Abstract
Keloid is a dermal fibroproliferative tissue of unknown etiology. Protein tyrosine kinases (PTKs) play an important role in the regulation of cell growth and differentiation. Activation of PTK cascades in keloid fibroblasts is thought to be closely linked to abnormal cell proliferation and migration. We determined the expression profile of PTK genes in normal skin and keloid fibroblasts using the homology cloning method with a degenerated primer. Eight PTK genes were expressed among a total of 46 receptor-type clones. The most abundant type of PTK receptors was the platelet-derived growth factor receptor in both fibroblasts. However, insulin-like growth factor-I receptor (IGF-IR) was overexpressed only in keloid-derived fibroblasts (9 of 24). Immunohistochemical analysis confirmed the high expression of IGF-IR in keloid fibroblasts, but not in normal fibroblasts. To examine the functional properties of the IGF-I/IGF-IR pathway, we investigated cell proliferation and invasion activities of both types of fibroblasts. The mitogenic effect of IGF-I on both fibroblasts was very weak compared with serum stimulation. In contrast, the invasive activity of keloid fibroblasts was markedly increased in the presence of IGF-I, and inhibited by a neutralizing antibody against IGF-IR. Our results indicate the involvement of activated IGF-I/IGF-IR in the pathogenesis of keloid by enhancing the invasive activity of fibroblasts.
Keloids are characterized by abnormal dermal fibroproliferative reaction during wound healing after skin injury. 1 Previous studies showed that the response to certain growth factors, such as platelet-derived growth factor (PDGF), epidermal growth factor (EGF), fibroblast growth factor (FGF), 2 and transforming growth factor-β, 3 differed in fibroblasts from keloid tissues compared with normal dermis. For example, Haisa et al 2 have demonstrated that stimulation by PDGF enhanced cell proliferation and migration of keloid fibroblasts compared with normal fibroblasts. The majority of these growth factors bind to receptors of the protein tyrosine kinase (PTK) family.
PTKs play a central role in the regulation of growth and differentiation of eukaryotic cells as components of the signal transduction pathway. 4 As more than 100 different PTKs have been cloned, 5 it would be difficult to identify the exact PTK involved in keloid formation. To break through this problem, we applied the PCR-based cloning technique using a degenerated primer 6 and compared the expression profile of receptor-type PTKs in fibroblasts and normal adult dermis and keloids. In addition to cell proliferation and differentiation function, activated PTK signals are closely related to malignant phenotypes, such as invasion and metastasis. Although keloid is a benign disorder, it is invasive beyond the margins of the original wound. 1 We speculated that the invasiveness of keloid fibroblasts is similar to some extent to that of malignant cells. To analyze the function of keloid-specific PTK signal, we used an invasion assay system usually used to estimate the invasive potential of malignant cells. 7
Thus, in this study, we first determined the expression profile of PTK genes both in normal and keloid fibroblasts of the same patient. We then investigated the expression of PTK in both specimens and primary cultured fibroblasts. To further examine the functional properties of the PTK signal pathway, we investigated the effect of application of certain growth factors or anti-growth factor receptor neutralizing antibodies on cell proliferation and invasion.
Materials and Methods
Subjects
The three keloid samples used in this investigation were obtained from three different Japanese female patients after undergoing surgical excision. Three control samples were obtained from two different Japanese male volunteers and a Japanese male patient with syndactly. The profile of each subject is listed in Table 1 ▶ . We also established primary cultures using normal skin around the keloid from one of the keloid patients (K-1). Informed consent was obtained from each subject.
Table 1.
Sources of Keloid Fibroblasts and Normal Control Fibroblasts
| Cell type | Sex | Age (years) | Biopsy site | Duration of keloid (years) |
|---|---|---|---|---|
| Keloid | ||||
| K-1 | F | 18 | Chest | 14 |
| NK-1 | F | 18 | Normal skin around the keloid | |
| K-2 | F | 73 | Chest | 6 |
| K-3 | F | 46 | Abdomen | 15 |
| Control | ||||
| N-1 | M | 30 | Back | |
| N-2 | M | 50 | Forearm | |
| N-3 | M | 5 | Foot* |
F, female; M, male.
*Surplus skin of graft for syndactly was used in this subject.
Cell Cultures and Tissue Preparation
Primary cultures of dermal fibroblasts were established as previously described. 8 Strains of fibroblasts at passages 3 to 7 were used in this study. Explants were maintained in 10% fetal bovine serum (FBS)/Dulbecco’s modified Eagle’s medium (DMEM), containing 100 U/ml penicillin, 100 μg/ml streptomycin at 37°C in a humidified incubator with 5% CO2. Tissue specimens were fixed in 4% paraformaldehyde, dehydrated, embedded in paraffin, and serially sectioned at 4 μm. Sections were prepared for histological examination, including hematoxylin and eosin (H&E) staining.
