Abstract
Membrane-associated guanylate kinase homologs (MAGUKs) may play a role in cellular functions preventing tumorigenesis as indicated by the neoplastic phenotype caused by genetic loss of the MAGUK Dlg in Drosophila. To test this possibility, we examined the expression and subcellular localization of the tight junction MAGUK ZO-1, as well as the cell adhesion molecule E-cadherin, in paraffin-embedded breast cancer samples, using immunohistochemistry and confocal microscopy. As expected, normal tissue showed intense staining for ZO-1 at the position of the epithelial tight junctions, but this staining was reduced or lost in 69% of breast cancers analyzed (n = 48). In infiltrating ductal carcinomas (n = 38) there was a reduction in staining in 42% of well differentiated, in 83% of moderately differentiated and 93% of poorly differentiated tumors. ZO-1 staining was positively correlated with tumor differentiation (P = .011) and more specifically with the glandular differentiation of tumors (P = .0019). Reduction in ZO-1 staining was strongly correlated with reduced E-cadherin staining (P = 4.9 × 10−5). The results suggest that down-regulation of ZO-1 expression and its failure to accumulate at cell junctions may be causally related to cancer progression. To detect loss of heterozygosity, the ZO-1 gene tjp-1 was mapped relative to other markers in 15q13 and polymorphic markers flanking tjp-1 were identified. The marker D15S1019 showed loss of heterozygosity in 23% of informative tumors (n = 13). Loss of a tjp-1-linked marker suggests that genetic loss may, in some cases, be responsible for the reduction in ZO-1 expression in breast cancer.
Drosophila genetics has provided a powerful tool to identify genes involved in the regulation of cell proliferation and many of these genes are highly conserved between Drosophila and mammals. 1,2 An example of such a gene is dlg, which regulates epithelial cell proliferation in the imaginal discs of Drosophila. dlg is the founding member of the MAGUK gene family. MAGUKs, membrane associated guanylate kinase homologs, constitute a family of proteins that share with Dlg significant amino acid sequence identify and domain structure including PDZ domains, an SH3 domain, and a guanylate kinase-homologous domain. They are essential components of cell-cell junctions and synapses. 3 For example, loss of Dlg protein in the fruit fly results in the loss of the septate junction, the invertebrate equivalent of the tight junction, and a lethal, neoplastic epithelial phenotype. 4,5 It also prevents complete development of the neuromuscular synapse. 6
ZO-1, a human MAGUK, is a critical regulator of epithelial tight junctions. 3 The structural and functional similarity of ZO-1 to Dlg suggests that it could play a significant role in cell proliferation and epithelial cancers. ZO-1 interacts directly with the transmembrane protein occludin, with ZO-2 (another MAGUK) and AF-6, a target of the ras oncogene which is involved in acute myeloid leukemia. 7-10 ZO-1 has been shown to be down-regulated in poorly differentiated, highly invasive breast cancer cell lines 11 and the gene encoding ZO-1, tjp-1, is found near a genomic interval showing frequent (70%) loss of heterozygosity (LOH) in metastatic breast tumors. 12,13
To explore the possible role of ZO-1 in breast cancer, we characterized ZO-1 protein expression and localization in paraffin-embedded tumor samples using immunohistochemistry, and tested for LOH of polymorphic markers flanking tjp-1. Our results indicate that the majority of breast tumors show reduction or loss of ZO-1 expression, but that this is associated with LOH near tjp-1 in only a fraction of cases. The expression of E-cadherin, another junction-associated molecule that is known to be down-regulated in breast cancer, 14 was also analyzed in the same samples.
Materials and Methods
Tissue Samples
Formalin-fixed, paraffin-embedded, archived breast tumor samples were collected from the University of California-Irvine Department of Medicine, Division of Epidemiology, the University of Michigan Department of Pathology, Ann Arbor, MI and the St. Joseph’s Hospital Department of Pathology (Orange, CA). Samples were accompanied by pathology reports, which were corroborated by a pathologist (S-YL).
Immunohistochemical Detection of ZO-1 and E-cadherin
To examine protein expression in situ, paraffin-embedded sections were analyzed by double labeling with fluorochrome-labeled secondary antibodies according to Harlow and Lane. 15 Additions to this protocol included deparaffination of tissue and antigen retrieval. For deparaffination, sequential washes in HemoD were used (Fischer, Pittsburgh, PA) followed by hydration with a decreasing ethanol series and a wash in TBS solution (100 mmol/L Tris, 0.9% NaCl, pH 7.5). For antigen retrieval, a pressure cooker was used to boil sections in 6.5 mmol/L of sodium citrate (pH 6.0) for 5 minutes (JM Anderson, personal communication).
