Abstract
Mantle-cell lymphomas are associated with a characteristic chromosomal translocation, t(11;14)(q13;q32). This translocation involves rearrangement of the bcl-1 proto-oncogene from chromosome 11 to the immunoglobulin heavy chain gene on chromosome 14, resulting in an overexpression of cyclin D1 mRNA (also known as bcl-1 and PRAD1). In the current study performed on paraffin-embedded tissue, cyclin D1 mRNA could be detected in 23 of 24 mantle-cell lymphomas by reverse transcription polymerase chain reaction (RT-PCR) whereas only 9 of 24 demonstrated a t(11;14) by PCR. However, we also found that cyclin D1 mRNA could be detected in the majority (11 of 17, 65%) of non-mantle-cell lymphomas and in a minority of atypical lymphoid hyperplasias (3 of 7, 43%). Cyclin D1 mRNA expression was not observed in floridly reactive lymph nodes (0 of 9) or in unstimulated lymph nodes (0 of 20), suggesting that it is a sensitive adjunct marker for malignant lymphoproliferative processes, but not specific for mantle-cell lymphoma. A semiquantitative RT-PCR assay was developed that compared the ratio of cyclin D1 to the constitutively expressed gene β2-microglobulin. Using this assay on a limited number of our specimens, cyclin D1 overexpression in mantle-cell lymphoma could be reliably distinguished from its expression in other non-Hodgkin’s lymphomas. This assay for cyclin D1 expression, designed for formalin-fixed, paraffin-embedded tissue, was a very sensitive and specific marker for mantle-cell lymphoma.
Mantle-cell lymphoma (MCL) is a non-Hodgkin’s B-cell lymphoma classically described as showing either a mantle zone, nodular, or diffuse pattern and being composed of a monotonous population of small to intermediately sized slightly cleaved B cells. Sometimes, however, there are overlapping features between mantle-cell lymphoma and other small-B-cell lymphomas, making histological distinction problematic. For example, these features may include cytology, which shows predominantly round cells or extreme nodularity of the architecture. Clinically, mantle-cell lymphoma is more aggressive than many other so-called small-B-cell lymphomas, necessitating the importance of an accurate diagnosis. 1-4 Recently developed antibodies for paraffin-embedded tissue, including cyclin D1 and CD5, are not always helpful in differentiating histologically low-grade B-cell lymphomas (because of varying staining with different fixation), and other modalities are sometimes necessary for definitive diagnosis. Specific genetic markers for various types of lymphomas and non-lymphoid neoplasms have proven useful for diagnosis and prognosis. The most recent classification scheme for lymphoid neoplasms, the revised European-American Classification of Lymphoid Neoplasms (REAL), 5 includes morphology, immunophenotype, and genetic features for defining neoplasms. Although immunophenotype of lymphomas has always been important, with the advent of the REAL classification, immunophenotype is no longer optional in diagnosing lymphoid neoplasms, and genetic studies are often necessary. The t(11;14)(q13;q32) translocation with its molecular counterpart, bcl-1 rearrangement, is seen in 50% to 80% of MCLs, using Southern blot or cytogenetics, 1-3,6-18 with even greater incidence (up to 95% of MCLs) using DNA fiber fluorescence in situ hybridization (FISH). 19 This rearrangement appears to be very specific, although occasionally other lymphoproliferative disorders have been reported to show t(11;14). 13,17,20-22 The expression of cyclin D1 mRNA has been examined with Northern blot 9-12,20,22 and in situ hybridization 23,24 and has been found to be present in MCL. Competitive reverse transcription polymerase chain reaction (RT-PCR) in fresh tissue has also been used to demonstrate the overexpression of cyclin D1 in MCL compared with expression patterns of cyclin D2 and cyclin D3. 25
In this paper we compared three different methods for detecting markers associated with MCL in formalin-fixed, paraffin-embedded (FFPE) tissue: immunohistochemical profile including immunoreactivity for cyclin D1 and CD5, PCR for bcl-1 rearrangement corresponding to t(11;14), and RT-PCR for cyclin D1 mRNA expression. We found that, although the RT-PCR assay for cyclin D1 was very sensitive for MCL, it was not specific in that cyclin D1 expression was observed in the majority of non-MCLs examined. To further characterize expression of cyclin D1 in MCL from its expression in other lymphomas, a semiquantitative RT-PCR assay was developed. This assay reliably distinguished high levels of cyclin D1 mRNA in MCL from the low levels found in other malignant lymphoproliferative processes.
Materials and Methods
Histological Classification
Cases diagnosed as either MCL, intermediately differentiated lymphocytic lymphoma, or diffuse small cleaved lymphoma from 1980 to 1997 were retrieved from the files of the Armed Forces Institute of Pathology Hematopathology Registry and classified according to the REAL classification. Lymphomas with classic, unequivocal histological features of MCL were used. 5,26 These showed either a mantle zone, nodular, or diffuse pattern with small to intermediately sized lymphocytes with slightly cleaved nuclei. No transformed cells or proliferation centers were seen. The blastic variant, which made up a subset of these lymphomas, occasionally showed a starry-sky appearance with diffuse effacement of the lymph node with medium-sized lymphocytes with finely dispersed chromatin. So-called variant forms (large-cell or anaplastic) were also accepted for this study. 14,27,28
Non-MCLs were selected, as available, and included examples of follicle center lymphoma, diffuse large-B-cell lymphoma, peripheral T-cell lymphoma, or anaplastic large-cell lymphoma. Atypical cases were selected, as available, and were defined as suspicious for malignant lymphoma, but they lacked definitive histological features of lymphoma (see Table 4 ▶ ). Nonstimulated lymph nodes from node-negative mastectomy specimens and florid reactive lymph nodes without suspicion of malignancy were used as controls.
Table 4.
Non-Mantle Cell Processes
| ID | Diagnosis | t(14;18) | t(11;14) | PRAD1 | IgH |
|---|---|---|---|---|---|
| 1 | Atypical | Neg | ND | Neg | Polyclonal |
| 2 | Atypical | Neg | ND | Neg | Polyclonal |
| 3 | Atypical | ND | ND | Neg | ND |
| 4 | Atypical | ND | Neg | Pos | Polyclonal |
| 5 | Atypical | ND | Neg | Pos | Polyclonal |
| 6 | Atypical | ND | Neg | Pos | Indeterminate* |
| 7 | Atypical | ND | Neg | Neg | Polyclonal |
| 8 | FCL | Pos | Neg | Pos | Monoclonal |
| 9 | FCL | Pos | Neg | Pos | Monoclonal |
| 10 | FCL | Neg | ND | Neg | Polyclonal |
| 11 | FCL | Neg | ND | Neg | ND |
| 12 | FCL | Pos | Neg | Pos | Polyclonal |
| 13 | FCL | Neg | ND | Neg | Polyclonal |
| 14 | FCL | Pos | Neg | Pos | Polyclonal |
| 15 | FCL | Pos | Neg | Pos | Polyclonal |
| 16 | FCL | Pos | Neg | Pos | Monoclonal |
| 17 | DLBCL | Neg | ND | Pos | Monoclonal |
| 18 | DLBCL | ND | ND | Neg | Non-Amplifiable |
| 19 | DLBCL | Neg | Neg | Pos | ND |
| 20 | PTCL | ND | Neg | Pos | ND |
| 21 | PTCL | ND | Neg | Pos | Polyclonal |
| 22 | PTCL | ND | ND | Neg | ND |
| 23 | ALCL | ND | ND | Neg | ND |
| 24 | ALCL | ND | Neg | Pos | ND |
FCL, follicle center lymphoma; DLBCL, diffuse large-B-cell lymphoma; PTCL, peripheral T-cell lymphoma; ALCL, anaplastic large-cell lymphoma; Neg, negative; ND, not done; Pos, positive immunoreaction.
