Abstract
Podocalyxin is a membrane protein of rat podocytes and endothelial cells. It has not been described in other cell types, and no amino acid or DNA sequence data are available about it. Here we show that podocalyxin antigens are present in rat platelets and megakaryocytes. In resting platelets, the antigens are mainly intracellular but become surface exposed after thrombin stimulation, as shown by immunofluorescence and flow cytometry. By Western blotting, platelet podocalyxin has an apparent Mr of 140,000. Cytocentrifuge slides of rat bone marrow show that anti-podocalyxin antibodies recognize large polyploid cells also expressing CD62P, indicating that the cells are megakaryocytes. From a rat glomerular cDNA library we isolated a clone covering the carboxyl-terminal nucleotides of rat podocalyxin. Its putative transmembrane or intracellular domains are 100% or >93% identical, respectively, with the human and rabbit podocalyxin-like proteins. The truncated extracellular domain extends to include two of the four conserved cysteines shared by podocalyxin-like proteins. By Northern blotting, a 5.5-kb renal cortical transcript is seen. By in situ hybridization, cRNA probes recognize podocytes, endothelial cells, and megakaryocytes, and by reverse transcription polymerase chain reaction, platelets are shown to contain podocalyxin mRNA. Our results show that rat podocalyxin is a homologue of the previously cloned podocalyxin-like proteins and suggest that also in mammals podocalyxin has a role in hematopoiesis, as previously shown in the chicken.
Podocalyxin was first described as an integral membrane protein covering the luminal membranes of glomerular podocytes and endothelial cells in the rat. 1-4 Thus far it has not been described in other cell types. It is highly glycosylated with N- and O-linked carbohydrates, both of which are sialylated and sulfated. 5 Its localization at the apical membranes and its strong anionic charge suggest that it may help to maintain open vascular lumens and functional glomerular filtration slits. 5-7 Podocalyxin-like proteins (PCLPs) have been cloned from chicken, 8 rabbit, 9 and man. 10 In all of these species the intracellular and transmembrane domains of the proteins are highly homologous. The extracellular parts are more heterogeneous, but all share a mucin-like structure and four conserved cysteins. Tissue distribution, biochemical characteristics, and genomic features 10 of these proteins resemble each other, but no sequence data of the rat protein have been published so far. The chicken PCLP thrombomucin is present in myeloid stem cells, megakaryocytes, and thrombocytes 8 and structurally resembles CD34 antigen, but the mammalian PCLPs have not been described in hematopoietic cells.
We have earlier characterized monoclonal antibodies specific for rat podocalyxin. 3,4 In preliminary experiments we noticed that after intravenous injections into rats the antibodies bound to endothelial cells 3 and sometimes were detected at the surface of platelets in glomeruli (A. Miettinen, unpublished observations). This prompted us to study whether podocalyxin is present in platelets. Here we show, using biochemical, immunological, and histological techniques, that podocalyxin is expressed in rat platelets and megakaryocytes. We have also partially cloned rat glomerular podocalyxin, present the first sequence data of it, and demonstrate using molecular biology techniques that podocalyxin mRNA is present in megakaryocytes and platelets.
Materials and Methods
Materials
All reagents were of analytical grade, and their sources, if not given below, were as given in previous publications. 3,4,11,12 Sprague-Dawley rats were from the Division of Bacteriology and Immunology (University of Helsinki, Finland). The use of experimental animals was approved by the Ethical Committee of the Haartman Institute (University of Helsinki).
Antibodies
The monoclonal mouse anti-rat glomerular podocalyxin antibodies of clones 5A (IgG1) and 1A (IgG2b) as well as the monoclonal antibodies against rat gp330/megalin (clone 20B, IgG1) and O-acetyl GD3 ganglioside (clone 27A, IgG3) 4,13 have been described. The controls included also monoclonal antibodies against rat annexin I (clone 34E11, IgG3; Tissari and A. Miettinen, unpublished observations). Mouse monoclonal LYP-20 antibodies against human CD62P (P-selectin) cross-reacting with rat CD62P 14 were obtained from Dr. E. Chignier (INSERM, Lyon, France).
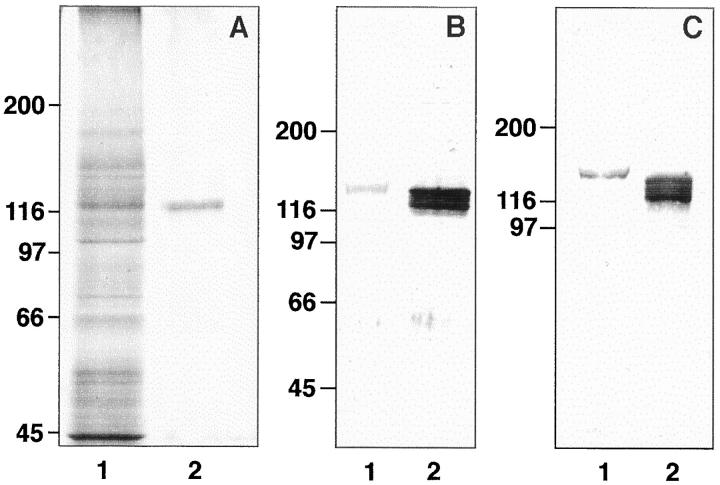
Polyclonal antibodies against podocalyxin were made by immunizing two rabbits with podocalyxin purified from isolated rat glomeruli 4 solubilized in 1% Triton X-100. Podocalyxin was obtained from the extract by sequential use of wheat germ agglutinin affinity chromatography, 1 preparative sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis (PAGE), and electroelution (Isco model 1750, Lincoln, NE). The purity of the isolated material was analyzed by silver staining of SDS-PAGE gels (Figure 3a ▶ ; see below) and by Western blotting with the monoclonal antibodies before it was used for immunizations or for amino acid sequencing (Dr. Marc Baumann, Department of Medical Biochemistry, University of Helsinki). The rabbits received 500 μg of the isolated protein divided into four injections given 1 month apart. Sera were collected before and 2 weeks after the last injection. The first immunization was given with Freund’s complete (Difco Laboratories, Detroit, MI) and the booster injections with incomplete adjuvant. The specificity of the antisera was tested by immunofluorescence (IF) on kidney sections (Figure 1a) ▶ , by Western blotting (Figure 3c) ▶ , and by immunoprecipitation of glomerular extracts (results not shown). IgG fractions from the hybridoma supernatants or one of the rabbit sera (290) were isolated by protein A affinity chromatography, 4 and the rabbit IgG was adsorbed exhaustively with rat blood cells depleted of platelets. For double-labeling experiments, 5A IgG was coupled with fluorescein isothiocyanate (FITC).