Antibodies used were rabbit anti-ZO-1 pAb (ZyMed, San Francisco, CA) at 1:400 dilution; mouse anti-E-cadherin pAb (ZyMed) at 1:400 dilution; DTAF-labeled donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) at 1:500 dilution; Cy3-labeled sheep anti-mouse IgG (Jackson ImmunoResearch) at 1:500 dilution. ToPro II (Molecular Probes Inc., Eugene, OR) was used to stain DNA.
The tissue sections were observed using an MRC 1024 Bio-Rad/Nikon Diaphot 200 laser-scanning confocal microscope and LaserSharp image analysis software (Bio-Rad Microscience Division, Cambridge, MA).
Evaluation of ZO-1 and E-cadherin Staining
Within each patient biopsy, the intensity of ZO-1 and E-cadherin staining in breast tumors was compared to that seen in normal glands or ducts present in the same tissue (Figure 1) ▶ . The level of expression was characterized semiquantitatively according to the number of positive cells. When >10% of cells stained positively the tumor was defined as Positive (Figure 1B) ▶ . When up to 10% of cells stained positively the tumor was defined as Reduced (Figure 1C) ▶ . When epithelial cells that normally stain showed no staining, the tumor was defined as Negative (Figure 1D) ▶ . Reduced and negative tumors were grouped together as Rd type for statistical analysis.
Figure 1.
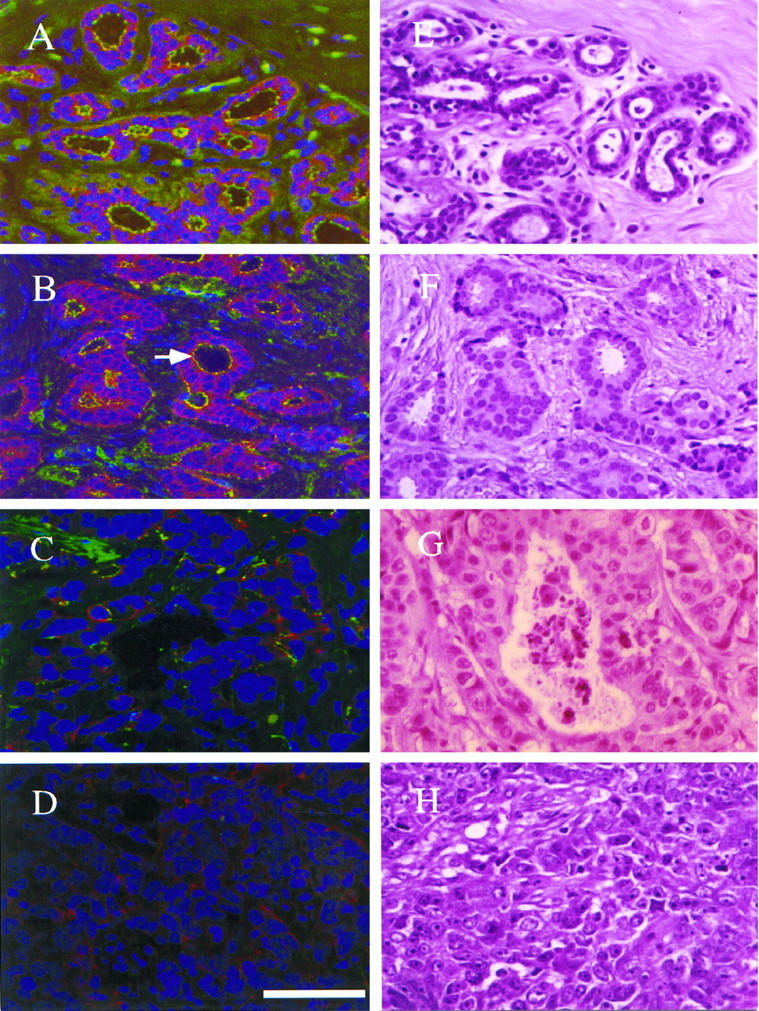
Immunoreactive ZO-1 expression in normal and neoplastic breast tissue. A–H: paired confocal and light micrographs of normal tissue and infiltrating ductal carcinomas from sequential sections. A–D: ZO-1 is in green, E-cadherin in red, DNA in blue. E–H: images of corresponding fields from sequential H&E sections. A: ZO-1 localizes apicolaterally in cells of normal breast epithelium and E-cadherin localizes basolaterally. Note staining of nonepithelial cells (A, upper right) . B and F: a well-differentiated ductal carcinoma classified as ZO-1 Positive and E-cadherin Positive. Arrow indicates normal ZO-1 staining in neoplastic ductal structure. Note colocalization of E-cadherin and ZO-1 protein (yellow color). C and G: moderately differentiated ductal carcinoma classified as ZO-1 Reduced and E-cadherin Positive. D and H: poorly differentiated ductal carcinoma classified as ZO-1 Negative and E-cadherin Reduced. Scale bar, 50 μm.
