Abstract
Monoclonal antibody Ki-A10 recognizes a nuclear antigen of 25 and 22 kd apparent molecular mass, which is abundantly expressed by immature gonocytes, spermatogonia, and spermatocytes, whereas it is absent in spermatids, spermatozoa, oocytes, and normal somatic tissues. In a broad spectrum of human cancers the antibody showed no reactivity except for a small subset of malignant lymphomas. Because of this restricted expression pattern, we examined 173 germ cell tumors and 18 sex cord stromal tumors immunohistochemically to assess the distribution of the Ki-A10 antigen. A strongly positive reaction was found in classic seminomas, dysgerminomas, spermatocytic seminomas, and the germ cell component of gonadoblastomas. Yolk sac tumors presented a heterogeneous reactivity pattern ranging from overall positivity to complete lack of antigen expression, and in three of eight choriocarcinomas, a few clusters of cytotrophoblast cells were strongly labeled. All other tumors, including Leydig and Sertoli cell tumors as well as placental tissue, were negative. Our findings suggest that specific germ cell antigens can be retained in germ cell tumors along particular differentiation pathways. Ki-A10 is the first marker that consistently labels spermatocytic seminoma, further confirming its germ cell origin and suggesting a close relationship to classic seminoma. The antibody may serve for diagnostic purposes and promises new insights into the process of germ cell differentiation and the development of germ cell-derived neoplasia.
A novel monoclonal antibody, Ki-A10, with a unique reactivity pattern, which recognizes a nuclear antigen of 25 and 22 kd molecular mass constitutively expressed in L428 cells 1 is described. In normal tissue, this antigen is consistently and exclusively expressed in the premeiotic precursors of spermatogenesis while being totally absent in normal mature somatic cells. Screening of a wide spectrum of solid cancers of adulthood and infancy showed no reactivity. Only a small subset of malignant lymphomas reacted with Ki-A10. Because of this exceptionally restrictive tissue distribution, we studied the expression of the antigen in various types of germ cell tumors (GCT).
It is now generally accepted that all GCT arise from a pluripotent stem cell, the primordial germ cell. In adults, the initial stage of malignant transformation presents as a preinvasive lesion known as carcinoma in situ (CIS) or intratubular germ neoplasia (IGCN), which is considered to be the precursor of other types of GCT in adolescents and adults. 2-4 The evolutionary pathways of GCT, however, remain largely unclear. We will show that the expression of the Ki-A10 antigen is abundant in the most immature forms of germ cell neoplasia, and that it is conserved along certain lines of differentiation. 4,5 Besides being of diagnostic use, our observations may provide new insights into the histogenetic relationship between different types of GCT.
Materials and Methods
Generation of the Antibody
Entire nuclei from L428 cells 1 lysed in hypotonic saline harvested by centrifugation and supplemented with incomplete Freund’s adjuvant were used to immunize female Balb/c mice as described previously. 6 After boostering, mouse splenocytes were fused by somatic hybridization with P3x63-Ag.8653 mouse myeloma cells. Hybrid clones were grown in RPMI 1640 medium with 10% fetal calf serum and subcloned. Supernatants were screened for immunoreactivity on cytospin preparations of L428 cells.
Tissue Samples
Both fresh-frozen and paraffin-embedded material from an exhaustive spectrum of normal human tissues was available; at least three samples of each tissue type were examined. Additional paraffin-embedded specimens included: normal placental tissue at various stages of maturity (n = 22), two hydatidiform moles, and 18 normal embryos at different ages of gestation (8 to 18 weeks); 152 carcinomas from various locations comprising 23 squamous cell carcinomas from the skin and the upper digestive and respiratory tracts, 16 cutaneous basal cell carcinomas, adenocarcinomas of the stomach (n = 7), colon (n = 12), pancreas (n = 3), liver (n = 3), lung (n = 6), prostate (n = 11), endometrium (n = 14), ovaries (n = 15), and female breast (n = 28, consisting of 18 ductal, seven lobular, and three medullary carcinomas), and five carcinomas of the thyroid (three papillary, two follicular, and one medullary); 57 melanocytic lesions (31 benign nevi and 26 malignant melanomas); 68 soft tissue sarcomas (leiomyosarcomas, rhabdomyosarcomas, liposarcomas, synovial sarcomas, fibrosarcomas, angiosarcomas, osteosarcomas, chondrosarcomas, and malignant fibrous histiocytomas); blastomatous tumors of infancy comprising three neuroblastomas, three nephroblastomas, three hepatoblastomas, and two lipoblastomas; seven testicular lymphomas (three lymphoblastic lymphomas, one immunoblastic lymphoma, and one centrocytic-centroblastic (CB-CC) lymphoma, 28 cases of Hodgkin’s disease (HD) and 52 non-Hodgkin lymphomas from other sites (six chronic lymphocytic leukemias (B-CLL, immunocytoma), 11 CB-CC, eight centroblastic (CB) lymphomas, 12 large-cell anaplastic lymphomas (LCAL), six lymphomas of mucosa-associated lymphoid tissue, five Burkitt’s lymphomas, and four T-immunoblastic lymphomas) as well as 17 plasmacytomas and 14 acute and 10 chronic myeloid leukemias.
Furthermore, 172 cases of testicular and ovarian GCT, comprising both monotypic tumors and malignant GCT of more than one histological type, as well as 11 testicular sex cord stromal tumors, four thecomas, and three granulosa cell tumors of the ovary were examined with the antibody Ki-A10 (Table 1) ▶ . Diagnosis was made by conventional histology and supported by immunohistochemistry using monoclonal antibodies against placenta-specific alkaline phosphatase, human beta-chorionic gonadotropin, and alpha-fetoprotein. The majority of the tumors were located in the testis, a smaller number in the ovary, and only a few were extragonadal. IGCN (CIS) was present in 102 of the specimens. To exclude false negative reactions, a sample of normal testicular tissue was carried with each series but, as a rule, a few seminiferous tubules at the tumor margin provided a reliable internal control.
Table 1.