Figure 3.
Podocalyxin proteins of rat platelets and glomeruli. A: SDS-PAGE analysis of a Triton X-100 extract from isolated rat glomeruli (lane 1) and of the purified podocalyxin used for amino acid analysis or immunizations (silver staining). B and C: Western blotting of extracts of freshly isolated platelets (lane 1) or of rat glomeruli (lane 2). with rabbit (290, B) or mouse monoclonal (5A, C) anti-podocalyxin antibodies. Note slower mobility of platelet podocalyxin as compared with the podocalyxin forms of the glomerular extract.
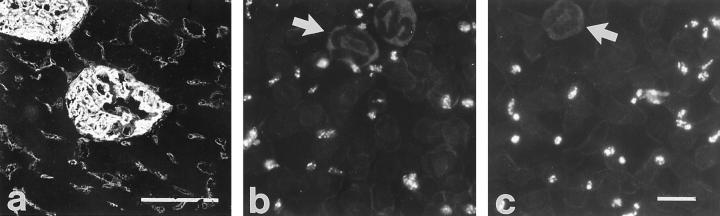
Figure 1.
Podocalyxin antigens in rat kidney (a) and platelets (b). Rabbit anti-podocalyxin antibodies (290) give a typical staining of glomerular podocytes and peritubular endothelial cells, as shown by indirect IF staining at cryostat sections of rat kidney (a). Smear slides of rat peripheral blood stained with monoclonal 5A anti-podocalyxin (b) or anti-CD62P (c) antibodies. The antibodies bind to platelets but not to red blood cells or leukocytes (arrows). Note the granular staining pattern of acetone-fixed platelets. Bar, 100 μm (a), 10 μm (b and c).
Rat anti-mouse and rat anti-rabbit IgG antibodies conjugated with FITC were from Boehringer (Mannheim, Germany) or Zymed Laboratories (South San Francisco, CA). Affinity-purified goat anti-mouse IgG conjugated with rhodamine isothiocyanate (TRITC), affinity-purified rabbit anti-mouse IgG, and goat anti-rabbit IgG (Fc fragment specific) labeled with horseradish peroxidase were from Jackson Immunoresearch Laboratories (West Grove, PA). Goat anti-rabbit IgG coupled to colloidal gold, 10-nm size, was from Zymed.
Platelets and Bone Marrow Cells
Blood samples for smear slides or platelet isolation were drawn via a polyethylene catheter to a polyvinyl syringe from the aorta of the rats under diethyl ether anesthesia. One milliliter of anticoagulant containing 85 mmol/L sodium citrate, 111 mmol/L dextrose, and 71 mmol/L citric acid per 7 ml of blood was used. Platelets were isolated by the technique of Fox et al 15 with slight modifications. To avoid platelet activation, iloprost, a prostacyclin analogue (Ilomedin, Schering Corp.; a generous gift from Leiras Pharmaceutical Co., Helsinki, Finland), 50 ng/ml, was added to the blood. After centrifugation at 200 × gmax for 20 minutes at room temperature, platelet-rich plasmas (PRPs) were obtained. These were further run at 900 × gmax for 20 minutes, and the upper layers of the pellets were resuspended in the original volume of a buffer containing 120 mmol/L sodium chloride, 13 mmol/L sodium citrate, and 30 mmol/L dextrose, pH 7.0. After addition of iloprost, the centrifugation step was repeated, and the pellets were resuspended into Tyrode’s buffer containing 138 mmol/L NaCl, 2.9 mmol/L KCl, 12 mmol/L NaHC03, 0.36 mmol/L NaH2PO4, 5.5 mmol/L glucose, 1.8 mmol/L CaCl2, and 0.4 mmol/L MgCl2, pH 7.4, with added iloprost. The suspension was divided into two tubes, and after recentrifugation, the cells were suspended in Tyrode’s buffer with added iloprost (nonactivated platelets) or thrombin (10 NIH/ml; Topostasin, Roche, Basel, Switzerland; activated platelets) and incubated at 37°C for 10 minutes. For flow cytometry, paraformaldehyde (final concentration, 3%) was added to the tubes for 10 to 20 minutes at room temperature before antibody incubations (see below). Cytocentrifuge slides were prepared from the nonactivated or activated platelets in a Shandon Cytospin 2 cytocentrifuge (Ashmoore, UK). For immunoblotting experiments, the cells were washed and boiled in the Laemmli sample buffer (see below). For immunoelectron microscopy, the platelets were centrifuged into tiny pellets of 1 mm 3 and treated as described below. Isolated platelets were also used for RNA isolation (see below).
Bone marrow tissues were obtained from the femur bones of rats (600 g) anesthetized with diethyl ether. For cytospin preparations, the tissue was dispersed in PBS containing 0.38% sodium citrate and passed three times through a 22-gauge needle before centrifugation. For in situ hybridization experiments, sterile buffer and silanated microscope slides were used.
Immunofluorescence and Flow Cytometry
For IF staining experiments, rat kidneys were frozen rapidly in liquid nitrogen and processed as described earlier. 4 Cryostat sections (5 μm thick), smear slides of rat peripheral blood, or cytocentrifuge slides of isolated platelets or bone marrow cells, were fixed in acetone at −20°C for 10 minutes, incubated with the primary and the labeled secondary antibodies at room temperature for 30 minutes each, and embedded in nonfading mounting medium, as described. 4 To visualize the nuclei of the marrow cells, Hoechst dye 33258 (0.5 μg/ml; Calbiochem-Behring Corp., La Jolla, CA) was added in the conjugate solution in some experiments. The slides were observed and photographed in an Olympus BX-50 fluorescence microscope equipped with epi-illumination and dichroic mirrors for FITC, TRITC, or UV light.
