Abstract
The factor(s) responsible for the reduced B cell number and increased T cell infiltrate in T-cell-rich large-B-cell lymphomas (TCRBCLs) have not been well characterized. We studied 18 TCRBCLs and 12 diffuse large-B-cell lymphomas (DLBCLs) to compare the 1) predominant T cell subpopulation(s), 2) expression of cytotoxic granule proteins (TIA-1 and granzyme B), 3) level of tumor cell apoptosis (Apoptag system, Oncor, Gaithersburg, MD), and 4) expression of Ki-67 (Mib-1) and apoptosis-related proteins (fas (CD95), bcl-2, and p53). T cells in TCRBCLs and DLBCLs were predominantly CD8+ T cells expressing αβ T-cell receptors and TIA-1 (16 of 18 TCRBCLs with >50% TIA-1+ small lymphocytes) but lacking granzyme B (16 of 18 TCRBCLs with <25% granzyme B+ small lymphocytes). Scattered apoptotic tumor cells (confirmed with CD20 co-labeling) were present in 15 of 18 TCRBCLs, with 14 of 15 cases having <10% apoptotic cells. No apoptotic cells were seen in 12 of 12 DLBCLs. In 16 of 16 immunoreactive TCRBCLs, <25% tumor cells were bcl-2+, whereas 6 of 12 DLBCLs had >50% bcl-2+ tumor cells. CD95 (fas) expression was also lower, with 3 of 18 (16.7%) TCRBCLs versus 4 of 12 (33%) DLBCLs having >25% CD95+ tumor cells. TCRBCLs and DLBCLs had similar levels of p53 and Ki-67 (Mib-1) expression. Thus, T cells in TCRBCLs are non-activated cytotoxic T lymphocytes (TIA-1+, granzyme B−). Tumor cell apoptosis (perhaps cytotoxic T cell mediated) may partly account for the decreased number of large (neoplastic) B cells in TCRBCLs, but other factors (ie, decreased bcl-2 expression) may also be needed.
T-cell-rich large-B-cell lymphoma (TCRBCL) is a term used to describe a group of diffuse large-B-cell lymphomas with a prominent infiltrate of small T lymphocytes. 1-6 The percentage of T cells needed to classify a lymphoma as a TCRBCL is somewhat controversial, but most authors would accept tumors as TCRBCL if at least 65% to 90% of the lymphoid infiltrate is T cells, with the remainder being large B cells. 7 The B cells have been shown to be a clonal, presumably neoplastic cell population by demonstration of light chain restriction and/or by the presence of immunoglobulin gene rearrangements. 2
Despite the abundance of T cells, most studies indicate the clinical behavior of TCRBCLs is not significantly different from other diffuse large-B-cell lymphomas (DLBCLs), which typically have few tumor-infiltrating lymphocytes (TILs). Previous studies by Chittal et al 5 and Delabie et al 6 have indicated that some TCRBCLs may follow a more aggressive clinical course. These studies examined a total of nine and six patients, respectively. Of the combined 15 patients, 6 followed a very aggressive clinical course with short survival (≤18 months). Of these six patients with an aggressive clinical course, it is interesting to note that four had been originally diagnosed and treated as Hodgkin’s disease rather than non-Hodgkin’s lymphoma. Therefore, the poor results in some of these patients may have been related to inappropriate initial therapy rather than due to an inherently more aggressive biological behavior. More recent studies by Greer et al 8 and Rodriguez et al 9 have examined larger series of 44 and 23 patients, respectively, and have shown no significant survival difference between TCRBCLs and DLBCLs. Finally, data examining bone marrow involvement in TCRBCLs 10 have shown no significant difference in overall 4-year survival in patients with TCRBCL involving bone marrow compared with a group of control patients with bone marrow involvement by histologically concordant DLBCL when treated with curative intent.
The few TCRBCLs analyzed by flow cytometry or frozen-section immunohistochemistry have shown a predominance of CD4+ T cells with a CD4:CD8 ratio compatible with a reactive infiltrate. 1,2 At least one case report with flow cytometric immunophenotyping has suggested that the predominant T cell subpopulation is CD8+. 11 However, no previous studies have fully characterized the T cells based on functional and T cell receptor (TCR) framework antigen expression. Recently, a number of antibodies have been developed to allow CD4 and CD8 subtyping of T cells in fixed, paraffin-embedded tissue sections to assess the type of TCR expressed and to evaluate the presence of cytotoxic-granule-associated proteins such as TIA-1 and granzyme B. 12-14
Furthermore, the factor(s) responsible for the increased number of T cells and/or the reduced number of neoplastic B cells in TCRBCLs have not been extensively studied. Previous studies from our group have suggested that interleukin (IL)-4 may play a role in the proliferation of T cells and/or the suppression of B cell growth. 15 However, the role of other proliferation-associated and/or apoptosis-associated proteins has not been investigated.
We therefore undertook the present study to compare TCRBCLs and DLBCLs to 1) define the predominant T cell phenotype in TCRBCLs in paraffin-embedded, fixed tissue sections, 2) to assess the expression of cytotoxic T lymphocyte (CTL)-associated proteins, namely TIA-1 and granzyme B, in the T cell infiltrate of TCRBCL and DLBCL, 3) to examine tissue sections for evidence of apoptosis in both TCRBCL and DLBCL using a commercially available terminal deoxynucleotidyl transferase (TdT) end-labeling technique (Apoptag, Oncor, Gaithersburg, MD), and 4) to examine and compare the expression of the proliferation-associated marker Ki-67 (Mib-1) and the apoptosis-associated proteins fas (CD95), bcl-2, and p53 in TCRBCL versus DLBCL.
Materials and Methods
Selection of Cases
Eighteen TCRBCLs and twelve DLBCLs were evaluated by paraffin immunoperoxidase staining for the antigens described below and for the presence of apoptotic tumor cells using the Apoptag in situ detection kit (Oncor). The clinicopathological features of the cases chosen for study have been previously published. 2,15 As defined in previous publications, 2,8,15 lymphomas were classified as TCRBCLs if the majority (ie, more than 50%) of cells were small T cells by paraffin immunoperoxidase studies for CD3 and/or CD45RO (UCHL-1). One TCRBCL (case 17 in Ref. 2 ) and three DLBCLs (see Ref. 15 ) described previously could not be evaluated in the present study because of insufficient tissue.
