Abstract
Inflammatory cells accumulate within the lungs of cigarette smokers. Current concepts suggest that these cells can induce protease-antiprotease and/or oxidant-antioxidant imbalance(s), which may damage the normal lung alveolar and interstitial structures. Because type II pneumocytes line the alveolar space, and because the inflammatory cells migrate and reside at the alveolus, we postulated that the type II pneumocytes might release chemotactic activity for neutrophils and monocytes in response to smoke extract. To test this hypothesis, A549 cells were cultured and the supernatant fluids were evaluated for the neutrophil and monocyte chemotactic activity (NCA and MCA) by a blind-well chamber technique. A549 cells released NCA and MCA in response to smoke extract in a dose- and time-dependent manner (P < 0.05). Checkerboard analysis showed that the activity was chemotactic. Partial characterization of NCA and MCA revealed that the activity was partly heat labile, trypsin sensitive, and ethyl acetate extractable. Lipoxygenase inhibitors and cycloheximide inhibited the release of NCA and MCA. Molecular sieve column chromatography showed multiple peaks for both NCA and MCA. NCA was inhibited by anti-human-interleukin (IL)-8 antibody, granulocyte colony-stimulating factor (G-CSF) antibody, or leukotriene (LT)B4 receptor antagonist. Monocyte chemoattractant protein (MCP)-1 antibody or LTB4 receptor antagonist inhibited MCA. Immunoreactive IL-8, G-CSF, MCP-1, and LTB4 significantly increased in the supernatant fluids in response to smoke extract. These data suggest that the type II pneumocytes may release NCA and MCA and modulate the inflammatory cell recruitment into the lung.
The association of cigarette smoke and bronchitis and pulmonary emphysema is well established. 1,2 Chronic exposure to cigarette smoke induces an influx of inflammatory cells into the lower respiratory tract. 3 The prevalent theory in the pathogenesis of the pulmonary emphysema is that the parenchymal damage is due to an imbalance between proteases and antiproteases and/or oxidants and antioxidants in the lung. 4 Studies in animal models have demonstrated that cigarette smoking is associated with the chronic accumulation of inflammatory cells in the lung. 5 Increased numbers of neutrophils and monocytes, activated by cigarette smoke, produce large amounts of proteases and oxidants. 6,7 The cigarette smoke can inactivate antiprotease protection. 8 Senior and co-workers reported that experimental emphysema was induced by intratracheal instillation of purified human neutrophil elastase in animals. 9 Thus, the cigarette smoke may influence both matrix damage and repair processes, leading to lung destruction by inflammatory processes.
Alveolar type II epithelial cells synthesize and secrete surfactant, control the volume and composition of the epithelial lining fluid, proliferate, and differentiate into type I alveolar epithelial cells after lung injury to maintain the integrity of the alveolar walls. 10 Lately they have been recognized to play a role in regulating the lung immune environment. It is reported that delipidated surfactant protein markedly augments the migration of alveolar macrophages in response to endotoxin-activated serum and that surfactant protein A expresses chemotactic activity for the monocytes. 11,12 Furthermore, the type II epithelial-like cell line, A549 cells, release monocyte chemoattractant activity (MCA) constitutively 13 and express interleukin (IL)-8 and monocyte chemoattractant protein (MCP)-1 in response to asbestos, tumor necrosis factor (TNF)-α, and IL-1β. 14-16 These cytokines have the potential to attract and activate inflammatory cells, leading to lung injury.
Cigarette smoke contains more than 4000 chemicals. 17 Among them, nicotine, one of the major components of cigarettes, is a chemotactic factor for neutrophils, and acrolein, one of the metabolites of cigarette smoking, stimulates the airway epithelial cells to release lipoxygenase products as neutrophil chemotactic factor (NCA). 18,19 Hunninghake and co-workers reported that smoke stimulates the alveolar macrophages to release NCA. 3 Kew et al have demonstrated that smoke extract can activate complements. 20 Robbins et al have shown that smoke activates the NCA of serum and inhibits the activity of chemotactic factor inactivator. 21 However, the possibility that the alveolar type II epithelial cells could interact with cigarette smoke to release the chemotactic activity remains to be elucidated.
Because neutrophils and monocytes play important roles in the pathogenesis of pulmonary emphysema and because type II epithelial cells participate in lung inflammatory responses, we hypothesized that smoke extract might stimulate type II epithelial cells to release NCA and MCA. The results demonstrate that a human alveolar epithelial-like cell line, A549 cells, released NCA and MCA in response to smoke extract, including IL-8, granulocyte colony-stimulating factor (G-CSF), MCP-1, and leukotriene (LT)B4.
Materials and Methods
Preparation of A549 Type II Alveolar Epithelial Cells
Because of difficulty in obtaining primary human type II epithelial cells of sufficient purity, A549 cells (passage 75; American Type Culture Collection, Rockville, MD), a pulmonary type II epithelial cell line derived from an individual with alveolar cell carcinoma, was used. 22 These cells retain many of the characteristics of the normal type II epithelial cells, such as surfactant production, cytoplasmic multilamellar inclusion bodies, and cuboidal appearance. 16 A549 cells were grown as monolayers on 100-mm-diameter tissue culture dishes. A549 cells were incubated in 100% humidity and 5% CO2 at 37°C with F-12 medium (GIBCO, Grand Island, NY) supplemented with penicillin (50 U/ml; GIBCO), streptomycin (50 μg/ml; GIBCO), fungizone (2 μg/ml; GIBCO), and 10% heat-inactivated fetal calf serum (FCS; GIBCO). The cells from monolayers were harvested with trypsin (0.25%) and EDTA (0.1%) in PBS (Sigma Chemical Co., St. Louis, MO), centrifuged at low speed (250 × g for 5 minutes), and resuspended in fresh medium at the concentration of 1.0 × 10 6 cells/ml in 35-mm-diameter tissue culture dishes. The cells were grown to confluence during 5 to 7 days of incubation. After the cells reached confluence, the cells were used for the experiment.
Preparation of Cigarette Smoke Extract
Smoke extract was prepared by a modification of the method of Carp and Janoff. 8 Briefly, two cigarettes without filters were combusted with a modified syringe-driven apparatus. The smoke was bubbled through 50 ml of Hanks’ balanced salt solution (HBSS; GIBCO). The resulting suspension was adjusted to pH 7.4 with concentrated NaOH and then filtered through a 0.20-μm pore filter (Lida Manufacturing Corp., Kenosha, WI) to remove bacteria and large particles. The resulting smoke extract was applied to A549 cell cultures within 30 minutes of preparation.