Provenance of Tumors
| Tumors | Testicular | Ovarian | Extragonadal | n |
|---|---|---|---|---|
| Seminoma | 38 | 38 | ||
| Metastatic seminoma | 7 | 7 | ||
| Spermatocytic seminoma | 2 | 2 | ||
| Dysgerminoma | 9 | 9 | ||
| Gonadoblastoma | 4 | 4 | ||
| Yolk sac tumor | 15 (14) | 9 (6) | 5 (3) | 28 |
| Embryonal carcinoma | 14 | 2 | 1 | 17 |
| Choriocarcinoma | 3 | 3 | ||
| Teratoma (mature) | 8 | 4 (3) | 12 | |
| Teratoma (immature) | 3 | 2 | 2 (2) | 7 |
| Mixed GCT | 29 (3) | 10 (2) | 6 (3) | 45 |
| Stromal tumors | 7 (4) | 11 (5) | 18 | |
| Total | 111 (21) | 55 (13) | 25 (11) | 191 (45) |
The number of infantile tumors is given in parentheses.
Immunohistochemistry
Immunohistochemical staining with Ki-A10 was done as described previously. 7 Briefly, paraffin sections were mounted on silane-coated slides, routinely processed, and incubated for 5 minutes in 3% (vol/vol) peroxide in methanol. Antigen retrieval was obtained by heating the section immersed in 0.01 mol of citric acid, pH 6.0, for 10 minutes in a microwave oven (Sharp, Berlin, Germany) at the highest power setting (950 W). The primary antibody Ki-A10 (undiluted cell culture supernatant) was then incubated on the tissue sections for 30 minutes, and the reaction was enhanced with the streptavidin-biotin complex using peroxidase and rabbit-antimouse antibody (all reagents purchased from DAKO, Hamburg, Germany). The tissue structures were visualized by brief counterstaining with Mayer’s hematoxylin.
Immunostaining was evaluated in a semi-quantitative manner as diffuse, focal, or negative.
Determination of Molecular Mass
Immunoprecipitation of the antigen was done with minor modifications as described elsewhere. 8 Briefly, cell homogenates from L428 cells were lysed in 2 ml of alkylation buffer supplemented with phenylmethylsulfonyl-fluoride (PMSF) and 20 μl of β-mercaptoethanol and further disintegrated by sonication (80 bursts). The suspension was dialyzed four times against 2-liter 4 mol/L urea/50 mmol/L Tris-buffer, pH 7.5, and once against 2-liter 0.1% sodium dodecyl sulfate (SDS). After addition of a neutralizing buffer (1.5% Nonidet P-40, 300 mmol/L Tris, pH 7.4, 60 mmol/L EDTA, 600 mmol/L NaCl, with PMSF) at a ratio of 1:5 (vol/vol), the dialysis product was admixed with 2 ml of antibody-containing cell culture supernatant and incubated for 6 hours at 4°C and 2 hours at room temperature. Incubation was resumed overnight at 4°C after addition of 100 μl of protein G sepharose (Biochrom, Berlin, Germany). Following brief sedimentation, the precipitate was washed twice with a buffer composed of 0.05% SDS, 0.25% Nonidet P-40, 50 mmol/L Tris, pH 7.4, 10 mmol/L EDTA, and 100 mmol/L NaCl, and PMSF. The pellet was then dissolved in loading buffer without β-mercaptoethanol and heated to 65°C for 20 minutes. After dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE, 10 to 20%) and transfer to polyvinylidendifluoride (PVDF) membranes by semidry blotting (50 minutes), the immunoreaction was enhanced with enhanced chemiluminescence (Amersham, Buckinghamshire, UK) according to the supplier’s instructions and controlled by India Ink staining. A molecular weight standard (Sigma, München, Germany) served as reference.
The results were verified by Western blot experiments using fresh frozen tissue from normal fertile testes and seminomas. The specimens were mechanically disrupted in lysis buffer (4 mol/L urea, 0.5% SDS, 62.5 mmol/L Tris, pH 6.8) with use of Ultraturrax (IKA Labortechnik, Germany), sonicated (40 bursts) for DNA fragmentation, and sedimented by centrifugation. Twenty μl of the supernatants were then diluted 1:5 in water, and the protein content was determined by means of bicinchoninic acid assay (Pierce, Rockford, IL) according to the suppliers’ instructions. After addition of 10% blue SDS (4 mol/L urea, 10% SDS, 62.5 mmol/L Tris with bromophenol-blue), protein denaturation was achieved by incubation with 1% (vol/vol) β-mercaptoethanol for 20 minutes at 65°C. Subsequent SDS-PAGE, blotting, and immunostaining with Ki-A10 were done as described above. Additionally, immunoprecipitated protein bands were excised from the membranes and further processed for protein sequencing.
Results
General Observations
The supernatant of one hybrid clone showed nuclear reactivity with L428 cells. This antibody of the IgG1 subclass was designated Ki-A10 and used for all further experiments. Immunostaining of frozen and paraffin sections yielded identical results. The reaction was confined to the nuclei, accentuating the nuclear membrane and the nucleoli but leaving mitotic figures unstained. Occasionally, the intensity of staining was variable, ranging from dark to light brown. A background staining of acellular tissue or a nonspecific cross-reaction was hardly ever observed but for a faint, purely cytoplasmic coreactivity of a few macrophages and plasma cells and a coarsely granular staining in the cytoplasm of eosinophils and hepatoblasts.
Ki-A10 Reactivity in Normal Tissues
The immunoreactivity of monoclonal antibody Ki-A10 was strictly confined to the nuclei of spermatogonia and spermatocytes. The staining intensity was invariably strong in spermatogonia and decreased in spermatocytes in proportion to their differentiation, whereas further maturation to spermatids resulted in an abrupt loss of reactivity (Figure 1a) ▶ . Other testicular cells, in particular Sertoli and Leydig cells, did not express the antigen. Ovarian tissue, inclusive of primordial and Graafian follicles, was also negative. In placental tissue including hydatidiform moles, neither trophoblastic nor stromal cells reacted with Ki-A10. No immunoreactivity was observed in any type of adult or fetal somatic tissue, whereas a strong immunoreaction was detected in some of the gonads of the embryos examined. The embryonal gonads were undifferentiated in five cases, four displayed early testicular and three early ovarian differentiation (in six cases, no gonadal tissue was apparent on histological sections). Only the germ cells of undifferentiated gonads and a portion of cells within the tubular structures of immature testes were reactive, whereas the antigen abruptly disappeared with the onset of ovarian differentiation.
Figure 1.
