Abstract
Splenic marginal zone lymphomas (MZLs) have been found to occur at a high frequency in NFS.N mice congenic for high-expressing ecotropic murine leukemia virus (MuLV) genes from AKR and C58 mice. Based on morphological, immunological, and molecular studies of these mice, MZL is clearly recognizable as a distinct disease with a characteristic clinical behavior. MZL was staged according to the degree of accumulation and morphological change of cells within the splenic marginal zone, as follows: 1) a moderate increase in normal-looking MZ cells, judged to be prelymphomatous, and 2) MZL in three variants: i) distinct enlargement of MZ by normal-looking cells (MZL), ii) distinct enlargement of MZ by basophilic centroblast-like cells (MZL+), and iii) extensive splenic involvement by centroblast-like cells (MZL++). The rate of mitosis and apoptosis increases with lymphoma grade. In most cases, emergence of a dominant IgH clonal pattern in paired splenic biopsy and necropsy samples was correlated with progression. MZLs were transplantable and homed to the spleen. MZL may constitute a commonly occurring lymphoma type unrecognized, in part, because of the centroblastic morphology of high-grade MZL and possible overgrowth of lower-grade MZL by more aggressive follicular lymphomas.
For nearly 100 years, it has been recognized that the lymph follicles of the spleen are limited by a lighter zone of lymphoid cells, 1 named the marginal zone (MZ) by Snook 2 in 1964. The lymphoid cells of the MZ are now considered by some to be memory B cells derived from recirculating precursors that migrate into germinal centers (GCs) when prompted by specific stimuli. 3,4 After mutations of their immunoglobulin (Ig) genes, they reappear in the MZ and are apparently a reservoir of postfollicular memory B cells. 5 MZs occur in the spleen of all mammals studied (reviewed in Ref. 6 ). In contrast to humans and most other animals, in rats, MZs are histologically prominent 3 and divided into an outer and inner layer of cells by a clearly defined sinusoid. In mice, the MZ is less prominent, and the separating sinusoid is often not discernible.
Human malignant lymphomas of the splenic MZ cells were described as a separate clinical-morphological entity 7-12 named marginal zone lymphoma (MZL). 13 It is thought by some to be distinct from MZL of other localizations, for instance of stomach, the so-called mucosa-associated lymphoid tissue (MALT) lymphomas. Although human MZL is now a well established entity, in mice there have been only two morphological descriptions, in strains NZB 14 and NFS.V+15 (NFS/N congenic for loci encoding infectious ecotropic murine leukemia viruses (MuLV)16). The present study of high-MuLV-expressing NFS.V+ mice indicates that splenic MZLs were, in fact, the most common type of B cell lymphoma, representing 36% of the hematopoietic neoplasms examined (J. W. Hartley, S. K. Chattopadhyay, M. R. Lander, L. Taddesse-Heath, Z. Nagashfar, H. C. Morse III, T. N. Fredrickson, manuscript in preparation).
Studies of 240 cases of MZL and a series of splenic biopsies compared with subsequently obtained necropsy specimens showed that some MZLs progressed to a higher grade with a change in cytology to a centroblastic (CB) type. This change was associated with complete infiltration of the red pulp and follicle, leaving only the periarteriolar lymphoid sheath (PALS) component of the white pulp intact. Furthermore, MZLs were sometimes supplanted by centroblastic-centrocytic lymphoma (CBCCL) when both types occurred within the same spleen. MZL may thus be a fairly common lymphoma type in mice that was not recognized previously due to progression or replacement masking its occurrence.
Materials and Methods
Mice
NFS.V+ mice (NFS/N congenic for ecotropic MuLV genes were bred and housed under conventional conditions at MA BioServices (Rockville, MD). These mice express infectious MuLV throughout life and have been described elsewhere. 16,17 Approximately equal numbers of males and females were studied.
Histology and Immunocytochemistry
Tissue samples fixed in 10% buffered formalin were embedded in paraffin and, in some cases, in plastic for sectioning and staining with hematoxylin and eosin (H&E) or Giemsa. Blood smears and cytospin preparations were stained with Giemsa or Wright-Giemsa. For immunocytochemistry, tissues were mounted in tissue-freezing medium (TBS; Triangle Biomedical Service, Durham, NC), quick-frozen in dry ice and 2-methylbutane (Fisher Scientific, Fair Lawn, NJ), and stored at −70°C until cryostat sectioned. Immunostaining was performed using a Techmate1000 automated stainer (Ventana Biotek Systems, Tucson, AZ) and the following biotinylated mouse monoclonal antibodies against lineage-specific differentiation antigens: CD4, CD8, and CD45R (B220) (Gibco-BRL, Gaithersburg, MD) and IgM, Igκ light chain (IgK), and IgD (PharMingen, San Diego, CA). Biotin-labeled antibody to proliferating cell nuclear antigen (PCNA) was from Vector Laboratories (Burlingame, CA).
