Abstract
We have previously shown that fibroblast growth factor (FGF)-1-, FGF-4-, or vascular endothelial growth factor (VEGF/VPF)-transfected MCF-7 breast carcinoma cells growing as tumors in nude mice are tamoxifen resistant and/or estrogen independent. These transfectants provide opportunity for study of in situ tumor-induced angiogenesis promoted by the individual angiogenic factors under growth-promoting versus growth-inhibiting hormonal conditions. In the present study, vessels in tumors harvested at varying times after tumor cell injection were immunohistochemically highlighted and vessel morphology and topography were scored on a scale of 0 to 4 by blinded observers. In tumors produced by all cell lines under all growth-promoting hormonal conditions, there was significantly increased abundance (P < 0.05) of edge-associated and intratumor microvessels, but not of stromally located microvessels, when compared with tumor nodules harvested under growth-inhibiting conditions, regardless of the identity of the angiogenic factor or the hormonal treatment. Image analysis of bromodeoxyuridine (BrdU)-labeled nuclei of tumors produced by all cell lines under all hormonal conditions harvested at early time points showed that mean labeling indices were highest for hormonal conditions that produced the most robust growth in that particular cell line, implying that a high BrdU labeling index is a predictor of future tumor growth in individual tumors. These results confirm previous studies that established the importance of neovascularization for tumor growth and provide validation for use of these cell lines to study the process of angiogenesis in vivo. Study of gene expression in endothelial cells in edge-associated or intratumor vessels using this model might reveal mechanisms important in tumor-induced angiogenesis in human breast cancer.
Strong evidence is now in place confirming the importance of neovascularization in many types of cancer. Multiple studies have shown that maximal microvessel density is tightly correlated with prognosis and/or metastasis. 1,2 However, the mechanism whereby tumors induce neovascularization has not been well studied in situ. Because of the plasticity of endothelial cells in culture, 3-6 in vitro systems using cultured endothelial cells may not be appropriate for study of gene expression in tumor-induced neovascularization. Moreover, endothelial cells that are present in tumors are phenotypically distinct 7-9 and may behave differently from cells commonly used in in vitro studies or those present at atypical in vivo sites, such as cornea or chick chorioallantoic membrane. In addition, the contribution of neovascularization to tumor progression and metastasis cannot be assessed in such settings. Because of our belief that various current models of angiogenesis are inadequate or inappropriate for study of tumor-induced angiogenesis, we have embarked on a project to study tumor-induced angiogenesis in situ.
We have developed a model tumor system in which stable transfection of a particular angiogenic growth factor, fibroblast growth factor (FGF)-4, (also called kFGF/hst-1), FGF-1 (acidic FGF), or vascular endothelial cell growth factor (VEGF/VPF), into MCF-7 breast carcinoma cells has resulted in the acquisition of a more tumorigenic and/or more metastatic phenotype. Whereas parental MCF-7 cells require estrogen supplementation for tumorigenicity in ovariectomized nude mice, the FGF transfectants are capable of estrogen-independent growth. Additionally, in contrast to parental MCF-7 cells, the FGF transfectants are capable of growth in antiestrogen-treated mice and are reliably metastatic to lymph nodes and lungs of tumor-bearing mice. 10-12 The VEGF/VPF-transfected MCF-7 cells do not form tumors in ovariectomized mice, but when injected with matrix material, they form large, well vascularized, metastatic tumors in both estrogen- and tamoxifen-treated mice. 13 As all of the transfectants are derived from common parents, genetic variation other than expression of the transfected angiogenic factor cDNA is minimized. Thus, this model tumor system may be useful to study the process of tumor-induced angiogenesis in situ. Elucidation of steps in the process of tumor-induced angiogenesis using this model might reveal differences between tumor-induced angiogenesis and other types of angiogenesis that might be used to target therapy specifically to tumor blood vessels.
Studies that link maximal microvessel density to prognosis in breast cancer have typically quantitated microvessel density in hot spots of tumor vasculature, most often in the stroma surrounding foci of tumor cells. 14 The correlation of density of these particular vessels with prognosis implies functional significance of these vessels in metastasis. Therefore, vessels that comprise hot spots might be the particular vessels in which to study molecular mechanistic features of angiogenesis in a human breast cancer specimen. Because xenografts in the mouse mammary fat pad do not have the same morphology as human breast tumors, there is no location of tumor-associated vessels corresponding to hot spots. Identification of particular vessels for study of gene expression in a xenograft model may therefore not be straightforward. Therefore, although our long-term goal in these studies is to uncover molecular mechanistic features of tumor-induced angiogenesis, we felt it was important to first precisely identify blood vessels of interest and develop ways to study tumors during their initial growth when differences in tumor size are not evident but when neoangiogenesis would be most active. Surprisingly, we found that regardless of the identity of the transfected angiogenic factor, edge-associated and intratumor microvessel density was increased under growth-promoting conditions when compared with growth-inhibiting conditions. Thus, we believe we have identified particular vessel types in the xenograft that are functionally important to the growth of these tumors and that might correspond to vessels in hot spots of human tumors. In addition, we show that the bromodeoxyuridine (BrdU) labeling index can identify particular tumors that are growing rapidly at early time points before differences in tumor size become apparent. Therefore, study of gene expression in edge-associated and intratumor microvessels, in particular, tumors with high BrdU labeling index using this xenograft model system, may identify genes of which expression is important in the functionality of hot spot blood vessels in affecting prognosis in human cancer.
Materials and Methods
Cell Lines
ML-20 cells, which have been previously described, 10,11 are a clonal line of wild-type MCF-7 cells transfected with an expression vector containing the cDNA for bacterial lacZ. We find their in vivo behavior to be indistinguishable from wild-type MCF-7 cells. MKL-F cells are ML-20 cells transfected with an expression vector encoding FGF-4. This clonal cell line forms large tumors in both ovariectomized and tamoxifen-treated mice, but larger tumors arise in estrogen-treated mice 15,16 (Table 1) ▶ . MKL-4 cells are a clonal line of wild-type MCF-7 cells first transfected with an expression vector encoding FGF-4 and subsequently transfected with an expression vector encoding bacterial lacZ. This clonal cell line also forms tumors in ovariectomized mice under all hormonal conditions (no treatment, estrogen, or tamoxifen) but the largest tumors arise in tamoxifen-treated mice 10,11 (Table 1) ▶ . Hence, the MKL-4 cells and MKL-F cells are both clonal cell lines derived from wild-type MCF-7 cells that contain the same two expression vectors but that are oppositely affected in vivo by estrogen or tamoxifen treatment (Table 1) ▶ . Clone 18 cells are a clonal line of ML-20 cells transfected with an expression vector encoding FGF-1. Clone 18 cells also form large tumors under all three treatment conditions, but the largest tumors arise in estrogen-treated mice 12 (Table 1) ▶ . MV165-14 cells are ML-20 cells transfected with an expression vector encoding the 165-kd form of VEGF/VPF. These cells do not form tumors in ovariectomized animals but are tumorigenic and metastatic in estrogen-treated animals or in tamoxifen-treated animals when injected with Matrigel 13 (Table 1) ▶ . MPCX cells are ML-20 cells transfected with the expression vector for VEGF/VPF lacking the VEGF/VPF sequences. Thus, this cell line is the control transfectant line for MV165-14. 13 In our studies of microvessel density in tumors and tumor nodules, we found its behavior to be quite similar to its parent line ML-20, and results for these two lines are pooled for these studies. The in vivo growth characteristics of these cell lines are summarized in Table 1 ▶ .
Table 1.
In Vivo Growth Characteristics of the Clonal Cell Lines Used in This Study
| Cell line (transfected factor) | Ovariectomized | Estrogen | Tamoxifen |
|---|---|---|---|
| ML-20 (a LacZ-transfected clonal line of MCF-7) | − | + | ± |
| MPCX (control transfection for MV165-14) | − | +++ | ± |
| MKL-4 (FGF-4) | +++ | ++ | +++++ |
| MKL-F (FGF-4) | +++ | ++++ | +++ |
| Clone 18 (FGF-1) | +++ | ++++ | +++ |
| MV165-14 (VEGF/VPF) | − | +++ | ++ |
The table summarizes results of experiments done previously with the indicated cell lines in which tumors were harvested after several months of tumor growth. 10,11,13,15,16 ML-20 and MPCX, lacZ-transfected MCF-7 cell lines, have growth characteristics typical of wild-type MCF-7 cells; ie, they are estrogen-dependent for tumor growth. The transfected angiogenic factors are indicated in parentheses following the names of the clonal cell lines. Symbols are interpreted as follows: −, no tumors are produced; ±, static tumor nodules are produced; + to +++++, actively growing tumors are produced with relative sizes indicated by the number of pluses. Approximate tumor sizes are as follows: +, <30 mm3; ++, 30 to 150 mm3; +++, 150 to 350 mm3; ++++, 350 to 750 mm3; +++++, >750 mm3 after 2 months of tumor growth.