Exposure of A549 Cells to Smoke Extract
A549 cells were washed twice with serum-free F-12, and the cells were incubated in the presence and absence of smoke extract. To determine the dose- and time-dependent release of NCA and MCA, the cultures were incubated at various concentrations of smoke extract (0%, 0.5%, 1%, 5%, and 10%) for 12, 24, 48, 72, and 96 hours at 37°C in a humidified 5% CO2 atmosphere. Smoke extract did not cause A549 cell injury (no deformity of cell shape and no detachment from tissue culture dish, and greater than 95% of cells were viable by trypan blue exclusion) after 96 hours of incubation at the maximal doses. The supernatant fluids were harvested and stored at −80°C until assayed. At least six separate A549 cell supernatant fluids were harvested from cultures for each experimental condition.
Measurement of NCA and MCA
Polymorphonuclear leukocytes were purified from heparinized normal human blood by the method of Boyum. 23 Briefly, 15 ml of venous blood was obtained from healthy volunteers and then sedimented with 3% dextran in isotonic saline for 45 minutes to separate white blood cells from red blood cells. The leukocyte-rich upper layer was collected, and neutrophils were separated from mononuclear cells by Ficoll-Hypaque density centrifugation (Histopaque 1077, Sigma). The contaminating red blood cells were removed by lysing solution with 0.1% KHCO3 and 0.83% NH4Cl. The suspension was then centrifuged at 400 × g for 5 minutes and washed three times in HBSS. The resulting cell pellet consisted of >96% neutrophils and >98% viable cells as determined by trypan blue and erythrosin exclusion. The cells were suspended in Gey’s balanced salt solution (GIBCO) containing 2% bovine serum albumin (BSA; Sigma) at pH 7.2 to give a final concentration of 3.0 × 10 6 cells/ml. This suspension was used for the neutrophil chemotaxis assay.
Mononuclear cells for the chemotaxis assay were obtained from normal human volunteers by Ficoll-Hypaque density centrifugation to separate the red blood cells and neutrophils from the mononuclear cells. The mononuclear cells were harvested at the interface. The suspension was then centrifuged at 400 × g for 10 minutes and washed three times in HBSS. The preparation routinely consisted of 30% large monocytes and 70% small lymphocytes determined by morphology and α-naphthyl acetate esterase staining (Sigma) with >98% viability as assessed by trypan blue and erythrosin exclusion. The cells were suspended in Gey’s balanced salt solution containing 2% BSA at pH 7.2 to give a final concentration of 5.0 × 10 6 cells/ml. This suspension was then used for the monocyte chemotaxis assay.
The chemotaxis assay was performed in a 48-well microchemotaxis chamber (NeuroProbe, Cabin John, MD) as previously described. 24 The bottom wells of the chamber were filled with 25 μl of fluid containing the chemotactic stimulus or media in duplicate. A 10-μm-thick polyvinylpyrrolidone-free polycarbonate filter, with a pore size of 3 μm for the neutrophil chemotaxis and 5 μm for the monocyte chemotaxis, was placed over the bottom wells. The silicon gasket and upper pieces of the chamber were applied, and 50 μl of the cell suspension was placed into the upper wells above the filter. The chambers were incubated in humidified air in 5% CO2 at 37°C for 30 minutes for the neutrophil chemotaxis and 90 minutes for the monocyte chemotaxis. Nonmigrated cells were wiped away from the filter. The filter was then immersed in methanol for 5 minutes, stained with Diff-Quik, and mounted on a glass slide. The cells that completely migrated through the filter were counted using light microscopy in 10 random high-power fields (HPF, ×1000) per well.
To ensure that monocytes, but not lymphocytes, were the primary cells that migrated in the monocyte chemotaxis assay, some membranes were stained with α-naphthyl acetate esterase according to the manufacturer’s directions (Sigma).
To determine whether the migration was due to movement along a concentration gradient (chemotaxis) or stimulation of random migration (chemokinesis), a checkerboard analysis was performed with A549 cell supernatant fluid harvested at 72 hours in response to 5% smoke extract. 25 To do this, various dilutions of A549 cell supernatant fluids (1:1, 1:4, 1:16, 1:64, and 1:256) were placed below the membrane and above the membrane with target cells.
Partial Characterization of NCA and MCA
Partial characterization of NCA and MCA released from A549 cells was performed with the supernatant fluids harvested after a 72-hour incubation with 5% smoke extract. Sensitivity to proteases was tested by incubating the supernatant fluids with trypsin (100 μg/ml; Sigma) for 30 minutes at 37°C followed by the addition of a 1.5 mol/L excess of soybean trypsin inhibitor to terminate the proteolytic activity, and then the chemotactic activity was evaluated. The lipid solubility was evaluated by mixing the supernatant fluids twice with ethyl acetate, decanting the lipid phase after each extraction, evaporating the ethyl acetate to dryness, and resuspending the extracted material in F-12 used for the cell culture before the chemotaxis assay. Both the extracted and extractant materials were evaluated for chemotactic activity. Heat sensitivity was determined by heating the supernatant fluids at 98°C for 15 minutes.
Molecular Sieve Column Chromatographic Findings of NCA and MCA
To determine the approximate molecular weight of the released activity in the supernatant fluids harvested at 72 hours in response to 5% smoke extract, molecular sieve column chromatography was performed using Sephadex G-200 (Pharmacia, Piscataway, NJ). At a flow rate of 6 ml/hour, A549 cell culture supernatant fluid was eluted with PBS, and fractions were evaluated for NCA and MCA in duplicate.