Flow Cytometry
Single-cell suspensions prepared from spleens of normal mice and mice with lymphomas were stained with a panel of antibodies for two-color or multicolor analyses performed using a FACScan, FACStar Plus, or a FACS Vantage (Becton Dickinson, San José, CA) by established techniques. 18 Cells were stained with antibodies to IgK, IgG, IgM, IgD, CD45R (B220), CD5, CD11b (Mac-1), CD90 (Thy-1), CD4, CD8, TCRα/β, CD23 (FcεRII), CD43, and CD44. Antibodies directly labeled with fluorochromes or with biotin were purchased from Becton Dickinson, PharMingen, Hybritech (San Diego, CA), or Zymed (South San Francisco, CA) or were prepared in our laboratory. Nonviable cells were excluded from study by combined gating on forward angle light scatter and staining with propidium iodide. Winlist and IsoContour software (Verity Software House, Topsham, MA) were used to reanalyze primary data.
Hemisplenectomy
Mice were digitally palpated, and those with an estimated splenic weight of 0.4 g were selected for biopsy. After anesthesia with ketamine HCl (Ketaset, Fort Dodge Laboratories, Fort Dodge, IA) and xylazine (Rompun, Mobay Corp., Shawnee, KS), an incision was made through skin and muscle to expose the spleen. The artery and vein were tied off, and an Abbey needle holder (Roboz Surgical Instrument Co., Rockville, MD) was used to clamp off the lower third of the spleen, which was then removed as the biopsy specimen. The skin incision was closed with clips (9-mm Autoclips, Clay Adams, Persippany, NJ). All surgery was performed in accordance with protocols approved by the National Institutes of Health.
Molecular Studies
High-molecular-weight DNAs were prepared from lymphoid tissues or tail samples 19 digested with restriction endonucleases, separated by electrophoresis in 0.7% agarose gels, transferred to nitrocellulose, and hybridized with 32P-labeled probes by established techniques. Detection of immunoglobulin heavy chain (IgH) rearrangements used EcoRI and the J11 JH probe; 20 for TCRβ rearrangements, HpaI and the CTβ probe were used. 21
Results
Occurrence and Morphological Description of MZL
Among 674 lymphomas in NFS.V+ mice, ∼90% B cell, 240 cases of MZL at early, intermediate, and advanced stages were diagnosed at an average age of ∼400 days. The spleen is the apparent site of origin, and dissemination beyond the splenic lymph nodes is rare. Affected spleens are symmetrically enlarged, without nodules or the coarse grayish mottling characteristic of lymphomas involving the follicles.
Histological and cellular cytological studies of these cases suggested tumor progression in which a staging of sequential changes could be followed.
Expanded Marginal Zone (EMZ)
As NFS.V+ mouse spleens normally do not have a distinct MZ, 22 its definite appearance around splenic follicles signals the first stage in MZL development. Because of the normal-looking morphology of MZ cells, lack of mitoses, and narrowness of the MZ, this is regarded as non-neoplastic hyperplasia.
MZL
At this stage, widening of the MZ is advanced, giving each follicle a distinct halo, sometimes confluent with neighboring MZs; however, cytology appears normal, and the number of mitoses is low (Figure 1, A and B) ▶ .
Figure 1.
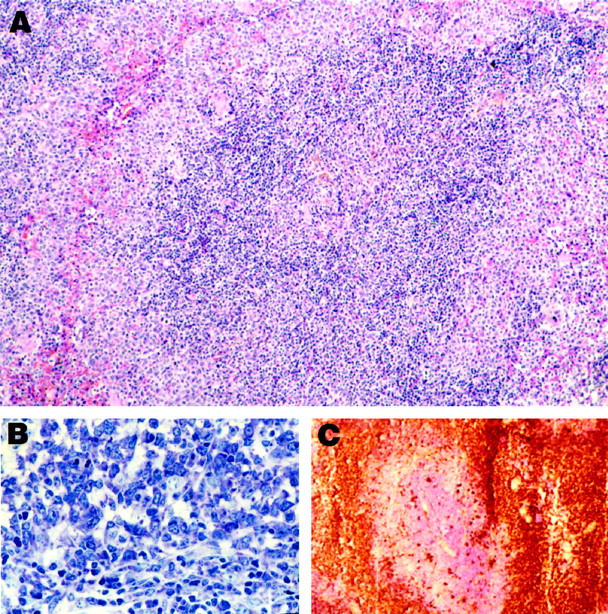
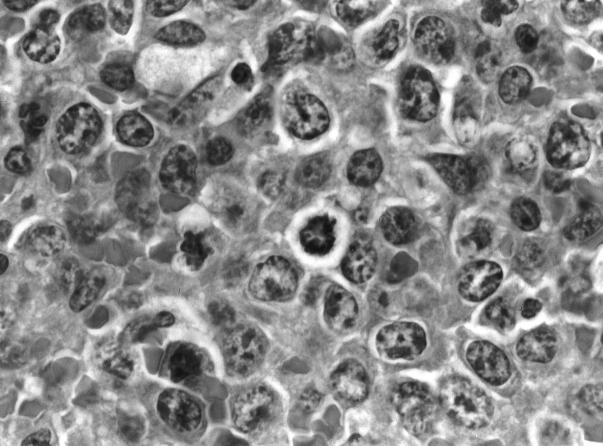
Histology and immunocytochemistry of MZL. A: MZL in low magnification. Note that the cells at the borders (marginal zones) are somewhat larger and more lightly stained than those in the center. H&E; magnification, ×110. B: Higher magnification of MZL, showing the high degree of basophilia in MZL cells. Giemsa; magnification, ×200. C: Frozen section, showing staining of MZL cells for IgM. The central portion, counterstained with hematoxylin, represents the T cells of the PALS. magnification, ×50.