For flow cytometry experiments, the fixed nonactivated or thrombin-activated platelets were washed twice in PBS, pH 7.2, containing 0.1% NaN3, and suspended in 5% fetal calf serum (FCS; Clone Fetal Calf Serum, PAA Labor- und Forschungges, Linz, Austria) in PBS (PBS-FCS) for 15 minutes to block the nonspecific binding of antibodies. After centrifugation, the cells were incubated in 1 ml of PBS-FCS or PBS-normal rabbit serum containing 1 to 3 μg of monoclonal IgG or 5 μg of adsorbed rabbit IgG, respectively, for 30 minutes, washed three times in PBS with azide, and resuspended in 100 μl of the FITC-anti-mouse or -rabbit IgG diluted in PBS-FCS or in PBS-normal rabbit serum, respectively, for 30 minutes. After three washes in PBS, the cells were suspended in 1 ml of PBS containing 1% paraformaldehyde and analyzed at a Becton-Dickinson FACScan flow cytometer equipped with a 15-mW argon laser emitting light at 480 nm, as described earlier. 11 In each analysis, 15,000 thrombocytes gated by their typical forward/side scatter were counted.
Electrophoresis and Western Blotting
For SDS-PAGE, the isolated platelets or detergent extracts of glomeruli were suspended in the Laemmli sample buffer, boiled for 5 minutes, and run under reducing conditions using 5% to 10% gels and a Protean Mini-gel electrophoresis system (Bio-Rad Laboratories, Richmond, CA). For Western blotting, the separated proteins were transferred to nitrocellulose sheets using a Novablot semidry blotting apparatus (Pharmacia, Uppsala, Sweden). The nitrocellulose strips were reacted with antibodies, washed, and incubated with the horseradish-peroxidase-coupled anti-mouse or -rabbit IgG, and the bound antibodies were visualized using the diaminobenzidine H2O2 reaction, as described. 4
Immunoelectron Microscopy
For post-embedding immunoelectron microscopy, 1-mm 3 pellets of isolated rat platelets were fixed in 0.1 mol/L phosphate containing 3% paraformaldehyde, 0.05% glutaraldehyde, and 0.5 mmol/L CaCl2, pH 7.4, for 2 hours at room temperature, and washed with five changes of 0.1 mol/L phosphate-CaCl2 buffer containing 3.5% sucrose at +4°C overnight. The free aldehyde groups were blocked by incubation in 50 mmol/L NH4Cl made in the same sucrose-CaCl2 buffer at 0°C for 1 hour, and the phosphate ions were washed away in a buffer containing 0.1 mol/L sodium maleate and 3.5% sucrose, pH 6.0, in an ice bath for 15 minutes. This was repeated four times. The pellets were post-fixed in 2% uranyl acetate made in the sucrose-maleate buffer, for 2 hours, dehydrated with acetone, and embedded at −20°C in LR Gold resin (London Resin Co., Hants, UK). 16 For antibody labeling, 100- to 150-nm thick sections were cut on 400-mesh nickel grids. To block nonspecific binding sites, the grids were placed on drops of 50 mmol/L TrisHCl buffer containing 150 mmol/L NaCl, pH 7.2 (TBS), and of TBS containing 5% fetal calf serum (TBS-FCS) and thereafter successively on drops of primary antibodies, affinity-purified rabbit anti-mouse IgG, and colloidal gold-labeled goat anti-rabbit IgG. All antibodies were diluted in TBS-FCS, which was used also for washing. After final washing, the grids were fixed in 2% glutaraldehyde for 1 hour, rinsed in water, stained for 15 minutes in 2% aqueous osmium tetroxide, and counterstained in Reynolds lead citrate or in uranyl acetate. The grids were dried and coated with Pioloform-F resin (Polaron Instruments, Hatfield, PA) and studied in a Jeol JEM 100B electron microscope.
Partial Cloning of Rat Podocalyxin
For the glomerular cDNA library, glomeruli were isolated from the kidneys of 10 1-month-old rats, using the sieving technique described earlier. 4 Total RNA was prepared from the glomeruli by a modification of the CsCl/guanidine thiocyanate method. 17 Polyadenylated mRNA was isolated using PolyATract mRNA isolation System III kit (Promega, Madison, WI) and used to construct a poly(T)-primed directional glomerular library in ZAP Express vector using ZAP Express cDNA Gigapack cloning kit (Stratagene, La Jolla, CA) according to the instructions of the manufacturer. The library was screened with the rabbit PCLP1 cDNA clone DP16 obtained from Dr. David B. Kershaw (University of Michigan, Ann Arbor, MI). The probe was an 868-bp-long ApaI fragment corresponding to nucleotides 1631 to 2499 of PCLP1 mRNA 9 and was labeled with [32P]-dCTP using a random priming kit (Boehringer Mannheim). One of the clones obtained (clone 51A) was approximately 4.5 kb and consisted of approximately 4 kb of the 3′-UTR and 555 nucleotides of the carboxyl-terminal region of rat podocalyxin. For Northern blotting experiments, a cDNA probe coding for the expressed sequence of rat podocalyxin (555 nucleotides) and 34 nucleotides of the 3′-UTR was made. For in situ hybridization experiments, 35S-labeled single-stranded antisense or control sense riboprobes, covering the same region as above, were prepared according to Wilcox 18 using [35S]-UTP label (>1200 Ci/mmol; DuPont NEN, Boston, MA) and an appropriate SP6/T7 transcription system (Promega). For Northern blotting, total RNA and mRNA were isolated from rat renal cortex. For control purposes, mRNA was isolated from cultured rat kidney epithelial NRK-52E cells (ATTC CRL-1571), not expressing podocalyxin antigens, as previously shown. 12
In Situ Hybridization and RT-PCR
For in situ hybridization, kidney tissues were fixed in 4% paraformaldehyde in PBS at +4°C for 4 hours, cryoprotected in 15% sucrose at +4°C in PBS for 16 hours, and frozen on dry ice. Cryostat sections, 5 μm thick, were cut on glass slides treated with 3′-(triethoxysilyl)propylamine (Sigma Chemical Co., St. Louis, MO). In situ hybridization was performed as described earlier 19 with slight modifications. Tissue sections or cytocentrifuge slides were fixed in 4% paraformaldehyde for 10 minutes, washed in PBS, and treated with 0.2 mol/L HCl for 5 minutes. After washing in PBS, the slides were incubated in proteinase K (0.25 μg/ml; Merck, Darmstadt, Germany) for 10 minutes, washed again in PBS and distilled water, and acetylated with 26 mmol/L acetic anhydride in 0.1 mol/L triethanolamine, pH 8.0, for 10 minutes. After washing in PBS, the slides were incubated in 50% formamide, 2X SSC for 10 minutes and prehybridized at hybridization conditions for 4 hours without probe. Hybridization with the [35S]-labeled probe (30,000 cpm/μl) was performed in 60% formamide, 5X SSC, 10% dextran sulfate, 1X Denhardt’s solution, 100 mmol/L dithiothreitol (DTT), and 0.5 mg/ml yeast tRNA at 53°C for 16 hours. After hybridization, the slides were washed first in 5X SSC, 10 mmol/L DTT at 55°C for 30 minutes followed by 50% formamide, 2X SSC, 10 mmol/L DTT at 65°C for 30 minutes. The slides were then washed three times at 37°C in NTE (0.5 mol/L NaCl, 10 mmol/L TrisHCl, 0.5 mol/L EDTA, pH 8.0) for 10 minutes each, treated with 20 μg/ml ribonuclease A (Merck) in NTE at 37°C for 30 minutes, and washed at 37°C in NTE for 15 minutes. After washing again in 50% formamide, 2X SSC, 10 mmol/L DTT followed by washes in 2X SSC and 0.1X SSC, the slides were dehydrated sequentially in 30%, 60%, 80%, and 94% ethanol containing 0.3 mol/L ammonium acetate and, finally, twice in 100% ethanol. After dipping them in NTB-2 nuclear track emulsion (Kodak), the slides were exposed at 4°C for 7 to 21 days. After development and counterstaining with hematoxylin (Shandon), the slides were investigated and photographed under dark- or bright-field illumination with an Olympus BX-50 microscope.