Effects of Metabolic Inhibitors on the Release of NCA and MCA
The effects of nonspecific lipoxygenase inhibitors, nordihydroguaiaretic acid (NDGA, 100 μmol/L; Sigma), diethylcarbamazine (DEC, 1 mmol/L; Sigma), and 5-lipoxygenase inhibitor AA-861 (100 μmol/L; Takeda Pharmaceutical Co., Tokyo, Japan) on the release of NCA and MCA in response to 5% smoke extract for a 72-hour incubation were evaluated. To further examine the involvement of protein synthesis in the release of the chemotactic activity, cycloheximide (20 μg/ml; Sigma) was added to inhibit protein synthesis. 26
Effects of LTB4 and PAF Receptor Antagonists on NCA and MCA
Because the release of NCA and MCA was blocked by 5-lipoxygenase inhibitors, and because NCA and MCA were extracted into ethyl acetate, LTB4 receptor antagonist (ONO 4057, ONO Pharmaceutical Co., Tokyo, Japan) and platelet-activating factor (PAF) receptor antagonist (TCV 309, Takeda Pharmaceutical Co.) at the concentration of 10−5 mol/L were used to evaluate the involvement of LTB4 and PAF for NCA and MCA. 27,28
Measurement of LTB4 and PAF in the Supernatant Fluid
The concentration of LTB4 in the supernatants was measured by radioimmunoassay (RIA) as previously described. 29-31 Anti-LTB4 serum, [5,6,8,9,11,12,14,15,3H(N)]-LTB4, and synthetic LTB4 were purchased from Amersham Co. (Arlington Heights, IL). Briefly, ethanol and supernatant mixtures were centrifuged at 5500 × g at 0°C. At a temperature of 37°C, the supernatants were evaporated under N2 gas to remove ethanol. To each sample, 10 ml of distilled water was added. These samples were acidified to pH 4.0 with 0.1 mol/L hydrochloric acid and applied to Sep-Pak C l8 columns (Waters Associates, Milford, MA). The columns were washed with a 10-ml mixture of distilled water and 20 ml of petroleum ether and then eluted with 15 ml of methanol. These eluates were dried with N2 gas at 37°C and then redissolved in 20 μl of methanol and 180 μl of RIA buffer (50 mmol/L Tris/HCl buffer containing 0.1% (w/v) gelatin, pH 8.6). [3H]LTB4 was diluted in RIA buffer (100 μl, containing approximately 4000 dpm) and mixed with 100 μl of standards or samples in disposable siliconized tubes. Anti-LTB4 serum, diluted by RIA buffer (100 μl), was added to siliconized tubes to give a total incubation volume of 400 μl. The mixture was incubated at 4°C for 18 hours. Free LTB4 was absorbed onto dextran-coated charcoal. The supernatant, containing the antibody-bound LTB4 was decanted into scintillation counter after centrifugation for 15 minutes at 2000 × g. Scintillation fluid (Aquazol 2, NEN Co., Boston, MA) was added, and radioactivity was counted by a scintillation counter (Tricarb-3255, Tackard Co., IL) for 4 minutes.
PAF in the supernatant fluids was evaluated via the scintillation proximity assay system. Briefly, this assay system combined the use of a high-specific-activity tritiated PAF tracer with an antibody specific for PAF and a PAF standard similar to the methods of measurement of LTB4.
Effects of Polyclonal Antibodies to IL-8, G-CSF, MCP-1, GM-CSF, RANTES, and TGF-β
The neutralizing antibodies to human IL-8, G-CSF, MCP-1, RANTES (regulated on activation, normal T cells, expressed and secreted), granulocyte-macrophage colony-stimulating factor (GM-CSF), and transforming growth factor (TGF)-β were purchased from Genzyme (Cambridge, MA). They were added to the A549 cell supernatant fluids that were harvested at 72 hours in response to 5% smoke extract at the suggested concentration to inhibit these cytokines and incubated for 30 minutes at 37°C. To evaluate the nonspecific effect of IgG, nonimmune IgG was added to the same supernatant fluids and incubated for 30 minutes at 37°C. These samples were then used for the chemotactic assay. These antibodies did not influence the chemotactic response to endotoxin-activated serum (data not shown).
Measurement of IL-8, G-CSF, MCP-1, GM-CSF, RANTES, and TGF-β in the Supernatant Fluids
The concentrations of IL-8, G-CSF, MCP-1, GM-CSF, RANTES, and TGF-β in A549 cell supernatant fluids cultured for 72 hours in response to 5% smoke extract were measured by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer’s directions. GM-CSF and RANTES kits were purchased from Amersham (Little Chalfont, UK), and the minimal concentration detected by these methods was 2.00 pg/ml for GM-CSF and 15.6 pg/ml for RANTES. IL-8, MCP-1, and TGF-β kits were purchased from R&D Systems (Minneapolis, MN), and the minimal detectable concentration of IL-8, MCP-1, and TGF-β was 10.0, 31.3, and 310 pg/ml, respectively. The G-CSF kit was obtained from Chugai Pharmaceutical Co., Tokyo, Japan. The minimal concentration of G-CSF detected by this kit was 1.0 pg/ml.
Statistics
In experiments where multiple measurements were made, differences between groups were tested for significance using one-way analysis of variance with Duncan’s multiple range test applied to data at specific time and dose points. In experiments where a single measurement was made, the differences between groups were tested for significance using Student’s paired t-test. In all cases, a P value less than 0.05 was considered significant. The data in the figures and tables are expressed as means ± SEM.
Results
Dose- and Time-Dependent Release of NCA and MCA from A549 Cells
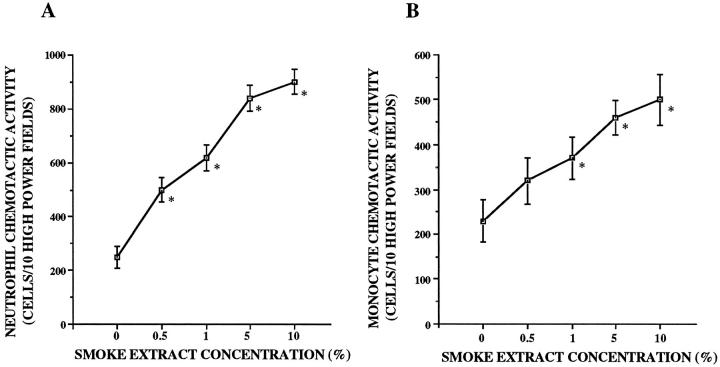
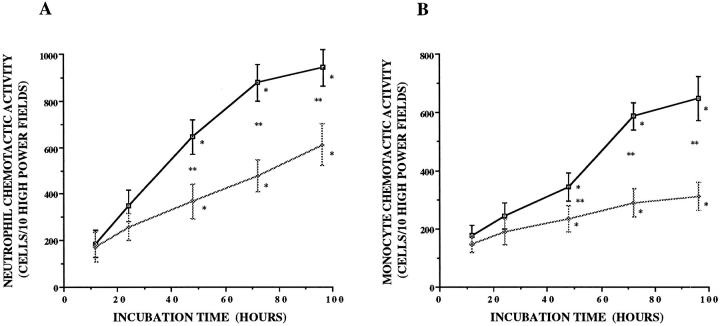
In response to smoke extract, A549 cells released NCA and MCA in a dose-dependent manner (P < 0.05; Figure 1, A and B ▶ ). The lowest doses of smoke extract to stimulate A549 cells were 0.5% for neutrophils and 1% for monocytes. Increasing concentrations of smoke extract progressively increased the release of chemotactic activity up to 10%. A549 cells released NCA and MCA in response to smoke extract in a time-dependent manner (P < 0.05; Figure 2, A and B ▶ ). After the exposure to smoke extract, the release of NCA and MCA was significant after 48 hours (P < 0.05; Figure 2, A and B ▶ ). Smoke extract itself was not chemotactic for neutrophils and monocytes (data not shown).
Figure 1.
Dose-dependent release of the neutrophil (A) and monocyte (B) chemotactic activity from A549 cell monolayers in response to smoke extract (n = 8). Chemotactic activity is on the ordinate, and the concentration of smoke extract is on the abscissa. *P < 0.05 compared with the control supernatant fluids.
Figure 2.
