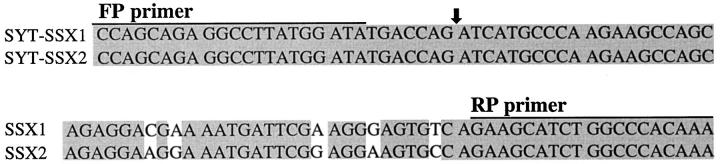Abstract
The reciprocal translocation t(X;18)(p11;q11) is known to be highly characteristic of synovial sarcoma, and its consequence, an SYT-SSX fusion gene, is expected to be a diagnostic molecular marker. In this study, we conducted a reverse transcription-polymerase chain reaction-based assay to detect the SYT-SSX fusion gene transcripts using archival formalin-fixed, paraffin-embedded tumor specimens from a series of 32 synovial sarcoma cases including 6 tumors found in unusual anatomical sites. The SYT-SSX fusion transcripts could be detected in 30 of 32 paraffin-embedded specimens (94%). A subsequent sequence analysis using the polymerase chain reaction products confirmed that the detected messages were derived from either the SYT-SSX1 (22 cases) or SYT-SSX2 (8 cases) fusion gene. Of 23 SYT-SSX-positive monophasic tumors, 16 tumors had an SYT-SSX1 fusion transcript. Fusion transcripts were detectable in all the 7 biphasic tumors analysed, one of which had an SYT-SSX2 fusion transcript. All of the six tumors at unusual locations (lung; 3, metastasis to the abdominal cavity from a tumor of retroperitoneal origin; 1, sacral region; 1, iliopsoas muscle; 1) contained detectable messages. Our results indicate that this molecular assay can be applied to archival formalin-fixed, paraffin-embedded tumor tissues as a feasible and reliable molecular technique for the diagnosis of synovial sarcoma.
Synovial sarcomas, which account for approximately 5 to 10% of soft tissue sarcomas, occur most commonly in young adults and arise mainly in the para-articular regions. There are two major histological subtypes of synovial sarcoma: the biphasic type with distinct epithelial and spindle cell components in varying proportions and the monophasic fibrous type. The monophasic epithelial type and the poorly differentiated type are rare variants of the tumor. 1 Because the differential diagnosis of synovial sarcoma, particularly of the monophasic fibrous type from other spindle cell sarcomas, such as fibrosarcomas, leiomyosarcomas and neurogenic sarcomas, is difficult, immunohistochemistry to demonstrate expressions of epithelial markers, such as cytokeratins and epithelial membrane antigen (EMA), is usually required. However, these markers are not universally present in synovial sarcomas and some other soft tissue sarcomas possess the epithelioid profile, as well as mesotheliomas and carcinomas. Thus, more reliable diagnostic aids are being sought.
Recent cytogenetic studies have shown that a characteristic t(X;18)(p11;q11) chromosomal translocation is associated with more than 90% of both subtypes of synovial sarcomas, and this translocation has been suggested as a diagnostic indicator of this tumor. 2 Cloning of the breakpoints of this translocation identified two novel genes, resulting in a fusion of the segments of the SSX gene on chromosome Xp11 and the SYT gene on chromosome 18q11. 3 Subsequent studies disclosed that the Xp11 breakpoint actually involves either of two closely related genes, SSX1 and SSX2, 4,5 located in the vicinity of ornithine aminotransferase-like pseudogenes 1 and 2, respectively. The SSX1 and SSX2 genes are presumably derived from a relatively recent duplication event and encode proteins with considerable homology (81%). 5 Because SYT contains a region similar to a transcription activation domain, this fusion may generate a novel transcriptional regulator. Thus, the fusion protein derived from t(X;18)(p11;q11) seems to aberrantly activate its target genes, and likely plays an important role in the oncogenesis of synovial sarcoma.
Identification of such specific chromosomal translocations or fusion genes known to characterize particular tumor types can facilitate or justify the tumor diagnosis, and may provide a prognostic implication. The cytogenetical and molecular approaches to detect these translocations or rearranged genes usually require fresh or snap-frozen samples of tumor tissues or cultured tumor cells. Because fresh or snap-frozen material or cultured tumor cells are not always available, methods applicable to paraffin-embedded material are necessary to identify such translocations or gene arrangements for diagnostic and prognostic purposes. So far, nested reverse transcription-polymerase chain reaction (RT-PCR) and fluorescence in situ hybridization are major tools for the detection of such molecular or genetic alterations in tumors using paraffin-embedded tissue. Most recently, molecular detection of the SYT-SSX fusion transcripts by a one-step RT-PCR using post-fixed, paraffin-embedded tumor specimens has been reported. 6 However, the examined cases were relatively limited, and the type of fusion transcript was not addressed in the reported study.