For RT-PCR, total RNA from isolated rat platelets was DNAse treated (DNAse RQ1, Promega) and reverse transcribed using random hexanucleotide primers (Boehringer Mannheim) and MMLV reverse transcriptase (Promega). The following primers were designed for rat podocalyxin: sense primer 5′-GGT GGC AGT GAA GAG AAT C corresponding to nucleotides 105 to 120 (aa 45 to 49) and antisense primer 5′-CTG TGA GTC GTT GTT GGT C corresponding to nucleotides 355 to 373 (aa 119 to 124). cDNA was amplified using 1 μmol/L primers, 0.2 mmol/L dNTPs, and 1.25 U of AmpliTaq DNA polymerase (Perkin-Elmer Cetus, Norwalk, CT) in buffer containing 10 mmol/L Tris-HCl, pH 8.3, 50 mmol/L KCl, 1.5 mmol/L MgCl2 in the total volume of 25 μl on a thermal cycler (PTC-200; MJ Research, Watertown, MA). The following program was used: first denaturation at 94°C for 3 minutes followed by 30 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds. The PCR product was analyzed by agarose gel electrophoresis and ethidium bromide staining, purified (Magic PCR Preps DNA purification and separation kit; Promega), and sequenced directly using gene-specific primers (ABIPrism 310, Perkin-Elmer Applied Biosystems, Foster City, CA).
Results
Podocalyxin in Platelets and Megakaryocytes
The monoclonal (5A and 1A) and the adsorbed polyclonal (290) anti-podocalyxin IgGs bound to endothelial cells and glomerular podocytes in a typical manner (Figure 1a) ▶ . The antibodies bound also to platelets but not to other blood cells on the smear slides of rat peripheral blood (Figure 1b) ▶ . The staining pattern was granular both in the resting and thrombin-treated platelets and resembled that obtained with the anti-CD62P antibodies (Figure 1c) ▶ . Monoclonal anti-annexin I antibodies gave an intensive staining at polymorphonuclear leukocytes (not shown) but not at platelets, whereas the anti-megalin or the anti-O-acetyl GD3 antibodies did not show any binding to rat blood cells.
On cytospin slides of bone marrow cells, the anti-podocalyxin antibodies bound to both large polyploid cells and to some smaller cells (Figure 2a) ▶ . The large cells also bound the anti-CD62P antibodies (Figure 2b) ▶ , as shown by double-labeling experiments, indicating that they were megakaryocytes. Most, but not all, cells positive for podocalyxin were also positive for CD62P. For both antigens, the staining intensity varied in individual cells.
Figure 2.
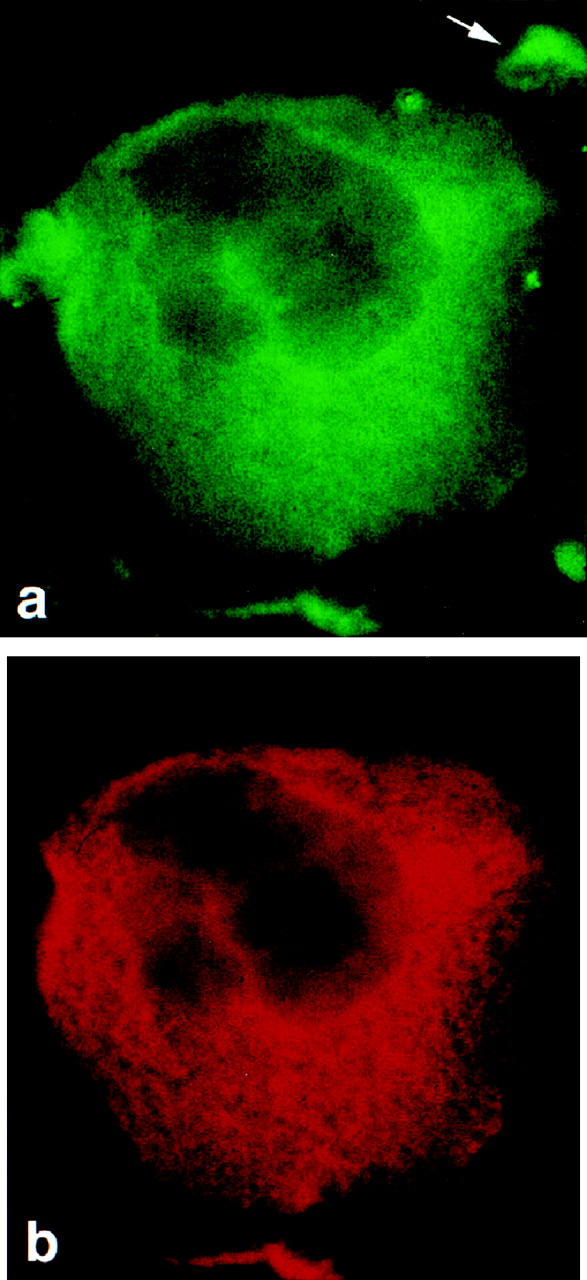
Podocalyxin (a) and CD62P antigens (b) in a megakaryocyte, shown by double IF staining of a cytospin slide of rat bone marrow cells first with monoclonal anti-CD62P and TRITC-conjugated goat anti-mouse IgG (b), followed by FITC-conjugated 5A IgG (a). Binding to the anti-mouse conjugate was blocked by pretreatment with normal mouse serum. The polyploid megakaryocyte expresses both antigens. Anti-podocalyxin antibodies detect also a small, unidentified cell (a, arrow). Magnification, ×600.
