Abstract
Fas ligand (FasL) exists in transmembrane and soluble forms and induces apoptosis on cross-linking with the Fas receptor. We evaluated the biological significance of FasL and Fas in 61 tumor tissues and 9 cell lines of the Ewing’s sarcoma family of tumors (ESFT). FasL was present in 62.5% and Fas in 79.4% of primary ESFT. Metastatic tumors had higher expression of FasL (95%), suggesting association with a metastatic phenotype. FasL was detected in the cytoplasm and membrane of ESFT cells by immunofluorescence. Western blotting revealed transmembrane and soluble FasL in cytosolic extracts and soluble FasL in conditioned media. Both transmembrane and soluble FasL induced apoptosis of Fas-sensitive Jurkat cells in co-culture experiments with ESFT cells or their media. Treatment with phenanthroline and the synthetic metalloproteinase inhibitor BB-3103 reduced the levels of soluble FasL in the media, suggesting that in ESFT, FasL is processed by a metalloproteinase and released in the extracellular milieu. The released soluble FasL may serve to attack cells of the immune system and/or interfere with the binding of transmembrane FasL with Fas, and results in down-regulation of transmembrane FasL. Synthetic metalloproteinase inhibitors may modify the ratio of transmembrane to soluble FasL.
FasL is a transmembrane protein of the tumor necrosis factor (TNF) family, with an amino-terminal cytoplasmic and a carboxy-terminal extracellular region (type II protein). Its molecular weight ranges from 37 to 42 kd, 1 probably reflecting differences in levels of glycosylation. In addition to the transmembrane FasL (tm-FasL), a soluble form has been described as well. The human soluble FasL (s-FasL) has a molecular weight of 23–26 kd and consists of the extracellular domain of the FasL molecule. 2 Recent studies have shown that s-FasL originates from cleavage of tm-FasL by an unidentified metalloproteinase-like enzyme. 3-5 Both tm- and s-FasL bind to the Fas (APO-1/CD95) receptor and induce apoptosis. 1-3,6,7 Fas is a type I transmembrane protein of the TNF/nerve growth factor receptor superfamily. 8 The Fas/FasL system plays an important role in immune homeostasis 9,10 and participates in T cell-mediated cytotoxicity. 11-13 According to a recently proposed model, FasL-expressing tumor cells use FasL as a cytolytic effector molecule to kill Fas-expressing activated lymphocytes (“counterattack” model). 14,15
The group of small round cell sarcomas in children and adolescents that is collectively referred to as the Ewing’s sarcoma family of tumors (ESFT) includes morphological variants of Ewing’s sarcoma and peripheral primitive neuroectodermal tumor (PNET). All ESFTs are characterized by specific chromosomal translocations. The most commonly encountered translocation, t(11;22) (q24;q12), results in the fusion of the EWS and Fli-1 genes. 16 Clinical studies have shown that despite a significant initial response to conventional treatment, a high percentage of patients with ESFT suffer a recurrence at metastatic sites. 16 Since the “counterattack” model suggests that the FasL may offer a survival advantage to tumors, we investigated the role of FasL in the biology of ESFT.
In this study, we show that ESFTs frequently express Fas and FasL. A significantly higher level of FasL expression in metastatic than primary ESFTs supports the theory that FasL-expressing clones survive and undergo expansion in metastatic sites. Our in vitro data demonstrate that ESFT express not only tm-FasL, but also s-FasL and release s-FasL in the media. Both tm- and s-FasL induce apoptosis in vitro. The levels of s-FasL in the media are reduced by metalloproteinase inhibitors, suggesting cleavage by a metalloproteinase. Collectively, these data support the hypothesis that there is a dynamic equilibrium between tm- and s-FasL that can be manipulated by agents such as metalloproteinase inhibitors.
Materials and Methods
Cell Lines
Seven previously described and one unpublished ESFT cell lines were used in this study. Specifically, the TC-71, TC-32, A4573, 5838, SK-N-MC, CHP100 (clone-S and -L), and TC-268 cell lines were shown to have the characteristic translocation and/or EWS/Fli-1 fusion gene product of the ESFT. 17-20 The unpublished cell line, TC-248, was established in our laboratory from an ESFT that exhibited the typical EWS/Fli-1 fusion product as well (data not shown). The T-cell leukemia cell line Jurkat (American Type Culture Collection, Manassas, VA) was used in functional experiments. All cells except Jurkat were grown in Dulbecco’s modified Eagle’s medium (DMEM) (BioWhittaker, Walkersville, MD) with 100 U/ml penicillin, 100 mg/ml streptomycin and 10% fetal calf serum (FCS) (GIBCO/BRL, Gaithersburg, MD), unless stated otherwise. Jurkat cells were grown in RPMI (GIBCO/BRL) with 10% FCS and antibiotics as above.
Tissue Sections
Sections from 61 formalin-fixed paraffin-embedded tumor tissue specimens obtained from 49 patients with ESFT and stored in the files of the Laboratory of Pathology at the National Cancer Institute (NCI) were stained for FasL. Forty of these tumors were primary and 21 were metastatic. In 12 cases, primary and metastatic tumor tissue from the same patient was available for comparison. In 34 primary and 19 metastatic tumors from which additional sections were available, immunocytochemical staining for Fas receptor was performed.
Antibodies
For immunofluorescence and immunoperoxidase staining, the anti-FasL rabbit polyclonal antibodies C-20 (against the extracellular C-terminus) and Q-20 (against the intracellular N-terminus) (Santa Cruz Biotechnology, Santa Cruz, CA) were used. Both antibodies were used in the presence or absence of the corresponding blocking peptide (amino acid residues 260–279 for C-20 and residues 2–19 for Q-20, Santa Cruz) to confirm specificity of staining. The monoclonal anti-FasL antibodies clone 33 (0.25 μg/ml) (Transduction Laboratories, Lexington, KY) and G247–4 (1:500 dilution) (Pharmingen, San Diego, CA), as well as the polyclonal antibodies Ab-3 (1:100 dilution) (Oncogene Research, Cambridge, MA) and C-20 (1:500 dilution) and Q-20 (1:500 dilution) were used in the immunoblotting experiments. Both monoclonal antibodies are directed against the extracellular portion of the FasL molecule. For detection of the Fas receptor on paraffin sections, the anti-Fas rabbit antibody Ab-1 was used (Oncogene Research) in the presence or absence of its blocking peptide (amino acid residues 321–335) (Oncogene Research). The neutralizing anti-FasL antibody NOK-2 (Pharmingen) and the cytotoxic anti-Fas monoclonal CH-11 antibody (Panvera, Madison, WI) were used in the cytotoxic cell assays.