ZO-1 and Polymorphic Marker Mapping
The ZO-1 gene tjp-1, was precisely mapped using the Stanford G3 and Whitehead G4 radiation hybrid panels (Research Genetics, Huntsville, AL). The following tjp-1-specific primers were used for amplification: 5′ primer ACCATCATTGTCGTCGCATGTAGATCC and 3′ primer GATGCTCTAGGTGCCTGTTCGTAACG. Reactions were done in duplicate with 20 ng of DNA, Taq DNA polymerase buffer (20 mmol/L Tris-HCl (pH 8.4), 50 mmol/L KCl, 2.5 mmol/L MgCl2), 2.5 mmol/L dNTPs, and 2.5 units recombinant Taq DNA polymerase (Gibco BRL, Gaithersburg, MD). The samples were subjected to 35 cycles of denaturation (30 seconds at 95°C), annealing (1 minute at 60°C), and extension (2 minutes at 72°C). The resulting binary code of positive and negative radiation hybrid clones was sent to Stanford (G3 hybrid panel; http://shgc.stanford.edu/) or the Whitehead Institute at MIT (G4 hybrid panel; http://www.genome.wi.mit.edu/) and a physical distance in centirads (cR) from specific DNA markers was returned. The same procedure was used to map several polymorphic markers (Table 1) ▶ and to determine their position relative to tjp-1. To determine the approximate physical distances in megabases (Mb) between tjp-1 and mapped markers, distances were determined using a published chromosome-specific centirad to Mb conversion. 16
Table 1.
Mapping Distance of Polymorphic Markers Relative to tjp-1 and LOH
| Polymorphic Marker | 5′ Primer | 3′ Primer | Distance from tjp-1 | LOH |
|---|---|---|---|---|
| D15S1048 | AGCCGTCTTTGTGCCA | TGCAGCCACTGTGGAA | 1.6 Mb centromeric | N.D.* |
| D15S165 | GTTTACGCCTCATGGATTTA | GGGCACACAGTCCCAC | 1.4 Mb centromeric | 0/14 |
| D15S1019 | TTCTGGACCACGCATACTA | ATCAGGCCATCTTTCATTGT | 0.7 Mb centromeric | 3/13 |
| D15S122 | GATAATCATGCCCCCCA | CCCAGTATCTGGCACGTAG | 5.4 Mb telomeric | 0/7 |
| D15S975 | CCAGTGTAGCACTTGTATGTATGTA | GCTATTGTTTGGTCCTTTGA | 6.5 Mb telomeric | N.D. |
N.D., not determined.
Loss of Heterozygosity
To test for LOH of polymorphic markers linked to tjp-1, DNA was extracted from 26 patient samples. With a razor blade, tumor and normal tissue were dissected from paraffin sections. A method using microwave and proteinase K treatment was used to extract DNA from the paired samples. 17 5′ primers of the polymorphic markers (Table 1) ▶ were labeled with FITC. An internal amplification control was used with primers specific for 5′ UTR of human skeletal α-actin (5′ primer FITC-FACTTTCCGTTGCTGCCATCGTAA and 3′ primer CACTCCCGCCCCAAGCAAATAAAC). The DNA was polymerase chain reaction (PCR)-amplified using approximately 20 ng of extracted DNA and thermocycled at either 33 cycles of 1 minute annealing and extension (D15S1019, D15S165, Actin) or 33 cycles at 57°C for 30 seconds annealing and 72°C for 30 seconds extension (D15S122, Actin 1). Amplified DNA was electrophoresed on an A.L.F. Pharmacia automated sequencer (Pharmacia Biotech, Uppsala, Sweden) and electropherograms 18 were generated by the A.L.F. Manager 2.6 Pharmacia software package (Pharmacia Biotech). Patients were scored as informative for a polymorphic marker if their DNA was effectively amplified and their normal DNA was heterozygous. LOH was characterized according to published methods. 18,19 A decrease of 50% or more in peak area indicated LOH. PCR and analysis of electropherograms was repeated four times for each sample.