Paraffin Immunoperoxidase Studies
Paraffin sections (4 μm) from B5-fixed tissues were stained by the streptavidin-biotin complex method and horseradish peroxidase (HRP) reacted with 2′,5′-diaminobenzidine (DAB) according to manufacturer’s instructions or previously published modifications 12-13,16 for expression of the following antigens: CD20 (L26), CD8, CD3 (polyclonal), p53, and bcl-2, all from Dako, Carpinteria, CA; CD56 (123C3) from Zymed Corp., South San Francisco, CA; CD4 from Vector Laboratories, Burlingame, CA; TCR-αβ (βF-1) and TCR-γδ (TCRδ1), both from Endogen, Woburn, MA; Ki-67 (Mib-1) from Immunotech, Westbrook, ME; and TIA-1 from Coulter Immunology, Hialeah, FL.
In addition, immunoperoxidase staining for CD95 (fas) expression was performed using a rabbit polyclonal antiserum (Santa Cruz Biotechnology, Santa Cruz, CA), with microwave epitope retrieval in 10 mmol/L sodium citrate, pH 6.0 (two 5-minute applications). Paraffin-embedded, B5-fixed sections (4 μm) were deparaffinized in 2 g% iodine in xylene (10 minutes at 25°C), followed by three washes (five minutes each at room temperature) in xylene alone. Slides were rehydrated by sequential washes (5 minutes each) in 100% ethanol (two times), 70% ethanol, and phosphate-buffered saline (PBS; 50 mmol/L sodium phosphate, 200 mmol/L sodium chloride, pH 7.4). Endogenous peroxidase activity was inactivated by incubating slides in 3% hydrogen peroxide in PBS (5 minutes at 25°C), followed by three washes in PBS (5 minutes each at 25°C). After blocking nonspecific antibody-binding sites (Protein Blocker, Research Genetics, Huntsville, AL) for 5 minutes at 25°C, slides were incubated with anti-CD95 at a 1:50 dilution in PBS for 60 minutes at 37°C and washed three times in PBS. Staining was then detected using biotinylated goat anti-rabbit antibody (Dako; 60 minutes at 37°C, followed by three washes in PBS), streptavidin-conjugated horseradish peroxidase (HRP; Research Genetics; 30 minutes at 25°C, followed by three washes in PBS), and DAB (Stable DAB, Research Genetics; two applications of 5 minutes each at 25°C). Sections were then washed three times in distilled water, stained with hematoxylin (Autohematoxylin, Research Genetics), and mounted onto coverglasses using standard techniques.
Immunoperoxidase staining for human granzyme B expression (1:20 dilution, clone GrB-7; Monosan, Uden, The Netherlands) was performed by the streptavidin-biotin complex method using HRP with DAB on B5-fixed paraffin-embedded sections (4 μm) pretreated by microwave epitope retrieval in 100 mmol/L sodium citrate, according to the manufacturer’s directions.
In all immunostaining procedures, the adequacy of staining was verified with appropriate positive controls, including tonsil (CD20, CD3, CD4, CD8, βF1, TIA-1, and Mib-1), hepatosplenic γδ T cell lymphoma (granzyme B, TCRδ1), retinoblastoma (CD56), follicular lymphoma (bcl-2), breast carcinoma (p53), and reactive lymph node (CD95).
Apoptosis Assay
Mercury salts and paraffin were removed from sections (4 μm) of B5-fixed tissues by soaking in 2 g% iodine in xylene for 10 minutes followed by three washes (5 minutes each) in xylene only. Sections were then rehydrated and processed using the Apoptag in situ detection kit (Oncor) according to the manufacturer’s instructions, except that tissues were counterstained with Gill’s hematoxylin (Fisher Scientific, Fair Lawn, NJ). Positive control sections (from a case of HIV-related lymphadenopathy) were run with each experiment. TCRBCL cases were also double labeled for CD20 expression (L26) followed by biotinylated goat anti-mouse antibody (Dako) and a streptavidin-biotin detection system with alkaline-phosphatase and fast red substrate (Research Genetics) according to the manufacturer’s instructions.
Evaluation of Staining
Antibody staining was evaluated independently and jointly by at least two pathologists (R.E. Felgar, K.R. Steward, and/or W.R. Macon). Staining of the small lymphocytes was scored as follows: 0, no staining; 1+, 1% to 25% small lymphocytes positive; 2+, 26% to 50% small lymphocytes positive; 3+, 51% to 75% small lymphocytes positive; 4+, 76% to 100% small lymphocytes positive. Staining of the large (neoplastic) lymphocytes was also evaluated using the following scale: 0, no staining; 1+, 1% to 10% large cells positive; 2+, 11% to 25% large cells positive; 3+, 26% to 50% large cells positive; 4+, >50% large cells positive.
Results
Patient Populations
The general demographic features of the patients from whom the TCRBCLs and DLBCLs were obtained for study were similar. The TCRBCL group consisted of 10 males and 8 females with a mean age of 52.2 years (median, 53.5 years); the DLBCL group consisted of 7 males and 5 females with a mean age of 58.7 years (median, 56.5 years). The age range of the TCRBCL group (18 to 92 years), however, was much broader than that seen in the DLBCL group (46 to 74 years).
Immunophenotype of Small Lymphocytes in TCRBCLs and DLBCL
Immunophenotyping data for the small lymphocytes in the TCRBCL group are summarized in Table 1 ▶ . In 16 of 18 TCRBCLs, more than 50% of the TILs were TIA-1+ (score of 3+ to 4+) with most of them also expressing CD8 and αβ TCRs (βF1+) (see Table 1 ▶ and Figure 1 ▶ ). One case (number 4 in Table 1 ▶ ) had a predominance of CD4+ and βF1+ TILs that were also TIA-1+. Staining for granzyme B showed a few cells staining in all cases but a much lower number staining in comparison with TIA-1 (16 of 18 cases with 25% or fewer granzyme B+ cells), suggesting a non-activated functional status. In most cases, few lymphocytes stained for CD4, CD56, or γδ TCRs (TCRδ1+). Staining of the DLBCLs showed only a few scattered small lymphocytes with a lower percentage of TIA-1+ cells (9 of 12 cases with <50% small lymphocytes TIA-1+) than in TCRBCLs; the percentage of small lymphocytes in DLBCLs expressing granzyme B was also generally lower in comparison with TIA-1 expression, with 8 of 12 cases showing <25% granzyme B+ cells (see Table 2 ▶ ). In general, most of the TILs in DLBCLs were also CD8+ and βF1+ (see Table 2 ▶ and Figure 2 ▶ ). Because most lymphocytes were CD8+ and βF1+, staining for CD56 and TCRδ1 was not evaluated in the DLBCLs.
