Abstract
Objective
To assess change in cognitive functioning following iron deficiency in infancy, depending on socio-economic status (middle- vs. low-SES).
Design
Longitudinal study.
Setting
Urban community in Costa Rica (infancy phase, 1981-1984, through 19-year follow-up, 2000-2002).
Participants
185 individuals enrolled at 12-23 months (no preterm/low birth weight or acute/chronic health problems), assessed in infancy and at 5, 11-14, 15-18, and 19 years. 97% evaluated at 5 or 11-14 years; 78% at 15-18 or 19 years.
Exposure
Infancy iron status: chronic iron deficiency (iron deficiency with hemoglobin (Hb) ≤ 10.0 g/dL or, with higher Hb, not fully corrected with 3 months of iron therapy) vs. good iron status (Hb ≥ 12.0 g/dL and normal iron measures before and/or after therapy). For middle-SES, 20 chronic-iron-deficiency compared to 67 good-iron-status group. For low-SES, 33 chroniciron-deficiency compared to 65 good-iron-status group.
Outcome Measures
Cognitive change over time (composite of standardized scores at each age).
Results
For middle-SES participants, scores averaged 101.2 in chronic-iron-deficiency vs. 109.3 in good-iron-status groups in infancy and remained 8-9 points lower through 19 years (95% CI -10.1 to -6.2). For low-SES participants, the gap widened from 10 points (93.1 v. 102.8; 95% CI for the difference, -12.8 to -6.6) to 25 points (70.4 vs. 95.3; 95% CI for the difference, 20.6 to 29.4).
Conclusions
The chronic-iron-deficiency group did not catch up to the good-iron-status group in cognitive scores over time. There was a widening gap for those in low-SES families. The results suggest the value of preventing iron deficiency in infancy.
Keywords: iron deficiency, cognitive function, longitudinal analysis, socio-economic status
INTRODUCTION
Infants with iron-deficiency anemia or other indications of chronic, severe iron deficiency have shown lower cognitive test scores than infants with good iron status in all but 1 of 14 studies that assessed overall cognitive functioning, from countries around the world.1,2 The few available follow-up studies at school-age or early adolescence report persisting lower scores, despite iron therapy in infancy.3-7 A recent meta-analysis estimated the long-term effects on intelligence quotient (IQ) to be 1.73 points lower for each 1.0 g/dL decrease in hemoglobin.8 Such differences in group means raise questions about stability and change over time. Is there evidence of catch-up or further decline? Does the impact of iron deficiency in infancy vary over time depending on socio-economic circumstances? These issues pertain to millions of children.8 An estimated 20-25% of babies worldwide have iron-deficiency anemia and more have iron deficiency without anemia.9-11 Poor, minority, and/or immigrant infants in industrialized countries are also at increased risk for iron deficiency.12
To address questions about change over time depending on iron status in infancy and socio-economic status, we applied techniques of longitudinal analysis13 to data from an ongoing study in Costa Rica. Previous cross-sectional analyses showed that cognitive test scores for the group with chronic, severe iron deficiency in infancy (see Methods) were lower than the group with good iron status in infancy14 and at 5 and 11-14 years.6,15 By 11-14 years, a higher proportion of children in the chronic-iron-deficiency group had repeated a grade in school and/or been referred for special services.6 The present study assessed change in cognitive test performance from the second year of life to the transition to adulthood (19 years).
METHODS
Sample
The analysis used data from a longitudinal study in Costa Rica that included evaluations in infancy and 4 subsequent follow-ups (5, 11-14, 15-18, and 19 years). Enrollment in the original infant study was conducted in 1981-1984 in an urban community near San Jose, the capital of Costa Rica.14 The 19-year evaluation was conducted in 2000 - 2002. The community was mixed middle- and lower-class, and parents of study infants averaged 8-10 years of education. Enrollment entailed door-to-door screening, inviting study participation for all 12- to 23-month-old infants who had a birth weight ≥ 2.5 kg and a singleton term uncomplicated birth, who were free of acute or chronic medical problems and had a normal physical examination. Refusal was 11.6%. Infants enrolled in the study had no evidence of growth failure or other nutrient deficiencies.14 The mean age at study entry was 17 months. Iron deficiency is thus likely to have lasted for some time, especially since the local feeding practice at the time was to introduce unmodified cow milk in the first months of life (together with breast feeding). Of the 191 infants in the initial study, 185 provided data for this longitudinal analysis (6 were excluded due to lack of information about their iron status following iron therapy).
