis in the immune system. Curr Opin Immunol 1996, 8:245-254 [DOI] [PubMed] [Google Scholar]
- 16.Billingham ME, Cary NRB, Hammond ME, Kemnitz J, Marboe C, McCallister HA, Snovar DC, Winters GL, Zerbe A: A working formulation for the standardization of nomenclature in the diagnosis of heart and lung rejection: heart rejection study group. J Heart Transplant 1990, 9:587-593 [PubMed] [Google Scholar]
- 17.Giordano C, Stassi G, De Maria R, Todaro M, Richiusa P, Papoff G, Ruberti G, Bagnasco M, Testi R, Galluzzo A: Potential involvement of Fas and its ligand in the pathogenesis of Hashimoto’s thyroiditis. Science 1997, 275:960-963 [DOI] [PubMed] [Google Scholar]
- 18.Gavrieli Y, Sherman Y, Ben-Sasson SA: Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol 1992, 119:493-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Hoffen E, Van Wichen D, Stuij I, De Jonge N, Klöpping C, Lahpor J, Van Den Tweel J, Gmelig-Meyling F, De Weger R: In situ expression of cytokines in human heart allografts. Am J Pathol 1996, 149:1991-2003 [PMC free article] [PubMed] [Google Scholar]
- 20.Armitage P, Berry G: Statistical Methods in Medical Research. ed 2 1987, :pp 411-417 Blackwell Scientific Publications, Oxford [Google Scholar]
- 21.Joshi A, Masek MA, Brown BW, Weiss LM, Billingham ME: “Quilty” revisited: a 10-year perspective. Hum Pathol 1995, 26:547-557 [DOI] [PubMed] [Google Scholar]
- 22.Boudet F, Lecoeur H, Gougeon ML: Apoptosis associated with ex vivo down-regulation of Bcl-2 and up-regulation of Fas in potential cytotoxic CD8+ T lymphocytes during HIV infection. J Immunol 1996, 156:2282-2293 [PubMed] [Google Scholar]
- 23.Dutra WO, Martins-Filho OA, Cançado JR, Pinto-Dias JC, Brener Z, Gazzinelli G, Carvalso JF, Colley DG: Chagasic patients lack CD28 expression on many of their circulating T lymphocytes. Scand J Immunol 1996, 43:88-93 [DOI] [PubMed] [Google Scholar]
- 24.Linsley PS, Bradshaw J, Urnes M, Grosmaire L, Ledbetter JA: CD28 engagement by B7/BB-1 induces transient down-regulation of CD28 synthesis and prolonged unresponsiveness to CD28 signaling. J Immunol 1993, 150:3161-3169 [PubMed] [Google Scholar]
- 25.Dhein J, Walczak H, Bäumler C, Debatin KM, Krammer PH: Autocrine T cell suicide mediated by APO-1/(Fas/CD95). Nature 1995, 373:438-441 [DOI] [PubMed] [Google Scholar]
- 26.Boehme SA, Zheng L, Lenardo MJ: Analysis of the CD4 coreceptor and activation-induced costimulatory molecules in antigen-mediated mature T lymphocyte death. J Immunol 1995, 155:1703-1712 [PubMed] [Google Scholar]
- 27.Bergese SD, Klenotic SM, Wakely ME, Sedmak DD, Orosz CG: Apoptosis in murine cardiac grafts. Transplantation 1997, 63:320-325 [DOI] [PubMed] [Google Scholar]
- 28.Laguens RP, Cabeza Meckert PM, San Martino J, Perrone S, Favaloro R: Identification of programmed cell death (apoptosis) in situ by means of specific labeling of nuclear DNA fragments in heart biopsy samples during acute rejection episodes. J Heart Lung Transplant 1996, 15:911-918 [PubMed] [Google Scholar]
- 29.Jollow KC, Sundstrom JB, Gravanis MB, Kanter K, Herskowitz A, Ansari AA: Apoptosis of mononuclear cell infiltrates in cardiac allograft biopsy specimens questions studies of biopsy-cultured cells. Transplantation 1997, 63:1482-1489 [DOI] [PubMed] [Google Scholar]
- 30.Dong C, Wilson JE, Winters GL, McManus BM: Human transplant coronary artery disease: pathological evidence for Fas-mediated apoptotic cytotoxicity in allograft artheriopathy. Lab Invest 1996, 74:921-931 [PubMed] [Google Scholar]
- 31.Ito H, Kasagi N, Shomori K, Osaki M, Adachi H: Apoptosis in the human allografted kidney: analysis by terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling. Transplantation 1995, 60:794-798 [PubMed] [Google Scholar]
- 32.Akbar AN, Salmon M: Cellular environments and apoptosis: tissue microenvironments control activated T cell death. Immunol Today 1997, 18:72-76 [DOI] [PubMed] [Google Scholar]