Table 1.
Immunophenotype of Small T Cells in T-Cell-Rich Large-B-Cell Lymphomas
| Case | Age (Years) | Sex | TIA-1 | GrB | CD4 | CD8 | CD56 | βF1 | TCRδ1 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 55 | M | 3+ | 1+ | 0 | 3+ | 1+ | 3+ | 1+ |
| 2 | 66 | M | 3+ | 1+ | 0 | 2+ | 1+ | 2+ | 1+ |
| 3 | 42 | F | 3+ | 1+ | 0 | 2–3+ | 1+ | 1+ | 1+ |
| 4 | 18 | F | 3+ | 1+ | 4+ | 1+ | 1+ | 4+ | 1+ |
| 5 | 68 | F | 3+ | 1+ | 0 | 1+ | 1+ | 2+ | 1+ |
| 6 | 74 | M | 3+ | 1+ | 0 | 4+ | 1+ | 2–3+ | 1+ |
| 7 | 44 | F | 3+ | 1+ | 1+ | 2–3+ | 1+ | 3+ | 1+ |
| 8 | 47 | M | 2+ | 1+ | 1+ | 2+ | 1+ | 3+ | 1+ |
| 9 | 69 | M | 3+ | 1+ | 0 | 2–3+ | 1+ | 3+ | 1+ |
| 10 | 56 | F | 4+ | 2+ | 0 | 4+ | 1+ | 1+ | 1+ |
| 11 | 67 | F | 3+ | 1+ | 1+ | 1+ | 1+ | 4+ | 1+ |
| 12 | 57 | M | 3+ | 1+ | 2+ | 3+ | 1+ | 4+ | 1+ |
| 13 | 52 | M | 4+ | 1+ | 2+ | 3+ | 1+ | 4+ | 1+ |
| 14 | 36 | M | 3+ | 1+ | 1+ | 4+ | 1+ | 3+ | 1+ |
| 15 | 36 | F | 4+ | 1+ | 0 | 2+ | 1+ | 4+ | 1+ |
| 16 | 18 | M | 1+ | 1+ | 1+ | 1+ | 1+ | 2–3+ | 1+ |
| 17 | 92 | F | 4+ | 1+ | 1+ | 3+ | 1+ | 2–3+ | 1+ |
| 18 | 43 | M | 4+ | 3+ | 1–2+ | 4+ | 1+ | 3+ | 1+ |
Patients 10 and 12 represent relapsed lymphomas with a TCRBCL pattern, occurring 8 and 7 years, respectively, after an initial diagnosis of DLBCL. (See also Tables 2 and 4 ▶ ▶ footnotes.) GrB, granzyme B; M, male; F, female. Results are shown based on the following scoring system: 0, no small cells marking; 1+, 1% to 25% small cells marking; 2+, 26% to 50% small cells marking; 3+, 51% to 75% small cells marking; 4+, 76% to 100% small cells marking.
Figure 1.
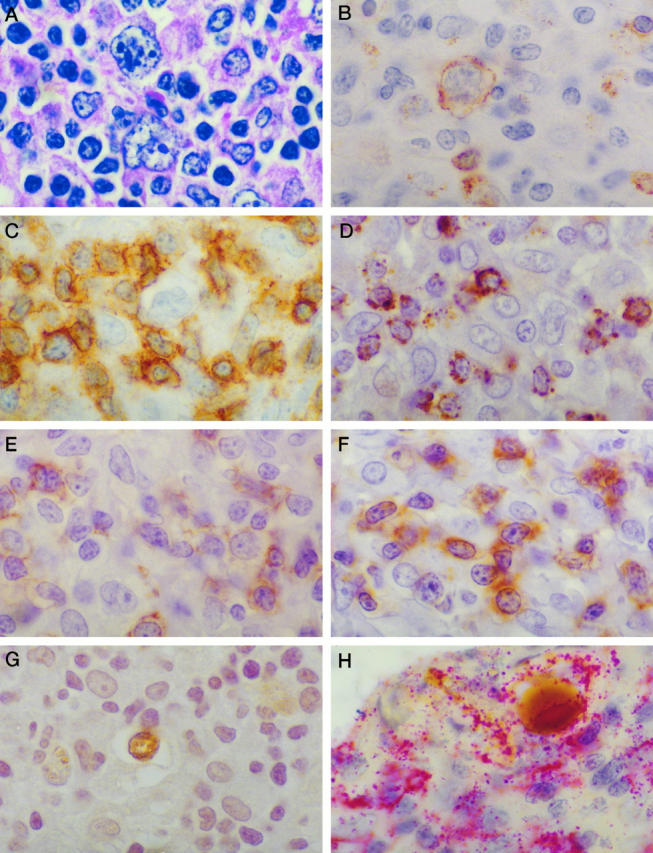
Staining pattern in a typical case of TCRBCL (case 15 in Tables 1 and 3 ▶ ▶ ). A: Typical H&E appearance. B to F: Immunostains for CD20 (B; L26), CD3 (C), TIA-1 (D), CD8 (E), and βF1 (F) demonstrating that most of the TILs are CD8+ and βF1+ T cells. G: Labeling of scattered large apoptotic cells with the Apoptag system that are also CD20+ on co-labeling studies. H: L26 antibody with alkaline phosphatase and fast red substrate. Magnification (oil immersion), ×1000.
Table 2.