MZL+
The most prominent change accompanying this stage is the appearance of MZ cells that have transformed to a CB-like morphology and display increased basophilia and numbers of mitotic figures. The follicles at this stage are intact, but much of the red pulp may be completely infiltrated.
MZL++
By this stage, almost the entire spleen is composed of basophilic CB-like or even immunoblast-like cells (Figure 2) ▶ , and the white pulp is reduced in size, leaving only the PALS intact. Lymphoma cells may be disseminated to splenic lymph nodes and, infrequently, the liver. Mitotic figures are frequent, PCNA labeling is extensive, and apoptotic bodies are numerous (not shown).
Figure 2.
Typical MZL++ composed of cells with CB-like morphology and with numerous mitotic figures. A biopsy taken from the same spleen 113 days earlier was diagnosed as EMZ because the marginal zone was only slightly enlarged and was composed of typical marginal zone cells. The biopsy had three IgH rearrangements, but only one remained at necropsy.
CBL Diffuse
A possible final stage, which cannot be verified as of MZ origin, is diffuse CBL, a condition in which the entire spleen is filled with CB, obliterating any morphological landmarks that would help to identify follicular or MZ origin.
On cytological criteria, we consider MZL as low-grade, MZL+ as intermediate, and MZL++ and CBL diffuse as high-grade lymphomas. Low-grade MZL was most often diagnosed in mice with spleen weights of 0.25 to 0.4 g. The number of cases with spleens of 0.8 g or more increased with progression in grade, being 5%, 30%, and 56% for MZL, MZL+, and MZL++.
Molecular and Immunophenotypic Profiles of MZL Lymphomas
MZLs were determined to be of B lymphocyte lineage based on prominent rearrangements of IgH genes (Table 1) ▶ . In the great majority of tests, the rearranged IgH bands were sharp and of a moderate to high intensity usually proportional to the extent of splenic infiltration as discerned histologically. They were of random size, within a range of 2.5 kb to ∼12 kb and often in association with a germline band of reduced intensity, and are considered to be clearly indicative of mono- or oligoclonality. Such bands were also present in tests of 29 of 35 EMZs (Table 1) ▶ , and although some bands were light, strong bands were found in 18 of the 29 clonal cases. Surprisingly, 9 of 32 morphologically normal or reactive spleens also showed evidence of oligoclonal or monoclonal B cell populations, although band intensity was usually weak. In some cases, other non-germline IgH bands were observed that were diffuse and of low intensity, and those in the range of 4.8 to 6.0 kb tended to co-migrate in samples from different mice with no substantial change in the intensities of the germline bands. We consider these repetitive bands to reflect the contribution of common DJH joins from many different cells and thus indicative of polyclonality.
Table 1.
Clonal Analysis of Spleens of NFS.V† Mice with Marginal Zone Lymphomas, Prelymphomatous Marginal Zone Lesions, or No Lymphoma
| Histopathological diagnosis | Number clonal/number tested* (%) | IgH rearrangements† | |||
|---|---|---|---|---|---|
| Mono | Oligo/Mono | Oligo | Poly | ||
| Lymphoma | |||||
| MZL | 47/47 (100) | 26 (55) | 9 (19) | 12 (26) | 0 |
| MZL+ | 37/37 (100) | 25 (68) | 10 (27) | 2 (5) | 0 |
| MZL++ | 80/80 (100) | 57 (71) | 18 (23) | 5 (6) | 0 |
| Total | 164/164 (100) | 108 (66) | 37 (23) | 19 (12) | 0 |
| Prelymphoma | |||||
| EMZ | 29/35 (83) | 7 (20) | 13 (37) | 9 (26) | 6 (17) |
| Normal or Reactive‡ | 9/32 (28) | 5 (16) | 0 | 4 (12) | 23 (72) |
*Number of splenic DNAs with mono- to oligoclonal IgH rearrangements divided by number tested.
†Number of cases and (percentage) of DNA samples with clonal IgH rearrangement in each diagnostic category. DNAs were prepared from spleen, digested with EcoRI, and hybridized with JH probe. Mono, monoclonal, or biclonal, represented by a single band or two of equal intensity; Oligo, oligoclonal, typically three to five bands; Oligo/Mono, represented by several bands of variable density but with a predominant monoclonal population; Poly, polyclonal, no distinct random-sized bands but a diffuse pattern and/or concentration in 4.8- to 6.0-kb size range.
‡Reactive spleens have normal architecture and red and white pulp activity but show one or more small germinal centers within the mantle zone.
The immunophenotype was uniform for MZL regardless of grade. By flow cytometry, the great majority were positive at low levels for CD5 and CD45R (B220), positive at low levels or negative for IgD, negative for CD23, positive at intermediate or most often high levels for IgM and IgK, and negative for IgG (Table 2 ▶ ; Figure 3 ▶ ). An equally consistent phenotype was revealed by immunocytochemistry: IgM (Figure 1C) ▶ and IgK bright positive (IgL was not examined), CD45R (B220) dull to normal, and CD5 negative. Both EMZ and MZL cells were uniformly negative for IgD, in contrast to the highly IgD-positive mantle zone cells.
Table 2.