Figure 1 shows a flow chart of the number and percent of participants at each subsequent assessment. From the 5-year follow-up study,15 161 children provided data for the longitudinal analysis. All but 15 received the comprehensive psychoeducational assessment within 2 weeks of their fifth birthday (age range 59-63 months). From the reevaluation at 11-14 years,6 162 children provided data (mean age, 12.3 years, range 10.9-13.7 years). Overall, 97% of the original sample participated in assessments either at 5 years or early adolescence. A brief follow-up in late adolescence provided data for 133 participants (mean age 16.4 years, range 15.0 -17.9). A comprehensive assessment at 19 years provided data for 121 participants (mean age 19.0 years, range 18.0 - 20.0). Seventy-eight percent (n = 145) of the original 185 participants were evaluated at 15-18 years and/or 19 years. Participants who were not tested at a given age often participated subsequently (Figure 1). Lack of participation was primarily due to difficulty in locating a family.
Figure 1.

Flow chart of study participation. The number (%) of participants at each time point is shown, indicating that participants who missed an assessment were often available subsequently.
Parental signed informed consent for each phase of the study was obtained by the project pediatrician. Assent/consent of the adolescent was obtained beginning with the early adolescent follow-up. The infancy and 5-year protocols were approved by the Institutional Review Board of Case Western Reserve University, Cleveland, and subsequent protocols were approved by the Institutional Review Board of the University of Michigan, Ann Arbor. All protocols were approved by ethics committees of the Hospital Nacional de Niños or Instituto Costarricense de Investigationes Clinicas (for the 19-year evaluation) and the Ministry of Health, Costa Rica.
Measures
Iron status
Iron status in infancy was determined by venous concentrations of hemoglobin (Hb), transferrin saturation, free erythrocyte protoporphyrin, and serum ferritin. Iron deficiency was defined as 2 or more abnormal iron measures16 (serum ferritin < 12 μg/L16 and either free erythrocyte protoporphyrin ≥ 100 μg/dL red blood cells17 or transferrin saturation < 10%.18 Iron sufficiency was defined as Hb ≥ 12.0 g/dL and normal values on all iron status measures. Hematologic response to iron therapy in infancy was excellent, with a mean Hb increase of 3.7 g/dL among iron-deficient infants with Hb ≤ 10.5 g/dL. All corrected their anemia with 3 months of iron therapy, but, as might be expected, those with indications of more severe or chronic iron deficiency still had biochemical alterations, such as elevated erythrocyte protoporphyrin values.14 At the subsequent follow-ups that included blood collection (5, 11-14, and 19 years), 6,15 iron deficiency was present in less than 5%, and no one had iron-deficiency anemia except for 4 women at 19 years, 2 of whom were pregnant. These data indicate that the Costa Rican diet at the time provided adequate iron to correct any iron parameters that had still been altered after treatment in infancy and maintain good iron status thereafter.
Following the approach in the 5-year follow-up and subsequent reports,6,15,19 we compared participants who had chronic, severe iron deficiency in infancy (with or without anemia) with the rest of the sample, who were iron-sufficient before and/or after iron therapy in infancy. For simplicity, the chronic, severe iron-deficient group will be referred to as “chronic iron deficiency” and the rest of the sample as “good iron status.” The chronic-iron-deficiency group consisted of participants who had marked iron-deficiency anemia in infancy (Hb ≤ 10.0 g/dL) and those with higher Hb concentrations and iron deficiency that did not fully correct after 3 months of iron therapy.15 Analyses compared the chronic-iron-deficiency (n = 53) and good-iron-status (n = 132) groups. There was no differential attrition; the chronic-iron-deficiency group constituted 28-29% of the sample in both infancy and the late adolescent follow-ups.