*Amplifiable but not clearly monoclonal.
Immunohistochemical Studies
Five-micron sections from paraffin-embedded tissue blocks were prepared for immunophenotypic analysis according to the standard avidin-biotin complex method of Hsu. 29 The antibody panel for each case included CD45RB, CD20, CD45RO, CD3, CD43, bcl-2, CD5, kappa (Ig light chain), lambda (Ig light chain), CD23 (BU38), and cyclin D1 (Table 1) ▶ . Of the above antibodies, kappa, lambda, CD3, and CD23 required predigestion for 20 minutes with 0.4% pepsin (P-7000, Sigma Chemical Co., St. Louis, MO) in 0.1 mol/L HCl buffer solution at pH 2.0 at 40°C to 42°C. Microwave antigen retrieval was necessary for CD5. 30
Table 1.
Antibodies
| Antibody | Source | Dilution |
|---|---|---|
| CD45RB(LCA)* | DAKO, Carpinteria, CA | 1:200 |
| CD20(L26)* | DAKO | 1:200 |
| CD45RO(UCHL-1)* | DAKO | 1:200 |
| CD3†‡ | DAKO | 1:500 |
| CD43(MT-1)* | Biotest, Denville, NJ | 1:50 |
| Kappa†‡ | DAKO | 1:25,000 |
| Lambda†‡ | DAKO | 1:50,000 |
| Bcl-2 | DAKO | 1:100 |
| CD23(BU38)*‡ | Binding Site Unlimited, Birmingham, UK | 1:200 |
| CD5 (NCL-CD5-4C7)*§ | Novocastra, Newcastle-upon-Tyne, UK | 1:100 |
| Cyclin D1/Bcl-1(5D4)* | Immunotech, Westbrook, ME | 1:3000 |
LCA, leukocyte common antigen.
*Mouse monoclonal.
†Rabbit polyclonal.
‡Required 20-minute predigestion with 0.4% pepsin.
§Microwave antigen retrieval.
Positive immunoreactivity for CD45RB and the B-cell marker CD20 (or kappa or lambda restriction) with negative CD45RO and/or CD3 was used to determined B-cell immunophenotype. Co-expression of CD43 in B-cell neoplasms was considered consistent with a B-cell malignancy. 31,32 CD23 was considered positive if individual cells showed cytoplasmic staining but negative if only reticular staining was seen. 33 Cyclin D1, a marker considered highly specific and sensitive for MCL, 8,13,34 was used to help confirm the diagnosis of MCL. We considered cyclin D1 to be positive if over 10% of the cells showed nuclear positivity. Cytoplasmic staining was not considered positive.
Molecular Diagnostic Studies
PCR for t(11;14) and Immunoglobulin Heavy Chain (IgH)
Molecular diagnostic studies for t(11;14) and IgH were performed by PCR. DNA was extracted from the FFPE sections and amplified as previously described. 35 A PCR master mix containing PCR buffer II, pH 8.3 (Perkin-Elmer, Norwalk, CT), MgCl2 (2.5 mmol/L final concentration), 1.25 U of AmpliTaq polymerase (Perkin-Elmer), and 10 pmol of appropriate primer, listed in Table 2 ▶ , was added for a final volume of 50 μl. The reaction mixture was amplified in a 9600 Gene Amp PCR system (Perkin-Elmer) programmed for a 5-minute denaturation at 94°C, followed by 40 cycles of 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute, with a final 7-minute extension at 72°C. The t(11;14) PCR products (18 μl) were separated by electrophoresis through a 2.5% agarose gel in Tris/borate/EDTA buffer, pH 8.4, and visualized by ethidium bromide staining. Specific products were detected by Southern blotting with end-labeled oligonucleotide probes, 36,37 as listed in Table 2 ▶ . End-labeling was performed with T4 polynucleotide kinase (Life Technologies) and [γ-32P]ATP (10 mCi/ml; Amersham, Arlington Hts, IL) or terminal transferase and digoxigenin-ddUTP (Boehringer Mannheim, Indianapolis, IN). A positive result for a major translocation cluster of t(11;14) produced a band of 180 to 280 bp. The positive control for the assay was the M02058 cell line. 38 PCR for IgH was performed according to previously described methods. 39 A monoclonal IgH rearrangement appeared as a dominant band of 105 to 120 bp.
Table 2.
Primers and Probe Sequences
| Name | Sequence |
|---|---|
| t(11;14) | |
| bcl-1 primer (22-mer) | 5′-ATTCGGTTAGACTGTGATTAGC-3′ |
| CFW-1 primer (24-mer) | 5′-ACCTGAGGAGACGGTGACCAGGG-3′ |
| bcl-1 probe | 5′-AAGTGGTTTTGTTAGATGTA-3′ |
| PRAD-1 | |
| PRAD 5′ | 5′-ATGCTGAAGGCGGAGGAGACC-3′ |
| PRAD 3′ (RT-primer) | 5′-TCCTCGCACTTCTGTTCCTCGC-3′ |
| Probe | 5′-CCTCGGTGTCCTACTTCAAA-3′ |
| IgH | |
| FR3A, V region primer | 5′-ACACGGC(C/T)TGTATTACTGT-3′ |
| CFW1, J region primer | 5′-ACCTGAGGAGGTGACCAGGGT-3′ |
RT-PCR for Cyclin D1 and β2-Microglobulin
All RT-PCR studies were performed as previously described 37 and according to the guidelines of the College of American Pathologists checklist for molecular pathology with appropriate positive and negative controls.