Immunophenotype of Small T Cells in Diffuse Large-B-Cell Lymphomas
| Case | Age (Years) | Sex | TIA-1 | GrB | CD4 | CD8 | βF1 |
|---|---|---|---|---|---|---|---|
| 1 | 62 | M | 2–3+ | 1+ | 0 | 2–3+ | NR |
| 2 | 74 | M | 3+ | 1+ | 0 | 3+ | 3+ |
| 3 | 46 | M | 2+ | 2+ | 0 | 2+ | 2+ |
| 4 | 73 | F | 2+ | 1–2+ | 0 | 3+ | 3+ |
| 5 | 58 | F | 2+ | 2+ | 1+ | 3+ | 3+ |
| 6 | 57 | M | 1+ | 1+ | 0–1+ | 3+ | 3+ |
| 7 | 56 | F | 3+ | 2–3+ | 0 | 3+ | 3+ |
| 8 | 66 | M | 1+ | 0 | 0 | 3+ | 3+ |
| 9 | 56 | M | 3+ | 1+ | 0 | 3+ | 3+ |
| 10 | 52 | M | 1+ | 0 | 0 | 1–2+ | 3+ |
| 11 | 50 | F | 2+ | 1+ | 0 | 2+ | 2–3+ |
| 12 | 54 | F | 1+ | 1+ | 0 | 2+ | 2+ |
Initial neoplasm in case number 6 was a DLBCL; patient had relapsed lymphoma 7 years later with a TCRBCL pattern (case 12 in Tables 1 and 3 ▶ ▶ ). The initial neoplasm in case 7 also was a DLBCL, with a relapse 8 years later with a TCRBCL pattern (case 10 in Tables 1 and 3 ▶ ▶ ). See Table 1 ▶ for scoring system. GrB, granzyme B; M, male; F, female.
Figure 2.
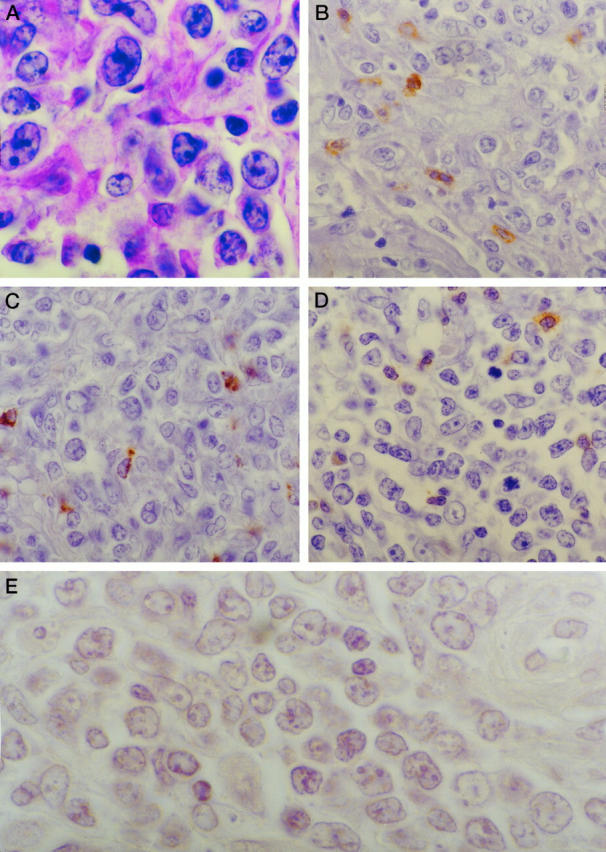
Staining pattern in a typical case of DLBCL (case 2 in Tables 2 and 4 ▶ ▶ ). A: Typical H&E appearance of large transformed lymphocytes. B: CD8 staining; C: TIA-1 staining; D: βF1 staining, showing that a few TILs are present that are also CD8+ and βF1+ T cells. E: No apoptotic tumor cells are detectable with the Apoptag assay system. Magnification (oil immersion), ×1000 (A and E) and ×600 (B to D).
Apoptosis Studies
TdT end-labeling studies using the Apoptag in situ detection kit highlighted the presence of apoptotic cells in 15 of 18 cases of TCRBCL (see Table 3 ▶ and Figure 1 ▶ ); double-labeling studies using L26 with an immunoalkaline phosphatase detection system showed that these cells were marking as CD20+ B cells (ie, consistent with the neoplastic cell population; see Figure 1 ▶ .) In general, the number of apoptotic cells in TCRBCLs was low, with 14 of 15 cases having <10% Apoptag-labeled cells. No apoptotic cells were seen in the DLBCL cases (Table 4 ▶ and Figure 2 ▶ ).
Table 3.
Immunophenotype of Large (Neoplastic) B Cells in T-Cell-Rich Large-B-Cell Lymphomas
| Case | Age (Years) | Sex | Apoptag | p53 | Bcl-2 | Mib-1 (Ki-67) | CD95 (fas) |
|---|---|---|---|---|---|---|---|
| 1 | 55 | M | 1+ | 0 | NR | 1+ | 3+ |
| 2 | 66 | M | 0 | 1+ | 1+ | 1+ | 3+ |
| 3 | 42 | F | 1+ | 1+ | 0 | 1+ | 2+ |
| 4 | 18 | F | 2+ | 3+ | 0 | 2–3+ | 1+ |
| 5 | 68 | F | 1+ | 1–2+ | 0 | 2+ | 1+ |
| 6 | 74 | M | 0 | 1+ | 1+ | 1+ | 1+ |
| 7 | 44 | F | 1+ | 3+ | 0 | 4+ | 3+ |
| 8 | 47 | M | 1–2+ | 1+ | 0 | 2+ | 1+ |
| 9 | 69 | M | 1+ | 0 | NR | 1+ | 1+ |
| 10 | 56 | F | 1+ | 0 | 0 | 1+ | 1+ |
| 11 | 67 | F | 0 | 1+ | 0 | 1+ | 1+ |
| 12 | 57 | M | 1+ | 3+ | 0 | 2+ | 1+ |
| 13 | 52 | M | 1+ | 0 | 1+ | 3+ | 1+ |
| 14 | 36 | M | 1+ | 2+ | 0 | 1+ | 1+ |
| 15 | 36 | F | 1+ | 1+ | 0 | 1+ | 1+ |
| 16 | 18 | M | 1+ | 1+ | 2+ | 4+ | 0 |
| 17 | 92 | F | 1+ | 1+ | 1+ | 1+ | 1+ |
| 18 | 43 | M | 1+ | 1+ | 1+ | 2+ | 1+ |
Patients 10 and 12 represent relapsed lymphomas with a TCRBCL pattern, occurring 8 and 7 years, respectively, after an initial diagnosis of DLBCL. (See also Tables 2 and 4 ▶ ▶ footnotes.) Results are shown based on the following scoring system: 0, no large cells marking; 1+, 1% to 10% large cells marking; 2+, 11% to 25% large cells marking; 3+, 26% to 50% large cells marking; 4+, >51% large cells marking. NR, tissue nonreactive with antibody (confirmed on repeat staining); M, male; F, female.
Table 4.