By Western blotting, both the monoclonal (Figure 3b) ▶ and the polyclonal (Figure 3c) ▶ anti-podocalyxin antibodies detected in platelet extracts a protein with an apparent Mr of 140,000. In glomerular extracts, the antibodies bound to three protein bands migrating slightly faster than the platelet protein (Figure 3b and c) ▶ .
At the ultrastructural level, podocalyxin antigens were seen in freshly isolated platelets mainly at intracellular membranes (Figure 4a) ▶ or at structures resembling α-granules (Figure 4c) ▶ , as shown by post-embedding immunoelectron microscopy. Anti-megalin or -O-acetyl GD3 IgGs did not show any binding to platelets (Figure 4b) ▶ .
Figure 4.
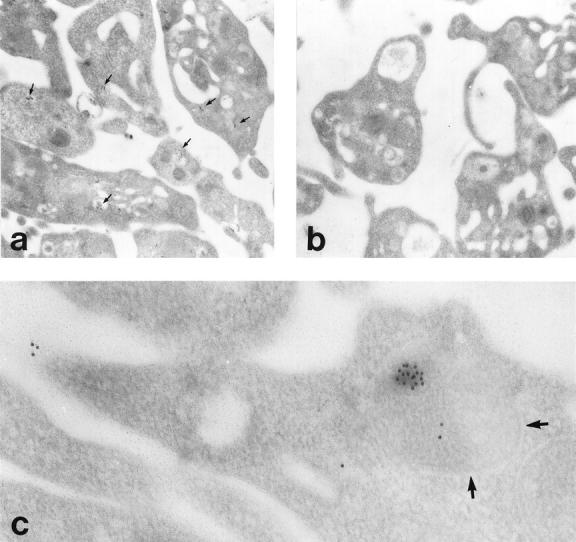
Ultrastructural localization of podocalyxin in freshly isolated platelets. Colloidal gold particles indicating the presence of podocalyxin are detected at intracellular membranes in all platelets after staining with monoclonal anti-podocalyxin (a, arrows) but not with the control anti-megalin (b). IgG. At higher magnification, gold particles are seen in an α-granule (c, arrows) and at the surface of a platelet. Post-embedding indirect immunogold staining; magnification, ×10,000 (a and b) and ×36,000 (c).
Surface Expression after Platelet Activation
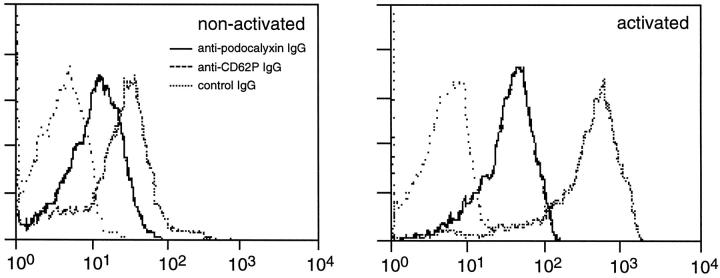
The anti-podocalyxin antibodies showed only limited binding to the surface of isolated, nonactivated platelets, but the antigens became detectable at the cell surface after thrombin activation (Figure 5) ▶ . Some platelet activation occurred during the isolation process, as demonstrated by the binding of the anti-CD62P antibodies to isolated platelets even before thrombin treatment.
Figure 5.
Surface expression of podocalyxin or CD62P antigens at freshly isolated (left panel) or thrombin-treated (right panel), nonpermeabilized platelets. Both anti-podocalyxin and anti-CD62P IgGs bind to some extent to the surface of isolated platelets, indicating slight activation of the cells. A significant increase in the expression of podocalyxin or CD62P is seen after thrombin stimulation. The x axis shows fluorescence intensity.
Partial Cloning of Podocalyxin
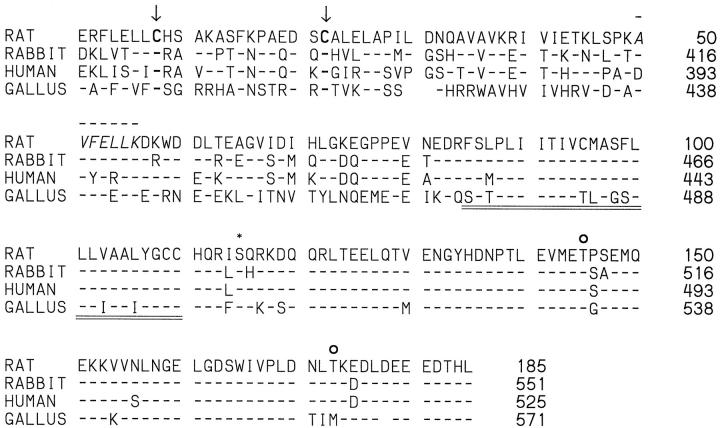
Using the rabbit PCLP1 cDNA clone DP16 from Dr. David Kershaw (University of Michigan, Ann Arbor, MI), we obtained from our rat glomerular cDNA library several clones. One of these, clone 51A, was ∼4.5 kb and consisted of ∼4 kb of 3′-UTR and 555 nucleotides corresponding to the carboxyl-terminal region of rat podocalyxin and coding for its putative intracellular, transmembrane, and membrane proximal extracellular domains (Figure 6) ▶ . The extracellular part included a seven-amino-acid sequence (AVFELLK) identical to a sequence found independently by amino acid sequencing of a tryptic peptide from isolated glomerular podocalyxin. As shown in Figure 6 ▶ , the transmembrane and intracellular domains of rat podocalyxin are 100% and 93% identical, respectively, with rabbit PCLP1, 96% and 95%, respectively, with human PCLP, and 69% and 89%, respectively, with chicken thrombomucin. The intracellular part of rat podocalyxin has one putative PKC and two CKII consensus phosphorylation sequences, and the partially cloned extracellular part includes two of the four conserved cysteins shared by all podocalyxin-like proteins (Figure 6) ▶ , but it does not extend to the region of the other two. By Northern blotting, the cDNA probes covering all expressed 185 amino acids of our rat podocalyxin clone (Figure 6) ▶ hybridized with a 5.5-kb transcript of rat cortical mRNA but not of the rat epithelial NRK cell mRNA (Figure 7) ▶ . By in situ hybridization, the corresponding 35S-labeled single-stranded antisense cRNA probes bound to podocytes and endothelial cells (Figure 8, a and b) ▶ , whereas the sense probes did not.