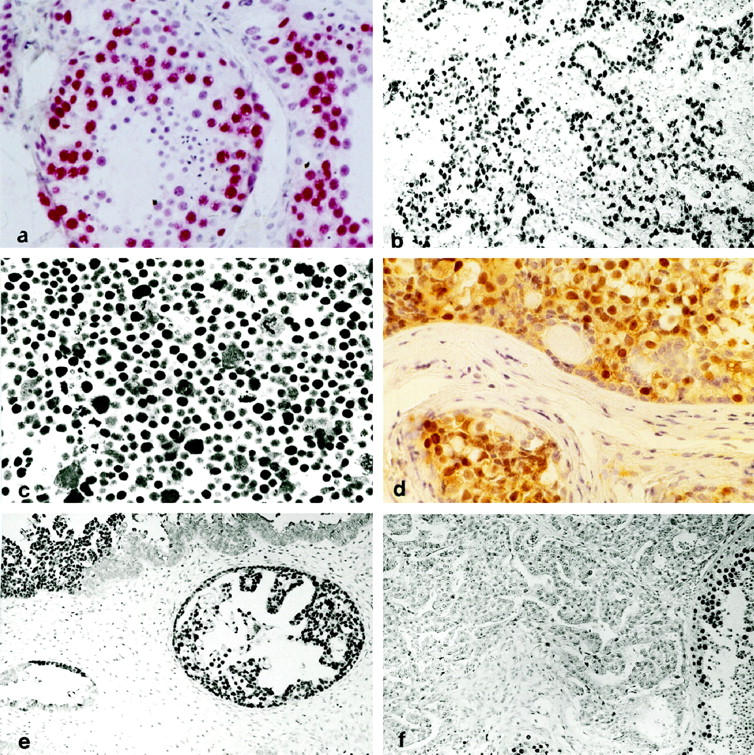
a: Section of a normal adult testis stained with Ki-A10; spermatogonia are consistently positive (red label); the staining intensity decreases in spermatocytes along with maturation and becomes undetectable in spermatids and spermatozoa. Sertoli cells show no reactivity (original magnification, ×350). b: Strongly Ki-A10-labeled tumor cell nuclei of testicular seminoma scattered in a lymphoid infiltrate negative to Ki-A10 (magnification, ×140). c: The totality of the bizarre cells of spermatocytic seminoma exhibit strong nuclear Ki-A10 reactivity (magnification, ×350). d: The germ cell component of gonadoblastoma stains with slightly variable intensity (brown label) for Ki-A10 (magnification, ×140). e: A large number of cells in YST of glandular type express the Ki-A10 antigen; focally, abrupt loss of antigenity is noted (upper right) (magnification, ×140). f: Embryonal carcinoma characteristically does not express the Ki-A10 antigen; in the preserved seminiferous tubules (bottom right), spermatogonia and spermatocytes are strongly positive (magnification, ×140; all sections peroxidase technique and hematoxylin counterstain).
Immunoreactivity of Ki-A10 in Tumors
All carcinomas, sarcomas, and melanocytic tumors as well as the testicular lymphomas were consistently negative. Also, none of the blastomas comprising partly differentiated and undifferentiated neuroblastomas and triphasic and blastema-rich nephroblastomas showed nuclear labeling by Ki-A10. However, in all three cases of hepatoblastoma, a nonspecific, coarsely granular cytoplasmic staining of the epithelial tumor cells was noted.
By contrast, nuclear immunoreactivity was present in a portion of malignant lymphomas. The highest percentage of positive tumors was found in HD (12 of 28), followed by LCAL (4 of 12), CB (2 of 10), and CB-CC (1 of 14). In HD, immunostaining was confined to Hodgkin and Sternberg-Reed cells exclusively, whereas in LCAL and in the positive CB the near totality of the neoplastic cells were stained; in the single positive CB-CC, only the blastic cell fraction was reactive. Other lymphoid and hematological malignancies, notably plasmacytomas and myeloid leukemias, did not express the antigen.
GCT displayed distinctive patterns of Ki-A10 reactivity (Table 2) ▶ . The cells of IGCN (CIS) consistently showed a strong reactivity comparable with that of spermatogonia. In virtually all seminomas, a strong or at least moderately intense nuclear staining of the majority tumor cells was observed (Figure 1b) ▶ . Syncytiotrophoblastic giant cells occasionally present in the tumors were consistently negative as were the lymphoid infiltrates and occasional granulomatous inflammatory reactions. A similar reactivity was encountered in all 12 lymph node metastases of seminoma. Remarkably, both cases of spermatocytic seminoma (SS) also displayed a strong overall immunoreaction (Figure 1c) ▶ . All 14 dysgerminomas showed a diffuse positive reaction for Ki-A10 in a pattern similar to that of seminomas. Also the intensity of staining was comparable, and even in areas with massive granulomatous inflammation the rare tumor cells present were highlighted by Ki-A10 immunostaining. Similarly, in all cases of gonadoblastoma, the well circumscribed nests of germ cells exhibited overall positivity albeit with variable intensity, whereas no staining was detected in the sex-cord derivatives (Figure 1d) ▶ .
Table 2.
Immunoreactivity of Ki-A10 in Germ Cell Tumors and Gonadal Stromal Tumors
| Parameters | Overall positive | Focally positive | Negative |
|---|---|---|---|
| Monotypic germ cell tumors | |||
| Seminoma [38] | 38 | 0 | 0 |
| Metastatic seminoma [7] | 7 | 0 | 0 |
| Dysgerminoma [9] | 9 | 0 | 0 |
| Gonadoblastoma [4] | 4 | 0 | 0 |
| Yolk sac tumor [29] | 7 | 9 | 13 |
| Choriocarcinoma [3] | 0 | 1 | 2 |
| Embryonal carcinoma [17] | 0 | 0 | 17 |
| Teratoma (mature) [12] | 1* | 0 | 11 |
| Teratoma (immature) [7] | 0 | 0 | 7 |
| Mixed germ cell tumors [45] (components) | |||
| Seminoma [17] | 17 | 0 | 0 |
| Dysgerminoma [11] | 11 | 0 | 0 |
| Yolk sac tumor [34] | 8 | 11 | 15 |
| Choriocarcinoma [6] | 0 | 2 | 4 |
| Embryonal carcinoma [26] | 0 | 0 | 26 |
| Teratoma (mature) [25] | 0 | 0 | 25 |
| Teratoma (immature) [10] | 0 | 0 | 10 |
| IGCN (all cases) [71] | 71 | 0 | 0 |
| Stromal tumors [18] | |||
| Sertoli cell tumor [7] | 0 | 0 | 7 |
| Leydig cell tumor [4] | 0 | 0 | 4 |
| Thecoma [4] | 0 | 0 | 4 |
| Granulosa cell tumor [3] | 0 | 0 | 3 |
Items in brackets indicate the numbers of cases.