Tumor Production, Harvest, and Processing
Ten million cells/0.2 ml were injected into the mammary fat pad of ovariectomized nude mice as previously described. 10-12 For the VEGF/VPF-transfected MV165-14 cells and their control line MPCX, cells were mixed 1:1 with matrix material (Matrigel, Collaborative Biomedical Products, Bedford, MA) before injection. Some mice were treated with 60-day sustained release pellets containing 17β-estradiol (0.72 mg) or tamoxifen (5 mg, Innovative Research, Sarasota, FL). Eight hours before sacrifice and tumor harvest, 1 mg of BrdU was injected intraperitoneally. At indicated time points, tumors were harvested with adjacent stroma by cutting through the skin and peritoneum in a circular incision circumscribing the tumor. The tumor was fixed for 48 hours in 10% buffered formalin and subjected to X-gal staining as previously described 10-12 to delineate borders of the tumor. Stained tumors were trimmed of extraneous skin and muscle, cut in half to reveal a section from skin to muscle, and fixed in 10% buffered formalin for 48 hours, followed by dehydration, paraffin embedding, and sectioning.
Immunohistochemistry
Immunohistochemistry for murine PECAM-1 was accomplished using the rat monoclonal antibody Mec 13.3, 17 which was initially generously provided by Elisabetta Dejana, Rolande Berthier, and Annunciata Vecchi. Subsequently, this antibody became available from Pharmingen (01951D), San Diego, CA. After deparaffination in xylenes and rehydration in graded alcohols, slides were digested in 0.1% trypsin in phosphate-buffered saline (PBS) for 30 minutes at 37° and washed in running distilled water for 10 minutes. Endogenous peroxidases were quenched in 0.3% H2O2 in methanol for 30 minutes. Blocking solution (2% normal rabbit serum, 5% bovine serum albumin (Sigma (St. Louis, MO) A7888) in PBS) was applied for 1 hour. Primary rat anti-murine PECAM-1 antibody was applied for 2 hours at room temperature in blocking solution at a concentration of 5 μg/ml. After PBS washes, secondary rabbit anti-rat antibody coupled to biotin (Vector (Burlingame, CA) BA4001) was applied for 1 hour. Slides were washed three times in PBS, and ABC reagent coupled to peroxidase (Vector Elite kit PK6001) was applied for 1 hour. After three PBS washes, slides were incubated with 0.05% diaminobenzidine and 0.01% H2O2 for 15 minutes. Slides were then briefly counterstained with Harris acidified hematoxylin, washed, dehydrated in graded alcohols, and coverslipped from xylenes. Immunohistochemistry for BrdU incorporation followed the same protocol as for PECAM-1 except that before the trypsin digestion, slides were placed in 2 N HCl for 30 minutes and washed thoroughly in deionized water. The primary antibody was a rat monoclonal anti-BrdU antibody (Accurate Antibodies, Westbury, NY, MAS250b) used at a dilution of 1:32 in blocking solution and incubated for 1 hour at room temperature. After incubation with the same secondary antibody as for PECAM-1, slides were incubated with an ABC reagent coupled to alkaline phosphatase (Vector AK5000). Slides were then incubated with the Vector red alkaline phosphatase substrate (Vector SK-5100) according to the manufacturer’s directions. For double PECAM-1 and BrdU immunohistochemistry, PECAM-1 staining with the peroxidase ABC kit and diaminobenzidine was performed first. After the wash step after diaminobenzidine disclosure, slides were left overnight in PBS and begun the next morning at the HCl step of the BrdU protocol.
Cell Death Assays
Terminal deoxynucleotidyl transferase (TdT) dUTP nick end labeling (TUNEL) was performed using the TACS 2 TdT kit (Trevigen, Gaithersburg, MD) according to the manufacturer’s instructions.
Analysis of Tumor Sections
Analysis of tumor sections was done in a blinded fashion. The parameters for inflammation or microvessel density were scored on a rating system of 0 to 4, with 4 being the highest and 0 denoting absence of the parameter. Inflammatory infiltrates were evaluated from hematoxylin and eosin (H&E)-stained sections and scored as acute (primarily neutrophilic) or chronic (primarily lymphocytic), in addition to being given a numerical rating. Neovascularization was assessed using slides stained with immunohistochemistry for PECAM-1, both singly and in combination with staining for BrdU incorporation. Microvessels were defined as small vessels without visible smooth muscle. The densities of four microvessel types were rated subjectively, using standard slides for each numerical rating and vessel type. Vessel types rated were 1) tumor edge-associated microvessels, 2) intratumor microvessels, 3) ectatic (dilated vessels without associated smooth muscle) stromal microvessels, and 4) normal stromal microvessels. (Because the density of each vessel type was rated separately, microvessel densities obtained here cannot be compared with microvessel counts or Chalkley scores obtained by others.) For the time courses depicted in Figures 1 and 2 ▶ ▶ and Table 1 ▶ , a single observer (P. R. Vezza) was used. For correlation of BrdU and TUNEL labeling indices with edge-associated or intratumor microvessel density, a second observer’s (A. C. Filie) scoring was added for sections harvested between 10 and 20 days after tumor cell injection.
Figure 1.
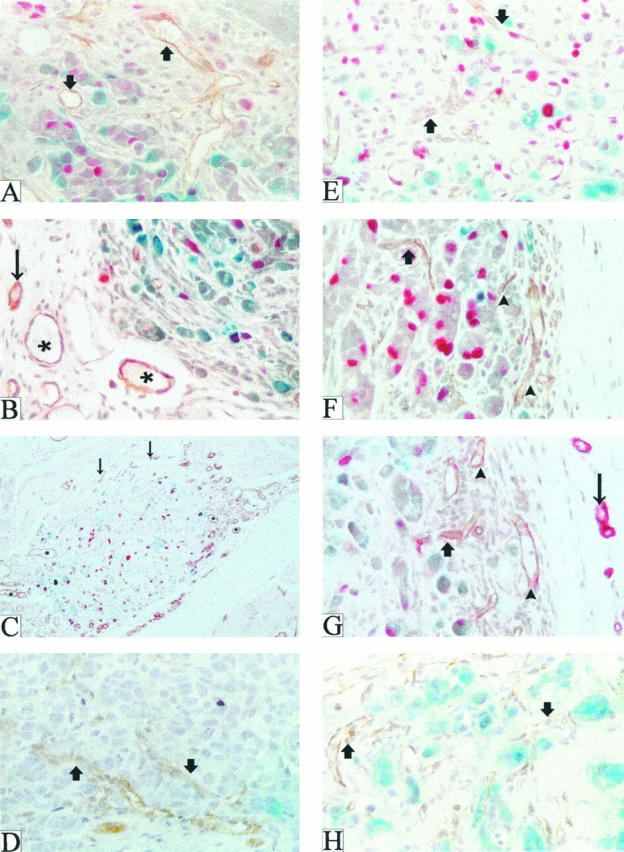
Patterns of neovascularization after injection of parental MCF-7 and FGF-transfected MCF-7 cells into the mammary fat pads of ovariectomized nude mice. Tumors or tumor nodules produced by injection of parental MCF-7 (A–C) or FGF-4 transfected (MKL-F, E–G) cells were harvested at days 6 (A and E), 15 (B and F), and 35 (C and G), stained with X-gal (blue) to reveal tumor margins, embedded in paraffin, sectioned, and subjected to double immunohistochemistry for BrdU (red) and murine PECAM-1 (brown or reddish brown). (Because the tumors were stained with X-gal when whole, only surface tumor cells stain blue.) D and H depict VEGF/VPF-transfected cell tumors (MV165-14) harvested 18 days after tumor cell injection. Arrowheads point to edge-associated microvessels, thick arrows to intratumor microvessels; asterisks denote ectatic stromal vessels, and thin arrows indicate normal stromal vessels. Magnification, ×400 (A, B, and D–H) and ×200 (C).
Figure 2.
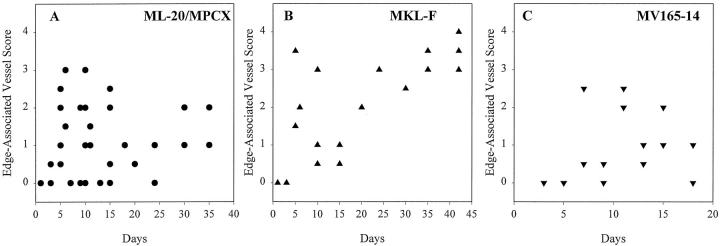
Edge-associated microvessels are more abundant in estrogen-independent tumors produced by FGF-4-transfected MCF-7 cells than in tumor nodules produced by parental or VEGF/VPF-transfected cells. Edge-associated vessel abundance for particular tumors was plotted against day of harvest. A: Parental/control cells (data from tumors produced by ML-20 and MPCX control lines are combined). B: FGF-4-transfected MKL-F cells (abundance of edge-associated vessels was significantly greater (P < 0.05) when compared with parental/control cell tumors). C: VEGF/VPF-165-transfected MVF165-14 cells (abundance of edge-associated vessels was not significantly greater than parental/control).