Time-related release of the neutrophil (A) and monocyte (B) chemotactic activity from A549 cell monolayers in response to 5% smoke extract (n = 8). The chemotactic activity is on the ordinate, and the incubation time is on the abscissa. □, chemotactic activity stimulated by smoke extract; ⋄, chemotactic activity without smoke extract. *P < 0.05 compared with F-12 medium; **P < 0.05 compared with the supernatant fluids without smoke extract.
The chemotactic responses to LTB4 at the concentration of 10−7 mol/L as positive control were 1020 ± 74 cells/10 HPF for neutrophils and 756 ± 34 cells/10 HPF for monocytes.
Checkerboard analysis revealed that the A549 cell supernatant fluids stimulated by smoke extract induced neutrophil and monocyte migration in the presence of a gradient across the membrane in a concentration-dependent manner. However, a smaller increase of the neutrophil and monocyte migration was observed in the absence of a gradient (Table 1) ▶ . Thus, the migration of neutrophils and monocytes was predominantly consistent with chemotactic rather than chemokinetic activity.
Table 1.
Checkerboard Analysis of the A549 Cell Culture Supernatant Fluid Harvested after 72 Hours in Response to 5% Smoke Extract
| Lower well | F-12 | Upper well | ||||
|---|---|---|---|---|---|---|
| 1:256 | 1:64 | 1:16 | 1:4 | 1:1 | ||
| Neutrophils | ||||||
| F-12 | 4 ± 2 | 6 ± 1 | 4 ± 1 | 5 ± 1 | 5 ± 1 | 4 ± 1 |
| 1:256 | 6 ± 3 | 6 ± 2 | 3 ± 1 | 6 ± 3 | 2 ± 1 | 4 ± 1 |
| 1:64 | 7 ± 1 | 4 ± 2 | 4 ± 3 | 3 ± 1 | 3 ± 3 | 9 ± 3 |
| 1:16 | 4 ± 2 | 6 ± 1 | 5 ± 2 | 2 ± 1 | 2 ± 1 | 3 ± 1 |
| 1:4 | 13 ± 2 | 16 ± 3 | 11 ± 1 | 6 ± 2 | 5 ± 2 | 3 ± 1 |
| 1:1 | 18 ± 3 | 44 ± 4 | 20 ± 4 | 3 ± 1 | 7 ± 1 | 4 ± 1 |
| Monocytes | ||||||
| F-12 | 5 ± 1 | 9 ± 1 | 9 ± 2 | 2 ± 4 | 4 ± 2 | 4 ± 1 |
| 1:256 | 6 ± 2 | 6 ± 1 | 9 ± 3 | 3 ± 2 | 3 ± 2 | 5 ± 3 |
| 1:64 | 7 ± 2 | 8 ± 3 | 10 ± 3 | 5 ± 3 | 5 ± 2 | 4 ± 2 |
| 1:16 | 9 ± 1 | 11 ± 1 | 11 ± 3 | 11 ± 4 | 10 ± 2 | 8 ± 1 |
| 1:4 | 9 ± 3 | 12 ± 1 | 11 ± 1 | 18 ± 2 | 11 ± 3 | 5 ± 3 |
| 1:1 | 18 ± 1 | 26 ± 3 | 12 ± 1 | 17 ± 3 | 11 ± 4 | 10 ± 3 |
The vertical column represents the dilution of A549 cell supernatant fluids in the lower wells, and the horizontal row represents the dilutions of supernatant fluids in upper wells with cells.
Confirmation that the migrated cells were monocytes was provided by the following lines of evidence: 1) >90% of the migrated cells appeared to be monocytes morphologically by light microscopy; 2) >90% of the migrated cells were esterase positive; and 3) lymphocytes purified by allowing the monocytes to attach to plastic and tested in the chemotaxis assay yielded 0% to 20% of the chemotactic activity of the monocyte preparation.
Partial Characterization of NCA and MCA
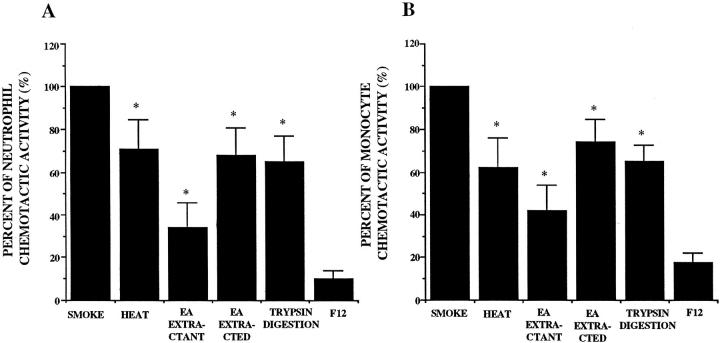
The NCA and MCA were heterogeneous in character. Both NCA and MCA were partially but significantly sensitive to heat, extractable into ethyl acetate, and partially digested by trypsin (Figure 3, A and B) ▶ .
Figure 3.
Partial characterization of the released neutrophil (A) and monocyte (B) chemotactic activity from A549 cell monolayer in response to 5% smoke extract after a 72-hour incubation (n = 6). Percentage of chemotactic activity is on the ordinate, and the experimental groups are on the abscissa. *P < 0.05 compared with the smoke extract exposed supernatant fluids. EA, ethyl acetate.
Molecular Sieve Column Chromatographic Findings of NCA and MCA
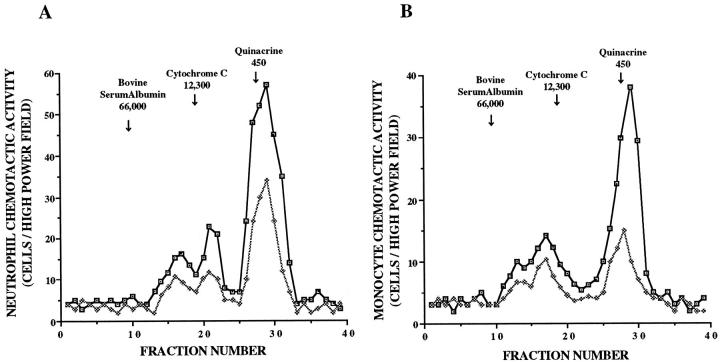
The released chemotactic activity of the supernatant fluids was evaluated by molecular sieve column chromatography using Sephadex G-200. These experiments revealed that NCA obtained from the unstimulated cells was heterogeneous in size (Figure 4A) ▶ . At least three peaks of activity were separated by column chromatography with two peaks near cytochrome c (molecular weight, 12,300) and an additional peak that eluted near quinacrine (molecular weight, 450). By stimulation of smoke extract, these peaks became prominent.
Figure 4.