Cognitive assessments
In infancy, the Mental Development Index (MDI) of the Bayley Scales of Infant Development20 was administered before and after iron treatment. At the 5-year follow-up, the overall tests of cognitive function were the Wechsler Preschool and Primary Scale of Intelligence21 and the Woodcock-Johnson PsychoEducational Battery.22 At 11-14 years, the general cognitive measures were the Wechsler Intelligence Scale for Children-Revised,23 the Wide Range Achievement Test-Revised arithmetic and reading,24 and the Directed Writing Task.25 At 15-18 years, the measures were arithmetic and reading achievement24 and the Directed Writing Task.25 At 19 years, the general cognitive measures were arithmetic achievement24 and 5 subscales of the Wechsler Intelligence Scale for Adults,26 prorated to estimate Verbal and Performance intelligence scores.27 All assessments were conducted by trained Costa Rican psychologists who were unaware of participants’ hematologic status at any age.
Factor analyses (principal components with varimax rotation28) showed that the general cognitive measures at each assessment were closely associated with each other. Each set of measures yielded a single factor that explained from 62% (5 years) to 78% (19 years) of the variance. This data reduction information warranted combining measures into a composite cognitive score for a given age. Since all tests were standardized or could be rescaled to a mean of 100 and a SD 15-16, analyses could be conducted across ages and differing tests. In the longitudinal model structure described below, all analyses adjusted for each individual’s exact age at each follow-up. Results report change relative to age-normed scores based on US standardization samples at each time point. Thus, “decline” is relative to norms for age, rather than in actual knowledge or absolute cognitive performance.
Environmental factors
Iron deficiency often goes along with other individual, family, and/or environmental disadvantages, some of which could affect cognitive development.1,29-31 The critical comparison of our study considers the impact of infant iron status depending on family socio-economic status (SES). Measures of family SES generally assess such factors as family structure, economic circumstances, education, and occupation. We used the Hollingshead Four Factor Index, 32 which considers parental education and occupation and father presence and is widely used. We compared individuals whose family SES in infancy was low (levels IV and V, unskilled and semi-skilled workers) or middle to above (levels I-III, professional, managerial, clerical, and skilled workers).32
Statistical analysis
Longitudinal analysis using hierarchical modeling (HLM) was the primary statistical approach.13,33 This class of analytic techniques has not previously been applied to change in cognitive scores over time with iron deficiency in infancy. By considering the within-individual correlations between measures, longitudinal analysis provides relatively unbiased estimates for each individual of the starting level (intercept), change over time (slope), and acceleration/deceleration (curvature). The study’s analyses were conducted with HLM software.33 HLM estimates the covariance structure appropriately in data sets that, like ours, have incomplete and unbalanced time parameters, with varying number of assessments and time intervals between assessments for different individuals, resulting in a varying variance structure.13 The analysis used measures nested within individuals (level one), comparing differences between individuals in the good-iron-status and chronic-iron-deficiency groups (level two). The level one model used individual age at testing as the time parameter, with the change estimated specific to the amount of time between assessments for each individual. We tested for the possibility of curvilinear components or multiple growth trajectories, as well as single linear change, and selected the most parsimonious and best-fitting model based on the lowest deviance relative to the degrees of freedom.13 The best model estimated two distinct slopes - one for change from infancy to 5 years and a second for change from 5 to 19 years. Models were anchored to actual test scores at the beginning and end of these intervals (infancy, 5 years, and 19 years).
We tested for an interaction between iron status in infancy and socio-economic status (middle vs. low) in an overall model. The interactions were statistically significant for intercept and both slopes (P values < .05). To facilitate interpretation, we present results comparing change over time in chronic-iron-deficiency and good-iron-status groups separately for middle- and low-SES families (for middle-SES, 67 good-iron-status vs. 20 chronic-iron-deficiency; for low-SES, 65 good-iron-status vs. 33 chronic-iron-deficiency). The estimated intercepts and change over time for these analyses match the estimates from the overall model. Where appropriate, we conducted post-hoc tests of common parameters for significant differences by examining the difference in parameter estimate relative to the pooled standard error of the estimate (a form of t-test comparison).13
RESULTS
A higher proportion of the chronic-iron-deficiency group was male (75% vs. 48% in the good-iron-status group, P < .01), as noted in previous reports.6,14 They also weighed 200 g less at birth (mean (SD) birth weight: 3.1 (0.3) kg vs. 3.3 (0.4) kg in the good-iron-status group, P = .02). Gender and birth weight were therefore covaried in all analyses. The sample was reasonably balanced between middle- and low-SES families (87 middle-SES and 98 low-SES). SES was lower in the chronic-iron-deficiency group when analyzed as a continuous variable (mean (SD) Hollingshead: 27.2 (10.8) vs. 31.0 (12.6) in the good-iron-status group, P = .02). However, the difference in the proportion in low-SES families did not reach statistical significance. Sixty-two percent of the chronic-iron-deficiency group came from low-SES families, compared to 49% of the good-iron-status group (P = .11).