*Positive immunoreaction confined to an undifferentiated gonad within a mature teratoma.
Of all nonseminomatous GCT, only yolk sac tumors (YST) presented an overall or focal immunoreactivity in approximately 50% of the cases, whereas the remainder were either completely negative or contained just a few labeled cells. Antigen expression did not correlate with a particular histological pattern. Occasionally, an abrupt transition from intense labeling to total negativity was observed, coinciding with a barely discernible change of cellular morphology (Figure 1e) ▶ . In two of five choriocarcinomas (CC), approximately 10% of the cytotrophoblast cells were strongly stained, whereas syncytiotrophoblastic cells did not react. Positive immunostaining was also seen in the germ cell component of a single case of a mixed germ cell-sex cord stromal tumor. Conversely, no immunoreactivity could be detected in the cells of embryonal carcinomas (EC; Figure 1f ▶ ), and none of the manifold tissue types in mature and immature teratoma ever exhibited nuclear labeling.
In germ cell tumors of more than one histological type, the different components exhibited a reactivity pattern in every way comparable with that of their monotypic counterparts. Briefly, areas with histological features of seminoma or dysgerminoma were strongly labeled; staining of YST was irregular, as described above, and again, in one of six choriocarcinomas, clusters of cytotrophoblast cells showed strong nuclear positivity. No reactivity was observed in EC, mature and immature teratoma, or the stromal component of these tumors.
In the group of sex cord stromal tumors, which comprised seven Sertoli cell and three Leydig cell tumors of the testes and four thecomas and three granulosa cell tumors of the ovaries, no positive reaction for Ki-A10 could be detected.
There was no difference in immunostaining between infants’ and adults’ tumors of the same histological type. For tabulation, the tumors were classified in three subgroups, namely monotypic GCT, mixed GCT, and sex cord stromal tumors. In GCT of more than one histological type, the different components were evaluated separately.
Molecular Mass of the Ki-A10 Protein
Immunoprecipitation of the immunoreactive antigen using nuclear extracts from L428 cells revealed a strong double band of 25 and 22 kd apparent molecular mass (Figure 2) ▶ . In addition, a weaker signal near 46 kd was observed in some experiments. Identical results were obtained with lysates from normal testicular tissue. Molecular mass was confirmed by Western blot experiments using normal adult testicular tissue and seminoma specimens. In these, however, the double band was seen only occasionally. More often, an intense signal at approximately 25 kd was apparent with an additional weaker band close to 46 kd (Figure 3) ▶ . Preliminary sequencing results showed no homology with any known protein.
Figure 2.
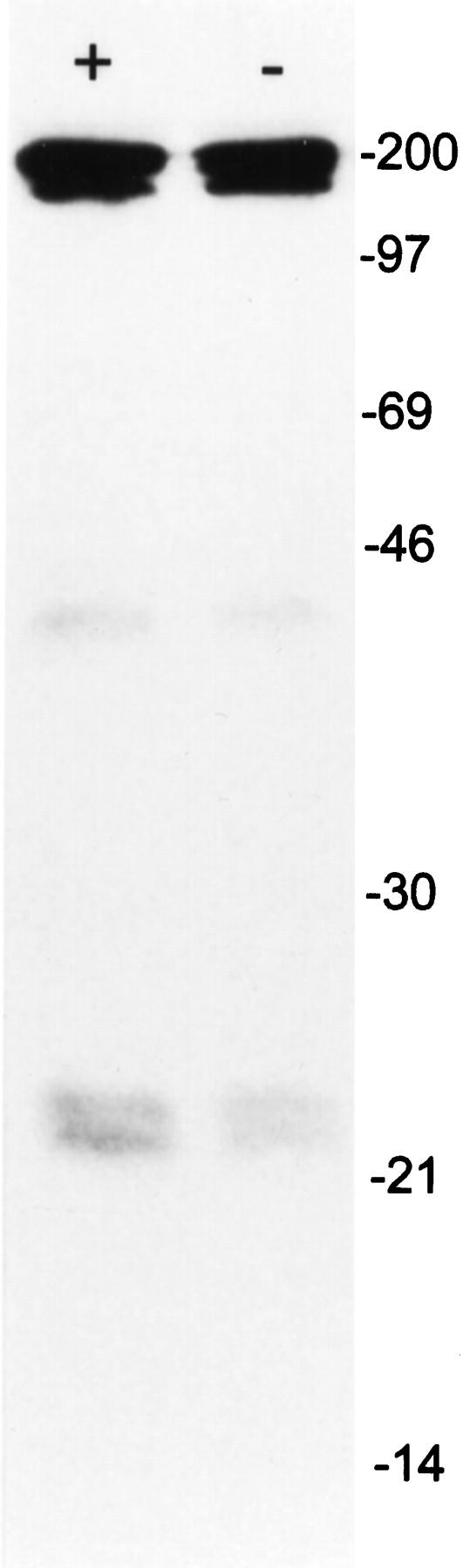
Immunoprecipitation of nuclear extracts of L428 cells with Ki-A10 at 4°C (left) and room temperature (right) showing two neat protein bands corresponding to approximately 25 and 22 kd and a faint band near 46 kd. The signal intensity was slightly weaker when incubation was performed at room temperature. The thick bands at approximately 180 to 200 kd represent the nonreduced antibody Ki-A10 with the molecular mass of an IgG molecule. Molecular weight reference is indicated on the right.
Figure 3.
