Abstract
Wound contraction is mediated by myofibroblasts, specialized fibroblasts that appear in large numbers as the wound matures and when resistance to contractile forces increases. We considered that the regulation of myofibroblast differentiation by wound-healing cytokines may be dependent on the resistance of the connective tissue matrix to deformation. We examined transforming growth factor-β1 (TGF-β1) induction of the putative fibroblast contractile marker, α-smooth muscle actin (α-SMA), and the regulation of this process by the compliance of collagen substrates. Cells were cultured in three different types of collagen gels with wide variations of mechanical compliance as assessed by deformation testing. The resistance to collagen gel deformation determined the levels of intracellular tension as shown by staining for actin stress fibers. For cells plated on thin films of collagen-coated plastic (ie, minimal compliance and maximal intracellular tension), TGF-β1 (10 ng/ml; 6 days) increased α-SMA protein content by ninefold as detected by Western blots but did not affect β-actin content. Western blots of cells in anchored collagen gels (moderate compliance and tension) also showed a TGF-β1-induced increase of α-SMA content, but the effect was greatly reduced compared with collagen-coated plastic (<3-fold increase). In floating collagen gels (high compliance and low tension), there were only minimal differences of α-SMA protein. Northern analyses for α-SMA and β-actin indicated that TGF-β1 selectively increased mRNA for α-SMA similar to the reported protein levels. In pulse-chase experiments, [35S]methionine-labeled intracellular α-SMA decayed most rapidly in floating gels, less rapidly in anchored gels, and not at all in collagen plates after TGF-β1 treatment. TGF-β1 increased α2 and β1 integrin content by 50% in cells on collagen plates, but the increase was less marked on anchored gels and was undetectable in floating gels. When intracellular tension on collagen substrates was reduced by preincubating cells with blocking antibodies to the α2 and β1 integrin subunits, TGF-β1 failed to increase α-SMA protein content in all three types of collagen matrices. These data indicate that TGF-β1-induced increases of α-SMA content are dependent on the resistance of the substrate to deformation and that the generation of intracellular tension is a central determinant of contractile cytoskeletal gene expression.
Wound contraction and remodeling of granulation tissue involve the differentiation of fibroblasts into myofibroblasts, cells that typically express high levels of α-smooth muscle actin (α-SMA1,2). The myofibroblasts form tight adhesions to the substrate, 3 and it appears that their differentiation is temporally associated with the resistance of the wound to contractile forces. 2 Myofibroblasts also exhibit the formation and arrangement of stress fibers along the long axis of the cells, 4,5 which in turn facilitates tissue contraction.
To model wound contraction, hydrated collagen lattices have been used as floating or anchored matrices 6-8 that resemble early and later stages of wound remodeling, respectively. Notably, the elevated breaking strength of mature skin wounds is due to increased reorganization and cross-linking of collagen, 9 which can be modeled by varying the biophysical properties of collagen gels. Thus, contraction of floating collagen matrices provides a model for a mechanically relaxed tissue with low tensile strength comparable to resting dermis 8 or to very early stages of wound healing, 9 whereas anchored matrices develop into a more stressed tissue that resembles granulation tissue. Notably, in two variations of the in vitro collagen matrix reorganization model, the morphology and the behavior of fibroblasts in the gels reflect intracellular tension levels. In floating collagen matrices, fibroblasts develop a stellate morphology with long processes and a well developed subcortical actin meshwork. 7 In marked contrast, cells in anchored matrices become bi- polar, orient along lines of tension, develop prominent stress fibers and fibronexus junctions, and resemble myofibroblasts. 10
The molecular mechanisms of collagen contraction are largely unknown. Previous studies have suggested an essential role for cell surface collagen receptors. 11-13 Indeed, a direct role for the collagen-binding integrin α2β1 in fibroblast-mediated collagen gel contraction has been demonstrated. 12,13 Members of the β1 integrin family are known to mediate fibroblast interactions with collagen fibers, 14,15 and wound-healing cytokines such as transforming growth factor (TGF)-β may enhance collagen gel contraction by increasing the expression of the α2β1 integrin collagen receptor. 16 This integrin-dependent force generation appears to require the cytoplasmic domain of the α2β1 integrin as the extracellular domain is insufficient to mediate contraction. 12 Several growth factors (eg, TGF-β, platelet-derived growth factor (PDGF), and fibroblast growth factor) can modulate contraction of collagen matrices. TGF-β1 is an extensively studied cytokine on the basis of its importance in wound healing 17-19 and matrix formation 19,20 and possibly in regulating α-SMA expression. 21 It can stimulate contraction of both floating and anchored collagen matrices in vitro, 22 but this mechanism is poorly understood.
We have assessed previously cell-mediated remodeling of floating collagen matrices and showed that α-SMA is functionally important for collagen gel contraction. 23 Although all fibroblasts probably exhibit some degree of contractile ability, fibroblast populations that express α-SMA are evidently more contractile. Furthermore, studies on the effects of TGF-β on fibroblasts from wounds at different stages of repair and normal skin show that cells from older granulation tissue contract collagen matrices faster than cells from younger granulation tissue or normal skin. 24 The source of this variability of contractility between fibroblast populations from different stages of wound development is not understood. In view of the increased mechanical strength and reduced compliance of tissues at later stages of wound healing, 9,25 and because of the importance of integrins in gel contraction, 12,13 we considered that fibroblast responsiveness to TGF-β is regulated by the compliance of the supporting matrix and modulation through integrins. Currently, it is unknown whether the compliance of the matrix affects TGF-β-induced up-regulation of α-SMA and how tension-dependent cell responses to TGF-β1 are regulated. Based on in vivo results showing that the timing of the switch from the fibroblastic to the myofibroblastic phenotype 2 is temporally associated with the resistance of the wound to contraction, 9 we studied the effect of TGF-β1 on α-SMA expression by human fibroblasts cultured in collagen matrices with three different levels of resistance to deformation. Culture of cells in these three types of collagen substrates promoted the development of different levels of intracellular tension and allowed us to test the hypothesis that intracellular tension modulates the TGF-β1-induced expression of cytoskeletal genes associated with wound contraction.
Materials and Methods
Cell Culture and Collagen Substrates
Primary cultures of human gingival fibroblasts were obtained from biopsies of normal gingiva in patients aged 10 to 16 years as described. 26 Cells at passages 3 to 12 were used for all experiments. For all three types of collagen matrices (see below), cells were initially plated as monolayer cultures in α-minimal essential medium (α-MEM) plus 15% v/v fetal bovine serum on plastic tissue culture dishes. The medium was removed and cells were incubated with α-MEM and TGF-β1 (R&D Systems, Minneapolis, MN) or vehicle (PBS) for 3 days. Cells were incubated with TGF-β1 at concentrations of 1, 5, 10, and 20 ng/ml. Immunoblotting for α-SMA (see below) showed a dose-dependent response to TGF-β1, and the optimal dosage was 10 ng/ml TGF-β1. Subsequently, all experiments were performed at this dose. Notably, others 17,27 have used similar doses for studies of TGF-β-induced actin gene expression that are equivalent to levels of TGF-β found in wound fluid. 28
TGF-β treatments consisted of two protocols. In the first protocol, cells on tissue culture plastic were treated with PBS vehicle (controls) or with TGF-β1 (experimentals) at 10 ng/ml for 3 days in α-MEM without serum. Cells were then tryspinized and replated on either tissue culture plastic coated with collagen or in anchored or floating gels (see below) and incubated with either vehicle or TGF-β1 for 3 to 4 days. In the second protocol, both controls and experimentals were treated with TGF-β1 for 3 days on tissue culture plastic and then were trypsinized, inoculated onto collagen-coated plates, anchored collagen gels, or floating gels and incubated with either vehicle (controls) or TGF-β1 (experimentals) for 3 to 4 days.