Detection of FasL Protein in ESFT Cells and Media by Immunoblotting
Cell Lysates
Cells (1 × 106) from all 9 ESFT cell lines were scraped, centrifuged briefly, and lysed for 30 minutes on ice in a lysis buffer (50 mmol/L Tris-HCl, pH 8.0, containing 120 mmol/L NaCl and 1% Igepal), supplemented with the Complete-TM mixture of proteinase inhibitors (Boehringer Mannheim, Indianapolis, IN). The samples were cleared by centrifugation (14,000 rpm for 30 minutes at 4°C) and assessed for protein concentration. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (12%) was performed (30 μg of protein per lane) and the proteins were electroblotted onto nitrocellulose membranes. After 1 hour incubation in blocking solution (20% IgG-free normal horse serum in PBS, GIBCO/BRL), the membranes were exposed to the primary antibody overnight at 4°C. After washing in PBS, the secondary peroxidase-labeled secondary antibody (Amersham, Arlington Heights, IL) was added at 1:10,000 dilution for 40 minutes at room temperature. The proteins were visualized with the enhanced chemiluminescence (ECL) technique (Amersham). An endothelial cell lysate, provided by Transduction Laboratories as a positive control for the anti-FasL antibody clone 33, was used along with the ESFT cell lysates in our immunoblotting experiments.
Conditioned Media
Conditioned media were generated from 3 of the 9 ESFT cell lines (TC-248, TC-268 and TC-71). These lines expressed the highest levels of FasL in the cell pellets and were selected for further analysis. Following brief washing in Hanks’ balanced salt solution (HBSS) (GIBCO/BRL) and a 4-hour washing in serum-free DMEM, ESFT cells were then incubated with fresh serum-free DMEM medium overnight. The overnight-conditioned media were collected and centrifuged for 5 minutes at 2,000 rpm. The supernatants were supplemented with a proteinase inhibitor cocktail of 10 μg/ml aprotinin (Sigma, St. Louis, MO), 1 mmol/L phenylmethylsulfonyl fluoride (Sigma) and 25 μmol/L leupeptin (Sigma). To increase the sensitivity of detection of s-FasL by Western blotting, the conditioned media were subjected to 50-fold concentration with Centricon-10 filters (Amicon, Beverly, MA). The concentrates were mixed with 5× Laemmli buffer, electrophoresed on 12% SDS-PAGE gels and immunoblotted with the anti-FasL monoclonal antibody G247–4 (Pharmingen) at 1:500 dilution (1 μg/ml).
Immunocytochemical Detection of FasL in ESFT Cells and Tissues
Immunofluorescence
Air-dried cytospins from cultured cells were fixed in −20°C acetone for 10 minutes. Subsequently, the cytospins were washed and blocked for 1 hour with 20% normal goat serum and 3% bovine serum albumin (Sigma) solution in PBS. The cytospins were then washed in PBS and incubated overnight at 4°C with the C-20 or Q-20 rabbit polyclonal anti-FasL antibodies at 1:100 dilution (1μg/ml), in the presence or absence of a 10-fold excess of the corresponding blocking peptide. Subsequently, the cytospins were washed in PBS and incubated with fluorescein isothiocyanate (FITC)-labeled anti-rabbit IgG (Boehringer Mannheim, Indianapolis, IN) (1:75 dilution) for 1 hour at room temperature. Fluorescent signals were visualized with a Zeiss standard fluorescence microscope equipped with an epifluorescence illuminator and FITC narrow-band filter.
Avidin Biotin Complex Peroxidase
Immunohistochemical detection of Fas and FasL was performed as previously described. 21 Briefly, 5-micron paraffin sections were deparaffinized, rehydrated, and subjected to antigen retrieval by incubation in 10 mmol/L citrate buffer for 15 minutes in a microwave oven. Endogenous peroxidase activity was quenched for 30 minutes in methanol containing 0.5% H2O2. The sections were washed in PBS and blocked for 1 hour in 20% normal goat serum in PBS. The primary antibodies, anti-FasL Q-20 (0.7 μg/ml) and anti-Fas Ab-1 (2.5 μg/ml) respectively, were applied overnight in the presence or absence of a 10-fold excess of the corresponding blocking peptides. Subsequently, the sections were washed in PBS and incubated with a biotinylated anti-rabbit antibody (1:500 dilution) for 1 hour at room temperature. After washing with PBS, the sections were covered with the Vectastain Elite Avidin Biotin Complex Reagent (Vector Laboratories, CA) for 30 minutes. The peroxidase reaction was developed with 3,3′-diaminobenzidine and the slides were counterstained with methyl green. Positive staining was evaluated subjectively by two independent observers for both percentage of positive cells and intensity of staining on a scale of 1 to 3 (interobserver agreement in 95% of cases). The product of the intensity of staining and percentage of positive cells was used for final classification into grade 0 (no staining), grade 1 (0.1–0.3), grade 2 (0.4–0.6), grade 3 (0.7–1), or grade 4 (>1).
Survival and Death Assays
3-(4,5-Dimethylthiazol-2-yl)−2,5-Diphenyltetrazolium Bromide (MTT) Colorimetric Assay
Following treatment as indicated below, the cells were incubated with 1 mg/ml MTT (Sigma) in fresh media for 4 hours at 37°C. Subsequently, a mixture of isopropanol and 1N HCl (24:1, v/v) was added under vigorous pipetting to dissolve the formazan crystals. Dye absorbance (A) in viable cells was measured at 570 nm, with 630 nm as a reference wavelength. Cell death was estimated with the formula:
 |
Cellular DNA Fragmentation ELISA
This method, a non-radioactive analogue of the [3H]-Thymidine-DNA fragmentation assay, was used to detect apoptosis in the co-culture experiments described below. The DNA fragmentation ELISA kit (Boehringer Mannheim) was used. Target cells were labeled overnight with 5′-bromo-2′-deoxy-uridine (BrdU) according to the manufacturer’s instructions and subsequently were co-cultured with effector cells. The amount of fragmented DNA in the target cells was quantified according to the manufacturer’s instructions. The results were expressed as percentages of the value in control cells.
Terminal Deoxynucleotidyl Transferase-Mediated dUTP End-Labeling (TUNEL) Method
Air-dried cytospins were labeled with the in situ cell death kit-Fluorescence (Boehringer Mannheim) following the instructions of the manufacturer and were viewed with a Zeiss standard fluorescence microscope equipped with an epifluorescence illuminator and FITC narrow-band filter.