Figure 6.
Amino acid sequence alignment of rat podocalyxin (derived from clone 51A) and the previously published podocalyxin-like proteins of rabbit, human, and chicken (gallus). Note the identical or highly homologous structure of the putative transmembrane (double underlining) and intracellular domains of all species. The intracellular domain of rat podocalyxin shows one protein kinase C(*) and two casein kinase II (○) consensus phosphorylation sites, and its partially cloned extracellular domain extends to include two of the four conserved cysteins (arrows). The extracellular seven-amino-acid sequence (aa 50 to 56, line above) was found independently by amino acid sequencing of tryptic peptides of isolated rat glomerular podocalyxin. The probes used for Northern blotting or in situ hybridization covered the whole area shown. Numbering is according to the sequences in GenBank. Accession numbers are U35239 rabbit PCPL-1, U97519 human PCLP, Y13978 Gallus gallus thrombomucin.
Figure 7.
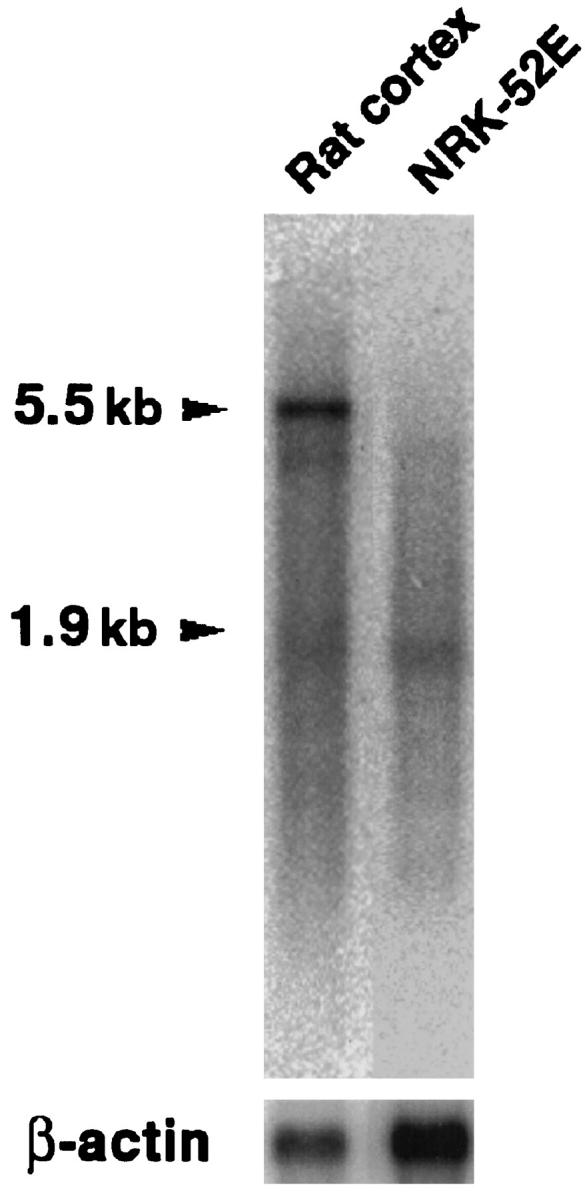
Northern blot of mRNA extracted from rat renal cortex (left lane) or cultured rat renal epithelial NRK cells (right lane) developed using the 589-bp rat-podocalyxin probe. The probe binds to a 5.5-kb transcript of renal cortical but not NRK mRNA. The lower arrowhead shows the position of the 1.9-kb β-actin mRNA. The size of the podocalyxin transcript is deduced from results obtained with renal cortical total RNA (not shown).
Figure 8.
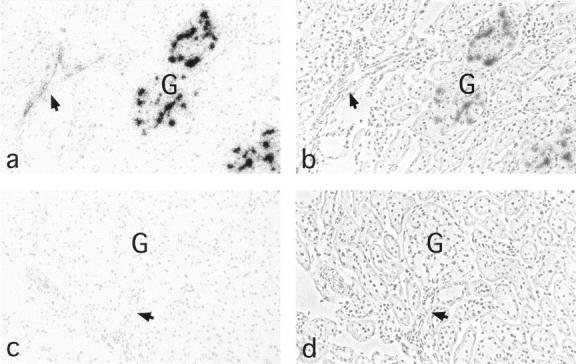
Micrographs demonstrating in situ hybridization of antisense (a and b) or sense (c and d) cRNA probes of rat podocalyxin to rat kidney sections. The antisense probe decorates glomerular (G) podocytes and all endothelial cells (arrow shows an arteriole), whereas the sense probes do not show any specific binding. a and c, direct light; b and d, phase contrast. Magnification, ×200.
Podocalyxin mRNA in Megakaryocytes and Platelets
By in situ hybridization, the antisense cRNA probe of rat podocalyxin bound to large megakaryocytic cells and also to some smaller cells (Figure 9, a and b) ▶ on cytospin preparations of rat bone marrow. With the sense RNA probes, no staining over background was seen (Figure 9, c and d) ▶ . Using primers specific for rat podocalyxin and RT-PCR technique, a PCR product of expected length (271 bp) was obtained from total RNA extracted from isolated platelets, but not from NRK cells. No DNA products were seen in the controls. The RT-PCR products were identical with glomerular podocalyxin, as shown by direct sequencing.
Figure 9.