Collagen solutions were prepared as described. 7 We used type 1 collagen as the substrate for all types of gels to validate comparisons based solely on the mechanical properties of the gels. Briefly, under sterile conditions, a collagen solution was prepared from 0.3 ml of 10X concentrated α-MEM, 0.3 ml of 0.26 mol/L NaHCO3 buffer, 0.3 ml of fetal bovine serum (serum), 0.12 ml of 0.1 mol/L NaOH, and 1.5 ml of Vitrogen 100 (Collagen Corp., Santa Clara, CA). For relatively low-compliance (ie, rigid) collagen substrates, films of collagen (∼10 μm thick) were prepared on tissue culture plastic and polymerized by neutralizing the collagen films to pH 7.4. To block nonspecific binding, plates were incubated with 1% (w/v) bovine serum albumin (BSA) for 4 hours at 4°C. Before plating the cells, dishes were rinsed with PBS to remove excess BSA. Cells were plated subsequently at 7.5 × 10 4 cells/cm 2 and incubated in α-MEM without serum in the presence or absence of TGF-β1 (see below). For anchored or floating gels, a cell suspension of 4 × 10 5 cells/ml in α-MEM without serum was added to the solution. Gel solutions were pipetted into tissue culture or non-tissue-culture dishes to obtain anchored or floating gels, respectively, as described previously. 29 Collagen gels were incubated at 37°C in 95% air and 5% CO2 until polymerization was completed. The gels were covered with α-MEM and incubated with TGF-β1 or vehicle for 3 to 10 days.
Physical assessments of gel compliance were performed with a mechanical deformation tester (Dynatek Dalta, Galena, MO) in which a pair of 5-mm-diameter disks was used to obtain measurements of gel compression in a 15-second deformation period. The tester was operated in the stroke mode, which in turn controlled the position of the actuator. For all gel types, the actuator function was a linear compression stroke of 1-mm displacement over a 15-second duration. The displacement of the compression disk was measured directly by a linearly variable differential transducer, and loads were measured by a load cell (50 g). The precision of the displacement transducer was ∼1 μm. Measurements were conducted at 30°C in α-MEM. All samples were representative 5-mm-diameter circles cut out of the different types of gels that were prepared. Displacement and load were plotted separately over each 15-second cycle for each sample (n = 5 separate samples for each gel type), and a best-fit curve over the duration of the compression stroke was obtained by linear regression analysis to estimate displacement versus time (mm/second) and load versus time (g/second) functions. Gel compliance was expressed in terms of the slope of the individual displacement versus time and load versus time functions and expressed as the average load versus gel compression over the entire 15-second sampling period (g/mm). A full 1-mm compression stroke was achieved for the anchored and floating gels, but because of the thin gels on the collagen-coated plates (∼10 μm), only minimal displacements were recorded.
Gel Contraction Assay
Cells on tissue culture plastic were serum starved for 48 hours, pretreated with TGF-β1 or vehicle for 3 days and were then incubated in the three different types of collagen matrices described above. We determined the effect of TGF-β1 on remodeling of anchored and floating gels using contraction assays conducted on triplicate cultures. 3H2O was added to the culture media, and the radioactivity of the gels at certain time points was measured. 30 Samples of cell/collagen solutions (400 μl, 4 × 10 5 cells/ml) were pipetted into 35-mm tissue and non-tissue-culture dishes. After polymerization, gels were covered with α-MEM without serum containing 1 μCi of 3H2O (1 mCi/g; Dupont, Boston, MA) with or without TGF-β1. Equilibration of radioactivity in the gels required 30 minutes. At the times indicated, the medium was removed, and the gels were rinsed quickly and dissolved in 0.5 ml of 1 mol/L NaOH. The samples were neutralized with HCl, mixed with 2 ml of scintillation solution, and counted in a Beckman scintillation spectrometer. The initial gel volume was determined in gels without fibroblasts.
Fluorescence Microscopy
The effect of TGF-β1 on development and reorganization of stress fibers was investigated using affinity labeling of filamentous actin. Cells were plated on collagen-coated coverslips in 24-well multichamber slides (7.5 × 10 4 cells/cm2) and treated with TGF-β1 as described above. Cells were washed with PBS and fixed with 1.5% paraformaldehyde, and F-actin was stained for 15 minutes with 5 × 10−6 mol/L tetramethylrhodamine isothiocyanate (TRITC)-phalloidin (Sigma Chemical Co., St. Louis, MO) in PBS containing 0.01% Nonidet. Finally, the coverslips were washed, air dried, and mounted with an anti-fade reagent. The total cell fluorescence due to (TRITC)-phalloidin was measured in a standardized area of cytoplasm (100 μm2) using a Leitz MVP-SP spectrofluorimeter (Wetzlar, Germany) and a 25× Plan Apo objective with excitation at 530/30 nm and emission monochromators set at 600/3 nm. The photomultiplier tube voltage was set to 599 V and the amplifier gain to 4×. The fluorescence of standardized unstained cell areas was subtracted from each sample measurement to correct for background and dark current. To stain for α-SMA, cells were incubated with a mouse anti-human α-SMA monoclonal antibody (1:50 dilution, clone 1A4, 31 Sigma) for 1 hour at 37°C followed by a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse antibody (1:100; Sigma) for 1 hour at 37°C. Preimmune mouse and goat sera were used to block nonspecific staining before antibody incubation. The cells were examined in a Leitz Orthoplan microscope equipped with specific filters for rhodamine and fluorescein. Cells were imaged on a confocal microscope (Leica CLSM, Heidelberg, Germany) with a 40× oil immersion lens (numerical aperture = 1.2). Optical sections were obtained at a nominal thickness of 1 μm. In some samples, computer-generated images were combined to reconstruct a composite image. For staining cells in collagen gels, fluorescence staining for α-SMA and F-actin was conducted as described for monolayer cultures.
Western Blotting
We quantified TGF-β1-induced modulation of α-SMA content by immunoblot analysis. For harvesting fibroblasts from the gels, each gel was rinsed thoroughly with Mg2+,Ca2+-free PBS and incubated for 10 minutes at 37°C with 0.3 ml of 0.05% trypsin/0.53 mmol/L EDTA solution, followed by a 20-to 30-minute incubation with 0.35 ml of collagenase (5 mg/ml). After cells were dispersed completely, enzymatic activity was blocked by the addition of 0.05 ml of serum. The dispersed cells were counted with a hemocytometer before collection by centrifugation at 14,000 RPM for 10 minutes at 22°C and 0.05 ml of extraction buffer (2% Triton X-100, 160 mmol/L KCl, 40 mmol/L Tris/HCl, 20 mmol/L EGTA, 10 mmol/L phenylmethylsulfonyl fluoride, 1 mmol/L leupeptin, 1 mmol/L benzamidine; Sigma). Protein content was assessed by the BioRad assay. Samples were boiled for 3 minutes at 95°C, and equal amounts of protein were electrophoresed on a 10% SDS gel. Separated proteins were transferred to nitrocellulose filters and probed with the mouse monoclonal antibody for α-SMA followed by a horseradish-peroxidase-conjugated second antibody and developed with ECL reagents (Amersham, Arlington Heights, IL). Blots were stripped and probed with a monoclonal antibody for β-actin (clone AC-15, Sigma) for comparison. Subsequently, X-OMAT Kodak films were exposed to the blots, and the density of the bands was estimated by Scan Analysis (Biosoft, Cambridge, UK). The ratio of the density of α-SMA to β-actin was calculated for each sample.