Surface Antigen and Immunoglobulin Profiles of MZL Cells Determined by Flow Cytometric Analysis
| Staining intensity | Number of cases with indicated antigen expression | |||||
|---|---|---|---|---|---|---|
| κ | IgM | B220 | CD5 | CD11b | CD23 | |
| Bright | 5 | 28 | 3 | 1 | 0 | 0 |
| Normal | 11 | 11 | 9 | 0 | 1 | 2 |
| Dull | 10 | 12 | 32 | 51 | 24 | 2 |
| Negative | 3 | 0 | 11 | 5 | 30 | 21 |
| Total Cases | 29 | 51 | 52 | 57 | 55 | 25 |
Figure 3.
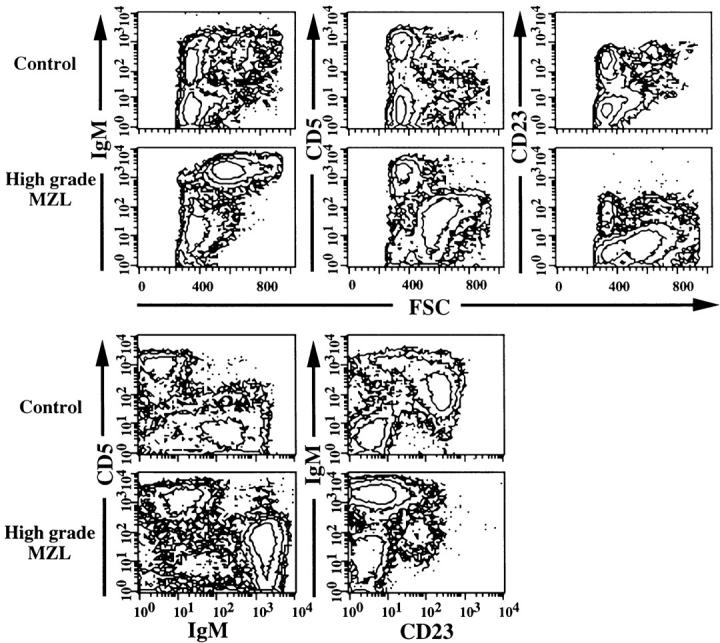
Phenotypic characteristics of high-grade MZL and EMZ in spleens as determined by multicolor flow cytometry. Control B cells had low forward angle light scatter (FSC) and expressed IgM at normal levels, were CD5−, and approximately 65% of B cells were CD23+. High-grade MZL cells were larger than control cells, as shown by increased FSC, were IgMbrightCD5dullCD23−, and constituted 60% of total spleen cells.
Using flow cytometric analysis, MZL+ or MZL++ cells could be distinguished from residual normal spleen cells by their larger size, as indicated by increased forward angle light scatter, by high-level expression of IgM and Igκ light chain, and by low-level expression of both CD5 and CD45R (B220), as shown for a high-grade case in Figure 3 ▶ . MZL cells with this phenotype could be distinguished at frequencies as low as 5% from normal CD5−B220+ B cells and CD5+B220− T cells of the PALS. Splenic cells of several MZL cases were sorted by flow cytometric selection of the IgMbrightCD5dull double-stained component. Cytospin preparations of sorted cells consisted of morphologically normal MZ and larger CB-like cells, consistent with the histological picture.
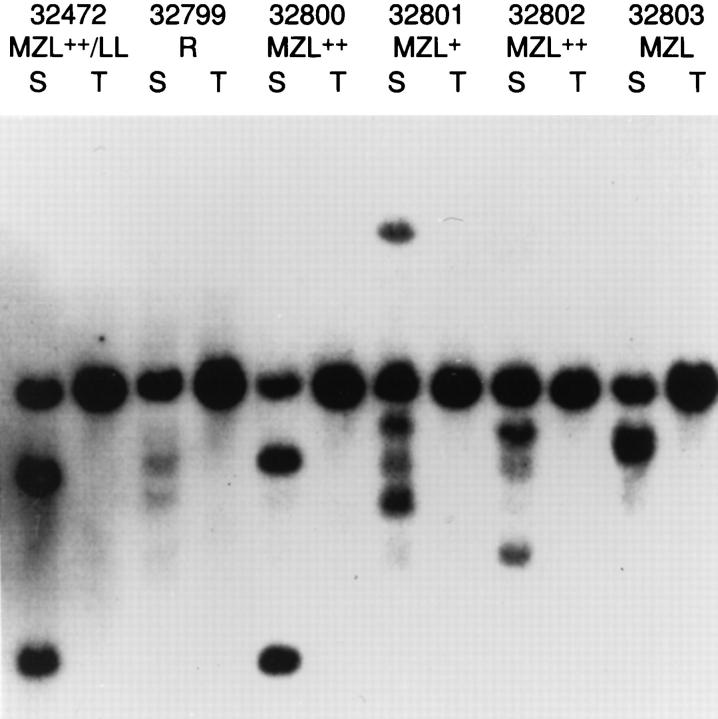
Characterization of low- to high-grade MZL by both histopathology and flow cytometry indicated the presence of lymphoma. Southern blot analysis of IgJH organization, illustrated in Figure 4 ▶ , indicated that lymphomas 32800 to 32803 were monoclonal populations with rearrangements of one or both alleles of the IgH locus, or were biclonal. The distinct bands in 32802 DNA suggest a relatively large population of lymphoma cells, in keeping with the histological picture. In the case of composite lymphoma 32472, it was not possible to distinguish clones representing either of the two morphological components. The low-density bands detected in spleen of the nonlymphomatous control 32799 are particularly distinct examples of the aforementioned repetitive-size bands in the 4.8- to 6.0-kb range sometimes detected in normal spleens.