Molecular sieve column chromatographic findings of the released neutrophil (A) and monocyte (B) chemotactic activity in response to 5% smoke extract harvested after a 72-hour incubation. Chemotactic activity is on the ordinate, and fraction numbers are on the abscissa. The data presented are representative of four experiments. □, chemotactic activity stimulated by smoke extract; ⋄, chemotactic activity without smoke extract.
The MCA from the unstimulated cells was also heterogeneous (Figure 4B) ▶ . At least three peaks of activity were separated by column chromatography with two peaks between BSA and cytochrome c, and an additional peak eluted near quinacrine. When stimulated with smoke extract, each peak became prominent.
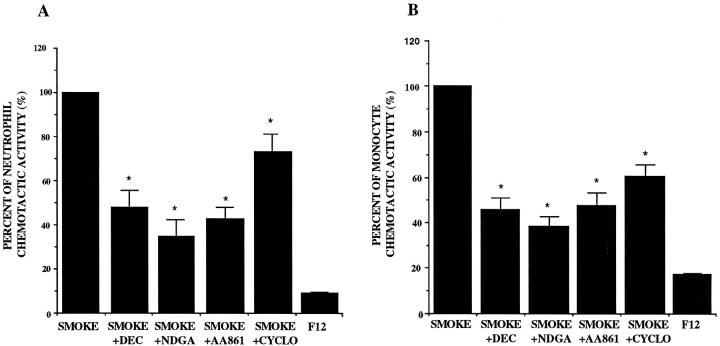
Effects of Metabolic Inhibitors on the Release of NCA and MCA
The 72-hour supernatant fluids incubated with 5% smoke extract in the presence of NDGA, DEC, and AA-861 showed a significant decrease in the release of NCA and MCA. Cycloheximide also inhibited the release of NCA and MCA (P < 0.05; Figure 5, A and B ▶ ).
Figure 5.
Effects of nordihydroguaiaretic acid (NDGA), diethylcarbamazine (DEC), AA-861, and cycloheximide (CYCLO) on the release of the neutrophil (A) and monocyte (B) chemotactic activity in response to 5% smoke extract harvested after a 72-hour incubation (n = 8). Percentage of chemotactic activity is on the ordinate, and the experimental groups are on the abscissa. *P < 0.05 compared with the smoke-extract-exposed supernatant fluids.
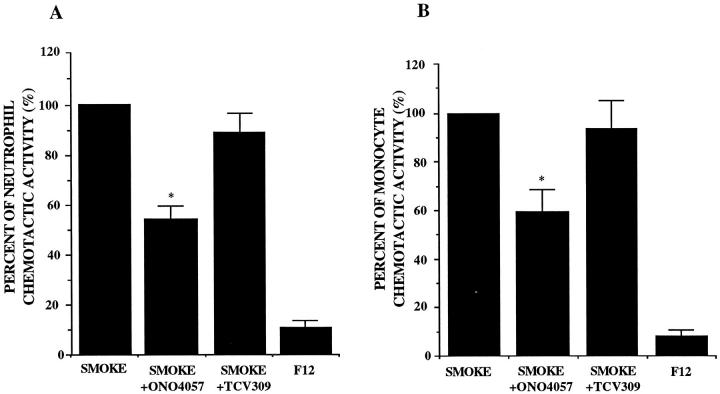
Effects of LTB4 and PAF Receptor Antagonists on NCA and MCA
NCA and MCA in the supernatant fluids were significantly inhibited by the addition of LTB4 receptor antagonist ONO4057, approximately 50% for NCA and 40% for MCA (Figure 6, A and B) ▶ . ONO4057 also inhibited the chromatography-separated lowest molecular weight peak more than 80% for both NCA and MCA (data not shown). The effects of PAF receptor antagonist TCV 309 on chemotactic activity were not significant for NCA and MCA. Each receptor antagonist at the concentration of 10−5 mol/L completely inhibited the neutrophil migration in response to 10−7 mol/L LTB4 and PAF, respectively, but showed no inhibitory effects on activated-serum-induced neutrophil and monocyte chemotaxis (data not shown).
Figure 6.
Effects of LTB4 (ONO 4057) and PAF receptor (TCV 309) antagonists on the released neutrophil (A) and monocyte (B) chemotactic activity obtained from A549 cell monolayers incubated with 5% smoke extract for 72 hours (n = 8). Percentage of the chemotactic activity is on the ordinate, and the experimental groups are on the abscissa. *P < 0.05 compared with the smoke-extract-exposed supernatant fluids.
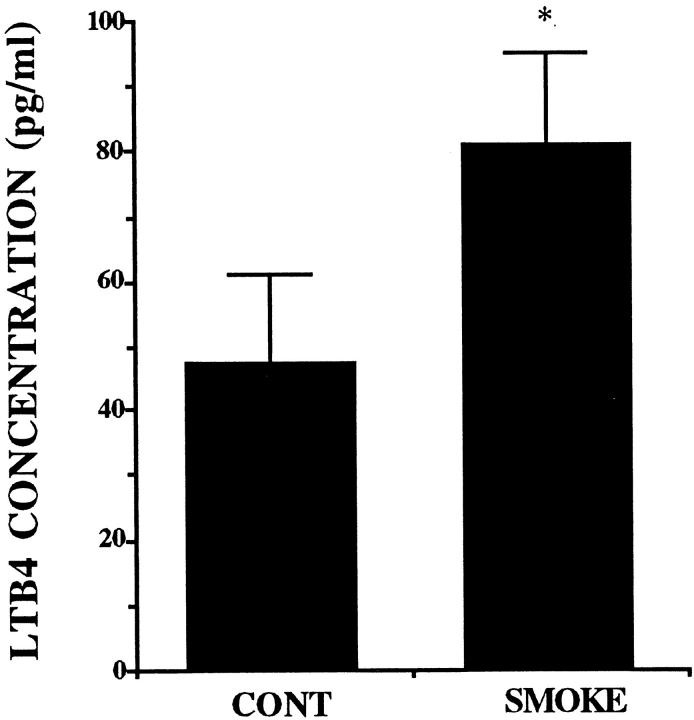
Effects of Smoke Extract on the Release of LTB4 and PAF
The measurement of LTB4 by RIA revealed that A549 cells released LTB4 in the baseline culture condition. The addition of smoke extract at the concentration of 5% for 72 hours induced a significant increase in LTB4 release from A549 cells (P > 0.05; Figure 7 ▶ ). In contrast, PAF was not detected in the baseline and smoke-extract-stimulated supernatant fluids.
Figure 7.
The release of LTB4 from A549 cell monolayer in response to 5% smoke extract harvested after a 72-hour incubation (n = 6). The concentration of LTB4 is on the ordinate, and the experimental groups are on the abscissa. *P < 0.05 compared with the supernatant fluids without smoke extract.