Integrins
We studied the effect of TGF-β1 on the expression of α2 and β1 integrin subunits. Immunoblotting for α2 and β1 integrins was performed with mouse monoclonal antibodies (clone P1E6 from Calbiochem, Temecula, CA, and 4B4-RD1 from Coulter, Burlington, Ontario, Canada). The importance of the α2β1 integrin on TGF-β1-induced expression of α-SMA was examined using mouse monoclonal antibodies to block β1 and/or α2 integrin subunits. Fibroblasts (7.5 × 10 4 cells/cm2) were preincubated with mouse monoclonal antibodies for β1 integrin (clone 4B4-RD1) and/or α2 integrin (clone P1E6) at room temperature, and the cells were allowed to adhere to collagen-coated plastic petri dishes. The next day media were removed, cultures were covered with α-MEM containing 10 ng/ml TGF-β1 and supplemented with β1 integrin (1:30) and/or α2 (1:50) integrin antibodies, and incubated for 3 days. The antibodies were replenished once during the 3-day incubation with the same concentrations used for preincubation. Each experimental condition was prepared in triplicate. To analyze the antibody-induced changes of cell morphology, cells were observed with a phase contrast microscope (Nikon, Mississauga, Ontario, Canada), and photographs were taken on the third day of incubation. At the end of treatment, immunoblotting for α-SMA was conducted as described before, and blot density was normalized to β-actin.
Flow Cytometry
Cell suspensions were prepared (0.01% trypsin), fixed with 3.7% formaldehyde, permeabilized in 0.02% Triton with PBS, and stained for α-SMA as described. 23 First (anti-α-SMA) and second antibody (FITC-conjugated goat anti-mouse) dilutions were 1:25 and 1:50, respectively. Cells were washed and resuspended in Mg2+,Ca2+-free PBS. Samples were analyzed on a FACStar Plus flow cytometer (Becton-Dickinson, Mississauga, Ontario, Canada) with 488-nm excitation and 530/30-nm band pass filter for FITC. For all flow cytometry analyses, at least 1 × 10 4 cells were assessed in each sample, and only cells with forward and orthogonal light scatter characteristics similar to whole, intact fibroblasts were included in the analysis by electronic gates previously established for fibroblasts. Mouse monoclonal antibodies for α2 (1:20 dilution, clone P1E6) and β1 (undiluted, clone 4B4-RD1) integrins were used for staining.
Northern Analysis
Northern blots were performed on monolayer cultures grown on plastic tissue culture dishes after 3 days of TGF-β1 treatment or vehicle. As described above for Western blots, cells were subsequently grown on either collagen-coated plates or anchored or floating gels in the presence or absence of TGF-β1 for 3 days to determine the effect of TGF-β1 on mRNA levels for α-SMA. The cells were isolated from gels as described for Western blotting, and total RNA was isolated as described. 29 RNA samples were separated in denaturing 1.3% formaldehyde-agarose gels, transferred to a nylon membrane (Bio-Rad) and cross-linked by ultraviolet light. The McMolly Tetra program (Soft Gene) was used to design 32-mer oligonucleotides (5′TCCACAGGACATTCACAGTTGTGTGCTAGAGA-3′ and 5′-CCATGCCAATCTCATCTTGTTTTCTGCGCAAG-3′) complementary to specific sequences of the α-SMA and β-actin mRNA 3′ untranslated region, respectively. The oligonucleotides were labeled with [γ32P]ATP (Dupont NEN) using 3′ end labeling. The blots were washed twice with 0.5% SSC plus 0.5% SDS in dH2O, each time for 30 minutes at 55°C, and exposed to Kodak X-OMAT films at −70°C between intensifying screens overnight. The blots were stripped and reprobed with [γ32P]ATP-labeled glyceraldehyde phosphate dehydrogenase cDNA.
35S Labeling and Immunoprecipitation
Confluent cell monolayers were serum starved for 24 hours and treated with vehicle or TGF-β1 for 72 hours. Cells were metabolically labeled for 24 hours with [35S]methionine (100 μCi/ml; ICN Biochemicals) in methionine-free α-MEM. Cells were plated on collagen-coated tissue culture plastic, anchored gels, or floating gels in the presence or absence of TGF-β1. Cells were isolated from collagen matrices as described above. Cell pellets were solubilized in 200 μl of lysis buffer. Insoluble material was removed by centrifugation at 10,000 × g for 5 minutes at 4°C. Radioactivity in cell lysates was counted, and equal amounts of radioactivity were used in immunoprecipitation assays. Supernatants were immunoprecipitated with α-SMA antibody overnight at 4°C. Immunocomplexes were recovered by binding to protein A-Sepharose (Zymed Laboratories, South San Francisco, CA) and washed four times with 25 mmol/L Tris-buffered saline (pH 7.4) containing 0.5% Triton X-100 and 1 mg/ml BSA and twice with 0.5 mmol/L NaCl and 25 mmol/L Tris-HCl (pH 7.4). The immunocomplexes were analyzed by electrophoresis on 10% polyacrylamide gels followed by fluorography and scanning for quantification for the density of the band. The data are expressed as percent remaining density for the different sample days, and these data provided an estimate of the percent decay of radioactivity due to nascent α-SMA.
TGF-β Quantification
The levels of active and total TGF-β1 produced by fibroblasts and the effect of exogenous TGF-β1 on these levels were measured with a human TGF-β1 immunoassay (Quantikine, R&D Systems) with a sensitivity of 5 pg/ml. Cultures of fibroblasts in floating and anchored gels or on collgen plates were prepared as described. Cells were incubated with α-MEM with or without 10 ng/ml TGF-β1. After 3 days of culture, supernatants were collected, triplicate samples and activated, and non-activated forms were assayed. Activation was performed by adding 0.1 ml of 1 N HCl to 0.5 ml of cell supernatants for 10 minutes followed by neutralization with 0.1 ml of 1.2 N NaOH. To determine the amount of adherence of TGF-β1 to collagen, 10 ng/ml TGF-β1 was incubated in collagen-coated plates without cells, and the medium was assayed after 3 days. The samples containing exogenous TGF-β1 were diluted 1:5 to adjust the concentration of TGF-β1 to the linear range of the kit. Samples were analyzed in a 96-well microtiter plate coated with recombinant human TGF-β1 soluble receptor type II. The optical density of wells was determined with a microtiter plate reader set to 450-nm absorbance. The readings at 570 nm were subtracted from readings at 450 nm to correct for nonspecific absorbance. A standard curve was constructed by plotting the mean absorbance for each standard against the concentration, and a best-fit curve was determined by regression analysis.
To determine the specificity of the effect of TGF-β1 on α-SMA expression, an anti-TGF-β neutralizing antibody was used to block the effect of TGF-β1. Cells were plated on 35-mm collagen-coated dishes and anchored and floating gels. After 24 hours, media were replaced by α-MEM with or without 10 ng/ml TGF-β1 and with or without anti-TGF-β1 neutralizing antibody (15 μl/ml; R&D Systems). After 3 days, α-SMA content was quantified by Western blot.