Immunophenotype of Large (Neoplastic) B Cells in Diffuse Large-B-Cell Lymphomas
| Case | Age (Years) | Sex | Apoptag | p53 | Bcl-2 | Mib-1 (Ki-67) | CD95 (fas) |
|---|---|---|---|---|---|---|---|
| 1 | 62 | M | 0 | 2+ | 4+ | 4+ | 0 |
| 2 | 74 | M | 0 | 1+ | 3+ | 2–3+ | 4+ |
| 3 | 46 | M | 0 | 1+ | 4+ | 1+ | 1+ |
| 4 | 73 | F | 0 | 4+ | 0 | 1+ | 3+ |
| 5 | 58 | F | 0 | 1+ | 4+ | 1+ | 0 |
| 6 | 57 | M | 0 | 2+ | 0 | 2+ | 4+ |
| 7 | 56 | F | 0 | 1+ | 1+ | 1+ | 0 |
| 8 | 66 | M | 0 | 2+ | 3+ | 3+ | 1+ |
| 9 | 56 | M | 0 | 1+ | 4+ | 2+ | 0 |
| 10 | 52 | M | 0 | 1+ | 4+ | 1+ | 0 |
| 11 | 50 | F | 0 | 4+ | 1+ | 2+ | 3+ |
| 12 | 54 | F | 0 | 3+ | 4+ | 2+ | 0 |
Initial neoplasm in case 6 was a DLBCL; patient had relapsed lymphoma 7 years later with a TCRBCL pattern (case 12 in Tables 1 and 3 ▶ ▶ ). The initial neoplasm in case 7 also was a DLBCL, with a relapse 8 years later with a TCRBCL pattern (case 10 in Tables 1 and 3 ▶ ▶ ). See Table 3 ▶ for scoring system. M, male; F, female.
Expression of Proliferation- or Apoptosis-Associated Antigens
Data regarding the expression of the proliferation-associated antigen Ki-67 (Mib-1 staining) and apoptosis-related antigens (p53, bcl-2, and CD95/fas) in the tumor (large B) cell population are summarized in Tables 3 and 4 ▶ ▶ .
TCRBCLs had a lower percentage of tumor cells expressing bcl-2 (16 of 16 immunoreactive cases had <25% bcl-2+ tumor cells) than DLBCLs (4 of 12 cases with <25% bcl-2+ tumor cells and 6 of 12 cases with >50% bcl-2+ tumor cells). CD95 (fas) expression was slightly lower in TCRBCLs, as 3 of 18 (16.7%) cases had >25% CD95+ tumor cells as compared with DLBCLs, which had 4 of 12 (33%) cases with >25% CD95+ tumor cells.
In general, there were no consistent differences in the percentage of tumor cells expressing Ki-67 (Mib-1+) or p53 between TCRBCLs and DLBCLs. In both TCRBCLs and DLBCLs, 83% of cases (15 of 18 TCRBCLs and 10 of 12 DLBCLs) stained positively for Mib-1 in <25% of tumor cells. There was also comparable p53 staining, as 15 of 18 (83%) TCRBCLs and 9 of 12 (75%) DLBCLs were positive in <25% of tumor cells.
Discussion
The present study was designed to address three basic issues: 1) to determine the phenotype of the T cell infiltrate in TCRBCL, with emphasis on the presence of cytotoxic-lymphocyte-associated markers, 2) to investigate the role of apoptosis, possibly CTL-mediated, in TCRBCL, and 3) to investigate possible mechanism(s) by which increased apoptosis or reduced cellular proliferation might account for the lower number of neoplastic large B cells in TCRBCL in comparison with DLBCL.
Previous flow cytometry studies of TCRBCLs, including previous analyses on material from five of the cases studied here, have suggested that the predominant T cell subpopulation is a CD4+ T cell. 1-2 Conversely, at least one case report using flow cytometric immunophenotyping has suggested that the predominant small lymphocyte may be a CD8+ T cell. 11 However, the number of cases studied in this manner is small, and such studies are hindered by the inability to directly correlate the flow cytometry data with morphology. In the present study, 8 of 18 TCRBCLs showed CD8 positivity in >50% of the small lymphocytes (score of 3+ or 4+; Table 1 ▶ ), and in an additional 3 cases, CD8+ lymphocytes appeared to be the predominant T cell subset (score of 2+ to 3+; Table 1 ▶ ). Previous studies on peripheral T cell lymphomas by our group 13 have estimated the sensitivity of CD8 immunostaining by this paraffin immunoperoxidase (PIP) technique to be 90% using flow cytometry as the comparative standard. PIP CD4 staining, in contrast, had an estimated sensitivty of 64%. Therefore, it is possible that we have underestimated the true CD4+ T cell subset in the present study. Indeed, in six cases (cases 2, 5, 9, 11, 13, and 16 in Table 1 ▶ ), the total percentage of CD4+ and CD8+ cells combined appears to be less than 75% of all T cells. However, in five of these cases (cases 2, 5, 9, 11, and 15), the percentage of TIA-1+ cells was scored as 3+ or greater, indicating that the majority of cells are cytotoxic lymphocytes. In most of the cases, TIA-1 expression appeared to correlate with a CD8+ CTL phenotype; it therefore seems probable that the true number of CD8+ T cells was underestimated in these five cases. Furthermore, in eight cases (Table 1) ▶ , no CD4+ cells were detected within the tumor. However, in nearly all of these cases, CD4+ cells were seen within adjacent lymphoid tissues not involved by tumor; so it is not likely that these results are solely attributable to a lack of tissue immunoreactivity. In toto, therefore, these results indicate that most of the infiltrating small lymphocytes are CD8+ T cells that express αβ TCR (ie, βF1+) as well as the cytotoxic lymphocyte marker TIA-1.
TIA-1 (T-cell intracellular antigen-1) is a 15-kd cytolytic-granule-associated protein 17,18 expressed in both cytotoxic T cells and natural killer (NK) cells 18 and is known to cause apoptosis in target cells. 19 TIA-1 is probably identical to GMP-17 (granule membrane protein-17). 20 In most cases (16 of 18), TIA-1 expression was detected in >50% of the T cell infiltrate in TCRBCL (Table 1) ▶ . In contrast, granzyme B was expressed in a much lower percentage of cells in the majority of TCRBCLs, with 16 of 18 cases having <25% of small lymphocytes expressing granzyme B. A few scattered TILs were also seen in the DLBCLs studied. These infiltrating T cells in DLBCLs also marked as CD8+ CTLs with expression of αβ TCR and TIA-1 and a lower level of granzyme B. TIA-1 has been shown to be expressed in both activated and non-activated cytolytic lymphocytes, 17,18 whereas granzyme B is seen predominantly in activated cytolytic cells. 21 These data would suggest that the lymphocytic infiltrate in TCRBCLs consists predominantly of non-activated CTLs, which may be only partially effective at mediating a host antitumor response. Furthermore, it indicates that although TILs in DLBCL are fewer in number, they are phenotypically similar to those seen in TCRBCL.