Induction of Jurkat-cell Apoptosis by ESFT: Cells and Media
ESFT Tumor Cell-Induced Apoptosis
The ability of the TC-248 and TC-32 ESFT effector cells to kill target lymphocytes in a Fas-dependent manner was evaluated as previously described 22 with minor modifications. Briefly, ESFT cells (5 × 10 5 cells/well grown up to 90% confluency) were fixed lightly (0.6% paraformaldehyde in PBS for 15 minutes) and, after adequate washing in HBSS, incubated with a Jurkat cell suspension (10 5 cells in 400 ml DMEM supplemented with 1% calf serum, GIBCO/BRL), at an effector to target ratio 10:1 and in the presence or absence of the FasL neutralizing NOK-2 antibody (10 μg/ml). After a 48-hour incubation, Jurkat cell death was evaluated with the MTT assay, as described above. The percentage of Jurkat cell death in each well was calculated with the equation: % cell death =
 |
ESFT cell-induced Jurkat cell death was also evaluated with a DNA fragmentation ELISA that measured DNA fragmentation of BrdU-labeled Jurkat cells co-cultured with viable TC-248 cell monolayers for 48 hours in the presence or absence of NOK-2 antibody (10 μg/ml). The Jurkat cell suspension was collected at the end of the experiment with vigorous pipetting.
ESFT Media-Induced Apoptosis
Media conditioned with TC-248 and TC-71 cells for 48 hours were concentrated 30-fold with Centriprep-10 filters (Amicon) and mixed with a Jurkat cell suspension at a 1:1 v:v ratio. Thus the final concentration of media was 15-fold, much less than the 50-fold concentration used to detect s-FasL by Western blotting. The Jurkat cell concentration in the final sample was 1.25 × 10 5 cells/ml in DMEM supplemented with 1% calf serum. Media conditioned with TC-248 and TC-71 were added in the presence or absence of NOK-2 FasL neutralizing antibody (10 μg/ml). Control wells consisted of Jurkat cells in fresh medium with 1% calf serum. Percentage of Jurkat cell death in each well was evaluated with the MTT assay and estimated with the equation: % cell death =
 |
In addition, cells from duplicate wells were centrifuged onto positively charged slides and stained for apoptosis with the TUNEL method. The percent of apoptotic cells was evaluated subjectively.
Assessment of Anti-Fas Antibody CH-11-Induced Apoptosis of ESFT Cells
Cells from the TC-248, TC-268, and TC-71 cell lines were grown to 70–80% confluence. Subsequently, the cells were washed in HBSS and incubated for 18 hours with the CH-11 anti-Fas antibody (500 ng/ml, in DMEM medium with 10% calf serum) at 37°C. Cells grown in the absence of CH-11 antibody were used as negative controls. The Fas-sensitive Jurkat cell line was used as a positive control. Cell survival was evaluated with the MTT assay.
Effect of Metalloproteinase Inhibitors on s-FasL Shedding in Vitro
TC-248 cells were grown in serum-containing medium for 2 days, then washed twice in HBSS and incubated for 4 hours in serum-free DMEM with the general zinc-chelating agent 1,10-phenanthroline (0.1 mmol/L, Sigma) or with one of the following metalloproteinase inhibitors: BB-3103 (10 μM, generous gift of British Biotech, Oxford, UK), TIMP-1 (1 μg/ml), and TIMP-2 (1 μg/ml). To eliminate any possible traces of pre-existing s-FasL, this medium was discarded and replaced with fresh DMEM containing the same agents as before, at the same concentrations. The final incubation was 18 hours for BB-3103, TIMP-1, and TIMP-2 and 8 hours for 1,10-phenanthroline. At the end of the incubation times, the media were collected, subjected to 50-fold concentration, and tested for s-FasL by immunoblotting, as discussed previously
Statistical Analysis
The χ 2 test was used to compare numbers of positive and negative primary versus metastatic tumors. Intensity of staining in total number of primaries versus total number of metastatic tumors was evaluated with a two-sided unpaired t-test. In cases with primary and metastatic tumors from the same patient available for comparison, the two-sided paired t-test was used. All other comparisons were examined with the one-factor analysis of variance repeated measures method.
Results
ESF Tumor Cell Lines Exhibit tm- and s-FasL Protein and Release s-FasL in the Media
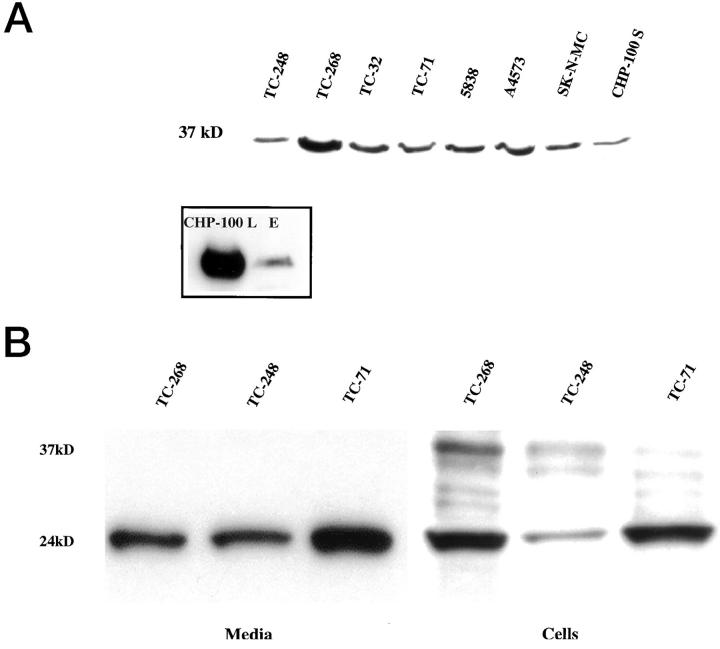
We investigated the presence of the tm- and s-FasL in ESFT cells and media by immunoblotting. All antibodies against FasL (G247–4, clone 33, Ab-3, C-20, and Q-20) identified a 37–40 kd band in the cell lysates of all 9 ESFT cell lines. This band co-migrated with the FasL band of the control endothelial cells and corresponds to the tm-FasL (Figure 1A ▶ and inset).
Figure 1.
Immunoblot analysis of FasL protein in cell pellets and media from ESFT cell lines. A: Cell lysates from 9 cell lines express a 37 kd protein detected with the clone 33 anti-FasL antibody. This band co-migrates with the FasL band of the control endothelial cells (E) that were electrophoresed and immunoblotted simultaneously with one of the ESFT cell lines, CHP100-L, for comparison (inset). B: Concentrated media from 3 ESFT cell lines demonstrate a 24-kd band that corresponds to s-FasL and is detected with the G247–4 anti-FasL antibody. The G247–4 antibody detects a similar band in cell lysates from the same cell lines, as well as the 37-kd band of tm-FasL and two other bands (approximately 31 kd) that correspond to nonglycosylated or partially glycosylated tm-FasL. The tm-FasL is less evident with G247–4 than with clone 33 anti-FasL antibody.