BrdU incorporation and TUNEL staining in tumor cells was analyzed utilizing image analysis, which counted numbers of positively stained nuclei in a given area (in square microns). Tumor cell density was determined by manually counting numbers of tumor cells in a given area and dividing by the area. Labeling indices for BrdU incorporation and TUNEL staining were then derived by converting areas to numbers of cells. Sections from mammary fat pads of mice injected with medium alone and harvested after 10 days were used to evaluate the effect of vehicle injections. No inflammatory infiltrates or microvessels of the types used for rating were observed in any sections thus evaluated.
RNAse Protection
Parental or transfected cells were grown to 60% confluence in medium containing 5% fetal bovine serum. RNA was harvested using Triazol (Life Technologies, Gaithersburg, MD), and ribosomal bands were determined to be intact by agarose gel electrophoresis followed by ethidium bromide staining. The riboprobe vector for human GAPDH has been previously described. 10 The riboprobe for human VEGF/VPF produces a 432-bp probe that contains plasmid sequences and a 389-bp fragment identical to the partial sequence of the mRNA that gives rise to the 165-amino-acid form of VEGF/VPF. When hybridized to VEGF/VPF-165 mRNA, a 389-bp protected fragment is produced. When hybridized to mRNA from VEGF/VPF-189 or -121, which share mRNA sequences with VEGF/VPF-165, a 216-bp fragment is protected. VEGF/VPF-189 transcripts also protect a 173-bp fragment. Thirty micrograms of total RNA was subjected to RNAse protection as previously described 10 using 32P-labeled antisense riboprobes for human VEGF/VPF and human GAPDH followed by denaturing polyacrylamide gel electrophoresis and autoradiography. The VEGF-165 isoform and the GAPDH band were quantitated by phosphorimaging, and VEGF-165/GAPDH ratios were calculated and compared.
Statistics
For analysis of data shown in Figures 2 and 3 ▶ ▶ and Table 2 ▶ , data were for the most part not normally distributed. In those cases, the Kruskal-Wallis test was used to compare microvessel scores in tumors produced by transfected cell lines with those produced by parental/control cell lines. In those data sets that were normally distributed, a one-way analysis of variance was performed. Scores for the various vessel types before day 5 were excluded from the analyses as those scores seemed atypical of the rest of the scores and probably reflect vessels formed in response to inflammation produced by injection of tumor cells. A Mann-Whitney rank sum test was used to compare vessel scores between estrogen- and tamoxifen-treated control tumors, as these data were also not normally distributed.
Figure 3.
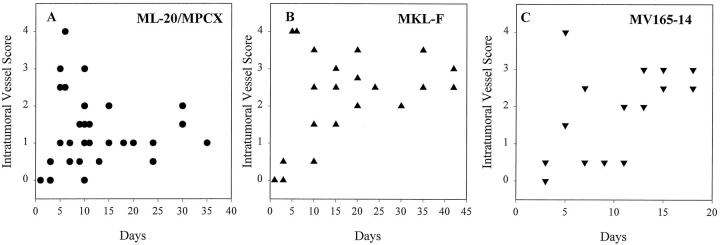
Intratumor microvessels are more abundant in estrogen-independent tumors produced by FGF-4-transfected MCF-7 cells and in tumor nodules produced by VEGF/VPF-transfected cells than in tumor nodules produced by parental cells. Intratumor vessel abundance was plotted against day of harvest. A: Parental/control cells. B: FGF-4-transfected MKL-F cells (abundance of intratumor vessels was significantly greater (P < 0.05) when compared with parental/control cell tumors). C: VEGF/VPF-165-transfected MV165-14 cells (abundance of intratumor vessels was not significantly greater than parental/control).
Table 2.
Abundance of Microvessel Types in Tumors Produced in Estrogen- or Tamoxifen-Treated Mice by Parental or Transfected MCF-7 Cells
| Vessel Type | Treatment | Parental/control | MKL-F | MKL-4 | Clone 18 | MV165-14 |
|---|---|---|---|---|---|---|
| Edge associated | Estradiol | 0.84 ± 0.18 | 2.60 ± 0.24* | 1.25 ± 0.35 | 2.42 ± 0.44* | 0.71 ± 0.14 |
| Tamoxifen | 0.36 ± 0.08† | 2.30 ± 0.33* | 1.17 ± 0.42 | 1.92 ± 0.41* | 1.14 ± 0.29 | |
| Intratumor | Estradiol | 1.23 ± 0.19 | 2.65 ± 0.66 | 2.07 ± 0.50 | 2.00 ± 0.49 | 1.86 ± 0.30 |
| Tamoxifen | 0.83 ± 0.13 | 3.05 ± 0.41* | 2.17 ± 0.42* | 2.07 ± 0.54* | 2.61 ± 0.30* | |
| Ectatic stromal | Estradiol | 1.09 ± 0.18 | 1.40 ± 0.53 | 1.14 ± 0.39 | 1.71 ± 0.51 | 1.29 ± 0.19 |
| Tamoxifen | 1.45 ± 0.25 | 1.90 ± 0.60 | 0.83 ± 0.31 | 1.86 ± 0.29 | 1.25 ± 0.21 | |
| Normal stromal | Estradiol | 1.75 ± 0.19 | 2.40 ± 0.51 | 1.96 ± 0.50 | 2.71 ± 0.43 | 1.68 ± 0.25 |
| Tamoxifen | 1.89 ± 0.25 | 2.70 ± 0.37 | 1.96 ± 0.57 | 2.21 ± 0.42 | 2.14 ± 0.27 |
Microvessel score averages ± SEM are given for the various microvessel types from tumor sections harvested from mice treated with estrogen or tamoxifen pellets from time points 5 days or later after tumor cell injection.
*Scores significantly different from the parental cell scores for a particular treatment.
†Scores of tumors in tamoxifen treated animals receiving control cells differed significantly from those receiving estrogen treatment.
For the correlation between BrdU labeling index (on a log scale) and edge-associated or intratumor vessel score, both variables were measured with error, which can make standard methods of correlation analysis too conservative. 18 Statistical methods to estimate the correlation when both variables are subject to measurement error have been proposed by several authors. 18,19 Because we desired conservative inferences, we conducted correlation analysis using the standard Pearson method found in SAS/PROC CORR. 20
Results
Estrogen-Independent Growth of Transfected MCF-7 Cells is Characterized by Increased Numbers of Tumor Edge-Associated Microvessels
We began these studies with the hypothesis that the phenotypic progression observed upon transfection of angiogenic factors into MCF-7 breast carcinoma cells to an estrogen-independent or tamoxifen-resistant phenotype could be explained, at least in part, by the angiogenic activity of the transfected factors. To summarize this phenotypic progression, in vivo growth characteristics of the cell lines used in this study are given in Table 1 ▶ . Because differences in tumorigenicity between the FGF transfectants and the parental and VEGF/VPF-transfected cells are most marked under conditions of estrogen-independent growth, we first examined characteristics of tumors in a time course from 1 day to 5 to 6 weeks after tumor cell injection. Under these conditions, the parental or VEGF/VPF-transfected cells form small nodules (∼15 mm 3 or less) that regress over ∼2 to 3 weeks, whereas the FGF-transfected cells form progressively growing tumors. For these initial studies, we used the parental line ML-20, the FGF-4-transfected cell line MKL-F, the VEGF/VPF-transfected line MV165-14, and its control line MPCX. We were able to harvest extremely small tumor nodules produced by the parental/control cells or VEGF/VPF-transfected cells at late time points because they express bacterial LacZ, enabling them to be identified in the mammary fat pad by X-gal staining.
At 1 to 10 days after tumor cell injection, neovessels were present in stroma surrounding and infiltrating the tumor cells in tumor nodules produced by all cell lines (examples of day 6 in Figure 1, A and E ▶ ). At later time points, however, in tumors produced by the FGF transfectants, small microvessels were closely associated with the tumor periphery and extended into the tumor (examples of day 15 and 35 in Figure 1, F and G ▶ ) whereas in tumor nodules produced by parental cell injection, blood vessels remained at the periphery of the nodule, in some cases separated from the edge of the nodule by an avascular space (examples of day 15 and 35 in Figure 1, B and C ▶ ). Moreover, some of the vessels in parental cell nodules gradually assumed a dilated, ectatic appearance with irregular contours (Figure 1, B and C) ▶ . At very late time points after tumor cell injection, tumors produced by the FGF transfectants continued to exhibit intratumor microvessels as well as large numbers of tumor edge-associated microvessels (Figure 1G) ▶ . Vessels in parental cell nodules at these time points were almost exclusively peripheral to the tumor (Figure 1C) ▶ .