Histopathological Findings and Statistical Analysis
An adjacent section from each tumor sample (Figure 1, E–H) ▶ was stained with hematoxylin-eosin for histological evaluation. Each tumor was accompanied by a patient clinical history and pathology report. A pathologist (S-YL) reviewed the reports and confirmed the reported findings. The clinicopathological stage of the tumors was classified according to the TNM classification system of the Union Internationale Contre le Cancer. 20 Their histological type was evaluated based on the World Health Organization classification 21 and their histological degree of differentiation was also graded using a modification of the Scarff and Handley/Bloom and Richardson system as described by Elston and Ellis. 22
The χ 2 test (Fischer’s exact test 2-tail) and trend analysis (with the well-differentiated grade and glandular differentiation of 1 as the baseline categories) were used to determine statistical significance.
Results
Immunohistochemical Reactivity of ZO-1
As expected because of its association with tight junctions, ZO-1 staining was intense at the apicolateral boundary of epithelial cells in normal mammary ducts and glands (Figure 1A) ▶ . Nonepithelial cells also expressed ZO-1 in their cytoplasm (Figure 1A) ▶ . In contrast to normal epithelium, cancer tissue showed a variety of ZO-1 staining levels (Figure 1, B–D) ▶ . Thirty-eight infiltrating ductal carcinomas (IDC), 5 ductal carcinomas in situ, 3 infiltrating lobular and 2 infiltrating lobular carcinomas in situ were analyzed. Of these 48 primary tumors, 15 (31.2%) were classified as positive and 33 (68.8%) were classified as showing the Rd type of expression (Table 2) ▶ .
Table 2.
Correlation of ZO-1 Immunostaining with Histopathology and E-Cadherin Immunostaining in Patients with Breast Cancer
| Total | ZO-1 Rd | P Value | ||||
|---|---|---|---|---|---|---|
| ZO-1 Positive | ZO-1 Reduced | ZO-1 Negative | Subtotal | |||
| Tumor type | *.0024 | |||||
| Infiltrating ductal | 38 | 10 (26.3%) | 14 | 14 | 28 (73.7%) | |
| Ductal carcinoma in situ | 5 | 5 (100%) | ||||
| Infiltrating lobular | 3 | 0 | 3 | 3 (100%) | ||
| Lobular carcinoma in situ | 2 | 0 | 2 | 2 (100%) | ||
| Total | 48 | 15 (31.2%) | 16 | 17 | 33 (68.8%) | |
| Tumor grade† | *.011 | |||||
| Well | 12 | 7 (58.3%) | 4 | 1 | 5 (41.7%) | |
| Moderate | 12 | 2 (16.7%) | 4 | 6 | 10 (83.3%) | |
| Poor | 14 | 1 (7.1%) | 6 | 7 | 13 (92.9%) | |
| Glandular differentiation† | *.0019 | |||||
| 1 | 10 | 7 (70.0%) | 3 | 0 | 3 (30.0%) | |
| 2 | 5 | 0 | 5 | 0 | 5 (100%) | |
| 3 | 22 | 3 (13.6%) | 6 | 13 | 19 (86.4%) | |
| E-cadherin staining | *‡4.9 × 10−5 | |||||
| Positive | 26 | 14 (53.8%) | 10 | 2 | 12 (46.2%) | |
| Reduced | 12 | 0 | 4 | 8 | 12 (100%) | |
| Negative | 8 | 0 | 0 | 8 | 8 (100%) | |
| Rd (reduced+ negative) | 20 | 0 | 4 | 16 | 20 (100%) |
*Statistically significant.
†Scarff-Bloom-Richardson histopathological staging.
‡Significance level of the correlation between E-cadherin and ZO-1 Positive and Rd type staining.