A band of approximately 24 kd that corresponds to the s-FasL was detected with the G247–4 antibody in concentrated conditioned media and cell lysates from ESFT cell lines (TC-248, TC-268, TC-71) (Figure 1B) ▶ . Tm-FasL was not detected in any of the media with either clone 33 or G247–4, thus ruling out cell contamination. Although both FasL-specific monoclonal antibodies used in the immunoblotting experiments were directed against the extracellular domain of FasL, clone 33 stained preferentially for tm-FasL and G247–4 exhibited higher affinity for s-FasL. Furthermore, the G247–4 antibody also detected an additional doublet of approximately 31 kd (Figure 1B) ▶ that corresponds to nonglycosylated or partially glycosylated tm-FasL. 2,5
FasL is Localized on the Surface and in the Cytoplasm of ESFT Cells
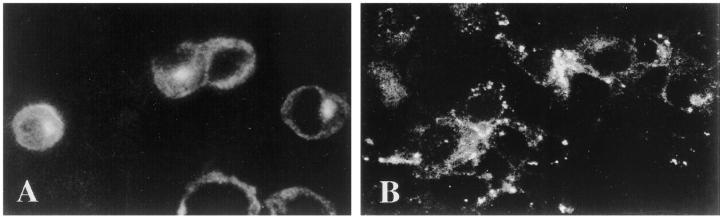
Having detected the presence of tm- and s-FasL in ESFT cells by immunoblotting, we assessed the cellular localization of these proteins by immunofluorescence staining of acetone-fixed cytospins from the TC-248, TC-268, and TC-71 cell lines. Both antibodies showed diffuse cytoplasmic and paranuclear dot-like staining that was more prominent with the C-20 (Figure 2A) ▶ than the Q-20 antibody (Figure 2B) ▶ . A granular pattern at the periphery of the cells, consistent with membranous staining, was additionally observed with the Q-20 antibody (Figure 2B) ▶ . These data show that FasL is expressed both on the surface and in the cytoplasm of ESFT cells. All staining was totally abolished in the presence of the corresponding blocking peptide that confirmed specificity (data not shown).
Figure 2.
Localization of FasL in ESFT cells by immunofluorescence. ESFT cells exhibit diffuse cytoplasmic staining that is more prominent with the C-20 antibody (A) (Magnification, ×360) than with the Q-20 antibody (B) (Magnification, ×340). A paranuclear dot-like pattern of staining is also evident with the C-20 antibody. The granular circular pattern of staining that is also observed with the Q-20 antibody at the periphery of the cells is consistent with membranous localization of FasL.
ESFT Tissues Express FasL and Fas at High Frequency and FasL Expression Is Significantly Higher in Metastatic Sites
To exclude the possibility that FasL expression in ESFT is a random trait acquired from prolonged propagation of tumor cells in vitro, we studied the expression of FasL by immunohistochemistry in sections of 61 primary and metastatic tumor tissues from 49 patients. Thirty-nine of 49 patients (79.6%) were found positive for FasL in at least one tumor specimen. Metastatic tumors exhibited a higher incidence of staining than primary tumors. Specifically, among the 61 tumor specimens examined, 25 of 40 (62.5%) primary and 20 of 21 (95%) metastatic were FasL-positive. This difference was statistically significant (P < 0.006). Metastatic tumors also exhibited a statistically significant higher grade of staining than primary tumors (P = 0.000467), as shown in tissue sections in Figure 3, A and B ▶ . Positive tumor cells demonstrated both diffuse cytoplasmic and peripheral membrane staining in a rim-like pattern. The staining for FasL disappeared when the antibody was applied simultaneously with the corresponding blocking peptide.
Figure 3.
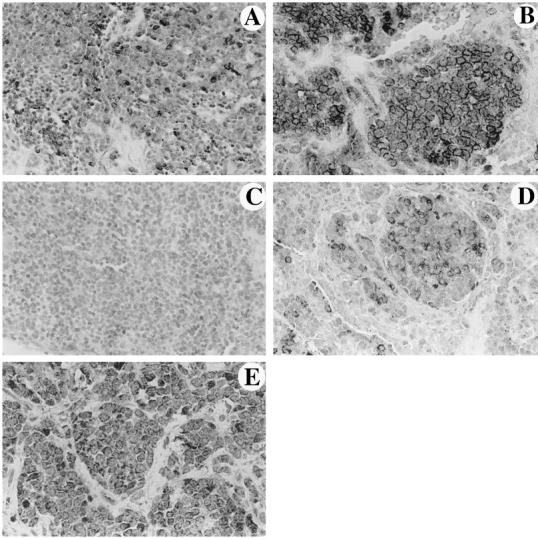
Primary (A) (Magnification, ×240) and metastatic (B) (Magnification, ×450) tumor specimens from different patients stained for FasL. Primary (C) (Magnification, ×165) and metastatic (D) (Magnification, ×250) tumor specimens from the same patient stained for FasL. FasL-positive cells exhibit diffuse cytoplasmic and/or peripheral membrane staining in all positive cases. The number of positive cells is higher in the metastatic tumors shown in B and D. In the paired specimens C and D, there is a definite increase in the number and immunoreactivity of FasL-expressing cells. E: ESFT cells exhibit diffuse cytoplasmic staining for Fas (Magnification, ×520).
To verify the significance of the observed higher FasL staining in metastatic ESFT, we also evaluated paired primary and metastatic tumor tissues from the same patients. A total of 12 pairs of tumor tissues were analyzed and in 8 of them a higher grade of tumor cell staining was observed in the metastasis (Figures 3C and D) ▶ . The remaining 4 showed no change. The difference in the grade of staining in the paired primary and metastatic tumor tissues was statistically significant (P = 0.0086). Positive endothelial cells within tumor tissues served as internal positive controls.
Because the apoptotic signal of FasL is transduced via the Fas receptor, we also investigated the presence of Fas in 53 ESFT tissues, 34 of which were primary and 19 metastatic. Fas was expressed in 40 (75.47%) ESFT in toto. Specifically, 27 of the primary (79.4%) and 13 of the metastatic (68%) tumors were found positive for the Fas receptor (Figure 3E) ▶ . However, the difference in the frequency and grade of staining for the Fas receptor in primary and metastatic ESFT was not statistically significant (P = 0.36 and P = 0.6425, respectively). Staining for Fas disappeared when the antibody was applied simultaneously with the corresponding blocking peptide.
The tm-FasL Is Functional
To evaluate the biological activity of the tm-FasL on the surface of ESFT cells, we used TC-248 or TC-32 cells as cytotoxic effectors in co-culture experiments with target Fas-expressing Jurkat cells. Two different assays were used for data confirmation: the MTT assay and a DNA fragmentation ELISA. Because the MTT assay is a cell viability test, the ESFT effector cells were lightly fixed to allow assessment of viability of target Jurkat cells. On the contrary, viable ESFT cells were used in the DNA fragmentation ELISA, in which quantification of specific DNA fragmentation of target cells was based on previous labeling of Jurkat cells with BrdU. With the MTT assay, Jurkat cells co-cultured with TC-248 cells exhibited 76 ± 2% cell death (mean ± SD). The Jurkat cell death was Fas/FasL-specific, because it was inhibited to 30.4 ± 0.9% in the presence of the neutralizing anti-FasL NOK-2 antibody (P < 0.000001). With the DNA fragmentation ELISA, Jurkat cells grown in the presence of TC248 cells exhibited 1721 ± 321% the amount of fragmented DNA seen in Jurkat cells grown in the absence of TC-248 cells (P < 0.000001). Treatment with NOK-2 antibody reduced the amount of fragmented DNA to 217 ± 105% of the control (P < 0.000001). Similar results were obtained for the TC-32 cell line.