Analysis of Expression Profile of PTKs in Normal and Keloid Cultured Fibroblasts of the Same Patient
Oligonucleotide primers were synthesized corresponding to the amino acids HRDLAARN and SDVWS(F/Y)G(V/I), which are highly conserved sequences in the catalytic subdomains VI and IX of receptor-type PTK catalytic domains. 5 The sense primer was 5′-CA(T/C)(C/A)GIGA(T/C)(C/T)TIGCIGCI(C/A)GIAA-3′, and the antisense primer was 5′-A(T/C)ICC(A/G)AT(A/I)(G/C)(A/T)CCAIAC(A/G)TC-3′. Total RNAs were extracted from normal and keloid fibroblasts of the same patient (NK-1 and K-1) by a modified acid/guanidine thiocyanate/phenol/chloroform method using Isogen (Wako Pure Chemical Co., Osaka, Japan) reagents according to the instructions provided by the manufacturer. First-strand cDNA was synthesized from 500 ng of total RNA using Moloney murine leukemia virus reverse transcriptase (Life Technologies, Rockville, MD) and random primers, a fraction of which was then amplified using 25 pmol of each primer, 200 mmol/L each deoxy-NTP, and 2 U of Taq polymerase (Takara Biochemical Co., Osaka, Japan) in 10 mmol/L Tris/HCl (pH 8.3), 50 mmol/L KCl, 1.5 mmol/L MgCl2, and 0.01% gelatin in a final volume of 50 ml. Thirty-five cycles of denaturation (94°C for 30 seconds), annealing (45°C for 30 seconds), and extension (72°C for 60 seconds) were conducted with an automated thermal cycler. All reactions were initiated with a 5-minute incubation at 95°C and terminated with a 7-minute incubation at 72°C. The products (approximately 210 bp) were gel purified with the Qiaex II Kit (Qiagen, Chatsworth, CA) and subcloned into pGEM-T Easy Vector (Promega, Madison, WI). Clones were sequenced by the dideoxynucleotide chain-termination methods using a Thermo Sequenase Core sequencing kit (Amersham, Arlington Heights, IL) and a Hitachi SQ-5500 DNA sequencer (Hitachi Electronics Engineering Co., Tokyo). Homology search of reverse transcription polymerase chain reaction products was performed using BLAST SEARCH DATA BANK (http//www.ncbi.nlm.nih.gov).
Immunohistochemistry
All collected tissues and fibroblasts (N-1–3 and K-1–3) were stained immunohistochemically. After immersion in 3% H2O2/methanol solution, deparaffinized sections were preincubated with 1% normal bovine serum albumin/phosphate-buffered saline (PBS). Sections were later incubated overnight at 4°C with an optimal dilution (1.0 μg/ml) of a primary antibody to IGF-IR (rabbit polyclonal antibody for the β-subunits of human IGF-IR; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Slides were sequentially incubated with biotinylated goat anti-rabbit immunoglobulin antibodies and the avidin-biotin complex (ABC kit; Vector Laboratories, Burlingame, CA). After the addition of 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB), used as a chromogen, slides were flooded with Tris/EDTA (TE; 10 mmol/L Tris/HCl (pH 7.5), 1 mmol/L EDTA) buffer, dehydrated, and mounted. Negative controls were prepared using the same serial sections but replacing the primary antibody with a nonimmunized serum.
Cell Proliferation Assay
We used normal and keloid fibroblasts (N-1–3 and K-1–3) in WST-1 assay. A colorimetric assay based on the cleavage of the tetrazolium salt WST-1 to a formazan dye by the mitochondrial dehydrogenase of viable cells was used. Cells suspended in DMEM containing 10% FBS were seeded at 3.0 × 10 3 cells/well into 96-well plates. The medium was changed to serum-free DMEM, and the culture was continued for 24 hours at 37°C. The medium was replaced with DMEM containing 0.2% FBS, 10% FBS, and IGF-I (1, 10, and 100 ng/ml, human recombinant IGF-I; Fujisawa Pharmaceutical Co., Tokyo, Japan) for 24 or 48 hours at 37°C. The ready-to-use WST-1 reagent was added to the cells and incubated during the last 2 hours at 37°C. The absorbance at 405 nm was measured by the multiwell spectrophotometer (IMMUNO-MINI NJ-2300 Inter Med, Tokyo, Japan).
Cell Invasive Assay
Normal fibroblasts (N-1) were seeded at 1.0 × 10 3 cells/well into 24-well plates and cultured in DMEM containing 10% FBS for 2 days at 37°C. The medium was changed to serum-free DMEM/F10 (DMEM/Ham’s F10 (Gibco BRL, Life Technologies) ratio, 1:1), and membrane culture inserts, 12-μm pore size (Iwaki Glass Co., Chiba, Japan), were placed in 24-well plates. Cell matrix (type IA collagen, Nitta Zeratin Co., Osaka, Japan), 10X F10, and buffer (NaOH 500 mmol/L, HEPES 260 mmol/L, and NaHCO3 200 mmol/L) were mixed in a volume ratio of 8:1:1. Gel mixture (50 μl) was applied to each chamber and incubated for 1 hour at 37°C. Normal and keloid fibroblasts (2.0 × 10 3 cells/well), suspended in serum-free DMEM/F10, were added to the upper chamber. The fibroblast strains were N-1–2 and K-1–2. IGF-I (100 ng/ml) and 2% FBS were added to the chamber after 3, 24, and 48 hours of incubation. The culture was continued for 72 hours. In another experiment, keloid fibroblasts (2.0 × 10 3 cells/well) suspended in serum-free DMEM/F10 were then added to the upper chamber. Keloid fibroblast strains were K-1 and K-2. Antibody to IGF-IR (1H7) (10 nmol/L, mouse monoclonal antibody for human IGF-IR, Santa Cruz Biotechnology) or the mouse IgG (10 nmol/L, normal mouse IgG, Santa Cruz Biotechnology) was added to the chamber after 3, 24, and 48 hours of incubation, and 2% FBS was added to the chamber after 4, 25, and 49 hours of incubation. The cultures were continued for 72 hours. At the end of the incubation period, the cells on the upper surface of the filter were completely removed by wiping with a cotton swab. The chambers were fixed in 70% ethanol and stained with H&E. Cells of the lower surface were photographed and counted.
Statistical Analysis
Data were expressed as mean ± SD. Differences between groups were examined for statistical significance using the Mann-Whitney U test. A P value less than 0.05 denoted the presence of a statistically significant difference.
Results
PTK cDNA in Normal and Keloid Fibroblasts
The frequency of each identified PTK cDNA is shown in Table 2 ▶ . In normal fibroblasts, the most abundant receptor-type PTK was PDGF-Rα, followed by ryk, axl, met, IGF-IR, and EGFR. In keloid fibroblasts, PDGF-Rα was also abundant, and axl, met, and Ror-1 were also identified. However, the proportion of the subclone for IGF-IR was markedly higher in keloid than normal fibroblasts (Table 2) ▶ .
Table 2.