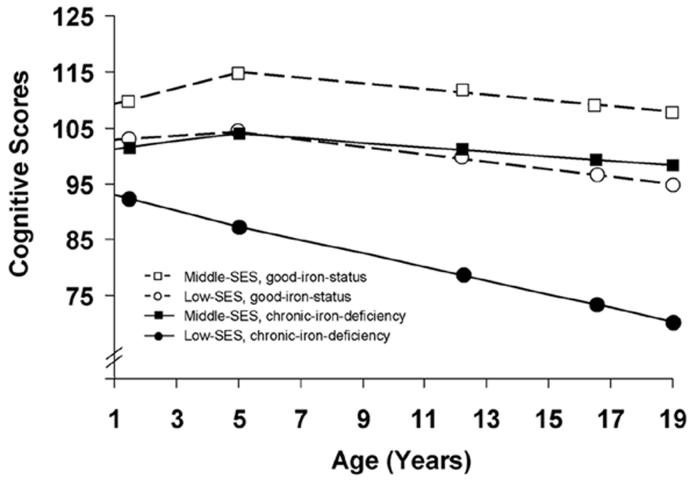
Figure 2 shows the relationship between iron deficiency and cognitive test scores over time depending on socio-economic status. In middle-SES families, initial cognitive scores for participants who had chronic iron deficiency in infancy averaged 8 points lower than those with good iron status (101.2 vs. 109.3; difference in infancy = -8.15, 95% CI -10.1 to -6.2, effect size .54 SD). There were no statistically significant differences between groups in change from infancy to 5 years or from 5 to 19 years. Thus, the magnitude of difference was maintained; at 19 years, their scores averaged 9 points lower (98.2 vs. 107.6, 95% CI for the difference -11.0 to -7.0).
Figure 2.
Cognitive composite scores over time, comparing infant iron status groups within middle and low socio-economic status (SES) families. Iron status group and SES level each affected initial scores (P values < .01). Change over time differed only for the chronic-iron-deficiency group in low-SES families (P < .05). Each participant is represented once: good iron status (n = 67) compared to chronic iron deficiency (n = 20) in middle-SES families and good iron status (n = 65) compared to chronic iron deficiency (n = 33) in low-SES families. Symbols are placed at the average age for each assessment.
In low-SES families, initial cognitive scores for participants with chronic iron deficiency in infancy averaged 10 points lower than those with good iron status (93.1 vs. 102.8; difference in infancy = -9.7, 95% CI -12.8 to -6.6, effect size .67 SD). Scores for the chronic-iron-deficiency group declined from infancy to 5 years (difference in rate of change = -1.8, 95% CI -2.6 to -1.1), whereas those for the good-iron-status group did not. While a pattern of decline in cognitive test scores from 5 to 19 years was generally observed, in low-SES families, the decline among individuals with chronic iron deficiency in infancy was steeper than for those in the good-iron-status-group (difference in rate of change = -0.6, 95% CI -0.8 to -0.3). This resulted in mean scores of 70.4 vs. 95.3 by 19 years - a 25-point gap (95% CI 20.6 to 29.4, effect size 1.67 SD) between chronic-iron-deficiency and good-iron-status groups with low-SES backgrounds.
In light of our interest in environmental disadvantage, we also compared iron status groups across SES levels in a post-hoc comparison of models. For the good-iron-status group, those in low-SES families had scores in infancy 7 points (95% CI 4.0 to 10.0, effect size .47 SD) lower than those in middle-SES families, increasing to 12 points (95% CI 8.4 to 15.6) by 19 years. For the chronic-iron-deficiency group, individuals from middle-SES families started with scores in infancy like those of the good-iron-status group in low-SES families - in between those with good iron status from middle-SES families and those with chronic iron deficiency from low-SES families. The chronic-iron-deficiency group from middle-SES families showed a pattern over time like that of the good-iron-status group in middle-SES families except 8-9 points lower (95% CI 5.2 to 11.8, effect sizes .53 to .60 SD). In contrast, the cognitive test score gap for individuals in the chronic-iron-deficiency group from low-SES families (compared to those in middle-SES families) widened from 8 points (95% CI 5.4 to 10.8) in infancy, to 28 points at 19 years (95% CI 23.6 to 32.4, effect size 1.87 SD).