In this study, we conducted an RT-PCR assay for the SYT-SSX gene using archival formalin-fixed, paraffin-embedded tumor specimens from 32 patients with synovial sarcomas to assess its feasibility in detecting the fusion gene transcript, and analyzed their molecular and clinicopathological features.
Materials and Methods
Materials
Thirty-two cases of synovial sarcoma were retrieved from the files of the Department of Pathology and Oncology, School of Medicine, University of Occupational and Environmental Health and the Department of Pathology, Kurume University Hospital, and from the consultation file of one of the authors (HH). The clinical data of these cases are summarized in Table 1 ▶ . The histological diagnosis of each case was confirmed by the authors (MH, YM, and HH) by reviewing the original and/or re-prepared hematoxylin-eosin (HE) staining sections of the tumor specimens, together with positive immunoreactivities to cytokeratin and/or epithelial membrane antigen, especially in the monophasic fibrous type. Six cases of the tumor at unusual anatomical sites, namely the lung (3 cases), sacral region (1 case), iliopsoas muscle (1 case), and abdominal cavity (1 case, metastasis from a tumor in the retroperitoneum) were included in this series. Two cases of malignant peripheral nerve sheath tumor (MPNST) were also added as negative controls. Information on the patient outcomes was obtained (Table 1) ▶ . Postoperative follow-up periods ranged from 2 to 163 months (mean, 40; median, 24).
Table 1.
Clinicopathological and Molecular Features of Synovial Sarcomas
| Case No. | Age (yr) | Sex | Site | Histological subtype | RT-PCR | Fusion type | Follow-up (months) | Outcome | ||
|---|---|---|---|---|---|---|---|---|---|---|
| β-actin | PBGD | SYT-SSX | ||||||||
| 1 | 24 | M | Hand | Biphasic | + | + | + | 1 | 41 | NED |
| 2 | 54 | M | Shoulder | Monophasic | + | + | + | 1 | 23 | DOD |
| 3 | 23 | M | Forearm | Monophasic | + | + | + | 1 | 22 | AWD |
| 4 | 30 | M | Sole | Monophasic | − | + | + | 1 | 12 | NED |
| 5 | 62 | F | Knee | Biphasic | + | + | + | 2 | 117 | NED |
| 6 | 39 | F | Thigh | Biphasic | − | − | + | 1 | 42 | NED |
| 7 | 45 | M | Shoulder | Monophasic | − | + | + | 1 | 23 | DOD |
| 8 | 14 | M | Chest wall | Monophasic | + | + | + | 1 | 27 | DOD |
| 9 | 28 | F | Shoulder | Monophasic | − | + | + | 1 | 156 | AWD |
| 10 | 13 | M | Sole | Monophasic | + | + | + | 1 | 25 | AWD |
| 11 | 61 | F | Lower leg | Monophasic | + | + | − | − | 21 | AWD |
| 12 | 13 | F | Knee | Biphasic | + | + | + | 1 | 36 | NED |
| 13 | 16 | M | Elbow | Monophasic | + | + | + | 2 | 7 | NED |
| 14 | 15 | F | Groin | Monophasic | + | + | + | 2 | 8 | DOD |
| 15 | 19 | F | Buttock | Monophasic | + | + | + | 1 | 35 | DOD |
| 16 | 64 | F | Upper arm | Monophasic | + | + | − | − | 14 | NED |
| 17 | 35 | F | Thigh | Monophasic | + | + | + | 1 | 3 | NED |
| 18 | 26 | M | Lower leg | Monophasic | + | + | + | 1 | 17 | DOD |
| 19 | 51 | M | Forearm | Biphasic | − | + | + | 1 | 153 | NED |
| 20 | 46 | M | Groin | Biphasic | − | + | + | 1 | 80 | DOD |
| 21 | 16 | M | Groin | Monophasic | − | + | + | 1 | 38 | DOD |
| 22 | 15 | F | Thigh | Biphasic | + | + | + | 1 | 83 | NED |
| 23 | 24 | M | Groin | Monophasic | − | − | + | 1 | 163 | AWD |
| 24 | 55 | M | Foot | Monophasic | − | − | + | 2 | 2 | NED |
| 25 | 28 | M | Great toe | Monophasic | + | + | + | 2 | 2 | NED |
| 26 | 59 | M | Chest wall | Monophasic | + | − | + | 2 | 20 | NED |
| Tumors occurring at unusual sites | ||||||||||
| 27 | 58 | F | Lung | Monophasic | + | − | + | 1 | 5 | NED |
| 28 | 53 | F | Lung | Monophasic | − | + | + | 2 | 24 | DOD |
| 29 | 50 | F | Lung | Monophasic | − | + | + | 1 | 46 | AWD |
| 30 | 22 | F | Abdominal cavity* | Monophasic | − | + | + | 2 | 25 | DOD |
| 31 | 56 | F | Sacral region | Monophasic | + | + | + | 1 | 2 | AWD |
| 32 | 55 | F | Iliopsoas muscle | Monophasic | + | + | + | 1 | 3 | DOD |
PBGD, porphobilinogen deaminase; NED, no evidence of disease; DOD, died of disease; AWD alive with disease.