Figure 4.
Southern blot analysis for clonal immunoglobulin heavy chain gene rearrangements in spleen (S) and tail (T) biopsy of NFS.V+ mice with the indicated histopathological findings. DNAs were digested with EcoRI and hybridized with the JH probe. MZL, MZL+, and MZL++ are progressive stages of MZ lymphoma; LL, lymphoblastic lymphoma; R, reactive changes. Tail DNA shows the 6.2-kb germline band.
Progression in Grade of Splenic MZL
To determine directly whether progression from low to high grade occurred and whether clonal populations would be maintained as grade advanced, splenic biopsies were obtained from 31 mice aged 8 to 19 months for comparison with samples taken at necropsy. Most mice were monitored for 1.5 to 7 months by palpation at regular intervals and killed on detection of clear splenic enlargement and/or lymphadenopathy. Early necropsy of several mice not included in the final tabulation verified that the procedure itself had not altered splenic morphology.
Biopsy diagnoses included normal morphology (2 cases), EMZ (12 cases), MZL (10 cases), and MZL+ to MZL++ (7 cases). Biopsy and necropsy samples were compared for extent of morphological change observed and occurrence and type of change in IgH clonal pattern or hybridization intensity. Shared bands were identified in 29 of 31 paired DNAs. Clonal changes were noted in the two normal biopsy cases and in six of the nine cases diagnosed as EMZ that progressed to MZL, MZL+, or MZL++. All involved loss of one or more of the clones seen in the biopsy splenic DNA, suggesting selection of a single clonal population by the time of necropsy. Progression from lower grade to MZL++ in three of four cases was accompanied by augmentation of band intensity or acquisition of new bands. When the biopsy diagnosis was of more advanced lymphoma, ie, MZL or higher stage, change in clonal pattern was rare. Progression was seen in 8 of 17 cases examined less than 2 months after biopsy, and clonal change was seen in only 2 of these. When the interval was 3 to 6 months, however, all of 13 cases progressed, with 9 showing clonal change. In an additional four cases that had been diagnosed as either EMZ or MZL at biopsy, necropsy revealed a complete replacement with another neoplasm, including CBCCL or lymphoblastic lymphoma (LL) or myelogenous leukemia (data not shown). In a fifth instance, a composite of CBCCL with advanced MZL developed from a low-grade MZL case.
Discussion
Comparison of Human and Mouse Marginal Zone Lymphomas
In mice, the primary site appeared to be the spleen, with peripheral spread largely restricted to draining lymph nodes and, exceptionally, the liver. Although traffic of normal MZ cells into the follicle after administration of certain immunological stimuli has been reported, 23 the first discernible expansion of the MZ is in the red pulp, but as disease progressed, the white pulp became increasingly disrupted. The cytology of human and mouse MZ cells appears to be very similar, as is the growth pattern. 8,11,24,25 The same is true for transformation into a higher-grade lymphoma; 26,27 however, it appears to be less frequent in humans. By immunohistochemistry, both human and mouse MZLs are negative for both IgD and CD5 expression, but by flow cytometry, we found mouse MZLs to be IgD negative or dull positive and CD5 dull positive. The much higher incidence of MZL in mice as compared with humans may reflect the life-long involvement of the mouse spleen in vigorous responses to repeated immunological challenges, this burden resting with lymph nodes rather than the spleen in humans. The recent detection, however, of early MZL in spleens of three individuals whose spleens were removed as a treatment for anemia or rupture 9 suggests that this type of lymphoma may be somewhat more frequent in humans than previously thought.
Diagnosis of MZL in Mice
MZL may be common in other strains of mice but not recognized because of progression to CBL-like cytology along with diffuse splenic infiltration or replacement of MZL by another lymphoma type. A case of reticulum cell sarcoma type B 28 in the study set forming the basis of the Dunn classification of 1954 29 is clearly MZL (T.N. Fredrickson, unpublished observation), so it does not appear to be a new disease. We have also observed MZL in both NZB and (NZB × DBA/2)F1 mice. 30 Now that this lymphoma has been described in detail, its true frequency can be addressed.
Do MZL Cells Derive from B1 B Cells?
The origins of MZ B cells in mice and humans are controversial. In both species, the great majority of cells are IgM+, suggesting that they have not passed through the germinal center to undergo isotype switching. Human MZ B cells, however, have mutated Ig VH genes, 5 which are characteristic of germinal-center-processed memory B cells responding to T-cell-dependent antigens. In keeping with these observations, Harris has suggested that MZ cells may be the progeny of antigen-selected centrocytes. 31 In contrast, studies of rodents indicated that in vivo Ig responses to T-independent type 2 antigens are mediated primarily by MZ cells not within germinal centers and that these cells can move from the MZ to the follicle soon after stimulation. 23,30 Another feature of rodent MZ cells is poor proliferative responses to Ig cross-linking, due in part to induction of apoptosis. 32 An apoptotic response to Ig cross-linking is also characteristic of CD5+ (B1) B cells, 33 a subset found at highest levels in the peritoneum. 34 B1 cells further resemble MZ cells in expressing IgM and responding vigorously to polysaccharide antigens, 34 suggesting a functional if not a lineage relationship between these B cell subsets. Parallels between normal B1 and MZ B cells could be of particular interest in view of the near uniform expression of CD5 at low levels, as detected by flow cytometry, by murine MZL.