CTLs mediate target cell death by a complex series of events, usually resulting in an apoptotic target cell death, with DNA cleavage into 200-bp oligomers, nuclear disintegration, and finally cell lysis. 22-24 Because CTLs mediate target cell death by apoptosis, we investigated the possibility that apoptosis might be occurring within the tumor cells of TCRBCL and, if so, whether or not it might account for the decreased tumor cell number in TCRBCLs. A number of techniques have been developed to label fragmented nuclear DNA in tissue sections, including in situ end labeling (using the Klenow fragment of DNA polymerase I to incorporate biotin- or digitonin-tagged nucleotides into nicked DNA strands) 25 and so-called TUNEL assays (which use TdT to add tagged nucleotides to the terminal ends of fragmented DNA strands). 26 All of the above techniques will label fragmented DNA from causes other than apoptosis, and correlation with histology is necessary to prevent misinterpretation. 27 The advantage of these methods is that they allow one to detect early apoptotic cells at a stage not obvious by routine histology. A variation on the TUNEL technique is currently available as the Apoptag kit (Oncor), which attaches digitonin-tagged nucleotides to the ends of fragmented DNA strands and detects them with an HRP-antidigitonin antibody (Fab fragment, sheep polyclonal) and DAB substrate. Previous studies 28 using tissues in which apoptosis is the known primary mechanism of cellular death have indicated that this technique is more specific for detecting apoptotic cells.
In the TCRBCLs studied, a small number of apoptotic tumor cells (confirmed by double labeling for CD20) was seen in the majority (15 of 18) of cases using the Apoptag detection kit (see Table 3 ▶ and Figure 1 ▶ ). No apoptotic cells were detected in the DLBCLs studied (Table 4) ▶ . Although CTLs mediate cellular destruction by inducing apoptosis in target cells, 22-24 the low percentage of apoptotic cells (<10% of large B cells in most cases) observed here suggests that this mechanism is only a partial factor in accounting for the decreased number of tumor cells in TCRBCLs.
Our data are consistent with several previous observations regarding TILs in B-cell non-Hodgkin’s lymphomas (NHLs). Diaz et al 29 studied B-cell NHLs not meeting the histological criteria for TCRBCL and showed an increased number of TIA-1+ TILs per unit area in intermediate- and high-grade NHLs in comparison with low-grade lymphomas and reactive lymphoid hyperplasias, suggesting an ineffective host antitumor response in DLBCLs. Additional studies demonstrated an apparent tyrosine phosphorylation and lck signaling defect in the TCR-CD3 complex within the T cells from patients with B-cell NHLs. 30 Our data on TCRBCLs further support the concept that TILs in intermediate- and high-grade NHLs are probably only partially effective at destroying the neoplastic B cell population and have a phenotype compatible with non-activated CTLs (TIA-1+, granzyme B−).
Other proteins known to be involved in the mediation of apoptosis include the fas (CD95)/fas ligand (CD95L) pathway and the oncoprotein p53. 31-34 CD95 expression has been shown in approximately one-third of DLBCLs. 35 We confirmed this finding and also showed a lower level of CD95 expression in TCRBCLs compared with DLBCLs with 3 of 18 (16.7%) TCRBCLs and 4 of 12 (33%) DLBCLs containing >25% CD95+ tumor cells. Thus, it is unlikely that CD95-induced apoptosis plays a significant role in explaining the difference in tumor cell number in TCRBCL as compared with DLBCL. Moreover, if at least some of the apoptosis present is the result of CTL-mediated cellular lysis, then CD95 might not be expected to play a prominent role. Most CD8+ CTLs destroy target cells via granule-mediated exocytosis and do not rely heavily on the fas/fas ligand pathway. The CD95/CD95L pathway appears to be more important in CD4+ CTL-mediated killing. 36,37
The wild-type p53 protein is known to be an important mediator of apoptosis in cells with irreparable DNA damage and is transiently overexpressed within DNA-damaged cells before apoptosis. 34 Mutations that inactivate the function of this gene and result in its overexpression are probably important in the oncogenesis of a number of malignancies. 38 Overexpression detected by paraffin immunoperoxidase methodologies are generally believed to be the result of nonfunctional gene mutations, resulting in a stable protein product. 39 Our data suggest that p53 is not likely an important mediator of either apoptosis or oncogenesis in TCRBCL or DLBCL, as the majority of each (80% of TCRBCLs and 75% DLBCLs) had detectable p53 expression in <25% of tumor cells.
An alternative explanation for the decreased number of tumor cells in TCRBCL in comparison with DLBCL is that the inhibition of apoptosis in DLBCLs allows for the accumulation of an increased number of neoplastic cells. Two findings support this hypothesis. First, no difference in proliferative activity was observed between TCRBCLs and DLBCLs as assessed by Mib-1 staining. Antibodies to Mib-1 recognize an epitope of the Ki-67 antigen that is preserved in formalin-fixed, paraffin-embedded tissues and is expressed in cells undergoing DNA replication or cellular division. 40 In both TCRBCLs and DLBCLs, 83% of cases contained <25% Mib-1+ tumor cells. Second, a higher percentage of DLBCLs stained positively for the bcl-2 protein (6 of 12 cases with >50% bcl-2+ tumor cells). In contrast 16 of 16 immunoreactive TCRBCLs had <25% bcl-2+ tumor cells. The protein product of the bcl-2 gene (located on chromosome 18) is known to inhibit apoptosis. 41,42 Overexpression of this protein as a result of translocation is well known to play a role in lymphomagenesis in more than 85% of follicular lymphomas, primarily those of low grade. 42-45 These data suggest that increased bcl-2 protein expression in DLBCLs may also play a role in the increased number of tumor cells in DLBCLs in comparison with TCRBCLs. Previous studies 2 by Southern blot analysis for the major and minor breakpoints of the t(14;18) translocation showed only 1 of 11 of these TCRBCLs had bcl-2 gene rearrangements, further supporting this hypothesis.