The s-FasL Is Also Functional
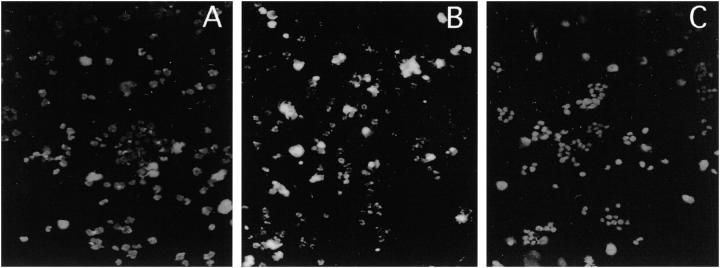
Having demonstrated that media conditioned with ESFT cells are positive for s-FasL, we studied whether they can induce apoptosis in Fas-sensitive cells. To address this question we incubated target Jurkat cells with TC-248 or TC-71 cell-conditioned media subjected to minimal (15-fold) concentration and evaluated Jurkat cell death with the MTT assay. Jurkat cells co-cultured with TC-248 media exhibited a 24 ± 6.5% (mean ± SD) cell death by MTT, that was decreased to 3.5 ± 0.4 in the presence of the neutralizing anti-FasL NOK-2 antibody (P < 0.005). These results were corroborated with TUNEL staining of cytospins generated from duplicate wells. Specifically, Jurkat cells treated with TC-248 medium showed many strongly stained apoptotic nuclei, which were absent or scarce in nontreated Jurkat cells and in Jurkat cells treated with TC-248 medium containing the NOK-2 neutralizing antibody (Figure 4, A ▶ -C). Similar results were obtained with TC-71 cell-conditioned media. These data show that s-FasL has the ability to induce apoptosis.
Figure 4.
Jurkat cells grown in nonconditioned media (A) and in media conditioned with TC-248 cells in the absence (B) or presence (C) of the FasL-neutralizing NOK-2 antibody were stained with the TUNEL method to detect apoptosis. Fluorescent nuclei corresponding to apoptotic nuclei are present in Jurkat cells incubated with TC-248 conditioned media, verifying the existence of functional s-FasL. Jurkat cells in non conditioned media (A) and in conditioned media with blocking antibody (C) show no apoptotic nuclei in these figures. Scant TUNEL-positive cells were only present in these conditions (Magnification, ×130).
ESFT Cells Resist Fas-Mediated Apoptosis
Because TC-268, TC-248, and TC-71 cells express functional tm- and s-FasL, we investigated their sensitivity to Fas-mediated apoptosis with the cytotoxic CH-11 anti-Fas antibody. All three ESFT cell lines showed minimal or no apoptosis, in contrast to the Fas-sensitive Jurkat cells used as a positive control.
Phenanthroline and the Synthetic Metalloproteinase Inhibitor BB-3103 Reduce Levels of s-FasL in the Media
Previously reported data have shown that the shedding of s-FasL by activated T-cells is dependent on metalloproteinase activity. 3-5 The detection of s-FasL in the media of ESFT cells led us to the hypothesis that ESFT cells may also have an active metalloproteinase-like enzyme that cleaves tm- to s-FasL. To test this hypothesis we treated TC-248 cells with various synthetic and natural tissue inhibitors of metalloproteinases. The zinc chelating agent 1,10-phenanthroline (Figure 5A) ▶ and the hydroxamic acid-based metalloproteinase inhibitor BB-3103 (Figure 5B) ▶ reduced the levels of s-FasL in the culture supernatant. On the contrary, treatment with the natural tissue metalloproteinase inhibitors TIMP-1 and TIMP-2 did not result in decreased levels of s-FasL in the supernatant (Figure 5C) ▶ . These results suggest that in ESFT cells, FasL is cleaved into s-FasL by a zinc-dependent metalloproteinase that is inhibited by BB-3103 but not by TIMP-1 or TIMP-2.
Figure 5.
Regulation of s-FasL release by metalloproteinases, detected by immunoblotting. Phenanthroline (A) and BB-3103 (B) inhibited the release of s-FasL in the media of TC-248 cells, whereas TIMP-1 and TIMP-2 did not (C).
Discussion
The expression of FasL in normal tissues is limited to activated lymphocytes, macrophages, 4 and endothelial cells, 23 and to a few immune-privileged tissues such as the testis, 24 eye, 25 brain 26 and placenta. 27 However, the list of tumors that express FasL is increasing and includes such histogenetically diverse tumors as hematopoietic neoplasms, 3,28-30 melanoma, 3 carcinomas (colon, hepatocellular, and lung), 14,22,31,32 and glioma. 33,34 Our finding of FasL in ESFT shows that FasL is not restricted to adult malignancies but is also expressed in pediatric sarcomas, namely ESFT. ESFT comprise a group of tumors with excellent initial response to chemotherapy and radiation, but with subsequent failures at metastatic sites. 16 Recently it has been proposed that FasL-expressing tumor cells use this molecule to overcome the host’s immune surveillance (“counterattack” model). 14 This concept was further supported by experiments in mice with and without mutation in the Fas gene. 15 Based on this model, we hypothesized that clones of ESFT resistant to Fas-mediated apoptosis and equipped with a cytolytic effector molecule such as FasL may enter the circulation and form metastatic colonies. Previous data have shown that only a small population of tumor cells succeed in forming metastases, 35-37 and individual metastases are likely clonal in origin. 38,39 Indeed, our data showed a statistically significant higher expression of FasL in a large number of paired and unpaired metastatic versus primary tumor tissue specimens. This finding supports selective survival and expansion of FasL-expressing clones in metastatic sites. The cytolytic property of FasL in ESFT cells was confirmed in co-culture experiments in which ESFT cells induced apoptosis of Jurkat cells. The apoptotic process was FasL-specific because it was inhibited by a neutralizing anti-FasL antibody.