Results
Effect of TGF-β1 on Collagen Gel Contraction
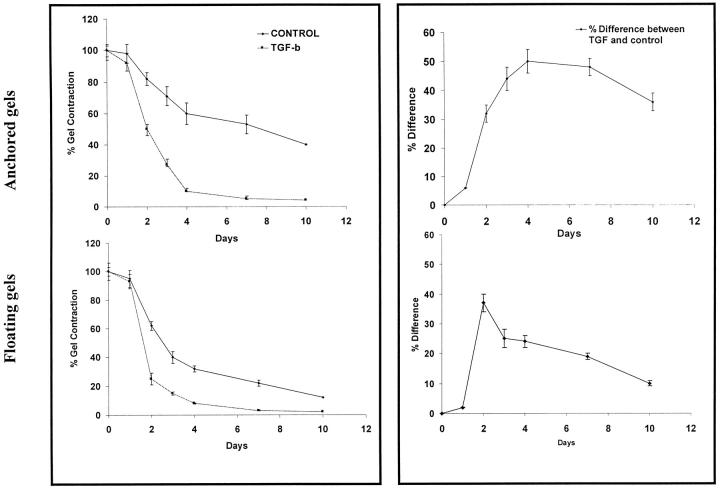
As several growth factors in serum potentially can influence collagen gel contraction, we have focused specifically on the effect of TGF-β1 in serum-free conditions. Contraction was determined by measuring gel volume based on the partitioning of 3H2O between the gel phase and the surrounding medium in time course studies. 32 If cells were not preincubated with TGF-β1 and the cytokine was added only from the beginning of the gel contraction assay, only small differences of gel contraction rate were observed between experimentals and controls from 0 to 10 days (data not shown). If, however, cells were preincubated with TGF-β1 for 3 days before incubation in the gels and the cytokine treatment was continued during the gel contraction phase, large and statistically significant (P < 0.01) differences were seen (Figure 1) ▶ . The floating gels showed a maximal difference of contraction between TGF-β1 and controls at day 2, which then decreased rapidly. In contrast, anchored gels exhibited a prolonged maximal difference from days 4 to 10. In view of these findings we assessed whether there was a temporal relationship between TGF-β1-induced up-regulation of α-SMA and the ability of the cells to contract collagen matrices. Cells on collagen plates were incubated for 1 to 3 days with TGF-β1 at a concentration of 10 ng/ml. Immunoblotting demonstrated that cells exposed to TGF-β1 for only 1 day showed little change in α-SMA content whereas incubation for 3 days showed an optimal increase in α-SMA. These data are consistent with the minimal effects of TGF-β1 on gel contraction without cytokine preincubation and greatly enhanced contraction with preincubation and provided a rationale for conducting all additional experiments with either a 3-day preincubation of cells with 10 ng/ml TGF-β1 or with vehicle.
Figure 1.
Collagen gel contraction and regulation by TGF-β1. Left panels: Cells in anchored (top) and floating (bottom) collagen gels were incubated in α-MEM containing 3H2O (1 μCi/ml) with or without TGF-β1 (10 ng/ml). At 1, 2, 3, 4, 7, and 10 days, gel volume was determined using scintillation counting of labeled gels. For each time point, n = 3 independent samples. Results are presented as mean percent gel volume ± SEM. Right panels: Data from left panels re-plotted to show percent differences between TGF-β1-treated cultures and controls.
Gel Compliance
The floating gels were highly compliant as determined by mechanical testing and offered little resistance to deformation (5.67 ± 0.56 mg/μm). As they were physically separated from the culture dish, this type of gel could contract in all three dimensions. In contrast, the anchored gels offered increased resistance to deformation (13.6 ± 2.25 mg/μm; P < 0.01), and as they were fixed to the sides and the bottom of the dish, these gels could only contract in the z axis. Collagen-coated plates showed high resistance to deformation (57.8 ± 20.4 mg/μm; P < 0.05) and were not free to contract significantly in any dimension.
TGF-β1 Enhancement of Stress Fibers Depends on Gel Compliance
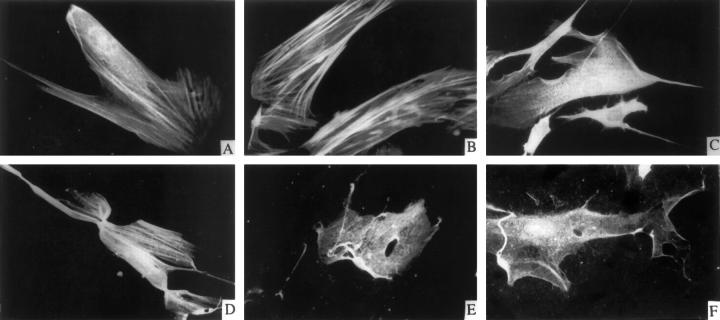
In collagen-coated plates (ie, low compliance), compared with controls, TGF-β1 promoted the development of stress fibers that were prominently stained for α-SMA (Figure 2) ▶ . As reorganization of stress fibers is promoted by the development of intracellular tension, 6 we assessed quantitatively whether TGF-β1 could regulate F-actin content. Affinity labeling of filamentous actin by rhodamine-phalloidin in cultures on collagen-coated plates was measured by single-cell photometry. There was a >3-fold increase in F-actin content compared with untreated controls (TGF-β1 = 74.3 ± 4.2 intensity units, and controls = 22.7 ± 5.8 intensity units; P < 0.01). Confocal imaging of cells stained for filamentous actin in monolayer cultures or anchored or floating gels revealed the development of prominent stress fibers parallel to the long axis of cells in monolayer cultures and to a lesser extent in anchored gels. Stress fibers were not detected in floating gels. As noted above, F-actin staining was significantly enhanced with TGF-β1 in monolayer (Figure 2, A and B) ▶ and to a lesser extent in anchored gels (Figure 2, C and D) ▶ ; however, this enhancement was not detectable in floating gels (Figure 2, E and F) ▶ , suggesting that stress fiber formation and the development of intracellular tension is dependent on the compliance of the substrate and may regulate TGF-β1-induced effects on α-SMA.
Figure 2.
Immunofluorescence of α-SMA in cells on different substrates. Confocal micrographs of cells on collagen-coated plastic (A and B), anchored collagen gels (C and D), and floating collagen gels (E and F) were incubated with vehicle (A, C, and E) or with TGF-β1 (10 ng/ml; B, D, and F) for 3 days. α-SMA was immunostained with anti-α-SMA antibody and FITC-conjugated goat anti-mouse antibody.
Effect of TGF-β1 on α-SMA Protein and mRNA Content
We determined quantitatively whether TGF-β1 induces changes of α-SMA protein content as a function of β-actin content. Western blotting was performed and β-actin content was used for comparison and for equilibration of protein loading as we found that in preliminary experiments, when lanes were loaded with equal amounts of protein, β-actin did not change appreciably under different experimental conditions. For cells grown on tissue culture plastic but without collagen coating, the amount of α-SMA in serum-free conditions was increased ∼threefold by TGF-β1 (10 ng/ml; 3 days) compared with controls (Figure 3A) ▶ . For cells grown on collagen gels, all data were normalized to β-actin content and are expressed as a ratio to overcome any variations in the efficiency of cell extraction and cytoskeletal protein isolation from the different substrates. After adjusting for equal protein loading by BioRad assay, β-actin did not show any change between the different matrices for controls and TGF-β1-treated cultures. Cells grown on collagen-coated plates showed ninefold increases in α-SMA:β-actin ratios after a 3-day TGF-β1 pretreatment and another 3 days of TGF-β1 treatment compared with controls. When cells were pretreated identically and then grown in anchored collagen gels and treated with TGF-β1, the α-SMA:β-actin ratio increased by 2.9-fold compared with controls. For floating gels, TGF-β1 induced only a 1.4-fold increase in the α-SMA:β-actin ratio (Figure 3B) ▶ . These findings are consistent with the results of immunofluorescence staining and also indicate that when TGF-β1-pretreated cells (with known increases of α-SMA content; Figure 3A ▶ ) are subsequently grown on different matrices (collagen-coated plastic, anchored gels, and floating gels), they continue to exhibit marked differences in their relative α-SMA content on different matrices. To separate the effect of TGF-β from that of gel compliance on α-SMA levels, we pretreated both experimentals and controls with TGF-β for 3 days, and then on inoculation into collagen gels, cells were treated with either vehicle or TGF-β (treatment protocol 2). Cells grown on collagen-coated plates showed a ∼sixfold increase of the α-SMA:β-actin ratio after TGF-β treatment. There were 2.3-fold and 1.2-fold increases for anchored gels and floating gels, respectively, after TGF-β treatment. These ratios were very similar to those observed without TGF-β pretreatment.