Thus, the T cell infiltrate in TCRBCLs is composed primarily of CD8+ CTLs, most of which have a non-activated phenotype (TIA-1+, granzyme B−). These data, along with the finding of apoptotic tumor cells in TCRBCLs, suggest that CTL-mediated lysis may play some role in explaining the reduced number of tumor (neoplastic B) cells in TCRBCL but probably does not play a major role. Other factors, such as increased bcl-2 expression in DLBCLs, may be more significant by providing resistance to apoptosis, thereby allowing more tumor cells to accumulate in DLBCLs. Previous studies have indicated that certain cytokines (principally IL-4) are expressed at increased levels in the neoplastic B cells of TCRBCLs. 15 It may be that local cytokine release results in increased CTL proliferation but that such cells are only partially effective at controlling tumor cell growth.
Footnotes
Address reprint requests to Dr. Raymond E. Felgar, Department of Pathology and Laboratory Medicine, University of Rochester Medical Center, 601 Elmwood Avenue, Box 626, Rochester, NY 14642. E-mail: raymond.felgar@gte.net.
This work was presented in part at the 87th Annual Meeting of the United States and Canadian Academy of Pathology, Boston, MA, March 3, 1998.
References
- 1.Ramsay AD, Smith WJ, Isaacson PG: T-cell-rich B-cell lymphoma. Am J Surg Pathol 1988, 12:433-443 [DOI] [PubMed] [Google Scholar]
- 2.Macon WR, Williams ME, Greer JP, Stein RS, Collins RD, Cousar JB: T-cell-rich B-cell lymphomas: a clinicopathologic study of 19 cases. Am J Surg Pathol 1992, 16:351-363 [PubMed] [Google Scholar]
- 3.Krishnan J, Wallberg K, Frizzera G: T-cell-rich large B-cell lymphoma: a study of 30 cases supporting its histologic heterogeneity and lack of clinical distinctiveness. Am J Surg Pathol 1994, 18:455-465 [PubMed] [Google Scholar]
- 4.Baddoura FK, Chan WC, Masih AS, Mitchell D, Sun NCJ, Weisenburger DD: T-cell-rich B-cell lymphoma: a clinicopathologic study of eight cases. Am J Clin Pathol 1995, 103:65-75 [DOI] [PubMed] [Google Scholar]
- 5.Chittal SM, Brousset P, Voigt J-J, Delsol G: Large B-cell lymphoma rich in T-cells and simulating Hodgkin’s disease. Histopathology 1991, 19:211-220 [DOI] [PubMed] [Google Scholar]
- 6.Delabie J, Vandenberghe E, Kennes C, Verhoef G, Foschini MP, Stul M, Cassiman JJ, De Wolf-Peeters C: Histiocyte-rich B-cell lymphoma: a distinct clinicopathologic entity possibly related to lymphocyte predominant Hodgkin’s disease, paragranuloma subtype. Am J Surg Pathol 1992, 16:37-48 [PubMed] [Google Scholar]
- 7.Farhi DC: T-cell-rich B-cell lymphoma: reflections on changes in hematopathology. Am J Clin Pathol 1995, 103:4-5 [DOI] [PubMed] [Google Scholar]
- 8.Greer JP, Macon WR, Lamar RE, Wolff SN, Stein RS, Flexner JM, Collins RD, Cousar JB: T-cell-rich B-cell lymphomas: diagnosis and response to therapy of 44 patients. J Clin Oncol 1995, 13:1742-1750 [DOI] [PubMed] [Google Scholar]
- 9.Rodriguez J, Pugh WC, Cabanillas F: T-cell-rich B-cell lymphoma. Blood 1993, 82:1586-1589 [PubMed] [Google Scholar]
- 10.Skinnider BF, Connors JM, Gascoyne RD: Bone marrow involvement in T-cell-rich B-cell lymphoma. Am J Clin Pathol 1997, 108:570-578 [DOI] [PubMed] [Google Scholar]
- 11.Kawada H, Watanabe S, Yoshida M, Fukuda R, Kobayashi N, Masumoto A, Ogawa Y, Ohbayashi Y, Yonekura S, Ichikawa Y: Flow cytometric analysis of T-cell-rich B-cell lymphoma. Acta Haematol 1994, 92:164-166 [DOI] [PubMed] [Google Scholar]
- 12.Felgar RE, Macon WR, Kinney MC, Roberts S, Pasha T, Salhany KE: TIA-1 expression in lymphoid neoplasms: identification of subsets with cytotoxic T lymphocyte or natural killer cell differentiation. Am J Pathol 1997, 150:1893-1900 [PMC free article] [PubMed] [Google Scholar]
- 13.Macon WR, Salhany KE: T-cell subset analysis of peripheral T-cell lymphomas by paraffin immunoperoxidase and correlation of CD4/CD8 results to flow cytometry. Am J Clin Pathol 1998, 109:610-617 [DOI] [PubMed] [Google Scholar]
- 14.Oudejans JJ, Kummer JA, Jiwa M, van der Valk P, Ossenkoppele GJ, Kluin PM, Kluin-Nelemans JC, Meijer CLM: Granzyme B expression in Reed-Sternberg cells of Hodgkin’s disease. Am J Pathol 1996, 148:233-240 [PMC free article] [PubMed] [Google Scholar]
- 15.Macon WR, Cousar JB, Waldron JA, Hsu S-M: Interleukin-4 may contribute to the abundant T-cell reaction and paucity of neoplastic B cells in T-cell-rich B-cell lymphomas. Am J Pathol 1992, 141:1031-1036 [PMC free article] [PubMed] [Google Scholar]
- 16.Shipley WR, Hammer RD, Lennington WJ, Macon WR: Paraffin immunohistochemical detection of CD56, a useful marker for neural cell adhesion molecule (NCAM), in normal and neoplastic fixed tissues. Appl Immunohistochem 1997, 5:87-93 [Google Scholar]
- 17.Anderson P, Nagler-Anderson C, O’Brien C, Levine H, Watkins S, Slayter HS, Blue M-L, Schlossman SF: A monoclonal antibody reactive with a 15-kd cytoplasmic granule-associated protein defines a subpopulation of CD8+ T lymphocytes. J Immunol 1990, 144:574-582 [PubMed] [Google Scholar]
- 18.Anderson P: TIA-1: Structural and functional studies on a new class of cytolytic effector molecule. Curr Top Microbiol Immunol 1995, 198:131-143 [DOI] [PubMed] [Google Scholar]