RNA was extracted from the formalin-fixed, paraffin-embedded tissue for RT-PCR as previously described. 37 RT-PCR for the ubiquitously expressed β2-microglobulin (β2M) gene was used as a control for the presence of amplifiable RNA. Reverse transcription was performed with a specific antisense primer, listed in Table 2 ▶ . The RT step was performed on 1- and 5-μl templates at 37°C for 60 minutes in 20-μl mixtures containing First Stand Buffer (Life Technologies, Gaithersburg, MD), 10 mmol/L dithiothreitol, 60 U of Moloney murine leukemia virus reverse transcriptase (Life Technologies), 0.2 mmol/L each deoxynucleotide triphosphate, and 15 pmol of antisense primer. A PCR master mix containing PCR buffer II, pH 8.3 (Perkin-Elmer), MgCl2 (2.5 mmol/L final concentration), 1.25 U of AmpliTaq polymerase (Perkin-Elmer), and 15 pmol of sense primer (Table 2) ▶ was added for a final volume of 50 μl. The reaction mixture was amplified in a 9600 Gene Amp PCR System (Perkin-Elmer) programmed for a 5-minute denaturation at 94°C, followed by 40 cycles of 94°C for 1 minute, 55°C for 1 minute, and 72°C for 1 minute, with a final 7-minute extension at 72°C. Cyclin D1 RT-PCR products (18 μl) were separated by electrophoresis through a 2.5% agarose gel in Tris/borate/EDTA buffer, pH 8.4, and visualized by ethidium bromide staining. Specific products were detected by Southern blotting with an end-labeled oligonucleotide probe as listed in Table 2 ▶ . A positive result for β2M produced a band of 158 bp. A positive result for cyclin D1 produced a band of 134 bp. The positive control for the cyclin D1 assay was RNA extracted from the t(11;14)-bearing cell line M02058. 38
For semiquantitative RT-PCR, 5 μl of lysate was reverse transcribed as described above for cyclin D1 and β2M. As absolute RNA amounts in FFPE lysates cannot be easily quantitated, a semiquantitative assay was designed in which the relative expression of cyclin D1 was compared with the expression of β2M. To determine the linear range of amplification, control lysates were amplified by RT-PCR for β2M with 20, 25, 30, and 40 cycles. It was determined that 30 cycles of PCR was still in the linear range and was optimal for this assay. For quantification, the amplified samples were subjected to gel electrophoresis and Southern blotting as above. Southern blots were visualized and bands were quantitated using a Storm 860 PhosphorImager equipped with ImageQuant software (Molecular Dynamics, Sunnyvale, CA). For cyclin D1 quantitation, the ratio of cyclin D1/β2M was calculated for each case. The resultant ratios were normalized to the cyclin D1/β2M ratio of the positive control, cell line M02058, arbitrarily set as 1.0.
Statistics
The Statistics Program for Social Sciences (STSS 7.5) for windows (STSS, Chicago, IL) was used for determining statistical significance between the cyclin D1/β2M ratios of MCLs and non-MCLs with a two-tailed t-test for equality of means.
Results
Twenty-four cases of lymphoma were collected prospectively and retrieved from the files at the Armed Forces Institute of Pathology that fit the criteria for MCL (Table 3) ▶ . Other cases examined included 17 non-mantle-cell lymphomas (9 follicle center lymphomas, 3 peripheral T-cell lymphomas, 3 diffuse large-B-cell lymphomas, and 2 anaplastic large-cell lymphomas) and 7 atypical lymphoid proliferations (Table 4) ▶ . Twenty axillary lymph nodes from node-negative breast carcinoma cases and nine floridly reactive lymph nodes were also examined for cyclin D1 expression and t(11;14).
Table 3.
Mantle Cell Lymphoma
| ID | Diagnosis | Age (years)/sex | Site | t(11;14) | PRAD1 | CCND1 | IgH | CD5 | CD43 | Bcl-2 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | MCL | 74 /M | LN, Rt axillary | Pos | Pos | Pos | Mo | Pos | Pos | Pos |
| 2 | MCL | 78 /M | LN, Rt groin; Lt/Rt tonsils | Neg | Pos | Neg | Mo | Pos | Pos | Pos |
| 3 | MCL-B | 50 /M | Nasopharynx | Pos | Pos | Pos | Mo | Pos | Pos | Pos |
| 4 | MCL | 76 /F | LN, Rt inguinal | Neg | Pos | Pos | Mo | Pos | Pos | Pos |
| 5 | MCL | 82 /M | Skin and LN, Rt inguinal | Pos | Pos | Neg | Mo | ND | ND | ND |
| 6 | MCL | 76 /M | LN, submental | Neg | Pos | Neg | Mo | Neg | Pos | Pos |
| 7 | MCL | 67 /F | LN, cervical | Neg | Pos | Neg | Mo | ND | Pos | ND |
| 8 | MCL | 53 /F | Mass, parotid | Pos | Pos | Pos | Mo | Pos | ND | Pos |
| 9 | MCL-B | 57 /M | LN, Rt inguinal | Neg | Pos | Pos | Mo | Pos | Pos | Pos |
| 10 | MCL-B | 77 /M | LN, Rt axillary | Pos | Pos | Pos | Mo | Pos | Pos | Pos |
| 11 | MCL | 63 /M | LN, Lt neck | Neg | Pos | Neg | Mo | Pos | Pos | Pos |
| 12 | MCL-MZ | 67 /M | LN, para-aortic | Pos | Pos | Pos | Mo | Neg | Pos | Pos |
| 13 | MCL-B | 70 /M | Lt tonsil | Neg | Pos | Pos | Mo | Neg | Neg | Pos |
| 14 | MCL | 47 /M | LN, Rt post cervical | Neg | Pos | Pos | Mo | Pos | Neg | Pos |
| 15 | MCL-B | 41 /M | LN, Lt axillary | Neg | Neg | Neg | Mo | Pos | Pos | Pos |
| 16 | MCL | 66 /M | LN, Rt femoral | Neg | Pos | Pos | Mo | Pos | Pos | Pos |
| 17 | MCL | 74 /F | LN, Rt neck | Neg | Pos | Pos | Mo | Pos | Pos | Pos |
| 18 | MCL | 57 /M | Mass, Lt parotid | Pos | Pos | Pos | Mo | Pos | Pos | Pos |
| 19 | MCL | 55 /M | Mass, Lt hard palate | Neg | Pos | Pos | Mo | Pos | Pos | Pos |
| 20 | MCL | 34 /M | LN, Lt inguinal | Neg | Pos | Neg | Mo | Pos | Pos | Pos |
| 21 | MCL | 84 /M | Mass, right neck | Pos | Pos | Pos | Mo | Pos | Pos | Pos |
| 22 | MCL-V | 73 /M | LN, cervical | Pos | Pos | Pos | Mo | Neg | Pos | Pos |
| 23 | MCL | 57 /M | LN, submandibular | Neg | Pos | Pos | Mo | ND | Pos | Pos |
| 24 | MCL | 70 /M | LN, axillary | Neg | Pos | Pos | Mo | ND | Pos | Pos |
MCL, mantle cell lymphoma; B, blastic; V, variant; M, male; F, female; LN, lymph node; Rt, right; Lt, left; post, posterior; Pos, positive; Neg, negative; Mo, monoclonal; ND, not done; CCND1, cyclin D1; IgH, immunoglobulin heavy chain.