*Metastasis to the abdominal cavity from a tumor of retroperitoneal origin.
RNA Extraction
Five sections, each 4 to 5 μm thick, were prepared from each representative paraffin-embedded tumor sample. To avoid cross-contamination of samples, a new microtome blade was used for each case. The area of the microtome around the blade was cleaned with 70% ethanol between cases. The sections were put into 1.5-ml sterile microtubes and dewaxed in two changes of xylene and three washes with 100% ethanol. After drying, tissue pellets were added by 200 μl of lysis buffer (20 mmol/L Tris-HCl, pH 8.0, 20 mmol/L EDTA, 2% sodium dodecyl sulfate). Each sample was homogenized with a hand homogenizer, and 10 μl of proteinase K solution (75 to 100 mg/ml) was added. After overnight incubation at 55°C, a 1-ml volume of Trizol reagent (Gibco BRL, Gaithersburg, MD) was added to the sample and total RNA was extracted according to the manufacturer’s instructions. The RNA pellet was reconstituted in 20 μl of DNase/RNase-free water.
RT-PCR
An aliquot of the extracted RNA was reverse-transcribed into cDNA using 1 μl of random primers and 200 U of reverse transcriptase (SuperScript II, Gibco BRL). PCR was carried out using the primer set, which was designed to specifically amplify both of the junctional regions of the SYT-SSX1 and SYT-SSX2 fusion gene transcripts reported previously (Fig. 1) ▶ .4> The × PCR buffer (Perkin Elmer), 200 mmol/L deoxynucleotide triphosphates, and 0.2 mmol/L each of forward and reverese primers. The amplification profile of the PCR consisted of 45 cycles of denaturation at 94°C for 50 seconds annealing at 61°C for 30 seconds and extension at 72°C for 1 minute. After 45 cycles, an aliquot of the PCR product was electrophoresed on a 3% agarose gel and stained with ethidium bromide. As positive controls for the integrity of mRNA in each sample, PCR for ubiquitously expressed β-actin and porphobilinogen deaminase (PBGD) gene transcripts was performed with the following primers: act-f (5′-AGGCCAACCGCGAGAAGATGACC-3′), act-r (5′-GAAGTCCAGGGCGACGTAGCAC-3′), PBGD-S (5′-TGTCTGGTAACGGCAATGCGGCTGCAAC-3′), PBGD-A (5′-TCAATGTTGCCACCACA-CTGTCCGTCT-3′). 7,8 These primers amplify a 343-bp fragment of β-actin mRNA and a 127-bp fragment of PBGD mRNA, respectively. A reaction mixture of reagents devoid of template cDNA was included in each PCR procedure as a negative control.
Figure 1.
Oligonucleotide sequences of primers used for SYT-SSX fusion transcripts. Partial nucleotide sequences flanking both types of fusion transcript of synovial sarcoma are shown. The SYT-SSX1 and SYT-SSX2 fusion points are indicated by an arrow.
To confirm the type of the fusion gene, the PCR product was cloned into a pCR2.1 vector (Invitrogen, San Diego, CA) by TA ligation and sequenced using an automated sequencing system, ALFexpress DNA sequencer (Pharmacia Biotech, Uppsala, Sweden).