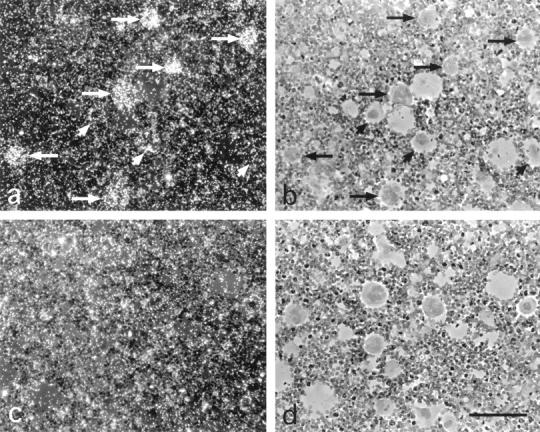
Micrographs demonstrating in situ hybridization of antisense (a and b) cRNA probes of rat podocalyxin to large megakaryocytic cells (arrows) at cytospin preparations of rat bone marrow. No binding over the relatively high background is seen with the sense probes (c and d). a and c, dark field; b and d, respective transmission light micrographs. Hematoxylin counterstaining; magnification, ×630.
Discussion
This is the first report to demonstrate that rat podocalyxin is a membrane protein of platelets and megakaryocytes. To our knowledge this is also the first report to show amino acid sequences of rat podocalyxin, and clearly demonstrates that the rat protein is a homologue of the previously cloned chicken, rabbit, and human podocalyxin-like proteins.
Podocalyxin was first discovered as the major sialoprotein of the glycocalyx of glomerular podocytes 1 and was soon found to be present in the endothelial cells, 2 but so far it has not been found in other rat cell types. Here we show using mono- and polyclonal antibodies that podocalyxin is present in megakaryocytes and platelets. These cells readily take up proteins from plasma 20 and store these in the α-granules, where podocalyxin was also detected. Podocalyxin is a membrane protein of endothelial cells and podocytes, but due to its high carbohydrate content the molecule is probably hydrophilic and may be present in plasma. However, our in situ hybridization results clearly show that podocalyxin mRNA is present in megakaryocytes, and by RT-PCR, podocalyxin mRNA could also be demonstrated in RNA extracted from platelets. Thus, it is evident that rat megakaryocytes make podocalyxin themselves.
Rat podocalyxin resembles the podocalyxin-like protein thrombomucin of chicken, present on podocytes, endothelial cells, and thrombocytes. 8 Thrombomucin is one of the earliest markers of hematopoietic cells in chicken embryos, and in adult bone marrow it is found on the surface of multipotent hematopoietic progenitors as well as on erythroid and thrombocyte precursors. 8 In rat bone marrow, anti-podocalyxin antibodies detected cells of different sizes and different staining intensity. All were not positive for CD62P, a marker for megakaryocytic cells (Figure 2a) ▶ . Whether the podocalyxin-positive and CD62P-negative cells are at an earlier megakaryocytic developmental stage or of different cell lineage remains to be studied. At present, no data of human or rabbit podocalyxin-like proteins in bone marrow cells are available, but our results suggest that podocalyxin may have a role in hematopoiesis also in mammals.
In glomeruli or lung tissues, intact podocalyxin has an apparent Mr of 140,000, but in detergent extracts only smaller, 110 to 140-kd molecular forms are usually detected by Western blotting. 1,2,4,5 The platelet protein migrates slightly slower than the glomerular forms in SDS-PAGE (Figure 3, B and C) ▶ . The different antigenic forms may be due to enzymatic degradation or post-translational modifications of the protein, or may indicate existence of different splice variants. Cell-type-specific differences in glycosylation have been reported with human PCLP, which may carry MECA-79 epitopes at the surface of high endothelia of peripheral lymph nodes, 21 whereas PCLP at other endothelia 21 or glomerular podocytes 22 do not express this epitope. Changes in the glycosylation pattern may have significant effects on the apparent Mr of the protein. The calculated molecular masses of the known chicken, rabbit or human PCLPs are 54 or 55 kd, but their apparent Mr values vary from 140,000 to 165,000/160,000. It is assumed that this is due to the rich mucin-type glycosylation of the molecules. Different splice variants of both chicken thrombomucin and rabbit PCLP1 apparently exist. Whether this is true also for rat podocalyxin is not known.
Rabbit podocalyxin-like protein 1, human podocalyxin like protein, and chicken thrombomucin do not show sequence homology to any previously described proteins, but the membrane spanning and intracellular domains of each are highly homologous, 8-10 indicating that they belong to the same protein family. The extracellular parts are less homologous but share a mucin-like structure and four conserved cysteins. All intracellular domains share PKC and casein kinase II phosphorylation consensus sequences, suggesting that the proteins may be involved in signaling events. Our data show that the putative transmembrane and intracellular parts of rat podocalyxin are almost identical with rabbit PCLP1 and human PCLP, including the phosphorylation consensus sequences, and the membrane proximal extracellular part has two of the conserved cysteins in their right positions (Figure 6) ▶ . This agrees with the earlier data of Kershaw et al, 10 who demonstrated antigenic cross-reactivity between the intracellular parts of human PCLP and rat podocalyxin. The cloned cDNA does not extend to the putative mucin-like domain, but earlier data have shown that ∼20% of glomerular podocalyxin consists of carbohydrates, both N- and O-linked side chains are present, and both of these may carry sialic acid and sulfate residues. 5,24 These are all features of mucin-type glycoproteins and clearly show that rat podocalyxin is a member of the PCLP family.
The functions of podocalyxin are not known. In the developing kidney it is detected in endothelial cells and differentiating podocytes already at the early S-shaped body stage. 6,7 As the podocytes mature, podocalyxin occupies the membranes above the occluding junctions, suggesting that its role may be to keep the urinary filtration slits open. 1,5,6 In endothelial cells it may also help to keep the vascular lumina open and/or repel circulating cells, although in some endothelia it may also have adhesive functions. 21 At present, only speculations on the functions of podocalyxin in megakaryocytes or platelets can be presented. Our finding that podocalyxin becomes detectable at the platelet surface first after thrombin stimulation suggests that it may have an activation-dependent function in these cells. The regulation of podocalyxin synthesis, glycosylation, and expression at the cell surface remains to be studied, as also its putative functions in hematopoiesis and/or in platelet-endothelial interactions.
Acknowledgments
We thank Dr. David B. Kershaw for generously providing us with the rabbit PCLP1 cDNA and Dr. E. Chignier for LYP-20 antibodies. Leiras Pharmaceutical Co., Helsinki, Finland, is acknowledged for giving us Ilomedin.
Footnotes
Address reprint requests to: Dr. Aaro Miettinen, Division of Bacteriology and Immunology, Haartman Institute, P.O. Box 21 (Haartmaninkatu 3), FIN-00014 University of Helsinki, Helsinki, Finland. E-mail: aaro.miettinen@helsinki.fi.