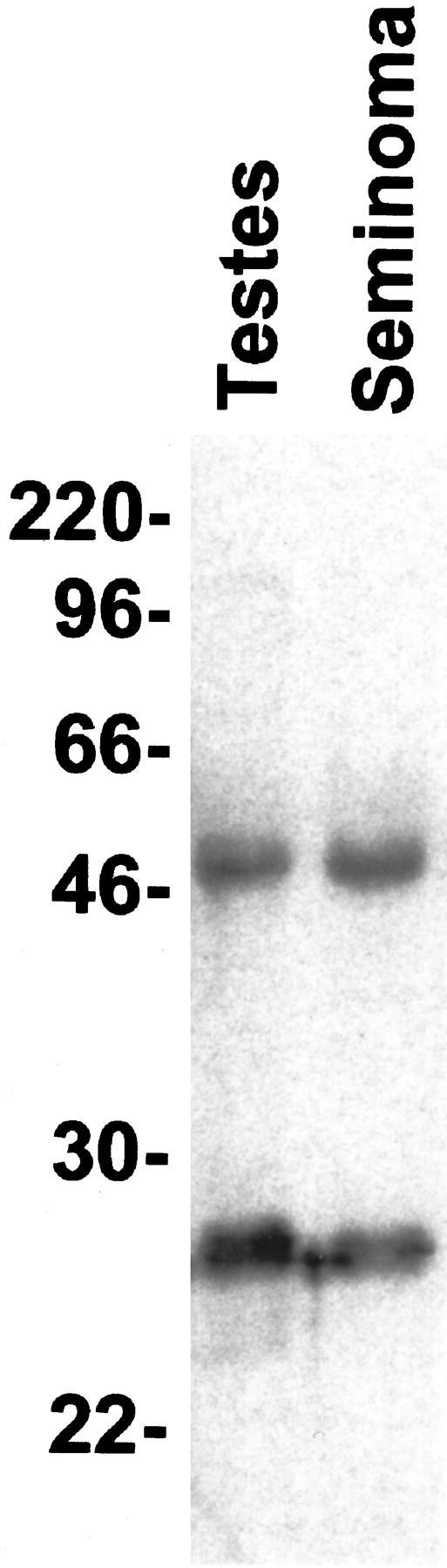
Western blot experiment using lysates from normal testicular tissue and seminoma. Both lanes show a strong signal at approximately 25 kd; a second band of lesser intensity is seen slightly above 46 kd, which corresponds to the upper band in Figure 2 ▶ . The apparent difference in molecular mass is probably because of slight variations in the migration of the molecular weight reference (indicated on the left).
Discussion
We have generated an antibody, Ki-A10, which has a high affinity to human germ cells but does not react with normal somatic tissues. The Ki-A10 antigen is consistently expressed in the nuclei of spermatogonia and spermatocytes, but absent in later stages of spermatogenesis. It could be immunohistochemically detected in immature gonads as early as the 8th week of gestation and disappears with the onset of morphologically assessable ovarian differentiation. These observations suggest that the Ki-A10 antigen is a germ cell-specific protein, which is lost by meiotic division, ie, in haploid maturation stages.
Germ cells have unique properties and are fundamentally distinct from somatic cells, but little is known about how this difference is established and maintained. There is nevertheless a growing body of evidence suggesting that the transactivation of specific genes plays important role in this process. 9,10 Considering its restrictive expression pattern, the Ki-A10 antigen might well be another yet unidentified member of the family of proteins responsible for germ cell development and differentiation. However, neither the molecular mass nor the distribution of the antigen allow conclusions as to its nature or function.
Immunoprecipitation of the Ki-A10 protein revealed two intense signals corresponding to approximately 25 and 22 kd and a much weaker band of approximately 46 kd apparent molecular mass. The latter may correspond to a heterodimer of the two former proteins or may represent a propeptide that is converted to active molecules by asymmetrical cleavage. The slightly different mass of the two smaller proteins is also suggestive of two isoforms generated by alternative splicing. However, as the lower signal was frequently lacking in Western blot experiments, it may also represent an early degradation product of the main 25 kd protein artifactually formed in the absence of denaturing conditions. Although the immunolocalization suggests a function within the nucleus, the consistent absence in cycling somatic cells implies that the Ki-A10 protein is unrelated to proliferation or cell cycle events.
The expression of the Ki-A10 protein in a subset of GCT provides novel clues as to their histogenetic relationship. Germ cell tumors represent the most frequent form of neoplasms in the male gonads, and the vast majority of them are malignant; in the ovary, they account for a smaller percentage of all tumors, and most of them are benign. The reasons for this gender-specific incidence are not clear, but they may be attributable to the conserved proliferative potential in male germ cells in contrast to the early haploid differentiation of oocytes.
All GCT are thought to arise from a common precursor, the primordial germ cell, 5,11 and there is evidence that malignant transformation may take place already during early embryogenesis. 12 A basic concept of germ cell differentiation in neoplasia was proposed by Teilum 13 who postulated an early and definitive bifurcation into separate tumor lineages, ie, seminomas, and nonseminomatous GCT (NSGCT) exemplified by EC. Today it is generally accepted that both groups originate from the primitive cells of IGCN (CIS), 2-4,14-16 which is encountered at high frequency adjacent to other germ cell tumors in adolescents and adults. 17 This concept is sustained by recent immunohistochemical and cytogenetic studies. 14,18,19 However, given the striking resemblance of seminoma to IGCN (CIS) cells, an alternative model has been proposed on the basis of morphological observations, 20-23 the relative DNA contents of the tumors, 4,24-27 and comparative cytogenetic studies, 24,28 which postulates that seminoma represents a transitional stage between IGCN (CIS) and other histological types of GCT.
Consistent with the latter idea, a strong expression of the Ki-A10 antigen was regularly detected in IGCN (CIS) and all types of germinoma, ie, seminoma, its metastases, and its histogenetically identical female counterpart, 29 dysgerminoma. Interestingly, our cases of spermatocytic seminoma also exhibited a similarly strong overall expression of the Ki-A10 antigen. On account of its preferential occurrence in older age groups, the absence of associated IGCN (CIS), its distinctive histological appearance, and its lack of metastatic potential contrasting with a strikingly high mitotic activity, SS is considered to be essentially distinct from classic seminoma. 30,31 It has even been suggested that the two entities are unrelated, which is seemingly corroborated by the lack of seminoma-specific surface antigens and the invariable PAS and PLAP negativity in SS. 32,33 The virtually identical Ki-A10 expression in both entities is nevertheless strong evidence in favor of a close histogenetic relationship. The invariably high staining intensity in the cells of SS, however, argues against a “spermatocytic” differentiation. Hence, it appears possible that SS directly arises from a transformed germ cell to become the endpoint of a putative “germinal” differentiation line. 22,34
A similarly consistent Ki-A10 reactivity was seen in the germ cell component of all gonadoblastomas although the cells stained with variable intensity. Whether gonadoblastoma is a true neoplasm or a dysgenetic malformation has been the subject of debates; however, it consists of primitive germ cells from which true germ cell neoplasms, especially germinomas but also YST and teratomas, can arise. 35-38 It has been shown recently that these germ cells are heterogeneous with respect to antigen expression and DNA ploidy, some fulfilling the criteria for IGCN (CIS) cells and others resembling normal spermatogonia. 39 This heterogeneity may account for the variations in the Ki-A10 staining intensity between individual cells in these tumors.