Summary of the Frequencies of PTK cDNAs Isolated from Normal and Keloid Fibroblasts in the Same Subject, as Determined by RT-PCR
| PTK cDNA | Fibroblasts | |
|---|---|---|
| Normal | Keloid | |
| PDGRF-α | 10 | 11 |
| IGF-IR | 1 | 9 |
| axl | 3 | 2 |
| met | 2 | 1 |
| ryk | 4 | |
| EGFR | 1 | |
| rptk | 1 | |
| Ror-1 | 1 | |
| Total | 22 | 24 |
Preferential Expression of IGF-IR in Keloid Fibroblasts
To confirm IGF-IR expression, we performed immunohistochemical studies using anti-IGF-IRβ antibody in cultured fibroblasts. Weak staining was present in normal cultured fibroblasts (Figure 1A) ▶ , but in contrast, strong staining was evident in cultured keloid fibroblasts (Figure 1B) ▶ . Histological examination of the dermis showed that the keloid contained numerous blood vessels and collagen fibers compared with normal skin (Figure 2, A and B) ▶ . Immunohistochemistry for IGF-IRβ subunit showed that the staining intensity in the epidermis and endothelial cells of normal specimens was almost similar to that of keloids. Strong staining for IGF-IR was detected in fibroblasts of the keloid specimens, but weak staining was seen in normal skin specimens (Figure 2, C and D) ▶ .
Figure 1.
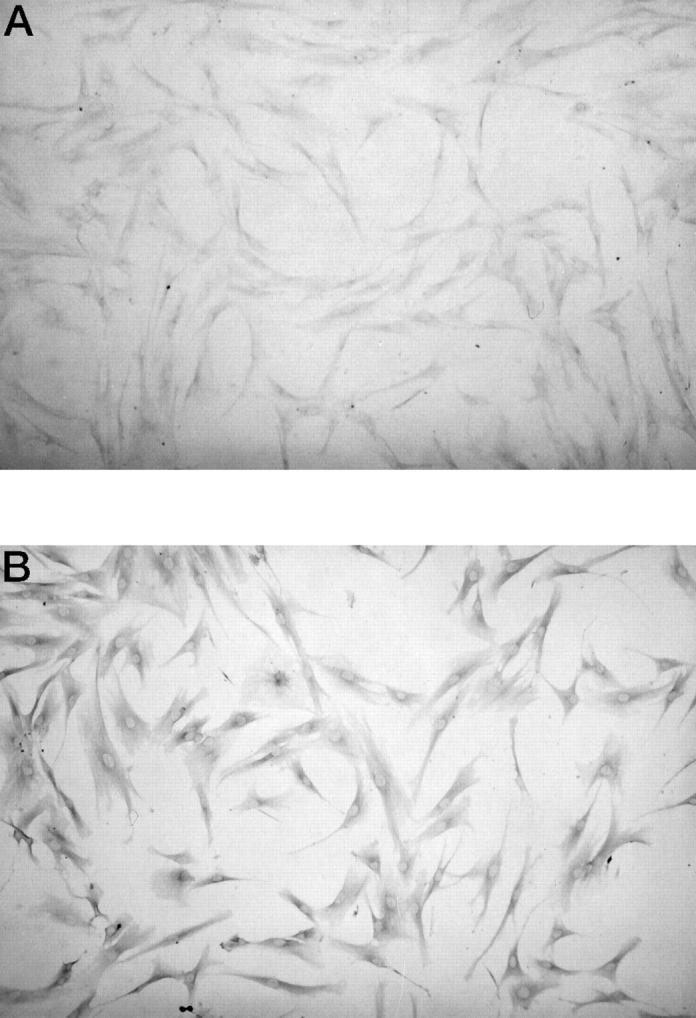
Immunohistochemistry of normal and keloid fibroblasts. (A) Note weak staining for IGF-IRβ subunits in normal fibroblasts (N-1). (B) Note strong staining for IGF-IRβ subunits in keloid fibroblasts (K-1). A, B; magnification × 100.
Figure 2.
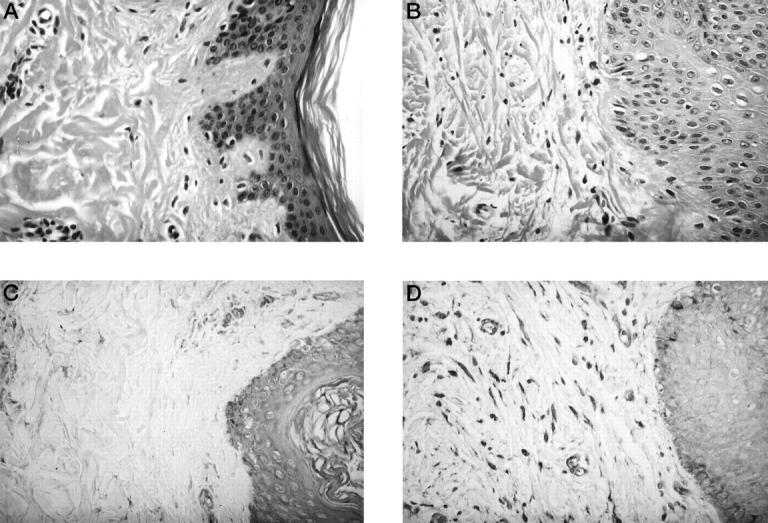
Histological comparison between normal and keloid tissues. A: H&E-stained sections of normal tissues (N-1). B: H&E-stained sections of keloid tissues (K-1). The keloid contains numerous blood vessels, collagen fibers, and fibroblasts. C: Immunohistochemistry of normal tissues (N-1) for IGF-IRβ subunits. Note the strong staining in the epidermis and endothelial cells and weak staining in the fibroblasts. D: Immunohistochemistry of keloid tissues (K-1) for IGF-IRβ subunits. Note the presence of strongly stained cells in the epidermis, endothelial cells, and fibroblasts. Magnification, ×400.