Statistical Analysis
Survival curves were estimated using the method of Kaplan and Meier. 9 We examined the effects of several variables on survival curves, including the patient’s age (older or younger than 30) and sex, tumor size (greater or less than 5 cm in diameter), location (extremity or others), histologic subtype (biphasic or monophasic), and type of fusion transcript (SYT-SSX1 or SYT-SSX2). Differences in the curves were analyzed with the log-rank test.
Results
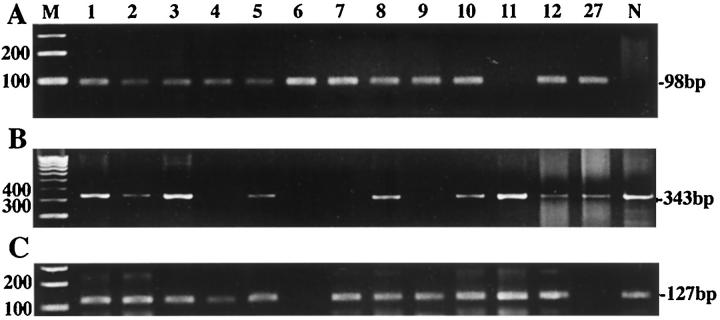
The transcript of the β-actin gene could be amplified in 20 (63%) of the 32 paraffin-embedded tumors, while that of the PBGD was detected in 27 (84%) samples (Figure 2) ▶ . Representative morphology of histologic subtypes are shown in Figure 3 ▶ . With use of the FP/RP primer set, the predicted 98-bp product of the SYT-SSX fusion transcript was identified in 30 (94%) of the 32 cases. Nucleotide sequences of these products were confirmed by sequence analysis. Twenty-two of the positive samples (73%) were proven to be SYT-SSX1 fusion type, whereas the other 8 (27%) were SYT-SSX2 fusion type. Partial sequences of both types of fragments amplified by this RT-PCR are shown in Figure 4 ▶ . Six of 7 biphasic and 16 of 25 monophasic tumors had an SYT-SSX1 fusion transcript, while 1 biphasic and 7 monophasic tumors had an SYT-SSX2 fusion transcript. All 6 tumors arising in unusual sites contained detectable SYT-SSX fusion transcripts. Two MPNSTs showed no PCR products of the SYT-SSX fusion transcript, although the messages of the β-actin and PBGD genes could be detected. The result of each case is shown in Table 1 ▶ .