In YST, expression of the Ki-A10 antigen was even more heterogeneous. This peculiar pattern and the abrupt alternations of strong immunoreactivity and negativity in some specimens may be correlated to the propensity of these tumors for multiple differentiation that might go along with a change of immunophenotype and possibly with the repression of a set of genes.
Except for a portion of choriocarcinomas, in which a small number of cytotrophoblast cells were reactive with Ki-A10, all other GCT as well as gonadal tumors of other histogenetic origin and placental tissue were negative. Assuming that expression of the Ki-A10 protein in different cell types is an evolutionary continuum, our results taken together suggest a germ cell-specificity and a loss of the antigen at later stages of differentiation. Although the exclusive expression of the Ki-A10 antigen in malignant tumors and its disappearance in benign GCT is in line with this concept, its absence in EC, which has been regarded as the most undifferentiated form of germ cell neoplasia capable of further differentiation, 13,40 is intriguing. This finding might be considered as an argument in favor of the concept that EC and seminoma arise from distinct subpopulations of IGCN (CIS) cells. 15,28 However, it does not contradict the model suggesting that seminoma may represent an intermediate stage between a primitive precursor cell and other types of germ cell-derived neoplasia. Indeed, an appealing explanation is provided by the observation that NSGCT, and notably EC, have a lesser median DNA content than seminomas, 4 corresponding to gradual chromosome losses that might account for the disappearance of the Ki-A10 antigen. GCT of more than one histological type were first considered as a separate group of tumors. Still, in their different components, the immunoreactivity pattern was analogous to that of their monotypic counterparts, so it seems justified to consider them as histogenetically equivalent. Interestingly, no difference of reactivity between adults’ and infants’ tumors was observed, although DNA analyses, immunohistochemistry, and cytogenetics have provided evidence that the latter have a different histogenesis. 4,12,41 Therefore, our findings only imply a common germ cell derivation and are in line with the detection of IGCN (CIS)-associated antigens in normal fetal germ cells. 42,43 Hence, it is conceivable that germ cell tumorigenesis in adults corresponds to a delayed process of tumor promotion allowing for DNA polyploidization 27 and a transit through an in situ stage, whereas these steps may be left out in most infantile GCT. The occasional finding of IGCN (CIS) associated with infantile GCT 44-47 seems to argue in favor of this hypothesis, but the reasons for these evolutionary differences are not known.
There is no doubt that Ki-A10 staining of malignant lymphoid cells represents a true reactivity because the labeling was typically nuclear and a lymphoma cell line was used to produce the antibody. Rather, it is surprising that an expression of the Ki-A10 antigen was chiefly encountered in germ cells and their derivatives, whereas it was undetectable in somatic cells including, notably, normal lymphoid tissue. It appears, however, that malignant transformation of lymphoid cells in some instances may be accompanied by a reactivation of the gene encoding the Ki-A10 antigen. That this occurs in lymphoid malignancies rather than in other types of cancer is well conceivable in light of the observation that germ cells share with cells of the hematopoietic system specific genes required for their development and proliferation, such as c-kit 48-51 or B94, 52 and restrictively distributed antigens detectable by immunohistochemistry. 53,54
In conclusion, monoclonal antibody Ki-A10 is likely to recognize a germ cell-specific antigen that is conserved during early spermatogenesis and retained in a subset of GCT. In addition, it may occasionally be expressed in malignant transformed lymphoid cells. In GCT, its expression seems to be restricted to tumors differentiated along the germ cell lineage. In this way, our findings provide evidence for a close histogenetic relationship between SS and classic seminomas. The persistence of the antigen also implies a direct evolution from a pluripotent stem cell into YST or choriocarcinoma, possibly with a transitional stage of seminoma, 23 whereas EC apparently follows a separate pathway. Although this observation does not necessarily contradict the thesis that most GCT originate from IGCN (CIS), it is consistent with the “tetrahedron” model of germ cell tumor histogenesis proposed by Srigley and colleagues. 22 In the extraembryonal pathway of differentiation, the Ki-A10 antigen apparently exhibits the longest persistence along the “yolk sac,” and to a lesser extent along the “trophoblastic” line of differentiation, whereas the acquisition of a “somatic” phenotype (teratoma) entails an early loss of antigenicity, which may be related to nonrandom gene deletions. 55 Because it was suggested 22 that EC in turn may evolve into CC or YST, the Ki-A10 negative CC and YST in our series may have developed along this pathway.
On account of its particular immunoreactivity pattern, the antibody Ki-A10 could be exploited as a diagnostic marker in germ cell derived neoplasms. Notably, it is the first and unique marker reactive with SS, a tumor notorious for its overall immunonegativity. 31,32 Provided that the Ki-A10 antigen is further identified, it may help to gain deeper insights into the physiology of germ cell differentiation and the histogenesis and evolution of GCT.
Footnotes
Address reprint requests to Dr. Pierre Rudolph, Department of Pathology, University of Kiel, Michaelisstr. 11, 24105 Kiel, Germany. E-mail: prudolph@path.uni-kiel.de.
This work was supported by a grant from the Kinder Krebs Initiative, Buchholz Holm-Seppensen, Germany.