Because it seemed that regressing tumor nodules produced by the parental cells were not lacking in blood vessels, but instead exhibited blood vessels with different characteristics when compared with those of the progressively growing tumors produced by the FGF transfectants, we further characterized these sections with regard to microvessel topography and morphology in tumor sections produced by the parental and transfected cells. Sections were rated by a blinded observer for abundance of four distinct morphologies of microvessels noted in the tumor sections. These were 1) tumor edge-associated microvessels (Figure 1, F and G) ▶ , 2) intratumor microvessels (Figure 1, A, E, F, and G) ▶ , 3) ectatic (dilated) stromal microvessels (Figure 1, B and C) ▶ , and 4) normal stromal microvessels (Figure 1, B, C, and G) ▶ .
When tumors or tumor nodules produced in ovariectomized mice by parental/control or transfected cells were compared over the entire time course with respect to abundance of these four vessel types, it was found that edge-associated microvessels were more abundant in the progressively growing tumors produced by the FGF-transfected cells (Figure 2B ▶ and not shown) than in tumor nodules produced by the parental or VEGF/VPF-transfected cells (Figure 2, A and C) ▶ . Statistical analysis of scores for edge-associated microvessels at time points 5 days or later after tumor cell injection revealed that scores for tumors produced by the tumorigenic FGF-4-transfected MKL-F cell line were significantly higher than those for tumor nodules produced by the ML-20 and MPCX control lines (P < 0.05), whereas edge-associated microvessel scores for tumor nodules produced by the nontumorigenic VEGF/VPF-transfected cell line MV165-14 were not different from control. Thus, edge-associated microvessels were significantly more abundant in tumors produced by tumorigenic MKL-F cells in ovariectomized nude mice than in tumor nodules produced by cell lines that are nontumorigenic under these conditions.
Intratumor microvessels also appeared more abundant in tumors produced by the FGF-transfected cells when compared with the parental cells (Figure 3) ▶ in ovariectomized mice. Statistical analysis of intratumor vessel scores at time points 5 days or later after tumor cell injection again revealed that tumors produced by the FGF-4-transfected cell line MKL-F had significantly higher intratumor vessel scores than the parental/control lines ML-20 and MPCX (P < 0.05). The intratumor vessel scores of tumor nodules produced by the VEGF/VPF-transfected line MV165-14 appeared higher than control, but this apparent difference did not achieve statistical significance. As the VEGF/VPF transfectants do not form tumors under these conditions, significant intratumor blood vessel abundance was correlated with estrogen-independent growth.
No significant differences were observed between tumors produced by FGF- or VEGF/VPF-transfected cells and parental cells in densities of ectatic or normal stromal microvessels (data not shown). Taken together, these data suggest that edge-associated and intratumor microvessels may be important in promoting estrogen-independent tumor growth in ovariectomtized nude mice.
Hormonal Treatments That Affect Tumor Growth also Produce Different Topography and Morphology of Tumor-Induced Blood Vessels
We extended our study to include tumors produced from injection of FGF- or VEGF/VPF-transfected or parental/control cells into ovariectomized mice treated with sustained-release pellets of 17β-estradiol or tamoxifen. To differentiate effects that were hormonally produced from those produced by tumor growth, we additionally used a clonal line of FGF-4-transfected MCF-7 cells (MKL-4), which is growth inhibited by estrogen treatment of tumor-bearing mice and growth stimulated by tamoxifen treatment of similar mice 10 (Table 1) ▶ .
Findings in this study confirmed a correlation of increased edge-associated microvessels with hormonal conditions that promote tumorigenicity or increased tumor size. Tumors produced by FGF-transfected cells grown under conditions of estrogen treatment exhibited increased abundance of edge-associated microvessels when compared with parental cell tumors (Table 2) ▶ . This effect was statistically significant for two of the three FGF-transfected cell lines (P < 0.05). These two lines (MKL-F and clone 18) produce larger tumors under conditions of estrogen treatment than the control lines (Table 1) ▶ . Tumors produced by VEGF/VPF-transfected cells with estrogen treatment had comparable scores for edge-associated microvessels compared with the parental cell tumors (Table 2) ▶ , which also correlates with their comparable eventual tumor size compared with the control line (Table 1) ▶ . Parental cell tumor nodules produced under conditions of tamoxifen treatment had a significantly reduced abundance of edge-associated microvessels when compared with estrogen-treated parental cell tumors (P = 0.045; Table 2 ▶ ). This was not true of tumors produced in tamoxifen-treated animals by the FGF or VEGF/VPF transfectants, which exhibit tamoxifen-resistant growth (Table 2) ▶ . Moreover, the edge-associated microvessel abundance in tumors produced by the same two transfected lines (MKL-F and clone 18) was significantly greater in tamoxifen-treated animals than that in tumors produced by control cells (P < 0.05). These data again imply that edge-associated vessels contribute positively to tumor growth under conditions of estrogen or tamoxifen treatment.
Intratumor vessel abundance was not significantly increased in the tumors produced by the FGF-transfected and VEGF/VPF-transfected cells when compared with the parental cells with estrogen treatment (Table 2) ▶ , a condition in which all lines are tumorigenic. Although there was a slightly decreased abundance of intratumor vessels in tamoxifen-treated parental cell tumor nodules, this effect did not reach statistical significance. However, intratumor microvessel abundance in tumors produced by all of the transfected cell lines was significantly increased when compared with control/parental cell tumor nodules under conditions of tamoxifen treatment (P < 0.05; Table 2 ▶ ). This was particularly evident in the VEGF/VPF-transfected cells (Figure 1, D and H) ▶ . Thus, these data and data on intratumor vessels under estrogen-free conditions suggest that intratumor vessels may have an important role in promoting growth under conditions where the parental cells are not tumorigenic, especially in the case of tamoxifen-resistant growth.
There were no differences in abundance of ectatic stromal or normal stromal vessels between different cell lines or treatment conditions. These data combined with the data from tumors produced in ovariectomized animals imply that these vessel types do not influence tumor growth.
Sustained Angigogenesis Produced by Tumor Cell Injection Is Not Due to a Prolonged Inflammatory Response
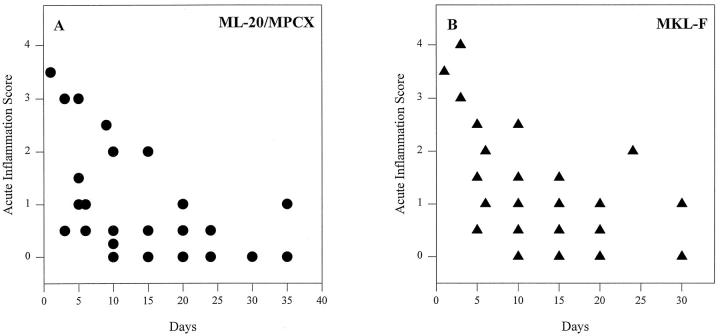
Although breast tumor xenografts in nude mice are a commonly used tumor model, injection of tumor cells into the mammary fat pad does not mimic early stages of growth of a spontaneously arising breast tumor. We were therefore concerned that the early angiogenic response might be due to inflammation as a result of the tumor cell injection and therefore would not be representative of spontaneous tumor angiogenesis. We examined inflammatory infiltrates over the entire time course, above, and found that, although in a few tumors high levels of acute, primarily neutrophilic, inflammation were initially present, after fewer than 10 days, this rapidly declined to baseline levels (Figure 4) ▶ . Chronic, primarily lymphocytic, inflammatory infiltrates remained constant throughout the time course (not shown). There was no difference in the degree of acute or chronic inflammatory reaction produced by different cell lines (Figure 4 ▶ and not shown). Moreover, injection of the medium used as a vehicle for the tumor cell injections did not produce neovascularization or inflammation when the injection sites were harvested 10 days later (data not shown). In addition, immunohistochemistry of tumor sections showed negligible expression of the inflammatory adhesion molecule ICAM-1 in tumor-associated blood vessels under all treatment conditions despite robust expression in several adjacent lymph nodes harvested coincidentally with the tumor (data not shown). In some tumors produced by all transfected cells, but more frequently in ones produced by VEGF/VPF-transfected cells, there was sporadic expression of ICAM-1 in blood vessels in adjacent tissues not intimately associated with the tumor, such as overlying skin (data not shown). As VEGF/VPF has been shown to increase ICAM-1 expression, 21 and FGF-2 (basic FGF) can either up-regulate or down-regulate ICAM-1 in cultured endothelial cells, 21,22 this finding may be due to an effect of diffusion of the transfected FGF or VEGF/VPF away from the tumor into surrounding tissues and actually argues for an ICAM-1-suppressing mechanism within the tumor. In any case, we conclude that sustained tumor-associated angiogenesis occurring after the initial period in xenografts produced by parental or transfected MCF-7 cells is not due to inflammation.
Figure 4.
Scores for acute inflammatory infiltrates are high initially but rapidly decline to baseline levels. Acute inflammation scores for tumors produced by the ML-20 and MPCX parental/control cell lines (A) or the FGF-4-transfected MKL-F cell line (B), plotted against the day of harvest. Other FGF and VEGF/VPF transfectants had similar levels of acute inflammation at comparable time points.