Mouse CD5+ B lymphoma cell lines were first described nearly 20 years ago, 35 and CD5+ primary B cell lymphomas have since been reported in several strains. 36-39 We have recently found, however, that almost all mouse B cell lymphomas, MZ, CB, CBCC, lymphoblastic, lymphocytic, or IB, are CD5+ by flow cytometry (H.C. Morse and T.N. Fredrickson, unpublished observations). These findings, together with the demonstration that CD5 is an activation marker expressed by normal CD5− B cells responding to cytokines and polyvalent antigens, 40 suggest that CD5 expression by the mouse lymphomas is a consequence of activation/transformation rather than a marker identifying lineage.
Description of MZL Prelymphoma and its Progression
A prelymphomatous state has been postulated but not directly proven for certain mouse lymphomas, particularly lymphoplasmacytic lymphoma in SJL mice. 41 We have used two approaches in the study of MZL to establish the existence of prelymphoma. First, consistent with our observation that MZL was entirely splenic in mice, we found that early changes in MZ morphology, including low-grade lymphoma, could be identified in spleens between 0.25 and 0.40 g in weight. This led to a second approach, the comparison of splenic tissue obtained by biopsy and at necropsy. Combining these approaches, we have been able to morphologically and molecularly follow progression from prelymphoma to lymphoma. Clonal populations were found in many cases of prelymphoma (EMZ), 20% being monoclonal. In such monoclonal cases, molecular indication of neoplasia is not reflected in morphology (Table 1) ▶ . In following the development of MZL in mice, morphological progression in most cases was associated with maintenance of a major clone, although clonal replacement was sometimes noted. Minor clones tended to be lost with progression, and clonal patterns did not change if the stage of MZL remained stable. Those EMZ cases and the small proportion of normal-appearing spleens that contained distinct clonal populations (Table 1) ▶ may represent clones that must undergo additional mutations to attain malignancy. Demonstration by others of lymphoma outgrowth from transplanted spleen cells of normal-appearing mice 36-39 supports the idea that the clones we detect in nonlymphomatous spleen represent potentially malignant populations.
Spleen cell suspensions of two cases of low-grade MZL grew out in the marginal zone of spleens of recipient mice, indicating the homing pattern of MZL cells (data not shown). Approximately 4% of primary lymphomas consisted of two morphologically distinct types within the spleen, most being a composite of MZL and CBL, CBCCL, or immunoblastic lymphoma. Although the clonality of most cases of MZL indicated the malignant nature of this lymphoma type, an observed overgrowth by follicle-derived lymphomas in composite splenic lymphomas suggested that MZL was not an aggressive type. Transplantation of three composites showed, however, that MZL was equally and even preferentially transplantable compared with other splenic types (data not shown).
The molecular basis for development and progression of MZL in humans or mice is not well understood, although a substantial proportion of human MZLs have mutations of p53, 42 with mutant lymphomas tending to be more aggressive or resistant to drug treatment than those with normal alleles of p53. 43,44 In addition, studies of mice with a null mutation of p53 have revealed a high incidence of MZL occurring as early as 3 months of life. 45
We conclude that mouse MZL provides remarkable parallels to human MZL in terms of morphology, cytology, immunophenotypic profile, progression, and molecular characteristics. Studies of the mouse model should provide unique opportunities to dissect the contribution of oncogenes and tumor suppressors to induction and progression of a neoplasm conserved so well between mouse and man.
Acknowledgments
We gratefully acknowledge the expert technical assistance of M. Lander in DNA preparation and analysis and K. Müller for histology preparations. We appreciate the assistance provided by Dr. K. L. Holmes in the performance and interpretation of flow cytometry. We thank B. R. Marshall for skillful preparation of the manuscript.
Footnotes
Address reprint requests to Dr. Janet W. Hartley, LIP, NIAID, NIH, Building 7, Room 302, 7 Center Drive MSC-0760, Bethesda, MD. E-mail: jhartley@atlas.niaid.nih.gov.
Supported in part by contract NO1-AI-45203 at MA BioServices, Rockville, MD.