DISCUSSION
Using longitudinal analytic techniques, this study showed no evidence catch-up in cognitive test performance for individuals with chronic iron deficiency in infancy and a widening gap for those in low-SES families. This was observed despite iron therapy in infancy sufficient to correct anemia for those who had been anemic and good iron status thereafter. A gap of the observed magnitude (25-28 points) is likely to correspond to major differences in life course.
The observed pattern appears to make sense in terms of the cumulative and transactional nature of cognitive development.34-36 Acquisition of new skills is intimately linked to mastery of skills at an earlier developmental level. If direct and indirect effects of early iron deficiency on the brain37 disrupted or delayed basic developmental processes, there could be a snowball effect. In an economically stressed family environment, there might not be the resources or capacity to help children compensate. Together, these factors could contribute to earlier school failure6 and less advanced cognitive processes in individuals with chronic iron deficiency in infancy in low-SES families. Thus, our results fit with the concept of “double jeopardy” or “double hazard,” 38,39 (i.e., worse outcome among individuals who experience both an early biologic insult or stressor and more disadvantaged background40).
As expected, individuals from disadvantaged backgrounds showed lower cognitive test scores. This was confirmed for both the good-iron-status and chronic-iron-deficiency groups. Even with good iron status in infancy, low-SES individuals showed a decline in test scores like that observed in the US.41 Our observation that SES differences in cognitive test performance appeared to be set by preschool-age and not improved by schooling has also been reported in the US and elsewhere.42,43 This has sometimes been called the Matthew effect,44,45 in reference to the biblical quotation “To all those who have, more will be given, and they will have an abundance, but from those who have nothing, even what they have will be taken away.” However, there was a differential effect of iron status on change over time in low-SES families. Individuals in the chronic-iron-deficiency group not only tested lower in infancy but also showed a more marked decline, and hence, an increasing gap, in subsequent cognitive test performance. Thus, good iron status before and/or after iron therapy in infancy appeared to attenuate the decline.
A major food supplementation trial in Guatemala found that early nutritional supplementation eliminated the decline in test scores associated with low SES.46 Although the interventions differed (single micronutrient in our study and energy plus multimicronutrients in the Guatemala study), both studies provide support for long-term cognitive benefits of improved nutrition in infancy. Several investigators have assessed the likelihood that improved nutrition contributes to the rising IQs observed in many countries.47 IQ tests and other such measures have had to be restandardized to adjust for rising scores. Because infant iron status has improved dramatically in the US and elsewhere in the past several decades,48,49 a reduction in iron deficiency might play a role in the continued phenomenon of rising IQs. If the pattern we observed among individuals with good iron status before or after iron therapy in infancy applies elsewhere (i.e., higher cognitive test scores later on), there might be corresponding population-level increases in cognitive test scores with improved iron status in infancy. If replicated, the results would suggest that, even in the face of stressed economic conditions, improving infant iron status has the potential for major societal impact in countries where iron deficiency is widespread.
Limitations
When the study started in 1981, there were few tests of specific cognitive functions in 1- to 2-year-old infants, and the only early cognitive measure was the mental development scale of the Bayley Scales of Infant Development. Consequently, this longitudinal analysis cannot provide evidence for specific central nervous system effects of early iron deficiency. The study also cannot determine the duration of iron deficiency; it could have started in the first year or even earlier (prenatally). The study is further limited by small sample size, potential confounding by measured and unmeasured factors, etc. Although missing data could also bias the results, losses were relatively low, considering follow-up from infancy to the transition to adulthood.