Figure 3.

Western blots of α-SMA protein content. A: Cells on plastic plates were incubated with vehicle control or TGF-β1 (10 ng/ml) for 3 days. Immunoblots of α-SMA and β-actin were scanned and the densities plotted (mean density ± SEM). B: Cells were preincubated for 3 days with vehicle or with TGF-β1 (10 ng/ml) on plastic plates as in A and then inoculated on collagen-coated plates or anchored collagen gels or in floating gels and incubated again with or without 10 ng/ml TGF-β1 for another 3 days. C: Both control and experimental cells were preincubated with TGF-β1 (10 ng/ml) for 3 days and then inoculated on collagen-coated plates or anchored or floating collagen gels for 3 days and treated either with vehicle (controls) or with TGF-β1. Western blotting and densitometry were used to assess α-SMA content and β-actin content in three independent samples. Data are expressed as a ratio of the blot densities of α-SMA to β-actin (mean ratio ± SEM).
Although these experiments were conducted without serum, it was possible that the increases of the α-SMA:β-actin ratios were due to TGF-β-mediated increases of cell numbers. However, cell counts at the end of the treatment periods showed only modest increases between experimentals and controls for all of the collagen gel types (collagen plates: controls, 1.0 × 10 6 cells/plate, and experimentals, 1.6 × 10 6 cells/plate; anchored gels: controls, 4.0 × 10 5 cells/ml of collagen gel, and experimentals, 4.8 × 10 5 cells/ml of collagen gel; floating gels: controls, 4.0 × 10 5 cells/ml of collagen gel, and experimentals, 4.2 × 10 5 cells/ml of collagen gel).
We also assessed whether TGF-β1 increased α-SMA at the mRNA level. Cells were grown and treated exactly as described for protein analysis. Before plating on collagen substrates, α-SMA mRNA in cells treated with TGF-β1 for 3 days on plastic plates was increased compared with untreated control cells (Figure 4A) ▶ whereas β-actin mRNA levels were unchanged. After a 3-day pretreatment with TGF-β1 and then 3 days of treatment in collagen matrices, cells on collagen-coated plates exhibited large increases of α-SMA mRNA. Cells in anchored gels showed much smaller increases in TGF-β1-induced α-SMA mRNA, whereas cells in floating gels showed no detectable increase in response to TGF-β1 treatment (Figure 4B) ▶ . Thus, at both the protein and mRNA levels, the ability of TGF-β1 to modulate α-SMA was dependent on the compliance of the substrate.
Figure 4.
Northern blots of α-SMA and β-actin. Cells were prepared as in Figure 3 ▶ . A: Fibroblasts were incubated with TGF-β1 or vehicle on tissue culture plastic for 3 days, and mRNA levels were measured by Northern hybridization using oligonucleotide probes specific for either human α-SMA or β-actin. B: Cells were preincubated with either vehicle or TGF-β as in A and then inoculated in collagen-coated plates, anchored collagen gels, or floating collagen gels and treated with or without TGF-β1 for 3 days. Cells were assayed for α-SMA and β-actin mRNAs by Northern hybridization. Note the lack of up-regulation of α-SMA by TGF-β1 in floating gels.
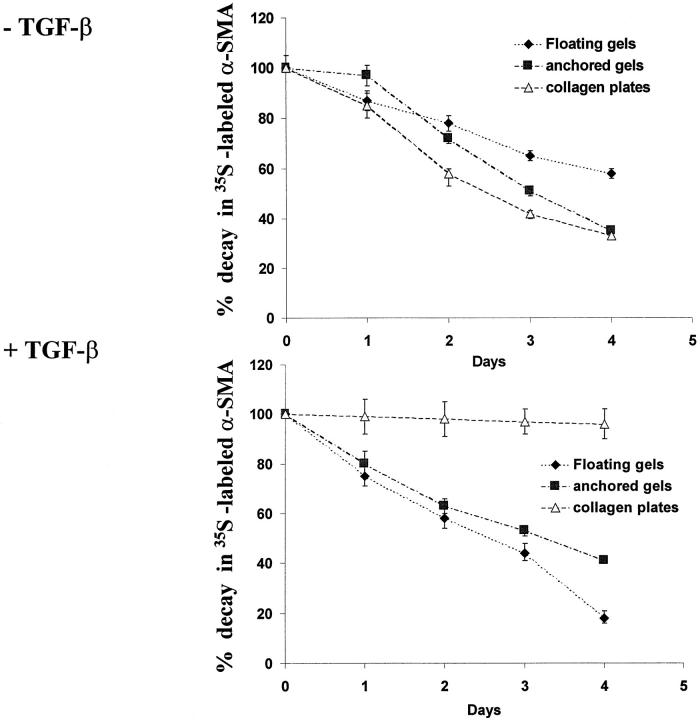
As α-SMA protein may have been either preferentially degraded or lost through transient membrane passages 33 in the different gel types, we measured loss of nascent α-SMA by metabolic labeling of cells for 24 hours on plastic plates with [35S]methionine followed by incubation of cells in gels for up to 4 days. In untreated cultures, cells on collagen-coated plates as well as anchored and floating gels showed similar reductions of radiolabeled α-SMA between 1 and 2 days whereas floating gels showed significantly less decay at days 3 and 4 (Figure 5 ▶ ; P < 0.01). In TGF-β1-treated cultures, nascent α-SMA content was relatively constant in cells in collagen-coated plates whereas large losses occurred in cells in anchored and particularly floating gels.
Figure 5.
Loss of nascent α-SMA by metabolic labeling analyses. Cells were preincubated with vehicle or TGF-β and labeled for the last 24 hours on plastic plates with [35S]methionine followed by inoculation of cells in different types of collagen gels for up to 4 days with or without TGF-β as indicated. Cells were released from gels and subjected to immunoprecipitation with anti-α-SMA antibody. The relative amounts of nascent α-SMA content were estimated from fluorographic blot densities and expressed as percentage of starting material (ie, day 0 of incubation in gels). Results are expressed as mean ± SEM percent starting material.