Correlation of ZO-1 Immunostaining with Histopathological Classification
The relationship between ZO-1 expression and histopathology is shown in Table 2 ▶ . There was a statistically significant trend toward reduced ZO-1 staining in more poorly differentiated tumors. Comparing well differentiated tumors to moderately differentiated tumors and moderately differentiated to poorly differentiated revealed a progressive reduction in the frequency of positive ZO-1 staining (Table 2 ▶ ; P = .0093). There was also a statistically significant difference in the numbers of ZO-1 Positive compared to Rd tumors in both moderate and poorly differentiated groups (Table 2 ▶ , P = .011). Thus, reduced ZO-1 staining was directly correlated with the loss of differentiation in breast cancer tumors.
Of the three aspects of tumor differentiation—glandular differentiation, mitotic index, and nuclear grade—only glandular differentiation was correlated with ZO-1 expression. Comparison of tumors with Scarff-Bloom-Richardson glandular differentiation scores of 1 to 2 to those with scores of 2 to 3 showed a statistically significant trend towards decreasing ZO-1 expression with greater loss of glandular differentiation (P = .0093). There was also a significant difference in the number of ZO-1 Positive and Rd tumors as a function of glandular differentiation score (Table 2 ▶ , P = .0019). This correlation was found within individual tumors as well. Glandular structures within tumors were positive for ZO-1 staining regardless of overall tumor differentiation (Figure 1B ▶ , arrow, and data not shown). All infiltrating lobular carcinomas examined in this study (n = 3) completely lacked ZO-1 expression (Table 2) ▶ . This tumor type lacks, in general, glandular structure. 23 Thus, Rd-type ZO-1 expression in tumors of poor differentiation correlated directly and specifically with decreased glandular differentiation. No significant correlations were found between tumor size or lymph node invasion and ZO-1 staining.
Correlation of ZO-1 and E-Cadherin Staining
E-cadherin expression in tumors was also analyzed (Table 2) ▶ . 80% of E-cadherin Rd tumors were ZO-1 negative and all E-cadherin negative tumors were ZO-1 negative (Table 2) ▶ . The correlation of ZO-1 Rd staining with E-cadherin Rd staining was statistically significant (P = 4.9 × 10−5). Furthermore, there was frequent overlap between ZO-1 and E-cadherin near the apicolateral cell borders in normal and tumor tissue. Whereas this was somewhat more pronounced in tumors, suggesting delocalization of one or both of the proteins, it was sometimes apparent in normal tissue as well (compare yellow color in Figure 1A and 1B ▶ ). Unlike ZO-1, E-cadherin immunostaining showed no significant correlation with tumor differentiation.
Precise ZO-1 Mapping and Loss of Heterozygosity
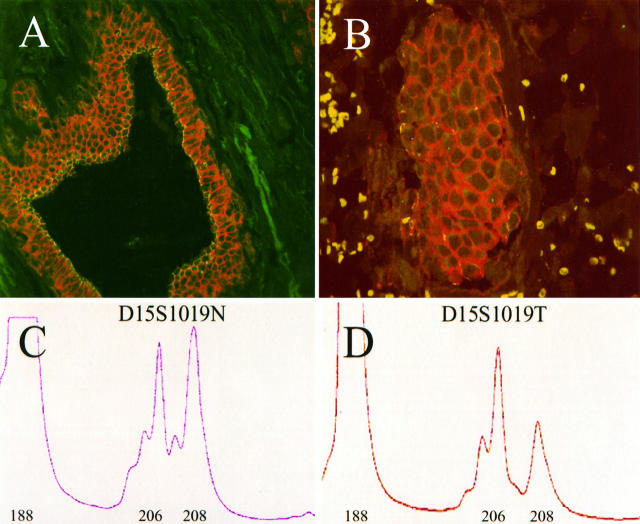
The ZO-1 locus tjp-1 had been physically mapped to chromosome band 15q13 before this study. 12 To test for LOH, we undertook more precise mapping by PCR using tjp-1-specific primers and two radiation-hybrid panels. tjp-1-positive radiation hybrid clones were reported to the Stanford and Whitehead Institute Genome Centers and a map position relative to other markers was provided by the Centers. tjp-1 mapped approximately 100 kb from WI-5590 (data not shown) and 1.4 Mb from the dinucleotide-repeat polymorphic marker D15S165 (Table 1) ▶ . Other polymorphic markers flanking tjp-1 were mapped to the radiation-hybrid panels and their relative positions were approximated in this manner (Table 1) ▶ . D15S165, D15S1019, and D15S122 were used to analyze LOH in the vicinity of tjp-1 in DNA extracted from 26 paired normal and tumor samples. Three of 13 informative cases (23%) showed LOH for the D15S1019 marker. In these three cases, there was a significant decrease in the peak size of one allele in the neoplastic component of the tumor (compare Figure 2C to F ▶ igure 2D). This peak size decrease indicates LOH of the marker. 18,19 All three cases with LOH had Rd type ZO-1 staining and poor glandular differentiation (Figure 2 ▶ and data not shown).