Socio-economic status may exert its effects in different ways in different societies and cultures. Thus, the relationships observed in this Costa Rican sample may not generalize to other parts of the world. Furthermore, study participants were full-term, free of chronic or acute illnesses, and growing normally by US standards. Children who are not in such good overall health might not show the same effects. Conversely, children who experience briefer or milder iron deficiency in infancy might not show the pattern of declining cognitive test scores we observed. However, most infants in the world are not tested for anemia or iron deficiency and thus may experience even more prolonged or severe and/or untreated iron deficiency. Their outcome might be poorer than we observed.
Conclusions
In this study in Costa Rica, participants who had chronic, severe iron deficiency in infancy (moderate iron-deficiency anemia or Hb ≥ 100 g/L with abnormal iron measure(s) after treatment) did not catch up in cognitive test scores over time to those who were iron-sufficient before and/or after treatment in infancy. For individuals from middle-SES families who had chronic iron deficiency in infancy, the magnitude of the gap remained the same from infancy to 19 years (8-9 points lower). However, those in lower-SES families seemed doubly burdened - the gap widened substantially from 10 points in infancy to 25 points at 19 years. Such a difference is likely to be functionally significant regarding educational attainment and career choices in adulthood. The analysis also suggested a protective effect of good iron status in infancy in low-SES families. In light of potential adverse effects at the level of the individual and the society in settings where iron deficiency is widespread, it seems reasonable to prevent iron deficiency in infancy and treat it before it becomes chronic or severe.
ACKNOWLEDGEMENTS
We are grateful to study families for commitment and continued participation; to the skilled psychologists who meticulously performed the cognitive tests over 2 decades; and to Agustin Calatroni, Niko Kaciroti, and Stephen Raudenbush for heated discussion and good advice on longitudinal analysis. B. Lozoff had full access to all study data and takes responsibility for data integrity and accuracy of data analysis. All phases of the study were supported by grants from the US National Institute of Child Health and Human Development. The longitudinal analysis was supported by a MERIT Award to B. Lozoff, R37 HD31606). Preliminary results were presented at the Pediatric Academic Societies meetings, San Francisco, 2004.
REFERENCES
- 1.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131:649S–668S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 2.Walker SP, Wachs TD, Gardner JM, Lozoff B, Wasserman GA, Pollitt E, Carter JA, Grantham-McGregor S, Engle P, Black M. Child development: risk factors for adverse outcomes in developing countries. Part of a 3 part series on child development. Lancet. doi: 10.1016/S0140-6736(07)60076-2. under revison. [DOI] [PubMed] [Google Scholar]
- 3.Cantwell RJ. The long term neurological sequelae of anemia in infancy. Pediatr Res. 1974;342:68. (abstract) [Google Scholar]
- 4.Palti H, Meijer A, Adler B. Learning achievement and behavior at school of anemic and non-anemic infants. Early Hum Dev. 1985;10:217–223. doi: 10.1016/0378-3782(85)90052-0. [DOI] [PubMed] [Google Scholar]
- 5.Hurtado EK, Claussen AH, Scott KG. Early childhood anemia and mild or moderate mental retardation. Am J Clin Nutr. 1999;69:115–119. doi: 10.1093/ajcn/69.1.115. [DOI] [PubMed] [Google Scholar]
- 6.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:E51. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- 7.Antunes H. Iron Deficiency Anaemia in Infants - A Prospective Neurodevelopment Evaluation. Faculty of Medicine, University of Portugal; 2004. [Google Scholar]