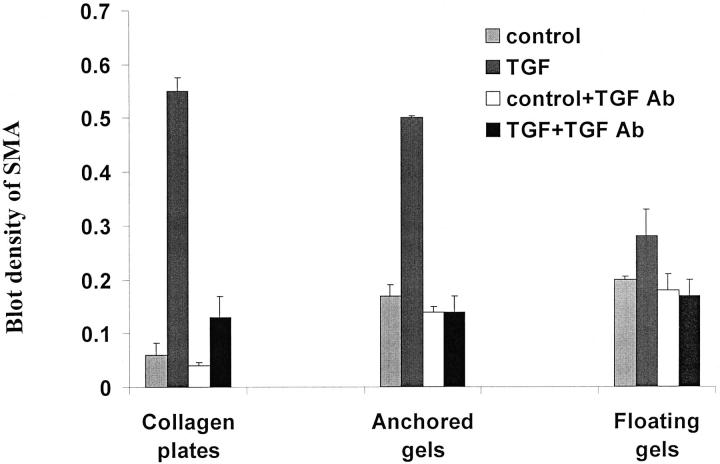
TGF-β1 Antibody Inhibition of α-Smooth Muscle Actin
Although TGF-β1 can regulate the synthesis of PDGF in some cell types, 34 PDGF does not induce α-SMA expression in dermal fibroblasts 35 or in the gingival fibroblasts studied here (data not shown); consequently, it is unlikely that TGF-β1 acted indirectly by inducing autocrine production of PDGF. To assess the specificity of TGF-β1 on the up-regulation of α-SMA we blocked TGF-β1 with a TGF-β neutralizing antibody that reacted with all TGF-β isoforms. Incubation of TGF-β1-treated cells on collagen-coated plates and anchored and floating matrices with the inhibiting antibody caused a large and significant reduction in α-SMA levels equivalent to that of untreated controls (Figure 6) ▶ . These results demonstrated the specificity of TGF-β1 in up-regulating α-SMA expression but also suggested a possible role for endogenous TGF-β1 in the induction of α-SMA. Therefore, we measured the actual concentrations of active and total TGF-β1 by ELISA in the culture conditions to which the cells were exposed. Addition of TGF-β1 (10 ng/ml) apparently stimulated cells to synthesize TGF-β1 for all three types of collagen substrates. More than half of the TGF-β1 produced by cells was in an active form. The levels of active TGF-β1 produced by cells in collagen-coated plates, anchored gels, and floating gels were 1.05 ± 0.3 ng/ml, 1.09 ± 0.23 ng/ml, and 1.06 ± 0.02 ng/ml, respectively, when exogenous TGF-β1 (10 ng/ml) was added. In control cultures, the active form of TGF-β1 was <0.005 ng/ml, and total TGF-β1 was 0.7 ng/ml (no significant difference between three different types of gels; P > 0.2). These data indicated that the type of gel did not significantly alter the endogenously produced levels of TGF-β1 and that cells were exposed to equivalent levels of TGF-β1 for all types of substrates.
Figure 6.
TGF-β antibody blockade of α-SMA induction. Cells were preincubated with vehicle or TGF-β1 for 3 days on plastic plates as in Figure 3 ▶ , inoculated into the three different types of collagen gels and incubated with PBS (control) or TGF-β1 (TGF) with or without 15 μg/ml anti-TGF-β neutralizing antibody (TGF Ab) for 3 days. Three independent samples were analyzed for the expression of α-SMA by Western blot. Results are expressed as mean density of α-SMA ± SEM. Values are normalized to β-actin densities for each lane.
Integrin Content
TGF-β1 increases α2β1 integrin expression in fibroblastic 36 and osteogenic cells, 16 and the α2β1 collagen receptor is important for collagen gel contraction. 12,13 We asked whether this increase was the result of TGF-β1-induced increase of integrin expression as has been reported earlier for certain fibroblastic cell lines cultured on plastic. 36 Flow cytometric analyses of the surface expression of α2 and β1 integrin subunits of untreated cells grown on collagen-coated plates, anchored gels, and floating gels showed mean staining intensities of 53.5 ± 10.7, 61.7 ± 4.8, and 154.9 ± 8.22, respectively, for α2 integrin subunits and 105.6 ± 9.8, 108.6 ± 7.5, and 121.3 ± 14.5, respectively, for β1 integrin subunits. As α2 integrin extracellular domains are insufficient for collagen gel contraction, it has been suggested that the cytoplasmic domain of the α2 integrin is involved in force generation required for contraction. 12 Consequently, we extended the flow cytometry results by immunoblotting whole-cell lysates for both α2 and β1 subunits in controls and TGF-β1-treated cultures. For controls, there was a twofold higher level of α2 integrin content and a 20% higher β1 content in cells in floating gels compared with cells grown on collagen-coated plates or anchored gels (Figure 7) ▶ , consistent with surface expression analyses by flow cytometry. TGF-β1 induced 1.5-fold and 2-fold increases in α2 integrin content and 1.5-fold and 1.2-fold increases in β1 content, respectively, for cells on collagen plates and anchored gels. TGF-β1 did not induce a significant change of α2 or β1 integrin expression in floating gels (Figure 7) ▶ . These results suggested to us that tension is required for TGF-β1-induced increases of collagen receptor expression.
Figure 7.
Integrin content of cells in collagen gels. Cells grown on tissue culture plastic were preincubated with vehicle or TGF-β and then inoculated into different types of collagen gels. Cells were incubated with or without TGF-β1 for another 3 days. Three independent samples were analyzed by Western blot for α2-integrin or β1-integrin by Western blots. β-actin was used for normalization of protein loading for each lane.
Loss of Tension Reverses TGF-β Induction of α-Smooth Muscle Actin
As intracellular tension was evidently required for TGF-β1-induced increase of α-SMA, we asked whether release of intracellular tension would abrogate the effect of TGF-β1 on α-SMA. We used blocking antibodies to induce cell rounding and to release intracellular tension in cells that remained adherent to the collagen substrate. Cells were preincubated with the monoclonal antibodies P1E6 (which blocks the α2 subunit14) and/or 4B4 (which blocks the β1 subunit37) and treated with TGF-β1 (10 ng/ml) for 3 days. To assess whether the antibodies were relieving intracellular tension as expected, cells on collagen-coated plates were examined by phase contrast microscopy. The cells were initially well spread with or without TGF-β1, but after treatment with antibodies for 3 days they exhibited rounding (Figure 8, A–E) ▶ and loss of stress fibers. These alterations were most prominent after β1 integrin antibody incubations and indicated that the anti-integrin antibodies greatly reduced intracellular tension. Quantitative analyses of α-SMA by Western blot demonstrated that preincubation with P1H5 alone reduced the level of α-SMA by 60% in TGF-β1 cells on collagen-coated plates. Treatment with 4B4 alone decreased TGF-β1-induced increases of α-SMA to nearly that of untreated controls without antibody. Preincubation with both antibodies reduced the effect of TGF-β1 to the level of controls (Figure 8F) ▶ . For cells on anchored gels and floating gels, the anti-integrin antibodies also reduced the TGF-β1-induced increase of α-SMA to control levels, although the amount of reduction was less for anchored gels and much less for floating gels. These results were not caused by cell death as the viability of cells preincubated with antibodies and tested at the end of the incubations (ie, 4 days) exceeded 95% as measured by trypan blue exclusion. These data indicate that TGF-β1 requires the development of intracellular tension and α2β1 integrins to induce increased α-SMA expression.
Figure 8.
A to E:Phase contrast photomicrographs of cells treated with α2- and/or β1-integrin inhibiting antibodies. Cells were incubated with vehicle control (A), TGF-β1 alone (B), TGF-β1 with anti-α2-integrin antibody (C), anti-β1-integrin antibody (D), or both anti-integrin antibodies (E). Micrographs were obtained on day 3 of treatment on collagen-coated plates. Magnification, ×150. F: Fibroblasts on collagen-coated plates, anchored gels, or floating gels were incubated with either no antibody (control) or with only anti-α2-integrin antibody or with only anti-β1-integrin in the presence or absence of TGF-β1 for 3 days. Antibodies were replenished once during the incubation period. Three independent samples were assayed by Western blot for expression of α-SMA and normalized against β-actin. Results are expressed as mean density ± SEM.