References
- 1.Diehl V, Kirchner HH, Schaadt M, Fonatsch C, Stein H, Gerdes J, Boie C: Hodgkin’s disease: establishment and characterization of four in vitro cell lines. J Cancer Res Clin Oncol 1981, 101:111-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skakkebaek NE: Possible carcinoma-in-situ of the testis. Lancet 1972, 2:516-517 [DOI] [PubMed] [Google Scholar]
- 3.Skakkebaek NE, Berthelsen JG, Giwercman A, Müller J: Carcinoma-in-situ of the testis: possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int J Androl 1987, 10:19-28 [DOI] [PubMed] [Google Scholar]
- 4.Oosterhuis JW, Castedo SM, de Jong B, Cornelisse CJ, Dam A, Sleijfer DT, Schraffordt Koops H: Ploidy of primary germ cell tumors of the testis: pathogenetic and clinical relevance. Lab Invest 1989, 60:14-21 [PubMed] [Google Scholar]
- 5.Damjanov I: Pathogenesis of testicular germ cell tumours. Eur Urol 1993, 23:2-5 [DOI] [PubMed] [Google Scholar]
- 6.Kellner U, Heidebrecht HJ, Rudolph P, Biersack H, Buck F, Dakowski T, Wacker HH, Domanowski M, Seidel A, Westergaard O, Parwaresch R: Detection of topoisomerase II alpha in cell lines and tissues: characterization of five novel monoclonal antibodies. J Histochem Cytochem 1997, 45:251-263 [DOI] [PubMed] [Google Scholar]
- 7.Rudolph P, Lappe T, Schubert C, Schmidt D, Parwaresch RM, Christophers E: Diagnostic assessment of two novel proliferation-specific antigens in benign and malignant melanocytic lesions. Am J Pathol 1995, 147:1615-1625 [PMC free article] [PubMed] [Google Scholar]
- 8.Anderson DJ, Blobel G: Immunoprecipitation of proteins from cell-free translations. Methods Enzymol 1983, 96:111-120 [DOI] [PubMed] [Google Scholar]
- 9.Hoog C: Expression of a large number of novel testis-specific genes during spermatogenesis coincides with the functional reorganization of the male germ cell. Int J Dev Biol 1995, 39:719-726 [PubMed] [Google Scholar]
- 10.Xu W, Cooper GM: Identification of a candidate c-mos repressor that restricts transcription of germ cell-specific genes. Mol Cell Biol 1995, 15:5369-5375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacobsen GK: Histogenetic considerations concerning germ cell tumours: morphological and immunohistochemical comparative investigation of the human embryo and testicular germ cell tumours. Virchows Arch A Pathol Anat Histopathol 1986, 408:509-525 [DOI] [PubMed] [Google Scholar]
- 12.Grigor KM, Skakkebaek NE: Pathogenesis and cell biology of germ cell neoplasia: general discussion. Eur Urol 1993, 23:46-53 [DOI] [PubMed] [Google Scholar]
- 13.Teilum G: Classification of endodermal sinus tumor (mesoblastoma vitellinum) and so called embryonal carcinoma of the ovary. Acta Pathol Microbiol Scand 1965, 64:407-429 [DOI] [PubMed] [Google Scholar]
- 14.van Echten J, van Gurp RJ, Stoepker M, Looijenga LH, de Jong J, Oosterhuis W: Cytogenetic evidence that carcinoma in situ is the precursor lesion for invasive testicular germ cell tumors. Cancer Genet Cytogenet 1995, 85:133-137 [DOI] [PubMed] [Google Scholar]
- 15.Rajpert De Meyts E, Kvist M, Skakkebaek NE: Heterogeneity of expression of immunohistochemical tumour markers in testicular carcinoma in situ: pathogenetic relevance. Virchows Arch 1996, 428:133-139 [DOI] [PubMed] [Google Scholar]
- 16.Pierce GB, Abell MR: Embryonal carcinoma of the testis. Pathol Annu 1970, 5:27-60 [PubMed] [Google Scholar]
- 17.Coffin CM, Ewing S, Dehner LP: Frequency of intratubular germ cell neoplasia with invasive testicular germ cell tumors: histologic and immunocytochemical features. Arch Pathol Lab Med 1985, 109:555-559 [PubMed] [Google Scholar]
- 18.Gregoire M, Schopperle WM, DeWolf WC: Distinct glycoforms of a tumor specific glycoprotein, gp200, in human testis and testicular tumors. J Urol 1995, 154:275-277 [PubMed] [Google Scholar]
- 19.Gillis AJ, Looijenga LH, de Jong B, Oosterhuis JW: Clonality of combined testicular germ cell tumors of adults. Lab Invest 1994, 71:874-878 [PubMed] [Google Scholar]
- 20.Walt H, Arrenbrecht S, Delozier-Blanchet CD, Keller PJ, Nauer R, Hedinger CE: A human testicular germ cell tumor with borderline histology between seminoma and embryonal carcinoma secreted beta-human chorionic gonadotropin and alpha-fetoprotein only as a xenograft [published erratum, Cancer 1986, 58: 1778]. Cancer 1986, 58:139-146 [DOI] [PubMed] [Google Scholar]
- 21.Oliver RT: HLA phenotype and clinicopathological behaviour of germ cell tumours: possible evidence for clonal evolution from seminomas to nonseminomas. Int J Androl 1987, 10:85-93 [DOI] [PubMed] [Google Scholar]
- 22.Srigley JR, Mackay B, Toth P, Ayala A: The ultrastructure and histogenesis of male germ neoplasia with emphasis on seminoma with early carcinomatous features. Ultrastruct Pathol 1988, 12:67-86 [DOI] [PubMed] [Google Scholar]
- 23.Czaja JT, Ulbright TM: Evidence for the transformation of seminoma to yolk sac tumor with histogenetic considerations. Am J Clin Pathol 1992, 97:468-477 [DOI] [PubMed] [Google Scholar]
- 24.de Jong B, Oosterhuis JW, Castedo SM, Vos A, te Meerman GJ: Pathogenesis of adult testicular germ cell tumors: a cytogenetic model. Cancer Genet Cytogenet 1990, 48:143-167 [DOI] [PubMed] [Google Scholar]
- 25.Müller J, Skakkebaek NE, Lundsteen C: Aneuploidy as a marker for carcinoma-in-situ of the testis. Acta Pathol Microbiol Scand A 1981, 89:67-68 [DOI] [PubMed] [Google Scholar]
- 26.Nistal M, Codesal J, Paniagua R: Carcinoma in situ of the testis in infertile men: a histological, immunocytochemical, and cytophotometric study of DNA content. J Pathol 1989, 159:205-210 [DOI] [PubMed] [Google Scholar]