- 8.Stoltzfus RJ, Mullany L, Black RE. Iron deficiency anaemia. In: Ezzati M, Lopez AD, Rodgers A, Murray CJL, editors. Comparative quantification of health risks: global and regional burden of disease attributable to selected major risk factors. I. World Health Organization; Geneva: 2004. pp. 163–209. [Google Scholar]
- 9.deMaeyer E, Adiels-Tegman M. The prevalence of anaemia in the world. World Health Stat Q. 1985;38:302–316. [PubMed] [Google Scholar]
- 10.Florentino RF, Guirriec RM. Prevalence of nutritional anemia in infancy and childhood with emphasis on developing countries. In: Steckel A, editor. Iron Nutrition in Infancy and Childhood. Raven Press; New York: 1984. pp. 61–74. [Google Scholar]
- 11.Mannar V, Bellamy C. Vitamin and mineral deficiency - a global progress report. UNICEF; New York: 2004. [Google Scholar]
- 12.Brotanek JM, Halterman J, Auinger P, Flores G, Weitzman M. Iron deficiency, prolonged bottle-feeding, and racial/ethnic disparities in young children. Arch Pediatr Adolesc Med. 2005;159:1038–1042. doi: 10.1001/archpedi.159.11.1038. [DOI] [PubMed] [Google Scholar]
- 13.Raudenbush SW, Bryk A. Hierarchical Linear Models Applications and Data Analysis Methods. 2nd ed. Sage Publications; Thousand Oaks: 2002. [Google Scholar]
- 14.Lozoff B, Brittenham GM, Wolf AW, McClish DK, Kuhnert PM, Jimenez E, Jimenez R, Mora LA, Gomez I, Krauskopf D. Iron deficiency anemia and iron therapy: effects on infant developmental test performance. Pediatrics. 1987;79:981–995. [PubMed] [Google Scholar]
- 15.Lozoff B, Jimenez E, Wolf AW. Long-term developmental outcome of infants with iron deficiency. N Engl J Med. 1991;325:687–694. doi: 10.1056/NEJM199109053251004. [DOI] [PubMed] [Google Scholar]
- 16.Cook JD, Finch CA. Assessing iron status of a population. Am J Clin Nutr. 1979;32:2115–2119. doi: 10.1093/ajcn/32.10.2115. [DOI] [PubMed] [Google Scholar]
- 17.Deinard AS, Schwartz S, Yip R. Developmental changes in serum ferritin and erythrocyte protoporphyrin in normal (nonanemic) children. Am J Clin Nutr. 1983;38:71–75. doi: 10.1093/ajcn/38.1.71. [DOI] [PubMed] [Google Scholar]
- 18.Saarinen UM, Siimes MA. Developmental changes in serum iron, total iron-binding capacity, and transferrin saturation in infancy. J Pediatr. 1977;91:875–877. doi: 10.1016/s0022-3476(77)80880-9. [DOI] [PubMed] [Google Scholar]
- 19.Felt B, Jimenez E, Smith J, Calatroni A, Kaciroti N, Wheatcroft G, Lozoff B. Iron deficiency in infancy predicts altered serum prolactin response 10 years later. Pediatr Res, doi: 10.1203/01.PDR.0000242848.45999.7b. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayley N. Bayley Scales of Infant Development. The Psychological Corporation; New York, NY: 1969. [Google Scholar]
- 21.Wechsler D. Manual for the Wechsler Preschool and Primary Scale of Intelligence. The Psychological Corporation; San Antonio: 1967. [Google Scholar]
- 22.Woodcock RW. Woodcock Spanish Psycho-educational Battery. DLM Teaching Resources; Allen: 1982. [Google Scholar]
- 23.Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. The Psychological Corporation; San Antonio: 1991. [Google Scholar]
- 24.Jastak S, Wilkinson GS. Wide Range Achievement Test-Revised. Jastak Associates, Inc; Wilmington, Delaware: 1984. [Google Scholar]
- 25.Wechsler D. Wechsler Individual Achievement Test. The Psychological Corporation; San Antonio: 1992. [Google Scholar]
- 26.Wechsler D. Manual for the Wechsler Adult Intelligence Scale-Revised. The Psychological Corporation; San Antonio: 1981. [Google Scholar]
- 27.Sattler JM. Assessment of Children. 3rd ed. Jerome M. Sattler, Publisher, Inc.; San Diego: 1992. Wechsler Intelligence Scale for Children--Revised (WISC-R): Description; pp. 119–143. [Google Scholar]
- 28.Brown TA. Confirmatory Factor Analysis for Applied Research: Methodology in the Social Sciences. The Guilford Press; New York, NY: 2006. [Google Scholar]
- 29.Lozoff B. Has iron deficiency been shown to cause altered behavior in infants. In: Dobbing J, editor. Brain, Behaviour, and Iron in Infant Diet. Springer-Verlag; New York: 1990. pp. 107–131. [Google Scholar]