FasL exists as tm-FasL and s-FasL. 1,2 We detected tm-FasL in all 9 and s-FasL in all 3 ESFT cell lines studied by immunoblotting and/or immunofluorescence staining. The tm-FasL was localized in the membrane, exhibiting a coarse granular pattern, and also in the cytoplasm, as reported previously. 6,7 The s-FasL was detected primarily in ESFT cell-conditioned media but also in cell pellets. Studies regarding the presence of s-FasL in human tumors have been limited. Specifically, s-FasL has been detected in cells and sera of patients with melanoma, 15 in sera of patients with hematological malignancies, 3,28 and in media conditioned with bleomycin-treated hepatocellular carcinoma cells. 32 The biological significance of s-FasL in solid tumors is currently unknown. Because it is released in the extracellular milieu and has been reported to have apoptotic properties, 2-5,28 s-FasL may induce apoptosis of activated lymphocytes beyond sites of tumor establishment, thus facilitating tumor cell propagation and successful homing of distant organs. However, the significance of the apoptotic properties of s-FasL in vivo has been recently questioned. Specifically, one study has shown that only aggregated s-FasL induces apoptosis in Jurkat cells 40 and another has suggested that various cells have preferentially lower sensitivity to s- than to tm-FasL depending on their levels of Fas expression. 41 We found that ESFT-conditioned media induced apoptosis in Jurkat cells at 15-fold concentration. These data support the hypothesis that s-FasL has apoptotic properties in vitro, but its apoptotic role in biological systems requires further confirmation. It has also been suggested that s-FasL may act as a negative regulator of cell death via binding to the Fas receptor and formation of Fas/s-FasL complexes, which are easily internalized, leading to secondary down-regulation of Fas. 41 Alternatively, s-FasL may simply be a catabolic product of FasL by which the cell down-regulates its surface levels of FasL, thus avoiding a suicidal death. To use the Fas/FasL system to their advantage, tumor cells must acquire resistance to Fas-mediated cytotoxicity. This is accomplished by one or both of the following mechanisms: decreased expression of the Fas receptor in tumor cells 15,32,33 or abrogation of transmission of the death signal. 14,15 The first mechanism does not apply to ESFT because these tumors frequently express Fas in both primary and metastatic sites, as shown in this study. In that respect, ESFT cells are similar to colon carcinoma cells that are resistant to Fas-mediated apoptosis but do not lack Fas expression. 14 Defective transmission of the cytotoxic signal may be partially responsible, because the 3 ESFT cell lines we studied were found to be resistant to the cytotoxic anti-Fas antibody. However, not all ESFT are resistant to Fas-mediated cytotoxicity, as we have shown in another study. 42 In the nonresistant cells, down-regulation of tm-FasL by cleavage may be important to their survival.
Recent studies have indicated that the FasL in activated human T-cells and FasL cDNA transfectants is processed by a metalloproteinase to yield a soluble form 3-5 similar to its homologous, TNF-α 43,44 that is cleaved by a recently cloned TNF-a converting enzyme. 45,46 The processing of the TNF-precursor is inhibited by hydroxamic acid-based synthetic metalloproteinase inhibitors that block zinc endopeptidases, 43,47 but not by exogenous TIMPs. These data suggested that a truncated metalloproteinase to which TIMPs are less potent may be responsible for TNF-a processing. 43 In this study we show that the tm-FasL in ESFT cells may also be cleaved into a soluble form via a peptidase that is a metalloproteinase, since both the general zinc chelator 1,10-phenanthroline and the more specific hydroxamic acid-based metalloproteinase inhibitor BB-3103 inhibited the shedding of s-FasL in the media. In agreement with the previous studies of TNF, TIMP-1 and TIMP-2 did not inhibit the release of s-FasL, supporting similarities between the metalloproteinases involved in cleavage of the two molecules. Recent data have shown that TNF-a and FasL are cleaved by distinct metalloproteinases that carry a similar active site. 41
Our data show that in solid tumors, namely ESFT, FasL may be cleaved by a metalloproteinase, because the release of s-FasL was inhibited by a metalloproteinase inhibitor (BB-3103) 48,49 as previously shown in lymphoid cells with older generation metalloproteinase inhibitors of the same family, such as batimastat (BB-94) and marimastat (BB-2516). 3,4,41 Batimastat and marimastat have been shown to reduce metastases and tumor growth in experimental assays and nude mice 50,51 and have been used as antineoplastic agents in phase II and III trials of a wide range of human solid tumors. 52-54 Their mode of action has been explained on the basis of their inhibition of extracellular matrix cleavage by metalloproteinases. However, new experimental evidence suggests that matrix metalloproteinases influence tumor progression not only via degradation of extracellular matrix, but also via growth regulation of primary and metastatic tumors. 50,55 Our finding that s-FasL levels in the media are reduced by the metalloproteinase inhibitor BB-3103 suggests that metalloproteinase inhibitors may reduce tumor growth by reversing the ratio of s- and tm-FasL. This may lead to tm-FasL accumulation, as reported in lymphoid and monocytic cells treated with metalloproteinase inhibitors, 4,5,41 and to tumor cell suicide in cells with a functional Fas pathway.
In conclusion, we have shown that ESFT generally express FasL and at significantly higher levels in their metastases. This suggests that FasL may help Fas-resistant tumor cells overcome the surveillance of the immune system. We have also shown that ESFT produce FasL in both transmembrane and soluble forms. The balance of these molecules may be important in tumor growth and metastasis and can be controlled by synthetic metalloproteinase inhibitors.
Footnotes
Address reprint requests to Nicholas Mitsiades, M.D., National Institutes of Health, Building 10, Room 2A10, 9000 Rockville Pike, Bethesda, MD 20892. E-mail: nmitsiad@box-n.nih.gov.