Discussion
The main finding of this study is that the ability of TGF-β1 to increase α-SMA expression in fibroblasts is determined by the physical resistance of the substrate to cell-generated forces. This finding is consistent with previous in vivo data showing a temporal association between the time of appearance of myofibroblasts and the time of increased resistance to wound contraction. 2 Although the modulatory role of TGF-β1 on myofibroblast differentiation and expression of α-SMA has been studied previously in monolayer cultures, 21,27,38 there are no reports on cellular responses to TGF-β1 under conditions of different tension levels. As healing tissues exhibit significant alterations in their biophysical properties over the time course of wound repair, 9 we considered that the three collagen substrates used here may provide models for connective tissues at different stages of maturation. For example, anchored gels resemble granulation tissue in later stages of remodeling with collagen fibers oriented parallel to fibroblasts whereas floating gels are more similar to resting dermis 8 and to very early stages of wound formation when resistance to intracellular tension is low. 9 Consistent with these proposed features, the three different types of collagen substrates showed large differences of compliance as measured by mechanical deformation studies, supporting the central notion of this study that the three collagen gel models exhibit large variations of compliance in response to cell-generated force. In turn, in the three culture systems used here, cells exhibited different levels of intracellular tension, as shown by the variation in the abundance and distribution of actin stress fibers in the different systems. Thus, cells in monolayer cultures on collagen-coated plastic developed high levels of intracellular tension, anchored gels developed moderate tension, and floating gels developed low tension. Indeed, fibroblasts grown on low-compliance substrates acquire characteristics of myofibroblasts that have high expression levels of α-SMA. 39
We designed experiments to separate the effects of TGF-β induction of α-SMA from the potential effects due to gel compliance. As shown in Figure 3, B and C ▶ , if cells were treated with TGF-β, then compared with controls, the α-SMA content was greatly increased, particularly in collagen plates. This effect was not due simply to the compliance of the gels because if both controls and experimental cultures were both preincubated with TGF-β for 3 days and then inoculated into the different gel types (Figure 3C) ▶ , the α-SMA content was again greatly increased in TGF-β-treated cultures. Thus, TGF-β not only induced α-SMA but also helped to maintain the cellular levels of this actin isoform. This point is emphasized by examination of [35S]methionine labeling experiments that showed TGF-β-treated cultures on collagen plates had virtually no loss of labeled α-SMA over a 4-day time period if TGF-β was present in the culture medium. If, on the other hand, TGF-β was not present, then the α-SMA content reduced sharply from the time of labeling. Thus, TGF-β both induces and maintains α-SMA content in fibroblasts, particularly in cells on collagen plates in which intracellular tension is high.
Separate experiments showed that TGF-β1-induced increases of α-SMA were clearly dependent on the maintenance of intracellular tension during the experimental period. Cell rounding induced by integrin-inhibiting antibodies reversed the effect of TGF-β1 on α-SMA content to levels of untreated controls for all three types of gel systems. Indeed, the increased decay rates of nascent, [35S]methionine-labeled α-SMA in floating gels indicate that the reduced α-SMA of cells in floating gels is due in part to increased loss of α-SMA, possibly through membrane pores. 33,40 However, the finding that TGF-β1 did not increase mRNA for α-SMA in floating gels also indicates that cytokine-mediated induction of α-SMA transcription is regulated in part by intracellular tension. The marked increase of α-SMA mRNA content induced by TGF-β1 in cells on low-compliance collagen gels is similar to previous observations for smooth muscle cells. 41 To express α-SMA in response to TGF-β1, smooth muscle cells require the binding of an undescribed factor to a TGF-β1 control element along with the binding of serum response factors to two CArG elements in the promoter region. Conceivably, the development of intracellular tension may serve to enhance transcription factor binding to the α-SMA promoter through the production and availability of mechanically coupled enzymes that in turn rely on well developed actin stress fibers traversing the cell. Indeed, mechanotranscriptional processes that regulate enzyme-substrate reactions are believed to be central elements of mechanically dependent transcription machinery 42 and likely rely on the ability of the cell to generate intracellular tension through the formation of highly adherent attachments to the matrix. In this context, a recent paper by Gabbiani and co-workers 43 indicates that a specialized fibronectin isoform (ED-A) is required for the TGF-β induction of the myofibroblastic phenotype. This paper suggests a mechanism by which a specialized extracellular matrix molecule, under the control of TGF-β, is required for outside-inside signaling and possibly for the development of the intracellular tension necessary for α-SMA induction.
Flow cytometric and Western blot analyses of fibroblasts grown on collagen-coated plastic or anchored gels showed a marked increase of the surface expression and total content of α2 and β1 subunits after TGF-β1 treatment, consistent with earlier data of cells grown on plastic dishes. 36 In contrast, cells in floating gels showed only very small increases in protein levels for both subunits. Evidently, TGF-β1 can increase α2β1 expression, but this effect is dependent on the compliance of the gels. We also showed a central role for α2β1 integrins in TGF-β1-induced regulation of α-SMA and maintenance of intracellular tension. These results further support the suggestion that generation of intracellular tension is a requirement for the regulation of α-SMA by TGF-β1. Collectively, this study supports the concept that in healing connective tissues, myofibroblastic differentiation is modulated locally by microenvironmental, interactive stimuli, including important interactions between wound-healing cytokines and mechanical forces. Conceivably, therapeutic strategies for enhancement of wound healing and reduction of scar formation could be developed using treatments that reduced generation of intracellular tension.
Footnotes
Address reprint requests to Dr. C.A.G. McCulloch, Room 244, Fitzgerald Building, University of Toronto, 150 College Street, Toronto, Ontario, Canada M5S 3E8. E-mail: christopher.mcculloch@utoronto.ca.
Supported by MRC of Canada Group and maintenance grants to C.A.G. McCulloch.