References
- 1.Weidenreich F: Das Gefäss-system der menschlichen Milz. Arch Mikr Anat 1901, 58:247-376 [Google Scholar]
- 2.Snook T: Studies on the perifollicular region of the rat’s spleen. Anat Rec 1964, 148:149-159 [DOI] [PubMed] [Google Scholar]
- 3.MacLennan ICM, Gray D, Kumararatne DS, Bazin H: The lymphocytes of the marginal zone: a distinct B lineage. Immunol Today 1982, 3:305-307 [DOI] [PubMed] [Google Scholar]
- 4.Gray D, Kumararatne DS, Lorton J, Khan M, MacLennan ICM: Relation of intra-splenic migration of marginal zone B cells to antigen localization on follicular dendritic cells. Immunology 1984, 52:659-669 [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn-Walters DK, Isaacson PG, Spencer J: Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J Exp Med 1995, 182:559-566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tischendorf F: Die Milz Handb Mikrosk Anatomie, vol 6, part 6. Berlin, Springer, 1969
- 7.Palutke M, Eisenberg L, Narano S, Han LL, Peeples TC, Kukuruga DL, Tabaczkna PM: B lymphocytic lymphoma (large cell) of possible splenic marginal zone origin presenting with prominent splenomegaly and unusual cordal red pulp distribution. Cancer 1988, 62:593-600 [DOI] [PubMed] [Google Scholar]
- 8.Schmid C, Kirkham N, Diss T, Isaacson PG: Splenic marginal zone cell lymphoma. Am J Surg Pathol 1992, 16:455-466 [DOI] [PubMed] [Google Scholar]
- 9.Rosso R, Neiman RS, Paulli M, Boveri E, Kindl S, Magrini U, Barosi G: Splenic marginal zone lymphoma: report of an indolent variant without massive splenomegaly presumably representing an early phase of the disease. Hum Pathol 1996, 26:39-46 [DOI] [PubMed] [Google Scholar]
- 10.Pawade J, Wilkins BS, Wright DH: Low-grade B cell lymphomas of the splenic marginal zone: a clinicopathological and immunohistochemical study of 14 cases. Histopathology 1995, 27:129-137 [DOI] [PubMed] [Google Scholar]
- 11.Hammer RD, Glick AD, Greer JP, Collins RD, Cousar JB: Splenic marginal zone lymphoma: a distinct B cell neoplasm. Am J Surg Pathol 1996, 20:613-626 [DOI] [PubMed] [Google Scholar]
- 12.Schmid U, Cogliatti SB, Diss TC, Isaacson PG: Monocytoid/marginal zone B-cell differentiation in follicle centre cell lymphoma. Histopathology 1996, 29:201-208 [DOI] [PubMed] [Google Scholar]
- 13.Harris NL, Jaffe ES, Stein H, Banks PM, Chan JKS, Cleary ML, Delsol G, deWolf-Peeters C, Falini B, Gatter KC, Grogan TM, Isaacson PG, Knowles DM, Mason DY, Muller-Hermelink H-K, Pileri SA, Piris MA, Ralfkiaer E, Warnke RA: A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood 1994, 84:1361-1392 [PubMed] [Google Scholar]
- 14.Yumoto T, Yoshida Y, Yoshida H, Ando K, Matsui K: Prelymphomatous and lymphomatous changes in splenomegaly of New Zealand Black mice. Acta Pathol Jpn 1980, 30:171-186 [DOI] [PubMed] [Google Scholar]
- 15.Fredrickson TN, Hartley JW, Morse HC, III, Chattopadhyay SK, Lennert K: Classification of mouse lymphomas. Curr Top Microbiol Immunol 1995, 194:109-116 [DOI] [PubMed] [Google Scholar]
- 16.Fredrickson TN, Morse HC, III, Rowe WP: Spontaneous tumors of NFS mice congenic for ecotropic murine leukemia virus induction loci. J Natl Cancer Inst 1984, 73:521-524 [DOI] [PubMed] [Google Scholar]
- 17.Rowe WP, Kozak CA: Germ-line reinsertions of AKR murine leukemia virus genomes in Akv-1 congenic mice. Proc Natl Acad Sci USA 1980, 77:4871-4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes KL, Lantz LM, Lee JS, Bedigian HG, Taubenberger JK: Characterization of a new antigen expressed by B and myeloid lineage cells identified by the monoclonal antibody LIP-6. Cell Immunol 1995, 166:131-140 [DOI] [PubMed] [Google Scholar]
- 19.Gross-Belland M, Oudet P, Chambon P: Isolation of high molecular weight DNA from mammalian cells. Eur J Biochem 1973, 36:32-37 [DOI] [PubMed] [Google Scholar]
- 20.Lang RB, Stanton LW, Marcu KB: On immunoglobulin heavy chain switching: two γ2b genes are rearranged via switch sequences in MPC-11 cells but only one is expressed. Nucleic Acids Res 1982, 10:611-630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedrick SM, Germain RN, Bevan MJ, Dorf M, Engel I, Fink P, Gascoigne N, Heber-Katz E, Kapp J, Kaufmann Y, Kaye J, Melchers F, Pierce C, Schwartz R, Sorenson C, Taniguchi M, Davis MM: Rearrangement and transcription of a T-cell receptor B-chain gene in different T cell subsets. Proc Natl Acad Sci USA 1985, 82:531-535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Koike R, Nishimura T, Tasumizu R, Tanaka H, Hataba Y, Watanabe T, Miyawaki S, Miyasaka M: The splenic marginal zone is absent in alymphoplastic aly mutant mice. Eur J Immunol 1996, 26:669-675 [DOI] [PubMed] [Google Scholar]
- 23.Groeneveld PHP, Erich T, Kraal G: In vivo effects of LPS on B lymphocyte populations, migration of marginal zone lymphocytes, and IgD-blast formation in the mouse spleen. Immunobiology 1985, 170:402-411 [DOI] [PubMed] [Google Scholar]