Figure 2.
Identification of SYT-SSX fusion transcripts (A) with RT-PCR and RT-PCR for β-actin gene (B) and PBGD gene (C) messages for assessment of the integrity of mRNA in each sample. Most tissue samples (Table 1) ▶ generated positive products of 98 bp corresponding to SYT-SSX1 or SYT-SSX2 fusion transcript of the SYT-SSX gene. The number of the lane is identical to the case number in Table 1 ▶ . M, 100-bp DNA ladder; N, negative control (malignant peripheral nerve sheath tumor).
Figure 3.
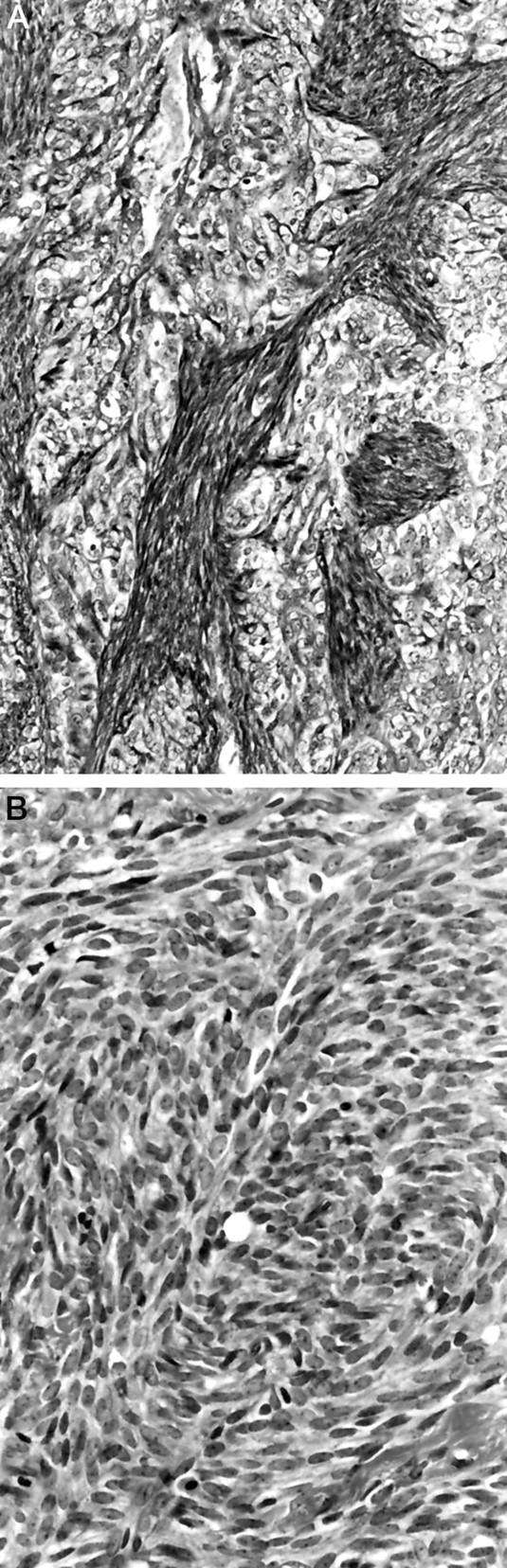
Histologic subtypes of synovial sarcoma. A: Biphasic synovial sarcoma (Case 19). The coexistence of epithelial and spindle cell components is recognized (hematoxylin and eosin, ×50). B: Monophasic fibrous synovial sarcoma (Case 4) (hematoxylin and eosin, ×100).
Figure 4.
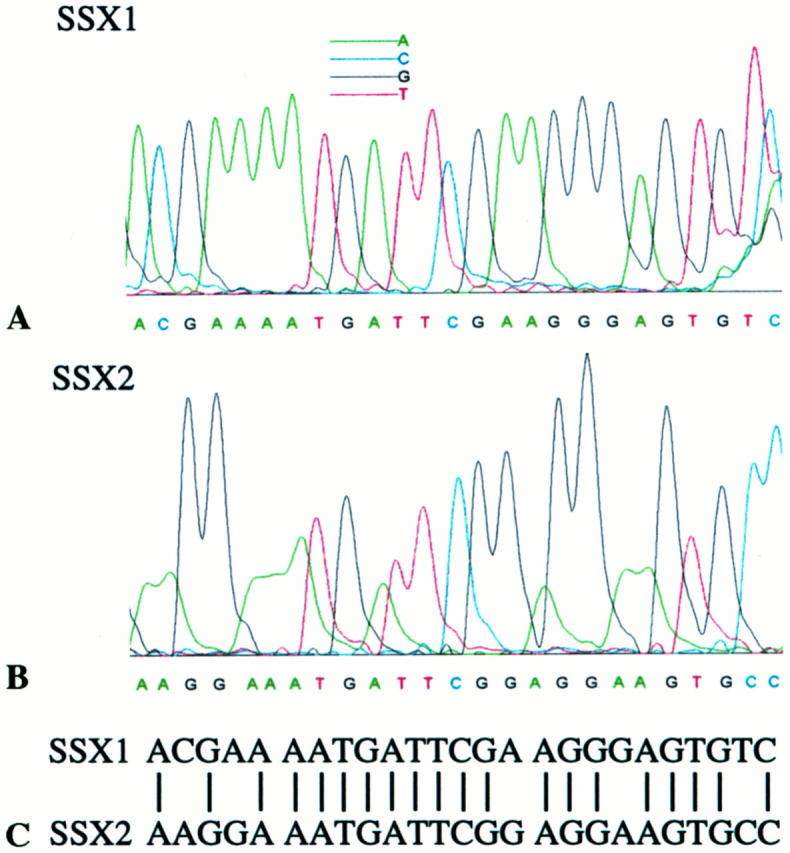
Partial sequences of SSX1 (A) and SSX2 (B) in the detected fusion transcripts. In these sequences, 5 nucleotides are distinctive (C).
The overall survival rate at five years for all 32 patients was 53 percent. By a log-rank test in the two subgroups, 18 patients with tumors in the extremities had a significantly longer survival than 14 patients who had a tumor in the trunk or a proximal site including the groin and the buttock (P = 0.013). Seven patients with a biphasic subtype also had a significantly longer survival than the 25 patients with a monophasic subtype (P = 0.013). The age and sex of patients, the size of tumor, and the type of fusion transcript (SYT-SSX1 or SYT-SSX2) did not significantly affect survival.