- 30.Martins S, Logan S, Gilbert R. The Cochrane Library. Update Software; Oxford: Oxford: 2003. Iron therapy for improving psychomotor development and cognitive function in children under the age of three with iron deficiency anaemia (Cochrane Review) [DOI] [PubMed] [Google Scholar]
- 31.Pollitt E. Developmental sequel from early nutritional deficiencies: conclusive and probability judgments. J Nutr. 2000;130:350S–353S. doi: 10.1093/jn/130.2.350S. [DOI] [PubMed] [Google Scholar]
- 32.Hollingshead AB. Four Factor Index of Social Status. Yale University; New Haven, CT: 1975. [Google Scholar]
- 33.Raudenbush SW, Bryk AS, Cheong YF, Congdon R. Hierarchical Linear and Nonlinear Modeling. SSI Scientific Software International; Lincolnwood, IL: 2004. [Google Scholar]
- 34.Sameroff A, MacKenzie MJ. Research strategies for capturing transactional models of development: the limits of the possible. Dev Psychopathol. 2003;15:613–640. doi: 10.1017/s0954579403000312. [DOI] [PubMed] [Google Scholar]
- 35.Williams WM, Ceci SJ. A person-process-context-time approach to understanding intellectual development. Rev Gen Psychol. 1997;1:288–310. [Google Scholar]
- 36.Rutter M. Statistical and personal interactions: facets and perspectives. In: Magnusson D, Allen VL, editors. Human Development An Interactional Perspective. Academic Press; New York: 1983. pp. 295–319. [Google Scholar]
- 37.Lozoff B, Beard J, Connor J, Felt B, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker S, Greer S, Zuckerman B. Double jeopardy: the impact of poverty on early child development. Pediatr Clin North Am. 1988;35:1227–1240. doi: 10.1016/s0031-3955(16)36580-4. [DOI] [PubMed] [Google Scholar]
- 39.Escalona SK. Babies at double hazard: early development of infants at biological and social risk. Pediatrics. 1982;70:670–676. [PubMed] [Google Scholar]
- 40.Sameroff AJ, Seifer R, Baldwin A, Baldwin C. Stability of intelligence from preschool to adolescence: the influence of social and family risk factors. Child Dev. 1993;64:80–97. doi: 10.1111/j.1467-8624.1993.tb02896.x. [DOI] [PubMed] [Google Scholar]
- 41.Breslau N, Chilcoat H, Susser ES, Matte T, Liang K, Peterson EL. Stability and change in children′s Intelligence Quotient scores: a comparison of two socioeconomically disparate communities. Am J Epidemiol. 2001;154:711–717. doi: 10.1093/aje/154.8.711. [DOI] [PubMed] [Google Scholar]
- 42.Bond GC. Social economic status and educational achievement: a review article. Anthropol Educ Quarterly. 1981;12:227–257. [Google Scholar]
- 43.Harris A. The contradictions of education policy: disadvantage and achievement. Br Educ Res J. 2005;31:571–587. [Google Scholar]
- 44.Merton RK. The Matthew effect in science. Science. 1968;159:56–63. [PubMed] [Google Scholar]
- 45.Shaywitz BA, Holford TR, Holahan JM, Fletcher JM, Stuebing KK, Francis DJ, Shaywitz SE. A Matthew effect for IQ but not for reading: results from a longitudinal study. Reading Research Quarterly. 1995;30:894–906. [Google Scholar]
- 46.Pollitt E, Gorman KS, Engle PL, Rivera JA, Martorell R. Nutrition in early life and the fulfillment of intellectual potential. J Nutr. 1995;125:1111S–1118S. doi: 10.1093/jn/125.suppl_4.1111S. [DOI] [PubMed] [Google Scholar]
- 47.Neisser U, editor. The Rising Curve Long-Term Gains in IQ and Related Measures. First ed. American Psychological Association; Washington, DC: 1998. [Google Scholar]
- 48.Sherry B, Bister D, Yip R. Continuation of decline in prevalence of anemia in low-income children: the Vermont experience. Arch Pediatr Adolesc Med. 1997;151:928–930. doi: 10.1001/archpedi.1997.02170460066011. [DOI] [PubMed] [Google Scholar]
- 49.Male C, Persson LA, Freeman V, Guerra A, van′t Hof MA, Haschke F, Euro-Growth Iron Study Group Prevalence of iron deficiency in 12-mo-old infants from 11 European areas and influence of dietary factors on iron status (Euro-Growth study) Acta Paediatr. 2001;90:492–498. doi: 10.1080/080352501750197601. [DOI] [PubMed] [Google Scholar]