Immunohistochemistry
The immunohistochemistry is summarized in Table 3 ▶ . All MCLs were B-cell immunophenotype with CD20 immunoreactivity and CD3 and/or CD45RO negativity. Cyclin D1 was immunoreactive in 16 of 23 cases (70%). Results of other immunohistochemical markers included the following: 20 of 22 showed CD43 coexpression (91%); 21 of 21 MCLs were bcl-2 immunoreactive (100%); and 15 of 19 MCL were CD5 positive (79%). All cases tested were CD23 negative. All cases of non-MCLs studied were negative with cyclin D1.
Molecular Diagnostic Studies
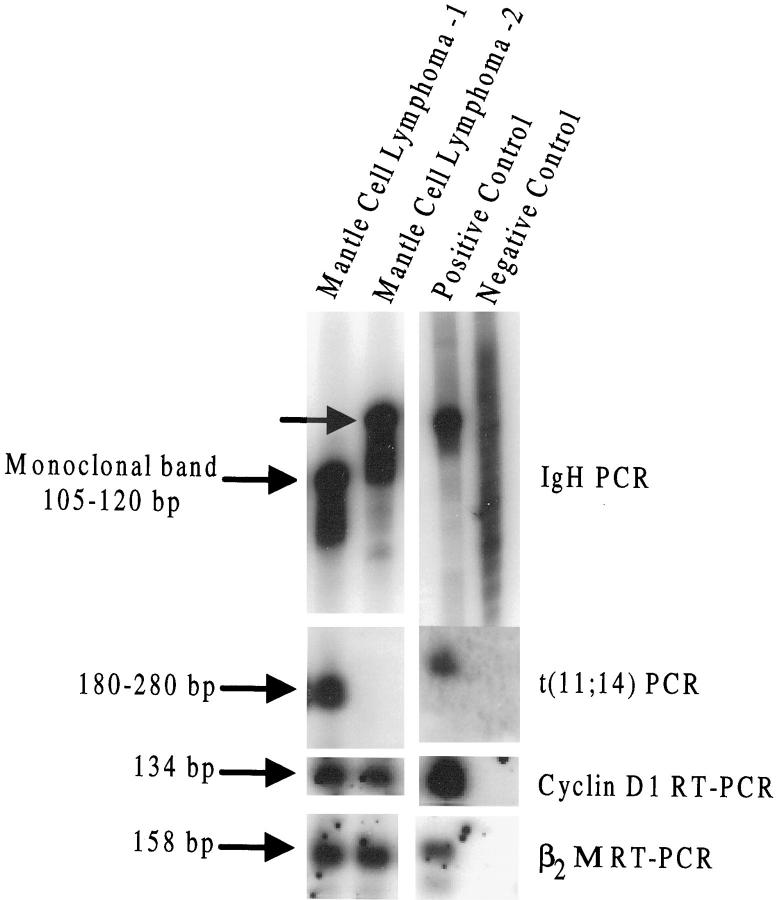
All cases of MCL showed a monoclonal IgH rearrangement by PCR (24 of 24, 100%). PCR for t(11;14) translocation was performed on all MCLs and showed a distinct band in 9 of 24 cases (38%); no band was seen in 15 of 24 cases. Cyclin D1 mRNA as detected by RT-PCR was expressed in 23 of 24 cases (96%) of MCL (Table 3) ▶ . All nine t(11;14)-positive MCL cases were cyclin D1 positive. Of the 15 MCL cases negative for t(11;14) by PCR, 14 (93%) were cyclin D1 positive. One case with a negative result for t(11;14), cyclin D1 mRNA and protein, had results showing CD20+, CD43+, CD5+, CD23−, and bcl-2 immunoreactivity and was considered MCL by morphology and immunohistochemistry. Results for two MCL cases are shown in Figure 1 ▶ .
Figure 1.
Examples of two mantle cell lymphomas both are cyclin D1 positive, but only one shows the t(11;14) by PCR (lane 1). Positive and negative controls for cyclin D1 are seen in the last two lanes. The negative control for IgH (immunoglobulin heavy chain) is reactive lymph node. For all other assays, the negative control is molecular grade water.
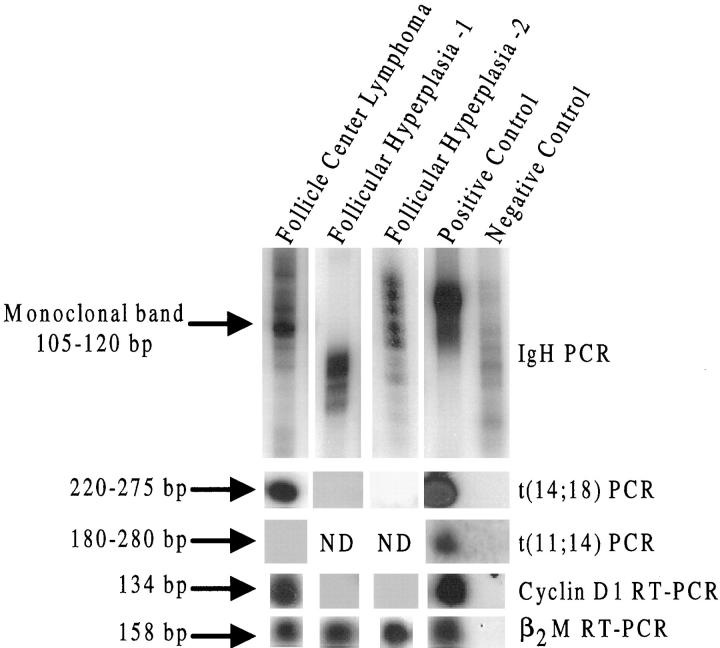
The majority (11 of 17, 65%) of non-MCLs also showed cyclin-D1 expression by RT-PCR. Cyclin-D1-positive cases by type included six of nine follicle center lymphomas (67%), two of three peripheral T-cell lymphomas (67%), two of three diffuse large-cell lymphomas (67%), and one of two anaplastic large-cell lymphomas (50%). Of the atypical lymphoid proliferations, three of seven (43%) were cyclin D1 positive. The floridly reactive lymph node cases were all negative for cyclin D1 mRNA (0 of 9). The cases of nonstimulated axillary lymph nodes from node-negative mastectomy specimens were also all cyclin D1 negative (0/20). Twelve cases of cyclin-D1-positive non-mantle-cell lymphomas were tested for t(11;14), and all were negative (Table 4) ▶ . Representative cases are also shown in Figure 2 ▶ .
Figure 2.
A follicle center lymphoma (FCL) expresses cyclin D1 (lane 1) whereas two follicular hyperplasias do not express cyclin D1 (lanes 2 and 3). The FCL also shows t(14;18) and is negative for t(11;14). Controls are seen in the last two lanes. The negative control for IgH is reactive lymph node. For all other assays, the negative control is molecular grade water.