Functional Effects of IGF-I on Cell Proliferation
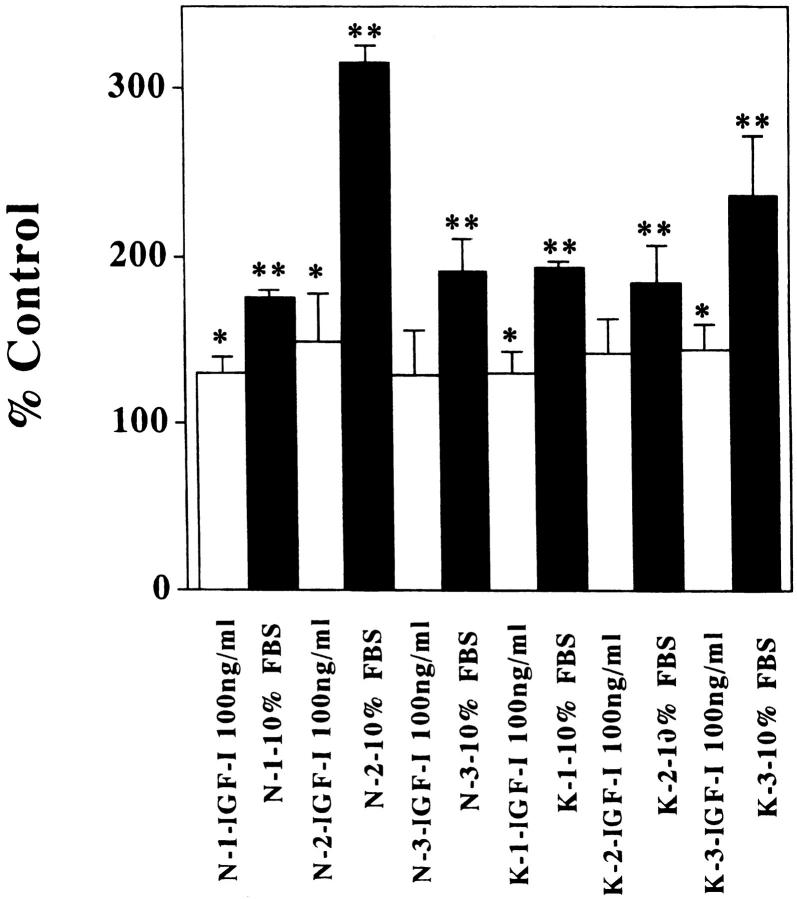
To examine the functional properties of IGF-I on cell proliferation, normal and keloid fibroblasts were cultured in DMEM containing 0.2% or 10% FBS or IGF-I (10, 100, or 1000 ng/ml) in the presence of 0.2% FBS. We examined each of the three cell lines. There were no significant differences between normal and keloid fibroblasts under all conditions in WST-1 assay (Figure 3, A and B) ▶ . The number of normal and keloid fibroblasts was significantly higher in the presence of 10% FBS than in the presence of 0.2% FBS at 48 hours (Figure 3, A and B ▶ , and Figure 4 ▶ ; P < 0.001). Furthermore, the number of some lines (N-1, N-2, K-1, and K-3) slightly increased in the presence of IGF-I (100 or 1000 ng/ml) compared with their number in DMEM containing 0.2% at 48 hours (Figure 3, A and B ▶ , and Figure 4 ▶ ; P < 0.005).
Figure 3.
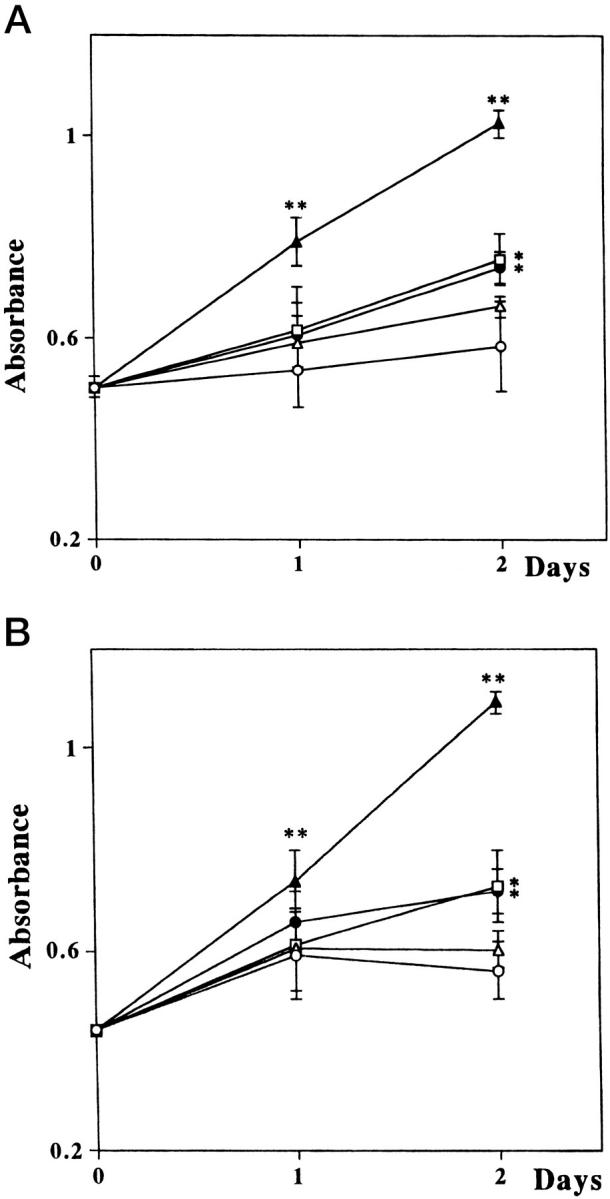
Growth of normal and keloid fibroblasts in different concentrations of serum and IGF-I. A: Normal fibroblasts (N-1); B: Keloid fibroblasts (K-1). The number of both fibroblasts significantly increased in the presence of 10% FBS. IGF-I at 100 and 1000 ng/ml resulted in a slight increase in the number of fibroblasts. ○, DMEM containing 0.2% FBS; ▵, 10 ng/ml IGF-I; □, 100 ng/ml IGF-I; •,1000 ng/ml IGF-I, solid triangles; 10% FBS. *P < 0.005; **P < 0.001.