Discussion
With the recent application of cytogenetic and molecular approaches to human sarcomas, many tumor-specific chromosomal translocations and their molecular consequences have been identified. t(X;18) or the SYT/SSX fusion gene in synovial sarcoma is a representative of such cytogenetic or molecular aberrations, and is expected to be an aid in the histological diagnosis as well as the elucidation of the basic biological mechanisms underlying tumorigenesis.
Molecular approaches using mRNA isolated from archival formalin-fixed, paraffin-embedded tissues have recently been carried out to detect messages derived from tumor-specific translocations or fusion genes in human sarcomas such as the Ewing’s sarcoma family, alveolar rhabdomyosarcoma, and desmoplastic small round cell tumor, 10-14 which suggested the feasibility and diagnostic utility of the RT-PCR technique in these tumors. Concerning synovial sarcoma, Argani et al recently reported one-step RT-PCR targeting of rather short fragments of the common junctional region of the SYT-SSX1 and SYT-SSX2 fusion gene transcripts. 6
Argani et al also demonstrated the application of their approach to problematic cases of synovial sarcoma in the thorax, neck, and subcutis. Our strategy conforms to their ideas and we can attest to the diagnostic utility of this approach in a larger series including three tumors in the lung, one metastasis to the abdominal cavity from a retroperitoneal tumor, one tumor in the iliopsoas muscle, and one which arose in one of the spinal nerve roots in the sacral region. All three pulmonary tumors occurred primarily in an intrapulmonary location. Although histological and immunohistochemical features of these tumors strongly suggested synovial sarcoma, the differential diagnosis of them was broad and included a wide variety of spindle cell tumors both primary and metastatic to the lung. Zeren et al 15 reported a series of 25 primary spindle cell sarcomas in the lung and suggested the intrapulmonary counterpart of synovial sarcoma of soft tissue. Kaplan et al 16 described a primary pulmonary spindle cell sarcoma with t(X;18), establishing synovial sarcoma as a subtype of pulmonary sarcomas. A small number of case reports on synovial sarcomas arising in the retroperitoneum 17,18 and the peripheral nerve 19-22 are present, although their observations were based only on clinicopathological features. Our results justify the diagnosis of lesions in such unusual sites and support these previous observations.
As three fusion-positive samples with neither detectable β-actin (343 bp) nor PBGD (127 bp) gene transcript in our series, the 98-bp fragment of the fusion gene transcript may still remain in paraffin-embedded tissues and seems to have been successfully amplified by RT-PCR. Therefore, the selection of small target sequences is important to improve the sensitivity of this detection method. We adopted a primer set amplifying 98-bp fragments of the SYT-SSX fusion transcripts, the size of which is comparable to that used by Argani et al. 6 In contrast to their method, the amplified fragments in our study contained either SSX1- or SSX2-specific sequence to facilitate analysis of the fusion type.
In the more recent series of 45 synovial sarcomas by Kawai et al, 16 (36%) tumors had an SYT-SSX2 transcript, which was reported to be confined to monophasic tumors, and patients with those tumors had a better prognosis than patients with tumors having an SYT-SSX1 transcript. 23 However, the present analysis showed no significant differences in prognoses between the two groups of patients, which might be due to the smaller number of cases in our study. One of our biphasic tumors had an SYT-SSX2 fusion transcript, indicating that the previously reported association between SSX2 fusions and monophasic histology is not absolute. Some other investigators have also reported the SYT-SSX2 fusion in biphasic synovial sarcoma. 5,24,25 The relationship between the molecular features and the tumor prognosis or histological subtype still remains to be determined in synovial sarcoma.
In two samples positive for both β-actin and PBGD gene transcript in our series, our molecular approach failed to detect the fusion gene transcript. At surgery, both fusion-negative tumors involved the peripheral nerves (the median nerve and common peroneal nerve, respectively) and were clinically considered MPNST. However, positive immunoreactivities to epithelial membrane antigen and cytokeratins reinforced synovial sarcoma rather than MPNST. Negative results would not always imply the absence of the fusion gene in this RT-PCR assay. Although total RNA was successfully extracted in both specimens, constituent messages derived from the tumor cells might be quite limited. Alternatively, an unrecognized variation of the junction in fusion genes or other types of gene rearrangement should be taken into consideration.