- 24.van Krieken JHJM, von Schilling C, Kluin M, Lennert K: Splenic marginal zone lymphocytes and related cells in the lymph node: a morphologic and immunohistochemical study. Hum Pathol 1989, 20:320-325 [DOI] [PubMed] [Google Scholar]
- 25.Wu CD, Jackson CL, Medeiros J: Splenic marginal zone cell lymphoma: an immunophenotypic and molecular study of five cases. Am J Clin Pathol 1996, 105:277-285 [DOI] [PubMed] [Google Scholar]
- 26.Ngan B-Y, Warnke RA, Wilson M, Takagi K, Cleary ML, Dorfman RF: Monocytoid B cell lymphoma: a study of 35 cases. Hum Pathol 1991, 22:409-421 [DOI] [PubMed] [Google Scholar]
- 27.Nathwani BN, Mohrmann RL, Brynes RK, Taylor CR, Hansmann ML, Sheibani K: Monocytoid B-cell lymphoma: an assessment of diagnostic criteria and a perspective on histogenesis. Hum Pathol 1992, 23:1061-1071 [DOI] [PubMed] [Google Scholar]
- 28.Dunn TB, Deringer MK: Reticulum cell neoplasm, type B, or the “Hodgkin’s-like lesion” of the mouse. J Natl Cancer Inst 1968, 40:771-820 [PubMed] [Google Scholar]
- 29.Dunn TB: Normal and pathologic anatomy of the reticular tissue in laboratory mice, with a classification and discussion of neoplasms. J Natl Cancer Inst 1954, 14:1281-1433 [PubMed] [Google Scholar]
- 30.Ramachandra A, Metcalf RA, Fredrickson T, Marti GE, Raveche E: Requirement for increased IL-10 in the development of B-1 lymphoproliferative disease in a murine model of CLL. J Clin Invest 1996, 98:1788-1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris NL: Low-grade B cell lymphoma of mucosa-associated lymphoid tissue and monocytoid B cell lymphoma. Arch Pathol Lab Med 1993, 117:771-775 [PubMed] [Google Scholar]
- 32.Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF: Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol 1997, 27:2366-2374 [DOI] [PubMed] [Google Scholar]
- 33.Bikah G, Carey J, Ciallella JR, Tarakhovsky A, Bondada S: CD5-mediated negative regulation of antigen receptor-induced growth signals in B-1 B cells. Science 1996, 274:1906-1909 [DOI] [PubMed] [Google Scholar]
- 34.Hardy RR, Hayakawa K: CD5 B cells, a fetal B cell lineage. Adv Immunol 1994, 55:297-229 [DOI] [PubMed] [Google Scholar]
- 35.Lanier LL, Warner NL, Ledbetter JA, Herzenberg LA: Expression of Lyt-1 antigen on certain murine B cell lymphomas. J Exp Med 1981, 153:998-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Houten N, Willoughby PB, Arnold LW, Haughton G: Early commitment to neoplasia in murine B- and T-cell lymphomas arising late in life. J Natl Cancer Inst 1989, 81:47-54 [DOI] [PubMed] [Google Scholar]
- 37.Davidson WF, Fredrickson TN, Rudikoff EK, Coffman RL, Hartley JW, Morse HC, III: A unique series of lymphomas related to the Ly-1+ lineage of B lymphocyte differentiation. J Immunol 1984, 133:744-753 [PubMed] [Google Scholar]
- 38.Rosner A, Peled A, Haran-Ghera N, Canaani E: Analysis of Ly-1+ B-cell populations and IgH rearrangements in “normal” spleens and in lymphomas of AKR/J and AKR Fv-1b mice. Cancer Res 1993, 53:2147-3153 [PubMed] [Google Scholar]
- 39.Stall AM, Farinas MC, Tarlinton DM, Lalor PA, Herzenberg LA, Strober S, Herzenberg LA: Ly-1 B-cell clones similar to human chronic lymphocytic leukemias routinely develop in older normal mice and young autoimmune (New Zealand Black-related) animals. Proc Natl Acad Sci USA 1988, 85:7312-7316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wortis HH, Teutsch M, Higer M, Zheng J, Parker DC: B-cell activation by crosslinking of surface IgM or ligation of CD40 involves alternative signal pathways and results in different B-cell phenotypes. Proc Natl Acad Sci USA 1995, 92:3348-3352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pattengale PK, Taylor DR: Experimental models of lymphoproliferative disease: the mouse as a model for human non-Hodgkin’s lymphomas and related leukemias. Am J Pathol 1983, 113:237-265 [PMC free article] [PubMed] [Google Scholar]
- 42.Baldini L, Fracchiolla NS, Cro LM, Trecca D, Romitti L, Polli E, Maiolo AT, Neri A: Frequent p53 gene involvement in splenic B-cell leukemia/lymphomas of possible marginal zone origin. Blood 1994, 84:270-278 [PubMed] [Google Scholar]
- 43.Baldini L, Guffanti A, Cro L, Fracchiolla NS, Colombi M, Motta M, Maiolo AT, Neri A: Poor prognosis in non-villous splenic marginal zone cell lymphoma is associated with p53 mutations. Br J Haematol 1997, 99:375-378 [DOI] [PubMed] [Google Scholar]
- 44.Du M, Peng H, Singh N, Isaacson PG, Pan L: The accumulation of p53 abnormalities is associated with progression of mucosa-associated lymphoid tissue lymphoma. Blood 1995, 86:4587-4593 [PubMed] [Google Scholar]
- 45.Ward JM, Taddesse-Heath L, Perkins SN, Chattopadhyay SD, Hursting SD, Morse HC III: Splenic marginal zone B cell and thymic T cell lymphomas in p53-deficient mice. Lab Invest 1999, in press. [PubMed]