Angiogenesis Produced by FGF-Transfected Cells Is Not Due to Increased Expression of VEGF/VPF
Recently it has been reported that transfection of mammary epithelial cells or MCF-7 cells with FGF-4 induces expression of VEGF/VPF. 23,24 In addition, FGF-2 induces VEGF expression in cultured endothelial cells. 25 As the validity of our model depends on our being able to ascribe angiogenic effects of the transfections to the transfected factors, we examined RNA from the parental and FGF-transfected cells for mRNA encoding VEGF/VPF. RNAse protection assays revealed that neither transfection with FGF-4 nor FGF-1 induced expression of any of the detected isoforms of VEGF/VPF (Figure 5) ▶ . We also examined RNA extracted from tumors produced by the same cell lines harvested 15 days after tumor cell injection. At this point, the tumors were actively growing but still small enough so there was no detectable necrosis. As with cells grown in tissue culture, there was no increase in VEGF mRNA expression in the tumors produced by the FGF-transfected cells when compared with a tumor produced by the control ML-20 cells (not shown). These data, in addition to the different in vivo phenotypes of the FGF-transfected and VEGF/VPF-transfected cell lines (Table 1) ▶ , argue that the angiogenic effects of FGF transfection in these cell lines are not due to increased expression of VEGF/VPF.
Figure 5.
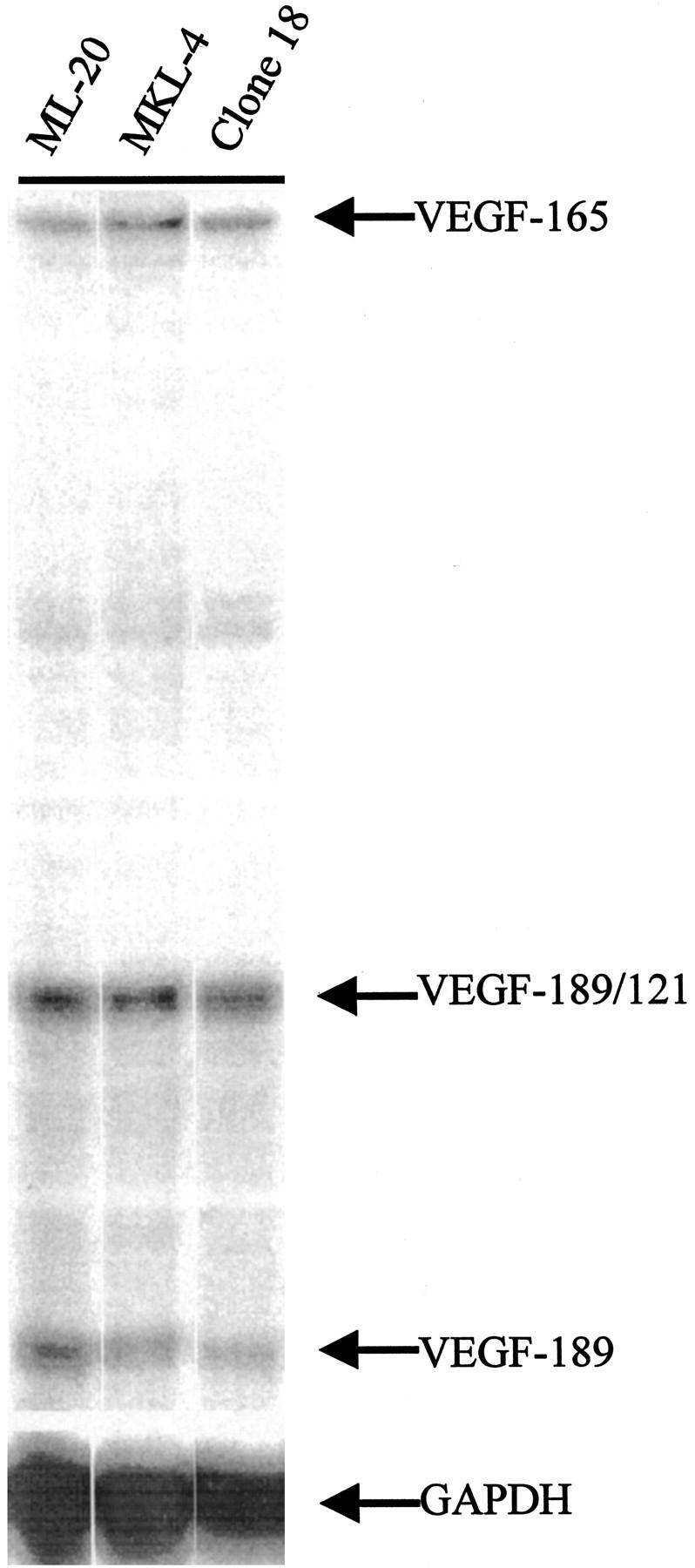
Expression of VEGF/VPF mRNA is not elevated in FGF-transfected cells. RNAse protection of 30 μg of RNA from the indicated cell lines shows that the parental ML-20 cells and the two FGF transfectants have similar levels of mRNA for all isoforms of VEGF/VPF detected. Isoforms detected by the riboprobe are as indicated. The VEGF-165 isoform and the GAPDH band were quantitated by phosphorimaging and VEGF-165/GAPDH ratios calculated and compared.
Identification of an In Situ Predictor of Future Tumor Growth in Tumor Sections from Transfected and Parental MCF-7 Cells
Our data, above, validate the importance of particular microvessels in producing active tumor growth. As mentioned, our goal is to study gene expression in these microvessels at early time points in tumor growth when we believe angiogenesis would be most active. From the time course experiments depicted in Figures 2 and 4 ▶ ▶ and from our previous work, 10,12,13,16,26 this would seem to be in the time range of 10 to 20 days after tumor cell injection, after the acute inflammatory response has subsided, but just before or concurrent with a substantial increase in tumor growth rate. Thus, we know which vessels to study and when to study them. However, tumor cell injection of any cell line under any conditions produces a range of tumor sizes or no tumor at all. Therefore, to correlate endothelial expression of particular genes with active tumor growth, it is important to know whether an individual early tumor is actively growing before differences in tumor size become apparent. In other words, we want to know which tumors to study. As tumor growth is thought to be due to the algebraic sum of cell proliferation and cell death, we asked whether measurements that estimate either of these parameters could identify actively growing tumors versus static or regressing ones. As the individual cell lines have different responses to hormonal treatment in vivo (Table 1) ▶ , we were able to use conditions of estrogen or tamoxifen treatment of tumor-bearing animals to answer this question.
For these studies, we used the FGF-4-transfected cell line MKL-4, which produces tumors that are stimulated by tamoxifen treatment of tumor-bearing mice, the FGF-1 transfected cell line clone 18, and the VEGF/VPF-transfected cell line MV165-14 as well as the ML-20/MPCX parental/control lines (Table 1) ▶ . Our hypothesis was that if proliferation, as measured by BrdU incorporation, 27 were a good in situ predictor for future tumor growth, the pattern of the mean BrdU labeling indices for tumors produced by a given cell line under conditions of estrogen or tamoxifen treatment would have the same relationship as the known growth pattern (measured at much later time points) for that cell line as influenced by estrogen or tamoxifen (Table 1) ▶ . Conversely, as TUNEL labeling is one measurement of cell death, if it were a good in situ predictor of future tumor growth, the mean TUNEL labeling index for a given cell line under conditions of estrogen or tamoxifen treatment would have the opposite pattern to the growth pattern produced by estrogen or tamoxifen treatment for that cell line. We were not interested in explaining growth characteristics of tumors produced by the different cell lines as the algebraic sums of proliferation and cell death. This would be a very difficult task because of the substantial differences in time sensitivity of the two assays, the probability that TUNEL, which is only one of several in situ measurements of apoptosis, identifies only a subset of apoptotic cells and may also stain necrotic cells, 28-30 and the possibility of entry of apoptotic cells into S phase, 31,32 which might produce positive staining for both BrdU incorporation and TUNEL in some cells.