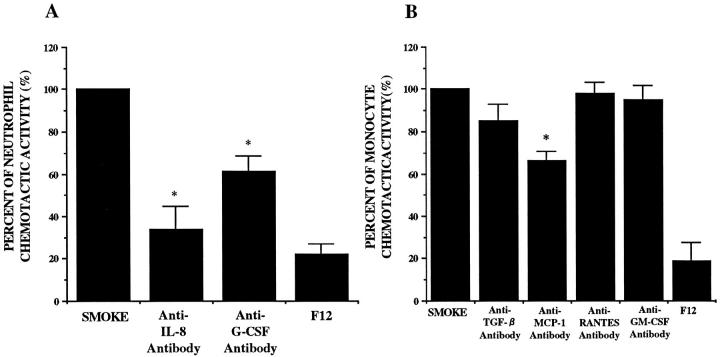
Effects of Polyclonal Antibodies to IL-8, G-CSF, MCP-1, GM-CSF, RANTES, and TGF-β
We evaluated the capacity of polyclonal blocking antibodies to IL-8, G-CSF, MCP-1, GM-CSF, RANTES, or TGF-β to reduce NCA and MCA. Anti-IL-8 and G-CSF antibodies significantly blocked NCA. Anti-MCP-1 antibody significantly reduced MCA (Figure 8) ▶ . Nonimmune IgG did not have any effects on NCA and MCA. We evaluated the effects of IL-8, G-CSF, and MCP-1 antibodies on the column-chromatography-separated high-molecular-weight peaks. These antibodies inhibited the chemotactic activity at the corresponding molecular weight peak (data not shown).
Figure 8.
Effects of anti-IL-8, G-CSF, GM-CSF, TGF-β, RANTES, and MCP-1 polyclonal antibodies on the released neutrophil (A) and monocyte (B) chemotactic activity obtained from A549 cell monolayers incubated with 5% smoke extract for 72 hours (n = 6). Percentage of the chemotactic activity is on the ordinate, and the experimental groups are on the abscissa. *P < 0.05 compared with the smoke-extract-exposed supernatant fluid.
Effects of Smoke Extract on the Release of IL-8, G-CSF, MCP-1, GM-CSF, RANTES, and TGF-β
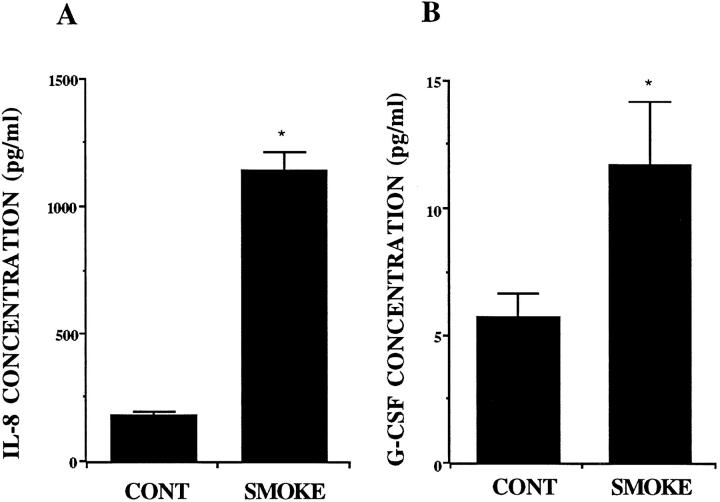
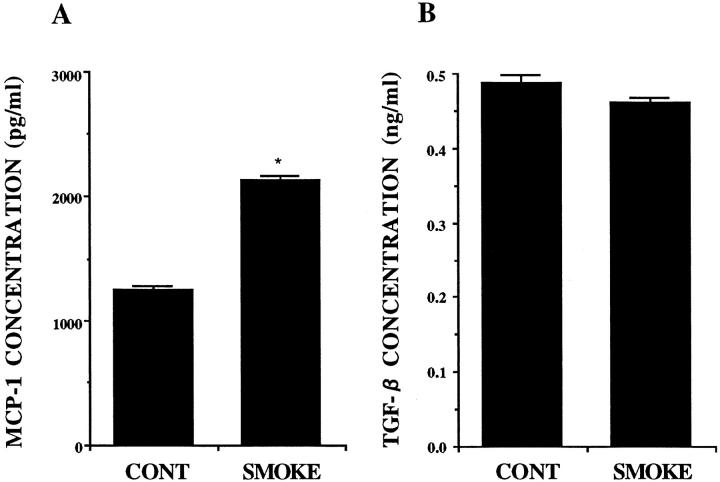
The measurement of chemokines by ELISA revealed that A549 cells released IL-8, G-CSF, MCP-1, and TGF-β constitutively. Smoke extract stimulated the release of IL-8 G-CSF, and MCP-1 significantly (Figures 9, A and B, and 10A) ▶ ▶ , but smoke extract did not stimulate the release of TGF-β (Figure 10B) ▶ . GM-CSF and RANTES were not detected in the A549 cell supernatant fluids.
Figure 9.
The release of IL-8 (A) and G-CSF (B) from A549 cell monolayers in response to 5% smoke extract harvested after a 72-hour incubation (n = 6). The concentration is on the ordinate, and the experimental groups are on the abscissa. *P < 0.05 compared with the supernatant fluids without smoke extract.
Figure 10.
The release of MCP-1 (A) and TGF-β (B) from A549 cell monolayers in response to 5% smoke extract after a 72-hour incubation (n = 6). The concentration is on the ordinate, and the experimental groups are on the abscissa. *P < 0.05 compared with the supernatant fluids without smoke extract.
Discussion
In the present study, A549 cells released NCA and MCA in response to smoke extract in a dose- and time-dependent manner. The released activity was heterogeneous. Anti-IL-8 and G-CSF antibodies and LTB4 receptor antagonist inhibited NCA. Anti-MCP-1 antibody and LTB4 receptor antagonist inhibited MCA. In response to smoke extract, LTB4, IL-8, G-CSF, and MCP-1 were significantly released. A549 cells are derived from alveolar cell carcinoma, and this cell line may be particularly susceptible to up-regulation of these factors. However, it is reported that the primary human alveolar type II cells and primary human bronchial epithelial cells release IL-8 in response to smoke extracts to a similar degree as our report. 32,33 Thus, these data suggest that the type II pneumocytes play a role in the pathogenesis of pulmonary emphysema by releasing NCA and MCA in response to smoke extract and may modulate the inflammatory cell recruitment to the lung.
It is recognized that destruction of the alveolar structures requires enzymes with elastolytic activity. A large amount of evidence suggests that neutrophils are the source of this elastolytic activity. Although a monocyte contains approximately 3% of elastase compared with a neutrophil, it is recently reported that the macrophage elastase is sufficient for the development of pulmonary emphysema. Macrophage elastase-deficient mice did not develop emphysema after chronic inhalation of cigarette smoke. 6 Thus, both the neutrophil and the monocyte may contribute to the etiology of pulmonary emphysema in cigarette smokers.