Figure 4.
Growth of normal and keloid fibroblasts in different concentrations of serum and IGF-I. Cultured fibroblasts were treated with IGF-I (100 ng/ml) or 10% FBS for 48 hours. Treatment with IGF-I resulted in a slight increase in some but not all fibroblasts (N-1, N-2, K-1, and K-3). Treatment with FBS resulted in a significant increase of all fibroblasts. *P < 0.005; **P < 0.001.
Functional Effects of IGF-I on Cell Invasion
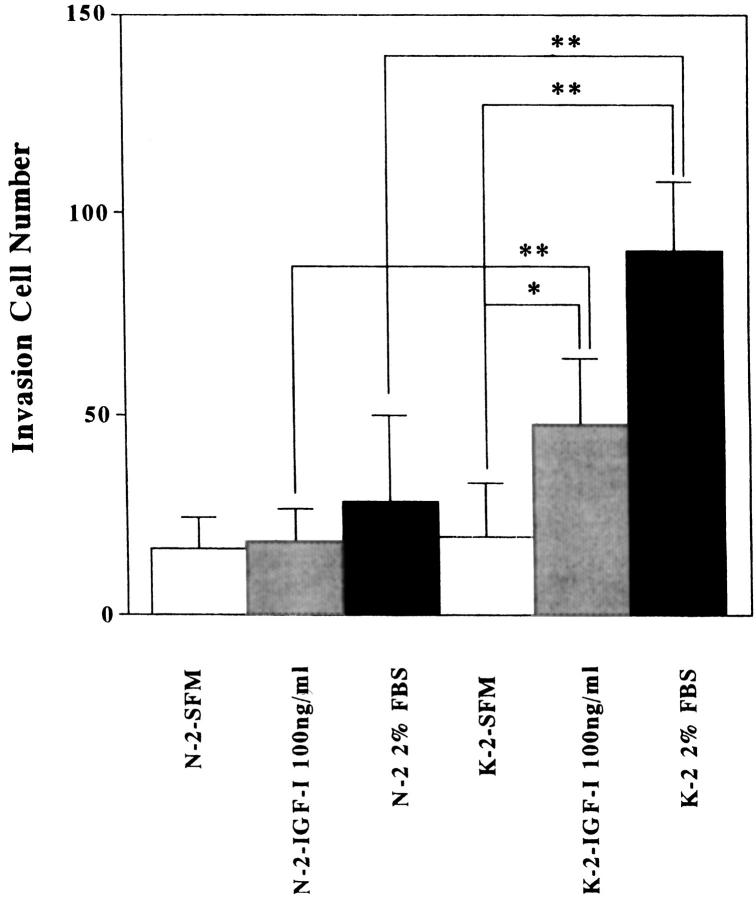
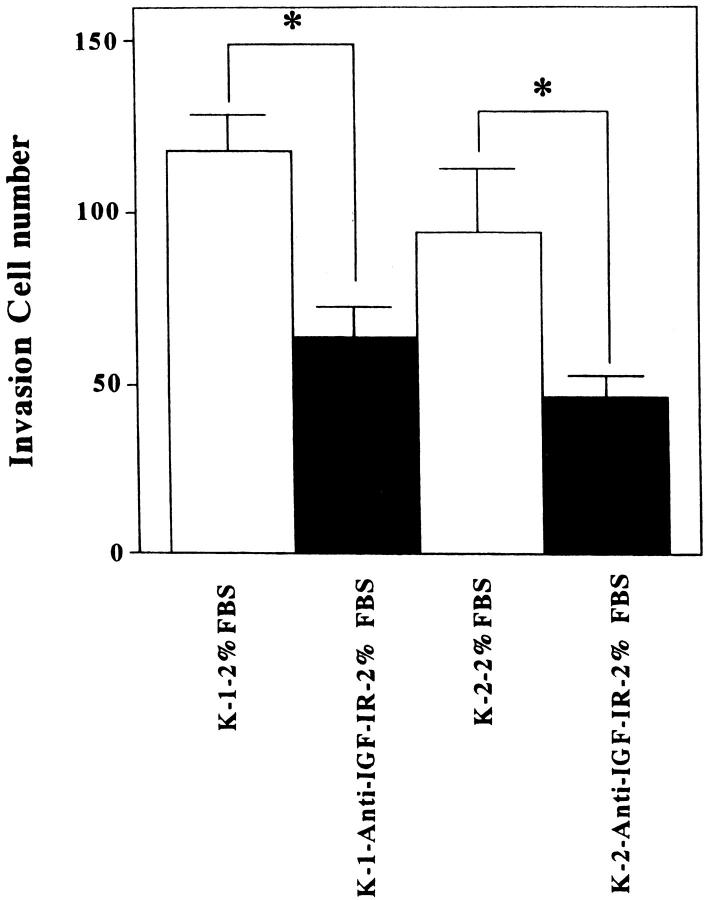
To elucidate the functional effects of IGF-I on cell invasion, normal and keloid fibroblasts were cultured in DMEM/F10 containing 0% or 2% FBS or IGF-I (100 ng/ml). The presence of IGF-I (100 ng/ml) or 2% FBS in the culture medium significantly increased the number of invading keloid fibroblasts (K-1 and K-2) compared with keloid fibroblasts in serum-free medium and normal fibroblasts (N-1 and N-2) under all conditions (Figure 5, A and B ▶ , and Figure 6 ▶ ; *P < 0.03, **P < 0.01, and data (K-1 and N-1) not shown.) When fibroblasts were not inoculated in the lower chamber, invasion was completely suppressed (data not shown). This phenomenon suggests that growth factor, including IGF-I, produced by normal fibroblasts stimulated keloid fibroblasts migration and invasion. To study how IGF-I influences the invasiveness of keloid fibroblasts in the presence of 2% FBS, we blocked the effect of IGF-I by using a neutralizing antibody against IGF-IR. The antibody inhibited 2% FBS-induced stimulation of invasion of K-1 and K-2 keloid fibroblasts (Figure 7 ▶ ; P < 0.03).