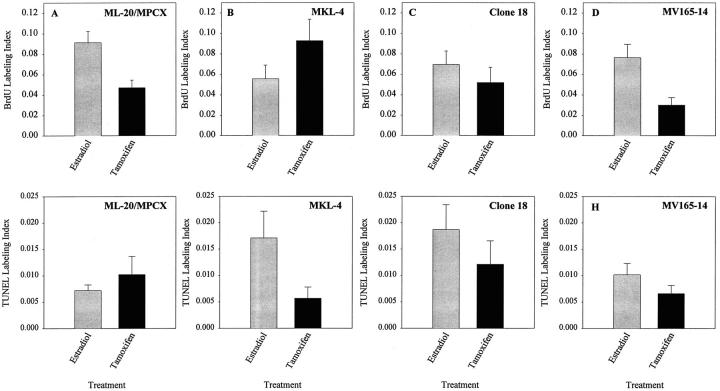
Representative tumor sections from tumors produced by FGF- or VEGF/VPF-transfected cells or parental cells in estrogen- or tamoxifen-treated mice harvested between 10 and 20 days after tumor cell injection were stained for BrdU incorporation or TUNEL labeling. Labeling indices for each section were determined by image analysis. For tumors produced by the ML-20/MPCX parental or control cell lines, the BrdU labeling index pattern showed the same trends as the tumor growth pattern, and the TUNEL labeling index pattern was weakly inverse to the growth pattern (Figure 6) ▶ . Thus, for these cell lines, BrdU labeling index is the best in situ predictor of future tumor growth. For the FGF-transfected MKL-4 cell line, the BrdU labeling index pattern again matched the tumor growth pattern and the TUNEL labeling index was opposite to the growth pattern, so that either a high BrdU labeling index or low TUNEL labeling index could serve as an in situ predictor of future tumor growth for this cell line (Figure 6) ▶ . For the clone 18 and MV165-14 cell lines, BrdU is a good predictor, but TUNEL is not, as the BrdU patterns correlate with the growth patterns, but the TUNEL patterns are not inverse. Thus, for the parental/control and all the transfected cells, BrdU incorporation is the best in situ predictor of tumor growth as it most consistently correlated with growth patterns of the transfected cell lines under conditions of estrogen and tamoxifen treatment. Moreover, rapidly growing tumors produced by individual cell lines can be identified as those for which BrdU labeling indices exceed means of the growth-stimulated tumors in these experiments, and slowly growing tumors can be identified as those for which BrdU labeling indices are lower than means of the growth-inhibited tumors in these experiments. These data will therefore assist us in assessing the effects of expression of particular genes on individual tumors as our studies of gene expression progress.
Figure 6.
Identification of an in situ marker for future tumor growth. Mean BrdU or TUNEL labeling indices from averages of two determinations of at least three different tumors produced by the indicated cell lines in mice treated with sustained release estrogen (gray bars) or tamoxifen (black bars) pellets are shown.
As BrdU can serve as an in situ predictor of tumor growth for the transfected cell lines, and edge-associated or intratumor microvessel abundance seemed to be increased in tumors with increased growth potential (above), we asked whether BrdU labeling were correlated with the abundance of edge-associated or intratumor microvessels in individual tumor sections harvested between 10 and 20 days after tumor cell injection. Statistical analysis such as this is difficult for several reasons. First, as both variables are the result of measurement (both are dependent variables), there is associated error present in both variables. Second, there must be a good spread of data across a range of values so that linear regression can be performed adequately. Third, the data must be normally distributed with a constant variance. To increase the validity of our analysis in this part of the study, we enlisted a second observer who performed ratings of microvessel abundance on the same sections as were evaluated by the first observer. Additionally, as we seek correlations for six individual cell lines and are thus conducting six linear regression analyses, we must divide the criterion for significance, a P value of less than 0.05, by 6 to rule out the possibility that one of our regressions will achieve a P < 0.05 by chance. Therefore, our criterion for significance in this analysis is a P value of less than 0.008. For tumor sections produced by the ML-20 or MPCX control cells, all but one of the sections examined had average edge-associated vessel scores of less than 2.5. Thus, these data did not meet the second criterion above, and we would not consider a linear regression analysis to be valid (data not shown). For tumors produced by one FGF-4-transfected cell line (MKL-4), average edge-associated vessel scores were highly correlated with BrdU labeling indices in the tumor sections analyzed (P = 0.0045), and intratumor vessel density showed a weak correlation (P = 0.07; data not shown). For the other transfected cell lines, there was no demonstrable correlation between BrdU labeling index and edge-associated or intratumor vessel scores. Thus, these data provide additional evidence, at least in one cell line, that increased tumor growth as identified by BrdU incorporation, is associated with increased abundance of edge-associated or intratumor vessels.
Discussion
This study has compared the morphological and topographical differences in microvessels formed in developing tumors produced by MCF-7 breast carcinoma cells or the same cells transfected with one of three different angiogenic growth factors, produced under estrogen-depleted conditions or with different hormonal treatments. The purpose of our study was to identify particular microvessels associated with active tumor growth for future study of the molecular mechanism of tumor-induced angiogenesis in a xenograft model. Surprisingly, differences between the tumors produced by parental or transfected cells with regard to microvessel morphology and topography were more clearly related to the growth status of the tumor than to the hormonal treatment or the transfected angiogenic factor. Abundance of microvessels at the edge of and within the tumor were found to be clearly associated with conditions that promote tumor growth. Although tumor nodules produced under growth-inhibiting conditions had abundant vessels, the vessels were not closely associated with the edge of the nodule and in many cases had an ectatic morphology. Thus, at least in this tumor system, it would seem that only certain vessels function to promote tumor growth. Although it is obvious that edge and intratumor microvessels perform this function by lying close to the growing tumor, it is possible that they have additional properties that are different from other vessels associated with the tumor, in addition to their topographic location. As the correlation between edge-associated and intratumor vessels and tumor growth was also operative for the parental cell tumors, albeit to a lesser extent than the transfectants, these data also imply that factors elaborated by the parental cells are able to influence the topography and morphology of neovessels. It has been shown that expression of some factors that may affect angiogenesis, such as transforming growth factor (TGF)-α or TGF-β, is regulated in MCF-7 cells by estrogen and tamoxifen. 33 Therefore, effects of hormonal treatment on microvessels may be due to factors elaborated by the tumor cells in response to the hormonal treatment. On the other hand, many endothelial cell types have been shown to have estrogen receptors 34-36 and to respond to estrogen by altering their gene expression, 36,37 so a direct effect of estrogen or tamoxifen on the endothelial cells comprising these microvessels cannot be ruled out.
The FGF-4-transfected MKL-4 cell line, the tumors of which are growth inhibited by estrogen treatment and growth stimulated by tamoxifen treatment, enables us to differentiate effects of hormonal treatment on blood vessels from those associated with growth. The correlation of BrdU labeling index and edge-associated vessel score for this cell line also underscores this point, as sections produced in untreated, estrogen-treated, and tamoxifen-treated animals were included in this analysis. We have also reported a second FGF-4-transfected MCF-7 cell line, MKS-13, which produces tamoxifen-resistant tumors and which is growth inhibited by estrogen treatment of tumor-bearing mice 10 (not shown). As these lines produce approximately equivalent or smaller amounts of mRNA for FGF-4 than the MKL-F cell line the tumors of which are not stimulated by tamoxifen 16 (not shown), the opposite responses to estrogen and tamoxifen in these clonal cell lines are probably not due to differences in FGF-4 production.
As mentioned, we wish to study molecular mechanisms of tumor-induced angiogenesis produced by the different transfected angiogenic factors. Therefore, the data of others showing that VEGF/VPF expression has been increased by virtue of FGF-4 transfection 23,24 or that FGF-2 can up-regulate VEGF/VPF expression in endothelial cells 25 were disturbing because they implied that the phenotypic changes of FGF transfections were due to induced VEGF/VPF expression. However, we do not find VEGF/VPF expression increased in any of our FGF transfectants (Figure 5) ▶ or in tumors produced by them (not shown). Because we observe necrosis in very few of our tumors after the first few days after tumor cell injection, there is probably not significant hypoxia that might induce VEGF/VPF expression. In addition, the growth patterns and patterns of neovascularization of the FGF- and VEGF/VPF-transfected cells are different (Tables 1 and 2 ▶ ▶ ; Figures 2 and 3 ▶ ▶ ), implying that the blood vessels are responding to different factors. Collectively, these data argue against substantial differences in VEGF/VPF expression in tumors produced by the FGF-transfected lines when compared with the parental cell tumors as being responsible for the estrogen-independent, tamoxifen-resistant phenotype of the FGF transfectants.
As others have reported anti-angiogenic activity of tamoxifen, 38-41 our findings in tamoxifen-treated animals are of interest. The patterns of increased edge-associated and intratumor vessels observed in the tumors produced by the transfected cell lines when compared with the parental cell tumors could be explained by hypothesizing that in this tumor system, there is no direct negative effect of tamoxifen on endothelial cells or that the transfected angiogenic factors can overcome that negative effect. In any case, we find abundant vascularization of tumors produced by transfected cells under conditions of tamoxifen treatment. Additionally, our collective data 10,12,13,16 (including the present study) imply that tamoxifen resistance in human breast cancer may come about because of increased angiogenic ability of the tumor.
The purpose of our measurements of BrdU and TUNEL labeling indices as in situ predictors of future growth was only to enable us to identify actively growing tumors for future study. However, it is of interest that others have also found increased TUNEL staining under angiogenic conditions, 42 as we showed with the clone 18 and MV165-14 cells (Figure 6, G and H) ▶ . Thus, the failure of TUNEL staining to qualify as a good predictor of future growth is probably due to the multifactorial nature of both growth and apoptosis, as well as the complexity of the apoptotic process and the inability of one test to identify all apoptotic cells.