References
- 1.Skalli O, Gabbiani G: The biology of the myofibroblast. Molecular and Cellular Biology of Wound Repair. Edited by Clark RAF, Henson PM. New York, Plenum, 1996
- 2.Darby I, Skalli O, Gabbiani G: α-Smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990, 63:21-29 [PubMed] [Google Scholar]
- 3.Singer II, Kawka DW, Kazazis DM, Clark RAF: The in vivo codistribution of fibronectin and actin fibers in granulation tissue: immunofluorescence and electron microscopic studies of the fibronexus at the myofibroblast surface. J Cell Biol 1984, 98:2091-2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burridge K: Are stress fibers contractile? Nature 1981, 249:61-62 [DOI] [PubMed] [Google Scholar]
- 5.Gabbiani G, Ryan GB, Majno G: Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971, 27:549-550 [DOI] [PubMed] [Google Scholar]
- 6.Bellows CG, Melcher AH, Aubin JE: Contraction and organization of collagen gels by cells cultured from periodontal differences between cell types. J Cell Sci 1981, 50:299-314 [DOI] [PubMed] [Google Scholar]
- 7.Bell E, Ivarsson B, Merrill C: Production of a tissue-like structure by contraction of collagen lattices by human fibroblasts of different proliferative potential in vitro. Proc Natl Acad Sci USA 1979, 76:1274-1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grinnell F: Mini-review on the cellular mechanism of disease: fibroblast, myofibroblast, and wound contraction. J Cell Biol 1994, 124:401-404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunphy JE: The fibroblast: a ubiquitous ally for the surgeon. N Engl J Med 1963, 268:1367-1377 [Google Scholar]
- 10.Tomasek JJ, Haaksma CJ, Eddy RJ, Vaughen ME: Fibroblast contraction occurs on release of tension in attached collagen lattices: dependency on an organized actin cytoskeleton and serum. Anat Rec 1992, 232:359-368 [DOI] [PubMed] [Google Scholar]
- 11.Gullberg D, Tingstrom A, Thuresson AC, Olsson L, Terracio L, Borg TK, Rubin K: β1-Integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res 1990, 186:264-272 [DOI] [PubMed] [Google Scholar]
- 12.Schiro JA, Chan BMC, Roswit WT, Kassner PD, Pentland AP, Hemler ME, Eisen AZ, Kupper TS: Integrin α2β1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell 1991, 67:403-410 [DOI] [PubMed] [Google Scholar]
- 13.Klein CE, Dressel D, Steinmayer T, Mauch C, Eckes B, Krieg T, Bankert RB, Weber L: Integrin α2β1 is up-regulated in fibroblasts and highly aggressive melanoma cells in three-dimensional collagen lattices and mediates the reorganization of collagen I fibrils. J Cell Biol 1991, 115:1427-1436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wayner EA, Carter WG: Identification of multiple cell adhesion receptors for type VI collagen and fibronectin in human fibrosarcoma cells possessing unique α and common β1 subunit. J Cell Biol 1987, 105:1873-1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirchofer D, Languino LR, Ruoslahti E, Pierschbacher MD: Alpha 2 beta 1 integrins from different cell types show different binding specificities. J Biol Chem 1990, 265:615-618 [PubMed] [Google Scholar]
- 16.Rikonen T, Koivisto L, Vihinen P, Heino J: Transforming growth factor-β regulates collagen gel contraction by increasing alpha 2 beta 1 integrin expression in osteogenic cells. J Biol Chem 1995, 270:376-382 [DOI] [PubMed] [Google Scholar]
- 17.Massague J: The transforming growth factors. Trends Biochem Sci 1985, 10:237-240 [Google Scholar]
- 18.Sporn MB, Roberts AB, Wakefield LM, de Crombrugghe B: Some recent advances in the chemistry and biology of transforming growth factor β. J Cell Biol 1987, 105:1039-1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roberts AB, Sporn MB, Assoian RK: Transforming growth factor β: rapid induction of fibrosis and angiogenesis and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 1986, 83:4167-4171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberts AB, McCune BK, Sporn MB: TGF-β: regulation of extracellular matrix. Kidney Int 1992, 41:557-559 [DOI] [PubMed] [Google Scholar]
- 21.Desmouliere A, Geiniz A, Gabbiani F, Gabbiani G: Transforming growth factor-β1 induces α-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J Cell Biol 1993, 122:103-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montesano R, Orci L: Transforming growth factor β stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA 1988, 85:4894-4897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arora PD, McCulloch CAG: Dependence of collagen remodeling on α-smooth muscle actin expression by fibroblasts. J Cell Physiol 1994, 159:161-175 [DOI] [PubMed] [Google Scholar]
- 24.Lin RY, Sullivan KM, Argenta PA, Meuli M, Lorenz HP, Adzick NS: Exogenous transforming growth factor-β amplifies its own expression and induces scar formation in a model of human fetal skin repair. Ann Surg 1995, 222:146-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schurch W, Seemayer TA, Gabbiani G: The myofibroblast. Sternberg SS eds. Histology for Pathologists. 1992, :pp 109-144 Raven Press, New York [Google Scholar]
- 26.McCulloch CAG, Knowles GC: Deficiencies in collagen phagocytosis by human fibroblasts in vitro: a mechanism for fibrosis? J Cell Physiol 1993, 155:461-471 [DOI] [PubMed] [Google Scholar]
- 27.Orlandi A, Roppaz P, Gabbiani G: Proliferative activity and α-smooth muscle actin expression in cultured rat aortic smooth muscle cells are differently modulated by transforming growth factor-β1 and heparin. Exp Cell Res 1994, 214:526-536 [DOI] [PubMed] [Google Scholar]
- 28.Cormack DT, Sporn MB, Roberts AB, Merino MJ, Dart LL, Norton JA: Transforming growth factor β levels in rat wound chambers. J Surg Res 1987, 42:622-628 [DOI] [PubMed] [Google Scholar]
- 29.Chomcyzinski P, Sacchi N: Single step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1987, 162:156-159 [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa S, Pawelek P, Grinnell F: Long-term culture of fibroblasts in contracted collagen gels: effect on cell growth and biosynthetic activity. J Invest Dermatol 1989, 93:792-798 [DOI] [PubMed] [Google Scholar]
- 31.Skalli O, Bloom WS, Ropraz P, Azzarone B, Gabbiani G: Cytoskeletal remodeling of rat aortic smooth muscle cells in vitro: relationships to culture conditions and analogies to in vivo situations. J Submicrosc Cytol 1985, 18:481-493 [PubMed] [Google Scholar]
- 32.Nakagawa S, Pawelek P, Grinnell F: Extracellular matrix organization modulates fibroblast growth factor responsiveness. Exp Cell Res 1989, 182:572-582 [DOI] [PubMed] [Google Scholar]
- 33.Lin Y-C, Ho C-H, Grinnell F: Fibroblasts contracting collagen matrices form transient plasma membrane passages through which the cells take up fluorescein isothiocyanate-dextran and Ca2+. Mol Biol Cell 1997, 8:59-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts AB, Sporn MB: Regulation of endothelial cell growth, architecture and matrix synthesis by TGF-β. Am Rev Respir Dis 1989, 140:1126-1128 [DOI] [PubMed] [Google Scholar]
- 35.Sappino AP, Schurch W, Gabbiani G: Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest 1990, 63:144-161 [PubMed] [Google Scholar]
- 36.Heino J, Ignotz RA, Hemler ME, Crouse C, Massague J: Regulation of cell adhesion receptors by transforming growth factor-β: concomitant regulation of integrins that share a common β1 subunit. J Biol Chem 1989, 264:380-388 [PubMed] [Google Scholar]
- 37.Shimuzu Y, van Serenter GA, Horgan K, Show S: Regulated expression and binding of three VLA (β1) integrin receptors on T cells. Nature 1990, 345:250-253 [DOI] [PubMed] [Google Scholar]
- 38.Bjorkerud S: Effects of transforming growth factor-β1 on human arterial smooth muscle cells in vitro. Arterioscler Thromb 1991, 11:892-902 [PubMed] [Google Scholar]
- 39.Schmitt-Graff A, Desmouliere A, Gabbiani G: Heterogeneity of myofibroblast phenotypic features: an example of fibroblastic cell plasticity. Virchows Arch 1994, 425:3-24 [DOI] [PubMed] [Google Scholar]
- 40.Moroianu J, Fett JW, Riordan JF, Vallee BL: Actin is a surface component of calf pulmonary artery endothelial cells in culture. Proc Natl Acad Sci USA 1990, 90:3815-3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hautmann MB, Madsen CS, Owens GK: A transforming growth factor β (TGF-β) control element derives TGF-β-induced stimulation of smooth muscle α-actin gene expression in concert with two CArG elements. J Biol Chem 1997, 272:10948-10956 [DOI] [PubMed] [Google Scholar]
- 42.Ingber DE: Tensegrity: the architectural basis of cellular mechanotransduction. Annu Rev Physiol 1997, 59:575-599 [DOI] [PubMed] [Google Scholar]
- 43.Serini G, Bochaton-Piallat M-L, Ropraz P, Geinoz A, Borsi L, Zardi L, Gabbiani G: The fibronectin domain ED-A is crucial for myofibroblastic phenotype induction by transforming growth factor β1. J Cell Biol 1998, 142:873-881 [DOI] [PMC free article] [PubMed] [Google Scholar]