Figure 5.
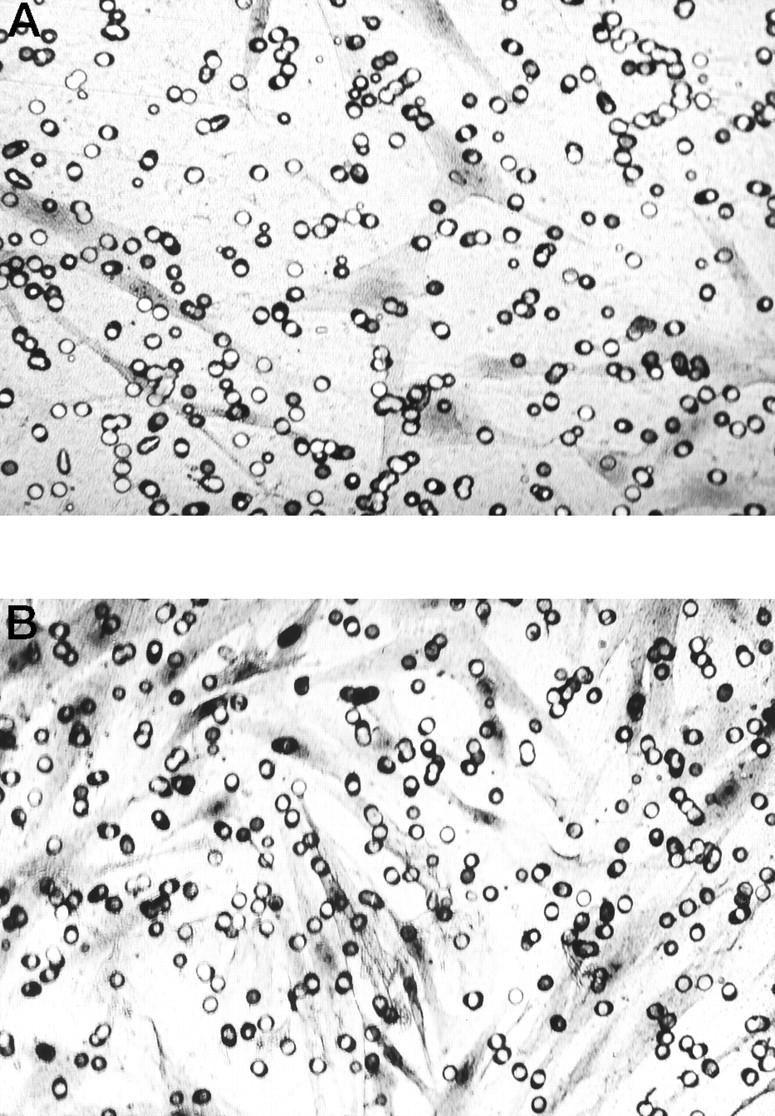
Lower surfaces of membrane culture insert when keloid fibroblasts (K-2) were assayed in the presence of different concentrations of serum or IGF-I. A: Fibroblasts were treated with SFM for 72 hours. B: Fibroblasts were treated with IGF-I (100 ng/ml) for 72 hours. Magnification, ×200.
Figure 6.
Invasion activities of normal and keloid fibroblasts (N-2 and K-2) in the presence of different concentrations of serum or IGF-I. The number of invasive fibroblasts increased in keloid fibroblasts in the presence of IGF-I (100 ng/ml) or 2% FBS. *P < 0.03; **P < 0.01.
Figure 7.
Effects of neutralizing antibody to IGF-IR on keloid fibroblasts. Treatment with a neutralizing antibody against IGF-IR reduced the number of invasive fibroblasts in both keloid lines (K-1 and K-2) in the presence of 2% FBS. *P < 0.03.
Discussion
In the present study, we first examined the expression profile of PTK genes in both normal and keloid fibroblasts of the same subject. PDGF-Rα was the most abundant receptor-type PTK in both types of fibroblasts, and there was no difference in the expression between normal and keloid fibroblasts. In sharp contrast to normal fibroblasts, IGF-IR was overexpressed in keloid fibroblasts. This finding was confirmed by immunohistochemistry in primary cultured cells. In the adult normal skin, IGF-IR was expressed in both the dermis and the epidermis. However, the dermal fibroblast was weakly stained. 9 The keloid fibroblast were predominantly stained by the IGF-IRβ subunit antibody, whereas normal fibroblasts did not show such staining. On the other hand, IGF-IR expression in dermis did not show any difference between normal and keloid tissues.
Insulin-like growth factors are peptides that are structurally similar to proinsulin. IGF-I mediates various actions of the growth hormone postnatally 10,11 and is thought to have a dual function, acting both as a mitogen and a differentiation factor. 12 In addition, several studies have suggested that IGF-I production is increased in various types of regenerating tissue, including subcutaneous tissue, skeletal muscle, 13,14 and peripheral nerves 15 after injury. Ghahary et al 16 reported increased expression of IGF-I mRNA in hypertrophic scar tissue. They also showed that IGF-I treatment increased the expression of mRNA for pro-α[I] chain of type I procollagen and pro-α[III] chain of type III procollagen in hypertrophic scar fibroblasts. 16 Furthermore, IGF-I reduced collagenase production by postburn hypertrophic scar fibroblasts compared with normal fibroblast. 17 In keloid fibroblasts, IGF-I may alter the process regulating the synthesis of extracellular matrix. Keloids are collagenous lesions acquired as a result of delayed wound healing. Other studies have demonstrated increased expression of IGF-I during the later stages of wound healing. 18 IGF-I/IGF-IR pathway may delay the wound-healing process and promote keloid formation.