The function of tumor-associated blood vessels is presumably to provide oxygen and nutrients to the tumor cells to support their metabolism and proliferation. It could be hypothesized that those tumors cells near blood vessels would be the ones proliferating most rapidly or that tumors with the highest microvessel density would have the highest rate of growth. However, correlation of microvessel density with tumor cell proliferation has been difficult to achieve. 43,44 Some studies have shown a correlation between tumor cell proliferation and position at the edge of a tumor or near a blood vessel, 45-48 although others have not. 44,49 We do observe areas of high and low labeling in our BrdU-labeled tumors, but highly labeled areas of tumor cells are not reliably associated with the tumor’s edge or with tumor cords surrounding intratumor blood vessels (not shown). It may be that intratumor vessels represent previously edge-associated vessels that have become surrounded by tumor and that their abundance is sufficient to support tumor cell proliferation in the tumor’s center. This would imply that intratumor vessels represent stable vessels. Indeed, BrdU labeling in endothelial cells has been shown to be more frequent at edges of tumors than at their centers, 44,46 supporting the idea that neoangiogenesis occurs first at the edge of the tumor. Although we have performed double-label immunohistochemistry for BrdU incorporation and PECAM-1 in our tumor sections, we observe a very low incidence of BrdU-positive endothelial cells (not shown), making such analyses in our tumors technically difficult. Thus, the processes that result in a high abundance of edge-associated or intratumor vessels in our model and their purpose in promoting the tumor’s growth are not clear. These questions will form the basis for further study.
The dilated, irregularly shaped, ectatic vessels we observed in all tumors have a morphology similar to that of regressing vessels associated with healing wounds. Thus, it is possible that the initial neovascularization observed in all our tumors and subsequent appearance of these morphologically distinct vessels are at least partially due to the initial acute inflammatory reaction and represent an attempt by the host to heal the wound of injection. A role for the immune system in the process of tumor regression is not ruled out, either by our inability to find a difference between inflammatory infiltrates in growth-stimulated versus growth-inhibited tumors or by the absence of ICAM-1 expression in tumor-associated vessels. Studies that examined natural killer cell activity in lymphocytes isolated from the tumor or assessed the production of various inflammatory cytokines within the tumor might be more enlightening. However, the contribution of the immune system to tumor regression in immunodeficient mice may be quite different from that in immunocompetent humans, making such studies of doubtful relevance to human breast cancer.
In conclusion, this study using a tumor system of FGF- or VEGF/VPF-transfected MCF-7 cells in ovariectomized or hormonally treated nude mice has provided morphological and topographic evidence for effective patterns of neovascularization that occur in actively growing tumors, regardless of the hormonal treatment or angiogenic growth factor. This evidence provides support for the dogma that the ability to stimulate a functionally effective network of neovessels is an extremely important attribute of a tumor and validates this xenograft model as a vehicle for studies of tumor-induced angiogenesis in breast cancer. Our identification of edge-associated and intratumor vessels as being associated with actively growing tumors paves the way for future studies that address gene expression in the endothelial cells that compose these particular neovessels. These future studies may in turn shed light on the mechanisms by which effective tumor neovascularization occurs in the presence of various forms of hormonal therapy in human breast cancer.
Acknowledgments
We are indebted to Elisabetta Dejana, Rolande Berthier, and Annunciata Vecchi for initial supplies of the rat monoclonal antibody to PECAM. Rachel Hannum, Justina Ju, and Grace Chu provided technical assistance. We thank Luyuan Li for critical reading of this manuscript. Animal protocols for this work were approved by the Georgetown University Animal Care and Use Committee.
Footnotes
Address reprint requests to Dr. Sandra W. McLeskey, E304 Research Building, Lombardi Cancer Center, Georgetown University Medical Center, Washington, DC 20007. E-mail: mcleskes@gunet.georgetown.edu.
Supported by American Cancer Society starter grant IRG-93 (S. W. McLeskey), NIH grant R29 CA66154 (S. W. McLeskey), a Career Development Award DAMD17-94-4173 from the U.S. Army Breast Cancer Program (S. W. McLeskey), and NIH grant R01 CA50376 (F. G. Kern). Animal work and image analysis were done in the Lombardi Center Animal Research Resource and the Microscopy and Imaging Shared Resource, respectively, supported by NCI Center grant P30 CA51008.
References
- 1.Gasparini G: Biological and clinical role of angiogenesis in breast cancer. Breast Cancer Res Treat 1995, 36:103-107 [DOI] [PubMed] [Google Scholar]
- 2.Baillie CT, Winslet MC, Bradley NJ: Tumour vasculature: a potential therapeutic target. Br J Cancer 1995, 72:257-267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speir E, Sasse J, Shrivastav S, Casscells W: Culture-induced increase in acidic and basic fibroblast growth factor activities and their association with the nuclei of vascular endothelial and smooth muscle cells. J Cell Physiol 1991, 147:362-373 [DOI] [PubMed] [Google Scholar]
- 4.Auerbach R, Auerbach W, Polakowski I: Assays for angiogenesis: a review. Pharmacol Ther 1991, 51:1-11 [DOI] [PubMed] [Google Scholar]
- 5.Liaw L, Schwartz SM: Comparison of gene expression in bovine aortic endothelium in vivo versus in vitro. Arterioscler Thromb 1993, 13:985-993 [DOI] [PubMed] [Google Scholar]
- 6.Craig LE, Spelman JP, Strandberg JD, Zink CM: Endothelial cells from diverse tissues exhibit differences in growth and morphology. Microvasc Res 1998, 55:65-76 [DOI] [PubMed] [Google Scholar]
- 7.McCarthy SA, Kuzu I, Gatter KC, Bicknell R: Heterogeneity of the endothelial cell and its role in organ preference of tumour metastasis. Trends Pharmacol Sci 1991, 12:462-467 [DOI] [PubMed] [Google Scholar]
- 8.Yuan F, Leunig M, Berk DA, Jain RK: Microvascular permeability of albumin, vascular surface area, and vascular volume measured in human adenocarcinoma LS174T using dorsal chamber in SCID mice. Microvasc Res 1993, 45:269-289 [DOI] [PubMed] [Google Scholar]
- 9.Martin GR, Jain RK: Fluorescence ratio imaging measurement of pH gradients: calibration and application in normal and tumor tissues. Microvasc Res 1993, 46:216-230 [DOI] [PubMed] [Google Scholar]
- 10.McLeskey SW, Kurebayashi J, Honig SF, Zwiebel J, Lippman ME, Dickson RB, Kern FG: Fibroblast growth factor 4 transfection of MCF-7 cells produces cell lines that are tumorigenic and metastatic in ovariectomized or tamoxifen-treated athymic nude mice. Cancer Res 1993, 53:2168-2177 [PubMed] [Google Scholar]
- 11.Kurebayashi J, McLeskey SW, Johnson MD, Lippman ME, Dickson RB, Kern FG: Quantitative demonstration of spontaneous metastasis by MCF-7 human breast cancer cells cotransfected with fibroblast growth factor 4 and LacZ. Cancer Res 1993, 53:2178-2187 [PubMed] [Google Scholar]
- 12.Zhang L, Kharbanda S, Chen D, Bullocks J, Miller DL, Ding IYF, Hanfelt J, McLeskey SW, Kern FG: MCF-7 breast carcinoma cells overexpressing FGF-1 form vascularized metastatic tumors in ovariectomized or tamoxifen-treated nude mice. Oncogene 1997, 15:2093-2108 [DOI] [PubMed] [Google Scholar]
- 13.Bullocks J, Zhang L, Ding IYF, McLeskey SW, Tobias CA, Miller DL, Kern FG: Overexpression of vascular endothelial cell growth factor (VEGF) in MCF-7 breast carcinoma cells facilitates growth in tamoxifen-treated nude mice and tumor cell dissemination. Proc Am Assoc Cancer Res 1997, 38:3521 [Google Scholar]
- 14.Weidner N: Current pathologic methods for measuring intratumoral microvessel density within breast carcinoma and other solid tumors. Breast Cancer Res Treat 1995, 36:169-180 [DOI] [PubMed] [Google Scholar]