- 19.Tian Q, Streuli M, Saito H, Schlossman SF, Anderson P: A polyadenylate binding protein localized to the granules of cytolytic lymphocytes induces DNA fragmentation in target cells. Cell 1991, 67:629-639 [DOI] [PubMed] [Google Scholar]
- 20.Medley QG, Kedersha N, O’Brien S, Tian Q, Schlossman SF, Streuli M, Anderson P: Characterization of GMP-17, a granule membrane protein that moves to the plasma membrane of natural killer cells following target cell recognition. Proc Natl Acad Sci USA 1996, 93:685-689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boulland M-L, Kanavaros P, Wechsler J, Casiraghi O, Gaulard P: Cytotoxic protein expression in natural killer cell lymphomas in αβ and γδ peripheral T-cell lymphomas. J Pathol 1997, 183:432-439 [DOI] [PubMed] [Google Scholar]
- 22.Berke G: The CTL’s kiss of death. Cell 1995, 81:9-12 [DOI] [PubMed] [Google Scholar]
- 23.Liu C-C, Young LHY, Young JD-E: Lymphocyte mediated cytolysis and disease. N Engl J Med 1996, 335:1651-1659 [DOI] [PubMed] [Google Scholar]
- 24.Young JD-E, Liu CC: Multiple mechanisms of lymphocyte-mediated killing. Immunol Today 1988, 9:140-144 [DOI] [PubMed] [Google Scholar]
- 25.Wijsman JH, Jonker RR, Keijzer R, van de Velde CJ, Cornelisse CJ, van Dierendonck JH: A new method to detect apoptosis in paraffin sections: in situ end labeling of fragmented DNA. J Histochem Cytochem 1993, 41:7-12 [DOI] [PubMed] [Google Scholar]
- 26.Thiry M: Highly sensitive immunodetection of DNA on sections with exogenous terminal deoxynucleotidyl transferase and non-isotopic nucleotide analogues. J Histochem Cytochem 1992, 40:411-419 [DOI] [PubMed] [Google Scholar]
- 27.Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R: In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology 1995, 21:1465-1468 [DOI] [PubMed] [Google Scholar]
- 28.Gold R, Schmied M, Giegrich G, Breitschopf H, Hartung HP, Toyka KV, Lassmann H: Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 1994, 71:219-225 [PubMed] [Google Scholar]
- 29.Diaz J, Tubbs R, Stoler M, Grogan T: Cytolytic (TIA-1+) tumor infiltrating lymphocytes in B cell non-Hodgkin’s lymphomas. Leuk Lymphoma 1993, 9:91-94 [DOI] [PubMed] [Google Scholar]
- 30.Wang Q, Stanley J, Kudoh S, Myles J, Kolenko V, Yi T, Tubbs R, Bukowski R, Finke J: T cells infiltrating non-Hodgkin’s B cell lymphomas show altered tyrosine phosphorylation pattern even though T cell receptor/CD3-associated kinases are present. J Immunol 1995, 155:1382-1392 [PubMed] [Google Scholar]
- 31.Yonehara S, Ishii A, Yonehara M: A cell-killing monoclonal antibody (anti-Fas) to a cell surface antigen co-downregulated with the receptor of tumor necrosis factor. J Exp Med 1989, 169:1747-1756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trauth BC, Klas C, Peters AMJ, Matzku S, Moller P, Flak E, Debatin KM, Krammer PH: Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science 1989, 245:301-305 [DOI] [PubMed] [Google Scholar]
- 33.Hanabuchi S, Koyanagi M, Kawasaki A, Shinohara N, Matsuzawa A, Nishimura Y, Kobayashi Y, Yonehara S, Yagita H, Okumura K: Fas and its ligand in a general mechanism of T-cell-mediated cytotoxicity. Proc Natl Acad Sci USA 1994, 91:4930-4934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oren M: Relationship of p53 to the control of apoptotic cell death. Semin Cancer Biol 1994, 5:221-227 [PubMed] [Google Scholar]
- 35.Nguyen PL, Harris NL, Ritz J, Robertson MJ: Expression of CD95 antigen and bcl-2 protein in non-Hodgkin’s lymphomas and Hodgkin’s disease. Am J Pathol 1996, 148:847-853 [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn S, Gehri R, Erb P: Mechanism and biological significance of CD4-mediated cytotoxicity. Immunol Rev 1995, 146:57-79 [DOI] [PubMed] [Google Scholar]
- 37.Takayama H, Kojima H, Shinohara N: Cytotoxic T lymphocytes: The newly identified fas (CD95)-mediated killing mechanism and a novel aspect of their biological functions. Adv Immunol 1995, 60:289-321 [DOI] [PubMed] [Google Scholar]
- 38.Vogelstein B, Kinzler KW: p53 function and dysfunction. Cell 1992, 70:523-526 [DOI] [PubMed] [Google Scholar]
- 39.Lane DP: p53 and human cancers. Br Med Bull 1994, 50:582-599 [DOI] [PubMed] [Google Scholar]
- 40.Cattoretti G, Becker MH, Key G, Duchrow M, Schluter C, Galle J, Gerdes J: Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB-1 and MIB-3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 1992, 168:357-363 [DOI] [PubMed] [Google Scholar]
- 41.Reed JC: Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol 1995, 7:541-546 [DOI] [PubMed] [Google Scholar]
- 42.Yang E, Korsmeyer SJ: Molecular thanatopsis: a discourse on the BCL-2 family and cell death. Blood 1996, 88:386-401 [PubMed] [Google Scholar]
- 43.Fukuhara S, Rowley JD, Variakojis D, Golomb HM: Chromosome abnormalities in poorly differentiated lymphocytic lymphoma. Cancer Res 1979, 39:3119-3128 [PubMed] [Google Scholar]
- 44.Yunis JJ, Frizzera G, Oken MM, McKenna J, Theologides A, Arnesen M: Multiple recurrent genomic defects in follicular lymphoma: a possible model for cancer. N Engl J Med 1987, 316:79-84 [DOI] [PubMed] [Google Scholar]
- 45.Cleary ML, Smith SD, Sklar J: Cloning and structural analysis of cDNAs for bcl-2 and a hybrid bcl-2/immunoglobulin transcript resulting from the t(14;18) translocation. Cell 1986, 47:19-28 [DOI] [PubMed] [Google Scholar]