- 27.de Graaff WE, Oosterhuis JW, de Jong B, Dam A, van Putten WL, Castedo SM, Sleijfer DT, Schraffordt Koops H: Ploidy of testicular carcinoma in situ. Lab Invest 1992, 66:166-168 [PubMed] [Google Scholar]
- 28.Oosterhuis JW, Gillis AJ, van Putten WJ, de Jong B, Looijenga LH: Interphase cytogenetics of carcinoma in situ of the testis: numeric analysis of the chromosomes 1, 12 and 15. Eur Urol 1993, 23:16-21 [DOI] [PubMed] [Google Scholar]
- 29.Gibas Z, Talerman A: Analysis of chromosome aneuploidy in ovarian dysgerminoma by flow cytometry and fluorescence in situ hybridization. Diagn Mol Pathol 1993, 2:50-56 [PubMed] [Google Scholar]
- 30.Eble JN: Spermatocytic seminoma. Hum Pathol 1994, 25:1035-1042 [DOI] [PubMed] [Google Scholar]
- 31.Ulbright TM: Germ cell neoplasms of the testis. Am J Surg Pathol 1993, 17:1075-1091 [DOI] [PubMed] [Google Scholar]
- 32.Cummings OW, Ulbright TM, Eble JN, Roth LM: Spermatocytic seminoma: an immunohistochemical study. Hum Pathol 1994, 25:54-59 [DOI] [PubMed] [Google Scholar]
- 33.Soosay GN, Bobrow L, Happerfield L, Parkinson MC: Morphology and immunohistochemistry of carcinoma in situ adjacent to testicular germ cell tumours in adults and children: implications for histogenesis. Histopathology 1991, 19:537-544 [DOI] [PubMed] [Google Scholar]
- 34.Talerman A, Fu YS, Okagaki T: Spermatocytic seminoma: ultrastructural and microspectrophotometric observations. Lab Invest 1984, 51:343-349 [PubMed] [Google Scholar]
- 35.Scully RE: Gonadoblastoma: a review of 74 cases. Cancer 1970, 25:1340-1356 [DOI] [PubMed] [Google Scholar]
- 36.Talerman A: The pathology of gonadal neoplasms composed of germ cells and sex cord stroma derivates. Pathol Res Pract 1980, 170:24-38 [DOI] [PubMed] [Google Scholar]
- 37.Hart WR, Burkons DM: Germ cell neoplasms arising in gonadoblastomas. Cancer 1979, 43:669-678 [DOI] [PubMed] [Google Scholar]
- 38.Parkash V, Carcangiu ML: Transformation of ovarian dysgerminoma to yolk sac tumor: evidence for a histogenetic continuum. Mod Pathol 1995, 8:881-887 [PubMed] [Google Scholar]
- 39.Jorgensen N, Müller J, Jaubert F, Clausen OP, Skakkebaek NE: Heterogeneity of gonadoblastoma germ cells: similarities with immature germ cells, spermatogonia and testicular carcinoma in situ cells. Histopathology 1997, 30:177-186 [DOI] [PubMed] [Google Scholar]
- 40.Hawkins EP: Pathology of germ cell tumors in children. Crit Rev Oncol Hematol 1990, 10:165-179 [DOI] [PubMed] [Google Scholar]
- 41.Summersgill B, Goker H, Weber-Hall S, Huddart R, Horwich A, Shipley J: Molecular cytogenetic analysis of adult testicular germ cell tumours and identification of regions of consensus copy number change. Br J Cancer 1998, 77:305-313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jorgensen N, Giwercman A, Müller J, Skakkebaek NE: Immunohistochemical markers of carcinoma in situ of the testis also expressed in normal infantile germ cells. Histopathology 1993, 22:373-378 [DOI] [PubMed] [Google Scholar]
- 43.Jorgensen N, Rajpert De Meyts E, Graem N, Müller J, Giwercman A, Skakkebaek NE: Expression of immunohistochemical markers for testicular carcinoma in situ by normal human fetal germ cells. Lab Invest 1995, 72:223-231 [PubMed] [Google Scholar]
- 44.Jacobsen GK, Henriques UV: A fetal testis with intratubular germ cell neoplasia (ITGCN). Mod Pathol 1992, 5:547-549 [PubMed] [Google Scholar]
- 45.Stamp IM, Jacobsen GK: Infant intratubular germ cell neoplasia. Am J Surg Pathol 1995, 19:489. [DOI] [PubMed] [Google Scholar]
- 46.Stamp IM, Barlebo H, Rix M, Jacobsen GK: Intratubular germ cell neoplasia in an infantile testis with immature teratoma. Histopathology 1993, 22:69-72 [DOI] [PubMed] [Google Scholar]
- 47.Hu LM, Phillipson J, Barsky SH: Intratubular germ cell neoplasia in infantile yolk sac tumor. Verification by tandem repeat sequence in situ hybridization. Diagn Mol Pathol 1992, 1:118-128 [PubMed] [Google Scholar]
- 48.Ratajczak MZ, Luger SM, Gewirtz AM: The c-kit proto-oncogene in normal and malignant human hematopoiesis. Int J Cell Cloning 1992, 10:205-214 [DOI] [PubMed] [Google Scholar]
- 49.Yoshinaga K, Nishikawa S, Ogawa M, Hayashi S, Kunisada T, Fujimoto T: Role of c-kit in mouse spermatogenesis: identification of spermatogonia as a specific site of c-kit expression and function. Development 1991, 113:689-699 [DOI] [PubMed] [Google Scholar]
- 50.Rolink A, Streb M, Nishikawa S, Melchers F: The c-kit-encoded tyrosine kinase regulates the proliferation of early pre-B cells. Eur J Immunol 1991, 21:2609-2612 [DOI] [PubMed] [Google Scholar]
- 51.Sorrentino V, Giorgi M, Geremia R, Besmer P, Rossi P: Expression of the c-kit proto-oncogene in the murine male germ cells. Oncogene 1991, 6:149-151 [PubMed] [Google Scholar]
- 52.Wolf FW, Sarma V, Seldin M, Drake S, Suchard SJ, Shao H, O’Shea KS, Dixit VM: B94, a primary response gene inducible by tumor necrosis factor-alpha, is expressed in developing hematopoietic tissues and the sperm acrosome. J Biol Chem 1994, 269:3633-3640 [PubMed] [Google Scholar]
- 53.Kirchhoff C: CD52 is the ‘major maturation-associated’ sperm membrane antigen. Mol Hum Reprod 1996, 2:9-17 [DOI] [PubMed] [Google Scholar]
- 54.Hutter H, Hammer A, Blaschitz A, Hartmann M, Mahnert W, Sedlmayr P, Primus G, Rosenkranz C, Gebru G, Henkel R, Dohr G: The monoclonal antibody GZS-1 detects a maturation-associated antigen of human spermatozoa that is also present on the surface of human mononuclear blood cells. J Reprod Immunol 1996, 30:115-132 [DOI] [PubMed] [Google Scholar]
- 55.Murty VV, Bosl GJ, Houldsworth J, Meyers M, Mukherjee AB, Reuter V, Chaganti RS: Allelic loss and somatic differentiation in human male germ cell tumors. Oncogene 1994, 9:2245-2251 [PubMed] [Google Scholar]