- 15.McLeskey SW, Zhang L, Kharbanda S, Kurebayashi J, Lippman ME, Dickson RB, Kern FG: Fibroblast growth factor overexpressing breast carcinoma cells as models of angiogenesis and metastasis. Breast Cancer Res Treat 1996, 39:103-117 [DOI] [PubMed] [Google Scholar]
- 16.McLeskey SW, Zhang L, El-Ashry D, Trock BJ, Lopez CA, Kharbanda S, Tobias CA, Lorant LA, Hannum RS, Dickson RB, Kern FG: Tamoxifen-resistant fibroblast growth factor-transfected MCF-7 cells are cross-resistant in vivo to the antiestrogen, ICI 182,780, and two aromatase inhibitors. Clin Cancer Res 1998, 4:697-711 [PubMed] [Google Scholar]
- 17.Vecchi A, Garlanda C, Lampugnani MG, Resnati M, Matteucci C, Stoppacciaro A, Schnurch H, Risau W, Ruco L, Mantovani A, Dejana E: Monoclonal antibodies specific for endothelial cells of mouse blood vessels: their application in the identification of adult and embryonic endothelium. Eur J Cell Biol 1994, 63:247-254 [PubMed] [Google Scholar]
- 18.Carroll RJ, Ruppert D, Stefanski LA: Measurement Error in Nonlinear Models. 1995. Chapman and Hall, London
- 19.Cheng C-L, Van Ness JW: On estimating linear relationships when both variables are subject to errors. J Royal Statistical Soc 1994, B56:167–183
- 20.SAS Institute: SAS. Release 6.07. Cary, NC, SAS Institute, 1998
- 21.Melder RJ, Koenig GC, Witwer BP, Safabakhsh N, Munn LL, Jain RK: During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nature Med 1996, 2:992-997 [DOI] [PubMed] [Google Scholar]
- 22.Griffioen AW, Damen CA, Martinotti S, Blijham GH, Groenewegen G: Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res 1996, 56:1111-1117 [PubMed] [Google Scholar]
- 23.Deroanne CF, Hajitou A, Calberg-Bacq C-M, Nusgens BV, Lapière CM: Angiogenesis by fibroblast growth factor 4 is mediated through an autocrine up-regulation of vascular endothelial growth factor expression. Cancer Res 1997, 57:5590-5597 [PubMed] [Google Scholar]
- 24.Hajitou A, Deroanne CF, Noël A, Colette J, Nusgens BV, Foidart JM, Calberg-Bacq C-M: FGF-3 increases tumorigenicity in MCF7 breast cancer cells but FGF-4 effect is more pronounced and associated with VEGF upregulation. Proc Am Assoc Cancer Res 1998, 39:34 [Google Scholar]
- 25.Seghezzi G, Patel S, Ren CJ, Gualandris A, Pintucci G, Robbins ES, Shapiro RL, Galloway AC, Rifkin DB, Mignatti P: Fibroblast growth factor-2 (FGF-2) induces vascular endothelial growth factor (VEGF) expression in the endothelial cells of forming capillaries: an autocrine mechanism contributing to angiogenesis. J Cell Biol 1998, 141:1659-1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McLeskey SW, Zhang L, Trock BJ, Kharbanda S, Liu Y, Gottardis MM, Lippman ME, Kern FG: Effects of AGM-1470 and pentosan polysulphate on tumorigenicity and metastasis of FGF-transfected MCF-7 cells. Br J Cancer 1996, 73:1053-1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weidner N, Moore DH, II, Ljung B-M, Waldman FM, Goodson WHI, Mayall B, Chew K, Smith HS: Correlation of bromodeoxyuridine (BRDU) labeling of breast carcinoma cells with mitotic figure content and tumor grade. Am J Surg Pathol 1993, 17:987-994 [DOI] [PubMed] [Google Scholar]
- 28.Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H: Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 1994, 71:219-225 [PubMed] [Google Scholar]
- 29.Mundle SD, Raza A: The two in situ techniques do not differentiate between apoptosis and necrosis but rather reveal distinct patterns of DNA fragmentation in apoptosis. Lab Invest 1995, 72:611-612 [PubMed] [Google Scholar]
- 30.Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H: The two in situ techniques do not differentiate between apoptosis and necrosis but rather reveal distinct patterns of DNA fragmentation in apoptosis: reply. Lab Invest 1995, 72:612-613 [Google Scholar]
- 31.Mundle S, Iftikhar A, Shetty V, Dameron S, Wright-Quinones V, Marcus B, Loew J, Gregory S, Raza A: Novel in situ double labeling for simultaneous detection of proliferation and apoptosis. J Histochem Cytochem 1994, 42:1533-1537 [DOI] [PubMed] [Google Scholar]
- 32.Raza A, Mundle S, Iftikhar A, Gregory S, Marcus B, Khan Z, Alvi S, Shetty V, Dameron S, Wright V, Adler S, Loew JM, Shott S, Ali SN, Preisler H: Simultaneous assessment of cell kinetics and programmed cell death in bone marrow biopsies of myelodysplastics reveals extensive apoptosis as the probable basis for ineffective hematopoiesis. Am J Hematol 1995, 48:143-154 [DOI] [PubMed] [Google Scholar]
- 33.Dickson RB, Bates SE, Valverius E, Knabbe C, Salomon D, Huff KK, Bronzert D, Walker-Jones D, Freter C, Favoni R, Yee D, Zugmaier G, Ennis B, Clarke R, Kern F, Rosen N, Lippman ME: Estrogen and antiestrogen regulation of mitogenic growth factors in human breast cancer cells. Prog Cancer Res Ther 1996, 35:217-222 [Google Scholar]
- 34.Morales DE, McGowan KA, Grant DS, Maheshwari S, Bhartiya D, Cid MC, Kleinman HK, Schnaper W: Estrogen promotes angiogenic activity in human umbilical vein endothelial cells in vitro and in a murine model. Circulation 1995, 91:755-763 [DOI] [PubMed] [Google Scholar]
- 35.Brandi ML, Crescioli C, Tanini A, Frediani U, Agnusdei D, Gennari C: Bone endothelial cells as estrogen targets. Calcif Tissue Int 1993, 53:312-317 [DOI] [PubMed] [Google Scholar]
- 36.Cid MC, Kleinman HK, Grant DS, Schnaper HW, Fauci AS, Hoffman GS: Estradiol enhances leukocyte binding to tumor necrosis factor (TNF)-stimulated endothelial cells via an increase in TNF-induced adhesion molecules E-selectin, intercellular adhesion molecule type 1, and vascular cell adhesion molecule type 1. J Clin Invest 1994, 93:17-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glaser-Caulin T, Watson CA, Pardi R, Bender JR: Effects of 17β-estradiol on cytokine-induced endothelial cell adhesion molecule expression. J Clin Invest 1996, 98:36-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gagliardi A, Collins DC: Inhibition of angiogenesis by antiestrogens. Cancer Res 1993, 53:533-535 [PubMed] [Google Scholar]
- 39.Gagliardi A, Hennig B, Collins DC: Antiestrogens inhibit endothelial cell growth stimulated by angiogenic growth factors. Anticancer Res 1996, 16:1101-1106 [PubMed] [Google Scholar]
- 40.Venkov CD, Rankin AB, Vaughan DE: Identification of authentic estrogen receptor in cultured endothelial cells: a potential mechanism for steroid hormone regulation of endothelial function. Circulation 1996, 94:727-733 [DOI] [PubMed] [Google Scholar]
- 41.Lindner DJ, Borden EC: Effects of tamoxifen and interferon-β or the combination on tumor-induced angiogenesis. Int J Cancer 1997, 71:456-461 [DOI] [PubMed] [Google Scholar]
- 42.Naik P, Karrim J, Hanahan D: The rise and fall of apoptosis during multistage tumorigenesis: down-modulation contributes to tumor progression from angiogeneic progenitors. Genes Dev 1996, 10:2105-2116 [DOI] [PubMed] [Google Scholar]
- 43.Rofstad EK: Growth and vascular structure of human melanoma xenografts. Cell Tissue Kinet 1984, 17:91-101 [DOI] [PubMed] [Google Scholar]
- 44.Fox SB, Gatter KC, Bicknell R, Going JJ, Stanton P, Cooke TG, Harris AL: Relationship of endothelial cell proliferation to tumor vascularity in human breast cancer. Cancer Res 1993, 53:4161-4163 [PubMed] [Google Scholar]
- 45.Porschen R, Classen S, Piontek M, Borchard F: Vascularization of carcinomas of the esophagus and its correlation with tumor proliferation. Cancer Res 1994, 54:587-591 [PubMed] [Google Scholar]
- 46.Brien SE, Zagzag D, Brem S: Rapid in situ cellular kinetics of intracerebral tumor angiogenesis using a monoclonal antibody to bromodeoxyuridine. Neurosurgery 1989, 25:715-719 [DOI] [PubMed] [Google Scholar]
- 47.Yoshii Y, Sugiyama K: Intercapillary distance in the proliferating area of human glioma. Cancer Res 1988, 48:2938-2941 [PubMed] [Google Scholar]
- 48.Tannock IF: Population kinetics of carcinoma cells, capillary endothelial cells, and fibroblasts in a transplanted mouse mammary tumor. Cancer Res 1970, 30:2470-2476 [PubMed] [Google Scholar]
- 49.Bennett MH, Wilson GD, Dische S, Saunders MI, Martindale CA, Robinson BM, O’Halloran AE, Leslie MD, Laing JHE: Tumour proliferation assessed by combined histological and flow cytometric analysis: implications for therapy in squamous cell carcinoma. Br J Cancer 1992, 65:870-878 [DOI] [PMC free article] [PubMed] [Google Scholar]