Clinically, keloids are most commonly observed in subjects 10 and 30 years of age. 19 Interestingly, plasma levels of IGF-I are also high during the same period. 20 IGF-IR is a membrane-bound heterotetramer receptor with an intrinsic tyrosine kinase activity. 4 The receptor is expressed in a variety of cell types, including tumor cells. 12,21 Activation of the IGF-IR by its ligands (IGF-I, IGF-II, and insulin) plays an important role in the control of cell growth and malignant transformation and is an important inhibitor of apoptosis. 22,23 Experiments using dominant negative mutants of IGF-IR, antibodies to IGF-IR, or antisense strategies directed against IGF-IR mRNA have shown that reduced or aberrant receptor expression is associated with a reversal of the transformed phenotype, with prevention of metastasis and induction of apoptosis. 24-26 Furthermore, cells lacking IGF-IR have prolonged cell cycle kinetics, 27 and IGF-IR-deficient mice show severely diminished growth. 28
How does the signaling pathway of the IGF-IR influence keloid formation? To examine the functional properties of IGF-I/IGF-IR pathway, we investigated cell proliferation and invasion in the presence of exogenous IGF-I or neutralizing antibody against IGF-IR. Our results showed that the mitogenic effect of IGF-I was weak, but FBS markedly enhanced cell proliferation activity on both normal and keloid fibroblasts. Sell et al 27 also indicated that the IGF-IR signaling pathway is not the absolute requirement for cell cycle progression but is necessary for optimal growth. In another study, mouse fibroblasts that overexpress IGF-IR were found to have reduced growth factor requirements in vitro and were less susceptible to apoptosis. 29 Combined together, our results and those of other investigators suggest that a complex network of various growth factors may regulate the growth of keloid fibroblasts. Other PTKs, such as c-met, axl, and ryk, might be also related to regulate the cell cycle in normal or keloid fibroblasts.
Our results also showed that the invasiveness of keloid fibroblasts in the presence of IGF-I (100 ng/ml) or 2% FBS was higher than that of keloid fibroblasts in serum-free medium and normal fibroblasts under all conditions. Furthermore, the neutralizing antibody against IGF-IR inhibited 2% FBS-induced stimulation of invasion of keloid fibroblasts. Previous studies have demonstrated that antisense RNA to the IGF-IR resulted in the suppression of tumor growth and prevented invasion of rat prostate cancer cells 30 or murine carcinoma, H59 cells 31 in vivo. Thus, the IGF-I/IGF-IR pathway may play a critical role in the regulation of migration and invasiveness of keloid fibroblasts. In this regard, IGF-I is also regulated by the IGF-binding proteins. 32 Additional studies are required to elucidate the molecular mechanism of increased expression of IGF-IR in keloid fibroblasts.
In summary, we analyzed the expression profile of PTKs in normal and keloid fibroblasts using a PCR-based cloning technique and identified overexpression of IGF-IR in keloid fibroblasts. Exogenously added IGF-I had a weak mitogenic effect on both normal and keloid fibroblasts, as shown by WST-1 assay. The number of invading cultured keloid fibroblasts increased in the presence of IGF-I (100 ng/ml) and 2% FBS, and a neutralizing antibody against IGF-IR inhibited 2% FBS-induced stimulation of invasive properties of keloid fibroblasts. In contrast, that of invading cultured normal fibroblasts did not have this effect in both IGF-I (100 ng/ml) and 2% FBS conditions. Our results showed that the IGF-I/IGF-IR pathway might be involved in the regulation of invasiveness of keloid fibroblasts.
Acknowledgments
Human recombinant IGF-I was gift from Fujisawa Pharmaceutical Co., Tokyo, Japan.
Footnotes
Address reprint requests to Dr. Shunichi Yamashita, Department of Nature Medicine, Nagasaki University School of Medicine, 1-12-4 Sakamoto, Nagasaki, 852-8523 Japan. E-mail: shun@net.nagasaki-u.ac.jp.
Supported by grant 10671682 from The Ministry of Education, Science, Sports, and Culture, Japan.
References
- 1.Tredget EE, Nedelec B, Scott PG, Ghahary A: Hypertrophic scars, keloids, and contractures: the cellular and molecular basis for therapy. Surg Clin North Am 1997, 77:701-730 [DOI] [PubMed] [Google Scholar]
- 2.Haisa M, Okochi H, Grotendorst GR: Elevated levels of PDGF-α receptors in keloid fibroblasts contribute to an enhanced response to PDGF. J Invest Dermatol 1994, 103:560-563 [DOI] [PubMed] [Google Scholar]
- 3.Babu M, Diegelmann R, Oliver N: Keloid fibroblasts exhibit an altered response to TGF-β. J Invest Dermatol 1992, 99:650-655 [DOI] [PubMed] [Google Scholar]
- 4.Ullrich A, Schlessinger J: Signal transduction by receptors with tyrosine kinase activity. Cell 1990, 61:203-212 [DOI] [PubMed] [Google Scholar]
- 5.Hanks SK, Quinn AM, Hunter T: The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science 1988, 241:42-52 [DOI] [PubMed] [Google Scholar]
- 6.Tanaka K, Nagayama Y, Nakano T, Takamura N, Namba H, Fukada S, Kuma K, Yamashita S, Niwa M: Expression profile of receptor-type protein tyrosine kinase genes in the human thyroid. Endocrinology 1998, 139:852-858 [DOI] [PubMed] [Google Scholar]