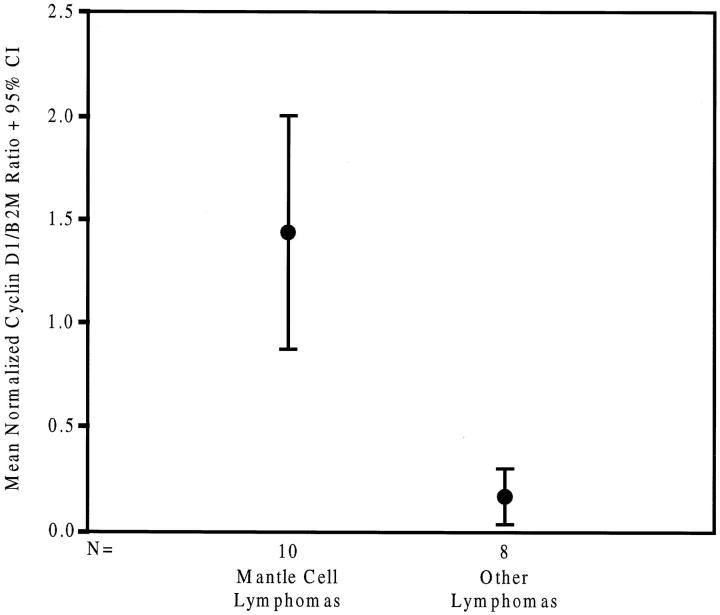
A semiquantitative RT-PCR assay was developed for use with FFPE tissues to establish whether cyclin D1 expression in MCLs and non-MCLs could be distinguished. The relative expression of cyclin D1 was compared with the expression of the constitutively expressed gene β2M. Relative cyclin D1 expression in 10 MCL cases (six t(11;14) positive and four t(11;14) negative) was compared with expression in 8 other cyclin-D1-positive non-MCLs and atypical hyperplasias, including 3 follicular center lymphomas, 2 peripheral T-cell lymphomas, 1 anaplastic large-cell lymphoma, and 2 atypical follicular hyperplasias. The calculated case ratios were normalized to the cyclin D1/β2M ratio of the positive control cell line, M02058, which was arbitrarily set at 1.0 (see Materials and Methods). As shown in Figure 3 ▶ , the MCL cases showed significantly higher expression of cyclin D1 than did other non-MCLs. There was no statistically significant difference between the cyclin D1/β2M ratio of MCLs that were t(11;14) positive and those that were t(11;14) negative. Mean relative cyclin D1/β2M ratio of MCL ± SEM was 1.44 ± 0.25. Mean relative cyclin D1/β2M ratio for non-MCLs was 0.17 ± 0.05 (P < 0.001, two-tailed t-test for equality of means).
Figure 3.
Mean normalized cyclin D1/β2M ratio. This represents a 95% confidence interval when comparing mantle cell lymphoma and non-mantle cell lymphoma. Positive control line, M02058, was arbitrarily set at 1.0.
Discussion
Mantle-cell lymphoma was first recognized in the 1970s as a lymphoma with intermediate differentiation that could not be readily classified as either follicle center lymphoma (poorly differentiated lymphoma) or small lymphocytic lymphoma (well differentiated lymphoma). 40-42 Lennert and Feller later included this entity in the Kiel classification as centrocytic lymphoma, 43 and in the United States the terms lymphocytic lymphoma of intermediate differentiation and intermediate lymphocytic lymphoma were used for what we now refer to as mantle-cell lymphoma. 40 Recent immunohistochemical, genetic, and clinical studies have confirmed the previously held views that MCL is a distinct clinicopathological entity. 5
With the advent of the REAL classification, there has been an augmentation of knowledge and treatment of lymphomas. With this expanding knowledge, there is increasing necessity to subclassify small-B-cell lymphomas. In the case of MCL, this is especially justified because the natural history of MCL, which has low-grade histological features, has proven to be worse than its more indolent simulators, and its clinical behavior is more similar to intermediate histological grade. 1-4 The recent development of immunohistochemical markers, such as CD5, 44 cyclin D1, 5,13,14 and CD23, 33 has made the classification of small-B-cell lymphomas considerably easier, although there are still cases that are difficult to classify unequivocally by immunohistochemistry alone.
MCL has been associated with the cytogenetic abnormality t(11;14)(q13;q32). This translocation, which involves the bcl-1 breakpoint and cyclin D1, appears to be restricted to MCLs with occasional exceptions. 13,17,20-22,45 This translocation leads to deregulation of the bcl-1 gene and plays a role in the overexpression of cyclin D1 mRNA and subsequent overexpression of the cyclin D1 protein. 1,8-10,12,13 Cyclin D1 mRNA overexpression in MCL has been demonstrated by in situ hybridization, by Northern blot, 11,12,23,24 and more recently by competitive RT-PCR, with cyclin D2 and D3, in fresh tissue. 25 The cyclin D1 protein overexpression has been demonstrated with both Western blot and immunohistochemical studies in fresh and fixed tissue. 13,14,17,34
The bcl-1 locus is rearranged in 50% to 80% of MCLs when using Southern blot or 75% to 80% using cytogenetics, 13,18 but using a direct visualization method with DNA fiber fluorescence in situ hybridization, this translocation can be seen in as many as 95% of MCLs. 19 As a result of the characteristic t(11;14), the bcl-1 locus, on chromosome 11, is juxtaposed to the immunoglobulin heavy chain (IgH) gene locus located on chromosome 14. The breakpoints are widely scattered on chromosome 11q13, but it has been shown that 70% to 80% of the breakpoints are localized to a 1-kb DNA segment known as the major translocation cluster (MTC). 18,24 Within the MTC, the breakpoints occur in a relatively small region of approximately 80 bp on chromosome 11 and the 5′ area of one of the IgH joining (JH) regions on chromosome 14. 46 Because of the localization of these breakpoints, the translocation is amenable to PCR techniques for detection, although some of the breakpoints falling outside the MTC cannot be identified with PCR. This technique has been demonstrated to be sensitive when the MTC is present, 46 although because of the low percentage of MCLs exhibiting the MTC, the technique has a low sensitivity for MCL overall. It is estimated that only in 33% to 50% of patients with MCL can the breakpoints be detected by PCR using primers in the region of the MTC. 18,47,48 Our findings in this study fall within this expected range of detection.
Although there is low sensitivity for the t(11;14) molecular counterpart of the bcl-1 rearrangement by PCR, 47 the expression of the cyclin D1 protein in MCL is similar to that of the presence of t(11;14) itself, approximately 50% to 90%. 13,16,17 This can be demonstrated by immunohistochemical studies. 13,49 In the current study, cyclin D1 protein expression was observed in 70% of MCLs, which may reflect the variability of fixation and processing of cases submitted to the Armed Forces Institute of Pathology. Cyclin D1 is associated with progression of the cell cycle through G1 but is generally not found in normal lymphoid tissue or B-cell lines without t(11;14). 50 As with the translocation, the presence of this protein is highly characteristic of MCL, although it has been demonstrated occasionally in other hematopoietic malignancies, including hairy cell leukemia, splenic marginal zone lymphoma, multiple myeloma, and plasmacytoma. 20-22,45 Nonhematopoietic neoplasms, such as parathyroid adenoma, 51 breast carcinoma, and squamous cell carcinomas 52 have also demonstrated cyclin D1. Although the weak nuclear expression of this protein, detected by the anti-cyclin-D1 antibody, and high background in some cases can hinder interpretation, we feel that in conjunction with other immunohistochemical stains and diagnostic modalities, it is extremely helpful in definitive classification of MCL.