We now have developed a method for detecting the SYT-SSX fusion gene characteristic of synovial sarcomas in archival formalin-fixed, paraffin-embedded tissues. In addition to its utility in the diagnosis of synovial sarcoma even with unusual clinical presentation, this assay will facilitate retrospective studies to correlate molecular findings with clinicopathological features in a large series of tumors.
Acknowledgments
We thank Professors Minoru Morimatsu and Masamichi Kojiro, Kurume University School of Medicine, Professor Teruo Iwamasa, Ryukyu University School of Medicine, and Dr. Shigeo Yokoyama, Oita Medical University, for providing paraffin-embedded tissues of synovial sarcomas, and Professor Yasuyuki Sasaguri, University of Occupational and Environmental Health, for serious and encouraging comments. We also thank Atsuko Tanaka and Megumi Katayama for technical assistance.
Footnotes
Address correspondence and reprint requests to Dr. Hiroshi Hashimoto, Department of Pathology and Oncology, School of Medicine, University of Occupational and Environmental Health, 1-1 Iseigaoka, Yahatanishi-ku, Kitakyushu 807-8555, Japan. E-mail: hiroshi\@med.uoeh-u.ac.jp.
Supported in part by 1997 Grants-in-Aid from the Ministry of Education, Science, Sports and Culture (08670229) and the Vehicle Racing Commemorative Foundation.
References
- 1.Enzinger FM, Weiss SW: Synovial sarcoma. Soft Tissue Tumors. 1995:pp 757-786 Mosby, Edited by FM Enzinger and SW Weiss. 3rd ed. St. Louis
- 2.Sreekantaiah C, Ladanyi M, Rodriguez E, Chaganti RSK: Chromosomal aberrations in soft tissue tumors: relevance to diagnosis, classification, and molecular mechanisms. Am J Pathol 1994, 144:1121-1134 [PMC free article] [PubMed] [Google Scholar]
- 3.Clark J, Rocques PJ, Crew AJ, Gill S, Shipley J, Chan AML, Gusterson BA, Cooper CS: Identification of novel genes, SYT and SSX, involved in the t(X;18)(p11.2;q11.2) translocation found in human synovial sarcoma. Nature Genet 1994, 7:502-508 [DOI] [PubMed] [Google Scholar]
- 4.de Leeuw B, Balemans M, Olde Weghuis D, Geurts van Kessel A: Identification of two alternative fusion genes, SYT-SSX1 and SYT-SSX2, in t(X;18)(p11.2;q11.2)-positive synovial sarcomas. Hum Mol Genet 1995, 4:1097-1099 [DOI] [PubMed] [Google Scholar]
- 5.Crew AJ, Clark J, Fisher C, Gill S, Grimer R, Chand A, Shipley J, Gusterson A, Cooper CS: Fusion of SYT to two genes, SSX1 and SSX2, encoding proteins with homology to the Kruppel-associated box in human synovial sarcoma. EMBO J 1995, 14:2333-2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argani P, Zakowski MF, Klimstra DS, Rosai J, Ladanyi M: Detection of the SYT-SSX chimeric RNA of synovial sarcoma in paraffin-embedded tissue and its application in problematic cases. Mod Pathol 1998, 11:65-71 [PubMed] [Google Scholar]
- 7.Finke J, Fritzen R, Ternes P, Lange W, Dölken G: An improved strategy and a useful housekeeping gene for RNA analysis from formalin-fixed, paraffin-embedded tissues by PCR. Biotechniques 1993, 14:448-453 [PubMed] [Google Scholar]
- 8.Fligman I, Lonardo F, Jhanwar SC, Gerald WL, Woodruff J, Ladanyi M: Molecular diagnosis of synovial sarcoma and characterization of a variant SYT-SSX2 fusion transcript. Am J Pathol 1995, 147:1592-1599 [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P: Nonparametric estimation for incomplete observations. J Am Stat Assoc 1958, 53:457-481 [Google Scholar]