- 33.Munn DH, Pressey J, Beall AC, Hudes R, Alderson MR: Selective activation-induced apoptosis of peripheral T cells imposed by macrophages: a potential mechanism of antigen-specific peripheral lymphocyte deletion. J Immunol 1996, 156:523-532 [PubMed] [Google Scholar]
- 34.Bofill M, Gombert W, Borthwick NJ, Akbar AN, McLaughlin JE, Lee CA, Johnson MA, Pinching AJ, Janossy G: Presence of CD3+CD8+Bcl-2low lymphocytes undergoing apoptosis and activated macrophages in lymph nodes of HIV1+ patients. Am J Pathol 1995, 146:1542-1555 [PMC free article] [PubMed] [Google Scholar]
- 35.Badley AD, Dockrell D, Simpson M, Schut R, Lynch DH, Leibson P, Paya CV: Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-infected individuals is mediated by FasL and tumor necrosis factor. J Exp Med 1997, 185:55-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Weger RA, Van Hoffen E, Van Wichen DF, Broekhuizen R, Parker J, Bredée JJ, Gmelig-Meyling FHJ: Cytokine receptor clinical studies: expression of cytokine receptors in acute rejection of human heart allografts. Kishimoto T Kikutani H Von Dem Borne AEGKr Goyeret SM Mason DY Miyasaka M Moretta L Okumura K Shaw S Springer TA Sugamura K Zola H eds. Leucocyte Typing VI, White Cell Differentiation Antigens. 1997, :pp 920-922 Garland Publishing, Inc., New York [Google Scholar]
- 37.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ: Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 1995, 377:348-351 [DOI] [PubMed] [Google Scholar]
- 38.Strasser A, Harris AW, Huang DCS, Krammer PH, Cory S: Bcl-2 and Fas/APO-1 regulate distinct pathways to lymphocyte apoptosis. EMBO J 1995, 14:6136-6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Itoh N, Tsujimoto Y, Nagata S: Effect of bcl-2 on Fas antigen-mediated cell death. J Immunol 1993, 151:621-627 [PubMed] [Google Scholar]
- 40.Schreier MH, Baumann G, Zenke G: Conventional immunosuppressive agents: cyclosporin. Inhibition of T cell signaling pathways by immunophilin drug complexes. Are side effects inherent to immunosuppressive properties? Transplant Proc 1993, 25:502-507 [PubMed] [Google Scholar]
- 41.Broome HE, Dargan CM, Krajewski S, Reed JC: Expression of Bcl-2, Bcl-x, and Bax after T cell activation and IL-2 withdrawal. J Immunol 1995, 155:2311-2317 [PubMed] [Google Scholar]
- 42.Pawelec G, Hambrecht A, Rehbein A, Adibzadeh M: Interleukin 10 protects activated human T lymphocytes against growth factor withdrawal-induced cell death but only anti-Fas antibody can prevent activation-induced cell death. Cytokine 1996, 8:877-881 [DOI] [PubMed] [Google Scholar]
- 43.Finn PW, He H, Wang Y, Wang Z, Guan G, Listman J, Perkins DL: Synergistic induction of CTLA-4 expression by costimulation with TCR plus CD28 signals mediated by increased transcription and messenger ribonucleic acid stability. J Immunol 1997, 158:4074-4081 [PubMed] [Google Scholar]
- 44.Amendola A, Lombardi G, Oliverio S, Colizzi V, Piacentini M: HIV-1 gp120-dependent induction of apoptosis in antigen-specific human T cell clones is characterized by ‘tissue’ transglutaminase expression and prevented by cyclosporin A. FEBS Lett 1994, 339:258-264 [DOI] [PubMed] [Google Scholar]
- 45.Prud’homme GJ, Vanier LE, Bocarro DC, Ste-Croix H: Effects of cyclosporin A, rapamycin, and FK520 on peripheral T cell deletion and anergy. Cell Immunol 1995, 164:47-56 [DOI] [PubMed] [Google Scholar]
- 46.Narula J, Haider N, Virmani R, DiSalvo TG, Kolodgie FD, Hajjar RJ, Schmidt U, Semigran MJ, Dec GW, Khaw BA: Apoptosis in myocytes in end-stage heart failure. N Engl J Med 1996, 335:1182-1189 [DOI] [PubMed] [Google Scholar]
- 47.Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara JA, Quaini E, Di Loreto C, Beltrami CA, Krajewski S, Reed JC, Anversa P: Apoptosis in the failing human heart. N Engl J Med 1997, 336:1131-1141 [DOI] [PubMed] [Google Scholar]