Because cyclin D1 protein appears helpful in diagnosis of MCL, we speculated that cyclin D1 mRNA would also be useful, even at low levels. We investigated the use of RT-PCR in FFPE tissue to demonstrate the presence of cyclin D1 mRNA and create a specific and sensitive test for MCL. The use of RT-PCR is often helpful in demonstrating the presence of low-level target mRNA in tissue, often lower than can be detected with Northern blot or in situ analysis. This was recently demonstrated in a study by Uchimaru et al in which competitive RT-PCR was used to detect cyclin D1 in MCL and lymphoid cell lines. 25 The finding of cyclin D1 in non-MCL cell lines was also demonstrated in the study by Uchimaru et al but was seen rarely and in conjunction with the presence of t(11;14). 25 Our study shows that cyclin D1 can be demonstrated in MCL in a high percentage of cases by RT-PCR from paraffin-embedded tissue. The presence of cyclin D1 mRNA by RT-PCR was highly sensitive, but the extensive presence of cyclin D1 in non-MCL was an unexpected finding. In contrast to our study of cyclin D1 expression by RT-PCR, other studies, in which cyclin D1 expression was examined, used Northern blot and demonstrated only rare non-MCLs to be positive: 3/122 (2.5%), 10 0/10 (0%), 53 and 1/50 (2%). 11 Only one study by De Boer et al also found low levels of expression in 36/56 (64%), a high percentage of non-MCLs. 9 In our current study and that of de Boer and colleagues, cyclin D1 expression (without quantitation) did appear to be a sensitive marker for lymphoid malignancy but not specific for MCL as has been previously reported. 25,11 Quantitation of the cyclin D1 expression was essential in differentiating MCL from non-MCL. Although Uchimaru used comparative quantitation, 25 to our knowledge, the present study is the first to use a semiquantitative, comparative method in paraffin-embedded tissue.
Cyclin D1 mRNA has also rarely been demonstrated in non-neoplastic lymphoid tissues by Northern blot. 11,17,22,50,53 Only a single study by de Boer et al 9 demonstrated low levels of cyclin D1 expression in widespread non-neoplastic lymphoid tissue, including normal lymph node, spleen, and tonsil. This finding is unique to the study of de Boer et al and has not been demonstrated by other authors, nor was it seen in our study. In our study, all of the non-neoplastic lymph nodes we examined, both nonstimulated and floridly reactive, were negative for cyclin D1 mRNA. The differences in sensitivity between the Northern blot assays developed by these different groups and our RT-PCR assay are difficult to assess.
Our study showed that the majority of non-MCLs expressed cyclin D1 mRNA, albeit at low levels. The presence of cyclin D1 expression in our study appears to be a sensitive adjunctive marker for malignancy in lymphoproliferative processes, but it is not specific for MCL. Because cyclin D1 expression is not seen in normal hematopoietic cells, the demonstrated expression of cyclin D1 in lymphomas other than MCL may indicate that cyclin D1 is a ubiquitous oncogene, however, at significantly lower levels than is seen in MCL. As the vast majority of these lymphomas do not possess the t(11;14) responsible for cyclin D1 overexpression in MCL, the mechanism for cyclin D1 expression/overexpression in these cases likely is different from that of MCL. However, using the semiquantitative assay described herein, cyclin D1 overexpression in MCL can be readily distinguished in paraffin-embedded tissues from its low expression/overexpression in non-MCLs. This finding may be helpful in diagnosis of morphologically and immunophenotypically ambiguous cases of suspected MCL. This method of examining differential expression of mRNA, although somewhat arduous, may also be helpful in other studies using paraffin-embedded fixed tissue where there is a necessity to quantitate mRNA expression.
Acknowledgments
We thank Sherman A. McCall, M.D., for the statistical analysis in this study.
Footnotes
Address reprint requests to Dr. Jeffery K. Taubenberger, Armed Forces Institute of Pathology, Division of Molecular Pathology, Department of Cellular Pathology, Room G-137, 14th Street and Alaska Avenue, NW, Washington DC, 20306-6000. E-mail: taubenbe@afip.osd.mil.
Supported by the intramural funds of the Armed Forces Institute of Pathology.
References
- 1.Weisenburger DD, Armitage JO: Mantle cell lymphoma: an entity comes of age. Blood 1996, 87:4483-4494 [PubMed] [Google Scholar]
- 2.Campo E, Jaffe ES: Mantle cell lymphoma: accurate diagnosis yields new clinical insights. Arch Pathol Lab Med 1996, 120:12-14 [PubMed] [Google Scholar]
- 3.Argatoff LH, Connors JM, Klasa RJ, Horsman DE, Gascoyne RD: Mantle cell lymphoma: a clinicopathologic study of 80 cases. Blood 1997, 89:2067-2087 [PubMed] [Google Scholar]
- 4.Zucca E, Roggero E, Pinotti G, Pedrinis G, Cappella C, Venco A, Cavalli F: Patterns of survival in mantle cell lymphoma. Ann Oncol 1995, 6:257-262 [DOI] [PubMed] [Google Scholar]
- 5.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKC, Cleary ML, Delsol G, De Wolf-Peters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Müller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 6.Pittaluga S, Wlodarska I, Stul MS, Thomas J, Verhoef G, Cassman JJ, Van Den Berghe H, De Wolf-Peeters C: Mantle cell lymphoma: a clinicopathological study of 55 cases. Histopatology 1995, 26:17-24 [DOI] [PubMed] [Google Scholar]