The type II alveolar epithelial cells had been regarded as passive bystanders in the immune interactions. However, previous studies have shown that A549 cells can release soluble chemotactic factors that direct the migration of neutrophils and monocytes into the alveolar space in response to TNF-α and IL-1β. 14,16 Koyama et al have reported that A549 cells released chemoattractant activity for monocytes spontaneously. 13 The present study demonstrated that A549 cells can also release NCA and MCA in response to smoke extract and suggested the possibility that the type II alveolar epithelial cells play a role in defining the lung inflammatory environment.
The present study demonstrates that several chemotactic factors were released by A549 cells that may contribute to the inflammatory cell recruitment. Partial characterization revealed that the released NCA and MCA were partly ethyl acetate extractable. Pretreatment with AA-861, NDGA, and DEC inhibited the release of NCA and MCA. Molecular sieve column chromatography showed that there was a large chemotactic peak in the lowest molecular range. The chemotactic activity in the lowest molecular peak was inhibited by LTB4 receptor antagonist. Furthermore, the concentration of LTB4 assessed by RIA is high enough to produce neutrophil and monocyte chemotactic activity. Smoke extract increased the release of LTB4 into A549 cell culture supernatant fluids. In this context, LTB4 may be the predominant chemotactic activity.
In contrast, the trypsin sensitivity of the chemotactic activity along with the inhibition of the release by cycloheximide suggests that the activity was at least partly dependent on protein synthesis. Molecular sieve column chromatography revealed increases in the high molecular weight peaks of chemotactic activity in response to smoke extract. The antibodies to IL-8, G-CSF, and MCP-1 inhibited the NCA and MCA. IL-8, G-CSF, and MCP-1 were significantly increased in the supernatant fluid in response to smoke extract. These concentrations of IL-8, G-CSF and MCP-1 were chemotactic for neutrophils and monocytes, respectively. These data suggest that these cytokines may play important roles in the recruitment of inflammatory cells into the lungs of smokers.
Early descriptions of cytokines focused on their production by immune and inflammatory effector cells. However, it is apparent that structural cells are also capable of releasing many cytokines. A549 cells are known to produce a variety of cytokines, including IL-8, 14,15 G-CSF, 34 TGF-β, 13 and MCP-1, 16 in response to a variety of stimuli. However, the relation between smoking and the release of these cytokines has not been established. The present study demonstrated that A549 cells released these cytokines as chemotactic factors in response to smoke extract and suggest that these cytokines may play a role in smoking-induced lung disease by recruiting inflammatory cells.
Although TGF-β was detected in the supernatant fluid, TGF-β antibody did not attenuate monocyte chemotactic activity. TGF-β induces monocyte chemotaxis at concentrations from 0.1 to 10 pg/ml. 35 At higher concentration, the chemotactic response of monocytes declines. It was reported that the biologically inactive form of TGF-β, which constitutes more than 98% of autocrine TGF-β, is secreted by 12 different cell types. TGF-β was unable to bind to the receptor without previous proteolytic activation. 36 The release of inactive TGF-β may account for the lack of inhibition of MCA in the A549 cell supernatant fluids by anti-TGF-β..
G-CSF could be an important factor determining the number and functional activity of neutrophils. G-CSF has been reported to induce neutrophil migration at concentrations of more than 10 to 100 U/ml (7 to 10 ng/ml). 37 The concentration of G-CSF in the supernatant fluids released from A549 cells was relatively low in the present study. However, the blocking antibody of G-CSF inhibited chemotactic response of neutrophils up to 46%. Recently, we have found that doses of 10 to 100 pg/ml G-CSF will induce significant NCA. 34 Although G-CSF may be facilitating the chemotactic response of other cytokines, the concentration of G-CSF in the culture supernatant fluids exceeded the lower chemotactic threshold observed in our laboratory.
In conclusion, A549 cells released chemotactic activity toward neutrophils and monocytes in response to cigarette smoke extract. The released activity was lipid and peptide in its nature and involved LTB4, IL-8, G-CSF, and MCP-1. These data suggest the possibility that the type II alveolar epithelial cells may play an important role in the recruitment of the inflammatory cells in the lung in response to cigarette smoke.
Footnotes
Address reprint requests to Dr. Sekiya Koyama, The First Department of Internal Medicine, Shinshu University School of Medicine, 3–1-1 Asahi Matsumoto, 390 Japan.
References
- 1.Auerbach O, Stout AP, Hammond EC, Garfinkel L: Smoking habits and age in relation to pulmonary changes: rupture of alveolar septums, fibrosis and thickening of walls of small arteries and arterioles. N Engl J Med 1963, 269:1045-1054 [DOI] [PubMed] [Google Scholar]
- 2.Auerbach O, Hammond EC, Garfinkel L, Benante C: Relation of smoking and age to emphysema: whole-lung section study. N Engl J Med 1972, 286:853-857 [DOI] [PubMed] [Google Scholar]
- 3.Hunninghake GW, Crystal RG: Cigarette smoking and lung destruction: accumulation of neutrophils in the lungs of cigarette smokers. Am Rev Respir Dis 1983, 128:833-838 [DOI] [PubMed] [Google Scholar]
- 4.Janoff A: Elastases and emphysema: current assessment of the protease-antiprotease hypothesis. Am Rev Respir Dis 1985, 132:417-433 [DOI] [PubMed] [Google Scholar]
- 5.Snider GL, Lucey EC, Stone PJ: Animal models of emphysema. Am Rev Respir Dis 1986, 133:149-169 [DOI] [PubMed] [Google Scholar]
- 6.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD: Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997, 277:2002-2004 [DOI] [PubMed] [Google Scholar]
- 7.Hinman LM, Stevens CA, Matthay RA, Gee JB: Elastase and lysozyme activities in human alveolar macrophages: effects of cigarette smoking. Am Rev Respir Dis 1980, 121:263-271 [DOI] [PubMed] [Google Scholar]