Figure 2.
Immunoreactive ZO-1 expression and D15S1019 loss of heterozygosity in a single case of infiltrating ductal carcinoma. A and B: confocal images of normal (A) and tumor (B) tissue from a single patient. ZO-1 is in green, E-cadherin in red. The tumor showed reduced ZO-1 expression. C and D: electropherograms of normal (D15S1019N) and tumor (D15S1019T) DNA amplified for the polymorphic marker D15S1019 (see Material and Methods). The DNA amplified in C and D was extracted from the same tissue shown in A and B, respectively. The numbers below the peaks represent the basepair sizes of the amplified products. Note the dramatic decrease in the size of the 208-basepair allele, indicating loss of heterozygosity of D15S1019. The 188-basepair peak is the internal amplification control, actin.
Discussion
Our findings indicate a statistically significant correlation between decreased tumor differentiation and decreased ZO-1 staining in breast cancer samples. Previously, a decrease in ZO-1 staining was shown in poorly differentiated and invasive breast cancer cell lines. 11 Because of the critical role ZO-1 plays in the function of the tight junction, 24 one possibility is that the entire tight junction may be lost with the loss of tumor differentiation. This is supported by a study in mice demonstrating the absence of the tight junction in breast adenocarcinomas. 25 Furthermore, preliminary studies indicate the loss of the tight junction transmembrane protein occludin 8 with loss of tumor differentiation (K. Hoover and L. Mitic, unpublished observations). The reduction does not affect all cell contact-associated proteins, because no correlation of E-cadherin loss and decreased tumor differentiation was found in this study. It would appear that loss of ZO-1, and possibly other tight junction-associated proteins, is specifically correlated with the loss of tumor differentiation.
The differentiation of infiltrating ductal carcinomas is defined routinely by characterizing three elements of the tumor phenotype: gland (tubule) differentiation, nuclear grade, and mitotic index. 22,26 In the present study, the decrease in ZO-1 staining was significantly and specifically correlated with a decrease in the glandular differentiation of the tumor. This correlation was reflected by the absence of ZO-1 staining in infiltrating lobular carcinomas, which largely lack glandular structure, and nearly two-thirds of IDC samples with poor glandular differentiation (Table 2) ▶ . The correlation is probably even stronger than suggested by the statistics because the Scarff-Bloom-Richardson staging system quantifies the amount of glandular structure for the entire tumor and does not account for the variability of glandular phenotype within the tumor. ZO-1 staining was evident in parts of the tumors that contain glandular structures, regardless of overall tumor differentiation (Figure 1B ▶ , arrow, and data not shown). This correlation of ZO-1 staining with the glandular phenotype of breast tumors is consistent with evidence showing that several properties of differentiated epithelium, including paracellular permeability and tight junction structure, are regulated by ZO-1. 24,27,28
We have also documented a significant correlation between reduced E-cadherin and reduced ZO-1 expression in breast cancer samples. All of those tumors that were E-cadherin negative were also ZO-1 negative (Table 2) ▶ . A role for E-cadherin in regulating ZO-1 is supported by the finding that E-cadherin-based adhesion is required for proper ZO-1 localization at the tight junction of kidney epithelial cell lines. 29 In this study we noted frequent overlap between ZO-1 and E-cadherin staining indicated by yellow signal in the confocal micrographs (Figure 1) ▶ . However, this does not necessarily indicate the presence of both proteins in the same domain of the plasma membrane, since each of our images represents stacked laser scans though a thickness of approximately 10 μm.
The interrelationship between ZO-1 and E-cadherin is potentially significant given the down-regulation and mutation of E-cadherin in breast cancer. 14,30 Because of E-cadherin’s crucial role in regulating cell-cell adhesion, it has been postulated that the loss of functional protein results in more invasive and metastatic tumor cells. 31 While no clear mechanism for this function of E-cadherin has been characterized, the interaction of E-cadherin with β-catenin, which is often mutated in colon cancer, could be involved. 32,33 A recent study showing that ZO-1 also interacts with the catenin proteins 29 raises the possibility that ZO-1 could function downstream of E-cadherin in an adhesion-dependent signaling pathway.