- 7.Segal GH, Masih AS, Fox AC, Jorgensen T, Scott M, Braylan RC: CD5-expressing B-cell non-Hodgkin’s lymphomas with bcl-1 gene rearrangement have a relatively homogeneous immunophenotype and are associated with an overall poor prognosis. Blood 1995, 85:1570-1579 [PubMed] [Google Scholar]
- 8.Ott MM, Helberg A, Ott G, Bartek J, Fischer L, Durr A, Kreipe H, Muller-Hermelink HK: bcl-1 rearrangement and cyclin D1 protein expression in mantle cell lymphoma. J Pathol 1996, 179:238-242 [DOI] [PubMed] [Google Scholar]
- 9.de Boer CJ, van Krieken JHJM, Kluin-Nelemans HC, Kluin PM, Schuurin E: Cyclin D1 messenger RNA overexpression as a marker for mantle cell lymphoma. Oncogene 1995, 10:1833-1840 [PubMed] [Google Scholar]
- 10.Bosch F, Jares P, Campo E, Lopez-Guillermo A, Piris MA, Villamor N, Tassies D, Jaffe ES, Monsterrat E, Rozman C, Cardesa A: PRAD-1/cyclin D1 gene overexpression in chronic lymphoproliferative disorders: a highly specific marker of mantle cell lymphoma. Blood 1994, 84:2726-2732 [PubMed] [Google Scholar]
- 11.Oka K, Ohno T, Kita K, Yamaguchi M, Takakura N, Nishii K, Miwa H, Shirakawa S: PRAD1 gene over-expression in mantle-cell lymphoma but not in other low-grade lymphomas, including extranodal lymphoma. Br J Haematol 1994, 86:786-791 [DOI] [PubMed] [Google Scholar]
- 12.Oka K, Ohno T, Yamaguchi M, Mahmud N, Miwa H, Kita K, Shiku H, Shirakawa S: PRAD1/cyclin D1 gene overexpression in mantle cell lymphoma. Leuk Lymphoma 1996, 21:37-42 [DOI] [PubMed] [Google Scholar]
- 13.Swerdlow SH, Yang W-I, Zukerberg LR, Harris NL, Arnold A, Williams ME: Expression of cyclin D1 protein in centrocytic/mantle cell lymphomas with and without rearrangements of the bcl-1/cyclin D1 gene. Hum Pathol 1995, 26:999-1004 [DOI] [PubMed] [Google Scholar]
- 14.Swerdlow SH, Zukerberg LR, Yang W-I, Harris NL, Williams ME: The morphologic spectrum of non-Hodgkin’s lymphoma with bcl-1/cyclin D1 gene rearrangements. Am J Surg Pathol 1996, 20:627-640 [DOI] [PubMed] [Google Scholar]
- 15.Velders GA, Kluin-Nelemans JC, De Boer CJ, Hermans J, Noodijk EM, Schuuring E, Kramer MHH, Van Deijk WA, Rahder JB, Kluin PM, Van Krieken JHJM: Mantle-cell lymphoma: a population-based clinical study. J Clin Oncol 1996, 14:1269-1274 [DOI] [PubMed] [Google Scholar]
- 16.Yatabe Y, Nakamura S, Seto M, Kuroda H, Kagami Y, Suzuki R, Ogura M, Kojima M, Koshikawa T, Ueda R, Suchi T: Clinicopathologic study of PRAD1/cyclin D1 overexpressing lymphoma with special reference to mantle cell lymphoma: a distinct molecular pathologic entity. Am J Surg Pathol 1996, 20:1110-1122 [DOI] [PubMed] [Google Scholar]
- 17.Yang W-I, Zukerberg LR, Motokura T, Arnold A, Harris NL: Cyclin D1(Bcl-1, PRAD1) protein expression in low-grade B-cell lymphomas and reactive hyperplasia. Am J Pathol 1994, 145:86-96 [PMC free article] [PubMed] [Google Scholar]
- 18.Rimokh R, Berger F, Delsol G, Digonnet I, Rouault JP, Togaud JD, Gadoux M, Coiffier B, Bryon PA, Magaud JP: Detection of chromosomal translocation t(11;14) by polymerase chain reaction in mantle cell lymphomas. Blood 1994, 83:1871-1875 [PubMed] [Google Scholar]
- 19.Vaandrager J-W, Schuuring E, Zwikstra E, de Boer CJ, Kleiverda KK, van Krieken JHJM, Kluin-Nelemans HC, van Ommen G-JB, Raap AK, Kluin PM: Direct visualization of dispersed 11q13 chromosomal translocations in mantle cell lymphoma by multicolor DNA fiber fluorescence in situ hybridization. Blood 1996, 88:1177-1182 [PubMed] [Google Scholar]
- 20.Jadayel D, Matutes E, Dyer MJS, Brito-Babapulle V, Khohkar MT, Oscier D, Catovsky D: Splenic lymphoma with villous lymphocytes: analysis of BCL-1 rearrangements and expression of the cyclin D1 gene. Blood 1994, 83:3664-3671 [PubMed] [Google Scholar]
- 21.Vasef MA, Medeiros LJ, Yospur LS, Sun NCJ, McCourty A, Brynes RK: Cyclin D1 protein in multiple myeloma and plasmacytoma: an immunohistochemical study using fixed, paraffin-embedded tissue sections. Mod Pathol 1997, 10:927-932 [PubMed] [Google Scholar]
- 22.Bosch F, Campo E, Jares P, Pittaluga S, Muñoz J, Nayach I, Piris MA, Dewolf-Peeters C, Jaffe ES, Rozman C, Montserrat E, Cardesa A: Increased expression of the PRAD-1/CCND1 gene in hairy cell leukemia. Br J Haematol 1995, 91:1025-1030 [DOI] [PubMed] [Google Scholar]
- 23.Delmer A, Ajchenbaum-Cymbalista F, Tang R, Ramond S, Faussat A-M, Marie J-P, Zittoun R: Over-expression of cyclin D1 in chronic B-cell malignancies with abnormality of chromosome 11q13. Br J Haematol 1995, 89:798-804 [DOI] [PubMed] [Google Scholar]
- 24.Williams ME, Nichols GE, Swerdlow SH, Stoler MH: In situ hybridization detection of cyclin D1 mRNA in centrocytic/mantle cell lymphoma. Ann Oncol 1995, 6:297-299 [DOI] [PubMed] [Google Scholar]
- 25.Uchimaru K, Taniguchi T, Yoshikawa M, Asano S, Arnold A, Fujita T, Motokura T: Detection of cyclin D1 (bcl-1, PRAD1) overexpression by a simple competitive reverse transcriptase-polymerase chain reaction assay in t(11;14)(q13;q32) bearing B-cell malignancies and/or mantle cell lymphoma. Blood 1997, 89:965-974 [PubMed] [Google Scholar]
- 26.Lardelli P, Bookman MA, Sundeen J, Longo DL, Jaffe ES: Lymphocytic lymphoma of intermediate differentiation: morphologic and immunophenotypic spectrum and clinical correlations. Am J Surg Pathol 1990, 14:752-763 [DOI] [PubMed] [Google Scholar]
- 27.Ott MM, Ott G, Kuse R, Porowski P, Gunzer U, Feller AC, Müller-Hermelink HK: The anaplastic variant of centrocytic lymphoma is marked by frequent rearrangements of the bcl-1 gene and high proliferation indices. Histopathology 1994, 24:329-334 [DOI] [PubMed] [Google Scholar]