- 8.Janoff A, Carp H: Possible mechanisms of emphysema in smokers: cigarette smoke condensate suppresses protease inhibition in vitro. Am Rev Respir Dis 1977, 116:65-72 [DOI] [PubMed] [Google Scholar]
- 9.Senior RM, Tegner H, Kuhn C, Ohlsson K, Starcher BC, Pierce JA: The induction of pulmonary emphysema with human leukocyte elastase. Am Rev Respir Dis 1977, 116:469-475 [DOI] [PubMed] [Google Scholar]
- 10.Hahon N, Castranova V: Interferon production in rat type II pneumocytes and alveolar macrophages. Exp Lung Res 1989, 15:429-445 [DOI] [PubMed] [Google Scholar]
- 11.Wright JR, Youmans DC: Pulmonary surfactant protein A stimulates chemotaxis of alveolar macrophage. Am J Physiol 1993, 264:L338-L344 [DOI] [PubMed] [Google Scholar]
- 12.Hoffman RM, Claypool WD, Katyal SL, Singh G, Rogers RM, Dauber JH: Augmentation of rat alveolar macrophage migration by surfactant protein. Am Rev Respir Dis 1987, 135:1358-1362 [DOI] [PubMed] [Google Scholar]
- 13.Koyama S, Sato E, Nomura H, Kubo K, Nagai S, Izumi T: Type II pneumocytes release chemoattractant activity for monocytes constitutively. Am J Physiol 1997, 272:L830-L837 [DOI] [PubMed] [Google Scholar]
- 14.Standiford TJ, Kunkel SL, Basha MA, Chensue SW, Lynch JP, Toews GB, Westwick J, Strieter RM: Interleukin-8 gene expression by a pulmonary epithelial cell line: a model for cytokine networks in the lung. J Clin Invest 1990, 86:1945-1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenthal GJ, Germolec DR, Blazka ME, Corsini E, Simeonova P, Pollock P, Kong LY, Kwon J, Luster MI: Asbestos stimulates IL-8 production from human lung epithelial cells. J Immunol 1994, 153:3237-3244 [PubMed] [Google Scholar]
- 16.Standiford TJ, Kunkel SL, Phan SH, Rollins BJ, Strieter RM: Alveolar macrophage-derived cytokines induce monocyte chemoattractant protein-1 expression from human pulmonary type II-like epithelial cells. J Biol Chem 1991, 266:9912-9918 [PubMed] [Google Scholar]
- 17.Huber GL, First MW, Grubner O: Marijuana and tobacco smoke gas-phase cytotoxins. Pharmacol Biochem Behav 1991, 40:629-336 [DOI] [PubMed] [Google Scholar]
- 18.Nowak D, Ruta U, Piasecka G: Nicotine increases human polymorphonuclear leukocytes chemotactic response: a possible additional mechanism of lung injury in cigarette smokers. Exp Pathol 1990, 39:37-43 [DOI] [PubMed] [Google Scholar]
- 19.Doupnik CA, Leikauf GD: Acrolein stimulates eicosanoid release from bovine airway epithelial cells. Am J Physiol 1990, 259:L222-L229 [DOI] [PubMed] [Google Scholar]
- 20.Kew RR, Ghebrehiwet B, Janoff A: The role of complement in cigarette smoke-induced chemotactic activity of lung fluids. Am Rev Respir Dis 1986, 133:478-481 [DOI] [PubMed] [Google Scholar]
- 21.Robbins RA, Gossman GL, Nelson KJ, Koyama S, Thompson AB, Rennard SI: Inactivation of chemotactic factor inactivator by cigarette smoke: a potential mechanism of modulating neutrophil recruitment to the lung. Am Rev Respir Dis 1990, 142:763-768 [DOI] [PubMed] [Google Scholar]
- 22.Lieber M, Smith B, Szakal A, Nelson-Rees W, Todaro G: A continuous tumor-cell line from a human lung carcinoma with properties of type II alveolar epithelial cells. Int J Cancer 1976, 17:62-70 [DOI] [PubMed] [Google Scholar]
- 23.Boyum A: Isolation of mononuclear cells and granulocytes from human blood: isolation of mononuclear cells by one centrifugation and of granulocytes by combining centrifugation, and sedimentation at 1 g. Scand J Clin Lab Invest 1968, 97:S77-S89 [PubMed] [Google Scholar]
- 24.Harvath L, Falk W, Leonard EJ: Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods 1980, 37:39-45 [DOI] [PubMed] [Google Scholar]
- 25.Zigmond SH, Hirsch JG: Leukocyte locomotion and chemotaxis: new methods for evaluation and demonstration of a cell-derived chemotactic factor. J Exp Med 1973, 137:387-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldberg IH: Mode of action of antibiotics: drugs affecting nucleic acid and protein synthesis. Am J Med 1965, 39:722-752 [DOI] [PubMed] [Google Scholar]
- 27.Kishikawa K, Tateishi N, Maruyama T, Seo R, Toda M, Miyamoto T: ONO-4057, a novel, orally active leukotriene B4 antagonist: effects on LTB4-induced neutrophil functions. Prostaglandins 1992, 44:261-75 [DOI] [PubMed] [Google Scholar]
- 28.Terashita Z, Kawamura M, Takatani M, Tsushima S, Imura Y, Nishikawa K: Beneficial effects of TCV-309, a novel potent and selective platelet activating factor antagonist in endotoxin and anaphylactic shock in rodents. J Pharmacol Exp Ther 1992, 260:748-755 [PubMed] [Google Scholar]
- 29.Poubelle PE, Borgeat P, Rola-Pleszczynski M: Assessment of leukotriene B4 synthesis in human lymphocytes by using high performance liquid chromatography and radioimmunoassay methods. J Immunol 1987, 139:1273-1277 [PubMed] [Google Scholar]
- 30.Powell WS: Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. Methods Enzymol 1982, 86:467-477 [DOI] [PubMed] [Google Scholar]
- 31.Salmon JA, Simmons PM, Palmer RM: A radioimmunoassay for leukotriene B4. Prostaglandins 1982, 24:225-235 [DOI] [PubMed] [Google Scholar]
- 32.Witherden IR, Goldstraw P, Pastroni U, Ratcliffe C, Tetley TD: Interleukin-8 release by primary human type II cells in vitro: effects of neutrophil elastase and cigarette smoke. Respir Med 1997, 91:A27 [Google Scholar]
- 33.Mio T, Romberger DJ, Thompson AB, Robbins RA, Heires A, Rennard SI: Cigarette smoke induces interleukin-8 release from human bronchial epithelial cells. Am J Respir Crit Care Med 1997, 155:1770-1776 [DOI] [PubMed] [Google Scholar]
- 34.Koyama S, Sato E, Masubuchi T, Takamizawa A, Kubo K, Nagai S, Izumi T: Alveolar type II cells (A549 cells) release granulocyte colony-stimulating factor as neutrophil chemotactic activity. Am J Physiol 1998 (in press) [DOI] [PubMed]
- 35.Wahl SM, Hunt DA, Wakefield LM, McCartney-Francis N, Wahl LM, Roberts AB, Sporn MB: Transforming growth factor type β induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA 1987, 84:5788-5792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wakefield LM, Smith DM, Masui T, Harris CC, Sporn MB: Distribution and modulation of the cellular receptor for transforming growth factor-β. J Cell Biol 1987, 105:965-975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang JM, Chen ZG, Colella S, Bonila MA, Welte K, Bordignon C, Mantovani A: Chemotactic activity of recombinant human granulocyte colony-stimulating factor. Blood 1988, 72:1456-1460 [PubMed] [Google Scholar]