To begin characterizing the mechanism for decreased ZO-1 staining in breast tumors, genetic changes in the ZO-1 locus, tjp-1, were analyzed using a PCR-based LOH assay. 18,19 One tjp-1-linked marker showed 23% LOH. This suggests LOH is one mechanism of ZO-1 protein loss. Other mechanisms could include point mutation and hypermethylation, both of which have been described for E-cadherin. 30,34 The percentage of LOH observed in this study is higher than that reported by Wick et al, 13 who found 11% LOH of markers near tjp-1 in nonmetastatic tumors of the breast. However, Wick et al also showed 70% LOH of the same markers in breast cancer metastases to the brain, suggesting the possibility that tjp-1 LOH and ZO-1 loss may be especially significant in allowing metastasis.
Acknowledgments
We thank Drs. David Peel and Hoda Anton-Culver for their assistance in completing the loss of heterozygosity analysis. For their assistance in tissue preparation and imaging we also thank Janine Roach and Shannon Curry. We acknowledge Sandya Upasni of Shared Statistical Resources at the UC Irvine Chao Family Clinical Cancer Center for assistance with statistical analysis and use of the Developmental Biology Center Confocal Microscopy Core Facility.
Footnotes
Address reprint requests to Kevin B. Hoover, 4340 Bio Sci II, Developmental Biology Center, University of California-Irvine, Irvine, CA 92697-2275. E-mail: khoover@meded.med.uci.edu.
Supported by U.S. Public Health Service-National Institutes of Health National Cancer Institute Grant CA-70557.
References
- 1.Rubin GM: Drosophila melanogaster as an experimental organism. Science 1988, 240:1453-1459 [DOI] [PubMed] [Google Scholar]
- 2.Merriam J, Ashburner M, Hartl DL, Kafatos FC: Toward cloning and mapping the genome of Drosophila. Science 1991, 254:221-225 [DOI] [PubMed] [Google Scholar]
- 3.Anderson JM: Cell signalling: MAGUK magic. Curr Biol 1996, 6:382-384 [DOI] [PubMed] [Google Scholar]
- 4.Woods DF, Bryant PJ: The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell 1991, 66:451-464 [DOI] [PubMed] [Google Scholar]
- 5.Noirot-Timothee C, Noirot C: Septate and scalariform junctions in arthropods. Int Rev Cytol 1980, 63:97-140 [DOI] [PubMed] [Google Scholar]
- 6.Budnik V, Koh Y, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M: Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron 1996, 17:627-640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jesaitis LA, Goodenough DA: Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol 1994, 124:949-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S: Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol 1993, 123:1777-1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prasad R, Gu Y, Alder H, Nakamura T, Canaani O, Saito H, Huebner K, Gale RP, Nowell PC, Kuriyama K: Cloning of the ALL-1 fusion partner, the AF-6 gene, involved in acute myeloid leukemias with the t(6;11) chromosome translocation. Cancer Res 1993, 53:5624-5628 [PubMed] [Google Scholar]
- 10.Yamamoto T, Harada N, Kano K, Taya S, Canaani E, Matsuura Y, Mizoguchi A, Ide C, Kaibuchi K: The Ras target AF-6 interacts with ZO-1 and serves as a peripheral component of tight junctions in epithelial cells. J Cell Biol 1997, 139:785-795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sommers CL, Byers SW, Thompson EW, Torri JA, Gelmann EP: Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res Treat 1994, 31:325-335 [DOI] [PubMed] [Google Scholar]
- 12.Mohandas TK, Chen XN, Rowe LB, Birkenmeier EH, Fanning AS, Anderson JM, Korenberg JR: Localization of the tight junction protein gene TJP1 to human chromosome 15q13, distal to the Prader-Willi/Angelman region, and to mouse chromosome 7. Genomics 1995, 30:594-597 [DOI] [PubMed] [Google Scholar]
- 13.Wick W, Petersen I, Schmutzler RK, Wolfarth B, Lenartz D, Bierhoff E, Hümmerich J, Müller DJ, Stangl AP, Schramm J, Wiestler OD, von Deimling A: Evidence for a novel tumor suppressor gene on chromosome 15 associated with progression to a metastatic stage in breast cancer. Oncogene 1996, 12:973-978 [PubMed] [Google Scholar]