References
- 1.Suda T, Takahashi T, Golstein P, Nagata S: Molecular cloning and expression of the Fas ligand, a novel member of the tumor necrosis factor family. Cell 1993, 75:1169-1178 [DOI] [PubMed] [Google Scholar]
- 2.Tanaka M, Suda T, Takahashi T, Nagata S: Expression of the functional soluble form of human fas ligand in activated lymphocytes. EMBO J 1995, 14:1129-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka M, Suda T, Haze K, Nakamura N, Sato K, Kimura F, Motoyoshi K, Mizuki M, Tagawa S, Ohga S, Hatake K, Drummond AH, Nagata S: Fas ligand in human serum. Nat Med 1996, 2:317-322 [DOI] [PubMed] [Google Scholar]
- 4.Kayagaki N, Kawasaki A, Ebata T, Ohmoto H, Ikeda S, Inoue S, Yoshino K, Okumura K, Yagita H: Metalloproteinase-mediated release of human Fas ligand. J Exp Med 1995, 182:1777-1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mariani SM, Matiba B, Baumler C, Krammer PH: Regulation of cell surface APO-1/Fas (CD95) ligand expression by metalloproteases. Eur J Immunol 1995, 25:2303-2307 [DOI] [PubMed] [Google Scholar]
- 6.Kiener PA, Davis PM, Rankin BM, Klebanoff SJ, Ledbetter JA, Starling GC, Liles WC: Human monocytic cells contain high levels of intracellular Fas ligand. Rapid release following cellular activation. J Immunology 1997, 159:1594-1598 [PubMed] [Google Scholar]
- 7.Martinez-Lorenzo MJ, Alava MA, Anel A, Pineiro A, Naval J: Release of preformed Fas ligand in soluble form is the major factor for activation-induced death of Jurkat T cells. Immunology 1996, 89:511-517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oehm A, Berhmann I, Falk W, Pawlita M, Maier G, Klas C, Li-Weber M, Richards S, Dhein J, Trauth BC, Ponstingl H, Krammer PH: Purification and molecular cloning of the APO-1 cell surface antigen, a member of the TNF/NGF receptor superfamily. J Biol Chem 1992, 267:10709-10715 [PubMed] [Google Scholar]
- 9.Brunner T, Mogil RJ, LaFace D, Yoo NJ, Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, Green DR: Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates activation-induced apoptosis in T-cell hybridomas. Nature 1995, 373:441-444 [DOI] [PubMed] [Google Scholar]
- 10.Ju ST, Panka DJ, Cui H, Ettinger R, el-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A: Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature 1995, 373:444-448 [DOI] [PubMed] [Google Scholar]
- 11.Kägi D, Vignaux F, Ledermann B, Bürki K, Depraetere V, Nagata S, Hentgartner H, Golstein P: Fas and perforin pathways as major mechanisms of T-cell mediated cytotoxicity. Science 1994, 265:528-530 [DOI] [PubMed] [Google Scholar]
- 12.Lowin B, Hahne M, Mattmann C, Tschopp J: Cytolytic T-cell cytotoxicity is mediated through perforin, and Fas lytic pathways. Nature 1994, 370:650-652 [DOI] [PubMed] [Google Scholar]
- 13.Rensing-Ehl A, Frei K, Flury R, Matiba B, Mariani S, Weller M, Aebischer P, Krammer P, Fontana A: Local Fas/Apo-1 (CD95) ligand-mediated tumor cell killing in vivo. Eur J Immunol 1995, 25:2253-2258 [DOI] [PubMed] [Google Scholar]
- 14.O’Connell J, O’Sullivan GC, Collins JK, Shanahan F: The Fas counterattack: Fas-mediated T cell killing by colon cancer cells expressing Fas ligand. J Exp Med 1996, 184:1075-1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French LE, Schneider P, Bornand T, Fontana A, Lienard D, Cerottini J, Tschopp J: Melanoma cell expression of Fas(Apo-1/CD95) ligand: Implications for tumor immune escape. Science 1996, 274:1363-1366 [DOI] [PubMed] [Google Scholar]
- 16.Horowitz ME, DeLaney TF, Malawar MM, Tsokos MG: Ewing’s sarcoma family of tumors: Ewing’s sarcoma of bone and soft tissue and the peripheral primitive neuroectodermal tumors. Pizzo PA Poplack DG eds. In Principles and Practice of Pediatric Oncology. 1993, :pp 795-821 JB Lippincott, Philadelphia [Google Scholar]
- 17.Cavazzana AO, Navarro S, Noguera R, Reynolds PC, Triche TJ: Olfactory neuroblastoma is not a neuroblastoma but is related to primitive neuroectodermal tumor (PNET). Prog Clin Biol Res 1988, 271:463-473 [PubMed] [Google Scholar]
- 18.Sorensen PHB, Liu XF, Delattre O, Rowland JM, Biggs CA, Thomas G, Triche TJ: Reverse transcriptase PCR amplification of EWS/FLI-1 fusion transcripts as a diagnostic test for peripheral primitive neuroectodermal tumors of childhood. Diagn Mol Pathol 1993, 2:147-157 [PubMed] [Google Scholar]
- 19.Sorensen PHB, Wu JK, Berean KW, Lim JF, Donn W, Frierson HF, Reynolds CP, L-pez-Terrada D, Triche TJ: Olfactory neuroblastoma is a peripheral primitive neuroectodermal tumor related to Ewing’s sarcoma. Proc Natl Acad Sci USA 1996, 93:1038-1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Whang-Peng J, Triche TJ, Miser J, Kao-Shan S, Tsai S, Israel M: Cytogenetic characterization of selected small round cell tumors of childhood. Cancer Genet Cytogenet 1986, 21:185-208 [DOI] [PubMed] [Google Scholar]
- 21.Mitsiades N, Poulaki V, Kotoula V, Mastorakos G, Tseleni-Balafouta S, Koutras DA, Tsokos M: Fas/Fas ligand up-regulation, and Bcl-2 down-regulation may be significant in the pathogenesis of Hashimoto’s thyroiditis. J Clin Endocrinol Metab 1998, 83:2199-2203 [DOI] [PubMed] [Google Scholar]
- 22.Shiraki K, Tsuji N, Shioda T, Isselbacher KJ, Takahashi H: Expression of fas ligand in liver metastases of human colonic adenocarcinomas. Proc Natl Acad Sci USA 1997, 94:6420-6425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sata M, Walsh K: TNFalpha regulation of Fas ligand expression on the vascular endothelium modulates leukocyte extravasation. Nat Med 1998, 4:415-420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC: A role for CD95 ligand in preventing graft rejection. Nature 1995, 377:630-632 [DOI] [PubMed] [Google Scholar]
- 25.Griffith TS, Brunner T, Fletcher SM, Green DR, Ferguson TA: Fas ligand induced apoptosis as a mechanism of immune privilege. Science 1995, 270:1189-1192 [DOI] [PubMed] [Google Scholar]
- 26.French LE, Hahne M, Viard I, Radlgruber G, Zanone R, Becker K, Muller C, Tschopp J: Fas and Fas ligand in embryos and adult mice: ligand expression in several immune-privileged tissues and coexpression in adult tissues characterized by apoptotic cell turnover. J Cell Biol 1996, 133:335-343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Runic R, Lockwood CJ, Ma Y, Dipasquale B, Guller S: Expression of Fas ligand by human cytotrophoblasts: implications in placentation and fetal survival. J Clin Endocrinol Metab 1996, 81:3119-3122 [DOI] [PubMed] [Google Scholar]