- 10.Adams V, Hany MA, Schmid M, Hassam S, Briner J, Niggli FK: Detection of t(11;22)(q24;q12) translocation breakpoint in paraffin-embedded tissue of the Ewing’s sarcoma family by nested reverse transcription-polymerase chain reaction. Diagn Mol Pathol 1996, 5:107-113 [DOI] [PubMed] [Google Scholar]
- 11.Edwards RH, Chatten J, Xiong Q-B, Barr FG: Detection of gene fusions in rhabdomyosarcoma by reverse transcriptase-polymerase chain reaction assay of archival samples. Diagn Mol Pathol 1997, 6:91-97 [DOI] [PubMed] [Google Scholar]
- 12.Dorsey BV, Benjamin LE, Rauscher FR, Klencke B, Benook AP, Warren RS, Weidner N: Intra-abdominal desmoplastic small round-cell tumor: expansion of the pathologic profile. Mod Pathol 1996, 9:703-709 [PubMed] [Google Scholar]
- 13.Hisaoka M, Hashimoto H, Murao T: Peripheral primitive neuroectodermal tumour with ganglioneuroma-like areas arising in the cauda equina. Virchows Arch 1997, 431:365-369 [DOI] [PubMed] [Google Scholar]
- 14.Scotlandi K, Serra M, Manara MC, Benini S, Sarti M, Maurici D, Lollini P-L, Picci P, Bertoni F, Baldini N: Immunostaining of the p30/32MIC2 antigen and molecular detection of EWS rearrangements for the diagnosis of Ewing’s sarcoma and peripheral neuroectodermal tumor. Hum Pathol 1996, 27:408-416 [DOI] [PubMed] [Google Scholar]
- 15.Zeren H, Moran CA, Suster S, Fishback NF, Koss MN: Primary pulmonary sarcomas with features of monophasic synovial sarcoma: A clinicopathological, immunohistochemical, and ultrastructural study of 25 cases. Hum Pathol 1995, 26:474-480 [DOI] [PubMed] [Google Scholar]
- 16.Kaplan MA, Goodman MD, Satish J, Bhagavan BS, Travis WD: Primary pulmonary sarcoma with morphologic features of monophasic synovial sarcoma and chromosome translocation t(X;18). Am J Clin Pathol 1996, 105:195-199 [DOI] [PubMed] [Google Scholar]
- 17.Shmookler BM: Retroperitoneal synovial sarcoma. A report of four cases. Am J Clin Pathol 1982, 77:686-691 [DOI] [PubMed] [Google Scholar]
- 18.Miyashita T, Imamura T, Ishikawa Y, Okinaga K, Kunii O, Miyashita H: Primary retroperitoneal synovial sarcoma. Internal Med 1994, 33:692-696 [DOI] [PubMed] [Google Scholar]
- 19.Cugola L, Pisa R: Synovial sarcoma: with radial nerve involvement. J Hand Surg [Br] 1985, 10:243-244 [DOI] [PubMed] [Google Scholar]
- 20.Rinehart GC, Mustoe TA, Weeks PM: Management of synovial sarcoma of the median nerve at the elbow. Plast Reconstr Surg 1989, 83:528-532 [DOI] [PubMed] [Google Scholar]
- 21.O’Connell JX, Brown WL, Groper PT, Berean KW: Intraneural biphasic synovial sarcoma: an alternative “glandular” tumor of peripheral nerve. Mod Pathol 1996, 9:738-741 [PubMed] [Google Scholar]
- 22.Spielmann A, Janzen DL, O’Connell JX, Munk PL: Intraneural synovial sarcoma. Skeletal Radiol 1997, 26:677-681 [DOI] [PubMed] [Google Scholar]
- 23.Kawai A, Woodruff J, Healey JH, Brennan MF, Antonescu CR, Ladanyi M: SYT-SSX gene fusion as a determinant of morphology and prognosis in synovial sarcoma. N Engl J Med 1998, 338:153-160 [DOI] [PubMed] [Google Scholar]
- 24.Shipley J, Crew J, Birdsall S, Gill S, Clark J, Fisher C, Kelsey A, Nojima T, Sonobe H, Cooper C, Gusterson B: Interphase fluorescence in situ hybridization and reverse transcription polymerase chain reaction as a diagnostic aid for synovial sarcoma. Am J Pathol 1996, 148:559-567 [PMC free article] [PubMed] [Google Scholar]
- 25.Kashima T, Matsushita H, Kuroda M, Takeuchi H, Udagawa H, Ishida T, Hara M, Machinami R: Biphasic synovial sarcoma of the peritoneal cavity with t(X;18) demonstrated by reverse transcriptase polymerase chain reaction. Pathol Int 1997, 47:637-641 [DOI] [PubMed] [Google Scholar]