- 14.Oka H, Shiozaki H, Kobayashi K, Inoue M, Tahara H, Kobayashi T, Takatsuka Y, Matsuyoshi N, Hirano S, Takeichi M, Mori T: Expression of E-cadherin cell adhesion molecules in human breast cancer tissues and its relationship to metastasis. Cancer Res 1993, 53:1696-1701 [PubMed] [Google Scholar]
- 15.Harlow E, Lane D: Antibodies: A Laboratory Manual. 1988. Cold Spring Harbor Laboratory Press, Cold Spring Harbor NY
- 16.Schuler GD, Boguski MS, Stewart EA, Stein LD, Gyapay G, Rice K, White RE, Rodriguez-Tome P, Aggarwal A, Bajorek E, Bentolila S, Birren BB, Butler A, Castle AB, Chiannilkulchai N, Chu A, Clee C, Cowles S, Day PJ, Dibling T, Drouot N, Dunham I, Duprat S, East C, Hudson TJ: A gene map of the human genome. Science 1996, 274:540-546 [PubMed] [Google Scholar]
- 17.Banerjee SK, Makdisi WF, Weston AP, Mitchell SM, Campbell DR: Microwave-based DNA extraction from paraffin-embedded tissue for PCR amplification. BioTechniques 1995, 18:768–770, 772–773 [PubMed]
- 18.Hampton GM, Larson AA, Baergen RN, Sommers RL, Kern S, Cavenee WK: Simultaneous assessment of loss of heterozygosity at multiple microsatellite loci using semi-automated fluorescence-based detection: subregional mapping of chromosome 4 in cervical carcinoma. Proc Natl Acad Sci USA 1996, 93:6704-6709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Canzian F, Salovaara R, Hemminki A, Kristo P, Chadwick RB, Aaltonen LA, de la Chapelle A: Semiautomated assessment of loss of heterozygosity and replication error in tumors. Cancer Res 1996, 56:3331-3337 [PubMed] [Google Scholar]
- 20.TNM Classification of Malignant Tumors. Berlin-Heidelberg, Germany, Springer-Verlag, 1987, pp 748–759
- 21.Histological Typing of Breast Tumors. Geneva, World Health Organization, 1981, pp 15–25
- 22.Elston CW, Ellis IO: Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991, 19:403-410 [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, Cotran RS, Robbins S: Basic Pathology. 1992:pp 636-641 W.B. Saunders, Philadelphia
- 24.Anderson JM, Van Itallie CM: Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol 1995, 269:G467-475 [DOI] [PubMed] [Google Scholar]
- 25.Weinstein RS, Merk FB, Alroy J: The structure and function of intercellular junctions in cancer. Adv Cancer Res 1976, 23:23-89 [DOI] [PubMed] [Google Scholar]
- 26.Page DL, Anderson TJ: Diagnostic Histopathology of the Breast. 1987. Churchill Livingstone, New York
- 27.Gumbiner B: Epithelial morphogenesis. Cell 1992, 69:385-387 [DOI] [PubMed] [Google Scholar]
- 28.Balda MS, Anderson JM: Two classes of tight junctions are revealed by ZO-1 isoforms. Am J Physiol Cell Physiol 1993, 264:C918-C924 [DOI] [PubMed] [Google Scholar]
- 29.Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E: Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol 1996, 132:451-463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berx G, Cleton-Jansen AM, Nollet F, De Leeuw WJF, Van de Vijver MJ, Cornelisse C, Van Roy F: E-cadherin is a tumour invasion suppressor gene mutated in human lobular breast cancers. EMBO J 1995, 14:6107-6115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birchmeier W, Behrens J: Cadherin expression in carcinomas: Role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta Rev Cancer 1994, 1198:11-26 [DOI] [PubMed] [Google Scholar]
- 32.Hülsken J, Behrens J, Birchmeier W: Tumor-suppressor gene products in cell contacts: the cadherin-APC-armadillo connection. Curr Opin Cell Biol 1994, 6:711-716 [DOI] [PubMed] [Google Scholar]
- 33.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW: Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science 1997, 275:1787-1790 [DOI] [PubMed] [Google Scholar]
- 34.Graff JR, Herman JG, Lapidus RG, Chopra H, Xu R, Jarrard DF, Isaacs WB, Pitha PM, Davidson NE, Baylin SB: E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res 1995, 55:5195-5199 [PubMed] [Google Scholar]