- 28.Zoldan MC, Inghirami G, Masuda Y, Vanekerckhove F, Raphael B, Amorosi E, Hymes K, Frizzera G: Large cell variants of mantle cell lymphoma: cytologic characteristics and p53 anomalies may predict poor outcome. Br J Haematol 1996, 93:475-486 [DOI] [PubMed] [Google Scholar]
- 29.Hsu S-M, Raine L, Fanger H: Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981, 29:577-580 [DOI] [PubMed] [Google Scholar]
- 30.Shi S-R, Key ME, Kalra KL: Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 1991, 39:741-748 [DOI] [PubMed] [Google Scholar]
- 31.Ngan B-Y, Picker LJ, Mederios LJ, Warnke RA: Immunophenotypic diagnosis of non-Hodgkin’s lymphoma in paraffin sections: co-expression of L60 (Leu-22) and L26 antigens correlates with malignant lymphoma in paraffin sections. Am J Clin Pathol 1989, 91:579-583 [DOI] [PubMed] [Google Scholar]
- 32.Contos MJ, Kornstein DJ, Ben-Ezra J: The utility of CD20 and CD43 in subclassification of low-grade B-cell lymphoma on paraffin sections. Mod Pathol 1992, 5:631-633 [PubMed] [Google Scholar]
- 33.Dorfman DM, Pinkus GS: Distinction between small lymphocytic and mantle cell lymphoma by immunoreactivity for CD23. Mod Pathol 1994, 7:326-331 [PubMed] [Google Scholar]
- 34.de Boer CJ, Schuuring E, Dreef E, Peters G, Bartek J, Kluin PM, van Krieken JHJM: Cyclin D1 protein analysis in the diagnosis of mantle cell lymphoma. Blood 1995, 86:2715-2723 [PubMed] [Google Scholar]
- 35.Wright CF, Reid AH, Tsai MM, Ventre KM, Murari PJ, Frizzera G, O’Leary TJ: Detection of Epstein-Barr virus sequences in Hodgkin’s disease by the polymerase chain reaction. Am J Pathol 1991, 139:393-398 [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook J, Fritsch EF, Maniatis T: Molecular Cloning: A Laboratory Manual, ed 2 1989, NY, Cold Spring Harbor Laboratory Press, Cold Spring Harbor
- 37.Krafft AE, Duncan W, Bijwaard KE, Taubenberger JK, Lichy JH: Optimization of the isolation and amplification of RNA from formalin-fixed, paraffin-embedded tissue: the Armed Forces Institute of Pathology experience and literature review. Mol Diagn 1997, 2:217-230 [DOI] [PubMed] [Google Scholar]
- 38.Meeker TC, Sellers W, Harvey R, Withers D, Carey K, Xiao H, Block AM, Dadey M, Han T: Cloning of the t(11;14)(q13;q32) translocation breakpoints from two human leukemia cell lines. Leukemia 1991, 5:733-737 [PubMed] [Google Scholar]
- 39.Abruzzo LV, Griffith LM, Nandedkar M, Aguilera NS, Taubenberger JK, Raffeld M, Stass SA, Abbondanzo SL, Jaffe ES: Histologically discordant lymphomas with B-cell and T-cell components. Am J Clin Pathol 1997, 108:316-323 [DOI] [PubMed] [Google Scholar]
- 40.Banks PM, Chan J, Cleary ML, Delsol G, De Wolf-Peeters C, Gatter K, Grogan TM, Harris NL, Isaacson PG, Jaffe ES, Mason D, Pileri S, Ralfkiaer E, Stein H, Warnke RA: Mantle cell lymphoma: a proposal for unification of morphologic, immunologic and molecular data. Am J Surg Pathol 1992, 16:637-640 [DOI] [PubMed] [Google Scholar]
- 41.Lennert K, Stein H, Kaiserling E: Cytological and functional criteria for classification of malignant lymphomata. Br J Cancer 1975, 31(Suppl 2):29-43 [PMC free article] [PubMed] [Google Scholar]
- 42.Berard CW, Dorfman RF: Histopathology of malignant lymphomas. Clin Haematol 1974, 3:39-76 [Google Scholar]
- 43.Lennert K, Feller A: Histopathology of Non-Hodgkin’s Lymphomas. ed 2 1992, :pp 93-102 Springer Verlag, New York [Google Scholar]
- 44.Dorfman DM, Shahsafaei A: Usefulness of a new CD5 antibody for the diagnosis of T-cell and B-cell lymphoproliferative disorders in paraffin sections. Mod Pathol 1997, 10:859-863 [PubMed] [Google Scholar]
- 45.Zukerberg LR, Yang W-I, Arnold A, Harris NL: Cyclin D1 expression in non-Hodgkin’s lymphomas. Am J Clin Pathol 1995, 103:756-760 [DOI] [PubMed] [Google Scholar]
- 46.Pinyol M, Campo E, Nadal A, Terol MJ, Jares P, Nayach I, Fernandez PL, Piris MA, Montserrat E, Cardesa A: Detection of the bcl-1 rearrangement at the major translocation cluster in frozen and paraffin-embedded tissues of mantle cell lymphomas by polymerase chain reaction. Am J Clin Pathol 1996, 105:532-537 [DOI] [PubMed] [Google Scholar]
- 47.Lim L-C, Segal GH, Wittwer CT: Detection of bcl-1 gene rearrangement and B-cell clonality in mantle cell lymphoma using formalin-fixed, paraffin-embedded tissues. Am J Clin Pathol 1995, 104:689-695 [DOI] [PubMed] [Google Scholar]
- 48.Lasota J, Franssila K, Koo CH, Miettinen M: Molecular diagnosis of mantle cell lymphoma in paraffin-embedded tissue. Mod Pathol 1996, 9:361-366 [PubMed] [Google Scholar]
- 49.Soslow RA, Zukerberg LR, Harris NL, Warnke RA: Bcl-1 (PRAD-1/cyclin D1) overexpression distinguishes the blastoid variant of mantle cell lymphoma from B-lineage lymphoblastic lymphoma. Mod Pathol 1997, 10:810-817 [PubMed] [Google Scholar]
- 50.Ott MM, Bartkova J, Bartek J, Dürr A, Fischer L, Ott G, Müller-Hermelink HK, Kreipe H: Cyclin D1 expression in mantle cell lymphoma is accompanied by downregulation of cyclin D3 and is not related to proliferation activity. Blood 1997, 90:3154-3159 [PubMed] [Google Scholar]
- 51.Motokura T, Arnold A: PRAD1/cyclin D1 proto-oncogene: genomic organization, 5′ DNA sequence, and sequence of tumor-specific rearrangement breakpoint. Genes Chromosomes & Cancer 1993, 7:89-95 [DOI] [PubMed] [Google Scholar]
- 52.Donnellan R, Chetty R: Cyclin D1 and human neoplasia. J Clin Pathol Mol Pathol 1998, 51:1-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rosenberg CL, Wong E, Petty EM, Bale AE, Tsuijmoto Y, Harris NL, Arnold A: PRAD1, a candidate BCL-1 oncogene: mapping and expression in centrocytic lymphoma. Proc Natl Acad Sci USA 1991, 88:9638-9642 [DOI] [PMC free article] [PubMed] [Google Scholar]