- 28.Sato K, Kimura F, Nakamura Y, Murakami H, Yoshida M, Tanaka M, Nagata S, Kamatani Y, Wakimoto N, Nagata N, Motoyoshi K: An aggressive lymphoma accompanied by high levels of soluble FasL. Br J Haematol 1996, 94:379-382 [DOI] [PubMed] [Google Scholar]
- 29.Perzova R, Loughran T: Constitutive expression of Fas ligand in large granular lymphocyte leukaemia. Br J Haematol 1997, 197:123-126 [DOI] [PubMed] [Google Scholar]
- 30.Villunger A, Egle A, Marschitz I, Kos M, Böck G, Ludwig H, Geley S, Kofler R, Greil R: Constitutive expression of Fas (Apo-1/CD95) ligand on multiple myeloma cells: A potential mechanism of tumor-induced suppression of immune surveillance. Blood 1997, 90:12-20 [PubMed] [Google Scholar]
- 31.Niehans GA, Brunner T, Frizelle SP, Liston JC, Salerno CT, Knapp DJ, Green DR, Kratzke RA: Human lung carcinomas express Fas ligand. Cancer Res 1997, 57:1007-1012 [PubMed] [Google Scholar]
- 32.Strand S, Hofmann WJ, Hug H, Muller M, Otto G, Strand D, Mariani SM, Stremmel W, Krammer PH, Galle PR: Lymphocyte apoptosis induced by CD95 (APO-1/Fas) ligand-expressing tumor cells: a mechanism of immune evasion? Nat Med 1996, 12:1361-1366 [DOI] [PubMed] [Google Scholar]
- 33.Saas P, Walker PR, Hahne M, Quiquerez AL, Schnuriger V, Perrin G, French L, Van Meir EG, de Tribolet N, Tschopp J, Dietrich PY: Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? J Clin Invest 1997, 99:1173-1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gratas C, Tohma Y, Van Meir EG, Klein M, Tenan M, Ishii N, Tachibana O, Kleihues P, Ohgaki H: Fas ligand expression in glioblastoma cell lines and primary astrocytic brain tumors. Brain Pathol 1997, 7:863-869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spremuli EN, Dexter DL: Human tumor cell heterogeneity and metastasis. J Clin Oncol 1983, 1:496-509 [DOI] [PubMed] [Google Scholar]
- 36.Fidler IJ: Origin and biology of cancer metastasis. Cytometry 1989, 10:673-680 [DOI] [PubMed] [Google Scholar]
- 37.Weiss L: Metastatic inefficiency. Adv Cancer Res 1990, 54:159-211 [DOI] [PubMed] [Google Scholar]
- 38.Talmadge JE, Wolman SR, Fidler IJ: Evidence for the clonal origin of spontaneous metastases. Science 1982, 217:361-363 [DOI] [PubMed] [Google Scholar]
- 39.Chambers AF, Wilson S: Use of NeoR B16F1 murine melanoma cells to assess clonality of experimental metastases in the immune-deficient chick embryo. Clin Exp Metastasis 1988, 6:171-182 [DOI] [PubMed] [Google Scholar]
- 40.Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J: Conversion of membrane-bound Fas (CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med 1998, 187:1205-1213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanaka M, Itai T, Adachi M, Nagata S: Downregulation of Fas ligand by shedding. Nat Med 1998, 4:31-36 [DOI] [PubMed] [Google Scholar]
- 42.Tsokos M, Vassilopoulos D, Szentendrei T, Quezado M, Karameris A, Tsokos G: Fas-mediated apoptosis in peripheral primitive neuroectodermal tumors (PNET) Ewing’s sarcoma (ES). FASEB J 1996, 10:A1009(Abstr) [Google Scholar]
- 43.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Woodley K: Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature 1994, 370:555-557 [DOI] [PubMed] [Google Scholar]
- 44.Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements JM, Crimmin M, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, Leber TM, Mangan M, Miller K, Nayee P, Owen K, Patel S, Thomas W, Wells G, Wood LM, Woolley K: Matrix metalloproteinases and processing of pro-TNF-α. J Leukoc Biol 1995, 57:774-777 [DOI] [PubMed] [Google Scholar]
- 45.Moss ML, Jin SL, Milla ME, Bickett DM, Burkhart W, Carter HL, Chen WJ, Clay WC, Didsbury JR, Hassle D, Hoffman CR, Kost TA, Lambert MH, Leesnitzer MA, McCauley P, McGeehan G, Mitchell J, Moyer M, Pahel G, Rocque W, Overton LK, Schoenen F, Seaton T, Su JL, Warner J, Willard D, Becherer JD: Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-α. Nature 1997, 385:733-736 [DOI] [PubMed] [Google Scholar]
- 46.Black RA, Rauch CT, Kozlosky CJ, Peschon JJ, Slack JL, Wolfson MF, Castner BJ, Stocking KL, Reddy P, Srinivasan S, Nelson N, Boiani N, Schooley KA, Gerhart M, Davis R, Fitzner JN, Johnson RS, Paxton RJ, March CJ, Cerretti DP: A metalloproteinase disintegrin that releases tumour-necrosis factor-α from cells. Nature 1997, 385:729-733 [DOI] [PubMed] [Google Scholar]
- 47.McGeehan GM, Becherer JD, Bast RC, Jr, Boyer CM, Champion B, Connolly KM, Conway JG, Furdon P, Karp S, Kidao S, McElroy AB, Nichols J, Pryzwansky KM, Schoenen F, Sekut L, Truesdale A, Verghese M, Warner J, Ways JP: Regulation of tumour necrosis factor-α processing by a metalloproteinase inhibitor. Nature 1994, 370:558-561 [DOI] [PubMed] [Google Scholar]
- 48.Middelhoven PJ, Ager A, Roos D, Verhoeven AJ: Involvement of a metalloprotease in the shedding of human neutrophil Fc gammaRIIIB. FEBS Lett 1997, 414:14-18 [DOI] [PubMed] [Google Scholar]
- 49.Ancuta P, Fahmi H, Pons JF, Le Blay K, Chaby R: Involvement of the membrane form of tumour necrosis factor-α in lipopolysaccharide-induced priming of mouse peritoneal macrophages for enhanced nitric oxide response to lipopolysaccharide. Immunology 1997, 92:259-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chambers AF, Matrisian LM: Changing views of the role of matrix metalloproteinases in metastasis. J Natl Cancer Inst 1997, 89:1260-1270 [DOI] [PubMed] [Google Scholar]
- 51.Rasmussen HS, McCann PP: Matrix metalloproteinase inhibition as a novel anticancer strategy: a review with special focus on batimastat and marimastat. Pharmacol Ther 1997, 75:69-75 [DOI] [PubMed] [Google Scholar]
- 52.Bramhall SR: The matrix metalloproteinases and their inhibitors in pancreatic cancer: from molecular science to a clinical application. Int J Pancreatol 1997, 21:1-12 [DOI] [PubMed] [Google Scholar]
- 53.Denis LJ, Verweij J: Matrix metalloproteinase inhibitors: present achievements and future projects. Invest New Drugs 1997, 15:175-185 [DOI] [PubMed] [Google Scholar]
- 54.Wojtowicz-Praga SM, Dickson RB, Hawkins MJ: Matrix metalloproteinase inhibitors. Invest New Drugs 1997, 15:61-75 [DOI] [PubMed] [Google Scholar]
- 55.Stettler-Stevenson WG: Dynamics of matrix turnover during pathologic remodelling of the extracellular matrix. Am J Pathol 1996, 148:1345-1350 [PMC free article] [PubMed] [Google Scholar]