- 7.Albini A, Iwamoto Y, Kleinman HK, Martin GR, Aaronson SA, Kozlowski JM, McEwan RN: A rapid in vitro assay for quantitating the invasive potential of tumor cells. Cancer Res 1987, 47:3239-3245 [PubMed] [Google Scholar]
- 8.Arakawa M, Hatamochi A, Takeda K, Ueki H: Increased collagen synthesis accompanying elevated m-RNA levels in cultured Werner’s syndrome fibroblasts. J Invest Dermatol 1990, 94:187-190 [DOI] [PubMed] [Google Scholar]
- 9.Rudman SM, Philpott MP, Thomas GA, Kealey T: The role of IGF-I in human skin and its appendages: morphogen as well as mitogen? J Invest Dermatol 1997, 109:770-777 [DOI] [PubMed] [Google Scholar]
- 10.Isaksson OG, Ohlsson C, Nilsson A, Isgaard J, Lindahl A: Regulation of cartilage growth by growth hormone and insulin-like growth factor I. Pediatr Nephrol 1991, 5:451-453 [DOI] [PubMed] [Google Scholar]
- 11.Baker J, Liu JP, Robertson EJ, Efstratiadis A: Role of insulin-like growth factors in embryonic and postnatal growth. Cell 1993, 75:73-82 [PubMed] [Google Scholar]
- 12.Sara VR, Hall K: Insulin-like growth factors and their binding proteins. Physiol Rev 1990, 70:591-614 [DOI] [PubMed] [Google Scholar]
- 13.Edwall D, Schalling M, Jennische E, Norstedt G: Induction of insulin-like growth factor I messenger ribonucleic acid during regeneration of rat skeletal muscle. Endocrinology 1989, 124:820-825 [DOI] [PubMed] [Google Scholar]
- 14.Jennische E, Skottner A, Hansson HA: Satellite cells express the trophic factor IGF-I in regenerating skeletal muscle. Acta Physiol Scand 1987, 129:9-15 [DOI] [PubMed] [Google Scholar]
- 15.Hansson HA, Dahlin LB, Danielsen N, et al: Evidence indicating trophic importance of IGF-I in regenerating peripheral nerves. Acta Physiol Scand 1986, 126:609-614 [DOI] [PubMed] [Google Scholar]
- 16.Ghahary A, Shen YJ, Nedelec B, Scott PG, Tredget EE: Enhanced expression of mRNA for insulin-like growth factor-1 in post-burn hypertrophic scar tissue and its fibrogenic role by dermal fibroblasts. Mol Cell Biochem 1995, 148:25-32 [DOI] [PubMed] [Google Scholar]
- 17.Ghahary A, Shen YJ, Nedelec B, Wang R, Scott PG, Tredget EE: Collagenase production is lower in post-burn hypertrophic scar fibroblasts than in normal fibroblasts and is reduced by insulin-like growth factor-1. J Invest Dermatol 1996, 106:476-481 [DOI] [PubMed] [Google Scholar]
- 18.Gartner MH, Benson JD, Caldwell MD: Insulin-like growth factors I and II expression in the healing wound. J Surg Res 1992, 52:389-394 [DOI] [PubMed] [Google Scholar]
- 19.Ketchum LD, Cohen IK, Masters FW: Hypertrophic scars and keloids: a collective review. Plast Reconstr Surg 1974, 53:140-154 [DOI] [PubMed] [Google Scholar]
- 20.Copeland KC, Colletti RB, Devlin JT, McAuliffe TL: The relationship between insulin-like growth factor-I, adiposity, and aging. Metabolism 1990, 39:584-587 [DOI] [PubMed] [Google Scholar]
- 21.Baserga R: Oncogenes and the strategy of growth factors. Cell 1994, 79:927-930 [DOI] [PubMed] [Google Scholar]
- 22.Baserga R: The insulin-like growth factor I receptor: a key to tumor growth? Cancer Res 1995, 55:249-252 [PubMed] [Google Scholar]
- 23.Macaulay VM: Insulin-like growth factors and cancer. Br J Cancer 1992, 65:311-320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prager D, Li HL, Asa S, Melmed S: Dominant negative inhibition of tumorigenesis in vivo by human insulin-like growth factor I receptor mutant. Proc Natl Acad Sci USA 1994, 91:2181-2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trojan J, Johnson TR, Rudin SD, Ilan J, Tykocinski ML, Ilan J: Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science 1993, 259:94-97 [DOI] [PubMed] [Google Scholar]
- 26.Pietrzkowski Z, Wernicke D, Porcu P, Jameson BA, Baserga R: Inhibition of cellular proliferation by peptide analogues of insulin-like growth factor 1. Cancer Res 1992, 52:6447-6451 [PubMed] [Google Scholar]
- 27.Sell C, Dumenil G, Deveaud C, Miura M, Coppola D, DeAngelis T, Rubin R, Efstratiadis A, Baserga R: Effect of a null mutation of theinsulin-like growth factor I receptor gene on growth and transformation of mouse embryo fibroblasts. Mol Cell Biol 1994, 14:3604-3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu JP, Baker J, Perkins AS, Robertson EJ, Efstratiadis A: Mice carrying null mutations of the genes encoding insulin-like growth factor I (Igf-1) and type 1 IGF receptor (Igf1r). Cell 1993, 75:59-72 [PubMed] [Google Scholar]
- 29.Sell C, Baserga R, Rubin R: Insulin-like growth factor I (IGF-I) and the IGF-I receptor prevent etoposide-induced apoptosis. Cancer Res 1995, 55:303-30l6 [PubMed] [Google Scholar]
- 30.Burfeind P, Chernicky CL, Rininsland F, Ilan J, Ilan J: Antisense RNA to the type I insulin-like growth factor receptor suppresses tumor growth and prevents invasion by rat prostate cancer cells in vivo. Proc Natl Acad Sci USA 1996, 93:7263-7268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Long L, Rubin R, Baserga R, Brodt P: Loss of the metastatic phenotype in murine carcinoma cells expressing an antisense RNA to the insulin-like growth factor receptor. Cancer Res 1995, 55:1006-1009 [PubMed] [Google Scholar]
- 32.Baxter RC: Insulin-like growth factor binding proteins in the human circulation: a review. Horm Res 1994, 42:140-144 [DOI] [PubMed] [Google Scholar]