Supported by grants from Helsinki University Central Hospital, The Sirkka and Sakari Sohlberg Foundation, The Paulo Foundation, and The Academy of Finland.
References
- 1.Kerjaschki D, Sharkey DJ, Farquhar MG: Identification and characterization of podocalyxin, the major sialoprotein of the renal glomerular epithelial cell. J Cell Biol 1984, 98:1591-1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horvat R, Hovorka A, Dekan G, Poczewski H, Kerjaschki D: Endothelial cell membranes contain podocalyxin, the major sialoglycoprotein of visceral glomerular epithelial cells. J Cell Biol 1986, 102:484-491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dekan G, Miettinen A, Schnabel E, Farquhar MG: Binding of monoclonal antibodies to glomerular endothelium, slit membranes, and epithelium after in vivo injection: Localization of antigens and bound IgGs by immunoelectron microscopy. Am J Pathol 1990, 137:913-927 [PMC free article] [PubMed] [Google Scholar]
- 4.Miettinen A, Dekan G, Farquhar MG: Monoclonal antibodies against membrane proteins of the rat glomerulus: immunochemical specificity and immunofluorescence distribution of the antigens. Am J Pathol 1990, 137:929-944 [PMC free article] [PubMed] [Google Scholar]
- 5.Dekan G, Gabel C, Farquhar MG: Sulfate contributes to the negative charge of podocalyxin, the major sialoglycoprotein of the glomerular filtration slits. Proc Natl Acad Sci USA 1991, 88:5398-5402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schnabel E, Dekan G, Miettinen A, Farquhar MG: Biogenesis of podocalyxin, the major glomerular sialoglycoprotein, in the newborn rat kidney. Eur J Cell Biol 1989, 48:313-326 [PubMed] [Google Scholar]
- 7.Sawada H, Stukenbrok H, Kerjaschki D, Farquhar MG: Epithelial polyanion (podocalyxin) is found on the sides but not the soles of the foot processes of the glomerular epithelium. Am J Pathol 1986, 125:309-318 [PMC free article] [PubMed] [Google Scholar]
- 8.McNagny KE, Pettersson I, Rossi F, Flamme I, Shevchenko A, Mann M, Graf T: Thrombomucin, a novel cell surface protein that defines thrombocytes and multipotent hematopoietic progenitors. J Cell Biol 1997, 138:1395-1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kershaw DB, Thomas PE, Wharram BL, Goyal M, Wiggins JE, Whiteside CI, Wiggins RC: Molecular cloning, expression, and characterization of podocalyxin-like protein 1 from rabbit as a transmembrane protein of glomerular podocytes and vascular endothelium. J Biol Chem 1995, 270:29439-29446 [DOI] [PubMed] [Google Scholar]
- 10.Kershaw DB, Beck SG, Wharram B, Wiggins JE, Goyal M, Thomas PE, Wiggins RC: Molecular cloning and characterization of human podocalyxin-like protein: orthologous relationship to rabbit and rat podocalyxin. J Biol Chem 1997, 272:15708-15714 [DOI] [PubMed] [Google Scholar]
- 11.Reivinen J, Holthöfer H, Miettinen A: O-acetyl GD3 ganglioside in human peripheral blood T-lymphocytes. Int Immunol 1994, 6:1409-1416 [DOI] [PubMed] [Google Scholar]
- 12.Tissari J, Holthöfer H, Miettinen A: Novel 13A antigen is an integral protein of the basolateral membrane of rat glomerular podocytes. Lab Invest 1994, 71:519-527 [PubMed] [Google Scholar]
- 13.Reivinen J, Holthöfer H, Miettinen A: A cell-type specific ganglioside of glomerular podocytes in rat kidney: an O-acetylated GD3. Kidney Int 1992, 42:624-631 [DOI] [PubMed] [Google Scholar]
- 14.Chignier E, Parise M, McGregor L, Delabre C, Faucompret S, McGregor J: A P-selectin/CD62P monoclonal antibody (LYP-20), in tandem with flow cytometry, detects in vivo activated circulating rat platelets in severe vascular trauma. Thromb Haemost 1994, 72:745-749 [PubMed] [Google Scholar]
- 15.Fox JEB, Reynolds CC, Boyles JK: Studying the platelet cytoskeleton in Triton X-100 lysates. Methods Enzymol 1992, 215:42-49 [DOI] [PubMed] [Google Scholar]
- 16.Berryman MA, Rodewald RD: An enhanced method for post-embedding immunocytochemical staining which preserves cell membranes. J Histochem Cytochem 1990, 38:159-170 [DOI] [PubMed] [Google Scholar]
- 17.Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ: Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 1979, 18:5294-5299 [DOI] [PubMed] [Google Scholar]
- 18.Wilcox JN: Fundamental principles of in situ hybridization. J Histochem Cytochem 1993, 41:1725-1733 [DOI] [PubMed] [Google Scholar]
- 19.Haltia A, Solin M-L, Jalanko H, Holmberg C, Miettinen A, Holthöfer H: Mechanisms of proteinuria: vascular permeability factor in congenital nephrotic syndrome of the Finnish type. Pediatr Res 1996, 40:652-657 [DOI] [PubMed] [Google Scholar]
- 20.Handagama PJ, Shuman MA, Bainton DF: Incorporation of intravenously injected albumin, immunoglobulin G, and fibrinogen in guinea pig megakaryocyte granules. J Clin Invest 1989, 84:73-82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD: Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: parallels to CD34. J Exp Med 1998, 187:1965-1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segawa C, Wada T, Takaeda M, Furuichi K, Matsuda I, Hisada Y, Ohta S, Takasawa K, Takeda SI, Kobayashi K-I, Yokoyama H: In situ expression and soluble form of P-selectin in human glomerulonephritis. Kidney Int 1997, 52:1054-1063 [DOI] [PubMed] [Google Scholar]
- 23.Kerjaschki D, Poczewski H, Dekan G, Horvat R, Balzar E, Kraft N, Atkins RC: Identification of a major sialoglycoprotein in the glycocalyx of human visceral glomerular epithelial cells. J Clin Invest 1986, 78:1142-1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerjaschki D, Vernillo AT, Farquhar MG: Reduced sialylation of podocalyxin, the major sialoprotein of the rat kidney glomerulus, in aminonucleoside nephrosis. Am J Pathol 1985, 118:343-349 [PMC free article] [PubMed] [Google Scholar]